Volume 10, Issue 4 (12-2022)
J. Pediatr. Rev 2022, 10(4): 277-286 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nasri P, Sadeghi S, Hovsepian S, Chegini R, Soltani Esmaeili S, Kiani G. COVID-19 in Children With Inflammatory Bowel Disease: A Systematic Review. J. Pediatr. Rev 2022; 10 (4) :277-286
URL: http://jpr.mazums.ac.ir/article-1-476-en.html
URL: http://jpr.mazums.ac.ir/article-1-476-en.html
Peiman Nasri1 
 , Somayeh Sadeghi2
, Somayeh Sadeghi2 
 , Silva Hovsepian1
, Silva Hovsepian1 
 , Rojin Chegini1
, Rojin Chegini1 
 , Shahrzad Soltani Esmaeili3
, Shahrzad Soltani Esmaeili3 
 , Gelareh Kiani *4
, Gelareh Kiani *4 



 , Somayeh Sadeghi2
, Somayeh Sadeghi2 
 , Silva Hovsepian1
, Silva Hovsepian1 
 , Rojin Chegini1
, Rojin Chegini1 
 , Shahrzad Soltani Esmaeili3
, Shahrzad Soltani Esmaeili3 
 , Gelareh Kiani *4
, Gelareh Kiani *4 


1- Metabolic Liver Disease Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Acquired Immunodeficiency Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Department of Pediatrics, School of Medicine, Imam Hossein Children’s Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Pediatrics, School of Medicine, Imam Hossein Children’s Hospital, Isfahan University of Medical Sciences, Isfahan, Iran. ,goli2291@yahoo.com
2- Acquired Immunodeficiency Research Center, Al-Zahra Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Department of Pediatrics, School of Medicine, Imam Hossein Children’s Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Department of Pediatrics, School of Medicine, Imam Hossein Children’s Hospital, Isfahan University of Medical Sciences, Isfahan, Iran. ,
Full-Text [PDF 539 kb]
(1414 Downloads)
| Abstract (HTML) (3523 Views)
Full-Text: (951 Views)
Introduction
The first case of pneumonia with the novel beta-corona virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was found in Wuhan, ChinaChina in the late 2019 and turned rapidly into a pandemic worldwide in March 2020 [1]. The course of the disease caused by SARS-CoV-2, named coronavirus disease 2019 (COVID-19) can range from asymptomatic or mild infection to severe and fatal illness with a variety of signs and symptoms related to different body organs [1].
Research has shown that patients with co-morbidities and the elderly are at greater risk for the developing of severe complications following infection with SARS-CoV-2 [2], possibly due to cytokine storm formation, which is an excessive and inappropriate immune response to the virus [3].
Patients with inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD), and ulcerative colitis (UC) are believed to be at increased risk of opportunistic bacterial or viral infections due to chronic chronic immunosuppressive medications [2, 4]. In the meantime, it is essential to assess whether these individuals are at risk for the development of more intense cases of the disease caused by the novel coronavirus [1, 5, 6].
According to the literature, taking immunosuppressive drugs is not correlated with increased intensity of COVID-19 [7]. However, the augmented risk of COVID-19 severity may be due to the increased cytokine production or angiotensin-converting enzyme (ACE-2) overexpression, the receptor for SARS-CoV-2 on the host cells, in IBD ppatients [8].
Since the beginning of this pandemic, many efforts have been made by various research teams to answer this question. For example, the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease database (SECURE-IBD) is an international registry recruiting IBD patients with SARS-CoV-2. European society for pediatric gastroenterology, hepatology, and nutrition also created a database for IBD patients with SARS-CoV-2. The data acquired from this registry so far have shown that the risk of being infected with this virus or developing severe complications following the infection in IBD patients is not different from the healthy population can increase the risk of complications from the virus [9]. However, there is still not enough information in the literature [6]. In addition, most studies in this field have been performed on adult patients and few studies have been performed on children. Accordingly, in the present study, we intended to collect data on COVID-19 in children with IBD through conducting a systematic literature review.
Methods
Search strategy and databases
This systematic review study is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020. The research question were as follows:
P: Children with IBD and COVID-19
I: IBD treatments
C: Children with COVID-19 and without IBD
O: COVID-19 outcome
The search was based on the keywords listed in Table 1 through Scopus, PubMed, and the Web of Science databases until October 16, 2021.
.jpg)
The search strategy in each database is listed in Table 1.
Inclusion and exclusion criteria
All English language original articles and meeting abstracts of any type including case reports, case series, cross-sectional, cohort, and case-control were included in the present systematic review study. The study population included patients with COVID-19 and IBD who were less than 19 years old. In this study, all studies that examined the course of COVID-19 in patients with IBD less than 19 years old were included. Unrelated studies, reviews, duplicates, and low-quality studies were excluded from the review process.
Study selection process
After removing duplicates, the title and the abstracts of the articles were evaluated for eligibility and then, the full text of the remaining articles was reviewed independently by two reviewers. Unrelated ones and studies that did not report COVID-19 outcomes in patients under 19 years of age were removed from the review process. Final articles entered the quality assessment.
Quality assessment
Newcastle Ottawa scale was used for quality assessment. A score of less than 14 was considered low quality. No studies were excluded at this stage. The methodological quality of the "final " studies .
Data extraction
A checklist with the following items was used for data extraction by two independent reviewers: author name, country, sample size, IBD type, medications, and COVID-19 outcomes.
Results
From the initially retrieved 2215 papers, 1127 were duplicates. Based on the titles and the abstracts, 1028 articles were irrelevant or review papers. Finally, 60 articles were assessed for eligibility. Twenty-four articles were irrelevant. Twenty articles did not include pediatric patients or did not report COVID-19 outcomes in this group. Finally, 16 studies (14 articles, 2 meeting abstracts) were eligible (Figure 1). Characteristics of the studies
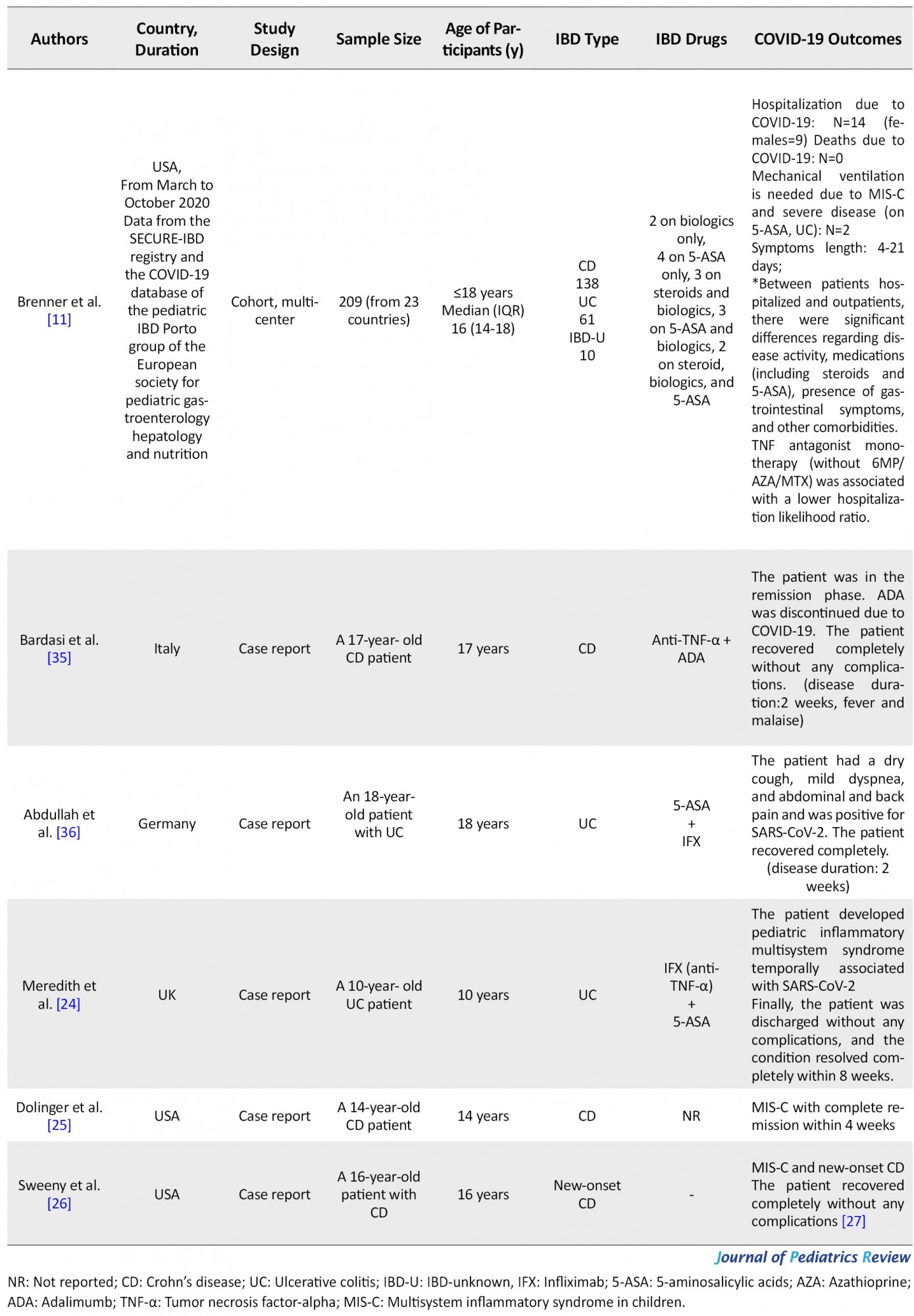
Nine articles had cohort design, five were case reports, and two were cross-sectional. Data from 1040 pediatric patients with IBD were reviewed. Regarding IBD type, 124 patients had UC, 234 had CD, 25 had IBD-unknown (IBD-U), and other patients not reported. Twenty-four patients were hospitalized due to COVID-19, but there were no related deaths or severe complications, and all patients recovered completely. Five patients (3 CD, 2 UC; 3 males and two females) developed Multisystem Inflammatory Syndrome in Children (MIS-C). One other patient also needed intensive care unit (ICU) due to secondary infection and disease severity. These patients were on biologics or 5-Aminosalicylic acids (5-ASA). Except for these five patients, others mainly reported mild signs and symptoms, including fatigue, fever, and dry cough, or were asymptomatic. One study showed that IBD activity status, steroid or 5-ASA use, presence of gastrointestinal symptoms, and other comorbidities increase the risk of hospitalization in children with COVID-19 and IBD.
In contrast, Tumor necrosis factor (TNF) antagonist monotherapy lowered hospitalization likelihood ratio. Another study also revealed no significant relation between biological agent use and the development of symptomatic COVID-19. Disease symptoms lasted from 4 days to 8 weeks to complete remission in an MIS-C patient (Table 2).
.jpg)

Discussion
According to the data from the SECURE-IBD registry and the COVID-19 database of the pediatric IBD Porto group of the European society for pediatric gastroenterology hepatology and nutrition, COVID-19 is a benign illness in the pediatric patients with IBD, please replace this part with the above phrase and disease, , and other reviewed studies, severity and complications will not be increased in these children compared to normal children. Symptoms of COVID-19 in pediatric IBD patients are not significantly different from the general population and mainly include fever, fatigue, dry cough, and gastrointestinal symptoms. However, limited data show that chronic steroid use may rise the COVID-19 severity [10, 11, 12]. Moreover, studies indicate that disease activity status is related to the patient’s outcomes when infected with SARS-CoV-2 [11, 13], particularly in younger individuals [14].
IBD patients show higher levels of ACE-2 expression. However, whether ACE-2 overexpression increases COVID-19 severity or has protective functions is not clear [15, 16]. Studies also have shown a benign course of COVID-19 in immunosuppressed adults with IBD [17, 18, 19] and even the protective roles of immunosuppressive agents [20]. However, one study showed a moderately increased risk of hospitalization in adult patients but a not severe disease or mortality [21]. Currently, it is recommended for these patients to stop their medications except for 5-ASA when infected with this virus [16]. In children, immunosuppressive drugs does not increase the risk of complications SARS-CoV-2 infection [22]. One study that evaluated the association between biological use in children with IBD and symptomatic COVID-19, found no association [23].
Preliminary reports have shown that steroid use, increasing age, and other comorbidities increase the risk of COVID-19 complications in adults with IBD. Although biological therapy is safe, thiopurine use is accompanied by increasing adverse events [9].
Brenner et al. studied a group of pediatric IBD patients hospitalized due to SARS-CoV-2 and compared them with outpatients. They concluded that the presence of other having comorbidities, gastrointestinal symptoms, more severe IBD activity, and steroid or 5-ASA use is accompanied by a higher risk for hospitalization. Anti-TNF monotherapy was shown to reduce the due to COVID-19 risk of hospitalization. They had two patients who required ICU admission, one due to MIS-C and the other one due to disease severity and secondary infections. Both patients were on 5-ASA and also suffered from asthma [11].
Several studies have reported MIS-C development following SARS-CoV-2 contracting in children with IBD [24, 25]. In these cases, the patient has recovered without any long-term complications and has been on biologic or 5-ASA before contracting COVID-19. Meredith et al. reported a 10-year-old female patient with UC who developed MIS-C. The patient was on 5-ASA and infliximab treatment [24]. Dolinger et al. also reported a 14-year-old boy with CD and MIS-C development following COVID-19. The patient was treated with two doses of infliximab and recovered [25]. Sweeny et al. reported a 16 years old boy with no known past medical history presenting with fever and gastrointestinal symptoms consistent with both new-onset IBD and MIS-C and with a hyperinflammatory state. The patient had a history of flu-like disease six weeks before to these symptoms. However, the nasopharyngeal swab test for SARS-CoV-2 was negative at admission. The patient had signs of IBD in endoscopy, but submucosal vasculitis was consistent with MIS-C. After receiving Intravenous immune globulin, the patient first showed clinical improvement. However, the clinical condition again worsened thereafter. Finally, he was successfully treated with infliximab [26]. Brenner et al. reported the other case of MIS-C; a six years old girl with UC and on 5-ASA who presented with respiratory failure and coagulopathy and needed ICU care and mechanical ventilation. The symptoms lasted for 19 days and the patient recovered after corticosteroid admission [11]. Other studies have reported a mild and benign course of COVID-19 in these patients. Literature reports no deaths related to infection with SARS-CoV-2 in pediatric IBD patients.
Compared to the hospital admission rate of 33 to 66% reported in adult IBD patients with SARS-CoV-2, Brenner et al. reported a rate of 7% in children. In total, COVID-19 in the pediatric population is usually asymptomatic [11].
Limitations
This study has several limitations. First, IBD type and medications are not reported in several included studies. Second, there is a lack of case-control studies that compare the outcomes of COVID-19 in healthy children and children with IBD. Third, we have only included English language articles. In general, current data suggest that that COVID-19 outcome is not significantly affected by IBD, disease activity status, steroid use, and other comorbidities affect the patients’ outcome following infection with SARS-CoV-2. However, further case-control studies with larger sample sizes are needed to confirm these results.
Conclusion
COVID-19 is a benign and self-limited disease in children with IBD even on immunosuppressive therapy. Corticosteroids, severe IBD activity, and other comorbidities increase the risk of disease severity. Case-control studies with larger sample sizes are needed to confirm these results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Medical University of Isfahan (Code: IR.MUI.MED.REC.1400.558). This article is a systematic review study with no human or animal sample.
Funding
This study was funded by Isfahan University of Medical Sciences (Code: 3400535).
Authors' contributions
All authors contributed the same in this study.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank the Vice-chancellor of Research at the Isfahan University of Medical Sciences.
References
The first case of pneumonia with the novel beta-corona virus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was found in Wuhan, ChinaChina in the late 2019 and turned rapidly into a pandemic worldwide in March 2020 [1]. The course of the disease caused by SARS-CoV-2, named coronavirus disease 2019 (COVID-19) can range from asymptomatic or mild infection to severe and fatal illness with a variety of signs and symptoms related to different body organs [1].
Research has shown that patients with co-morbidities and the elderly are at greater risk for the developing of severe complications following infection with SARS-CoV-2 [2], possibly due to cytokine storm formation, which is an excessive and inappropriate immune response to the virus [3].
Patients with inflammatory bowel diseases (IBDs), comprising Crohn’s disease (CD), and ulcerative colitis (UC) are believed to be at increased risk of opportunistic bacterial or viral infections due to chronic chronic immunosuppressive medications [2, 4]. In the meantime, it is essential to assess whether these individuals are at risk for the development of more intense cases of the disease caused by the novel coronavirus [1, 5, 6].
According to the literature, taking immunosuppressive drugs is not correlated with increased intensity of COVID-19 [7]. However, the augmented risk of COVID-19 severity may be due to the increased cytokine production or angiotensin-converting enzyme (ACE-2) overexpression, the receptor for SARS-CoV-2 on the host cells, in IBD ppatients [8].
Since the beginning of this pandemic, many efforts have been made by various research teams to answer this question. For example, the Surveillance Epidemiology of Coronavirus Under Research Exclusion for Inflammatory Bowel Disease database (SECURE-IBD) is an international registry recruiting IBD patients with SARS-CoV-2. European society for pediatric gastroenterology, hepatology, and nutrition also created a database for IBD patients with SARS-CoV-2. The data acquired from this registry so far have shown that the risk of being infected with this virus or developing severe complications following the infection in IBD patients is not different from the healthy population can increase the risk of complications from the virus [9]. However, there is still not enough information in the literature [6]. In addition, most studies in this field have been performed on adult patients and few studies have been performed on children. Accordingly, in the present study, we intended to collect data on COVID-19 in children with IBD through conducting a systematic literature review.
Methods
Search strategy and databases
This systematic review study is based on the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020. The research question were as follows:
P: Children with IBD and COVID-19
I: IBD treatments
C: Children with COVID-19 and without IBD
O: COVID-19 outcome
The search was based on the keywords listed in Table 1 through Scopus, PubMed, and the Web of Science databases until October 16, 2021.
.jpg)
The search strategy in each database is listed in Table 1.
Inclusion and exclusion criteria
All English language original articles and meeting abstracts of any type including case reports, case series, cross-sectional, cohort, and case-control were included in the present systematic review study. The study population included patients with COVID-19 and IBD who were less than 19 years old. In this study, all studies that examined the course of COVID-19 in patients with IBD less than 19 years old were included. Unrelated studies, reviews, duplicates, and low-quality studies were excluded from the review process.
Study selection process
After removing duplicates, the title and the abstracts of the articles were evaluated for eligibility and then, the full text of the remaining articles was reviewed independently by two reviewers. Unrelated ones and studies that did not report COVID-19 outcomes in patients under 19 years of age were removed from the review process. Final articles entered the quality assessment.
Quality assessment
Newcastle Ottawa scale was used for quality assessment. A score of less than 14 was considered low quality. No studies were excluded at this stage. The methodological quality of the "final " studies .
Data extraction
A checklist with the following items was used for data extraction by two independent reviewers: author name, country, sample size, IBD type, medications, and COVID-19 outcomes.
Results
From the initially retrieved 2215 papers, 1127 were duplicates. Based on the titles and the abstracts, 1028 articles were irrelevant or review papers. Finally, 60 articles were assessed for eligibility. Twenty-four articles were irrelevant. Twenty articles did not include pediatric patients or did not report COVID-19 outcomes in this group. Finally, 16 studies (14 articles, 2 meeting abstracts) were eligible (Figure 1). Characteristics of the studies
Nine articles had cohort design, five were case reports, and two were cross-sectional. Data from 1040 pediatric patients with IBD were reviewed. Regarding IBD type, 124 patients had UC, 234 had CD, 25 had IBD-unknown (IBD-U), and other patients not reported. Twenty-four patients were hospitalized due to COVID-19, but there were no related deaths or severe complications, and all patients recovered completely. Five patients (3 CD, 2 UC; 3 males and two females) developed Multisystem Inflammatory Syndrome in Children (MIS-C). One other patient also needed intensive care unit (ICU) due to secondary infection and disease severity. These patients were on biologics or 5-Aminosalicylic acids (5-ASA). Except for these five patients, others mainly reported mild signs and symptoms, including fatigue, fever, and dry cough, or were asymptomatic. One study showed that IBD activity status, steroid or 5-ASA use, presence of gastrointestinal symptoms, and other comorbidities increase the risk of hospitalization in children with COVID-19 and IBD.
In contrast, Tumor necrosis factor (TNF) antagonist monotherapy lowered hospitalization likelihood ratio. Another study also revealed no significant relation between biological agent use and the development of symptomatic COVID-19. Disease symptoms lasted from 4 days to 8 weeks to complete remission in an MIS-C patient (Table 2).
.jpg)


Discussion
According to the data from the SECURE-IBD registry and the COVID-19 database of the pediatric IBD Porto group of the European society for pediatric gastroenterology hepatology and nutrition, COVID-19 is a benign illness in the pediatric patients with IBD, please replace this part with the above phrase and disease, , and other reviewed studies, severity and complications will not be increased in these children compared to normal children. Symptoms of COVID-19 in pediatric IBD patients are not significantly different from the general population and mainly include fever, fatigue, dry cough, and gastrointestinal symptoms. However, limited data show that chronic steroid use may rise the COVID-19 severity [10, 11, 12]. Moreover, studies indicate that disease activity status is related to the patient’s outcomes when infected with SARS-CoV-2 [11, 13], particularly in younger individuals [14].
IBD patients show higher levels of ACE-2 expression. However, whether ACE-2 overexpression increases COVID-19 severity or has protective functions is not clear [15, 16]. Studies also have shown a benign course of COVID-19 in immunosuppressed adults with IBD [17, 18, 19] and even the protective roles of immunosuppressive agents [20]. However, one study showed a moderately increased risk of hospitalization in adult patients but a not severe disease or mortality [21]. Currently, it is recommended for these patients to stop their medications except for 5-ASA when infected with this virus [16]. In children, immunosuppressive drugs does not increase the risk of complications SARS-CoV-2 infection [22]. One study that evaluated the association between biological use in children with IBD and symptomatic COVID-19, found no association [23].
Preliminary reports have shown that steroid use, increasing age, and other comorbidities increase the risk of COVID-19 complications in adults with IBD. Although biological therapy is safe, thiopurine use is accompanied by increasing adverse events [9].
Brenner et al. studied a group of pediatric IBD patients hospitalized due to SARS-CoV-2 and compared them with outpatients. They concluded that the presence of other having comorbidities, gastrointestinal symptoms, more severe IBD activity, and steroid or 5-ASA use is accompanied by a higher risk for hospitalization. Anti-TNF monotherapy was shown to reduce the due to COVID-19 risk of hospitalization. They had two patients who required ICU admission, one due to MIS-C and the other one due to disease severity and secondary infections. Both patients were on 5-ASA and also suffered from asthma [11].
Several studies have reported MIS-C development following SARS-CoV-2 contracting in children with IBD [24, 25]. In these cases, the patient has recovered without any long-term complications and has been on biologic or 5-ASA before contracting COVID-19. Meredith et al. reported a 10-year-old female patient with UC who developed MIS-C. The patient was on 5-ASA and infliximab treatment [24]. Dolinger et al. also reported a 14-year-old boy with CD and MIS-C development following COVID-19. The patient was treated with two doses of infliximab and recovered [25]. Sweeny et al. reported a 16 years old boy with no known past medical history presenting with fever and gastrointestinal symptoms consistent with both new-onset IBD and MIS-C and with a hyperinflammatory state. The patient had a history of flu-like disease six weeks before to these symptoms. However, the nasopharyngeal swab test for SARS-CoV-2 was negative at admission. The patient had signs of IBD in endoscopy, but submucosal vasculitis was consistent with MIS-C. After receiving Intravenous immune globulin, the patient first showed clinical improvement. However, the clinical condition again worsened thereafter. Finally, he was successfully treated with infliximab [26]. Brenner et al. reported the other case of MIS-C; a six years old girl with UC and on 5-ASA who presented with respiratory failure and coagulopathy and needed ICU care and mechanical ventilation. The symptoms lasted for 19 days and the patient recovered after corticosteroid admission [11]. Other studies have reported a mild and benign course of COVID-19 in these patients. Literature reports no deaths related to infection with SARS-CoV-2 in pediatric IBD patients.
Compared to the hospital admission rate of 33 to 66% reported in adult IBD patients with SARS-CoV-2, Brenner et al. reported a rate of 7% in children. In total, COVID-19 in the pediatric population is usually asymptomatic [11].
Limitations
This study has several limitations. First, IBD type and medications are not reported in several included studies. Second, there is a lack of case-control studies that compare the outcomes of COVID-19 in healthy children and children with IBD. Third, we have only included English language articles. In general, current data suggest that that COVID-19 outcome is not significantly affected by IBD, disease activity status, steroid use, and other comorbidities affect the patients’ outcome following infection with SARS-CoV-2. However, further case-control studies with larger sample sizes are needed to confirm these results.
Conclusion
COVID-19 is a benign and self-limited disease in children with IBD even on immunosuppressive therapy. Corticosteroids, severe IBD activity, and other comorbidities increase the risk of disease severity. Case-control studies with larger sample sizes are needed to confirm these results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Medical University of Isfahan (Code: IR.MUI.MED.REC.1400.558). This article is a systematic review study with no human or animal sample.
Funding
This study was funded by Isfahan University of Medical Sciences (Code: 3400535).
Authors' contributions
All authors contributed the same in this study.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
The authors thank the Vice-chancellor of Research at the Isfahan University of Medical Sciences.
References
- Marín-Jiménez I, Zabana Y, Rodríguez-Lago I, Marín L, Barreiro-de Acosta M, Esteve M. COVID-19 and inflammatory bowel disease: Questions arising from patient care and follow-up during the initial phase of the pandemic (February-April 2020). Gastroenterología y Hepatología. 2020; 43(7):408-13. [DOI:10.1016/j.gastre.2020.07.001] [PMID] [PMCID]
- Anikhindi SA, Kumar A and Arora A. COVID-19 in patients with inflammatory bowel disease. Expert Rev Gastroenterol Hepatol. 2020; 14:1187-93. [DOI:10.1080/17474124.2020.1816822] [PMID]
- Ribaldone DG, Astegiano M, Actis GC, Pellicano R. Management of inflammatory bowel disease during COVID-19 pandemic. Govaresh. 2020; 25:44-50. [Link]
- Cardamone C and Donatiello I. Management of COVID-19 in comorbidities. Ital J Med. 2020; 14:223-7. [DOI:10.4081/itjm.2020.1406]
- D’Amico F, Danese S, and Peyrin-Biroulet L. Systematic review on inflammatory bowel disease patients with coronavirus disease 2019: It is time to take stock. Clin Gastroenterol Hepatol. 2020; 18:2689. [DOI:10.1016/j.cgh.2020.08.003] [PMID] [PMCID]
- Ashton JJ, Batra A, Coelho TA, Afzal NA, Beattie RM. Challenges in chronic paediatric disease during the COVID-19 pandemic: Diagnosis and management of inflammatory bowel disease in children. Arch Dis Child. 2020; 105(7):706. [DOI:10.1136/archdischild-2020-319751] [PMID]
- Papa A, Gasbarrini A and Tursi A. Epidemiology and the impact of therapies on the outcome of COVID-19 in patients with inflammatory bowel disease. Am J Gastroenterol. 2020; 115:1722-4. [DOI:10.14309/ajg.0000000000000830] [PMID] [PMCID]
- Corrias A, Cortes GM, Bardanzellu F, Melis A, Fanos V, Marcialis MA. Risk, course, and effect of SARS-CoV-2 infection in children and adults with chronic inflammatory bowel diseases. Children. 2021; 8(9):753. [DOI:10.3390/children8090753] [PMID] [PMCID]
- Horst S. COVID-19 and patients with IBD: Who is at highest risk for severe complications? Dig Dis Sci. 2021:1-2. [DOI:10.1007/s10620-021-07106-y] [PMID] [PMCID]
- Fragoso RP, Rodrigues M. COVID-19 and pediatric inflammatory bowel disease: How to manage it? Clinics. 2020; 75. [DOI:10.6061/clinics/2020/e1962] [PMID] [PMCID]
- Brenner EJ, Pigneur B, Focht G, Zhang X, Ungaro RC, Colombel JF, et al. Benign evolution of SARS-Cov2 infections in children with inflammatory bowel disease: Results from two international databases. Clin Gastroenterol Hepatol. 2021; 19(2):394-6. [DOI:10.1016/j.cgh.2020.10.010] [PMID] [PMCID]
- Marcela V, Marina M, Caio F, Fabio T, Liliana C, Rogerio SH, et al. P059 Corticosteroids, Aminosalicylates and Gastrointestinal Symptoms Are Associated With the Need of Hospitalization in Patients With Inflammatory Bowel Diseases and COVID-19. Am J Gastroenterol. 2020; 115:S15. [DOI:10.14309/01.ajg.0000723032.51671.1a]
- Macaluso FS, Giuliano A, Fries W, Viola A, Abbruzzese A, Cappello M, et al. Severe activity of inflammatory bowel disease is a risk factor for severe COVID-19. Inflamm Bowel Dis. 2022:izac064 [DOI:10.1016/S1590-8658(21)00463-1]
- Ricciuto A, Lamb C, Kuenzig E, Griffiths AM, Kaplan G, Walker GJ, et al. Fr493 Disease activity is associated with COVID-19 outcomes in IBD patients with effect modification by age. Gastroenterology. 2021; 160(6):S-330. [DOI:10.1016/S0016-5085(21)01514-6]
- Ferreira-Duarte M, Estevinho MM, Duarte-Araújo M, Magro F, Morato M. Unraveling the role of ACE2, the binding receptor for SARS-CoV-2, in inflammatory bowel disease. Inflamm Bowel Dis. 2020; 26(12):1787-95. [DOI:10.1093/ibd/izaa249] [PMID] [PMCID]
- Gutin LS, Lam AY, Velayos FS, Santos SA. Going Viral: Management of IBD in the Era of the COVID-19 Pandemic. Dig Dis Sci. 2020; 65(6):1571-5. [DOI:10.1007/s10620-020-06299-y] [PMID] [PMCID]
- Aziz M, Fatima R, Haghbin H, Lee-Smith W, Nawras A. The incidence and outcomes of COVID-19 in IBD patients: A rapid review and meta-analysis. Inflamm Bowel Dis. 2020:e132-e33. [DOI:10.1093/ibd/izaa170] [PMID] [PMCID]
- Bossa F, Carparelli S, Latiano A, Palmieri O, Tavano F, Panza A, et al. Impact of the COVID-19 outbreak and the serum prevalence of SARS-CoV-2 antibodies in patients with inflammatory bowel disease treated with biologic drugs. Dig Liver Dis. 2021; 53(3):277-82. [DOI:10.1016/j.dld.2020.12.120] [PMID] [PMCID]
- Gilissen LP, Heinen SG, Rijpma-Jacobs L, Schoon E, Schreuder RM, Wensing AM, et al. Neither inflammatory bowel disease nor immunosuppressants are associated with an increased risk of severe COVID-19: An observational Dutch cohort study. Clin Exp Med. 2022; 22(3):465-76. [DOI:10.1007/s10238-021-00755-3] [PMID] [PMCID]
- Hormati A, Ghadir MR, Zamani F, Khodadadi J, Khodadust F, Afifian M, et al. Are there any association between COVID-19 severity and immunosuppressive therapy? Immunol Lett. 2020; 224:12. [DOI:10.1016/j.imlet.2020.05.002] [PMID] [PMCID]
- Ludvigsson JF, Axelrad J, Halfvarson J, Khalili H, Larsson E, Lochhead P, et al. Inflammatory bowel disease and risk of severe COVID-19: A nationwide population-based cohort study in Sweden. UEG J. 2021; 9(2):177-92. [DOI:10.1002/ueg2.12049] [PMID] [PMCID]
- Demir F, Ulu K, Çağlayan Ş, Coşkuner T, Sözeri B. Clinical course of COVID-19 in children with rheumatic disease under biologic therapy. Clin Rheumatol. 2021; 39:36-37. [Link]
- Spencer EA, Klang E, Dolinger MT, Dubinsky M. 784 clinical course of pediatric inflammatory bowel disease patients with positive COVID-19 antibody testing at a single, tertiary care center. Gastroenterology. 2021; 160:S-158. [DOI:10.1016/S0016-5085(21)01129-X]
- Meredith J, Khedim CA, Henderson P, Wilson DC, Russell RK. Paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 [PIMS-TS] in a patient receiving infliximab therapy for inflammatory bowel disease. Journal of Crohn's and Colitis. 2021; 15(4):687-91. [DOI:10.1093/ecco-jcc/jjaa201] [PMID] [PMCID]
- Dolinger MT, Person H, Smith R, Jarchin L, Pittman N, Dubinsky MC, et al. Pediatric Crohn's disease and multisystem inflammatory syndrome in children (MIS-C) and COVID-19 treated with infliximab. J Pediatr Gastroenterol Nutr. 2020. [DOI:10.1097/MPG.0000000000002809] [PMID] [PMCID]
- Sweeny KF, Zhang YJ, Crume B, Martz CA, Blessing MM, Kahn SA. Inflammatory Bowel Disease Presenting With Concurrent COVID-19 Multisystem Inflammatory Syndrome. Pediatrics. 2021. [DOI:10.1542/peds.2020-027763] [PMID] [PMCID]
- Brenner EJ, Ungaro RC, Gearry RB, Kaplan GG, Kissous-Hunt M, Lewis JD, et al. Corticosteroids, but not TNF antagonists, are associated with adverse COVID-19 outcomes in patients with inflammatory bowel diseases: Results from an international registry. Gastroenterology. 2020; 159(2):481-91. [DOI:10.1053/j.gastro.2020.07.020] [PMCID]
- Queiroz NS, Martins CD, Quaresma AB, Hino AA, Steinwurz F, Ungaro RC, Kotze PG. COVID-19 outcomes in patients with inflammatory bowel diseases in Latin America: Results from SECURE-IBD registry. J Gastroenterol Hepatol. 2021; 36(11):3033-40. [Link]
- Turner D, Huang Y, Martín-de-Carpi J, Aloi M, Focht G, Kang B, Zhou Y, Sanchez C, Kappelman MD, Uhlig HH, Pujol-Muncunill G. Corona virus disease 2019 and paediatric inflammatory bowel diseases: Global experience and provisional guidance (March 2020) from the paediatric IBD Porto group of European Society of paediatric gastroenterology, hepatology, and nutrition. J Pediatr Gastroenterol Nutr. 2020; 70(6):727. [DOI:10.1097/MPG.0000000000002729] [PMID] [PMCID]
- D’Arcangelo G, Distante M, Raso T, Rossetti D, Catassi G, Aloi M. Safety of biological therapy in children with inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2021; 72(5):736-41. [DOI:10.1097/MPG.0000000000003044] [PMID]
- Sansotta N, Norsa L, Zuin G, Panceri R, Dilillo D, Pozzi E, et al. Children with inflammatory bowel disease in the COVID-19 main endemic focus: The lombardy experience. Front Pediatr. 2021; 9:607285. [DOI:10.3389/fped.2021.607285] [PMID] [PMCID]
- Ruan W, Ihekweazu F, Walsh S, Karam L, Wyatt A, Nguyen H, et al. SARS-COV-2 infection and seroconversion in pediatric inflammatory bowel disease patients. Inflamm. Bowel Dis. 2021; 27(Suppl 1):S44-5. [DOI:10.1093/ibd/izaa347.108] [PMCID]
- Koletzko L, Klucker E, Le Thi TG, Breiteneicher S, Rubio-Acero R, Neuhaus L, et al. Following pediatric and adult IBD patients through the COVID-19 pandemic: Changes in psychosocial burden and perception of infection risk and harm over time. J Clin Med. 2021; 10(18):4124. [DOI:10.3390/jcm10184124] [PMID] [PMCID]
- Arrigo S, Alvisi P, Banzato C, Bramuzzo M, Celano R, Civitelli F, et al. Impact of COVID-19 pandemic on the management of paediatric inflammatory bowel disease: An Italian multicentre study on behalf of the SIGENP IBD Group. Dig Liver Dis. 2021; 53(3):283-8. [DOI:10.1016/j.dld.2020.12.011] [PMCID]
- Bardasi G, Alvisi P. SARS-CoV-2 infection in severe pediatric Crohn's disease. What about anti-tumor necrosis factor α therapy? Dig Liver Dis. 2020; 52(11):1244-5. [DOI:10.1016/j.dld.2020.06.047] [PMID] [PMCID]
- Abdullah A, Neurath MF, Atreya R. Mild COVID-19 symptoms in an infliximab-treated ulcerative colitis patient: Can ongoing anti-TNF therapy protect against the viral hyperinflammatory response and avoid aggravated outcomes? Visc Med. 2020; 36(4):338-42. [DOI:10.1159/000508740] [PMID] [PMCID]
Type of Study: Systematic Review |
Subject:
Pediatric Gastroenterology
Received: 2022/06/6 | Accepted: 2022/08/10 | Published: 2022/12/19
Received: 2022/06/6 | Accepted: 2022/08/10 | Published: 2022/12/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





.jpg)
