Volume 8, Issue 3 (7-2020)
J. Pediatr. Rev 2020, 8(3): 201-208 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Salehi Omran M, Valiollahi S, Parsian H, Mosapour A, Bijani A, Pournasrollah M. The Relationship Between Serum and Cerebrospinal Fluid Concentrations of Zinc in Febrile Seizure. J. Pediatr. Rev 2020; 8 (3) :201-208
URL: http://jpr.mazums.ac.ir/article-1-238-en.html
URL: http://jpr.mazums.ac.ir/article-1-238-en.html
Mohammadreza Salehi Omran1 

 , Saleh Valiollahi
, Saleh Valiollahi 

 2, Hadi Parsian3
2, Hadi Parsian3 

 , Abbas Mosapour4
, Abbas Mosapour4 

 , Ali Bijani1
, Ali Bijani1 

 , Mohammad Pournasrollah5
, Mohammad Pournasrollah5 




 , Saleh Valiollahi
, Saleh Valiollahi 

 2, Hadi Parsian3
2, Hadi Parsian3 

 , Abbas Mosapour4
, Abbas Mosapour4 

 , Ali Bijani1
, Ali Bijani1 

 , Mohammad Pournasrollah5
, Mohammad Pournasrollah5 


1- Non-Communicable Pediatric Diseases Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
2- Student Research Committee, Babol University of Medical Sciences, Babol, Iran. , salehvalielahi@yahoo.com
3- Cellular and Molecular Biology Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran. & Department of Clinical Biochemistry, Babol University of Medical Sciences, Babol, Iran.
4- Department of Clinical Biochemistry, Babol University of Medical Sciences, Babol, Iran.
5- Clinical Research Development Unit of Amirkola Children’s Hospital, Babol University of Medical Sciences, Babol, Iran.
2- Student Research Committee, Babol University of Medical Sciences, Babol, Iran. , salehvalielahi@yahoo.com
3- Cellular and Molecular Biology Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran. & Department of Clinical Biochemistry, Babol University of Medical Sciences, Babol, Iran.
4- Department of Clinical Biochemistry, Babol University of Medical Sciences, Babol, Iran.
5- Clinical Research Development Unit of Amirkola Children’s Hospital, Babol University of Medical Sciences, Babol, Iran.
Full-Text [PDF 455 kb]
(1857 Downloads)
| Abstract (HTML) (3886 Views)
Full-Text: (856 Views)
1. Introduction
Febrile Seizures (FSs) are among the major causes of seizures in young kids. Almost 2%-4% of children aged ≤five years suffer from FS; however, its incidence in some populations has been reported to be 15% (1-3). The pathophysiology of the FS is not well understood. Different mechanisms have been suggested for its pathology, including genetic predisposition, immunological factors, altered levels of Trace Minerals (TMs), low Gamma-Aminobutyric Acid (GABA) levels, and so on (4).
TMs importantly influence growth, structural tissue maintenance, and the Central Nervous System (CNS) development. The importance of TMs in the physiology of the CNS is well established in the literature; their concentration alternations in biological fluids were investigated in neurological diseases (5-7). Zinc (Zn), as one of the most crucial TMs in the body, significantly impacts numerous metalloenzymes, i.e., essential in the CNS function (8). The role of GABA is well-documented in previous studies. It works as a crucial inhibitory neurotransmitter in the pathophysiology of seizures. Decreased CSF concentration of GABA is associated with a group of seizure disorders, including febrile convulsions (9). Furthermore, Zn, as a potent modulator of the glutaminergic synaptic transmission, regulates the activity of glutamic acid decarboxylase in the GABA synthesis process (10). Low levels of Zn have been detected in the serum and CSF of epileptic patients (11). Besides, experimental studies have suggested that fever (i.e., infections) may temporarily reduce serum Zn concentrations (12). Data from animal studies and preliminary clinical studies have suggested a possible role of cytokine network, like interleukin 1, influencing neuronal excitability. Accordingly, it may influence the pathogenesis of FS (13-15).
According to the particular circumstances in the CSF and the protective role of the blood-brain barrier, the concentrations of TMs in the CSF are lower but steadier than serum. Additionally, the CSF composition reflects the biological activities in the brain more closely than the blood (16). The comparison between serum and CSF Zn among patients with FS is available; however, data are controversial in this area. Therefore, the present study aimed to evaluate the relationship between serum and CSF levels of Zn in patients with FS.
2. Methods
This cross-sectional study was conducted in Amirkola Children’s Hospital affiliated to Babol University of Medical Sciences in 2013-2014. Fifty consecutive children (using the sample size formula) who were admitted with the primary diagnosis of FS and aged 6 months to 6 years were selected as the study samples. They were selected by non-random and convenience sampling techniques. The study protocol was approved by the Institutional Research Review Board and Ethics Committee of Babol University of Medical Sciences. After explaining the study purposes, informed consent was obtained from the studied children’s parents. Then, 1.5 cc peripheral venous blood samples were obtained and 1.5 cc CSF samples were collected by Lumbar Puncture performed in the lateral recumbent position with a stainless-steel needle. Next, the collected samples were transferred to the -800c refrigerator. The CSF samples with signs of traumatic spinal tap were excluded at this stage. A checklist, including age, weight, medical and family history, relevant data, body temperature at the admission, seizure duration, and seizure etiology was completed for each patient.
The exclusion criteria of the present study were children with cerebral palsy, metabolic disorders affecting CNS, the anatomical and structural malformation of CNS, neurodegenerative disorders, acute meningitis, previous Zn supplementation, and serum electrolyte disorders detected in biochemistry. In addition, to omit potential confounder effects, we initially ruled out these conditions; chronic diseases, inadequate absorption, bowel disease, alcohol intake, and histories of radiation therapy and surgery. For the Determination of Zn levels of serum and CSF by atomic absorption spectrophotometer, we followed the below procedure.
Samples: After collecting blood and CSF samples for measuring Zn level in all children, blood samples were centrifuged to remove cells (3000-4000 rpm for 10 min at 4°C), and the supernatants were transferred to new polystyrene tubes. The samples were stored at –80°C until the final analysis. The serum and CSF levels of Zn were assessed using flame and graphite furnace Atomic Absorption Spectrometry (AAS) method, respectively (PG-990, china). The normal range of Zn in serum was considered as 0.7-1.2 micg/L; however, constant value in CSF was not clear in resources.
Reagents and instrument: A stock standard solution of Zn at a concentration of 1000 mg/l (ppm) was prepared in a solution, containing 0.5 mol HNO3 (ultrapure), supplied by Merck. The calibration curve was developed using standard solutions. PG990 atomic absorption spectrophotometry equipped with deuterium background correction, HGA graphite furnace attachment, and Zn hollow-cathode lamps as the radiation source, were implemented for analysis. The operating parameters were set as recommended by the manufacturer.
Flame Procedure for analysis of Zn in serum: Zn levels in the serum were evaluated by flame atomic absorption spectroscopy. In this method, working standard solutions were used. Analysis of each sample was conducted three times with the integration time of 3s. This measure helped us to obtain a Relatively Standard Deviation (RSD) of ≤5% within the calibration range. Zn concentrations were determined with a deuterium background correction. Working standard solutions for Zn were 2.50, 1.25, 0.625, 0.312, and 0.156 ppm, prepared from the stock standard of 1000 mg/l, obtained from Merck. A linear calibration was obtained from the working standard (R=0.996). All serum samples were diluted 3 times with distilled water, and the concentration of Zn was delineated in the diluted samples. The analytical characteristics of Zn are listed in Table 1.
Furnace graphite for analysis of Zn levels in CSF: For the quantification of Zn levels in CSF, a 4-point calibration curve of Zn was applied in the concentration range of 0-30 (0, 5, 10, 30) µg/L (calibration equation, R=0.996). The samples and standards were dried for 20 s at 120˚C, charred (ashed) for 20 s at 300˚C, and atomized for 4 s at 1300 ˚C. The Zn levels of CSF were evaluated according to a standard curve. Furnace temperature settings are represented in tables 2 and 3.
Statistical analysis was performed using SPSS. Chi-squared test and Independent Samples t-test was used to compare qualitative and quantitative variables between the study groups. Pearson correlation coefficient was applied to assess the correlation between the changes in serum and CSF levels of Zn. P<0.05 was considered as the significance level.
3. Results
In total, 50 (28 male [56%] and 22 female [44%]) children with FC met the research inclusion criteria and were entered into the present study. The Mean±SD age of the study participants was 12.78±8.38 months; age range: 6-48 months. The Mean±SD birth weight and latest weight of study participants were 3.36±0.82 and 9.31±1.72 kg, respectively. Respiratory infections were the most frequent cause of fever in them (Figure 1).
Febrile Seizures (FSs) are among the major causes of seizures in young kids. Almost 2%-4% of children aged ≤five years suffer from FS; however, its incidence in some populations has been reported to be 15% (1-3). The pathophysiology of the FS is not well understood. Different mechanisms have been suggested for its pathology, including genetic predisposition, immunological factors, altered levels of Trace Minerals (TMs), low Gamma-Aminobutyric Acid (GABA) levels, and so on (4).
TMs importantly influence growth, structural tissue maintenance, and the Central Nervous System (CNS) development. The importance of TMs in the physiology of the CNS is well established in the literature; their concentration alternations in biological fluids were investigated in neurological diseases (5-7). Zinc (Zn), as one of the most crucial TMs in the body, significantly impacts numerous metalloenzymes, i.e., essential in the CNS function (8). The role of GABA is well-documented in previous studies. It works as a crucial inhibitory neurotransmitter in the pathophysiology of seizures. Decreased CSF concentration of GABA is associated with a group of seizure disorders, including febrile convulsions (9). Furthermore, Zn, as a potent modulator of the glutaminergic synaptic transmission, regulates the activity of glutamic acid decarboxylase in the GABA synthesis process (10). Low levels of Zn have been detected in the serum and CSF of epileptic patients (11). Besides, experimental studies have suggested that fever (i.e., infections) may temporarily reduce serum Zn concentrations (12). Data from animal studies and preliminary clinical studies have suggested a possible role of cytokine network, like interleukin 1, influencing neuronal excitability. Accordingly, it may influence the pathogenesis of FS (13-15).
According to the particular circumstances in the CSF and the protective role of the blood-brain barrier, the concentrations of TMs in the CSF are lower but steadier than serum. Additionally, the CSF composition reflects the biological activities in the brain more closely than the blood (16). The comparison between serum and CSF Zn among patients with FS is available; however, data are controversial in this area. Therefore, the present study aimed to evaluate the relationship between serum and CSF levels of Zn in patients with FS.
2. Methods
This cross-sectional study was conducted in Amirkola Children’s Hospital affiliated to Babol University of Medical Sciences in 2013-2014. Fifty consecutive children (using the sample size formula) who were admitted with the primary diagnosis of FS and aged 6 months to 6 years were selected as the study samples. They were selected by non-random and convenience sampling techniques. The study protocol was approved by the Institutional Research Review Board and Ethics Committee of Babol University of Medical Sciences. After explaining the study purposes, informed consent was obtained from the studied children’s parents. Then, 1.5 cc peripheral venous blood samples were obtained and 1.5 cc CSF samples were collected by Lumbar Puncture performed in the lateral recumbent position with a stainless-steel needle. Next, the collected samples were transferred to the -800c refrigerator. The CSF samples with signs of traumatic spinal tap were excluded at this stage. A checklist, including age, weight, medical and family history, relevant data, body temperature at the admission, seizure duration, and seizure etiology was completed for each patient.
The exclusion criteria of the present study were children with cerebral palsy, metabolic disorders affecting CNS, the anatomical and structural malformation of CNS, neurodegenerative disorders, acute meningitis, previous Zn supplementation, and serum electrolyte disorders detected in biochemistry. In addition, to omit potential confounder effects, we initially ruled out these conditions; chronic diseases, inadequate absorption, bowel disease, alcohol intake, and histories of radiation therapy and surgery. For the Determination of Zn levels of serum and CSF by atomic absorption spectrophotometer, we followed the below procedure.
Samples: After collecting blood and CSF samples for measuring Zn level in all children, blood samples were centrifuged to remove cells (3000-4000 rpm for 10 min at 4°C), and the supernatants were transferred to new polystyrene tubes. The samples were stored at –80°C until the final analysis. The serum and CSF levels of Zn were assessed using flame and graphite furnace Atomic Absorption Spectrometry (AAS) method, respectively (PG-990, china). The normal range of Zn in serum was considered as 0.7-1.2 micg/L; however, constant value in CSF was not clear in resources.
Reagents and instrument: A stock standard solution of Zn at a concentration of 1000 mg/l (ppm) was prepared in a solution, containing 0.5 mol HNO3 (ultrapure), supplied by Merck. The calibration curve was developed using standard solutions. PG990 atomic absorption spectrophotometry equipped with deuterium background correction, HGA graphite furnace attachment, and Zn hollow-cathode lamps as the radiation source, were implemented for analysis. The operating parameters were set as recommended by the manufacturer.
Flame Procedure for analysis of Zn in serum: Zn levels in the serum were evaluated by flame atomic absorption spectroscopy. In this method, working standard solutions were used. Analysis of each sample was conducted three times with the integration time of 3s. This measure helped us to obtain a Relatively Standard Deviation (RSD) of ≤5% within the calibration range. Zn concentrations were determined with a deuterium background correction. Working standard solutions for Zn were 2.50, 1.25, 0.625, 0.312, and 0.156 ppm, prepared from the stock standard of 1000 mg/l, obtained from Merck. A linear calibration was obtained from the working standard (R=0.996). All serum samples were diluted 3 times with distilled water, and the concentration of Zn was delineated in the diluted samples. The analytical characteristics of Zn are listed in Table 1.
Furnace graphite for analysis of Zn levels in CSF: For the quantification of Zn levels in CSF, a 4-point calibration curve of Zn was applied in the concentration range of 0-30 (0, 5, 10, 30) µg/L (calibration equation, R=0.996). The samples and standards were dried for 20 s at 120˚C, charred (ashed) for 20 s at 300˚C, and atomized for 4 s at 1300 ˚C. The Zn levels of CSF were evaluated according to a standard curve. Furnace temperature settings are represented in tables 2 and 3.
Statistical analysis was performed using SPSS. Chi-squared test and Independent Samples t-test was used to compare qualitative and quantitative variables between the study groups. Pearson correlation coefficient was applied to assess the correlation between the changes in serum and CSF levels of Zn. P<0.05 was considered as the significance level.
3. Results
In total, 50 (28 male [56%] and 22 female [44%]) children with FC met the research inclusion criteria and were entered into the present study. The Mean±SD age of the study participants was 12.78±8.38 months; age range: 6-48 months. The Mean±SD birth weight and latest weight of study participants were 3.36±0.82 and 9.31±1.72 kg, respectively. Respiratory infections were the most frequent cause of fever in them (Figure 1).
The mean±SD serum Zn level with the minimum and maximum serum levels of 0.13 and 3.40 µg/L, respectively, was equal to 0.68±0.62 µg/L. Of these 50 children, 34 (68%) patients presented Zn deficiency in FS (serum Zn level<0.7µg/L). The mean±SD CSF Zn level with the minimum and maximum CSF levels of 14.33 and 54.48 µg/L, respectively, were measured as 33.29± 11.84 µg/L. As per Figure 2, there was no association between the serum and CSF levels of Zn in the investigated patients with FC (r=0.173, P=0.23).
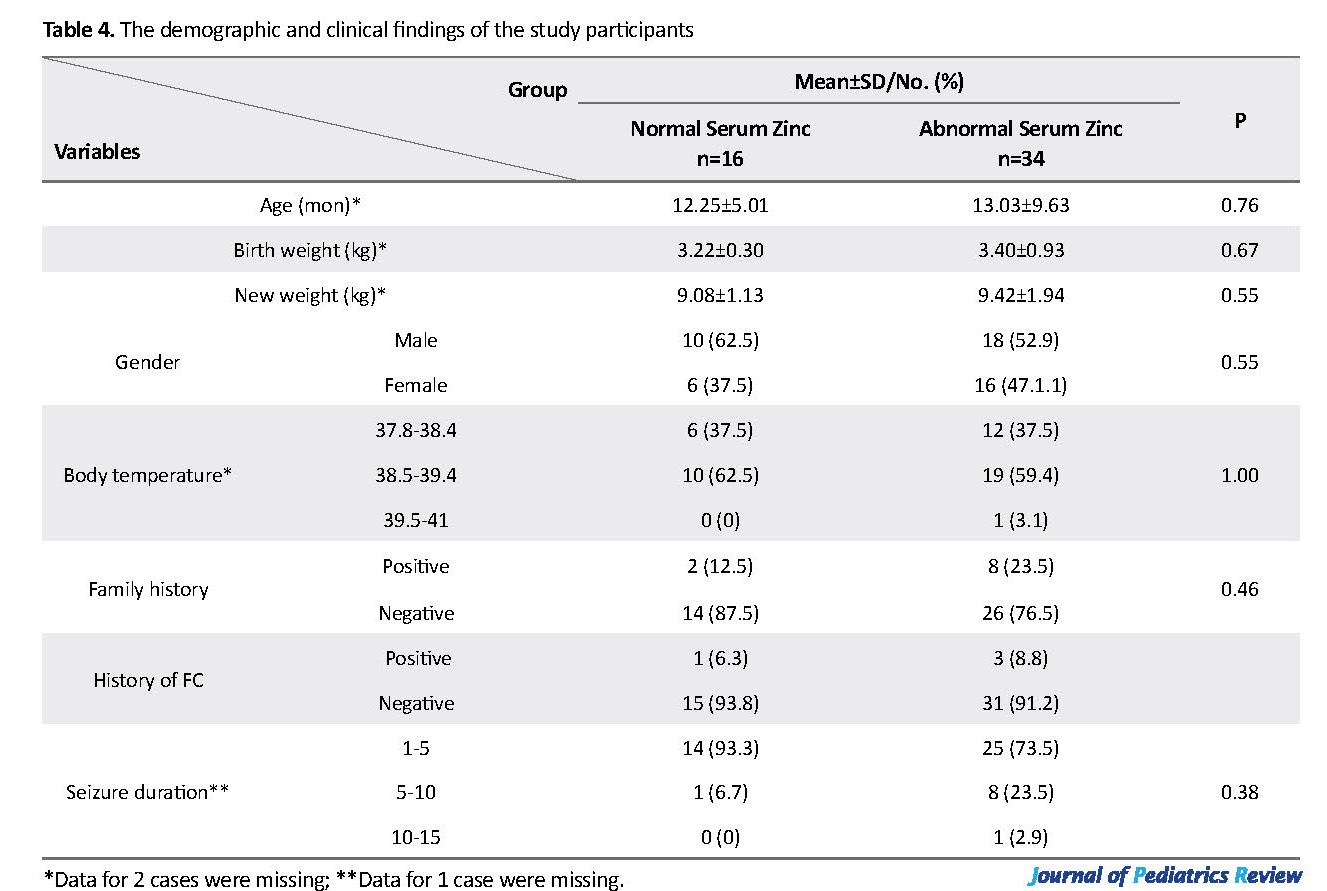
In addition, the Mean±SD CSF Zn levels in the normal and abnormal serum Zn groups were 37.71±10.82 and 31.21±11.87, respectively. Although the mean CSF level was lower in patients with decreased serum Zn, there was no significant difference in the mean CSF Zn level in patients with normal and decreased levels of serum Zn (P=0.07). As indicated in Table 4, no significant difference was found in the demographic/clinical characteristics of the studied patients with FC and normal/abnormal serum levels of Zn (P>0.05).
4. Discussion
According to our findings, the mean±SD serum Zn level was obtained as 0.68±0.62 µg/L and the mean±SD CSF Zn level was equal to 33.29± 11.84µg/L. Although 68% of the studied patients with FS presented abnormal levels of serum Zn, no correlation was found in Zn levels between serum and CSF.
Ehsanipour et al. revealed that children with FS had significantly lower serum Zn levels, compared to their counterparts with convulsion and those with fever but without convulsion (17). This report was consistent with other studies in Korea (18), Indonesia (19), and Iran (20, 21). Zn seems to be a predicting factor for FS; accordingly, a low level of this element may be considered as a contributing factor for this disease among children (22). However, in a study on 45 children with FS in Iran, their Mean±SD serum Zn level was obtained a 184.04±65.5 µg/dL, and their Mean±SD CSF Zn level was equal to 87.06±28.2 µg/dL. Besides, no clear abnormality was observed in serum and CSF Zn of the study samples (23). Cho et al., in Korea, compared febrile convulsive patients with healthy children. As a result, no significant differences were observed in serum Zn concentration of the studied subjects (24).
4. Discussion
According to our findings, the mean±SD serum Zn level was obtained as 0.68±0.62 µg/L and the mean±SD CSF Zn level was equal to 33.29± 11.84µg/L. Although 68% of the studied patients with FS presented abnormal levels of serum Zn, no correlation was found in Zn levels between serum and CSF.
Ehsanipour et al. revealed that children with FS had significantly lower serum Zn levels, compared to their counterparts with convulsion and those with fever but without convulsion (17). This report was consistent with other studies in Korea (18), Indonesia (19), and Iran (20, 21). Zn seems to be a predicting factor for FS; accordingly, a low level of this element may be considered as a contributing factor for this disease among children (22). However, in a study on 45 children with FS in Iran, their Mean±SD serum Zn level was obtained a 184.04±65.5 µg/dL, and their Mean±SD CSF Zn level was equal to 87.06±28.2 µg/dL. Besides, no clear abnormality was observed in serum and CSF Zn of the study samples (23). Cho et al., in Korea, compared febrile convulsive patients with healthy children. As a result, no significant differences were observed in serum Zn concentration of the studied subjects (24).
Studies that evaluated Zn levels in both serum and CSF are scarce. Mollah et al. investigated the Zn concentration changes in serum and CSF among the children with FS, compared to their matched Non-Seizure Febrile (NSF) peers. They concluded that serum and CSF Zn simultaneously decreased in children with FS, compared to their matched NSF children (25); i.e., inconsistent with the study conducted in Turkey (26), that presented a linear relationship between serum and CSF Zn. This finding is inconsistent with those of ours and Mollah et al.’s study.
It is assumed that blood metal levels have no or limited impact on the levels of metals in the CSF. This is because the trace element environment of the brain is controlled through the blood-brain-barrier. Generally, the concentrations of the trace elements in the blood are considerably higher than their respective concentrations in the CSF (16). Regional changes in CSF constituents could reflect local cerebral pathology and might not be detected by lumbar spinal fluid analysis. Therefore, for trace elements, the analysis of lumbar CSF concentrations and blood/CSF ratios are complex, and the expected correlations cannot be generally found (6). In the mouse model and in vitro studies, the mechanism underlying the role of Zn levels has been examined in seizures. Various studies have demonstrated that Zn modulates specific GABA receptors, and this mechanism could inhibit the seizure. Nevertheless, despite some disagreement, human studies revealed that the FS group presented lower Zn levels, compared to the controls with simple fever, (18).
It is assumed that blood metal levels have no or limited impact on the levels of metals in the CSF. This is because the trace element environment of the brain is controlled through the blood-brain-barrier. Generally, the concentrations of the trace elements in the blood are considerably higher than their respective concentrations in the CSF (16). Regional changes in CSF constituents could reflect local cerebral pathology and might not be detected by lumbar spinal fluid analysis. Therefore, for trace elements, the analysis of lumbar CSF concentrations and blood/CSF ratios are complex, and the expected correlations cannot be generally found (6). In the mouse model and in vitro studies, the mechanism underlying the role of Zn levels has been examined in seizures. Various studies have demonstrated that Zn modulates specific GABA receptors, and this mechanism could inhibit the seizure. Nevertheless, despite some disagreement, human studies revealed that the FS group presented lower Zn levels, compared to the controls with simple fever, (18).
The limitations of the present study were as follows: a matched control group could not be recruited due to the exposure of the control group to the risks of unnecessary lumbar puncture; all children received some medications, such as antibiotics and antipyretic agents before and or after admission to the hospital. Such interventions could have affected the measurements of serum or possible CSF levels of Zn.
Remarkably, the present study patients had seizures despite high levels of Zn in their serum or CSF. There exist doubts about the role of Zn in FS and its preventive use in all geographical areas. Further studies with large sample sizes and different control groups in various geographical areas are recommended. Such investigations could help to support the existing hypothesis that low serum and CSF Zn play major roles in FS.
5. Conclusion
The results of the study suggested that in the children with FS, serum zinc level was significantly decreased. No association was identified between serum and CSF zinc concentration levels.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; moreover, they were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This study was supported by a research grant and residency thesis of Dr. Saleh Valiollahi from the Non-Communicable Pediatric Diseases Research Center of Babol University of Medical Sciences (Grant No.: 9337715).
Authors' contributions
All authors were equally contributed in preparing this article.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
We are grateful to the Clinical Research Development Committee of Amirkola Children’s Hospital, Health Research Institute, Non-Communicable Pediatric Diseases Research Center of Babol University of Medical Sciences, and Mrs. Faeze Aghajanpour for their contribution to this study. The authors also would like to appreciate all personnel of Amirkola Children’s Hospital for their contribution to this study.
References
Berg AT, Jallon P, Preux PM. The epidemiology of seizure disorders in infancy and childhood: Definitions and classifications. Handbook of Clinical Neurology. 2013; 111:391-8. [DOI:10.1016/B978-0-444-52891-9.00043-9] [PMID]
Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994; 35:S1-6. [DOI:10.1111/j.1528-1157.1994.tb05932.x] [PMID]
Patterson JL, Carapetian SA, Hageman JR, Kelley KR. Febrile seizures. Pediatric Annals. 2013; 42(12):249-54. [DOI:10.3928/00904481-20131122-09] [PMID]
Pavlidou E, Hagel C, Panteliadis C. Febrile seizures: recent developments and unanswered questions. Child’s Nervous System. 2013; 29(11):2011-7. [DOI:10.1007/s00381-013-2224-3] [PMID]
Gerhardsson L, Lundh T, Minthon L, Londos E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2008; 25(6):508-15. [DOI:10.1159/000129365] [PMID]
Bogden JD, Troiano RA, Joselow MM. Copper, zinc, magnesium, and calcium in plasma and cerebrospinal fluid of patients with neurological diseases. Clinical Chemistry. 1977; 23(3):485-9. [DOI:10.1093/clinchem/23.3.485] [PMID]
Amiri M, Farzin L, Moassesi ME, Sajadi F. Serum trace element levels in febrile convulsion. Biological Trace Element Research. 2010; 135(1-3):38-44. [DOI:10.1007/s12011-009-8487-6] [PMID]
Kumar S, Kumar V, Mittal R, Jain D. Trace elemental analysis in epileptic children. Open Journal of Applied Sciences. 2013; 3:449-76. [DOI:10.4236/ojapps.2013.38056]
Garty BZ, Olomucki R, Lerman-Sagie T, Nitzan M. Cerebrospinal fluid zinc concentrations in febrile convulsions. Archives of Disease in Childhood. 1995; 73(4):338-41. [DOI:10.1136/adc.73.4.338] [PMID] [PMCID]
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Mueller RN, Zeng Y, et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Experimental Neurology. 2006; 198(2):285-93. [DOI:10.1016/j.expneurol.2005.08.030] [PMID]
Barbeau A, Donaldson J. Zinc, taurine, and epilepsy. JAMA Neurology. 1974; 30(1):52-8. [DOI:10.1001/archneur.1974.00490310054009] [PMID]
Izumi Y, Ishii K, Akiba K, Hayashi T. Hypozincemia during fever may trigger febrile convulsion. Medical Hypotheses. 1990; 32(1):77-80. [DOI:10.1016/0306-9877(90)90073-N]
Van Zeijl JH, Mullaart RA, Galama JM. The pathogenesis of febrile seizures: Is there a role for specific infections? Reviews in Medical Virology. 2002; 12(2):93-106. [DOI:10.1002/rmv.346] [PMID]
Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002; 43(8):920-3. [DOI:10.1046/j.1528-1157.2002.02002.x] [PMID]
Waruiru C, Appleton R. Febrile seizures: An update. Archives of Disease Childhood. 2004; 89(8):751-6. [DOI:10.1136/adc.2003.028449] [PMID] [PMCID]
Melo TM, Larsen C, White LR, Aasly J, Sjobakk TE, Flaten TP, et al. Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biological Trace Element Research. 2003; 93(1-3):1-8. [DOI:10.1385/BTER:93:1-3:1]
Ehsanipour F, Talebitaher M, Harandi NV, Kani K. Serum Zinc Level in Children with Febrile Convulsion and its Comparison with that of Control Group. Iranian Journal of Pediatrics. 2009; 19(1):65-8.
Lee J-H, Jeong Hyun Kim. Comparison of serum zinc levels measured by inductively coupled plasma mass spectrometry in preschool children with febrile and afebrile seizures. Annals of Laboratory Medicine. 2012; 32:190-3. [DOI:10.3343/alm.2012.32.3.190] [PMID] [PMCID]
Margaretha L, Masloman N. Correlation between serum zinc level and simple febrile seizure in children. Paediatrica Indonesiana. 2010; 50(6):326-30. [DOI:10.1111/j.1526-4610.2009.01539.x] [PMID]
Salehiomran MR, Mahzari M. Zinc Status in Febrile Seizure: A case-control study. Iranian Journal of Child Neurology. 2013; 7(4):20-3.
Mahyar A, Pahlavan A, Varasteh-Nejad A. Serum zinc level in children with febrile seizure. Acta Medica Iranica. 2008; 46(6):477-80.
Nasehi M, Sakhaei R, Moosazadeh M, Aliramzany M. Comparison of serum zinc levels among children with simple febrile seizure and control group: A systematic review. Iranian Journal of Child Neurology. 2015; 9(1):17-24.
Sadeghzadeh M, Nabi S, Khoshnevisasl P, Mousavinasab N. The correlation between cerebrospinal fluid and serum zinc and calcium in children with febrile seizure. Journal of Comprehensive Pediatrics. 2013; 3(5):179-83. [DOI:10.17795/compreped-12133]
Cho W, Son B, Kim S. Levels of Sodium and Zinc Concentration in Febrile Convulsion. Journal of the Korean Child Neurology Society. 1999; 7(2):214-19.
Mollah MA, Rakshit SC, Anwar KS, Arslan MI, Saha N, Ahmed S, et al. Zinc concentration in serum and cerebrospinal fluid simultaneously decrease in children with febrile seizure: Findings from a prospective study in Bangladesh. Acta Paediatrica. 2008; 97(12):1707-11. [DOI:10.1111/j.1651-2227.2008.01001.x] [PMID]
Burhanoglu M, Tutuncuoglu S, Coker C, Tekgul H, Ozgur T. Hypozincaemia in febrile convulsion. European Journal of Pediatrics. 1996; 155(6):498-501. [DOI:10.1007/BF01955189] [PMID]
Remarkably, the present study patients had seizures despite high levels of Zn in their serum or CSF. There exist doubts about the role of Zn in FS and its preventive use in all geographical areas. Further studies with large sample sizes and different control groups in various geographical areas are recommended. Such investigations could help to support the existing hypothesis that low serum and CSF Zn play major roles in FS.
5. Conclusion
The results of the study suggested that in the children with FS, serum zinc level was significantly decreased. No association was identified between serum and CSF zinc concentration levels.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; moreover, they were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This study was supported by a research grant and residency thesis of Dr. Saleh Valiollahi from the Non-Communicable Pediatric Diseases Research Center of Babol University of Medical Sciences (Grant No.: 9337715).
Authors' contributions
All authors were equally contributed in preparing this article.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
We are grateful to the Clinical Research Development Committee of Amirkola Children’s Hospital, Health Research Institute, Non-Communicable Pediatric Diseases Research Center of Babol University of Medical Sciences, and Mrs. Faeze Aghajanpour for their contribution to this study. The authors also would like to appreciate all personnel of Amirkola Children’s Hospital for their contribution to this study.
References
Berg AT, Jallon P, Preux PM. The epidemiology of seizure disorders in infancy and childhood: Definitions and classifications. Handbook of Clinical Neurology. 2013; 111:391-8. [DOI:10.1016/B978-0-444-52891-9.00043-9] [PMID]
Hauser WA. The prevalence and incidence of convulsive disorders in children. Epilepsia. 1994; 35:S1-6. [DOI:10.1111/j.1528-1157.1994.tb05932.x] [PMID]
Patterson JL, Carapetian SA, Hageman JR, Kelley KR. Febrile seizures. Pediatric Annals. 2013; 42(12):249-54. [DOI:10.3928/00904481-20131122-09] [PMID]
Pavlidou E, Hagel C, Panteliadis C. Febrile seizures: recent developments and unanswered questions. Child’s Nervous System. 2013; 29(11):2011-7. [DOI:10.1007/s00381-013-2224-3] [PMID]
Gerhardsson L, Lundh T, Minthon L, Londos E. Metal concentrations in plasma and cerebrospinal fluid in patients with Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2008; 25(6):508-15. [DOI:10.1159/000129365] [PMID]
Bogden JD, Troiano RA, Joselow MM. Copper, zinc, magnesium, and calcium in plasma and cerebrospinal fluid of patients with neurological diseases. Clinical Chemistry. 1977; 23(3):485-9. [DOI:10.1093/clinchem/23.3.485] [PMID]
Amiri M, Farzin L, Moassesi ME, Sajadi F. Serum trace element levels in febrile convulsion. Biological Trace Element Research. 2010; 135(1-3):38-44. [DOI:10.1007/s12011-009-8487-6] [PMID]
Kumar S, Kumar V, Mittal R, Jain D. Trace elemental analysis in epileptic children. Open Journal of Applied Sciences. 2013; 3:449-76. [DOI:10.4236/ojapps.2013.38056]
Garty BZ, Olomucki R, Lerman-Sagie T, Nitzan M. Cerebrospinal fluid zinc concentrations in febrile convulsions. Archives of Disease in Childhood. 1995; 73(4):338-41. [DOI:10.1136/adc.73.4.338] [PMID] [PMCID]
Frederickson CJ, Giblin LJ, Krezel A, McAdoo DJ, Mueller RN, Zeng Y, et al. Concentrations of extracellular free zinc (pZn)e in the central nervous system during simple anesthetization, ischemia and reperfusion. Experimental Neurology. 2006; 198(2):285-93. [DOI:10.1016/j.expneurol.2005.08.030] [PMID]
Barbeau A, Donaldson J. Zinc, taurine, and epilepsy. JAMA Neurology. 1974; 30(1):52-8. [DOI:10.1001/archneur.1974.00490310054009] [PMID]
Izumi Y, Ishii K, Akiba K, Hayashi T. Hypozincemia during fever may trigger febrile convulsion. Medical Hypotheses. 1990; 32(1):77-80. [DOI:10.1016/0306-9877(90)90073-N]
Van Zeijl JH, Mullaart RA, Galama JM. The pathogenesis of febrile seizures: Is there a role for specific infections? Reviews in Medical Virology. 2002; 12(2):93-106. [DOI:10.1002/rmv.346] [PMID]
Virta M, Hurme M, Helminen M. Increased plasma levels of pro- and anti-inflammatory cytokines in patients with febrile seizures. Epilepsia. 2002; 43(8):920-3. [DOI:10.1046/j.1528-1157.2002.02002.x] [PMID]
Waruiru C, Appleton R. Febrile seizures: An update. Archives of Disease Childhood. 2004; 89(8):751-6. [DOI:10.1136/adc.2003.028449] [PMID] [PMCID]
Melo TM, Larsen C, White LR, Aasly J, Sjobakk TE, Flaten TP, et al. Manganese, copper, and zinc in cerebrospinal fluid from patients with multiple sclerosis. Biological Trace Element Research. 2003; 93(1-3):1-8. [DOI:10.1385/BTER:93:1-3:1]
Ehsanipour F, Talebitaher M, Harandi NV, Kani K. Serum Zinc Level in Children with Febrile Convulsion and its Comparison with that of Control Group. Iranian Journal of Pediatrics. 2009; 19(1):65-8.
Lee J-H, Jeong Hyun Kim. Comparison of serum zinc levels measured by inductively coupled plasma mass spectrometry in preschool children with febrile and afebrile seizures. Annals of Laboratory Medicine. 2012; 32:190-3. [DOI:10.3343/alm.2012.32.3.190] [PMID] [PMCID]
Margaretha L, Masloman N. Correlation between serum zinc level and simple febrile seizure in children. Paediatrica Indonesiana. 2010; 50(6):326-30. [DOI:10.1111/j.1526-4610.2009.01539.x] [PMID]
Salehiomran MR, Mahzari M. Zinc Status in Febrile Seizure: A case-control study. Iranian Journal of Child Neurology. 2013; 7(4):20-3.
Mahyar A, Pahlavan A, Varasteh-Nejad A. Serum zinc level in children with febrile seizure. Acta Medica Iranica. 2008; 46(6):477-80.
Nasehi M, Sakhaei R, Moosazadeh M, Aliramzany M. Comparison of serum zinc levels among children with simple febrile seizure and control group: A systematic review. Iranian Journal of Child Neurology. 2015; 9(1):17-24.
Sadeghzadeh M, Nabi S, Khoshnevisasl P, Mousavinasab N. The correlation between cerebrospinal fluid and serum zinc and calcium in children with febrile seizure. Journal of Comprehensive Pediatrics. 2013; 3(5):179-83. [DOI:10.17795/compreped-12133]
Cho W, Son B, Kim S. Levels of Sodium and Zinc Concentration in Febrile Convulsion. Journal of the Korean Child Neurology Society. 1999; 7(2):214-19.
Mollah MA, Rakshit SC, Anwar KS, Arslan MI, Saha N, Ahmed S, et al. Zinc concentration in serum and cerebrospinal fluid simultaneously decrease in children with febrile seizure: Findings from a prospective study in Bangladesh. Acta Paediatrica. 2008; 97(12):1707-11. [DOI:10.1111/j.1651-2227.2008.01001.x] [PMID]
Burhanoglu M, Tutuncuoglu S, Coker C, Tekgul H, Ozgur T. Hypozincaemia in febrile convulsion. European Journal of Pediatrics. 1996; 155(6):498-501. [DOI:10.1007/BF01955189] [PMID]
Type of Study: Original Article |
Subject:
Neurology
Received: 2019/05/7 | Accepted: 2020/02/15 | Published: 2020/07/1
Received: 2019/05/7 | Accepted: 2020/02/15 | Published: 2020/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |