Volume 7, Issue 4 (10-2019)
J. Pediatr. Rev 2019, 7(4): 229-238 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rodriguez-Gonzalez M, Matamala-Morillo M, Castellano-Martinez A. Sudden Cardiac Death as the First Clinical Manifestation in Infants With Asymptomatic Ventricular Pre-excitation: Two Case Reports and Review of the Literature. J. Pediatr. Rev 2019; 7 (4) :229-238
URL: http://jpr.mazums.ac.ir/article-1-186-en.html
URL: http://jpr.mazums.ac.ir/article-1-186-en.html
1- Pediatric Cardiology Department of Puerta, del Mar Universitary Hospital, Cadiz, Spain. , moirogo@gmail.com
2- Pediatric Cardiology Department of Puerta, del Mar Universitary Hospital, Cadiz, Spain.
2- Pediatric Cardiology Department of Puerta, del Mar Universitary Hospital, Cadiz, Spain.
Full-Text [PDF 2052 kb]
(2249 Downloads)
| Abstract (HTML) (4612 Views)
No treatment was initiated and a close clinical and ECG follow-up was scheduled. He did well until six months of age. Parents reported that he was crying vigorously at home and suddenly stopped breathing and became unresponsive. They started basic Cardiopulmonary Resuscitation (CPR) while waiting for the emergency services that found him collapsed and continued with advanced CPR. Unfortunately, the CPR was unsuccessful, and he died on the way to the hospital. No ECG was recorded, but we believe that the most probable cause of death was VF.
2.2. Case 2
An 8-year-old previously healthy female consulted our Pediatric Cardiology Clinic for self-limited episodes of palpitations. Family history was negative. Her personal account revealed a sudden infant death episode at four months old. Her parents reported that hearing throaty sounds, they went to look at the cradle and found her blue, stiff, and unreactive. The emergency services found her unconscious, not breathing, and pulseless at home. After 10 minutes of advanced resuscitation, normal vital signs were recovered, and she was taken to our hospital. All the tests performed (including ECG) were reported as normal, and she was discharged.
The current research of palpitations shows baseline electrocardiogram with ventricular pre-excitation (Figure 2 A), resulting in the diagnosis of WPW syndrome. Echocardiography was normal. Her 24-h Holter monitoring revealed a continuous WPW pattern with no episodes of tachyarrhythmia. The treadmill test was unremarkable, but pre-excitation did not disappear with higher heart rates. We reviewed the ECG traces performed by the emergency medical services after resuscitation at the patient´s home and found a striking VPE pattern (Figure 2 B).
3. Discussion and Review of the Literature
The best clinical approach for managing patients with AWPW syndrome has yet to be determined. The clinical benefit of identifying and treating these patients who are at risk of SCD has been debated since RFA became effective and safe even in small children.
3.1. The real risk of sudden cardiac death in asymptomatic patients
Traditionally the management depends entirely on the patient’s clinical picture because symptomatic patients have a higher risk of SCD, and a more aggressive approach is preferred for them. The overall incidence of SCD in WPW syndrome is between 0 to 0.6% per year (2-4). In symptomatic patients, the risk is 3-4% over a lifetime (approximately 0.25% per year). Although most asymptomatic patients have a good prognosis, there is also a lifetime risk of malignant arrhythmias and SCD, estimated to be 0.1% per patient-year. It is a very low incidence of SCD (similar to that observed in the general population). A recent meta-analysis has shown that the risks associated with an invasive procedure such as RFA are identical to the risk of SCD in asymptomatic individuals (11). This finding argues against routine invasive management in most asymptomatic individuals with the WPW ECG pattern. Recently there have been advocates for performing invasive electrophysiologic assessment and catheter ablation therapy in asymptomatic individuals based on the finding by some investigators (Italian studies) of higher mortality rates in these individuals (12, 13). Thus, conflicting opinions are reported depending on the risk of SCD deemed, with some physicians aiming at the conservative approach and others advocating the first ablation approach.
Particular care must be taken with infants in whom it is challenging to determine the absence of symptoms and therefore, to ascertain the actual risk by the clinical history alone remains a dilemma. Remarkably, the incidence of SCD seems to be higher in children than adult patients (1.93 versus 0.86 per 1000 person-year), and SCD may be the first symptom in up to 53% of cases (5-8, 11, 14, 15). Also, it is essential to bear in mind that the unavailability of ECG during resuscitation could underestimate the low incidence of infants presenting with SCD. So the absence of symptoms does not necessarily connote low risk in infants, and risk stratification in this population is a matter of concern.
In 2012, the Pediatric and Congenital Electrophysiology Society (PACES) position statement for risk stratification in the young (aged 8-21 years) patients with AWPW syndrome (16) recommended an EPS as a Class IIA (level of evidence B/C) indication when non-invasive testing is ambiguous or uncertain regarding the risk, or there is a coexistent cardiac abnormality, and or multiple accessory pathways are suspected. Catheter ablation is a Class IIA (level of evidence B/C) indication for young patients (aged 8-21 years) with AWPW syndrome when high-risk electrophysiological properties of the AP at EPS are observed, regardless of the risk of the procedure. Of note, no clear recommendations have been given for infants.
3.2. Risk factors for sudden cardiac death
In the absence of any reliable test, some clinical variables such as male sex, younger age, family history of WPW syndrome, structural heart disease and septal localization of AP, have been associated with a higher risk of SCD (2-4, 14, 15). However, all these indexes have a modest power to identify these patients, thus, risk stratification has focused on the electrophysiological properties of the AP (17-19).
As mentioned previously, the obligatory condition for VF is an AP with a short antegrade refractory period, as reflected by the shortest R-R interval between pre-excited (SPERRI) QRS complexes during AF≤220 ms or the AERP measured during EP study ≤250 ms. Other electrophysiological risk-factors are inducibility (the ability to sustain an atrioventricular reciprocating tachycardia or AF for >1 min), and the presence of multiple APs.
The goal of risk stratification is to identify individuals with these high-risk AP features (17-19). Non-invasive and invasive tests are used for this purpose, but none alone is the best option for infants. Then, the researchers focus on what is the best strategy to asses these EP properties and therefore, to avoid the rare but definite risk of mortality in infants.
3.3. Invasive risk stratification
Electrophysiological Study (EPS) is the examination that offers the best cost/benefit ratio for risk stratification in asymptomatic patients. During the EPS, the inducibility of tachycardias is assessed as well as the conduction characteristics (SPERRI and AERP) of the AP. Long anterograde AERP/SPERRI, >250 ms of the accessory pathway, indicates the limited capability of anterograde conduction via the accessory pathway and therefore the low risk for VF and SCD. The sensitivity and negative predictive value are high and well established (near 100%), but its specificity and positive predictive values of predicting SCD are low (11, 15-19). The very low event rates of VF challenge the accuracy of EPS to predict SCD, so many patients are unnecessarily treated and exposed to the risks of EPS and RFA if all such asymptomatic patients were treated.
According to recent surveys, most pediatric electrophysiologists (84%) use some form of EPS for risk stratification of asymptomatic children with WPW syndrome, with high rates of successful RFA (>90%) (16-19). In asymptomatic patients, ablation of the AP decreases the incidence of potential future symptomatic arrhythmias (4). A randomized clinical trial that evaluated the results of prophylactic catheter ablation in children (aged 5-12 years) with AWPW syndrome showed that the absence of prophylactic ablation was an independent predictor of arrhythmic events (12). However, EPS is an invasive procedure with a risk of complications (5-15%), with major ones reported in 0.9-4.2% (death 0.12%), being higher in infants less than 15 kg of weight or 18 months of age. Also, prolonged exposure to radiation and high recurrence rates of arrhythmia after successful procedures (7-17%) are of particular concern (5-8, 16-19).
So, referral of every infant for an EPS or RFA could result in severe and potentially life-threatening complications, that possibly surpass the number of deaths caused by untreated disease, and usually, infants with AWPW syndrome (less than 15 kg) are not considered to be an indication for invasive risk stratification or RFA (20). An EPS to stratify risk and RFA procedure in small infants should only be considered when accepted high-risk factors determined non-invasively are present and whenever the risk of complications, judged mainly by localization and body surface area of the patient, is low.
3.3.1. Transesophageal EPS (TEPS)
In the current guidelines, TEPS is still considered as a suitable option for evaluating asymptomatic WPW syndrome (16-19). It has been shown that TEPS is useful to determine the EP properties of the AP and to manage the risk stratification in children because of its high correlation with EPS (21-23). It can easily be performed in low sophisticated facilities and on small children. Furthermore, it is a less-expensive, semi-invasive, and safe technique avoiding potential vascular complications and radiation exposure of EPS. These advantages could make considering TEPS before EPS and ablation a favorable risk stratification approach in small infants.
However, some limitations must be taken into account when using TEPS (21-23). First, the accuracy of TEPS to locate the AP and to discern multiple APs is low. Second, it could be painful and requires the use of sedation. More importantly, the values of the AERP and SEPRRI during AF are higher than those determined by EPS, and inducibility of AF is more difficult during TEPS compared to that in EPS. This condition is important because some cases could be wrongly classified as a low-risk patient. To avoid this condition, lower cut-off values (<280-300 ms) may be selected in risk stratification for WPW pattern using TEPS; this would lead to the determination of risky WPW patients with higher sensitivity.
3.3.2. Isoproterenol challenge
Observational data have shown that isoproterenol can modify the EP properties of APs and inducibility of supraventricular arrhythmia in patients with ventricular pre-excitation (24, 25). Kubus et al. identified an additional 36.4% of high-risk patients with isoproterenol when high-risk parameters were absent at baseline EP study in a group of 85 asymptomatic pediatric patients (24). Thus, use of intravenous infusion of isoproterenol during EPS or TEPS in children has been advocated as a possible surrogate of adrenergic stimulation, and it would be used as a pharmacologic stress test in infants who cannot perform an exercise test looking for a sudden loss of pre-excitation.
3.4. Non-invasive risk stratification (ECG, Holter Monitoring, Treadmill Testing)
In general, these tests look for evidence of an AP that fails to conduct at rapid rates, either in sinus rhythm or during AF (16-19, 22, 23). Intermittent pre-excitation is present when 2 consecutive sinus beats show the presence and absence of pre-excitation. This finding indicates a long antegrade refractory period of the accessory pathway resulting in a very low risk of sudden cardiac death. The appearance of different pre-excited morphologies on ECG or Holter monitoring is suggestive of multiple AP, which has been identified as a risk factor for ventricular fibrillation and SCD.
The best indicator of low risk is the sudden disappearance of pre-excitation during exercise, that indicates a long antegrade effective refractory period of the accessory pathway (16-19, 22, 23). Sympathetic stimulation occurring during exercise will shorten the duration of the AERP of the AP. When the AERP is achieved during exercise, as manifested by sudden block in the accessory pathway and normalization of the ECG, it is a good indicator that the patient is not at risk for VF even during sympathetic stimulation. The inability to demonstrate the sudden and absolute loss of manifest pre-excitation during exercise warrants invasive EPS.
Although these techniques have been reasonably applied as a part of routine clinical practice for risk assessment in WPW patients, non-invasive risk assessment itself may be problematic in infants for many reasons. First, it was recently observed that intermittent pre-excitation in children does not connote a lower risk AP by EP criteria (12, 13). Second, sudden and complete loss of preexcitation during exercise occurs in only 15% of a predominantly pediatric group of patients. Third, in children with subtle pre-excitation, an exercise test may be challenging to interpret. Finally, the child must be old enough to comply with the exercise test.
Remarkably, they are not as successful as the TEPS or EPS to predict EP AP properties, and up to 40% of the patients with intermittent pre-excitation on Holter and up to 30% of patients with the sudden loss of pre-excitation on an exercise test, will have high-risk accessory pathway conduction at TEEPS or EPS (22, 23). Therefore, risk stratification with non-invasive methods is relatively nonspecific, non-sensitive, incomplete, and difficult to perform in infants, and better methods are warranted.
3.5. Prophylactic pharmacological approach
Because of the well-known SCD risk in young patients with symptomatic WPW syndrome, it makes sense to treat symptomatic patients with antiarrhythmic drugs until ablation of the AP can be performed. Flecainide, an IC class antiarrhythmic drug, has proven to be safe and effective in controlling supraventricular arrhythmias in children, even infants, neonates, and fetuses (26-29). It is a sodium channel-blocking agent that decreases the velocity of conduction in fast-response cells, with minimal effects on action potential duration and repolarization.
Flecainide reduces the conductivity of the AP and has a stabilizing effect on the atria, thus preventing and reverting episodes of paroxysmal AF (26-29). Hence, its use in patients with WPW can prevent SVT and AF episodes, and, if AF develops, its effects on the properties of the AP can prevent fast ventricular responses and consequently VF. Remarkably, there is an approximately 4% risk of lethal proarrhythmia when using flecainide in children that is always related to the presence of structural heart diseases (29); thus, before starting flecainide, an echocardiographic study is warranted in these patients.
In asymptomatic infants, that can develop a potentially life-threatening arrhythmic event during follow-up and are not able to verbalize symptoms, the low accuracy of non-invasive methods, the risk of complications of EPS, the need of sedation of TEPS and EPS, and the lack of availability of EPS and TEPS in all centers, make difficult an appropriate risk-stratification. In this situation, an alternative and judicious approach could be a prophylactic treatment with flecainide (1-2 mg/kg/d) to minimize the risk of malignant arrhythmias at least until the age at which the patient can describe well the presence of symptoms and can comply with an exercise test, or a TEPS or EPS can be performed safely. The choice to observe asymptomatic infants should be preceded by the parents being informed of the low but real risk of life-threatening arrhythmias developing in the absence of treatment.
AWPW syndrome in infants is not rare. It is also a challenging condition that implies a very low but real risk of SCD, which is very difficult to ascertain with current diagnostic methods. The main argument against studying and treating asymptomatic patients has been the poor predictive accuracy (low specificity and low positive predictive value) of non-invasive and invasive risk stratification methods due to the low event rate of SCD. Non-invasive risk stratification is of limited value in infants, so it is not recommendable to make decisions based on these tests.
Invasive risk-stratification by EPS is the most accurate method, but it is a high-risk procedure in infants. When the complications of both electrophysiological studies are considered, the desired situation is using only TEPS with isoproterenol challenging and higher cut-off values of the refractory period of the AP to gain an accurate risk determination in infants with WPW syndrome pattern. EPS and RFA should follow TEPS for the cases considered with high-risk during TEPS. The cases considered to be without risk by applying TEPS alone should undoubtedly be followed up. It may be favorable to reevaluate these patients with EPS in the presence of clinical necessities.
If it is not possible or safe to perform an invasive risk stratification technique, prophylactic treatment with flecainide could be started to minimize the risk of malignant arrhythmias. The choice to observe without treatment of asymptomatic infants should be preceded by the parents being informed of the small but real risk of life-threatening arrhythmias developing in the absence of treatment.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualizing, designing, drafting the initial manuscript, and approving the final manuscript: Rodriguez-Gonzalez, Matamala-Morillo; Reviewing, revising manuscript and approving the final manuscript: Castellano-Martinez; Approving the final manuscript as submitted and agreeing to be accountable for all aspects of the work: All authors.
Conflict of interests
The authors declared no conflict of interests.
References
Full-Text: (1873 Views)
1. Introduction
Wolff-Parkinson-White (WPW) syndrome is a type of Ventricular Pre-excitation (VPE) and is caused by the existence of atrioventricular Accessory Pathways (AP). Epidemiological data indicate that it is observed in 0.1 to 0.3% of routine ECG performed in the general population, and 0.55% among the first-degree relatives of an index case (1-4). Although it is the most common cause of Supraventricular Tachycardia (SVT) in children, usually as orthodromic Atrioventricular Reentrant Tachycardia (AVRT), the majority of children are asymptomatic. Also, up to 60% of the asymptomatic patients with VPE are estimated to be children and adolescents (2-4).
Asymptomatic WPW (AWPW) syndrome is not rare and sometimes causes conflicting clinical scenario in pediatrics. The patient’s history is usually benign with spontaneous arrhythmia observed during the follow-up ranging from 8 to 21% (2-4); moreover, pre-excitation may spontaneously disappear. The anterograde conduction through the AP disappears in 40% of the patients in the first year of life, and in a similar percentage of cases, SVT becomes non-inducible, suggesting the loss of retrograde conductison. In children and adolescents, the probability of losing pre-excitation varies from 0 to 26% (5, 6).
Remarkably, Sudden Cardiac Death (SCD) may occur and be the first symptom in these patients (5-8). The assumed mechanism is Ventricular Fibrillation (VF) secondary to rapid stimulation of the ventricles due to Atrial Fibrillation (AF) quickly conducted through the AP. Of note, a prospective study of 184 asymptomatic children with WPW followed for 5 years with 2 Holter monitor per year showed that 12% had AF, an incidence significantly higher than seen in asymptomatic adults with WPW (9).
SCD can occur when the AP has a short Anterograde Effective Refractory Period (AERP), allowing many atrial impulses during AF to be conducted to the ventricle (10). However, the existing evidence about the real risk of SCD is weak, and advice on whether or not to invasively stratify the risk of SCD and ablate the AP through Electrophysiological Study (EPS) is not clear. In the present article, we present two cases of SCD in infants with AWPW syndrome, and review the literature and discuss the best risk-stratification strategy in infants with asymptomatic WPW.
2. Case Presentation
2.1. Case 1
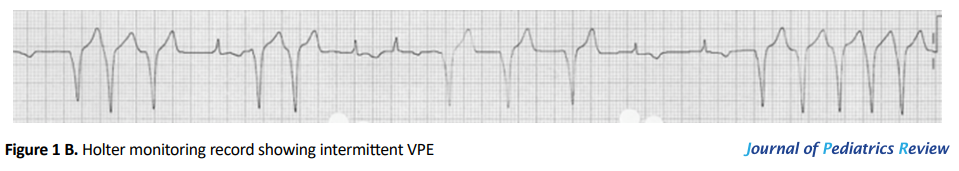
A 2-month-old previously healthy male was evaluated in our Pediatric Cardiology Clinic for a heart murmur. His family and personal history were unremarkable. He was asymptomatic. Physical exam was normal. ECG revealed a VPE pattern (Figure 1A). Echocardiography showed no anomalies. His 24-h Holter monitoring revealed sinus rhythm with loss of pre-excitation at higher heart rates (Figure 1B). No episodes of tachyarrhythmia were detected. He was diagnosed with AWPW syndrome.
Wolff-Parkinson-White (WPW) syndrome is a type of Ventricular Pre-excitation (VPE) and is caused by the existence of atrioventricular Accessory Pathways (AP). Epidemiological data indicate that it is observed in 0.1 to 0.3% of routine ECG performed in the general population, and 0.55% among the first-degree relatives of an index case (1-4). Although it is the most common cause of Supraventricular Tachycardia (SVT) in children, usually as orthodromic Atrioventricular Reentrant Tachycardia (AVRT), the majority of children are asymptomatic. Also, up to 60% of the asymptomatic patients with VPE are estimated to be children and adolescents (2-4).
Asymptomatic WPW (AWPW) syndrome is not rare and sometimes causes conflicting clinical scenario in pediatrics. The patient’s history is usually benign with spontaneous arrhythmia observed during the follow-up ranging from 8 to 21% (2-4); moreover, pre-excitation may spontaneously disappear. The anterograde conduction through the AP disappears in 40% of the patients in the first year of life, and in a similar percentage of cases, SVT becomes non-inducible, suggesting the loss of retrograde conductison. In children and adolescents, the probability of losing pre-excitation varies from 0 to 26% (5, 6).
Remarkably, Sudden Cardiac Death (SCD) may occur and be the first symptom in these patients (5-8). The assumed mechanism is Ventricular Fibrillation (VF) secondary to rapid stimulation of the ventricles due to Atrial Fibrillation (AF) quickly conducted through the AP. Of note, a prospective study of 184 asymptomatic children with WPW followed for 5 years with 2 Holter monitor per year showed that 12% had AF, an incidence significantly higher than seen in asymptomatic adults with WPW (9).
SCD can occur when the AP has a short Anterograde Effective Refractory Period (AERP), allowing many atrial impulses during AF to be conducted to the ventricle (10). However, the existing evidence about the real risk of SCD is weak, and advice on whether or not to invasively stratify the risk of SCD and ablate the AP through Electrophysiological Study (EPS) is not clear. In the present article, we present two cases of SCD in infants with AWPW syndrome, and review the literature and discuss the best risk-stratification strategy in infants with asymptomatic WPW.
2. Case Presentation
2.1. Case 1
A 2-month-old previously healthy male was evaluated in our Pediatric Cardiology Clinic for a heart murmur. His family and personal history were unremarkable. He was asymptomatic. Physical exam was normal. ECG revealed a VPE pattern (Figure 1A). Echocardiography showed no anomalies. His 24-h Holter monitoring revealed sinus rhythm with loss of pre-excitation at higher heart rates (Figure 1B). No episodes of tachyarrhythmia were detected. He was diagnosed with AWPW syndrome.
No treatment was initiated and a close clinical and ECG follow-up was scheduled. He did well until six months of age. Parents reported that he was crying vigorously at home and suddenly stopped breathing and became unresponsive. They started basic Cardiopulmonary Resuscitation (CPR) while waiting for the emergency services that found him collapsed and continued with advanced CPR. Unfortunately, the CPR was unsuccessful, and he died on the way to the hospital. No ECG was recorded, but we believe that the most probable cause of death was VF.
2.2. Case 2
An 8-year-old previously healthy female consulted our Pediatric Cardiology Clinic for self-limited episodes of palpitations. Family history was negative. Her personal account revealed a sudden infant death episode at four months old. Her parents reported that hearing throaty sounds, they went to look at the cradle and found her blue, stiff, and unreactive. The emergency services found her unconscious, not breathing, and pulseless at home. After 10 minutes of advanced resuscitation, normal vital signs were recovered, and she was taken to our hospital. All the tests performed (including ECG) were reported as normal, and she was discharged.
The current research of palpitations shows baseline electrocardiogram with ventricular pre-excitation (Figure 2 A), resulting in the diagnosis of WPW syndrome. Echocardiography was normal. Her 24-h Holter monitoring revealed a continuous WPW pattern with no episodes of tachyarrhythmia. The treadmill test was unremarkable, but pre-excitation did not disappear with higher heart rates. We reviewed the ECG traces performed by the emergency medical services after resuscitation at the patient´s home and found a striking VPE pattern (Figure 2 B).
We thought that the sudden infant death episode could have been secondary to a VF in the context of an undiagnosed WPW syndrome, although it cannot be demonstrated because of the absence of ECG recording during the resuscitation. She underwent EPS and satisfactory Radiofrequency Ablation (RFA) of AP without complications. At 1 year follow-up, she remained asymptomatic with normal ECG and echocardiography studies (Figure 2 C).
3. Discussion and Review of the Literature
The best clinical approach for managing patients with AWPW syndrome has yet to be determined. The clinical benefit of identifying and treating these patients who are at risk of SCD has been debated since RFA became effective and safe even in small children.
3.1. The real risk of sudden cardiac death in asymptomatic patients
Traditionally the management depends entirely on the patient’s clinical picture because symptomatic patients have a higher risk of SCD, and a more aggressive approach is preferred for them. The overall incidence of SCD in WPW syndrome is between 0 to 0.6% per year (2-4). In symptomatic patients, the risk is 3-4% over a lifetime (approximately 0.25% per year). Although most asymptomatic patients have a good prognosis, there is also a lifetime risk of malignant arrhythmias and SCD, estimated to be 0.1% per patient-year. It is a very low incidence of SCD (similar to that observed in the general population). A recent meta-analysis has shown that the risks associated with an invasive procedure such as RFA are identical to the risk of SCD in asymptomatic individuals (11). This finding argues against routine invasive management in most asymptomatic individuals with the WPW ECG pattern. Recently there have been advocates for performing invasive electrophysiologic assessment and catheter ablation therapy in asymptomatic individuals based on the finding by some investigators (Italian studies) of higher mortality rates in these individuals (12, 13). Thus, conflicting opinions are reported depending on the risk of SCD deemed, with some physicians aiming at the conservative approach and others advocating the first ablation approach.
Particular care must be taken with infants in whom it is challenging to determine the absence of symptoms and therefore, to ascertain the actual risk by the clinical history alone remains a dilemma. Remarkably, the incidence of SCD seems to be higher in children than adult patients (1.93 versus 0.86 per 1000 person-year), and SCD may be the first symptom in up to 53% of cases (5-8, 11, 14, 15). Also, it is essential to bear in mind that the unavailability of ECG during resuscitation could underestimate the low incidence of infants presenting with SCD. So the absence of symptoms does not necessarily connote low risk in infants, and risk stratification in this population is a matter of concern.
In 2012, the Pediatric and Congenital Electrophysiology Society (PACES) position statement for risk stratification in the young (aged 8-21 years) patients with AWPW syndrome (16) recommended an EPS as a Class IIA (level of evidence B/C) indication when non-invasive testing is ambiguous or uncertain regarding the risk, or there is a coexistent cardiac abnormality, and or multiple accessory pathways are suspected. Catheter ablation is a Class IIA (level of evidence B/C) indication for young patients (aged 8-21 years) with AWPW syndrome when high-risk electrophysiological properties of the AP at EPS are observed, regardless of the risk of the procedure. Of note, no clear recommendations have been given for infants.
3.2. Risk factors for sudden cardiac death
In the absence of any reliable test, some clinical variables such as male sex, younger age, family history of WPW syndrome, structural heart disease and septal localization of AP, have been associated with a higher risk of SCD (2-4, 14, 15). However, all these indexes have a modest power to identify these patients, thus, risk stratification has focused on the electrophysiological properties of the AP (17-19).
As mentioned previously, the obligatory condition for VF is an AP with a short antegrade refractory period, as reflected by the shortest R-R interval between pre-excited (SPERRI) QRS complexes during AF≤220 ms or the AERP measured during EP study ≤250 ms. Other electrophysiological risk-factors are inducibility (the ability to sustain an atrioventricular reciprocating tachycardia or AF for >1 min), and the presence of multiple APs.
The goal of risk stratification is to identify individuals with these high-risk AP features (17-19). Non-invasive and invasive tests are used for this purpose, but none alone is the best option for infants. Then, the researchers focus on what is the best strategy to asses these EP properties and therefore, to avoid the rare but definite risk of mortality in infants.
3.3. Invasive risk stratification
Electrophysiological Study (EPS) is the examination that offers the best cost/benefit ratio for risk stratification in asymptomatic patients. During the EPS, the inducibility of tachycardias is assessed as well as the conduction characteristics (SPERRI and AERP) of the AP. Long anterograde AERP/SPERRI, >250 ms of the accessory pathway, indicates the limited capability of anterograde conduction via the accessory pathway and therefore the low risk for VF and SCD. The sensitivity and negative predictive value are high and well established (near 100%), but its specificity and positive predictive values of predicting SCD are low (11, 15-19). The very low event rates of VF challenge the accuracy of EPS to predict SCD, so many patients are unnecessarily treated and exposed to the risks of EPS and RFA if all such asymptomatic patients were treated.
According to recent surveys, most pediatric electrophysiologists (84%) use some form of EPS for risk stratification of asymptomatic children with WPW syndrome, with high rates of successful RFA (>90%) (16-19). In asymptomatic patients, ablation of the AP decreases the incidence of potential future symptomatic arrhythmias (4). A randomized clinical trial that evaluated the results of prophylactic catheter ablation in children (aged 5-12 years) with AWPW syndrome showed that the absence of prophylactic ablation was an independent predictor of arrhythmic events (12). However, EPS is an invasive procedure with a risk of complications (5-15%), with major ones reported in 0.9-4.2% (death 0.12%), being higher in infants less than 15 kg of weight or 18 months of age. Also, prolonged exposure to radiation and high recurrence rates of arrhythmia after successful procedures (7-17%) are of particular concern (5-8, 16-19).
So, referral of every infant for an EPS or RFA could result in severe and potentially life-threatening complications, that possibly surpass the number of deaths caused by untreated disease, and usually, infants with AWPW syndrome (less than 15 kg) are not considered to be an indication for invasive risk stratification or RFA (20). An EPS to stratify risk and RFA procedure in small infants should only be considered when accepted high-risk factors determined non-invasively are present and whenever the risk of complications, judged mainly by localization and body surface area of the patient, is low.
3.3.1. Transesophageal EPS (TEPS)
In the current guidelines, TEPS is still considered as a suitable option for evaluating asymptomatic WPW syndrome (16-19). It has been shown that TEPS is useful to determine the EP properties of the AP and to manage the risk stratification in children because of its high correlation with EPS (21-23). It can easily be performed in low sophisticated facilities and on small children. Furthermore, it is a less-expensive, semi-invasive, and safe technique avoiding potential vascular complications and radiation exposure of EPS. These advantages could make considering TEPS before EPS and ablation a favorable risk stratification approach in small infants.
However, some limitations must be taken into account when using TEPS (21-23). First, the accuracy of TEPS to locate the AP and to discern multiple APs is low. Second, it could be painful and requires the use of sedation. More importantly, the values of the AERP and SEPRRI during AF are higher than those determined by EPS, and inducibility of AF is more difficult during TEPS compared to that in EPS. This condition is important because some cases could be wrongly classified as a low-risk patient. To avoid this condition, lower cut-off values (<280-300 ms) may be selected in risk stratification for WPW pattern using TEPS; this would lead to the determination of risky WPW patients with higher sensitivity.
3.3.2. Isoproterenol challenge
Observational data have shown that isoproterenol can modify the EP properties of APs and inducibility of supraventricular arrhythmia in patients with ventricular pre-excitation (24, 25). Kubus et al. identified an additional 36.4% of high-risk patients with isoproterenol when high-risk parameters were absent at baseline EP study in a group of 85 asymptomatic pediatric patients (24). Thus, use of intravenous infusion of isoproterenol during EPS or TEPS in children has been advocated as a possible surrogate of adrenergic stimulation, and it would be used as a pharmacologic stress test in infants who cannot perform an exercise test looking for a sudden loss of pre-excitation.
3.4. Non-invasive risk stratification (ECG, Holter Monitoring, Treadmill Testing)
In general, these tests look for evidence of an AP that fails to conduct at rapid rates, either in sinus rhythm or during AF (16-19, 22, 23). Intermittent pre-excitation is present when 2 consecutive sinus beats show the presence and absence of pre-excitation. This finding indicates a long antegrade refractory period of the accessory pathway resulting in a very low risk of sudden cardiac death. The appearance of different pre-excited morphologies on ECG or Holter monitoring is suggestive of multiple AP, which has been identified as a risk factor for ventricular fibrillation and SCD.
The best indicator of low risk is the sudden disappearance of pre-excitation during exercise, that indicates a long antegrade effective refractory period of the accessory pathway (16-19, 22, 23). Sympathetic stimulation occurring during exercise will shorten the duration of the AERP of the AP. When the AERP is achieved during exercise, as manifested by sudden block in the accessory pathway and normalization of the ECG, it is a good indicator that the patient is not at risk for VF even during sympathetic stimulation. The inability to demonstrate the sudden and absolute loss of manifest pre-excitation during exercise warrants invasive EPS.
Although these techniques have been reasonably applied as a part of routine clinical practice for risk assessment in WPW patients, non-invasive risk assessment itself may be problematic in infants for many reasons. First, it was recently observed that intermittent pre-excitation in children does not connote a lower risk AP by EP criteria (12, 13). Second, sudden and complete loss of preexcitation during exercise occurs in only 15% of a predominantly pediatric group of patients. Third, in children with subtle pre-excitation, an exercise test may be challenging to interpret. Finally, the child must be old enough to comply with the exercise test.
Remarkably, they are not as successful as the TEPS or EPS to predict EP AP properties, and up to 40% of the patients with intermittent pre-excitation on Holter and up to 30% of patients with the sudden loss of pre-excitation on an exercise test, will have high-risk accessory pathway conduction at TEEPS or EPS (22, 23). Therefore, risk stratification with non-invasive methods is relatively nonspecific, non-sensitive, incomplete, and difficult to perform in infants, and better methods are warranted.
3.5. Prophylactic pharmacological approach
Because of the well-known SCD risk in young patients with symptomatic WPW syndrome, it makes sense to treat symptomatic patients with antiarrhythmic drugs until ablation of the AP can be performed. Flecainide, an IC class antiarrhythmic drug, has proven to be safe and effective in controlling supraventricular arrhythmias in children, even infants, neonates, and fetuses (26-29). It is a sodium channel-blocking agent that decreases the velocity of conduction in fast-response cells, with minimal effects on action potential duration and repolarization.
Flecainide reduces the conductivity of the AP and has a stabilizing effect on the atria, thus preventing and reverting episodes of paroxysmal AF (26-29). Hence, its use in patients with WPW can prevent SVT and AF episodes, and, if AF develops, its effects on the properties of the AP can prevent fast ventricular responses and consequently VF. Remarkably, there is an approximately 4% risk of lethal proarrhythmia when using flecainide in children that is always related to the presence of structural heart diseases (29); thus, before starting flecainide, an echocardiographic study is warranted in these patients.
In asymptomatic infants, that can develop a potentially life-threatening arrhythmic event during follow-up and are not able to verbalize symptoms, the low accuracy of non-invasive methods, the risk of complications of EPS, the need of sedation of TEPS and EPS, and the lack of availability of EPS and TEPS in all centers, make difficult an appropriate risk-stratification. In this situation, an alternative and judicious approach could be a prophylactic treatment with flecainide (1-2 mg/kg/d) to minimize the risk of malignant arrhythmias at least until the age at which the patient can describe well the presence of symptoms and can comply with an exercise test, or a TEPS or EPS can be performed safely. The choice to observe asymptomatic infants should be preceded by the parents being informed of the low but real risk of life-threatening arrhythmias developing in the absence of treatment.
AWPW syndrome in infants is not rare. It is also a challenging condition that implies a very low but real risk of SCD, which is very difficult to ascertain with current diagnostic methods. The main argument against studying and treating asymptomatic patients has been the poor predictive accuracy (low specificity and low positive predictive value) of non-invasive and invasive risk stratification methods due to the low event rate of SCD. Non-invasive risk stratification is of limited value in infants, so it is not recommendable to make decisions based on these tests.
Invasive risk-stratification by EPS is the most accurate method, but it is a high-risk procedure in infants. When the complications of both electrophysiological studies are considered, the desired situation is using only TEPS with isoproterenol challenging and higher cut-off values of the refractory period of the AP to gain an accurate risk determination in infants with WPW syndrome pattern. EPS and RFA should follow TEPS for the cases considered with high-risk during TEPS. The cases considered to be without risk by applying TEPS alone should undoubtedly be followed up. It may be favorable to reevaluate these patients with EPS in the presence of clinical necessities.
If it is not possible or safe to perform an invasive risk stratification technique, prophylactic treatment with flecainide could be started to minimize the risk of malignant arrhythmias. The choice to observe without treatment of asymptomatic infants should be preceded by the parents being informed of the small but real risk of life-threatening arrhythmias developing in the absence of treatment.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualizing, designing, drafting the initial manuscript, and approving the final manuscript: Rodriguez-Gonzalez, Matamala-Morillo; Reviewing, revising manuscript and approving the final manuscript: Castellano-Martinez; Approving the final manuscript as submitted and agreeing to be accountable for all aspects of the work: All authors.
Conflict of interests
The authors declared no conflict of interests.
References
- Page RL, Joglar JA, Caldwell MA, Calkins H, Conti JB, Deal BJ, et al. 2015 ACC/AHA/HRS guideline for the management of adult patients with supraventricular tachycardia: A report of the American College of Cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society. Circulation. 2016; 133(14):e506-e74. [DOI:10.1161/CIR.0000000000000311] [PMCID]
- Cain N, Irving C, Webber S, Beerman L, Arora G. Natural history of Wolff-Parkinson-White syndrome diagnosed in childhood. The American Journal of Cardiology. 2013; 112(7):961-5. [DOI:10.1016/j.amjcard.2013.05.035] [PMID]
- Munger TM, Packer DL, Hammill SC, Feldman BJ, Bailey KR, Ballard DJ, et al. A population study of the natural history of Wolff-Parkinson-White syndrome in Olmsted County, Minnesota, 1953-1989. Circulation. 1993; 87(3):866-73. [DOI:10.1161/01.CIR.87.3.866] [PMID]
- Santinelli V, Radinovic A, Manguso F, Vicedomini G, Gulletta S, Paglino G, et al. The natural history of asymptomatic ventricular pre-excitation. Journal of the American College of Cardiology. 2009; 53(3):275-80. [DOI:10.1016/j.jacc.2008.09.037] [PMID]
- Fazio G, Mossuto C, Basile I, Gennaro F, D’angelo L, Visconti C, et al. Asymptomatic ventricular pre-excitation in children. Journal of Cardiovascular Medicine. 2009; 10(1):59-63. [DOI:10.2459/JCM.0b013e32831a98c2] [PMID]
- Deal BJ, Keane JF, Gillette PC, Garson A. Wolff-Parkinson-White syndrome and supraventricular tachycardia during infancy: Management and follow-up. Journal of the American College of Cardiology. 1985; 5(1):130-5. [DOI:10.1016/S0735-1097(85)80095-4]
- Hoeffler CD, Krenek ME, Brand MC. Wolff-Parkinson-White syndrome in a term infant presenting with cardiopulmonary arrest. Advances in Neonatal Care. 2016; 16(1):44-51. [DOI:10.1097/ANC.0000000000000246] [PMID]
- Kruser TJ, Frank JL, Maginot KR. Recurrent syncope secondary to asystole in an infant with Wolff-Parkinson-White syndrome. Clinical Pediatrics. 2008; 47(7):701-4. [DOI:10.1177/0009922808316994] [PMID]
- Pietersen AH, Andersen ED, Sandoe E. Atrial fibrillation in the Wolff-Parkinson-White syndrome. The American Journal of Cardiology. 1992; 70(5):38A-43A. [DOI:10.1016/0002-9149(92)91076-G]
- Bromberg BI, Lindsay BD, Cain ME, Cox JL. Impact of clinical history and electrophysiologic characterization of accessory pathways on management strategies to reduce sudden death among children with Wolff-Parkinson-White syndrome. Journal of the American College of Cardiology. 1996; 27(3):690-5. [DOI:10.1016/0735-1097(95)00519-6]
- Obeyesekere MN, Leong-Sit P, Massel D, Manlucu J, Modi S, Krahn AD, et al. Risk of arrhythmia and sudden death in patients with asymptomatic preexcitation: A meta-analysis. Circulation. 2012; 125(19):2308-15. [DOI:10.1161/CIRCULATIONAHA.111.055350] [PMID]
- Pappone C Manguso F, Santinelli R, Vicedomini G, Sala S, Paglino G, et al. Radiofrequency ablation in children with asymptomatic Wolff-Parkinson-White syndrome. The New England Journal of Medicine. 2004; 351(12):1197-205. [DOI:10.1056/NEJMoa040625] [PMID]
- Di Mambro C, Russo MS, Righi D, Placidi S, Palmieri R, Silvetti MS, et al. Ventricular pre-excitation: Symptomatic and asymptomatic children have the same potential risk of sudden cardiac death. Ep Europace. 2014; 17(4):617-21. [DOI:10.1093/europace/euu191] [PMID]
- Della Bella P, Brugada P, Talajic M, Lemery R, Torner P, Lezaun R, et al. Atrial fibrillation in patients with an accessory pathway: Importance of the conduction properties of the accessory pathway. Journal of the American College of Cardiology. 1991; 17(6):1352-6. [DOI:10.1016/S0735-1097(10)80146-9]
- Timmermans C, Smeets JL, Rodriguez LM, Vrouchos G, van den Dool A, Wellens HJ. Aborted sudden death in the Wolff-Parkinson-White syndrome. The American Journal of Cardiology. 1995; 76(7):492-4. [DOI:10.1016/S0002-9149(99)80136-2]
- Cohen MI, Triedman JK, Cannon BC, Davis AM, Drago F, Janousek J, et al. PACES/HRS expert consensus statement on the management of the asymptomatic young patient with a Wolff-Parkinson-White (WPW, ventricular preexcitation) electrocardiographic pattern. Heart Rhythm. 2012; 9(6):1006-24. [DOI:10.1016/j.hrthm.2012.03.050] [PMID]
- Delise P. Asymptomatic children with the Wolff-Parkinson-White pattern: What to do to stratify the risk of serious arrhythmias? Journal of Cardiovascular Medicine. 2009; 10(1):6-7. [DOI:10.2459/JCM.0b013e32831cbd48] [PMID]
- Campbell RM, Strieper MJ, Frias PA, Collins KK, Van Hare GF, Dubin AM. Survey of current practice of pediatric electrophysiologists for asymptomatic Wolff-Parkinson-White syndrome. Pediatrics. 2003; 111(3):e245-7. [DOI:10.1016/S1062-1458(03)00220-4] [PMID]
- Brembilla-Perrot B. When and how to assess an asymptomatic ventricular pre-excitation syndrome? Archives of Cardiovascular Diseases. 2008; 101(6):407-11. [DOI:10.1016/j.acvd.2008.05.006] [PMID]
- Sarubbi B, D’Alto M, Vergara P, Calvanese R, Mercurio B, Russo MG, et al. Electrophysiological evaluation of asymptomatic ventricular pre-excitation in children and adolescents. International Journal of Cardiology. 2005; 98(2):207-14. [DOI:10.1016/j.ijcard.2003.10.017] [PMID]
- Toni L, Blaufox AD. Transesophageal evaluation of asymptomatic Wolff‐Parkinson‐White syndrome. Pacing and Clinical Electrophysiology. 2012; 35(5):519-23. [DOI:10.1111/j.1540-8159.2012.03339.x] [PMID]
- Brembilla-Perrot B, Chometon F, Groben L, Ammar S, Bertrand J, Marcha C, et al. Interest of noninvasive and semi-invasive testings in asymptomatic children with preexcitation syndrome. Europace. 2007; 9(9):837-43. [DOI:10.1093/europace/eum153] [PMID]
- Sharma AD, Yee R, Guiraudon G, Klein G. Sensitivity and specificity of invasive and non-invasive testing for risk of sudden death in Wolff-Parkinson-White syndrome. Journal of the American College of Cardiology. 1987; 10(2):373-81. [DOI:10.1016/S0735-1097(87)80021-9]
- Kubuš P, Vít P, Gebauer RA, Materna O, Janoušek J. Electrophysiologic profile and results of invasive risk stratification in asymptomatic children and adolescents with the Wolff-Parkinson-White electrocardiographic pattern. Circulation: Arrhythmia and Electrophysiology. 2014; 7(2):218-23. [DOI:10.1161/CIRCEP.113.000930] [PMID]
- Wellens HJ, Brugada P, Roy D, Weiss J, Bär FW. Effect of isoproterenol on the anterograde refractory period of the accessory pathway in patients with the Wolff-Parkinson-White syndrome. The American Journal of Cardiology. 1982; 50(1):180-4. [DOI:10.1016/0002-9149(82)90026-1]
- Kiger ME, McCanta AC, Tong S, Schaffer M, Runciman M, Collins KK. Intermittent versus persistent Wolff-Parkinson-White syndrome in children: Electrophysiologic properties and clinical outcomes. Pacing and Clinical Electrophysiology. 2016; 39(1):14-20. [DOI:10.1111/pace.12732] [PMID]
- Ebenroth ES, Cordes TM, Darragh RK. Second-line treatment of fetal supraventricular tachycardia using flecainide acetate. Pediatric Cardiology. 2001; 22(6):483-7. [DOI:10.1007/s002460010279] [PMID]
- Ferlini M, Colli AM, Bonanomi C, Salvini L, Galli MA, Salice P, et al. Flecainide as first-line treatment for supraventricular tachycardia in newborns. Journal of Cardiovascular Medicine. 2009; 10(5):372-5. [DOI:10.2459/JCM.0b013e328329154d] [PMID]
- Perry JC, Garson Jr A. Flecainide acetate for treatment of tachyarrhythmias in children: Review of world literature on efficacy, safety, and dosing. American Heart Journal. 1992; 124(6):1614-21. [DOI:10.1016/0002-8703(92)90081-6]
Type of Study: Case & Review |
Subject:
Pediatric Cardiology
Received: 2018/06/30 | Accepted: 2018/07/3 | Published: 2019/10/1
Received: 2018/06/30 | Accepted: 2018/07/3 | Published: 2019/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |