Volume 7, Issue 1 (1-2019)
J. Pediatr. Rev 2019, 7(1): 1-10 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mohammadi G, Ghazanfarpour M, Kargarfard L, Babakhanian M. Effectiveness of Herbal Medicines Containing Phytoestrogens to Treat Infantile Colic: A Meta-analysis Review
. J. Pediatr. Rev 2019; 7 (1) :1-10
URL: http://jpr.mazums.ac.ir/article-1-165-en.html
URL: http://jpr.mazums.ac.ir/article-1-165-en.html
1- Velayat Hospital of Damghan, Semnan University of Medical Sciences, Semnan, Iran.
2- Department of Midwifery, Razi School of Nursing and Midwifery, Kerman University of Medical Sciences, Kerman, Iran. , masumeh.ghazanfarpour@yahoo.com
3- Department of Midwifery, Fatemeh School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran.
2- Department of Midwifery, Razi School of Nursing and Midwifery, Kerman University of Medical Sciences, Kerman, Iran. , masumeh.ghazanfarpour@yahoo.com
3- Department of Midwifery, Fatemeh School of Nursing and Midwifery, Shiraz University of Medical Sciences, Shiraz, Iran.
4- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran.
Full-Text [PDF 563 kb]
(3510 Downloads)
| Abstract (HTML) (8840 Views)

Full-Text: (2974 Views)
1. Context
The infantile colic, also known as baby colic, is defined as behavioral episodes of persistent and excessive crying and paroxysmal irritability (1, 2), especially during the first three months of life. It is observed in 15% –30% of infants (1). It should be noted that the problem accounts for 10%–20% of all visits in the first four months of infantile life to pediatricians (3-5). Mothers complaining of baby colic reported more depression within two and six months of postpartum (6). The infantile colic is scientifically and clinically diagnosed according to the Wessel criteria of crying for over three hours a day, three days a week, or over three weeks (6). The parental fatigue, anxiety, and even impaired parental relationship are some of the consequences of the persistent disease (7). According to previous studies, maternal (anxiety and depression during pregnancy or postnatal period), infant (personal infant’s mood, developmental milestones and microbial causes) and environmental (exposure to tobacco smoke and food hypersensitivity) factors are seriously associated with the baby colic (1, 7). There are a variety of strategies to manage the disease, including behavioral, pharmaceutical, and dietary approaches (8-12).
However due to dissatisfaction with the conventional therapeutic methods to control the infantile colic, the mothers are currently interested in herbal medicines to calm their babies colic (13). Also, herbal medicines used in the treatment of infantile colic are associated with fewer side effects. Nevertheless, the previous systematic reviews have investigated the fennel effects on the infantile colic and limited studies are available on the complications of herbal medicines among the newborns. To the best of our knowledge on the effect of herbal medicines on the infantile colic, there is an urgent need for a new systematic review and meta-analysis in this regard. Hence, the present systematic review and meta-analysis aimed at investigating the efficacy and safety of phytoestrogens.
2. Objective
The present systematic review and meta-analysis was conducted to evaluate the effect of herbal medicines in the treatment of infantile colic.
3. Data Sources
The systematic electronic search was performed on the databases of the MEDLINE, Scopus and the Cochrane Central Register Trials inception up to July 2018 to assess the effectiveness of herbal medicines in the treatment of infantile colic. No determined limitation was considered and the search keywords were colic AND infant OR baby OR newborn OR child.
The infantile colic, also known as baby colic, is defined as behavioral episodes of persistent and excessive crying and paroxysmal irritability (1, 2), especially during the first three months of life. It is observed in 15% –30% of infants (1). It should be noted that the problem accounts for 10%–20% of all visits in the first four months of infantile life to pediatricians (3-5). Mothers complaining of baby colic reported more depression within two and six months of postpartum (6). The infantile colic is scientifically and clinically diagnosed according to the Wessel criteria of crying for over three hours a day, three days a week, or over three weeks (6). The parental fatigue, anxiety, and even impaired parental relationship are some of the consequences of the persistent disease (7). According to previous studies, maternal (anxiety and depression during pregnancy or postnatal period), infant (personal infant’s mood, developmental milestones and microbial causes) and environmental (exposure to tobacco smoke and food hypersensitivity) factors are seriously associated with the baby colic (1, 7). There are a variety of strategies to manage the disease, including behavioral, pharmaceutical, and dietary approaches (8-12).
However due to dissatisfaction with the conventional therapeutic methods to control the infantile colic, the mothers are currently interested in herbal medicines to calm their babies colic (13). Also, herbal medicines used in the treatment of infantile colic are associated with fewer side effects. Nevertheless, the previous systematic reviews have investigated the fennel effects on the infantile colic and limited studies are available on the complications of herbal medicines among the newborns. To the best of our knowledge on the effect of herbal medicines on the infantile colic, there is an urgent need for a new systematic review and meta-analysis in this regard. Hence, the present systematic review and meta-analysis aimed at investigating the efficacy and safety of phytoestrogens.
2. Objective
The present systematic review and meta-analysis was conducted to evaluate the effect of herbal medicines in the treatment of infantile colic.
3. Data Sources
The systematic electronic search was performed on the databases of the MEDLINE, Scopus and the Cochrane Central Register Trials inception up to July 2018 to assess the effectiveness of herbal medicines in the treatment of infantile colic. No determined limitation was considered and the search keywords were colic AND infant OR baby OR newborn OR child.


3.1. The inclusion criteria
1- Randomized Controlled Trials (RCTs) comparing oral red clover in either alone or combined prepared compounds with the placebo; and 2- Available information on at least one of the psychological symptoms.
4. Study Selection
Six studies assessed the effect of herbal medicine on colic infantile. Figure 1 shows the process of selection of articles for this systematic review.
1- Randomized Controlled Trials (RCTs) comparing oral red clover in either alone or combined prepared compounds with the placebo; and 2- Available information on at least one of the psychological symptoms.
4. Study Selection
Six studies assessed the effect of herbal medicine on colic infantile. Figure 1 shows the process of selection of articles for this systematic review.
5. Data Extraction
The data extraction and quality assessment of the trials were performed by two separate individuals based on standardized predefined checklist, including first author, year of publication, countries, sample size, duration of treatment, dosage and outcome values for both the intervention and the control groups with the mean and standard deviation of pre- and post-treatment or mean difference from the baseline.
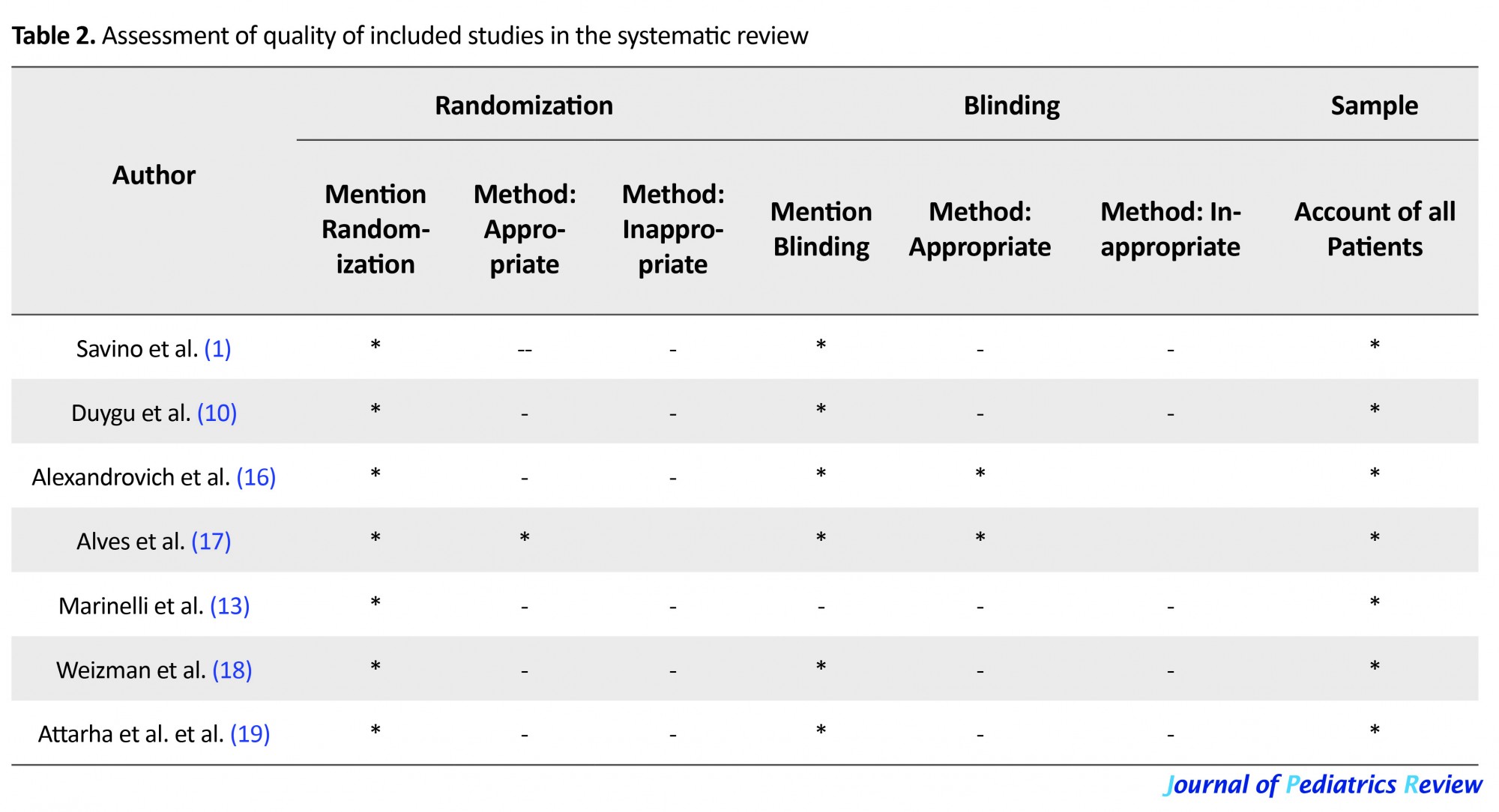
5.1. Quality assessment of the included studies
Two reviewers using Jade scale (14) (Table 2) independently rated the quality of RCTs. We added some other important items from Oxford Center for Evidence-based Medicine checklist for therapeutic studies to scale Jade, namely comparability of the treatment and control groups, Intention-To-Treat reporting (ITT), and suitable randomization technique (15).
The data extraction and quality assessment of the trials were performed by two separate individuals based on standardized predefined checklist, including first author, year of publication, countries, sample size, duration of treatment, dosage and outcome values for both the intervention and the control groups with the mean and standard deviation of pre- and post-treatment or mean difference from the baseline.
5.1. Quality assessment of the included studies
Two reviewers using Jade scale (14) (Table 2) independently rated the quality of RCTs. We added some other important items from Oxford Center for Evidence-based Medicine checklist for therapeutic studies to scale Jade, namely comparability of the treatment and control groups, Intention-To-Treat reporting (ITT), and suitable randomization technique (15).
5.2. Standardized Mean Difference (SMD)
The SMD of each study was calculated as the main effect in our analysis. The result of trials had a high heterogeneity. Random effect model (DerSimonian and Laird method) was used to estimate the pooled effect size of the herbal medicine of fennel on the duration of crying time. Cochrane Q test was applied to test the heterogeneity of the studies included in the meta-analysis (P<0.05 as statistically significance level). The I2 index showed the extent to which we could rely on variance across studies, excluding sampling errors. All statistical analyses were carried out by Comprehensive Meta-analysis version 2 (Biostat, Englewood, NJ, USA). Sensitive analysis was done to exclude outlier trials when there was a high heterogeneity among the trials.
6. Results
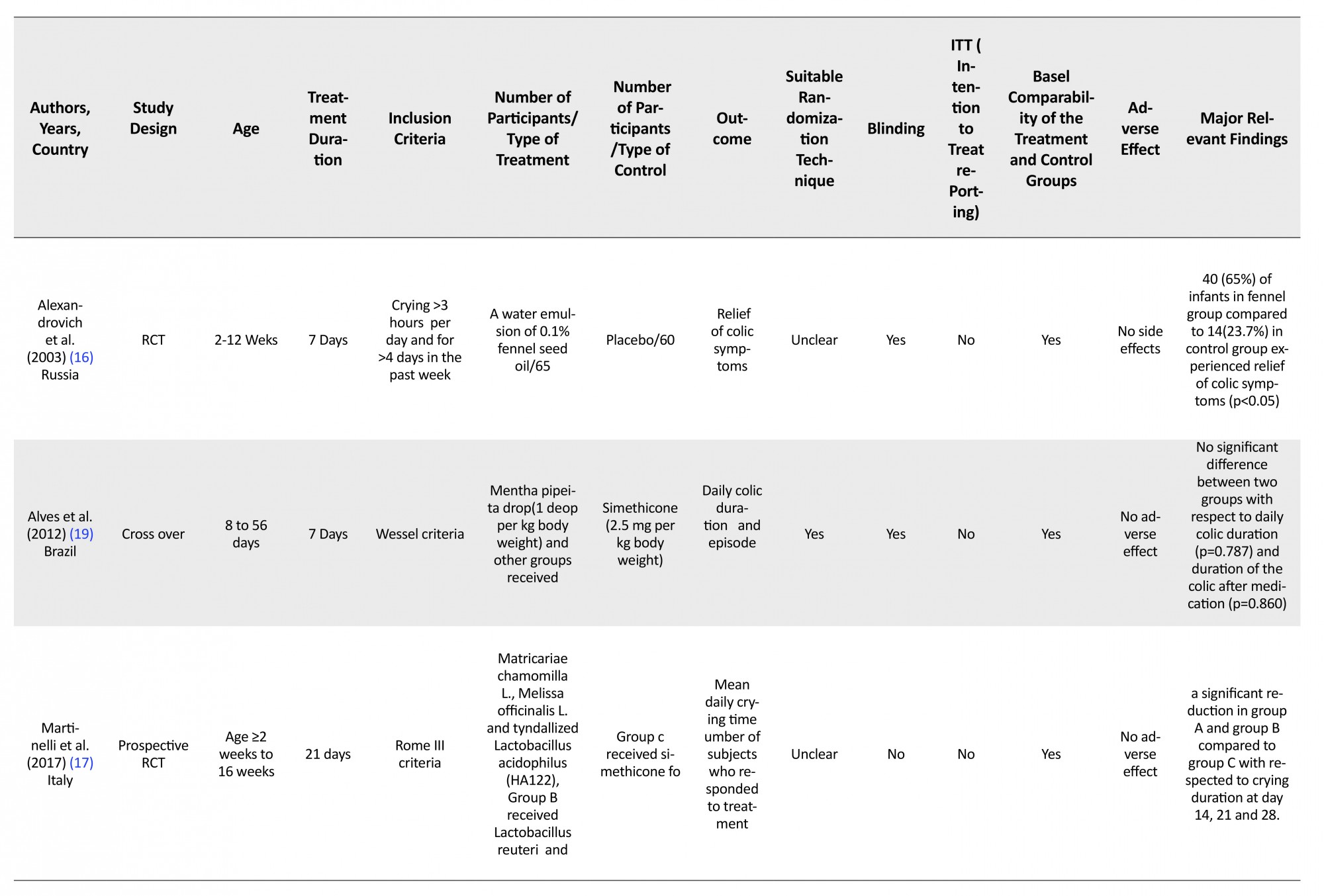
Savino et al. (1) conducted a prospective, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy of a phytotherapeutic agent containing Matricariae recutita, Foeniculum vulgare and Melissa officinalis in the treatment of infantile colic for 21 days (3 days before the treatment, 7 days during the treatment, 11 days after the treatment). After a 3-day observation period without treatment, 93 infants who met modified Wessel criteria were randomized into two groups: the intervention group (n=41) received a phytotherapeutic agent and placebo group (n=47). The average crying duration was similar at day -1 (3 days before starting intervention) and day 0 (start of treatment) was similar. Gradual decrease in the average crying times per day was observed in the intervention group (199.1, 204.5, 201.2, 182.1, 145.5, 112.4, 99.6, 86.3, 76.9, 82.1) and placebo group (198.1, 199.8, 198.7, 176.7, 153.7, 154.3, 157.5, 161.4, 168.6, 169.9 and 165.3) for 10-time point’s day -2 (P=0.790), -1 (P=0.226), 0 (P=0.507), 1 (P=0.189), 2 (P=0.079), 3 (P<0.005), 4 (P<0.005), 5 (P<0.005), 6 (P<0.005), 7 (P<0.005), 21 (P<0.005) days, respectively. Crying time was significantly reduced in 85.4% infants in the intervention group compared to 48.9% infants in placebo group (P<0.005).
Alexandrovich et al. (16), in a randomized, double-blinded, placebo-controlled trial, assessed the efficacy of fennel on infantile colic. Diagnosis was made according to the Wessel criteria. This study included 125 infants with colic aged 2 to 12 weeks. The intervention group (n=65) received either a water emulsion of 0.1% fennel seed oil or 0.4% polysorbate 80 and the control group (n=60) received a placebo containing 0.4% polylobate in water. Two groups were similar in terms of age, gender, birth number, the feeding method, and house crying. Parents were asked to complete diary for 21 days (7 days before the treatment, 7 days during the treatment, 7 days after the treatment). Forty (65%) infants in fennel group experienced relief from colic symptoms as compared to 14 (23.7%) in the control group (P<0.05). Cumulative crying time at the end of treatment decreased from 13.5±1.18 to 8.8±1 hours/week in the treatment group and 12.9±1.6 to 12.3±1.5 hours/week in placebo group. Comparison of two groups was significant (P<0.05).Duygu et al. performed a randomized, five arms, and double-blinded clinical trial (10). The five arms were as follows: massage, i.e. chiropractic spinal manipulation (n=35), sucrose (n=35), herbal tea (n=35), formula (n=35), and control group (n=35). Data were analyzed by analysis of variance (ANOVA) followed by Dunnett’s t test. In herbal tea group, the parents were instructed to give 35 mL of fennel tea (maximum dose of 150 mL) to their infants. Duration of crying time decreased significantly from 5.11±1.43 to 3.20±1.23 in herbal tea (t=8.78, P<0.001), but insignificantly decreased from 4.60±1.40 to 4.51±1.50 in control group (t=1.78, P>0.05). ANOVA test showed a significant difference among these five groups with respect to the duration of crying. Dunnett’s t test revealed a significant difference between the control and herbal tea group (P<0.01).
Alves et al. in a crossover, double-blinded, and placebo-controlled trial, investigated 30 infants aged 8 to 56 days (33±11.1 d) who met the Wessel criteria (17). They were randomized into two groups: the intervention group received leaves of the Mentha pipeita drop (1 drop/kg body weight) and other group received simethicone (2.5 mg/kg body weight) daily for a 14-days period. After 7 days washout, the groups were switched for another 7 days. According to Fisher’s exact test results, no statistical difference was found between the groups regarding daily colic episodes. The Mann-Whitney test showed no significant difference between two groups with respect to daily colic duration (P=0.787) and duration of the colic after medication (P=0.860).
Martinelli et al. performed a prospective, multicenter, open-label, randomized, and controlled trial (13). Diagnosis was made according to the Rome III criteria. Hundred and eighty infants were randomized into one of three groups as follows: group A (n=60) received a standardized extract of Matricariae chamomilla L., Melissa officinalis L. and tyndallized Lactobacillus acidophilus (HA122); Group B (n=60) received Lactobacillus reuteri; and Group C (n=60) received simethicone for 28 days. Response to treatment was considered as a 50% reduction in the daily average crying time. Follow-up visits were performed at day 7, 14, 21, and 28. There was a significant reduction in crying time during day 14, 21, and 28 in group A and group B compared to group C. The only difference between group A and group C was significant from day 7 while difference between group B and group C was significant from day 14. No significant difference was seen between group A and group B.
Weizman et al. performed a prospective, randomized, double-blinded, and placebo-controlled study (18). In this study, 72 infants aged 2-8 weeks were randomized into two groups: intervention group (n=36) that received herbal tea containing Verbena officinalis fennel (Foenieulum vulgare), licorice (glycyrrhiza glabra), chamomile (Matricaria chamomilla), and balm-mint (Melissa officinalis), and another group received placebo (n=36). Follow-up visits were performed at day -7, 0, and 7. After the 7 days of treatment, the colic improvement score showed a significant improvement in the intervention group (1.7±0.3) compared to the control group (0.7±0.5) (P<0.05). A total of 19 (57%) infants in herbal tea compared to 9 (26%) in placebo group reported to be colic free (P<0.01). Comparison of herbal tea group (1.9±0.9) and placebo tea (2.2±0.6) regarding the number of night waking times was not significant.
6.1. Comparison of efficacy of fennel and gripe in treatment of infantile colic
Attarha et al. compared the effect of fennel extract and gripe water syrup in a randomized clinical trial. In this study, 80 infants were divided into two groups: fennel extract (n=40) and gripe (n=40) (19). Gripe water syrup contains sodium bicarbonate and varing types of herbs (Anethum graveolens and fennel). Main outcome was duration of crying (hours per days) at day 3 and 7 after the treatment. Time of crying was categorized into three groups as follows: 1) less than 60 minutes, 2) 60-120 minutes, and 3) more than 120 minutes. Both fennel (P=0.004) and gripe water syrup (P=0.005) groups revealed a statistically significant decrease. Intragroup comparison before and after using paired t test showed that duration of crying dropped significantly at day 3 and 7 in both group fennel (P=0.004) and gripe water syrup (P=0.005). However, comparision of two groups were insignificant at all times.
6.2. Meta–analysis
Five trials assessed the effect of herbal preparation containing phytoestrogens on infantail colic. Measured outcomes included duration of crying, the colic improvement score, and the number of night waking times. Four trials (1, 10, 13, 16) assessed the duration of crying. A preparation containing phytoestrogens decreased the duration of crying compared to placebo (-0.536; 95% CI=-0.848 to 0.224; P=0.001; Figure 2). Heterogeneity was moderate but not statistically significant (I2=56%; P=0.07). Also, one study measured colic improvement score and the number of night waking times and showed a significant decrease in the parameters. To sum up, phytoestrogens can significantly improve infantile colic. Three trials (10, 16, 17) assessed the effect of the preparation containing fennel. A significant improvement regarding duration of crying was observed compared to the control group (SMD=0.712; 95% CI=-1.005 to -0.420; P<0.001). Heterogeneity was 0% among studies. SMD of mono preparation containing fennel reduced duration of crying less than -0.6 (95% CI=-0.940 to -0.456; P<0.001; Figure 3). Heterogeneity was moderate but not statistically significant (I2=0%; P=0.669).
The SMD of each study was calculated as the main effect in our analysis. The result of trials had a high heterogeneity. Random effect model (DerSimonian and Laird method) was used to estimate the pooled effect size of the herbal medicine of fennel on the duration of crying time. Cochrane Q test was applied to test the heterogeneity of the studies included in the meta-analysis (P<0.05 as statistically significance level). The I2 index showed the extent to which we could rely on variance across studies, excluding sampling errors. All statistical analyses were carried out by Comprehensive Meta-analysis version 2 (Biostat, Englewood, NJ, USA). Sensitive analysis was done to exclude outlier trials when there was a high heterogeneity among the trials.
6. Results
Savino et al. (1) conducted a prospective, randomized, double-blinded, placebo-controlled trial to evaluate the efficacy of a phytotherapeutic agent containing Matricariae recutita, Foeniculum vulgare and Melissa officinalis in the treatment of infantile colic for 21 days (3 days before the treatment, 7 days during the treatment, 11 days after the treatment). After a 3-day observation period without treatment, 93 infants who met modified Wessel criteria were randomized into two groups: the intervention group (n=41) received a phytotherapeutic agent and placebo group (n=47). The average crying duration was similar at day -1 (3 days before starting intervention) and day 0 (start of treatment) was similar. Gradual decrease in the average crying times per day was observed in the intervention group (199.1, 204.5, 201.2, 182.1, 145.5, 112.4, 99.6, 86.3, 76.9, 82.1) and placebo group (198.1, 199.8, 198.7, 176.7, 153.7, 154.3, 157.5, 161.4, 168.6, 169.9 and 165.3) for 10-time point’s day -2 (P=0.790), -1 (P=0.226), 0 (P=0.507), 1 (P=0.189), 2 (P=0.079), 3 (P<0.005), 4 (P<0.005), 5 (P<0.005), 6 (P<0.005), 7 (P<0.005), 21 (P<0.005) days, respectively. Crying time was significantly reduced in 85.4% infants in the intervention group compared to 48.9% infants in placebo group (P<0.005).
Alexandrovich et al. (16), in a randomized, double-blinded, placebo-controlled trial, assessed the efficacy of fennel on infantile colic. Diagnosis was made according to the Wessel criteria. This study included 125 infants with colic aged 2 to 12 weeks. The intervention group (n=65) received either a water emulsion of 0.1% fennel seed oil or 0.4% polysorbate 80 and the control group (n=60) received a placebo containing 0.4% polylobate in water. Two groups were similar in terms of age, gender, birth number, the feeding method, and house crying. Parents were asked to complete diary for 21 days (7 days before the treatment, 7 days during the treatment, 7 days after the treatment). Forty (65%) infants in fennel group experienced relief from colic symptoms as compared to 14 (23.7%) in the control group (P<0.05). Cumulative crying time at the end of treatment decreased from 13.5±1.18 to 8.8±1 hours/week in the treatment group and 12.9±1.6 to 12.3±1.5 hours/week in placebo group. Comparison of two groups was significant (P<0.05).Duygu et al. performed a randomized, five arms, and double-blinded clinical trial (10). The five arms were as follows: massage, i.e. chiropractic spinal manipulation (n=35), sucrose (n=35), herbal tea (n=35), formula (n=35), and control group (n=35). Data were analyzed by analysis of variance (ANOVA) followed by Dunnett’s t test. In herbal tea group, the parents were instructed to give 35 mL of fennel tea (maximum dose of 150 mL) to their infants. Duration of crying time decreased significantly from 5.11±1.43 to 3.20±1.23 in herbal tea (t=8.78, P<0.001), but insignificantly decreased from 4.60±1.40 to 4.51±1.50 in control group (t=1.78, P>0.05). ANOVA test showed a significant difference among these five groups with respect to the duration of crying. Dunnett’s t test revealed a significant difference between the control and herbal tea group (P<0.01).
Alves et al. in a crossover, double-blinded, and placebo-controlled trial, investigated 30 infants aged 8 to 56 days (33±11.1 d) who met the Wessel criteria (17). They were randomized into two groups: the intervention group received leaves of the Mentha pipeita drop (1 drop/kg body weight) and other group received simethicone (2.5 mg/kg body weight) daily for a 14-days period. After 7 days washout, the groups were switched for another 7 days. According to Fisher’s exact test results, no statistical difference was found between the groups regarding daily colic episodes. The Mann-Whitney test showed no significant difference between two groups with respect to daily colic duration (P=0.787) and duration of the colic after medication (P=0.860).
Martinelli et al. performed a prospective, multicenter, open-label, randomized, and controlled trial (13). Diagnosis was made according to the Rome III criteria. Hundred and eighty infants were randomized into one of three groups as follows: group A (n=60) received a standardized extract of Matricariae chamomilla L., Melissa officinalis L. and tyndallized Lactobacillus acidophilus (HA122); Group B (n=60) received Lactobacillus reuteri; and Group C (n=60) received simethicone for 28 days. Response to treatment was considered as a 50% reduction in the daily average crying time. Follow-up visits were performed at day 7, 14, 21, and 28. There was a significant reduction in crying time during day 14, 21, and 28 in group A and group B compared to group C. The only difference between group A and group C was significant from day 7 while difference between group B and group C was significant from day 14. No significant difference was seen between group A and group B.
Weizman et al. performed a prospective, randomized, double-blinded, and placebo-controlled study (18). In this study, 72 infants aged 2-8 weeks were randomized into two groups: intervention group (n=36) that received herbal tea containing Verbena officinalis fennel (Foenieulum vulgare), licorice (glycyrrhiza glabra), chamomile (Matricaria chamomilla), and balm-mint (Melissa officinalis), and another group received placebo (n=36). Follow-up visits were performed at day -7, 0, and 7. After the 7 days of treatment, the colic improvement score showed a significant improvement in the intervention group (1.7±0.3) compared to the control group (0.7±0.5) (P<0.05). A total of 19 (57%) infants in herbal tea compared to 9 (26%) in placebo group reported to be colic free (P<0.01). Comparison of herbal tea group (1.9±0.9) and placebo tea (2.2±0.6) regarding the number of night waking times was not significant.
6.1. Comparison of efficacy of fennel and gripe in treatment of infantile colic
Attarha et al. compared the effect of fennel extract and gripe water syrup in a randomized clinical trial. In this study, 80 infants were divided into two groups: fennel extract (n=40) and gripe (n=40) (19). Gripe water syrup contains sodium bicarbonate and varing types of herbs (Anethum graveolens and fennel). Main outcome was duration of crying (hours per days) at day 3 and 7 after the treatment. Time of crying was categorized into three groups as follows: 1) less than 60 minutes, 2) 60-120 minutes, and 3) more than 120 minutes. Both fennel (P=0.004) and gripe water syrup (P=0.005) groups revealed a statistically significant decrease. Intragroup comparison before and after using paired t test showed that duration of crying dropped significantly at day 3 and 7 in both group fennel (P=0.004) and gripe water syrup (P=0.005). However, comparision of two groups were insignificant at all times.
6.2. Meta–analysis
Five trials assessed the effect of herbal preparation containing phytoestrogens on infantail colic. Measured outcomes included duration of crying, the colic improvement score, and the number of night waking times. Four trials (1, 10, 13, 16) assessed the duration of crying. A preparation containing phytoestrogens decreased the duration of crying compared to placebo (-0.536; 95% CI=-0.848 to 0.224; P=0.001; Figure 2). Heterogeneity was moderate but not statistically significant (I2=56%; P=0.07). Also, one study measured colic improvement score and the number of night waking times and showed a significant decrease in the parameters. To sum up, phytoestrogens can significantly improve infantile colic. Three trials (10, 16, 17) assessed the effect of the preparation containing fennel. A significant improvement regarding duration of crying was observed compared to the control group (SMD=0.712; 95% CI=-1.005 to -0.420; P<0.001). Heterogeneity was 0% among studies. SMD of mono preparation containing fennel reduced duration of crying less than -0.6 (95% CI=-0.940 to -0.456; P<0.001; Figure 3). Heterogeneity was moderate but not statistically significant (I2=0%; P=0.669).
6.3. Assessment of side effect of phytomedine on infantile colic
One trial did not assess the safety of treatment and rest of studies (five) included in the systematic review reported no side effects for phytoestrogens.
7. Discussion
The results of the current systematic review showed that one week of treatment significantly reduced the duration of crying time in the infants receiving the fennel and the compound containing phytoestrogens as compared to the control group. According to a trial, Mentha piperita was more efficient than simethicone in reducing daily colic duration. Almost all trials included in the systematic review reported no side effects.
7.1. Comparison of the current systematic review with pervious reviews
A systematic review with 17 studies was recently performed on the effect of intervention on infantile colic. A subgroup analysis was conducted among the patients who received the fennel. The pooled mean difference in the crying time decreased significantly to less than 72.1 min/d (7). A systematic review published in 2010 showed that the fennel alone and in combination with herbal tea had beneficial effects on attenuating the infantile colic (20).
Firstly, some studies relied on parents’ memory instead of use of diary. The colic rate was recorded to be 2.5 when researchers used the diary for resisting colic symptom compared to the parent’s rememberance (21). Some of studies reported no compliance to treatment. There are several limitations in the systematic review that should be addressed, including small sample size and relatively short-term treatment duration, insufficient explanation, randomization method and blinding, use of polyhedral preparation, and lack of Intent-To-Treatment (ITT) analysis. Considering the above-mentioned limitations, there is a need to conduct well-designed trials to address these subjects. Given the fact that the infantile colic can be improved with time and several factors such as environmental, emotional and maternal habits may affect it, future trials should have double-blinded crossover design to control these conditions.
Some studies in our systematic review showed a high placebo response. Future trials should regard at least one of several methods to manage the conditions. The infants with high placebo response (more than 30% reduction in colic score) should be excluded from trials or replaced by other infants. Other methods include the use of run-in period design, longer treatment duration, and the use of sequential parallel design.
8. Conclusions
According to the results, the fennel, Mentha piperita, and phytoestrogen-containing compounds are useful and safe to alleviate the infantile colic. Moreover, further trials with larger sample size and longer treatment duration are needed to draw definite conclusions.
Ethical Considerations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
References
One trial did not assess the safety of treatment and rest of studies (five) included in the systematic review reported no side effects for phytoestrogens.
7. Discussion
The results of the current systematic review showed that one week of treatment significantly reduced the duration of crying time in the infants receiving the fennel and the compound containing phytoestrogens as compared to the control group. According to a trial, Mentha piperita was more efficient than simethicone in reducing daily colic duration. Almost all trials included in the systematic review reported no side effects.
7.1. Comparison of the current systematic review with pervious reviews
A systematic review with 17 studies was recently performed on the effect of intervention on infantile colic. A subgroup analysis was conducted among the patients who received the fennel. The pooled mean difference in the crying time decreased significantly to less than 72.1 min/d (7). A systematic review published in 2010 showed that the fennel alone and in combination with herbal tea had beneficial effects on attenuating the infantile colic (20).
Firstly, some studies relied on parents’ memory instead of use of diary. The colic rate was recorded to be 2.5 when researchers used the diary for resisting colic symptom compared to the parent’s rememberance (21). Some of studies reported no compliance to treatment. There are several limitations in the systematic review that should be addressed, including small sample size and relatively short-term treatment duration, insufficient explanation, randomization method and blinding, use of polyhedral preparation, and lack of Intent-To-Treatment (ITT) analysis. Considering the above-mentioned limitations, there is a need to conduct well-designed trials to address these subjects. Given the fact that the infantile colic can be improved with time and several factors such as environmental, emotional and maternal habits may affect it, future trials should have double-blinded crossover design to control these conditions.
Some studies in our systematic review showed a high placebo response. Future trials should regard at least one of several methods to manage the conditions. The infants with high placebo response (more than 30% reduction in colic score) should be excluded from trials or replaced by other infants. Other methods include the use of run-in period design, longer treatment duration, and the use of sequential parallel design.
8. Conclusions
According to the results, the fennel, Mentha piperita, and phytoestrogen-containing compounds are useful and safe to alleviate the infantile colic. Moreover, further trials with larger sample size and longer treatment duration are needed to draw definite conclusions.
Ethical Considerations
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declared no conflict of interest.
References
- Savino F, Cresi F, Castagno E, Silvestro L, Oggero R. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil®) in the treatment of breastfed colicky infants. Phytotherapy Research. 2005; 19(4):335-40. [DOI:10.1002/ptr.1668]
- Engler AC, Hadash A, Shehadeh N, Pillar G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. European Journal of Pediatrics. 2012; 171(4):729-32. [DOI:10.1007/s00431-011-1659-3]
- Iacovou M, Ralston RA, Muir J, Walker KZ, Truby H. Dietary management of infantile colic: A systematic review. Maternal and Child Health Journal. 2012; 16(6):1319-31. [DOI:10.1007/s10995-011-0842-5]
- Brazelton TB. Crying in infancy. Pediatrics. 1962; 29(4):579-88. [PMID]
- Lucassen P, Assendelft W, van Eijk JTM, Gubbels J, Douwes A, Van Geldrop W. Systematic review of the occurrence of infantile colic in the community. Archives of Disease in Childhood. 2001; 84(5):398-403. [DOI:10.1136/adc.84.5.398]
- Reust CE, Blake Jr RL. Diagnostic workup before diagnosing colic. Archives of Family Medicine. 2000; 9(3):282-3. [DOI:10.1001/archfami.9.3.282]
- Harb T, Matsuyama M, David M, Hill RJ. Infant Colic—what works: a systematic review of interventions for breast-fed infants. Journal of Pediatric Gastroenterology and Nutrition. 2016; 62(5):668-86. [DOI:10.1097/MPG.0000000000001075]
- Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics. 2000; 106(Supplement 1):184-90. [PMID]
- Lucassen P, Assendelft W, Gubbels J, van Eijk J, Van Geldrop W, Neven AK. Effectiveness of treatments for infantile colic: Systematic review. BMJ. 1998; 316(7144):1563-8. [DOI:10.1136/bmj.316.7144.1563]
- Duygu A, Handan A, Gözüm S, Orbak Z, Karaca Çifçi E. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing. 2008; 17(13):1754-61. [DOI:10.1111/j.1365-2702.2007.02093.x]
- Blumenthal I. The gripe water story. Journal of the Royal Society of Medicine. 2000; 93(4):172-4. [DOI:10.1177/014107680009300404]
- Forsyth BW. Colic and the effect of changing formulas: A double-blind, multiple-crossover study. The Journal of Pediatrics. 1989; 115(4):521-6. [DOI:10.1016/S0022-3476(89)80274-4]
- Martinelli M, Ummarino D, Giugliano F, Sciorio E, Tortora C, Bruzzese D, et al. Efficacy of a standardized extract of Matricariae chamomilla L., Melissa officinalis L. and tyndallized Lactobacillus acidophilus (HA122) in infantile colic: An open randomized controlled trial. Neurogastroenterology & Motility. 2017; 29(12):e13145. [DOI:10.1111/nmo.13145]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996; 17(1):1-12. [DOI:10.1016/0197-2456(95)00134-4]
- Center for Evidence Based Medicine. Critical Appraisal tools. Oxford: Center for Evidence Based Medicine; 2018.
- Alexandrovich I, Rakovitskaya O, Kolmo E, Sidorova T, Shushunov S. The effect of fennel (Foeniculum vulgare) seed oil emulsion in infantile colic: A randomized, placebo-controlled study. Alternative Therapies in Health and Medicine. 2003; 9(4):58-61. [PMID]
- Alves JGB, de Brito RdCCM, Cavalcanti TS. Effectiveness of Mentha piperita in the treatment of infantile colic: a crossover study. Evidence-Based Complementary and Alternative Medicine. 2012; 2012:1–4. [DOI:10.1155/2012/981352]
- Weizman Z, Alkrinawi S, Goldfarb D, Bitran C. Efficacy of herbal tea preparation in infantile colic. The Journal of Pediatrics. 1993; 122(4):650-2. [DOI:10.1016/S0022-3476(05)83557-7]
- Attarha M, Rosbahani N, Youssefi P. [Comparison of the effect of fennel essence and gripe water syrup in infantile colic (Persian)]. Scientific Journal of Kurdistan University of Medical Sciences. 2008; 13(1):28-35.
- Perry R, Hunt K, Ernst E. Nutritional supplements and other complementary medicines for infantile colic: a systematic review. Pediatrics. 2011; 127(4):720-33. [DOI:10.1542/peds.2010-2098]
- Canivet C, Hagander B, Jakobsson I, Lanke J. Infantile colic—less common than previously estimated? Acta Paediatrica. 1996; 85(4):454-8. [DOI:10.1111/j.1651-2227.1996.tb14060.x]
Type of Study: Meta-analysis Review |
Received: 2017/10/17 | Accepted: 2018/03/13 | Published: 2019/01/1
Received: 2017/10/17 | Accepted: 2018/03/13 | Published: 2019/01/1
References
1. Savino F, Cresi F, Castagno E, Silvestro L, Oggero R. A randomized double-blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil®) in the treatment of breastfed colicky infants. Phytotherapy Research. 2005; 19(4):335-40. [DOI:10.1002/ptr.1668] [DOI:10.1002/ptr.1668]
2. Engler AC, Hadash A, Shehadeh N, Pillar G. Breastfeeding may improve nocturnal sleep and reduce infantile colic: Potential role of breast milk melatonin. European Journal of Pediatrics. 2012; 171(4):729-32. [DOI:10.1007/s00431-011-1659-3] [DOI:10.1007/s00431-011-1659-3]
3. Iacovou M, Ralston RA, Muir J, Walker KZ, Truby H. Dietary management of infantile colic: A systematic review. Maternal and Child Health Journal. 2012; 16(6):1319-31. [DOI:10.1007/s10995-011-0842-5] [DOI:10.1007/s10995-011-0842-5]
4. Brazelton TB. Crying in infancy. Pediatrics. 1962; 29(4):579-88. [PMID] [PMID]
5. Lucassen P, Assendelft W, van Eijk JTM, Gubbels J, Douwes A, Van Geldrop W. Systematic review of the occurrence of infantile colic in the community. Archives of Disease in Childhood. 2001; 84(5):398-403. [DOI:10.1136/adc.84.5.398] [DOI:10.1136/adc.84.5.398]
6. Reust CE, Blake Jr RL. Diagnostic workup before diagnosing colic. Archives of Family Medicine. 2000; 9(3):282-3. [DOI:10.1001/archfami.9.3.282] [DOI:10.1001/archfami.9.3.282]
7. Harb T, Matsuyama M, David M, Hill RJ. Infant Colic—what works: a systematic review of interventions for breast-fed infants. Journal of Pediatric Gastroenterology and Nutrition. 2016; 62(5):668-86. [DOI:10.1097/MPG.0000000000001075] [DOI:10.1097/MPG.0000000000001075]
8. Garrison MM, Christakis DA. A systematic review of treatments for infant colic. Pediatrics. 2000; 106(Supplement 1):184-90. [PMID] [PMID]
9. Lucassen P, Assendelft W, Gubbels J, van Eijk J, Van Geldrop W, Neven AK. Effectiveness of treatments for infantile colic: Systematic review. BMJ. 1998; 316(7144):1563-8. [DOI:10.1136/bmj.316.7144.1563] [DOI:10.1136/bmj.316.7144.1563]
10. Duygu A, Handan A, Gözüm S, Orbak Z, Karaca Çifçi E. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. Journal of Clinical Nursing. 2008; 17(13):1754-61. [DOI:10.1111/j.1365-2702.2007.02093.x] [DOI:10.1111/j.1365-2702.2007.02093.x]
11. Blumenthal I. The gripe water story. Journal of the Royal Society of Medicine. 2000; 93(4):172-4. [DOI:10.1177/014107680009300404] [DOI:10.1177/014107680009300404]
12. Forsyth BW. Colic and the effect of changing formulas: A double-blind, multiple-crossover study. The Journal of Pediatrics. 1989; 115(4):521-6. [DOI:10.1016/S0022-3476(89)80274-4] [DOI:10.1016/S0022-3476(89)80274-4]
13. Martinelli M, Ummarino D, Giugliano F, Sciorio E, Tortora C, Bruzzese D, et al. Efficacy of a standardized extract of Matricariae chamomilla L., Melissa officinalis L. and tyndallized Lactobacillus acidophilus (HA122) in infantile colic: An open randomized controlled trial. Neurogastroenterology & Motility. 2017; 29(12):e13145. [DOI:10.1111/nmo.13145] [DOI:10.1111/nmo.13145]
14. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials. 1996; 17(1):1-12. [DOI:10.1016/0197-2456(95)00134-4] [DOI:10.1016/0197-2456(95)00134-4]
15. Center for Evidence Based Medicine. Critical Appraisal tools. Oxford: Center for Evidence Based Medicine; 2018.
16. Alexandrovich I, Rakovitskaya O, Kolmo E, Sidorova T, Shushunov S. The effect of fennel (Foeniculum vulgare) seed oil emulsion in infantile colic: A randomized, placebo-controlled study. Alternative Therapies in Health and Medicine. 2003; 9(4):58-61. [PMID] [PMID]
17. Alves JGB, de Brito RdCCM, Cavalcanti TS. Effectiveness of Mentha piperita in the treatment of infantile colic: a crossover study. Evidence-Based Complementary and Alternative Medicine. 2012; 2012:1–4. [DOI:10.1155/2012/981352] [DOI:10.1155/2012/981352]
18. Weizman Z, Alkrinawi S, Goldfarb D, Bitran C. Efficacy of herbal tea preparation in infantile colic. The Journal of Pediatrics. 1993; 122(4):650-2. [DOI:10.1016/S0022-3476(05)83557-7] [DOI:10.1016/S0022-3476(05)83557-7]
19. Attarha M, Rosbahani N, Youssefi P. [Comparison of the effect of fennel essence and gripe water syrup in infantile colic (Persian)]. Scientific Journal of Kurdistan University of Medical Sciences. 2008; 13(1):28-35.
20. Perry R, Hunt K, Ernst E. Nutritional supplements and other complementary medicines for infantile colic: a systematic review. Pediatrics. 2011; 127(4):720-33. [DOI:10.1542/peds.2010-2098] [DOI:10.1542/peds.2010-2098]
21. Canivet C, Hagander B, Jakobsson I, Lanke J. Infantile colic—less common than previously estimated? Acta Paediatrica. 1996; 85(4):454-8. [DOI:10.1111/j.1651-2227.1996.tb14060.x] [DOI:10.1111/j.1651-2227.1996.tb14060.x]
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |