Volume 9, Issue 3 (7-2021)
J. Pediatr. Rev 2021, 9(3): 175-196 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Vard B, Mahdieh M, Riahi R, Heidari-Beni M, Kelishadi R. The Association Between Antioxidant Status and Excess Weight in Children: A Systematic Review and Meta-analysis. J. Pediatr. Rev 2021; 9 (3) :175-196
URL: http://jpr.mazums.ac.ir/article-1-392-en.html
URL: http://jpr.mazums.ac.ir/article-1-392-en.html
1- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Pediatrics, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran. ,mari.mahdieh@gmail.com
3- Department of Pediatrics, Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Pediatrics, School of Medicine, Isfahan University of Medical Sciences, Isfahan, Iran. ,
3- Department of Pediatrics, Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
Full-Text [PDF 758 kb]
(1483 Downloads)
| Abstract (HTML) (5847 Views)
Full-Text: (1954 Views)
1. Introduction
Obesity and overweight are dramatically increasing worldwide [1]. Obesity is associated with several chronic lifelong diseases [2]. During the last three decades, the prevalence of obesity in children has increased worldwide [3], including in Iran, which is the seventh country in terms of having obese children [4]. The prevalence rates of overweight and obesity among Iranian children have reached 10.8% and 5.1%, respectively, between 2007 and 2012 [5]. Childhood obesity increases the risk of morbidity due to obesity in adulthood [6]. Obesity in children is associated with various factors such as genetics, environment, nutrition, and physical inactivity, especially screen time [7].
Reactive species of oxygen and nitrogen are unstable and highly reactive molecules. These molecules oxidize various macromolecules to reach a stable state [8, 9]. Free radicals destroy fats, proteins, and cellular DNA [10]. Antioxidants are generally regenerative substances found inside and outside the cell and can react with free radicals and active species. Antioxidants reduce or prevent oxidative stress by reacting with free radicals and active species [10, 11]. Antioxidants are synthesized both in the body and absorbed through food. Antioxidants are generally divided into two categories: enzymatic and non-enzymatic. The most important antioxidants are Superoxide Dismutase (SOD), Glutamine Peroxidase (GPX), and Catalase (CAT) [10, 11]. Vitamins C, A, E, and β-carotene are non-enzymatic antioxidants, and magnesium, zinc, selenium, and copper are mineral antioxidants [12].
Oxidative stress might change the balance between the production of free radicals and the antioxidant defense system in favor of free radicals, which would cause oxidative damage and worsen the pathological status [13]. On the one hand, obesity is usually associated with micronutrient deficiency as a result of an unhealthy diet and low antioxidants intakes, and on the other hand, it can change the balance towards oxidative stress [14].
Magnesium is related to the enzymatic activity of cells and glucose metabolism; a significant relationship is reported between magnesium deficiency and obesity [15]. Zinc is one of the most important elements in several health issues. Some studies found a significant relationship between low levels of zinc and high levels of leptin [15]. Selenium is a mineral antioxidant that prevents the damaging effects of radicals and strengthens the immune system and metabolic processes, and decreases obesity status [16]. Copper is a mineral and one of the components of antioxidant enzymes in the body that plays an essential role in upsetting the balance in favor of antioxidants and has an inverse relationship with obesity [17].
In recent years, many studies assessed the relationship between antioxidant levels and obesity in adults [18], but few studies have been conducted in the pediatric age group [19, 20]. These studies have reported controversial findings. Therefore, this study aims to assess the current literature on the relationship between childhood obesity and antioxidant status through systematic review and meta-analysis.
2. Materials and Methods
In this systematic review, the following keywords and their equivalents were searched separately and together: “Vitamin C”, “Vitamin E”, “Vitamin A”, “Carotenoids”, “Selenium”, “Magnesium”, “Copper”, “Zinc”, “Ascorbic acid”, “Tocopherol”, “Antioxidants”, “Childhood”, “Pediatric Adolescence”, “Overweight”, and “Obesity”. These words were searched in international databases of Google Scholar, PubMed, Web of Science, Scopus, Medline, and Cochrane. This research was conducted without time or gender restrictions until September 2020.
The search was performed by two researchers independently. Examining the agreement between the search results was performed by a third person, where eventually, duplicates were omitted. This study includes case-control, cross-sectional, and prospective articles with control groups. These English language full-text articles evaluated the association between antioxidant status and overweight/obesity. Overweight and obesity were confirmed by anthropometric indices: Body Mass Index (BMI), weight for height, and waist circumference. Research on animals, interventional studies, case studies, case reports, and irrelevant studies were excluded.
Data extraction
Initially, 1255 articles were extracted from the mentioned databases. Then, 360 duplicate articles were removed by reviewing the titles (those extracted by the two researchers whose titles of the authors and the published journal are the same). Next, the abstracts of the remaining 895 articles were reviewed, where 380 articles were omitted because the studies were case study, animal research, or their interventions were irrelevant. Afterward, the full texts of the remaining 515 articles from the previous step were read, and unrelated articles were excluded because of lacking the inclusion criteria. Finally, 46 articles were included in the systematic review after examining the inclusion and exclusion criteria, and 19 competent articles in the meta-analysis (Figure 1).

Quality of studies
All articles included in the current review were extracted using a pre-prepared checklist. It included the author’s last name, year of publication, country of origin, age, sex, and sample size. After reviewing the inclusion and exclusion criteria and determining the related studies, the quality of the articles was independently assessed by two researchers using the checklist of STROBE (strengthening the reporting of observational studies in epidemiology). This checklist has 22 sections and various methodological aspects, including sampling and measuring methods, variables, statistical analysis, and study objectives. In this checklist, the minimum and maximum scores were 1 and 22. The studies with a score of 11 and above were entered into this review study, and the data related to them were selected for the meta-analysis process. A third researcher examined any disagreement between the two researchers (Figure 1).
Data analyses
The desired pooled effect size was considered a mean difference with a 95% Confidence Interval (CI). We used the forest plot to investigate the association between antioxidants levels and obesity among children and adolescents. The fixed-effects model was used according to the nonexistence of significant heterogeneity. The z-statistics was used to assess the significance of the pooled effect size; a P value of less than 0.05 was considered as statistically significant. Heterogeneity between the included studies was assessed by Cochran’s Q statistic, which was quantified by calculating the inconsistency index (I2). In cases with high heterogeneity among studies (substantial heterogeneity considered as I2>50%), the random-effects model with Der Simonian and Liard method was used. We assessed potential publication bias using funnel plots (not shown) and both Begg’s and Egger’s tests. P values of less than 0.05 from both Begg’s and Egger’s tests and the asymmetrical shape of the funnel plot showed statistically significant publication bias. The sensitivity analysis was conducted to assess the extent of the influence of omitting individual studies on the pooled mean difference. The analysis was conducted by the Stata software, version 11.2 (STATA Corp, College Station, TX, USA).
3. Results
Figure 1 shows that 46 articles were reviewed, and finally, 19 articles met the inclusion criteria and were entered into the analysis. Table 1 shows a summary of reviewed articles.

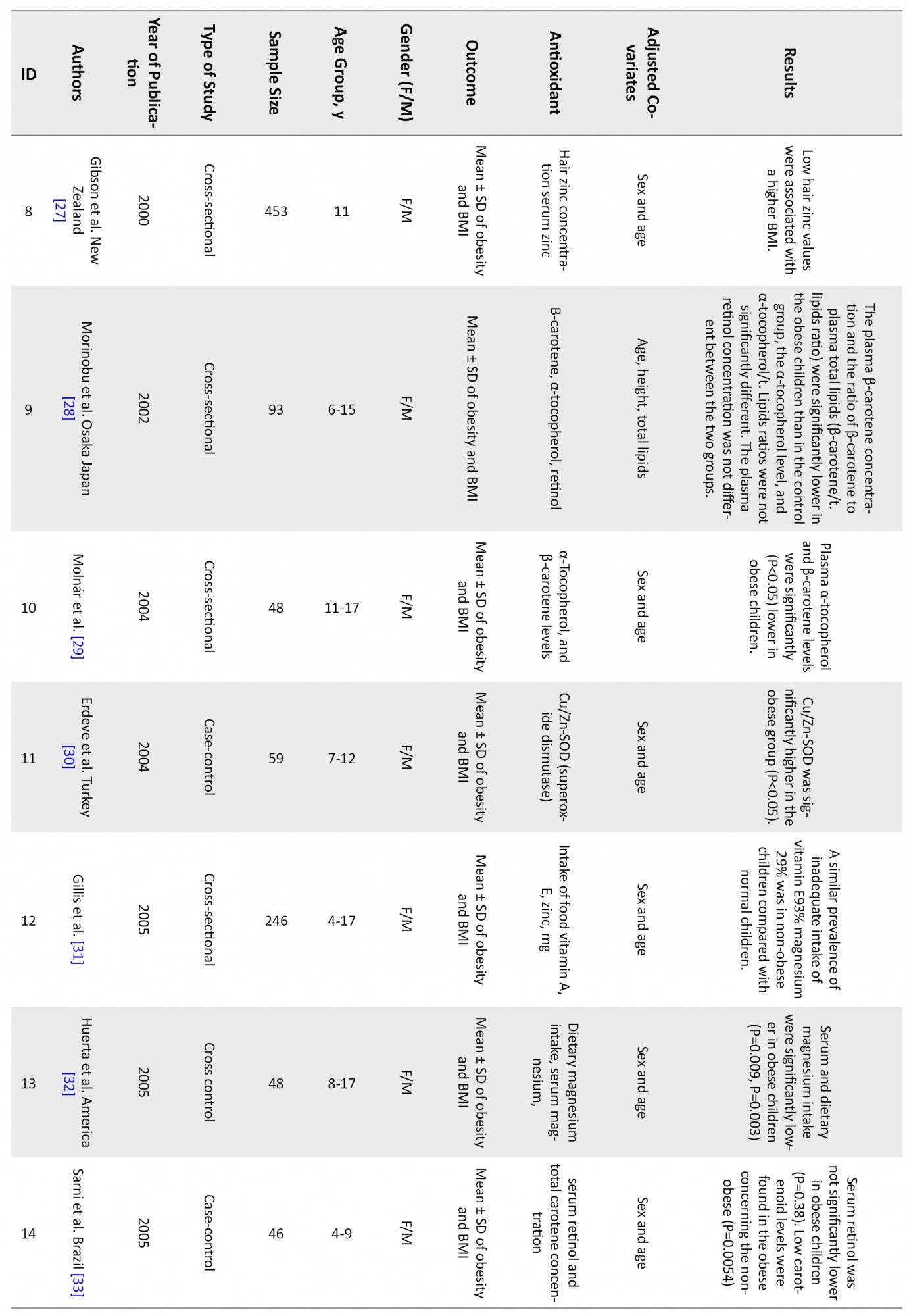
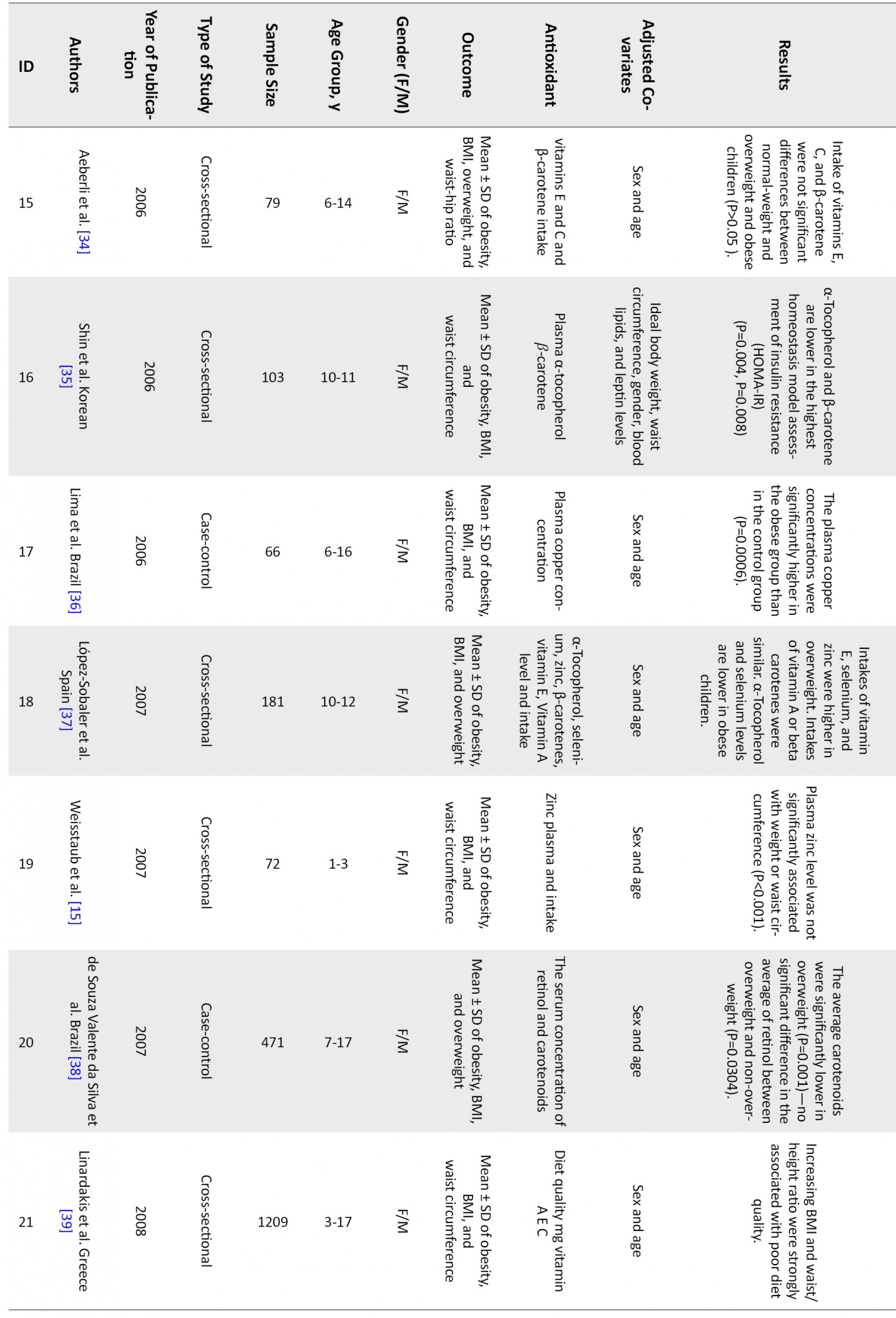
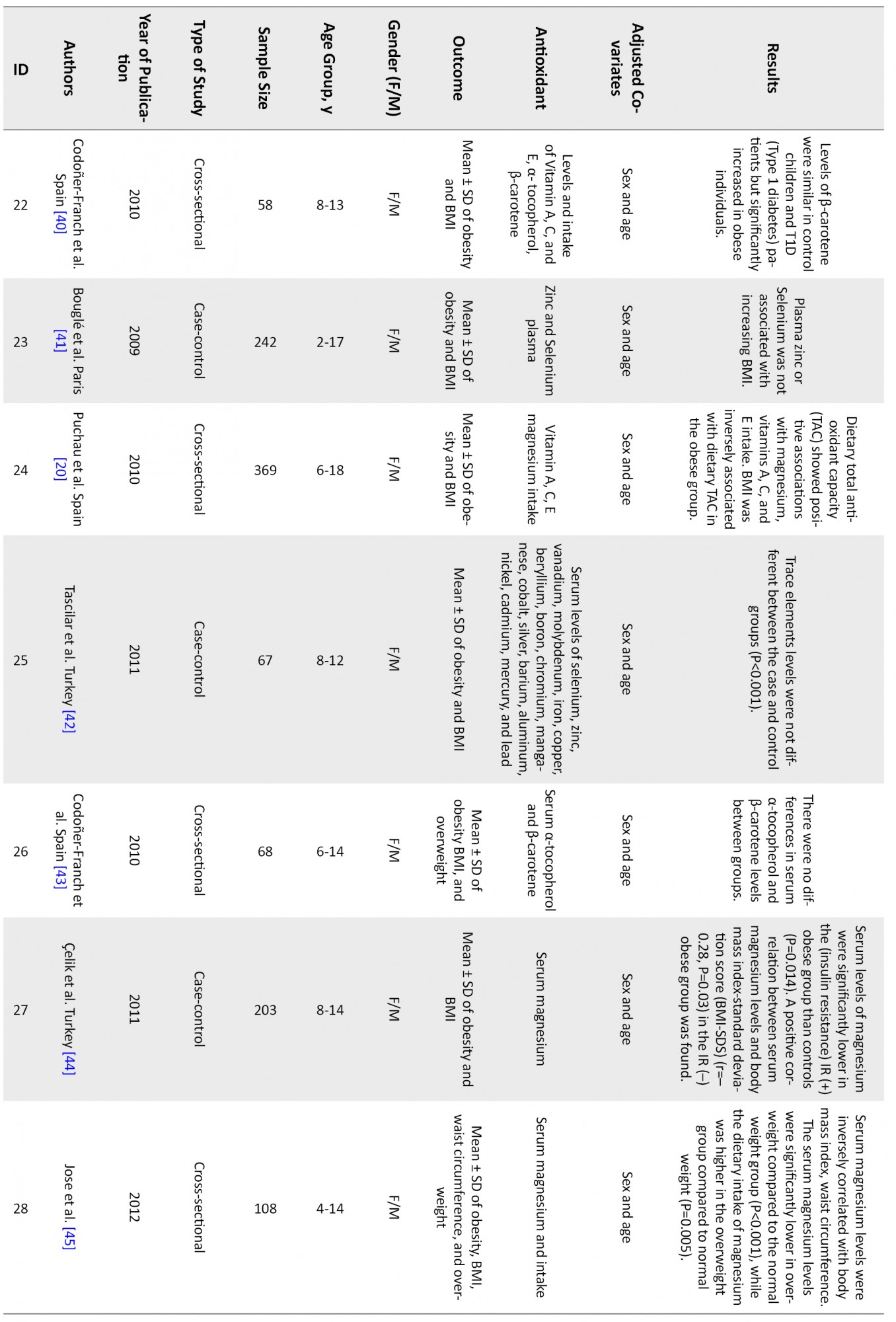
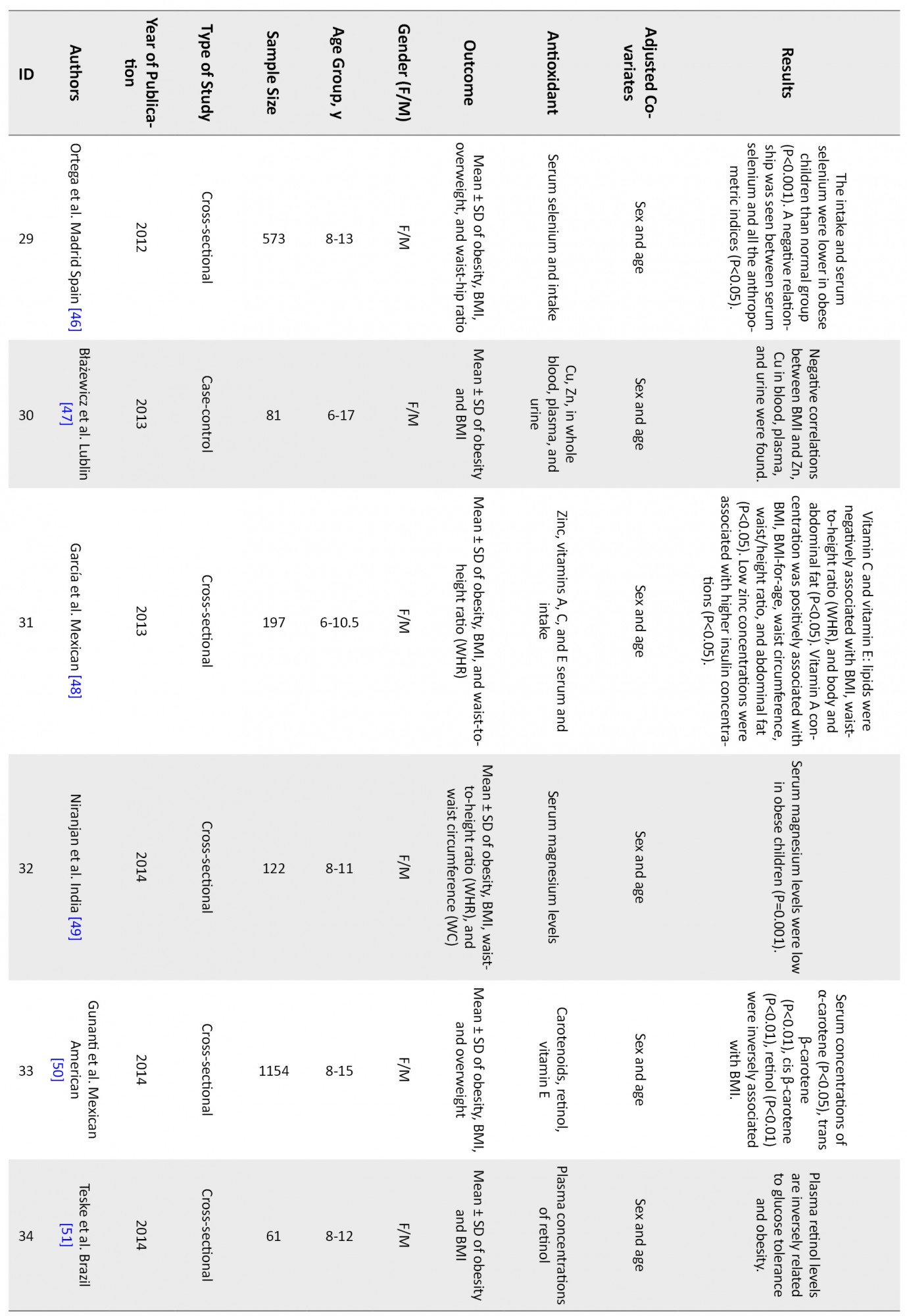


The associations of following antioxidants and childhood obesity were assessed:
Association between obesity and β-carotene
The forest plot for the association between antioxidants level and obesity according to the type of antioxidants is shown in Figures 2, 3, 4, 5, 6 and 7. The pooled mean difference of β-carotene level between obese and non-obese children was significantly different (mean difference: 0.13, 95% CI: 0.09-0.16, P<0.001), with significant heterogeneity (P<0.001, I2=85%) (Figure 2).

We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: P=0.27).
Subgroup meta-analysis according to α-tocopherol type
Results of subgroup meta-analysis according to α-tocopherol type are shown in Figure 3.

There was a significant difference between obese and non-obese children in both α-tocopherol (pooled mean difference respectively: 0.36, 95%CI: 0.04-0.96, P<0.001) with non-significant heterogeneity (P>0.05, I2=0.0%) and α-tocopherol per lipoid (pooled mean difference: 0.42, 95%CI: 0.28-0.55, P<0.001), with significant heterogeneity (P=0.048, I2=58.8%) (Figure 3).
We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: P=0.91).
Association between obesity and vitamin E
The forest plot for the association between vitamin E level and obesity is shown in Figure 4.

There was no significant association between vitamin E level and obesity (pooled mean difference: 0.40, 95%CI: -0.05-0.85, P>0.05), with significant heterogeneity (P<0.001, I2=84.5%) (Figure 4). We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: P=0.18).
Association of obesity with zinc, magnesium, copper, and selenium
The forest plot for the association between obesity and variables of zinc, magnesium, copper, and selenium level are shown in Figures 5, 6 and 7.
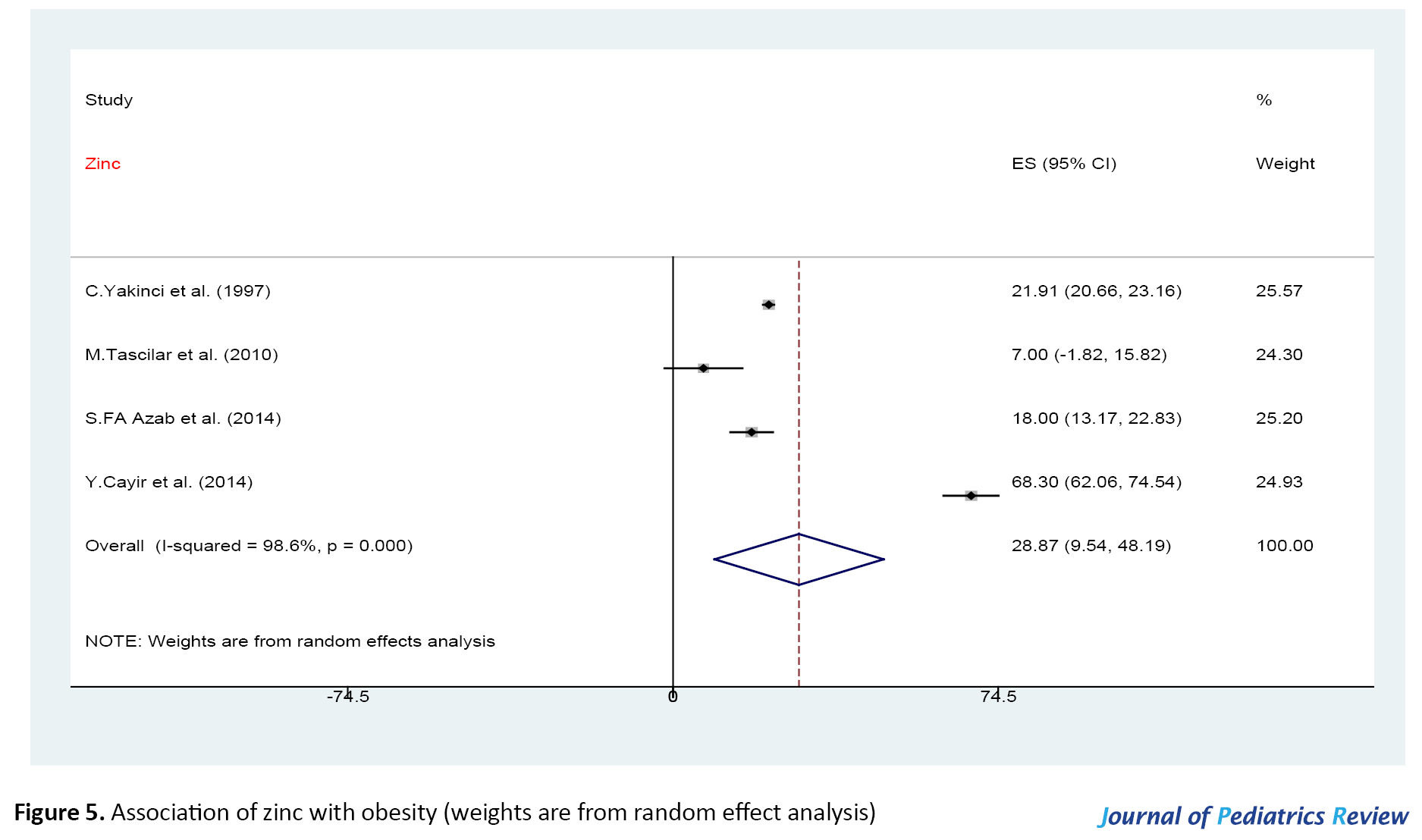


There were significant associations between zinc, magnesium, copper, and selenium level and obesity (P>0.05), with significant heterogeneity (P<0.001). We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: zinc studies, P=0.65; magnesium studies, P=0.07; selenium and copper studies, P=0.98).
Sensitivity analysis
The sensitivity analysis showed that eliminating any individual studies did not significantly change the pooled effect size and heterogeneity among magnesium, copper, and selenium studies (P>0.05). Regarding other antioxidant studies, the results of sensitivity analysis are presented in Table 2.

DiscussionThis systematic review and meta-analysis study was performed to determine the relationship between childhood obesity and anthropometric indices with antioxidant status. After completing the search, 46 articles were systematically reviewed (Table 1), and 19 were entered into the meta-analysis. The results showed that vitamins A, C, and their components, including α-tocopherol and β-carotene, as well as selenium, copper, magnesium, and zinc, were inversely related to childhood obesity. But vitamin E is not significantly associated with obesity.
Eighteen studies examined the relationship between vitamins A, E, C and obesity separately or together. In these studies, dietary intake of antioxidants or their serum levels or both have been investigated. In some studies, dietary intake of antioxidants predicted their serum levels, but in some studies, despite dietary intake of antioxidants, their serum levels were low, which suggests that serum levels of antioxidants may depend on various factors. In each article, a different hypothesis is addressed.
Strauss et al. reported that serum levels of α-tocopherol and β-carotene were significantly lower in obese children compared with the control group (P<0.001). It is hypothesized that the breakdown of α-tocopherol from adipose tissue in obese children is limited, leading to reduced access to other organs and thus its reduced serum levels [25]. Gillis et al. analyzed the diets of 156 obese children and 90 non-obese controls aged 4-17 years. The results showed that inadequate intake of vitamins E, A, magnesium, and zinc did not differ significantly between the two groups; however, in obese children, this inadequate intake was higher, indicating that all children should receive adequate vitamins as they grow [31].
Obese children may have inadequate food intake due to low intake of vitamins A, E, and C but may not have a poor diet due to overeating. Therefore, controversial results have been obtained regarding the relationship between serum levels of antioxidants and intake.
Aeberli et al. examined the food intake of the last 48 hours of 3 groups of normal, overweight, and obese children. They found that the intake of vitamins C, E, and carotene in the three groups was not significantly different, and only the amount of protein and meat intake was different between the two groups. The researchers proposed that mentioning food items in the food questionnaire in children may have some shortcomings and is not effective as adults [34]. Lopez-Sobaler et al. studied the 3-day dietary intake of 10-12 years old children. They found that the intake of vitamin E, selenium, and zinc is higher in obese and overweight children, but vitamin A or β-carotene intake did not differ between obese and control groups. Serum levels of α-tocopherol and selenium were lower in obese children [37]. Codone R-Franch et al. compared the dietary intake of two groups of children with Type 1 diabetes and obese children without disease with the control group. They showed that the intake of vitamins A, E, C, and fruits and vegetables was not different, and contrary to expectations, serum β-carotene levels were similar in diabetic children and the control group, but it was higher in the obese children. This finding may be because the obese children in this study had a normal diet [40].
Food questionnaires and their items and also the duration of recall varied in different articles, which makes it difficult to compare their results and draw conclusions.
Puchau et al. showed that dietary Total Antioxidant Capacity (TAC) is directly related to vitamins A.E, C and is inversely related to BMI [20]. Linardakis et al. reported that the group that received a nutritious diet of vitamins A, E, C, and minerals had lower metabolic syndrome indices such as BMI and waist/height ratio [39]. Ford et al. conducted a study on participants in the Third National Health and Nutrition Examination Survey (NHANES III). They found that all carotenoids from vitamin A compounds except lycopene were inversely related to BMI (P<0.001). There was also a direct relationship between dietary intake of fruits and vegetables and carotenoids [26].
In some reviewed articles, as expected, the levels of β-carotene and α-tocopherol and other components of vitamins A and E are inversely related to obesity and anthropometric indices, but in some articles, no significant relationship was found. In our study, we found no significant relationship between vitamin E with obesity, but α-tocopherol is a significant component of vitamin E and is significantly lower in obese children.
Because obese individuals are prone to hyperlipidemia and insulin resistance, Kljno et al. measured plasma levels of β-carotene and α-tocopherol in plasma and LDL. They wanted to assess antioxidant intake capacity and showed that the levels of β-carotene and α-tocopherol are lower in plasma and LDL of obese girls, and this low amount can make obese girls more prone to future atherosclerotic events [19]. A study by Gunanti et al. on children aged 8-15 years showed that plasma concentrations of α-tocopherol (β=–0.88, P<0.05), trans β-carotene (β=–2.21, P<0.01), cis β-carotene (β=–2.10, P<0.01), and α-tocopherol (β=–3.66, P<0.01) were inversely related to BMI [50]. Molnar et al. found that plasma β-carotene and α-tocopherol were significantly lower in obese children (P<0.005) [29]. Shin et al. showed a significant inverse relationship between HOMA-IR (Homeostasis Model Assessment of Insulin Resistance) and β-carotene (r=-0.233, P<0.05) and α-tocopherol (r=-0.370, P<0.0001) [35].
Different hypotheses have been suggested for the association of vitamin A and E with obesity. There is a hypothesis that in obesity, antioxidant levels rise due to an increase in the levels of oxidative stress. In other words, the defense system in the body is activated against antioxidants. Contrary to expectations, it leads to increase levels of antioxidants in obesity [64].
Codoñer-Franch et al. reported that β-carotene and α-tocopherol were not significantly associated with obesity. Because these two vitamins are fat-soluble, obese children in this study had normal lipid profiles to justify the result [43]. García et al. studied 197 children aged 6-10.5 years and found that vitamin A was positively correlated with BMI and Waist to Height Ratio (WHR) (P<0.05). Vitamins E and C were inversely related to BMI waist/height ratio (P<0.05). This finding may result from the direct relation of vitamin A, which is a fat-soluble vitamin, to the storage of adipose tissue [48]. Because vitamins A and E are fat-soluble and obese children have high-fat tissues, these vitamins can increase with obesity [65].
Another component of vitamin A is retinol, which has an antioxidant role [66], but different results are published in various articles on obesity. Mobinobu et al. showed that levels of plasma β-carotene were significantly lower in obese children compared with control groups. However, no significant differences were found in plasma levels of α-tocopherol and retinol, which may result from closer relation of β-carotene levels to adipose tissue than retinol [28]. Sarni et al. reported that serum carotene levels were significantly lower in obese children compared with the control group but did not differ significantly in retinol levels, although they were lower in obese children [33]. de Souza Valente da Silva et al. argued that overweight children had lower carotenoid serum (P<0.001), but there was no significant difference between BMI and retinol (P=0.304). This fact may indicate that retinol plays a lower role in antioxidant activity [38]. Teske et al. showed that plasma retinol levels were inversely related to obesity and glucose tolerance (fasting blood sugar and 2 hours later). In other words, it measured the association of retinol with metabolic syndrome [51]. However, few studies have linked retinol to obesity. Future studies may further consider the role of retinol in antioxidants activities.
Regarding the relationship between childhood obesity and variables of copper and selenium, zinc, magnesium status, we found 29 articles on the relationship of zinc, copper, magnesium, and selenium to obesity separately or in combinations. Some articles revealed an inverse relationship between obesity and the levels of these elements, which might be because of the antioxidant properties of these elements that can be reduced in obesity.
Various studies have been performed on the role of zinc in obesity, and the association between zinc and leptin has been considered. Leptin is a hormone produced by adipose tissue. In zinc deficiency, a decrease in zinc is associated with a decrease in leptin. Decreased leptin leads to increased appetite and obesity [15].
Magnesium, copper, selenium are essential trace elements. They play an essential role in the growth and regulation of various enzymes in metabolic processes [67]. Some studies have shown that obesity is associated with reduced trace elements.
Vivek et al. showed that zinc and copper levels are significantly lower in obesity among Indian children aged 6-16 years [63]. In a study by Perrone et al. serum zinc and copper levels were lower in obese, overweight children [24]. Błażewicz et al. reported that levels of zinc and copper of plasma are inversely related to BMI [47]. Erdeve et al. reported that Cu/Zn-SOD (superoxide dismutase) enzymes in obese children are significantly higher, indicating that the balance is changed in favor of the oxidative status in obesity (P<0.05) [30].
In some studies, the levels of antioxidants were searched in other body components like hair, nail, and urine. Gibson et al. showed that obese children with higher BMIs in New Zealand had lower hair zinc levels [27]. Xu et al. showed that nail selenium levels are lower in obese people, although there is no significant difference [59]. Błażewicz et al. found that obese people had lower serum and urine selenium [55].
In the Huerta et al. study, serum magnesium levels, as well as magnesium intake from food, were significantly lower in obese children (P=0.009) [32]. Zaakouk et al. concluded that serum magnesium levels were significantly lower in obese people (r=-0.8, P<0.001) [54]. Niranjan et al. also showed that obese people had lower magnesium levels [49]. Ul Hassan et al. reported that serum magnesium levels were significantly lower in obese and overweight individuals (P<0.001) [57]. Ortega et al. showed that dietary selenium intake was directly related to selenium levels and was lower in obese children and inversely related to BMI. This finding indicates that obese children had worse antioxidant status than other children [46].
However, in several reviewed articles, different results were obtained. They reported that the levels of zinc, copper, magnesium, and selenium are directly related to obesity, or at least there is no significant difference. Gonoodi et al. examined 408 Iranian girls aged 12-18 years, found that serum levels and intake of zinc and copper were not associated with obesity [60]. Studies show a correlation between serum zinc level and appetite which can lead to obesity. In other words, there is a direct link between zinc and increased appetite [68]. Hongo et al. conducted a study of 66 boys and girls in Tokyo, Japan. They reported that dietary zinc intake in the 3-day diet questionnaire was not related to serum zinc concentration. In other words, zinc food intake was not a predictor of serum level, and zinc status was not related to weight and height (P<0.01). That is, this study, which showed zinc deficiency in Japanese children, hypothesized that borderline serum deficiency could not affect children’s height and BMI [21].
In the study of Weisstaub et al. on 18- to 36-month-old children of Chile, no relationship was found between zinc of plasma, height, and waist circumference, which may be due to borderline zinc deficiency [15]. A study by Yakinci et al. on children aged 7 to 11 years in Turkey showed that zinc and copper levels in obese children were significantly higher than in control groups (P<0.01), which may be due to the high appetite of obese children. Dietary intake of zinc and copper was associated with serum levels, but magnesium levels were lower in obese children (P<0.01) [23].
Zinc is involved in the cellular defense of lymphocytes. In this regard, Marotta et al. examined two groups of obese children and control groups of 5 to 17 years old in Nepal and found that the level of zinc content in lymphocytes was lower in obese children, but there was a significant difference in plasma zinc and erythrocytes and polymorphonuclear. According to the hypothesis of this article, zinc deficiency in lymphocytes can be an indicator of zinc deficiency in obese children [22].
Bouglé et al. study did not find any significant relationship between zinc and selenium levels and BMI. In other words, this study showed that obese children are not always deficient in micronutrients [41]. Tascilar et al. who studied the serum levels of various trace elements in obese children, concluded that serum zinc, copper, and selenium were lower in obese people but did not differ significantly from the control group. This finding may be due to the diet of obese children, which is not nutritious and in nutrients such as trace elements, and obese children are deficient in these substances [42].
Çelik et al. reported that serum magnesium levels in obese people with insulin resistance are very low, but the two obese groups without insulin resistance and the control group are not different. On the other hand, serum magnesium levels are directly related to BMI. The researchers may believe that this finding is due to the study’s limitations in measuring total magnesium, including intracellular magnesium, but in this study, serum magnesium was measured [44]. Jose et al. concluded that although dietary magnesium intake was higher in obese Indian children, serum magnesium levels were lower in obese children and were inversely related to BMI due to decreased reabsorption or increased urinary magnesium excretion. Magnesium excretion increase has been previously reported in obese adults and hypertension and Type 2 diabetes [45]. However, several studies have shown that copper levels are directly related to obesity. One of the hypotheses is that copper levels rise in obesity due to the reduction of zinc and the antagonist properties of zinc and copper in the body.
Chen et al. concluded that levels of plasma copper are directly related to BMI [62]. Gaber et al. found that obesity is associated with increased copper levels and decrease zinc and magnesium levels [61]. In the study of Lima et al. plasma copper levels in obese children were higher than that in the control group, but the copper concentration of erythrocytes did not differ [36]. In the study of Azab et al. serum levels of zinc and selenium are lower in obese people, but copper levels were higher in obese people due to the antagonist effect of zinc and copper in the body [52]. In the study by Habib et al. plasma zinc levels were lower in obese Egyptian children (P<0.001), but plasma copper levels were significantly higher in obese individuals, possibly due to zinc and copper interactions. In other words, dietary zinc intake may be associated with some degree of copper deficiency [56].
In the study by Fan et al. zinc and selenium levels were inversely related to the BMI of American children, but serum copper levels were directly related to BMI, which could still be due to zinc-copper interactions [58]. Cayir et al. showed that selenium and copper are higher in obese people, but zinc levels are lower, again due to the antagonist effects of zinc and copper. The study researchers suggested that this is due to the specific eating habits of the participants in this article, which is not included in this study. On the other hand, selenium in obese people increases due to its antioxidant defense mechanism against oxidative stress and is reduced if the balance gets upset in favor of the oxidative status [53].
Articles in this study have case-control or cross-sectional designs. The absence of a cohort study is one of the limitations of this study. Food questionnaire collection for evaluation of intake antioxidants was different in the studies. The definition of obesity and overweight, measurement parameters, how to choose the control and case group, and size of them were different in studies. The main strength in this study is the consideration of the most antioxidants, while in the previous review studies, only one or two antioxidants had been discussed. This study examined the population of children. While few review studies have researched individual children.
4. Conclusion
We showed that antioxidants decrease obesity and are inversely related to each other by reviewing existing studies. Because of the increasing prevalence of obesity in children and its complications, interventional and cohort studies are suggested in the future. Future studies based on cohort will show cause and effect relationship between obesity and antioxidants. That relationship can be used to prevent or plan intervention strategies to deal with obese children.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
References
Obesity and overweight are dramatically increasing worldwide [1]. Obesity is associated with several chronic lifelong diseases [2]. During the last three decades, the prevalence of obesity in children has increased worldwide [3], including in Iran, which is the seventh country in terms of having obese children [4]. The prevalence rates of overweight and obesity among Iranian children have reached 10.8% and 5.1%, respectively, between 2007 and 2012 [5]. Childhood obesity increases the risk of morbidity due to obesity in adulthood [6]. Obesity in children is associated with various factors such as genetics, environment, nutrition, and physical inactivity, especially screen time [7].
Reactive species of oxygen and nitrogen are unstable and highly reactive molecules. These molecules oxidize various macromolecules to reach a stable state [8, 9]. Free radicals destroy fats, proteins, and cellular DNA [10]. Antioxidants are generally regenerative substances found inside and outside the cell and can react with free radicals and active species. Antioxidants reduce or prevent oxidative stress by reacting with free radicals and active species [10, 11]. Antioxidants are synthesized both in the body and absorbed through food. Antioxidants are generally divided into two categories: enzymatic and non-enzymatic. The most important antioxidants are Superoxide Dismutase (SOD), Glutamine Peroxidase (GPX), and Catalase (CAT) [10, 11]. Vitamins C, A, E, and β-carotene are non-enzymatic antioxidants, and magnesium, zinc, selenium, and copper are mineral antioxidants [12].
Oxidative stress might change the balance between the production of free radicals and the antioxidant defense system in favor of free radicals, which would cause oxidative damage and worsen the pathological status [13]. On the one hand, obesity is usually associated with micronutrient deficiency as a result of an unhealthy diet and low antioxidants intakes, and on the other hand, it can change the balance towards oxidative stress [14].
Magnesium is related to the enzymatic activity of cells and glucose metabolism; a significant relationship is reported between magnesium deficiency and obesity [15]. Zinc is one of the most important elements in several health issues. Some studies found a significant relationship between low levels of zinc and high levels of leptin [15]. Selenium is a mineral antioxidant that prevents the damaging effects of radicals and strengthens the immune system and metabolic processes, and decreases obesity status [16]. Copper is a mineral and one of the components of antioxidant enzymes in the body that plays an essential role in upsetting the balance in favor of antioxidants and has an inverse relationship with obesity [17].
In recent years, many studies assessed the relationship between antioxidant levels and obesity in adults [18], but few studies have been conducted in the pediatric age group [19, 20]. These studies have reported controversial findings. Therefore, this study aims to assess the current literature on the relationship between childhood obesity and antioxidant status through systematic review and meta-analysis.
2. Materials and Methods
In this systematic review, the following keywords and their equivalents were searched separately and together: “Vitamin C”, “Vitamin E”, “Vitamin A”, “Carotenoids”, “Selenium”, “Magnesium”, “Copper”, “Zinc”, “Ascorbic acid”, “Tocopherol”, “Antioxidants”, “Childhood”, “Pediatric Adolescence”, “Overweight”, and “Obesity”. These words were searched in international databases of Google Scholar, PubMed, Web of Science, Scopus, Medline, and Cochrane. This research was conducted without time or gender restrictions until September 2020.
The search was performed by two researchers independently. Examining the agreement between the search results was performed by a third person, where eventually, duplicates were omitted. This study includes case-control, cross-sectional, and prospective articles with control groups. These English language full-text articles evaluated the association between antioxidant status and overweight/obesity. Overweight and obesity were confirmed by anthropometric indices: Body Mass Index (BMI), weight for height, and waist circumference. Research on animals, interventional studies, case studies, case reports, and irrelevant studies were excluded.
Data extraction
Initially, 1255 articles were extracted from the mentioned databases. Then, 360 duplicate articles were removed by reviewing the titles (those extracted by the two researchers whose titles of the authors and the published journal are the same). Next, the abstracts of the remaining 895 articles were reviewed, where 380 articles were omitted because the studies were case study, animal research, or their interventions were irrelevant. Afterward, the full texts of the remaining 515 articles from the previous step were read, and unrelated articles were excluded because of lacking the inclusion criteria. Finally, 46 articles were included in the systematic review after examining the inclusion and exclusion criteria, and 19 competent articles in the meta-analysis (Figure 1).

Quality of studies
All articles included in the current review were extracted using a pre-prepared checklist. It included the author’s last name, year of publication, country of origin, age, sex, and sample size. After reviewing the inclusion and exclusion criteria and determining the related studies, the quality of the articles was independently assessed by two researchers using the checklist of STROBE (strengthening the reporting of observational studies in epidemiology). This checklist has 22 sections and various methodological aspects, including sampling and measuring methods, variables, statistical analysis, and study objectives. In this checklist, the minimum and maximum scores were 1 and 22. The studies with a score of 11 and above were entered into this review study, and the data related to them were selected for the meta-analysis process. A third researcher examined any disagreement between the two researchers (Figure 1).
Data analyses
The desired pooled effect size was considered a mean difference with a 95% Confidence Interval (CI). We used the forest plot to investigate the association between antioxidants levels and obesity among children and adolescents. The fixed-effects model was used according to the nonexistence of significant heterogeneity. The z-statistics was used to assess the significance of the pooled effect size; a P value of less than 0.05 was considered as statistically significant. Heterogeneity between the included studies was assessed by Cochran’s Q statistic, which was quantified by calculating the inconsistency index (I2). In cases with high heterogeneity among studies (substantial heterogeneity considered as I2>50%), the random-effects model with Der Simonian and Liard method was used. We assessed potential publication bias using funnel plots (not shown) and both Begg’s and Egger’s tests. P values of less than 0.05 from both Begg’s and Egger’s tests and the asymmetrical shape of the funnel plot showed statistically significant publication bias. The sensitivity analysis was conducted to assess the extent of the influence of omitting individual studies on the pooled mean difference. The analysis was conducted by the Stata software, version 11.2 (STATA Corp, College Station, TX, USA).
3. Results
Figure 1 shows that 46 articles were reviewed, and finally, 19 articles met the inclusion criteria and were entered into the analysis. Table 1 shows a summary of reviewed articles.







The associations of following antioxidants and childhood obesity were assessed:
Association between obesity and β-carotene
The forest plot for the association between antioxidants level and obesity according to the type of antioxidants is shown in Figures 2, 3, 4, 5, 6 and 7. The pooled mean difference of β-carotene level between obese and non-obese children was significantly different (mean difference: 0.13, 95% CI: 0.09-0.16, P<0.001), with significant heterogeneity (P<0.001, I2=85%) (Figure 2).

We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: P=0.27).
Subgroup meta-analysis according to α-tocopherol type
Results of subgroup meta-analysis according to α-tocopherol type are shown in Figure 3.

There was a significant difference between obese and non-obese children in both α-tocopherol (pooled mean difference respectively: 0.36, 95%CI: 0.04-0.96, P<0.001) with non-significant heterogeneity (P>0.05, I2=0.0%) and α-tocopherol per lipoid (pooled mean difference: 0.42, 95%CI: 0.28-0.55, P<0.001), with significant heterogeneity (P=0.048, I2=58.8%) (Figure 3).
We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: P=0.91).
Association between obesity and vitamin E
The forest plot for the association between vitamin E level and obesity is shown in Figure 4.

There was no significant association between vitamin E level and obesity (pooled mean difference: 0.40, 95%CI: -0.05-0.85, P>0.05), with significant heterogeneity (P<0.001, I2=84.5%) (Figure 4). We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: P=0.18).
Association of obesity with zinc, magnesium, copper, and selenium
The forest plot for the association between obesity and variables of zinc, magnesium, copper, and selenium level are shown in Figures 5, 6 and 7.



There were significant associations between zinc, magnesium, copper, and selenium level and obesity (P>0.05), with significant heterogeneity (P<0.001). We used the funnel plot and Egger’s test to assess the publication bias. Based on the funnel plot (figures not shown) and Egger’s test, there was no evidence of publication bias (Egger’s test: zinc studies, P=0.65; magnesium studies, P=0.07; selenium and copper studies, P=0.98).
Sensitivity analysis
The sensitivity analysis showed that eliminating any individual studies did not significantly change the pooled effect size and heterogeneity among magnesium, copper, and selenium studies (P>0.05). Regarding other antioxidant studies, the results of sensitivity analysis are presented in Table 2.

DiscussionThis systematic review and meta-analysis study was performed to determine the relationship between childhood obesity and anthropometric indices with antioxidant status. After completing the search, 46 articles were systematically reviewed (Table 1), and 19 were entered into the meta-analysis. The results showed that vitamins A, C, and their components, including α-tocopherol and β-carotene, as well as selenium, copper, magnesium, and zinc, were inversely related to childhood obesity. But vitamin E is not significantly associated with obesity.
Eighteen studies examined the relationship between vitamins A, E, C and obesity separately or together. In these studies, dietary intake of antioxidants or their serum levels or both have been investigated. In some studies, dietary intake of antioxidants predicted their serum levels, but in some studies, despite dietary intake of antioxidants, their serum levels were low, which suggests that serum levels of antioxidants may depend on various factors. In each article, a different hypothesis is addressed.
Strauss et al. reported that serum levels of α-tocopherol and β-carotene were significantly lower in obese children compared with the control group (P<0.001). It is hypothesized that the breakdown of α-tocopherol from adipose tissue in obese children is limited, leading to reduced access to other organs and thus its reduced serum levels [25]. Gillis et al. analyzed the diets of 156 obese children and 90 non-obese controls aged 4-17 years. The results showed that inadequate intake of vitamins E, A, magnesium, and zinc did not differ significantly between the two groups; however, in obese children, this inadequate intake was higher, indicating that all children should receive adequate vitamins as they grow [31].
Obese children may have inadequate food intake due to low intake of vitamins A, E, and C but may not have a poor diet due to overeating. Therefore, controversial results have been obtained regarding the relationship between serum levels of antioxidants and intake.
Aeberli et al. examined the food intake of the last 48 hours of 3 groups of normal, overweight, and obese children. They found that the intake of vitamins C, E, and carotene in the three groups was not significantly different, and only the amount of protein and meat intake was different between the two groups. The researchers proposed that mentioning food items in the food questionnaire in children may have some shortcomings and is not effective as adults [34]. Lopez-Sobaler et al. studied the 3-day dietary intake of 10-12 years old children. They found that the intake of vitamin E, selenium, and zinc is higher in obese and overweight children, but vitamin A or β-carotene intake did not differ between obese and control groups. Serum levels of α-tocopherol and selenium were lower in obese children [37]. Codone R-Franch et al. compared the dietary intake of two groups of children with Type 1 diabetes and obese children without disease with the control group. They showed that the intake of vitamins A, E, C, and fruits and vegetables was not different, and contrary to expectations, serum β-carotene levels were similar in diabetic children and the control group, but it was higher in the obese children. This finding may be because the obese children in this study had a normal diet [40].
Food questionnaires and their items and also the duration of recall varied in different articles, which makes it difficult to compare their results and draw conclusions.
Puchau et al. showed that dietary Total Antioxidant Capacity (TAC) is directly related to vitamins A.E, C and is inversely related to BMI [20]. Linardakis et al. reported that the group that received a nutritious diet of vitamins A, E, C, and minerals had lower metabolic syndrome indices such as BMI and waist/height ratio [39]. Ford et al. conducted a study on participants in the Third National Health and Nutrition Examination Survey (NHANES III). They found that all carotenoids from vitamin A compounds except lycopene were inversely related to BMI (P<0.001). There was also a direct relationship between dietary intake of fruits and vegetables and carotenoids [26].
In some reviewed articles, as expected, the levels of β-carotene and α-tocopherol and other components of vitamins A and E are inversely related to obesity and anthropometric indices, but in some articles, no significant relationship was found. In our study, we found no significant relationship between vitamin E with obesity, but α-tocopherol is a significant component of vitamin E and is significantly lower in obese children.
Because obese individuals are prone to hyperlipidemia and insulin resistance, Kljno et al. measured plasma levels of β-carotene and α-tocopherol in plasma and LDL. They wanted to assess antioxidant intake capacity and showed that the levels of β-carotene and α-tocopherol are lower in plasma and LDL of obese girls, and this low amount can make obese girls more prone to future atherosclerotic events [19]. A study by Gunanti et al. on children aged 8-15 years showed that plasma concentrations of α-tocopherol (β=–0.88, P<0.05), trans β-carotene (β=–2.21, P<0.01), cis β-carotene (β=–2.10, P<0.01), and α-tocopherol (β=–3.66, P<0.01) were inversely related to BMI [50]. Molnar et al. found that plasma β-carotene and α-tocopherol were significantly lower in obese children (P<0.005) [29]. Shin et al. showed a significant inverse relationship between HOMA-IR (Homeostasis Model Assessment of Insulin Resistance) and β-carotene (r=-0.233, P<0.05) and α-tocopherol (r=-0.370, P<0.0001) [35].
Different hypotheses have been suggested for the association of vitamin A and E with obesity. There is a hypothesis that in obesity, antioxidant levels rise due to an increase in the levels of oxidative stress. In other words, the defense system in the body is activated against antioxidants. Contrary to expectations, it leads to increase levels of antioxidants in obesity [64].
Codoñer-Franch et al. reported that β-carotene and α-tocopherol were not significantly associated with obesity. Because these two vitamins are fat-soluble, obese children in this study had normal lipid profiles to justify the result [43]. García et al. studied 197 children aged 6-10.5 years and found that vitamin A was positively correlated with BMI and Waist to Height Ratio (WHR) (P<0.05). Vitamins E and C were inversely related to BMI waist/height ratio (P<0.05). This finding may result from the direct relation of vitamin A, which is a fat-soluble vitamin, to the storage of adipose tissue [48]. Because vitamins A and E are fat-soluble and obese children have high-fat tissues, these vitamins can increase with obesity [65].
Another component of vitamin A is retinol, which has an antioxidant role [66], but different results are published in various articles on obesity. Mobinobu et al. showed that levels of plasma β-carotene were significantly lower in obese children compared with control groups. However, no significant differences were found in plasma levels of α-tocopherol and retinol, which may result from closer relation of β-carotene levels to adipose tissue than retinol [28]. Sarni et al. reported that serum carotene levels were significantly lower in obese children compared with the control group but did not differ significantly in retinol levels, although they were lower in obese children [33]. de Souza Valente da Silva et al. argued that overweight children had lower carotenoid serum (P<0.001), but there was no significant difference between BMI and retinol (P=0.304). This fact may indicate that retinol plays a lower role in antioxidant activity [38]. Teske et al. showed that plasma retinol levels were inversely related to obesity and glucose tolerance (fasting blood sugar and 2 hours later). In other words, it measured the association of retinol with metabolic syndrome [51]. However, few studies have linked retinol to obesity. Future studies may further consider the role of retinol in antioxidants activities.
Regarding the relationship between childhood obesity and variables of copper and selenium, zinc, magnesium status, we found 29 articles on the relationship of zinc, copper, magnesium, and selenium to obesity separately or in combinations. Some articles revealed an inverse relationship between obesity and the levels of these elements, which might be because of the antioxidant properties of these elements that can be reduced in obesity.
Various studies have been performed on the role of zinc in obesity, and the association between zinc and leptin has been considered. Leptin is a hormone produced by adipose tissue. In zinc deficiency, a decrease in zinc is associated with a decrease in leptin. Decreased leptin leads to increased appetite and obesity [15].
Magnesium, copper, selenium are essential trace elements. They play an essential role in the growth and regulation of various enzymes in metabolic processes [67]. Some studies have shown that obesity is associated with reduced trace elements.
Vivek et al. showed that zinc and copper levels are significantly lower in obesity among Indian children aged 6-16 years [63]. In a study by Perrone et al. serum zinc and copper levels were lower in obese, overweight children [24]. Błażewicz et al. reported that levels of zinc and copper of plasma are inversely related to BMI [47]. Erdeve et al. reported that Cu/Zn-SOD (superoxide dismutase) enzymes in obese children are significantly higher, indicating that the balance is changed in favor of the oxidative status in obesity (P<0.05) [30].
In some studies, the levels of antioxidants were searched in other body components like hair, nail, and urine. Gibson et al. showed that obese children with higher BMIs in New Zealand had lower hair zinc levels [27]. Xu et al. showed that nail selenium levels are lower in obese people, although there is no significant difference [59]. Błażewicz et al. found that obese people had lower serum and urine selenium [55].
In the Huerta et al. study, serum magnesium levels, as well as magnesium intake from food, were significantly lower in obese children (P=0.009) [32]. Zaakouk et al. concluded that serum magnesium levels were significantly lower in obese people (r=-0.8, P<0.001) [54]. Niranjan et al. also showed that obese people had lower magnesium levels [49]. Ul Hassan et al. reported that serum magnesium levels were significantly lower in obese and overweight individuals (P<0.001) [57]. Ortega et al. showed that dietary selenium intake was directly related to selenium levels and was lower in obese children and inversely related to BMI. This finding indicates that obese children had worse antioxidant status than other children [46].
However, in several reviewed articles, different results were obtained. They reported that the levels of zinc, copper, magnesium, and selenium are directly related to obesity, or at least there is no significant difference. Gonoodi et al. examined 408 Iranian girls aged 12-18 years, found that serum levels and intake of zinc and copper were not associated with obesity [60]. Studies show a correlation between serum zinc level and appetite which can lead to obesity. In other words, there is a direct link between zinc and increased appetite [68]. Hongo et al. conducted a study of 66 boys and girls in Tokyo, Japan. They reported that dietary zinc intake in the 3-day diet questionnaire was not related to serum zinc concentration. In other words, zinc food intake was not a predictor of serum level, and zinc status was not related to weight and height (P<0.01). That is, this study, which showed zinc deficiency in Japanese children, hypothesized that borderline serum deficiency could not affect children’s height and BMI [21].
In the study of Weisstaub et al. on 18- to 36-month-old children of Chile, no relationship was found between zinc of plasma, height, and waist circumference, which may be due to borderline zinc deficiency [15]. A study by Yakinci et al. on children aged 7 to 11 years in Turkey showed that zinc and copper levels in obese children were significantly higher than in control groups (P<0.01), which may be due to the high appetite of obese children. Dietary intake of zinc and copper was associated with serum levels, but magnesium levels were lower in obese children (P<0.01) [23].
Zinc is involved in the cellular defense of lymphocytes. In this regard, Marotta et al. examined two groups of obese children and control groups of 5 to 17 years old in Nepal and found that the level of zinc content in lymphocytes was lower in obese children, but there was a significant difference in plasma zinc and erythrocytes and polymorphonuclear. According to the hypothesis of this article, zinc deficiency in lymphocytes can be an indicator of zinc deficiency in obese children [22].
Bouglé et al. study did not find any significant relationship between zinc and selenium levels and BMI. In other words, this study showed that obese children are not always deficient in micronutrients [41]. Tascilar et al. who studied the serum levels of various trace elements in obese children, concluded that serum zinc, copper, and selenium were lower in obese people but did not differ significantly from the control group. This finding may be due to the diet of obese children, which is not nutritious and in nutrients such as trace elements, and obese children are deficient in these substances [42].
Çelik et al. reported that serum magnesium levels in obese people with insulin resistance are very low, but the two obese groups without insulin resistance and the control group are not different. On the other hand, serum magnesium levels are directly related to BMI. The researchers may believe that this finding is due to the study’s limitations in measuring total magnesium, including intracellular magnesium, but in this study, serum magnesium was measured [44]. Jose et al. concluded that although dietary magnesium intake was higher in obese Indian children, serum magnesium levels were lower in obese children and were inversely related to BMI due to decreased reabsorption or increased urinary magnesium excretion. Magnesium excretion increase has been previously reported in obese adults and hypertension and Type 2 diabetes [45]. However, several studies have shown that copper levels are directly related to obesity. One of the hypotheses is that copper levels rise in obesity due to the reduction of zinc and the antagonist properties of zinc and copper in the body.
Chen et al. concluded that levels of plasma copper are directly related to BMI [62]. Gaber et al. found that obesity is associated with increased copper levels and decrease zinc and magnesium levels [61]. In the study of Lima et al. plasma copper levels in obese children were higher than that in the control group, but the copper concentration of erythrocytes did not differ [36]. In the study of Azab et al. serum levels of zinc and selenium are lower in obese people, but copper levels were higher in obese people due to the antagonist effect of zinc and copper in the body [52]. In the study by Habib et al. plasma zinc levels were lower in obese Egyptian children (P<0.001), but plasma copper levels were significantly higher in obese individuals, possibly due to zinc and copper interactions. In other words, dietary zinc intake may be associated with some degree of copper deficiency [56].
In the study by Fan et al. zinc and selenium levels were inversely related to the BMI of American children, but serum copper levels were directly related to BMI, which could still be due to zinc-copper interactions [58]. Cayir et al. showed that selenium and copper are higher in obese people, but zinc levels are lower, again due to the antagonist effects of zinc and copper. The study researchers suggested that this is due to the specific eating habits of the participants in this article, which is not included in this study. On the other hand, selenium in obese people increases due to its antioxidant defense mechanism against oxidative stress and is reduced if the balance gets upset in favor of the oxidative status [53].
Articles in this study have case-control or cross-sectional designs. The absence of a cohort study is one of the limitations of this study. Food questionnaire collection for evaluation of intake antioxidants was different in the studies. The definition of obesity and overweight, measurement parameters, how to choose the control and case group, and size of them were different in studies. The main strength in this study is the consideration of the most antioxidants, while in the previous review studies, only one or two antioxidants had been discussed. This study examined the population of children. While few review studies have researched individual children.
4. Conclusion
We showed that antioxidants decrease obesity and are inversely related to each other by reviewing existing studies. Because of the increasing prevalence of obesity in children and its complications, interventional and cohort studies are suggested in the future. Future studies based on cohort will show cause and effect relationship between obesity and antioxidants. That relationship can be used to prevent or plan intervention strategies to deal with obese children.
Ethical Considerations
Compliance with ethical guidelines
This article is a meta-analysis with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
References
- Visscher TL, Seidell JC. The public health impact of obesity. Annual Review of Public Health. 2001; 22:355-75. [DOI:10.1146/annurev.publhealth.22.1.355] [PMID]
- Schwimmer JB, Burwinkle TM, Varni JW. Health-related quality of life of severely obese children and adolescents. JAMA. 2003; 289(14):1813-9. [DOI:10.1001/jama.289.14.1813] [PMID]
- Anderson PM, Butcher KE. Childhood obesity: Trends and potential causes. The Future of Children. 2006; 16(1):19-45. [DOI:10.1353/foc.2006.0001] [PMID]
- Kelishadi R, Pour MH, Sarraf-Zadegan N, Sadry GH, Ansari R, Alikhassy H, et al. Obesity and associated modifiable environmental factors in Iranian adolescents: Isfahan Healthy Heart Program− heart health promotion from childhood. Pediatrics International. 2003; 45(4):435-42. [DOI:10.1046/j.1442-200X.2003.01738.x] [PMID]
- Kelishadi R, Haghdoost AA, Sadeghirad B, Khajehkazemi R. Trend in the prevalence of obesity and overweight among Iranian children and adolescents: A systematic review and meta-analysis. Nutrition. 2014; 30(4):393-400. [DOI:10.1016/j.nut.2013.08.011] [PMID]
- Abraham S, Collins G, Nordsieck M. Relationship of childhood weight status to morbidity in adults. HSMHA Health Reports. 1971; 86(3):273-84. [DOI:10.2307/4594149] [PMID]
- Vos MB, Welsh J. Childhood obesity: Update on predisposing factors and prevention strategies. Current Gastroenterology Reports. 2010; 12(4):280-7. [DOI:10.1007/s11894-010-0116-1] [PMID]
- Gomes VA, Casella-Filho A, Chagas AC, Tanus-Santos JE. Enhanced concentrations of relevant markers of nitric oxide formation after exercise training in patients with metabolic syndrome. Nitric Oxide. 2008; 19(4):345-50. [DOI:10.1016/j.niox.2008.08.005] [PMID]
- Roberts RA, Smith RA, Safe S, Szabo C, Tjalkens RB, Robertson FM. Toxicological and pathophysiological roles of reactive oxygen and nitrogen species. Toxicology. 2010; 276(2):85-94. [DOI:10.1016/j.tox.2010.07.009] [PMID]
- Rizzo AM, Berselli P, Zava S, Montorfano G, Negroni M, Corsetto P, et al. Endogenous antioxidants and radical scavengers. In: Giardi MT, Rea G, Berra B, editors. Bio-Farms for Nutraceuticals. Advances in Experimental Medicine and Biology. Vol. 698. Boston, MA: Springer; 2010. pp. 52-67. [DOI:10.1007/978-1-4419-7347-4_5]
- Limón-Pacheco J, Gonsebatt ME. The role of antioxidants and antioxidant-related enzymes in protective responses to environmentally induced oxidative stress. Mutation Research. 2009; 674(1-2):137-47. [DOI:10.1016/j.mrgentox.2008.09.015] [PMID]
- Farbstein D, Kozak-Blickstein A, Levy AP. Antioxidant vitamins and their use in preventing cardiovascular disease. Molecules. 2010; 15(11):8098-110. [DOI:10.3390/molecules15118098] [PMID]
- Malhotra, JD, Kaufman RJ. Endoplasmic reticulum stress and oxidative stress: A vicious cycle or a double-edged sword? Antioxidants & Redox Signaling. 2007; 9(12):2277-94. [DOI:10.1089/ars.2007.1782]
- Reedy J, Krebs-Smith SM. Dietary sources of energy, solid fats, and added sugars among children and adolescents in the United States. Journal of the American Dietetic Association. 2010; 110(10):1477-84. [DOI:10.1016/j.jada.2010.07.010] [PMID]
- Weisstaub G, Hertrampf E, López de Romaña D, Salazar G, Bugueño C, Castillo-Duran C. Plasma zinc concentration, body composition and physical activity in obese preschool children. Biological Trace Element Research. 2007; 118(2):167-74. [DOI:10.1007/s12011-007-0026-8] [PMID]
- Jiao HT, Liu P, Lu WT, Qiao M, Ren XF, Zhang Z. Correlation study between simple obesity and serum concentrations of essential elements. Trace Elements & Electrolytes. 2014; 31(2):53-9. [DOI:10.5414/TEX01319]
- Wintergerst ES, Maggini S, Hornig DH. Contribution of selected vitamins and trace elements to immune function. Annals of Nutrition and Metabolism. 2007; 51(4):301-23. [DOI:10.1159/000107673] [PMID]
- Vincent HK, Bourguignon CM, Weltman AL, Vincent KR, Barrett E, Innes KE, et al. Effects of antioxidant supplementation on insulin sensitivity, endothelial adhesion molecules, and oxidative stress in normal-weight and overweight young adults. Metabolism. 2009; 58(2):254-62. [DOI:10.1016/j.metabol.2008.09.022] [PMID]
- Kljno T, Hozumi M, Morinobu T, Murata T, Mingci Zh, Tamai H. Antioxidant vitamin levels in plasma and low density lipoprotein of obese girls. Free Radical Research. 1998; 28(1):81-6. [DOI:10.3109/10715769809097878] [PMID]
- Puchau B, Ochoa MC, Zulet MA, Marti A, Martínez JA, Members G. Dietary total antioxidant capacity and obesity in children and adolescents. International Journal of Food Sciences and Nutrition. 2010; 61(7):713-21. [DOI:10.3109/09637481003757860] [PMID]
- Hongo T, Suzuki T, Ohba T, Karita K, Dejima Y, Yoshinaga J, et al. Nutritional assessment of a group of Japanese elementary school children in Tokyo: With special emphasis on growth, anemia, and obesity. Journal of Nutritional Science and Vitaminology. 1992; 38(2):177-96. [DOI:10.3177/jnsv.38.177] [PMID]
- Marotta A, Todisco N, Di Toro A, Toraldo R, Ponte G, Perrone L. Zinc content of lymphomonocytes in obese children. Nutrition Research. 1995; 15(10):1411-5. [DOI:10.1016/0271-5317(95)02013-L]
- Yakinci C, Paç A, Küçükbay FZ, Tayfun M, Gül A. Serum zinc, copper, and magnesium levels in obese children. Acta paediatrica Japonica. 1997; 39(3):339-41. [DOI:10.1111/j.1442-200X.1997.tb03748.x] [PMID]
- Perrone L, Gialanella G, Moro R, Feng SL, Boccia E, Palombo G, et al. Zinc, copper, and iron in obese children and adolescents. Nutrition Research. 1998; 18(2):183-9. [DOI:10.1016/S0271-5317(98)00011-6]
- Strauss RS. Comparison of serum concentrations of alpha-tocopherol and beta-carotene in a cross-sectional sample of obese and nonobese children (NHANES III). National Health and Nutrition Examination Survey. The Journal of Pediatrics. 1999; 134(2):160-5. [DOI:10.1016/S0022-3476(99)70409-9] [PMID]
- Ford ES, Gillespie C, Ballew C, Sowell A, Mannino DM. Serum carotenoid concentrations in US children and adolescents. The American Journal of Clinical Nutrition. 2002; 76(4):818-27. [DOI:10.1093/ajcn/76.4.818] [PMID]
- Gibson RS, Skeaff M, Williams S. Interrelationship of indices of body composition and zinc status in 11-yr-old New Zealand children. Biological Trace Element Research. 2000; 75(1-3):65-77. [DOI:10.1385/BTER:75:1-3:65] [PMID]
- Morinobu T, Murata T, Takaya R, Tamai H. Nutritional status of beta-carotene, alpha-tocopherol and retinol in obese children. International Journal for Vitamin and Nutrition Research. 2002; 72(3):119-23. [DOI:10.1024/0300-9831.72.3.119] [PMID]
- Molnár D, Decsi T, Koletzko B. Reduced antioxidant status in obese children with multimetabolic syndrome. International Journal of Obesity and Related Metabolic Disorders. 2004; 28(10):1197-202. [DOI:10.1038/sj.ijo.0802719] [PMID]
- Erdeve O, Siklar Z, Kocaturk PA, Dallar Y, Kavas GO. Antioxidant superoxide dismutase activity in obese children. Biological Trace Element Research. 2004; 98(3):219-28. [DOI:10.1385/BTER:98:3:219] [PMID]
- Gillis L, Gillis A. Nutrient inadequacy in obese and non-obese youth. Canadian Journal of Dietetic Practice and Research. 2005; 66(4):237-42. [DOI:10.3148/66.4.2005.237] [PMID]
- Huerta MG, Roemmich JN, Kington ML, Bovbjerg VE, Weltman AL, Holmes VF, et al. Magnesium deficiency is associated with insulin resistance in obese children. Diabetes Care. 2005; 28(5):1175-81. [DOI:10.2337/diacare.28.5.1175] [PMID]
- Sarni ROS, de Souza FIS, Ramalho RA, de Oliveira Schoeps D, Kochi C, Catherino P, et al. Serum retinol and total carotene concentrations in obese pre-school children. Medical Science Monitor. 2005; 11(11):CR510-4. [PMID]
- Aeberli I, Molinari L, Spinas G, Lehmann R, l'Allemand D, Zimmermann MB. Dietary intakes of fat and antioxidant vitamins are predictors of subclinical inflammation in overweight Swiss children. The American Journal of Clinical Nutrition. 2006; 84(4):748-55. [DOI:10.1093/ajcn/84.4.748] [PMID]
- Shin MJ, Park E. Contribution of insulin resistance to reduced antioxidant enzymes and vitamins in nonobese Korean children. Clinica Chimica Acta. 2006; 365(1-2):200-5. [DOI:10.1016/j.cca.2005.08.019]
- Lima SC, Arrais RF, Sales CH, Almeida MG, de Sena KC, Oliveira VT, et al. Assessment of copper and lipid profile in obese children and adolescents. Biological Trace Element Research. 2006; 114(1-3):19-29. [DOI:10.1385/BTER:114:1:19] [PMID]
- López-Sobaler AM, AparicioA, Andres P, Bermejo LM, Rodríguez-Rodríguez E, Ortega RM. Lipid peroxidation, and antioxidant status in normal weight or overweight/obesity children from Madrid (Spain). Annals of Nutrition and Metabolism. 2007; 51(Suppl 1):360. https://www.karger.com/Article/Pdf/105121
- de Souza Valente da Silva L, da Veiga GV, Ramalho RA. Association of serum concentrations of retinol and carotenoids with overweight in children and adolescents. Nutrition. 2007; 23(5):392-7. [DOI:10.1016/j.nut.2007.02.009] [PMID]
- Linardakis M, Bertsias G, Sarri K, Papadaki A, Kafatos A. Metabolic syndrome in children and adolescents in Crete, Greece, and association with diet quality and physical fitness. Journal of Public Health. 2008; 16(6):421-8. [DOI:10.1007/s10389-008-0191-z]
- Codoñer-Franch P, Pons-Morales S, Boix-García L, Valls-Bellés V. Oxidant/antioxidant status in obese children compared to pediatric patients with type 1 diabetes mellitus. Pediatric Diabetes. 2010; 11(4):251-7. [DOI:10.1111/j.1399-5448.2009.00565.x] [PMID]
- Bouglé DL, Bureau F, Laroche D. Trace element status in obese children: Relationship with metabolic risk factors. e-SPEN, the European e-Journal of Clinical Nutrition and Metabolism. 2009; 4(2):e98-100. [DOI:10.1016/j.eclnm.2009.01.012]
- Tascilar ME, Ozgen IT, Abaci A, Serdar M, Aykut O. Trace elements in obese Turkish children. Biological Trace Element Research. 2011; 143(1):188-95. [DOI:10.1007/s12011-010-8878-8] [PMID]
- Codoñer-Franch P, Boix-García L, Simó-Jordá R, Del Castillo-Villaescusa C, Maset-Maldonado J, Valls-Bellés V. Is obesity associated with oxidative stress in children? International Journal of Pediatric Obesity. 2010; 5(1):56-63. [DOI:10.3109/17477160903055945] [PMID]
- Çelik N, Andiran N, Yilmaz AE. The relationship between serum magnesium levels wıth childhood obesity and insulin resistance: A review of the literature. Journal of Pediatric Endocrinology and Metabolism. 2011; 24(9-10):675-8. [DOI:10.1515/JPEM.2011.255] [PMID]
- Jose B, Jain V, Vikram NK, Agarwala A, Saini S. Serum magnesium in overweight children. Indian Pediatrics. 2012; 49(2):109-12. [DOI:10.1007/s13312-012-0024-6] [PMID]
- Ortega RM, Rodríguez-Rodríguez E, Aparicio A, Jiménez-Ortega AI, Palmeros C, Perea JM, et al. Young children with excess of weight show an impaired selenium status. International Journal for Vitamin and Nutrition Research. 2012; 82(2):121-9. [DOI:10.1024/0300-9831/a000101] [PMID]
- Błażewicz A, Klatka M, Astel A, Partyka M, Kocjan R. Differences in trace metal concentrations (Co, Cu, Fe, Mn, Zn, Cd, and Ni) in whole blood, plasma, and urine of obese and nonobese children. Biological Trace Element Research. 2013; 155(2):190-200. [DOI:10.1007/s12011-013-9783-8] [PMID]
- García OP, Ronquillo D, del Carmen Caamaño M, Martínez G, Camacho M, López V, et al. Zinc, iron and vitamins A, C and E are associated with obesity, inflammation, lipid profile and insulin resistance in Mexican school-aged children. Nutrients. 2013; 5(12):5012-30. [DOI:10.3390/nu5125012] [PMID]
- Niranjan G, Anitha D, Srinivasan AR, Velu VK, Venkatesh C, Babu MS, et al. Association of inflammatory sialoproteins, lipid peroxides and serum magnesium levels with cardiometabolic risk factors in obese children of South Indian population. International Journal of Biomedical Science: IJBS. 2014; 10(2):118-23. [PMID] [PMCID]
- Gunanti IR, Marks GC, Al-Mamun A, Long KZ. Low serum concentrations of carotenoids and vitamin E are associated with high adiposity in Mexican-American children. The Journal of Nutrition. 2014; 144(4):489-95. [DOI:10.3945/jn.113.183137] [PMID]
- Teske M, Melges APB, de Souza FIS, Fonseca FLA, Sarni ROS. Plasma concentrations of retinol in obese children and adolescents: Relationship to metabolic syndrome components. Revista Paulista de Pediatria. 2014; 32(1):50-4. [DOI:10.1590/S0103-05822014000100009] [PMID] [PMCID]
- Azab SF, Saleh SH, Elsaeed WF, Elshafie MA, Sherief LM, Esh AM. Serum trace elements in obese Egyptian children: A case-control study. Italian Journal of Pediatrics. 2014; 40:20. [DOI:10.1186/1824-7288-40-20] [PMID]
- Cayir Y, Cayir A, Turan MI, Kurt N, Kara M, Laloglu E, et al. Antioxidant status in blood of obese children: The relation between trace elements, paraoxonase, and arylesterase values. Biological Trace Element Research. 2014; 160(2):155-60. [DOI:10.1007/s12011-014-0038-0] [PMID]
- Zaakouk AM, Hassan MA, Tolba OA. Serum magnesium status among obese children and adolescents. Egyptian Pediatric Association Gazette. 2016; 64(1):32-7. [DOI:10.1016/j.epag.2015.11.002]
- Błażewicz A, Klatka M, Astel A, Korona-Glowniak I, Dolliver W, Szwerc W, et al. Serum and urinary selenium levels in obese children: A cross-sectional study. Journal of Trace Elements in Medicine and Biology. 2015; 29:116-22. [DOI:10.1016/j.jtemb.2014.07.016] [PMID]
- Habib SA, Saad EA, Elsharkawy AA, Attia ZR. Pro-inflammatory adipocytokines, oxidative stress, insulin, Zn and Cu: Interrelations with obesity in Egyptian non-diabetic obese children and adolescents. Advances in Medical Sciences. 2015; 60(2):179-85. [DOI:10.1016/j.advms.2015.02.002] [PMID]
- Ul Hassan SA, Ahmed I, Nasrullah A, Haq S, Ghazanfar H, Sheikh AB, et al. Comparison of serum magnesium levels in overweight and obese children and normal weight children. Cureus. 2017; 9(8):e1607. [DOI:10.7759/cureus.1607] [PMID]
- Fan Y, Zhang Ch, Bu J. Relationship between selected serum metallic elements and obesity in children and adolescent in the U.S.. Nutrients. 2017; 9(2):104. [DOI:10.3390/nu9020104] [PMID]
- Xu R, Chen C, Zhou Y, Zhang X, Wan Y. Fingernail selenium levels in relation to the risk of obesity in Chinese children: A cross-sectional study. Medicine. 2018; 97(9):e0027. [DOI:10.1097/MD.0000000000010027] [PMID]
- Gonoodi K, Moslem A, Darroudi S, Ahmadnezhad M, Mazloum Z, Tayefi M, et al. Serum and dietary zinc and copper in Iranian girls. Clinical Biochemistry. 2018; 54:25-31. [DOI:10.1016/j.clinbiochem.2018.02.006] [PMID]
- Gaber HAH, Eldahshan TAK, Ghanem SM, Abdel Fatah MAA. Value of serum zinc, magnesium and copper in obese and normal weight children. The Egyptian Journal of Hospital Medicine. 2019; 76(1):3254-9. [DOI:10.21608/ejhm.2019.36887]
- Chen G, Li Y, Deng G, Shrestha S, Chen F, Wei Y, et al. Associations of plasma copper, magnesium, and calcium levels with blood pressure in children: A cross-sectional study. Biological Trace Element Research. 2021; 199:815-24. [DOI:10.1007/s12011-020-02201-z]
- Vivek SM, Dayal D, Khaiwal R, Bharti B, Bhalla A, Singh S, et al. Low serum copper and zinc concentrations in North Indian children with overweight and obesity. Pediatric Endocrinology Diabetes and Metabolism. 2020; 26(2):79-83. [DOI:10.5114/pedm.2020.95627] [PMID]
- Andersen LF, Jacobs Jr DR, Gross MD, Schreiner PJ, Dale Williams O, Lee DH. Longitudinal associations between body mass index and serum carotenoids: The CARDIA study. British Journal of Nutrition. 2006; 95(2):358-65. [DOI:10.1079/BJN20051638] [PMID]
- Wallström P, Wirfält E, Lahmann PH, Gullberg B, Janzon L, Berglund G. Serum concentrations of β-carotene and α-tocopherol are associated with diet, smoking, and general and central adiposity. The American Journal of Clinical Nutrition. 2001; 73(4):777-85. [DOI:10.1093/ajcn/73.4.777] [PMID]
- Botella-Carretero JI, Balsa JA, Vázquez C, Peromingo R, Díaz-Enriquez M, Escobar-Morreale HF. Retinol and α-tocopherol in morbid obesity and nonalcoholic fatty liver disease. Obesity Surgery. 2010; 20(1):69-76. [DOI:10.1007/s11695-008-9686-5] [PMID]
- Ghayour-Mobarhan M, Taylor A, New SA, Lamb DJ, Ferns GA. Determinants of serum copper, zinc and selenium in healthy subjects. Annals of Clinical Biochemistry. 2005; 42(Pt 5):364-75. [DOI:10.1258/0004563054889990] [PMID]
- El-Mashad GM, El-Gebally ES, El-Hefnawy SM, El-Sayed Saad AM. Effect of zinc supplementation on serum zinc and leptin levels in children on regular hemodialysis. Menoufia Medical Journal. 2018; 31(2):664-70. http://www.mmj.eg.net/text.asp?2018/31/2/664/239739
Type of Study: Systematic Review |
Subject:
Pediatrics
Received: 2021/03/3 | Accepted: 2021/05/11 | Published: 2021/07/1
Received: 2021/03/3 | Accepted: 2021/05/11 | Published: 2021/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









