Volume 8, Issue 3 (7-2020)
J. Pediatr. Rev 2020, 8(3): 153-162 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Soori M, Mohammadi Y, Goodarzi M T, Mahmoodi M. Relationship Between Breast Milk Ghrelin and Infants’ Serum Ghrelin and Growth in Breastfeeding Infants: A Systematic Review and Meta-Analysis. J. Pediatr. Rev 2020; 8 (3) :153-162
URL: http://jpr.mazums.ac.ir/article-1-276-en.html
URL: http://jpr.mazums.ac.ir/article-1-276-en.html
1- Department of Biology, Hamedan Branch, Islamic Azad University, Hamedan, Iran.
2- Department of Epidemiology, School of Health, Hamadan University of Medical Sciences, Hamadan, Iran.
3- Department of Biochemistry, Shahrood Branch, Islamic Azad University, Shahrood, Iran. , mtgoodarzi@yahoo.com
2- Department of Epidemiology, School of Health, Hamadan University of Medical Sciences, Hamadan, Iran.
3- Department of Biochemistry, Shahrood Branch, Islamic Azad University, Shahrood, Iran. , mtgoodarzi@yahoo.com
Full-Text [PDF 760 kb]
(2089 Downloads)
| Abstract (HTML) (3896 Views)
Full-Text: (2067 Views)
1. Introduction
Obesity and weight gain are health-related risk factors with short- and long-term effects in infants (1). The increase in Body Mass Index (BMI) enhances the risk of diabetes (2), metabolic syndrome (3), cardiovascular disease (4), and numerous other complications. Prior research suggested that nourishment is effective in controlling weight gain and appetite in short and long terms. Comparing formula-fed and breast-fed infants indicated a better control of weight in the former (5, 6). Previous studies inferred the presence of adipokines in the breast milk, which protect and modulate weight and appetite in the infants (7, 8). Ghrelin is an adipokine in the breast milk and the human body. This adipokine predominantly contains 28 amino acids; it is a natural antagonist for leptin (9). There are two forms of ghrelin, i.e., acylated and de-acylated (10). Ghrelin is a Growth Hormone (GH) secretion stimulant, mainly secreted from the abdomen and the epsilon cells of the pancreas (11). In addition, ghrelin is among the most orexigenic peptides that stimulates hunger (12). The concentration of this hormone increases before eating and decreases after that (12, 13). Ghrelin is involved in regulating energy metabolism (14), carbohydrates and lipids (15), body growth and development, and food intake (16). Studies have investigated the relationship between breast milk feeding and infants’ serum ghrelin.
According to Aydin et al., there was a significant correlation between breast milk and infants’ serum ghrelin (17). Yesim Ozarda Ilcol and Banu Hizli observed a positive association between breast milk ghrelin and serum ghrelin in infants (r=0.331, P<0.05) (18). Moreover, there exist different reports regarding the effect of serum ghrelin on the growth and weight gain of infants. Based on F. Savino et al., serum ghrelin levels were negatively correlated with weight gain (r=-0.302, P=0.003) (19). Furthermore, Gokhan Cesur indicated a significant and positive relationship between breast milk, and serum ghrelin and weight gain in infants (r=0.576, P=0.025) (20). Ghrelin plays different roles; one of which is in controlling appetite and energy consumption and consequently, body weight. This hormone has been the subject of numerous research studies in various fields, including obesity and food intake. Breastfed infants receive ghrelin hormone through breast milk. However, data on the effects of serum and breast milk ghrelin on infants’ growth and weight gain are contradictory. Thus, this study investigated the relationship between breast milk ghrelin, serum ghrelin, and growth in infants that fed with breast milk, exclusively. This investigation was performed through a systematic review and meta-analysis.
The present study aimed to review the articles that illustrated the correlation between breast milk ghrelin concentration, weight gain, and growth in the infants through a systematic review. Moreover, we explored the relationship between serum ghrelin concentration and the growth, weight gain, and anthropometric characteristics of the infants through a meta-analysis study.
2. Methods
Electronic resources considered in the current search were Scopus, Cochrane Library, Science Direct, PubMed, and EMBASE. The articles in English and the articles with an English abstract published from 1999 to July 2019 were collected. The search mesh terms in PubMed and Scopus and text words included the following: “infant, BMI, growth, weight gain, anthropometric parameters, ghrelin, serum, adipokines, and breast milk”. Moreover, the references of the extracted articles were re-examined to find uncited papers by an electronic search.
Only the articles were reviewed that investigated infants <2 years of age and receiving breast milk, exclusively. Therefore, the main source of ghrelin in these infants was breast milk. Papers related to healthy mothers and infants were enrolled in this study. The studied mothers did not smoke, consume alcohol, or use medications. All analyses were conducted based on the information of previous published articles. Two authors collected the following data: authors’ names, journal’s name, the country of research conduction, the year of publication, the sample size, and the mean concentration value of the hormone level in breast milk and serum. The ghrelin level in breast milk and serum of infants was measured by one of the following approaches: Enzyme-Linked Immunosorbent Assay (ELISA), Radio-Immunoassay (RIA), and MILLIPLEX MAP Human Metabolic Hormone Magnetic Bead Panel. The relevance between breast milk, serum ghrelin, and the anthropometric (BMI & weight gain) properties of the studied infants was expressed by correlation coefficient (r).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and the Cochrane Recommendations were used in this meta-analysis and systematic review study. Three authors selected articles in accordance with the meta-analysis standard guidelines. Two authors independently collected all research papers’ data. Disagreements between the researchers were solved by consultation with a third author.
The quality of the articles was determined using the Newcastle-Ottawa Scale (NOS) checklist. The Review Manager Software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used in this study. The Standardized Incidence Rate (SIR)/Relative Risk (RR), and 95% CI were estimated to evaluate the effect of serum ghrelin on the anthropometric factors of the studied infants. Cochran’s test and random effects model were used, as well. Begg’s funnel plot was used to detect publication bias.
3. Results
In this study, in section A, the correlation between breast milk ghrelin and infants’ weight gain was determined by a systematic review method. In section B, a meta-analysis was performed to evaluate the effect of infant serum ghrelin on the growth and weight gain of the studied infants.
Initially, the relationship between breast milk ghrelin and weight gain was investigated. The characteristics of the studies are listed (in form of three papers) in Table 1. Khodabakhshi et al. (2014) evaluated 40 healthy-weight infants and 40 obese infants in 2-5 months (21). They observed that the mean breast milk ghrelin value in the mothers with healthy-weight infants was higher than those with obese infants (137.5 vs. 132 pg/mL). Infants’ weight and breast milk ghrelin level were measured at birth, as well as at 2, 4, and 6 months of age. The correlation between breast milk ghrelin and healthy-weight infants was evaluated at birth (r=-0.108), 2 (0.195), 4 (0.012), and 6 months (-0.053) of age (21).
Obesity and weight gain are health-related risk factors with short- and long-term effects in infants (1). The increase in Body Mass Index (BMI) enhances the risk of diabetes (2), metabolic syndrome (3), cardiovascular disease (4), and numerous other complications. Prior research suggested that nourishment is effective in controlling weight gain and appetite in short and long terms. Comparing formula-fed and breast-fed infants indicated a better control of weight in the former (5, 6). Previous studies inferred the presence of adipokines in the breast milk, which protect and modulate weight and appetite in the infants (7, 8). Ghrelin is an adipokine in the breast milk and the human body. This adipokine predominantly contains 28 amino acids; it is a natural antagonist for leptin (9). There are two forms of ghrelin, i.e., acylated and de-acylated (10). Ghrelin is a Growth Hormone (GH) secretion stimulant, mainly secreted from the abdomen and the epsilon cells of the pancreas (11). In addition, ghrelin is among the most orexigenic peptides that stimulates hunger (12). The concentration of this hormone increases before eating and decreases after that (12, 13). Ghrelin is involved in regulating energy metabolism (14), carbohydrates and lipids (15), body growth and development, and food intake (16). Studies have investigated the relationship between breast milk feeding and infants’ serum ghrelin.
According to Aydin et al., there was a significant correlation between breast milk and infants’ serum ghrelin (17). Yesim Ozarda Ilcol and Banu Hizli observed a positive association between breast milk ghrelin and serum ghrelin in infants (r=0.331, P<0.05) (18). Moreover, there exist different reports regarding the effect of serum ghrelin on the growth and weight gain of infants. Based on F. Savino et al., serum ghrelin levels were negatively correlated with weight gain (r=-0.302, P=0.003) (19). Furthermore, Gokhan Cesur indicated a significant and positive relationship between breast milk, and serum ghrelin and weight gain in infants (r=0.576, P=0.025) (20). Ghrelin plays different roles; one of which is in controlling appetite and energy consumption and consequently, body weight. This hormone has been the subject of numerous research studies in various fields, including obesity and food intake. Breastfed infants receive ghrelin hormone through breast milk. However, data on the effects of serum and breast milk ghrelin on infants’ growth and weight gain are contradictory. Thus, this study investigated the relationship between breast milk ghrelin, serum ghrelin, and growth in infants that fed with breast milk, exclusively. This investigation was performed through a systematic review and meta-analysis.
The present study aimed to review the articles that illustrated the correlation between breast milk ghrelin concentration, weight gain, and growth in the infants through a systematic review. Moreover, we explored the relationship between serum ghrelin concentration and the growth, weight gain, and anthropometric characteristics of the infants through a meta-analysis study.
2. Methods
Electronic resources considered in the current search were Scopus, Cochrane Library, Science Direct, PubMed, and EMBASE. The articles in English and the articles with an English abstract published from 1999 to July 2019 were collected. The search mesh terms in PubMed and Scopus and text words included the following: “infant, BMI, growth, weight gain, anthropometric parameters, ghrelin, serum, adipokines, and breast milk”. Moreover, the references of the extracted articles were re-examined to find uncited papers by an electronic search.
Only the articles were reviewed that investigated infants <2 years of age and receiving breast milk, exclusively. Therefore, the main source of ghrelin in these infants was breast milk. Papers related to healthy mothers and infants were enrolled in this study. The studied mothers did not smoke, consume alcohol, or use medications. All analyses were conducted based on the information of previous published articles. Two authors collected the following data: authors’ names, journal’s name, the country of research conduction, the year of publication, the sample size, and the mean concentration value of the hormone level in breast milk and serum. The ghrelin level in breast milk and serum of infants was measured by one of the following approaches: Enzyme-Linked Immunosorbent Assay (ELISA), Radio-Immunoassay (RIA), and MILLIPLEX MAP Human Metabolic Hormone Magnetic Bead Panel. The relevance between breast milk, serum ghrelin, and the anthropometric (BMI & weight gain) properties of the studied infants was expressed by correlation coefficient (r).
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist and the Cochrane Recommendations were used in this meta-analysis and systematic review study. Three authors selected articles in accordance with the meta-analysis standard guidelines. Two authors independently collected all research papers’ data. Disagreements between the researchers were solved by consultation with a third author.
The quality of the articles was determined using the Newcastle-Ottawa Scale (NOS) checklist. The Review Manager Software (The Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark) was used in this study. The Standardized Incidence Rate (SIR)/Relative Risk (RR), and 95% CI were estimated to evaluate the effect of serum ghrelin on the anthropometric factors of the studied infants. Cochran’s test and random effects model were used, as well. Begg’s funnel plot was used to detect publication bias.
3. Results
In this study, in section A, the correlation between breast milk ghrelin and infants’ weight gain was determined by a systematic review method. In section B, a meta-analysis was performed to evaluate the effect of infant serum ghrelin on the growth and weight gain of the studied infants.
Initially, the relationship between breast milk ghrelin and weight gain was investigated. The characteristics of the studies are listed (in form of three papers) in Table 1. Khodabakhshi et al. (2014) evaluated 40 healthy-weight infants and 40 obese infants in 2-5 months (21). They observed that the mean breast milk ghrelin value in the mothers with healthy-weight infants was higher than those with obese infants (137.5 vs. 132 pg/mL). Infants’ weight and breast milk ghrelin level were measured at birth, as well as at 2, 4, and 6 months of age. The correlation between breast milk ghrelin and healthy-weight infants was evaluated at birth (r=-0.108), 2 (0.195), 4 (0.012), and 6 months (-0.053) of age (21).
Gokhan Cesur et al. assessed the relationship between breast milk ghrelin and weight gain in infant among 25 mothers and their infants in 2012. Six mothers and infants were excluded from the analysis at 4 months after baseline. The mean breast milk ghrelin score was 2876.75-1626.35 pg/mL; the correlation coefficient between breast milk ghrelin level and weight gain in infants was 0.157 at 4 months of age (20). Huang Li-Liand et al. explored 67 infants and concluded that ghrelin level in breast milk was a key indicator related to body weight in them (β=0.161) (22). The Newcastle-Ottawa Scale (NOS) score was equal to 7 in two articles.
In this study, PRISMA checklist was used for the qualitative evaluation of the collected articles (Figure 1). Four keywords, namely: “infant, ghrelin, growth, and serum infant” were used to search the articles. Keywords were selected based on Patient, Intervention, Comparison, Outcome, Time, Study (PICOTS) method. One hundred eight articles were extracted while searching the databases. After studying the titles and abstracts of the collected articles, 29 full-texts were assessed and 23 articles were excluded from the study. Six articles met the criteria for meta-analysis (19, 20, 23-26). The excluded articles from the study (n=23) included the following: 5 articles presented the effect of breast milk ghrelin on growth in infants (6, 18, 21, 22, 27), 8 articles expressed no correlation between infants’ serum ghrelin and growth (2, 17, 28-33), 3 articles were of descriptive nature (34-36), and 6 articles had an intervention group (37-42).
This study considered a correlation between serum ghrelin and growth in infants. The characteristics of the selected studies are listed in Table 2. This study included three cohort (20, 23, 26) articles and three cross-sectional (19, 24, 25) studies on infants aged <2 years for meta-analysis. Immunoassay methods were used for measuring ghrelin in the serum of infants. BMI, weight gain, and weight-for-length ratio were considered for infant growth index. The correlation between growth index and infants serum ghrelin was expressed with Spearman’s correlation coefficient in 4 studies along with Pearson’s correlation coefficient.
The total number of investigated study participants was 554. A correlation coefficient (r) was used to express the relationship between infants’ serum ghrelin and growth. The quality assessment data by the NOS were ≥7 scores (Table 1). Sensitivity analysis and emission bias analysis were performed using funnel diagrams and Begg’s test. The publications indicated no significant bias in this meta-analysis (Figure 2).
The total number of investigated study participants was 554. A correlation coefficient (r) was used to express the relationship between infants’ serum ghrelin and growth. The quality assessment data by the NOS were ≥7 scores (Table 1). Sensitivity analysis and emission bias analysis were performed using funnel diagrams and Begg’s test. The publications indicated no significant bias in this meta-analysis (Figure 2).
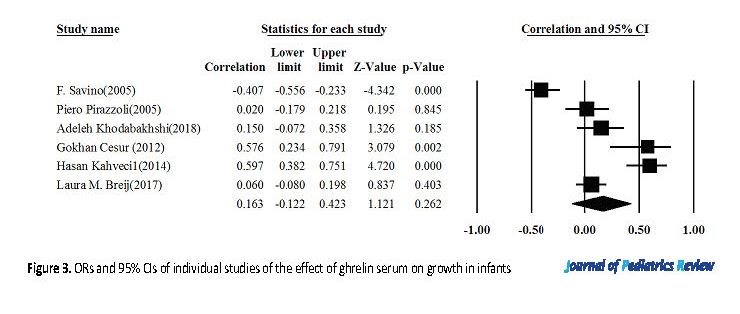
The heterogeneity of the selected studies was assessed using Q-test and 12 index (I-Squared=90). Due to the heterogeneity of the collected studies, the random effects model was used to combine the results of the investigations. According to Figure 3, the results of random-effect model demonstrated a direct and significant correlation between the serum ghrelin and growth of infants (r=0.163).
4. Discussion
The present study evaluated the effect of infants’ serum ghrelin on growth by a meta-analysis approach. The obtained data suggested a direct and significant effect in this area. Contradictory results were obtained regarding the effect of breast milk ghrelin on the studied infants’ weight gain.
Studies have reported that adipokines are essential hormones in regulating energy, appetite, and weight in the short and long term (43). Ghrelin (the first known controlling appetite hormone) is an adipokine discovered in 1999; its’ mechanism remains undiscovered (44). Prior research suggested that the main activities of ghrelin include growth hormone secretion, appetite stimulation, food intake, changes in gastric acid secretion, and changes in endocrine and exocrine pancreatic secretions. Furthermore, it directly affects corticotropin and lactotrophic hormone secretion, controlling the axons of male hypogonadism, polycystic ovary syndrome, and H-pylori gastric helicobacter pylori (44-46]. Research has suggested that ghrelin impacts short-term energy regulation; it is reduced in positive energy balance conditions (e.g., satiety time), and increased in negative energy balance conditions (e.g., hunger). Further observed was the effect of ghrelin on long-term energy regulation. The results of several studies have demonstrated that ghrelin is higher in obese individuals with a usual positive energy balance, and lower in those with anorexia nervosa with a frequent negative energy balance (47-49).
4. Discussion
The present study evaluated the effect of infants’ serum ghrelin on growth by a meta-analysis approach. The obtained data suggested a direct and significant effect in this area. Contradictory results were obtained regarding the effect of breast milk ghrelin on the studied infants’ weight gain.
Studies have reported that adipokines are essential hormones in regulating energy, appetite, and weight in the short and long term (43). Ghrelin (the first known controlling appetite hormone) is an adipokine discovered in 1999; its’ mechanism remains undiscovered (44). Prior research suggested that the main activities of ghrelin include growth hormone secretion, appetite stimulation, food intake, changes in gastric acid secretion, and changes in endocrine and exocrine pancreatic secretions. Furthermore, it directly affects corticotropin and lactotrophic hormone secretion, controlling the axons of male hypogonadism, polycystic ovary syndrome, and H-pylori gastric helicobacter pylori (44-46]. Research has suggested that ghrelin impacts short-term energy regulation; it is reduced in positive energy balance conditions (e.g., satiety time), and increased in negative energy balance conditions (e.g., hunger). Further observed was the effect of ghrelin on long-term energy regulation. The results of several studies have demonstrated that ghrelin is higher in obese individuals with a usual positive energy balance, and lower in those with anorexia nervosa with a frequent negative energy balance (47-49).
Ghrelin is the only known peptide that can cross the blood-brain barrier. The human ghrelin gene is on the short arm of chromosome 3 at position P 26-25 (50). The ghrelin precursor is in the form of pre-pro-ghrelin that becomes active with post-translation changes (the acidification of third serine residue hydroxyl group by 8-carbon fatty acid by ghrelin – O- acyl transferase [GOAT]) (13). Mainly, ghrelin is in des-acyl form (90%), and to lesser extent, in acyl form; moreover, it is very restricted to the C-terminal of progeline peptide form in the bloodstream (46, 50). Acyl and des-acyl forms of it are created by the cells of the hypothalamic arcuate nucleus (50). Ghrelin is a ligand for the Growth Hormone Secretagogue Receptor (GHS-R). The major Ghrelin Receptor (GHS-R) is mainly expressed from the hippocampus, pituitary, and hypothalamus whose gene is on chromosome 3 at position q26-27 (47).
There are two ghrelin receptor types: GHS-R type 1a, a seven-domain GPCR, acting as a receptor for ghrelin receptor, and GHS-R cDNA, type 1b; the second type receiver, i.e., pharmacologically inactive (51, 52). Ghrelin is mainly produced by the pancreatic cells in the fetus; however, after birth, ghrelin is mainly secreted from gastric endocrine X/A cells (13). Furthermore, ghrelin is expressed in numerous tissues of the body, including the lung, colon, duodenum, jejunum, ileum, heart, testis, pancreas, and the pituitary gland in adolescence (50). Ghrelin, secreted from the stomach, enters the bloodstream, then the hypothalamus and pituitary. Ghrelin binds to its receptor on the surface of pituitary somatotroph cells, which activates phospholipase C and produces IP3 and diacyl glycerol; accordingly increasing intracellular calcium levels (53). Increased intracellular calcium enhances pituitary GH production, appetite, and food absorption. Growth Hormone-Releasing Hormone (GHRH) is a natural analog of ghrelin in the human body. Besides, it activates adenylate cyclase and increases intracellular cAMP by binding to its receptor on the surface of pituitary cells.
GHRH is valuable as a secondary messenger for activating protein kinase A. Moreover, increased intracellular cAMP leads to the enhanced production of pituitary GH hormones as well as body metabolism (54). GHSs are synthetic analogs for ghrelin with similar mechanisms. In humans, 133 homologs have been identified for ghrelin (50). Studies have revealed that ghrelin, by affecting the arcuate nucleus of the hypothalamus, produces NPY/AgRP neurons; orexigenic neurons reduce fatty acid oxidation, and increase hunger and food absorption in the body. On the other hand, NPY neurons, by affecting the Paraventricular Nucleus (PVN) cells of the hypothalamus, suppress the activity of gamma-aminobutyric acid neurotransmitters, increase the production of Corticotropin-Releasing Hormones (CRH), and eventually enhance the production of cortisol and Adrenocorticotropic Hormones (ACTH) (50, 53, 54). POMC is a hormone in the body that suppresses appetite. The effect of ghrelin on hypothalamic cells activates NPY/AgRP neurotransmitter neurons. The NPY/AgRP neurons suppress POMC, consequently increasing appetite and the absorption of nutrients in the body (53).
The analyzed studies investigated the effect of breast milk ghrelin on infants’ growth. In some studies, the exact level of correlation was not mentioned or a correlation was not observed; therefore, they were excluded from this study. An example of these articles was Yis U et al.’s research in 2010 that demonstrated higher serum ghrelin in breast-fed infants. They also indicated its’ role in their faster growth rate during the first three months of life; however, they concluded that breast milk ghrelin was not correlated with the respective serum ghrelin or anthropometric data in the breast-fed group (28). Francesco Savino et al. measured ghrelin, leptin, and IGF-I levels in breast-fed infants in the first year of life (33). They expressed IGF-I, leptin, and ghrelin in breast milk were effective in the growth of infants up to 4 months of age; however, the exact correlation was disregarded (33).
A limitation of this study was the limited number of articles on the subject. In addition, the articles were in English language, and the relationship between breast milk, serum ghrelin, growth, and weight gain was not addressed in some published articles. It is suggested that more studies be conducted to evaluate the effect of breast milk ghrelin and serum ghrelin on growth and weight gain in infants.
5. Conclusion
According to the present meta-analysis study data, serum ghrelin had a direct and significant correlation with the growth of infants under two years of age. Increased ghrelin in infants’ serum level enhanced appetite, food absorption, and growth. Three articles with contradictory results were found regarding the relationship between breast milk and weight gain in infants; thus, more studies are required to provide further relevant details.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
Hamedan Islamic Azad University supported this study as the research project (Code: 1445908) as a PhD dissertation at.
Authors' contributions
All authors contributed in designing, running, and writing all parts of the research.
Conflicts of interest
The authors declared no conflicts of interest.
References
Savino F, Fissore MF, Liguori SA, Oggero R. Can hormones contained in mothers’ milk account for the beneficial effect of breast‐feeding on obesity in children? Clinical Endocrinology. 2009; 71(6):757-65. [DOI:10.1111/j.1365-2265.2009.03585.x] [PMID]
Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Gunderson EP, et al. Duration of lactation and maternal adipokines at 3 years postpartum. Diabetes. 2011; 60(4):1277-85. [DOI:10.2337/db10-0637] [PMID] [PMCID]
Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iranian Journal of Basic Medical Sciences. 2015; 18(5):430.
Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. Journal of Cardiology. 2014; 63(4):250-9. [DOI:10.1016/j.jjcc.2013.11.006] [PMID] [PMCID]
Gillman MW, Mantzoros CS. Breast-feeding, adipokines, and childhood obesity. Epidemiology. 2007; 18(6):730-2. [DOI:10.1097/EDE.0b013e3181571df0] [PMID]
Kon IY, Shilina NM, Gmoshinskaya MV, Ivanushkina TA. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Annals of Nutrition and Metabolism. 2014; 65(4):317-23. [DOI:10.1159/000367998] [PMID]
Oddy WH. Infant feeding and obesity risk in the child. Breastfeeding Review. 2012; 20(2):7.
Teague AM, Fields DA, Aston CE, Short KR, Lyons TJ, Chernausek SD. Cord blood adipokines, neonatal anthropometrics and postnatal growth in offspring of Hispanic and Native American women with diabetes mellitus. Reproductive Biology and Endocrinology. 2015; 13(1):68. [DOI:10.1186/s12958-015-0061-9] [PMID] [PMCID]
Kratzsch J, Bae YJ, Kiess W. Adipokines in human breast milk. Best Practice & Research Clinical Endocrinology & Metabolism. 2018; 32(1):27-38. [DOI:10.1016/j.beem.2018.02.001] [PMID]
Godlewski G, Cinar R, Coffey N, Liu J, Jourdan T, Mukhopadhyay B, et al. Endocannabinoids promote ethanol drinking via cb 1 receptor-mediated increase in ghrelin acylation and signalling in the stomach. https://www.researchgate.net/journal/1556-5068_SSRN_Electronic_Journal.3316795. 2019.
Andrews Z. Ghrelin: What’s the function? Journal of neuroendocrinology. 2019:e12772. [DOI:10.1111/jne.12772]
Sim A, Lim E, Leow M, Cheon B. Low subjective socioeconomic status stimulates orexigenic hormone ghrelin-A randomised trial. Psychoneuroendocrinology. 2018; 89:103-12. [DOI:10.1016/j.psyneuen.2018.01.006] [PMID]
Cowley MA, Grove KL. Ghrelin-satisfying a hunger for the mechanism. Endocrinology. 2004; 145(6):2604-6. [DOI:10.1210/en.2004-0346] [PMID]
Mani BK, Osborne-Lawrence S, Metzger N, Zigman M. Lowering oxidative stress in ghrelin cells stimulates ghrelin secretion. American Journal of Physiology-Endocrinology and Metabolism. 2020. [DOI:10.1152/ajpendo.00119.2020]
Poher AL, Tschöp MH, Müller TD. Ghrelin regulation of glucose metabolism. Peptides. 2018; 100:236-42. [DOI:10.1016/j.peptides.2017.12.015] [PMID] [PMCID]
Álvarez-Castro P, Pena L, Cordido F. Ghrelin in obesity, physiological and pharmacological considerations. Mini Reviews in Medicinal Chemistry. 2013; 13(4):541-52. [DOI:10.2174/1389557511313040007] [PMID]
Aydin S, Ozkan Y, Erman F, Gurates B, Kilic N, Colak R, et al. Presence of obestatin in breast milk: Relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition. 2008; 24(7-8):689-93. [DOI:10.1016/j.nut.2008.03.020] [PMID]
Ilcol YO, Hizli B. Active and total ghrelin concentrations increase in breast milk during lactation. Acta Pædiatrica. 2007; 96(11):1632-9. [DOI:10.1111/j.1651-2227.2007.00493.x] [PMID]
Savino F, Liguori SA, Fissore MF, Oggero R, Silvestro L, Miniero R. Serum ghrelin concentration and weight gain in healthy term infants in the first year of life. Journal of Pediatric Gastroenterology and Nutrition. 2005; 41(5):653-9. [DOI:10.1097/01.mpg.0000181856.54617.04] [PMID]
Cesur G, Ozguner F, Yilmaz N, Dundar B. The relationship between ghrelin and adiponectin levels in breast milk and infant serum and growth of infants during early postnatal life. The Journal of Physiological Sciences. 2012; 62(3):185-90. [DOI:10.1007/s12576-012-0193-z] [PMID]
Khodabakhshi A, Ghayour-Mobarhan M, Rooki H, Vakili R, Hashemy S, Mirhafez S, et al. Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. European Journal of Clinical Nutrition. 2015; 69(5):614. [DOI:10.1038/ejcn.2014.205] [PMID]
Huang L, Yang F, Xiong F. Association of leptin, adiponectin, and ghrelin in breast milk with the growth of infants with exclusive breastfeeding. Chinese Journal of Contemporary Pediatrics. 2018; 20(2):91-6.
Pirazzoli P, Lanari M, Zucchini S, Gennari M, Pagotto U, De Iasio R, et al. Active and total ghrelin concentrations in the newborn. Journal of Pediatric Endocrinology and Metabolism. 2005; 18(4):379-84. [DOI:10.1515/JPEM.2005.18.4.379] [PMID]
Khodabakhshi A, Mehrad-Majd H, Vahid F, Safarian M. Association of maternal breast milk and serum levels of macronutrients, hormones, and maternal body composition with infant’s body weight. European Journal of Clinical Nutrition. 2018; 72(3):394. [DOI:10.1038/s41430-017-0022-9] [PMID]
Kahveci H, Laloglu F, Kilic O, Ciftel M, Kara M, Laloglu E, et al. Fasting and postprandial glucose, insulin, leptin, and ghrelin values in preterm babies and their mothers: relationships among their levels, fetal growth, and neonatal anthropometry. The Journal of Maternal-Fetal & Neonatal Medicine. 2015; 28(8):916-21. [DOI:10.3109/14767058.2014.937693] [PMID]
Breij LM, Mulder MT, Hokken-Koelega AC. Appetite-regulating hormones in early life and relationships with type of feeding and body composition in healthy term infants. European Journal of Nutrition. 2017; 56(4):1725-32. [DOI:10.1007/s00394-016-1219-8] [PMID] [PMCID]
Savino F, Petrucci E, Lupica MM, Nanni GE, Oggero R. Assay of ghrelin concentration in infant formulas and breast milk. World Journal of Gastroenterology. 2011; 17(15):1971. [DOI:10.3748/wjg.v17.i15.1971] [PMID] [PMCID]
Yis U, Öztürk Y, Sisman AR, Uysal S, Soylu ÖB, Büyükgebiz B. The relation of serum ghrelin, leptin and insulin levels to the growth patterns and feeding characteristics in breast-fed versus formula-fed infants. The Turkish Journal of Pediatrics. 2010; 52(1):35.
Savino F, Benetti S, Lupica M, Petrucci E, Palumeri E, Di Montezemolo LC. Ghrelin and obestatin in infants, lactating mothers and breast milk. Hormone Research in Paediatrics. 2012; 78(5-6):297-303. [DOI:10.1159/000345876] [PMID]
Kierson JA, Dimatteo DM, Locke RG, MacKley AB, Spear ML. Ghrelin and cholecystokinin in term and preterm human breast milk. Acta Paediatrica. 2006; 95(8):991-5. [DOI:10.1080/08035250600669769] [PMID]
Weyermann M, Brenner H, Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: A prospective cohort study. Epidemiology. 2007; 18(6):722-9. [DOI:10.1097/EDE.0b013e3181567ed4] [PMID]
Karatas Z, Aydogdu SD, Dinleyici EC, Colak O, Dogruel N. Breast milk ghrelin, leptin, and fat levels changing foremilk to hindmilk: Is that important for self-control of feeding? European Journal of Pediatrics. 2011; 170(10):1273-80. [DOI:10.1007/s00431-011-1438-1] [PMID]
Savino F, Fissore MF, Grassino EC, Nanni GE, Oggero R, Silvestro L. Ghrelin, leptin and IGF‐I levels in breast‐fed and formula‐fed infants in the first years of life. Acta Paediat. 2005; 94(5):531-7. [DOI:10.1111/j.1651-2227.2005.tb01934.x] [PMID]
Savino F, Lupica MM, Liguori SA, Fissore MF, Silvestro L. Ghrelin and feeding behaviour in preterm infants. Early Human Development. 2012; 88:S51-S5. [DOI:10.1016/j.earlhumdev.2011.12.028] [PMID]
Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. International Journal of Pediatric Endocrinology. 2009; 2009(1):327505. [DOI:10.1186/1687-9856-2009-327505] [PMID] [PMCID]
Savino F, Benetti S, Liguori SA, Sorrenti M, Cordero Di Montezemolo L. Advances on human milk hormones and protection against obesity. Molecular and Cellular Biology. 2013; 59(1):89-98.
Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: A longitudinal study. British Journal of Nutrition. 2018; 120(12):1380-7. [DOI:10.1017/S0007114518002933] [PMID]
Patro-Małysza J, Trojnar M, Skórzyńska-Dziduszko KE, Kimber-Trojnar Ż, Darmochwał-Kolarz D, Czuba M, et al. Leptin and Ghrelin in Excessive Gestational Weight Gain-Association between Mothers and Offspring. International journal of molecular sciences. 2019; 20(10):2398. [DOI:10.3390/ijms20102398] [PMID] [PMCID]
Darendeliler F, Poyrazoglu S, Bas F, Sancakli O, Gokcay G. Ghrelin levels are decreased in non-obese prepubertal children born large for gestational age. European Journal of Endocrinology. 2009; 160(6):951-6. [DOI:10.1530/EJE-08-0924] [PMID]
Chiesa C, Osborn JF, Haass C, Natale F, Spinelli M, Scapillati E, et al. Ghrelin, leptin, IGF-1, IGFBP-3, and insulin concentrations at birth: Is there a relationship with fetal growth and neonatal anthropometry? Clinical Chemistry. 2008; 54(3):550-8. [DOI:10.1373/clinchem.2007.095299] [PMID]
Slupecka-Ziemilska M, Wolinski J, Herman A, Romanowicz K, Dziegelewska Z, Borszewska-Kornacka M. Influence of preterm delivery on ghrelin and obestatin concentrations in maternal plasma, milk and their expression in mammary epithelial cells. Journal of Physiology and Pharmacology. 2017; 68:693-8.
Chen X, Du X, Zhu J, Xie L, Zhang Y, He Z. Correlations of circulating peptide YY and ghrelin with body weight, rate of weight gain, and time required to achieve the recommended daily intake in preterm infants. Brazilian Journal of Medical and Biological Research. 2012; 45(7):656-64. [DOI:10.1590/S0100-879X2012007500062] [PMID] [PMCID]
Yeung E, Sundaram R, Xie Y, Lawrence D. Newborn adipokines and early childhood growth. Pediatric Obesity. 2018; 13(8):505-13. [DOI:10.1111/ijpo.12283] [PMID] [PMCID]
Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003; 37(4):649-61. [DOI:10.1016/S0896-6273(03)00063-1]
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001; 50(4):707-9. [DOI:10.2337/diabetes.50.4.707] [PMID]
Delporte C. Structure and physiological actions of ghrelin. Scientifica. 2013; 2013. [DOI:10.1155/2013/518909] [PMID] [PMCID]
Klok M, Jakobsdottir S, Drent M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obesity Reviews. 2007; 8(1):21-34. [DOI:10.1111/j.1467-789X.2006.00270.x] [PMID]
Guo Z-F, Zheng X, Qin Y-W, Hu J-Q, Chen S-P, Zhang Z. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. The Journal of Clinical Endocrinology & Metabolism. 2007; 92(5):1875-80. [DOI:10.1210/jc.2006-2306] [PMID]
Kraemer RR, Castracane VD. Exercise and humoral mediators of peripheral energy balance: Ghrelin and adiponectin. Experimental Biology and Medicine. 2007; 232(2):184-94.
Kojima M, Kangawa K. Ghrelin: Structure and function. Physiological Reviews. 2005; 85(2):495-522. [DOI:10.1152/physrev.00012.2004] [PMID]
Pradhan G, Samson SL, Sun Y. Ghrelin: Much more than a hunger hormone. Current Opinion in Clinical Nutrition and Metabolic Care. 2013; 16(6):619. [DOI:10.1097/MCO.0b013e328365b9be] [PMID] [PMCID]
Bender BJ, Vortmeier G, Ernicke S, Bosse M, Kaiser A, Els-Heindl S, et al. Structural model of ghrelin bound to its g protein-coupled receptor. Structure. 2019; 27(3):537-44. [DOI:10.1016/j.str.2018.12.004] [PMID]
Kojima M, Kangawa K. Structure and function of ghrelin. In: Civelli O., Zhou QY. (eds) Orphan G Protein-Coupled Receptors and Novel Neuropeptides. Results and Problems in Cell Differentiation, vol 46. Springer, Berlin, Heidelberg; 2008. https://doi.org/10.1007/400_2007_0492008.
Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of ghrelin. Cell Metabolism. 2018; 27(4):786-804. [DOI:10.1016/j.cmet.2018.02.008] [PMID]
There are two ghrelin receptor types: GHS-R type 1a, a seven-domain GPCR, acting as a receptor for ghrelin receptor, and GHS-R cDNA, type 1b; the second type receiver, i.e., pharmacologically inactive (51, 52). Ghrelin is mainly produced by the pancreatic cells in the fetus; however, after birth, ghrelin is mainly secreted from gastric endocrine X/A cells (13). Furthermore, ghrelin is expressed in numerous tissues of the body, including the lung, colon, duodenum, jejunum, ileum, heart, testis, pancreas, and the pituitary gland in adolescence (50). Ghrelin, secreted from the stomach, enters the bloodstream, then the hypothalamus and pituitary. Ghrelin binds to its receptor on the surface of pituitary somatotroph cells, which activates phospholipase C and produces IP3 and diacyl glycerol; accordingly increasing intracellular calcium levels (53). Increased intracellular calcium enhances pituitary GH production, appetite, and food absorption. Growth Hormone-Releasing Hormone (GHRH) is a natural analog of ghrelin in the human body. Besides, it activates adenylate cyclase and increases intracellular cAMP by binding to its receptor on the surface of pituitary cells.
GHRH is valuable as a secondary messenger for activating protein kinase A. Moreover, increased intracellular cAMP leads to the enhanced production of pituitary GH hormones as well as body metabolism (54). GHSs are synthetic analogs for ghrelin with similar mechanisms. In humans, 133 homologs have been identified for ghrelin (50). Studies have revealed that ghrelin, by affecting the arcuate nucleus of the hypothalamus, produces NPY/AgRP neurons; orexigenic neurons reduce fatty acid oxidation, and increase hunger and food absorption in the body. On the other hand, NPY neurons, by affecting the Paraventricular Nucleus (PVN) cells of the hypothalamus, suppress the activity of gamma-aminobutyric acid neurotransmitters, increase the production of Corticotropin-Releasing Hormones (CRH), and eventually enhance the production of cortisol and Adrenocorticotropic Hormones (ACTH) (50, 53, 54). POMC is a hormone in the body that suppresses appetite. The effect of ghrelin on hypothalamic cells activates NPY/AgRP neurotransmitter neurons. The NPY/AgRP neurons suppress POMC, consequently increasing appetite and the absorption of nutrients in the body (53).
The analyzed studies investigated the effect of breast milk ghrelin on infants’ growth. In some studies, the exact level of correlation was not mentioned or a correlation was not observed; therefore, they were excluded from this study. An example of these articles was Yis U et al.’s research in 2010 that demonstrated higher serum ghrelin in breast-fed infants. They also indicated its’ role in their faster growth rate during the first three months of life; however, they concluded that breast milk ghrelin was not correlated with the respective serum ghrelin or anthropometric data in the breast-fed group (28). Francesco Savino et al. measured ghrelin, leptin, and IGF-I levels in breast-fed infants in the first year of life (33). They expressed IGF-I, leptin, and ghrelin in breast milk were effective in the growth of infants up to 4 months of age; however, the exact correlation was disregarded (33).
A limitation of this study was the limited number of articles on the subject. In addition, the articles were in English language, and the relationship between breast milk, serum ghrelin, growth, and weight gain was not addressed in some published articles. It is suggested that more studies be conducted to evaluate the effect of breast milk ghrelin and serum ghrelin on growth and weight gain in infants.
5. Conclusion
According to the present meta-analysis study data, serum ghrelin had a direct and significant correlation with the growth of infants under two years of age. Increased ghrelin in infants’ serum level enhanced appetite, food absorption, and growth. Three articles with contradictory results were found regarding the relationship between breast milk and weight gain in infants; thus, more studies are required to provide further relevant details.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
Hamedan Islamic Azad University supported this study as the research project (Code: 1445908) as a PhD dissertation at.
Authors' contributions
All authors contributed in designing, running, and writing all parts of the research.
Conflicts of interest
The authors declared no conflicts of interest.
References
Savino F, Fissore MF, Liguori SA, Oggero R. Can hormones contained in mothers’ milk account for the beneficial effect of breast‐feeding on obesity in children? Clinical Endocrinology. 2009; 71(6):757-65. [DOI:10.1111/j.1365-2265.2009.03585.x] [PMID]
Stuebe AM, Mantzoros C, Kleinman K, Gillman MW, Rifas-Shiman S, Gunderson EP, et al. Duration of lactation and maternal adipokines at 3 years postpartum. Diabetes. 2011; 60(4):1277-85. [DOI:10.2337/db10-0637] [PMID] [PMCID]
Esfahani M, Movahedian A, Baranchi M, Goodarzi MT. Adiponectin: an adipokine with protective features against metabolic syndrome. Iranian Journal of Basic Medical Sciences. 2015; 18(5):430.
Nakamura K, Fuster JJ, Walsh K. Adipokines: a link between obesity and cardiovascular disease. Journal of Cardiology. 2014; 63(4):250-9. [DOI:10.1016/j.jjcc.2013.11.006] [PMID] [PMCID]
Gillman MW, Mantzoros CS. Breast-feeding, adipokines, and childhood obesity. Epidemiology. 2007; 18(6):730-2. [DOI:10.1097/EDE.0b013e3181571df0] [PMID]
Kon IY, Shilina NM, Gmoshinskaya MV, Ivanushkina TA. The study of breast milk IGF-1, leptin, ghrelin and adiponectin levels as possible reasons of high weight gain in breast-fed infants. Annals of Nutrition and Metabolism. 2014; 65(4):317-23. [DOI:10.1159/000367998] [PMID]
Oddy WH. Infant feeding and obesity risk in the child. Breastfeeding Review. 2012; 20(2):7.
Teague AM, Fields DA, Aston CE, Short KR, Lyons TJ, Chernausek SD. Cord blood adipokines, neonatal anthropometrics and postnatal growth in offspring of Hispanic and Native American women with diabetes mellitus. Reproductive Biology and Endocrinology. 2015; 13(1):68. [DOI:10.1186/s12958-015-0061-9] [PMID] [PMCID]
Kratzsch J, Bae YJ, Kiess W. Adipokines in human breast milk. Best Practice & Research Clinical Endocrinology & Metabolism. 2018; 32(1):27-38. [DOI:10.1016/j.beem.2018.02.001] [PMID]
Godlewski G, Cinar R, Coffey N, Liu J, Jourdan T, Mukhopadhyay B, et al. Endocannabinoids promote ethanol drinking via cb 1 receptor-mediated increase in ghrelin acylation and signalling in the stomach. https://www.researchgate.net/journal/1556-5068_SSRN_Electronic_Journal.3316795. 2019.
Andrews Z. Ghrelin: What’s the function? Journal of neuroendocrinology. 2019:e12772. [DOI:10.1111/jne.12772]
Sim A, Lim E, Leow M, Cheon B. Low subjective socioeconomic status stimulates orexigenic hormone ghrelin-A randomised trial. Psychoneuroendocrinology. 2018; 89:103-12. [DOI:10.1016/j.psyneuen.2018.01.006] [PMID]
Cowley MA, Grove KL. Ghrelin-satisfying a hunger for the mechanism. Endocrinology. 2004; 145(6):2604-6. [DOI:10.1210/en.2004-0346] [PMID]
Mani BK, Osborne-Lawrence S, Metzger N, Zigman M. Lowering oxidative stress in ghrelin cells stimulates ghrelin secretion. American Journal of Physiology-Endocrinology and Metabolism. 2020. [DOI:10.1152/ajpendo.00119.2020]
Poher AL, Tschöp MH, Müller TD. Ghrelin regulation of glucose metabolism. Peptides. 2018; 100:236-42. [DOI:10.1016/j.peptides.2017.12.015] [PMID] [PMCID]
Álvarez-Castro P, Pena L, Cordido F. Ghrelin in obesity, physiological and pharmacological considerations. Mini Reviews in Medicinal Chemistry. 2013; 13(4):541-52. [DOI:10.2174/1389557511313040007] [PMID]
Aydin S, Ozkan Y, Erman F, Gurates B, Kilic N, Colak R, et al. Presence of obestatin in breast milk: Relationship among obestatin, ghrelin, and leptin in lactating women. Nutrition. 2008; 24(7-8):689-93. [DOI:10.1016/j.nut.2008.03.020] [PMID]
Ilcol YO, Hizli B. Active and total ghrelin concentrations increase in breast milk during lactation. Acta Pædiatrica. 2007; 96(11):1632-9. [DOI:10.1111/j.1651-2227.2007.00493.x] [PMID]
Savino F, Liguori SA, Fissore MF, Oggero R, Silvestro L, Miniero R. Serum ghrelin concentration and weight gain in healthy term infants in the first year of life. Journal of Pediatric Gastroenterology and Nutrition. 2005; 41(5):653-9. [DOI:10.1097/01.mpg.0000181856.54617.04] [PMID]
Cesur G, Ozguner F, Yilmaz N, Dundar B. The relationship between ghrelin and adiponectin levels in breast milk and infant serum and growth of infants during early postnatal life. The Journal of Physiological Sciences. 2012; 62(3):185-90. [DOI:10.1007/s12576-012-0193-z] [PMID]
Khodabakhshi A, Ghayour-Mobarhan M, Rooki H, Vakili R, Hashemy S, Mirhafez S, et al. Comparative measurement of ghrelin, leptin, adiponectin, EGF and IGF-1 in breast milk of mothers with overweight/obese and normal-weight infants. European Journal of Clinical Nutrition. 2015; 69(5):614. [DOI:10.1038/ejcn.2014.205] [PMID]
Huang L, Yang F, Xiong F. Association of leptin, adiponectin, and ghrelin in breast milk with the growth of infants with exclusive breastfeeding. Chinese Journal of Contemporary Pediatrics. 2018; 20(2):91-6.
Pirazzoli P, Lanari M, Zucchini S, Gennari M, Pagotto U, De Iasio R, et al. Active and total ghrelin concentrations in the newborn. Journal of Pediatric Endocrinology and Metabolism. 2005; 18(4):379-84. [DOI:10.1515/JPEM.2005.18.4.379] [PMID]
Khodabakhshi A, Mehrad-Majd H, Vahid F, Safarian M. Association of maternal breast milk and serum levels of macronutrients, hormones, and maternal body composition with infant’s body weight. European Journal of Clinical Nutrition. 2018; 72(3):394. [DOI:10.1038/s41430-017-0022-9] [PMID]
Kahveci H, Laloglu F, Kilic O, Ciftel M, Kara M, Laloglu E, et al. Fasting and postprandial glucose, insulin, leptin, and ghrelin values in preterm babies and their mothers: relationships among their levels, fetal growth, and neonatal anthropometry. The Journal of Maternal-Fetal & Neonatal Medicine. 2015; 28(8):916-21. [DOI:10.3109/14767058.2014.937693] [PMID]
Breij LM, Mulder MT, Hokken-Koelega AC. Appetite-regulating hormones in early life and relationships with type of feeding and body composition in healthy term infants. European Journal of Nutrition. 2017; 56(4):1725-32. [DOI:10.1007/s00394-016-1219-8] [PMID] [PMCID]
Savino F, Petrucci E, Lupica MM, Nanni GE, Oggero R. Assay of ghrelin concentration in infant formulas and breast milk. World Journal of Gastroenterology. 2011; 17(15):1971. [DOI:10.3748/wjg.v17.i15.1971] [PMID] [PMCID]
Yis U, Öztürk Y, Sisman AR, Uysal S, Soylu ÖB, Büyükgebiz B. The relation of serum ghrelin, leptin and insulin levels to the growth patterns and feeding characteristics in breast-fed versus formula-fed infants. The Turkish Journal of Pediatrics. 2010; 52(1):35.
Savino F, Benetti S, Lupica M, Petrucci E, Palumeri E, Di Montezemolo LC. Ghrelin and obestatin in infants, lactating mothers and breast milk. Hormone Research in Paediatrics. 2012; 78(5-6):297-303. [DOI:10.1159/000345876] [PMID]
Kierson JA, Dimatteo DM, Locke RG, MacKley AB, Spear ML. Ghrelin and cholecystokinin in term and preterm human breast milk. Acta Paediatrica. 2006; 95(8):991-5. [DOI:10.1080/08035250600669769] [PMID]
Weyermann M, Brenner H, Rothenbacher D. Adipokines in human milk and risk of overweight in early childhood: A prospective cohort study. Epidemiology. 2007; 18(6):722-9. [DOI:10.1097/EDE.0b013e3181567ed4] [PMID]
Karatas Z, Aydogdu SD, Dinleyici EC, Colak O, Dogruel N. Breast milk ghrelin, leptin, and fat levels changing foremilk to hindmilk: Is that important for self-control of feeding? European Journal of Pediatrics. 2011; 170(10):1273-80. [DOI:10.1007/s00431-011-1438-1] [PMID]
Savino F, Fissore MF, Grassino EC, Nanni GE, Oggero R, Silvestro L. Ghrelin, leptin and IGF‐I levels in breast‐fed and formula‐fed infants in the first years of life. Acta Paediat. 2005; 94(5):531-7. [DOI:10.1111/j.1651-2227.2005.tb01934.x] [PMID]
Savino F, Lupica MM, Liguori SA, Fissore MF, Silvestro L. Ghrelin and feeding behaviour in preterm infants. Early Human Development. 2012; 88:S51-S5. [DOI:10.1016/j.earlhumdev.2011.12.028] [PMID]
Savino F, Liguori SA, Fissore MF, Oggero R. Breast milk hormones and their protective effect on obesity. International Journal of Pediatric Endocrinology. 2009; 2009(1):327505. [DOI:10.1186/1687-9856-2009-327505] [PMID] [PMCID]
Savino F, Benetti S, Liguori SA, Sorrenti M, Cordero Di Montezemolo L. Advances on human milk hormones and protection against obesity. Molecular and Cellular Biology. 2013; 59(1):89-98.
Yu X, Rong SS, Sun X, Ding G, Wan W, Zou L, et al. Associations of breast milk adiponectin, leptin, insulin and ghrelin with maternal characteristics and early infant growth: A longitudinal study. British Journal of Nutrition. 2018; 120(12):1380-7. [DOI:10.1017/S0007114518002933] [PMID]
Patro-Małysza J, Trojnar M, Skórzyńska-Dziduszko KE, Kimber-Trojnar Ż, Darmochwał-Kolarz D, Czuba M, et al. Leptin and Ghrelin in Excessive Gestational Weight Gain-Association between Mothers and Offspring. International journal of molecular sciences. 2019; 20(10):2398. [DOI:10.3390/ijms20102398] [PMID] [PMCID]
Darendeliler F, Poyrazoglu S, Bas F, Sancakli O, Gokcay G. Ghrelin levels are decreased in non-obese prepubertal children born large for gestational age. European Journal of Endocrinology. 2009; 160(6):951-6. [DOI:10.1530/EJE-08-0924] [PMID]
Chiesa C, Osborn JF, Haass C, Natale F, Spinelli M, Scapillati E, et al. Ghrelin, leptin, IGF-1, IGFBP-3, and insulin concentrations at birth: Is there a relationship with fetal growth and neonatal anthropometry? Clinical Chemistry. 2008; 54(3):550-8. [DOI:10.1373/clinchem.2007.095299] [PMID]
Slupecka-Ziemilska M, Wolinski J, Herman A, Romanowicz K, Dziegelewska Z, Borszewska-Kornacka M. Influence of preterm delivery on ghrelin and obestatin concentrations in maternal plasma, milk and their expression in mammary epithelial cells. Journal of Physiology and Pharmacology. 2017; 68:693-8.
Chen X, Du X, Zhu J, Xie L, Zhang Y, He Z. Correlations of circulating peptide YY and ghrelin with body weight, rate of weight gain, and time required to achieve the recommended daily intake in preterm infants. Brazilian Journal of Medical and Biological Research. 2012; 45(7):656-64. [DOI:10.1590/S0100-879X2012007500062] [PMID] [PMCID]
Yeung E, Sundaram R, Xie Y, Lawrence D. Newborn adipokines and early childhood growth. Pediatric Obesity. 2018; 13(8):505-13. [DOI:10.1111/ijpo.12283] [PMID] [PMCID]
Cowley MA, Smith RG, Diano S, Tschöp M, Pronchuk N, Grove KL, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003; 37(4):649-61. [DOI:10.1016/S0896-6273(03)00063-1]
Tschöp M, Weyer C, Tataranni PA, Devanarayan V, Ravussin E, Heiman ML. Circulating ghrelin levels are decreased in human obesity. Diabetes. 2001; 50(4):707-9. [DOI:10.2337/diabetes.50.4.707] [PMID]
Delporte C. Structure and physiological actions of ghrelin. Scientifica. 2013; 2013. [DOI:10.1155/2013/518909] [PMID] [PMCID]
Klok M, Jakobsdottir S, Drent M. The role of leptin and ghrelin in the regulation of food intake and body weight in humans: A review. Obesity Reviews. 2007; 8(1):21-34. [DOI:10.1111/j.1467-789X.2006.00270.x] [PMID]
Guo Z-F, Zheng X, Qin Y-W, Hu J-Q, Chen S-P, Zhang Z. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. The Journal of Clinical Endocrinology & Metabolism. 2007; 92(5):1875-80. [DOI:10.1210/jc.2006-2306] [PMID]
Kraemer RR, Castracane VD. Exercise and humoral mediators of peripheral energy balance: Ghrelin and adiponectin. Experimental Biology and Medicine. 2007; 232(2):184-94.
Kojima M, Kangawa K. Ghrelin: Structure and function. Physiological Reviews. 2005; 85(2):495-522. [DOI:10.1152/physrev.00012.2004] [PMID]
Pradhan G, Samson SL, Sun Y. Ghrelin: Much more than a hunger hormone. Current Opinion in Clinical Nutrition and Metabolic Care. 2013; 16(6):619. [DOI:10.1097/MCO.0b013e328365b9be] [PMID] [PMCID]
Bender BJ, Vortmeier G, Ernicke S, Bosse M, Kaiser A, Els-Heindl S, et al. Structural model of ghrelin bound to its g protein-coupled receptor. Structure. 2019; 27(3):537-44. [DOI:10.1016/j.str.2018.12.004] [PMID]
Kojima M, Kangawa K. Structure and function of ghrelin. In: Civelli O., Zhou QY. (eds) Orphan G Protein-Coupled Receptors and Novel Neuropeptides. Results and Problems in Cell Differentiation, vol 46. Springer, Berlin, Heidelberg; 2008. https://doi.org/10.1007/400_2007_0492008.
Yanagi S, Sato T, Kangawa K, Nakazato M. The homeostatic force of ghrelin. Cell Metabolism. 2018; 27(4):786-804. [DOI:10.1016/j.cmet.2018.02.008] [PMID]
Type of Study: Meta-analysis Review |
Subject:
Endocrinology
Received: 2019/12/10 | Accepted: 2020/02/4 | Published: 2020/07/1
Received: 2019/12/10 | Accepted: 2020/02/4 | Published: 2020/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |