Volume 11, Issue 3 (7-2023)
J. Pediatr. Rev 2023, 11(3): 251-260 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Aina N, Dhayalan I, Vasudevan J, Mannu A, Thiagarajan K S. Case Series of Prolonged Febrile Illness in Pediatric Age Group: A Diagnostic Challenge. J. Pediatr. Rev 2023; 11 (3) :251-260
URL: http://jpr.mazums.ac.ir/article-1-511-en.html
URL: http://jpr.mazums.ac.ir/article-1-511-en.html
Noorul Aina1 

 , Indumathi Dhayalan *2
, Indumathi Dhayalan *2 

 , Jaishree Vasudevan1
, Jaishree Vasudevan1 

 , Alexander Mannu1
, Alexander Mannu1 

 , Kathir Subramanian Thiagarajan1
, Kathir Subramanian Thiagarajan1 




 , Indumathi Dhayalan *2
, Indumathi Dhayalan *2 

 , Jaishree Vasudevan1
, Jaishree Vasudevan1 

 , Alexander Mannu1
, Alexander Mannu1 

 , Kathir Subramanian Thiagarajan1
, Kathir Subramanian Thiagarajan1 


1- Department of Pediatrics, Chettinad Hospital And Research Institute, Chettinad Academy of Research Education, Tamil Nadu, India.
2- Department of Pediatrics, Chettinad Hospital And Research Institute, Chettinad Academy of Research Education, Tamil Nadu, India. ,indu.ich@gmail.com
2- Department of Pediatrics, Chettinad Hospital And Research Institute, Chettinad Academy of Research Education, Tamil Nadu, India. ,
Full-Text [PDF 550 kb]
(1749 Downloads)
| Abstract (HTML) (3408 Views)
Full-Text: (3574 Views)
Background
Fever, defined as a rectal temperature of 100.4oF or more, is a physiological response characterized by an elevation of body temperature above normal [1]. Fever is one of the common causes of medical consultation in children, responsible for 15%–25% of consultations in pediatrics practice [2]. Children with prolonged fever can worry parents and pediatricians alike. The causes of fever can be classified under 4 main categories: Infectious, inflammatory, neoplastic, and miscellaneous causes. The exact duration of prolonged fever is undefined. However, children with a temperature more than 38°C (100.4°F) recorded by a healthcare professional without any determined reason after at least 8 days of evaluation should be classified as having a “fever of unknown origin” (FUO). When the initial physical evaluation does not reveal any associated or distinctive findings, and all laboratory values are inconclusive or normal, the definitive diagnosis can be made only after extensive evaluation and observation. Most fevers with unknown causes usually are atypical presentations of common illnesses [3, 4, 5, 6]. Infections, connective tissue conditions, and neoplasms are the 3 most frequent causes of prolonged fever in children. The infectious diseases are usually salmonellosis, tuberculosis, malaria, rickettsia, and so on. The connective tissue disorder commonly linked to prolonged fever is juvenile rheumatoid arthritis. The third most common cause of prolonged fever in children is malignancy. The persistence of fever raises clinical queries towards diagnosis, especially in patients without identifiable cause. Here, we present a series of children with prolonged fever who presented as a diagnostic challenge.
Case Presentation
We reviewed the case records of 9 children admitted to our pediatric ward with prolonged fever episodes from December 2021 to October 2022 that were diagnostic challenges for clinicians. The demographic, clinical, laboratory parameters and examination findings are listed in Table 1.

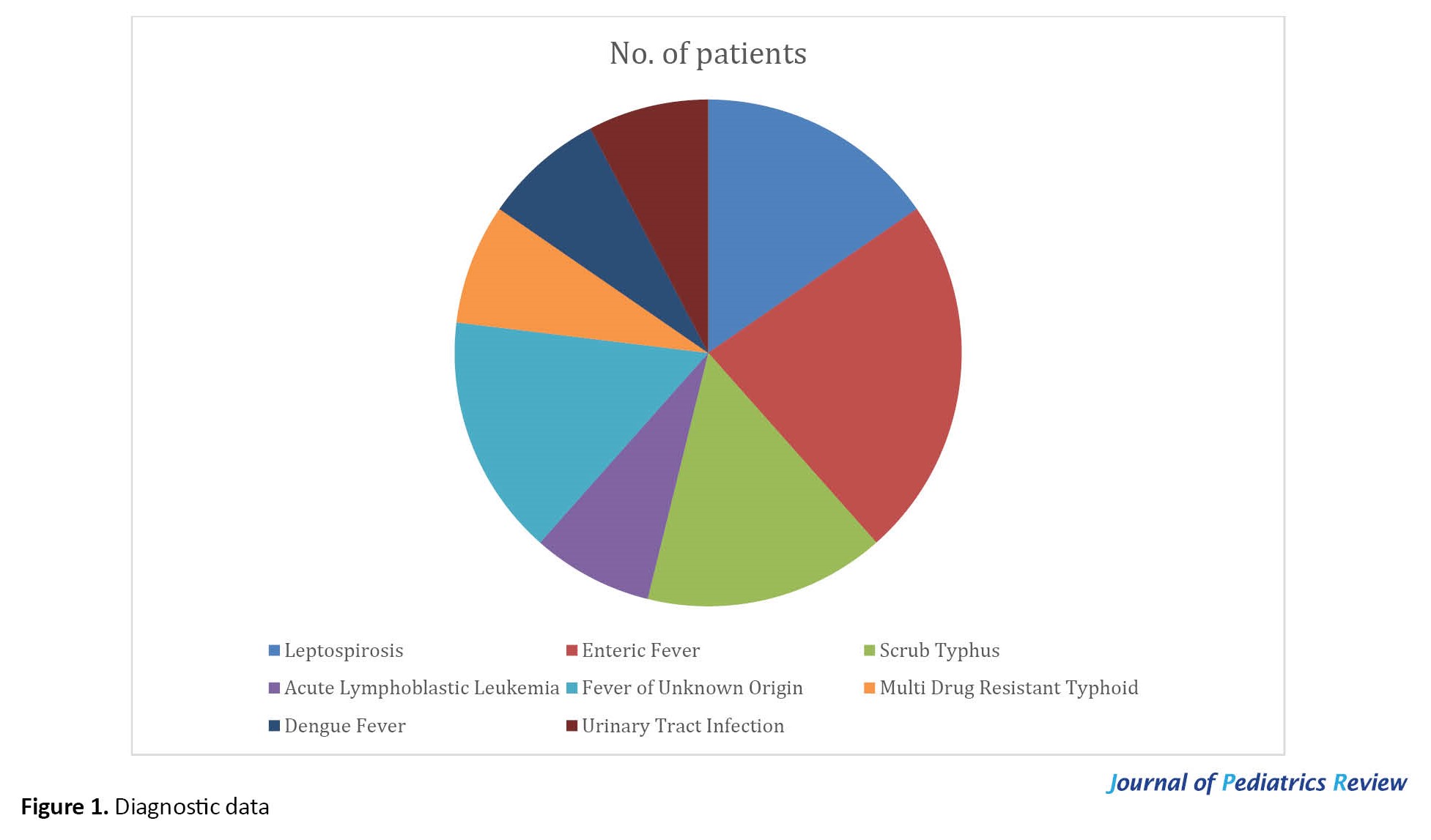
Figure 1 displays a comprehensive overview and diagnosis of all our cases. Two children had scrub typhus, 1 acute lymphoblastic leukemia, and 1 multidrug-resistant typhoid fever. However, two cases remained as FUO. Three children were found to have coinfections and diagnosed with enteric fever with other coinfections like dengue, leptospirosis, and urinary tract infection.
Discussion
We described 9 children admitted to our pediatric ward with prolonged fever. All cases were evaluated primarily based on the clues given through history, physical examination, and routine investigations. First-line investigations included complete blood count, acute phase reactants (C-reactive protein), and urinalysis. In addition, imaging studies like abdominal ultrasound and chest radiographs were done as clinically indicated. Further specific microbiological or pathological investigations were done on case-to-case basis. We started on empirical antibiotics in patients suspected of bacterial infections after taking blood and urine cultures and sensitivity. Further screening for tuberculosis workup like chest x-ray, Mantoux test, and gene-xpert has also been done in suspected cases, especially those with a history of significant contact with tuberculosis. Infective workups for scrub typhus, enteric fever, leptospirosis, and malaria were also done in cases of clinical suspicion.
Case 1 was a 14-year-old boy who presented with complaints of high-grade fever, headache and abdominal pain, loose stools, and non-bilious vomiting for 10 days. In addition, there were complaints of headaches intermittently for 10 days. This case had a history of travel, outside food consumption, and a history of playing in rainwater. He was initially treated in another hospital with a course of oral antibiotics, but the fever did not seem to settle, and was referred to our hospital on day 10 of fever. Examination revealed right hypochondriac tenderness and hepatosplenomegaly. The child was started on empiric antibiotics of ceftriaxone and doxycycline. Initial investigations showed raised transaminase levels, reactive inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]), and an elevated Widal test titer. Hence, the child was continued on injection of ceftriaxone. An infective workup was also done, and Leptospira IgM was reactive (32.94 Panbio units). Although the initial blood culture and sensitivity showed no growth of organisms, extended blood culture and sensitivity showed significant growth of Salmonella typhi. Hence a diagnosis of enteric fever and leptospirosis was made. Workup for other infective etiologies was negative. There were still persistent high-grade fever spikes; hence on day 8 of admission, the child was started on oral azithromycin. Hence on day 10 of admission, antibiotics were escalated due to the persistence of fever spikes to IV ofloxacin according to sensitivity pattern. The fever subsided within 48 hours on day 12 of admission, and the child was discharged with oral ofloxacin to be continued for 5 more days.
Case 2 had a similar picture to case 1. She was a 13-year-old girl, referred with complaints of high-grade fever, vomiting, abdominal pain for 12 days, and occasional complaints of high-colored urine. On examination, right hypochondriac, and epigastric tenderness were present. The child was initially treated in an outside hospital with oral fluoroquinolones and continued with oral azithromycin. However, fever episodes persisted even after 3 days of initial management. Hence child was evaluated extensively, and investigations revealed raised transaminase levels, reactive inflammatory markers (CRP and ESR), and an elevated Widal test titer. An infective workup was also done, and Leptospira IGM was reactive. On day 6 of admission, Given persistent fever spikes, intravenous ceftriaxone was started. In addition to that, COVID-19 RT-PCR, real-time reverse transcription polymerase chain reaction (RT-PCR) was also reactive. Hence, she was diagnosed with leptospirosis and enteric fever with COVID-19 infection, and the child responded to the treatment and became afebrile from day 10 of admission.
Cases 1 and 2 had persistent fever episodes despite appropriate antibiotic therapy based on sensitivity patterns, which was a significant challenge. Apart from serological evidence, the modified Faine’s clinical criteria confirmed leptospirosis in both cases. Leptospirosis coinfection is more common with pathogenic agents causing dengue, malaria, and scrub typhus [7], and recently coinfection has also been observed with coronavirus [8]. However, coinfection with Leptospira and Salmonella is rare [9]. In a study conducted by Parker et al. to determine the prevalence of coinfections from 100 fever cases, there was only one coinfection with leptospirosis, typhoid, and malaria [10].
Cases 3 and 6 had initial diagnostic deviations due to their atypical presentations. Case 3 was a 10-year-old boy presented with 12 days of fever along with arthritis and left ankle swelling. The swelling was unilateral and associated with tenderness. Initially, a clinical suspicion of acute rheumatic fever and reactive arthritis was made. Autoimmune workup was also done and found to be negative. Inflammatory markers (ESR and CRP) were mildly elevated; ASO titers and ANA were non-reactive. Because of thrombocytopenia at admission, scrub typhus serology was done, which turned positive. The child was started on oral doxycycline, and his fever defervescence started on day 4 of admission, on day 16 of his illness. Reactive arthritis, as in numerous infectious diseases, is still a potential localization with scrub typhus and may be mistaken for septic arthritis. Monoarthritis is a rare presenting feature of scrub typhus, and to our knowledge, only one pediatric case has been previously reported in India [11].
Case 6 was a 9-year-old girl who presented with complaints of fever for 10 days and swelling behind the right postauricular region for 10 days. On examination, the child was febrile with an ulcer with a white base measuring 1x1 cm beneath the ear lobe. Usually, a typical eschar starts with a primary papular lesion, which eventually becomes a black ulcer with central necrosis. However, the appearance of this lesion was atypical, presenting an ulcer with a white base. Due to the site of the lesion and onset of eschar associated with fever, we suspected scrub typhus. Hence, scrub typhus serology was sent, which turned out to be reactive. The child responded well with oral doxycycline drug, and fever defervescence started on day 6 of admission. Eschar is the most helpful diagnostic evidence and is pathognomonic for Orientia tsutsugamushi. However, it is only present in fewer than 10% of patients in the Indian subcontinent. Clinical and laboratory characteristics of scrub typhus are non-specific [12]. Scrub typhus is commonly underdiagnosed in India due to the disease’s non-specific presentation, the lack of eschar, and the scarcity of confirming diagnostic testing [13]. Hence, it is important to recognize various clinical presentations of scrub typhus, which will aid in prompt diagnosis and treatment. Failure to do so can result in serious complications [14].
In case 4, a 12-year-old boy presented with a fever for 1 week and weight loss for 2 months. On examination, the child had hepatosplenomegaly, and blood investigations showed bicytopenia with normal peripheral smear, and CRP was elevated. Initial differential diagnoses included acute infectious illnesses (typhoid, malaria, and tropical diseases), acute viral illnesses, viral hepatitis, Epstein–Barr virus (EBV), and tuberculosis (TB). TB workup was negative, and the blood culture was sterile. Uric acid, phosphorous, and calcium were normal. Because of suspicion of hemophagocytic lymphohistiocytosis (HLH), further workup was done with serum ferritin and fibrinogen, which ruled out HLH. Viral panel studies were also done, which ruled out viral etiology. Given persistent bicytopenia, hematological malignancy was suspected. Bone marrow aspiration was done as a next step which showed evidence of blast cells. Flow cytometry confirmed the diagnosis of T-cell acute lymphoblastic leukemia. Bone marrow evaluation is important to peripheral smear blood analysis since some patients do not have circulating peripheral blast cells [15]. Children with leukemia frequently present with non-specific symptoms of illness, such as fever and fatigue. Fever is the most prevalent and obvious sign of infection. Acute lymphoblastic leukemia (ALL) comprised 77% of childhood leukemia cases and was the first widely prevalent malignancy to be proven to be treatable [16].
In case 8, a 12-year-old boy presented with high-grade fever, headache, and vomiting, which was non-bilious for 1 week, and there were no significant findings in the examination. Along with baseline investigations, a blood culture was sent, and the child was started on empirical antibiotics (injection of ceftriaxone). C-reactive protein was non-reactive, and another infective workup was also normal. During the initial treatment, the boy had no clinical response even with 5 days of empirical antibiotics administration, and the blood culture and sensitivity turned out to be sterile. The child persistently had a high-grade and intermittent fever, even 1 week after hospital management. Hence with clinical evidence and after suspecting multi-drug resistant typhoid, the child was further upgraded from antibiotics to intravenous azithromycin. The child demonstrated clinical response well, and the temperature gradually subsided. Fever defervescence started on day 6 of hospitalization.
Case 9 was a 3.5-year-old girl who had the longest hospital stay in our series. She initially presented with complaints of high-grade fever, vomiting with abdominal pain for 6 days, and examination findings revealed hepatomegaly. The child had an initial provisional diagnosis of Dengue fever with positive serology and was managed symptomatically. However, the child continued febrile with high-temperature spikes during the hospital stay. Although blood culture and sensitivity were sterile, urine culture showed significant growth of Klebsiella, and hence we upgraded antibiotics (amikacin and piperacillin+tazobactum) according to the sensitivity pattern. However, the repeated urine culture was sterile. Also, fever spikes continued to persist. Hence, we proceeded with an evaluation with bone marrow culture, which showed growth of Salmonella. The child was started ceftriaxone, based on the sensitivity pattern to which she responded by day 7. Fever episodes began to subside within 48 hours of antibiotic therapy, and fever defervescence started on the second week of the hospital stay.
When a child exhibits considerable pyrexia without localizing symptoms in endemic locations, typhoid fever should be considered a differential diagnosis. Typhoid fever should be promptly diagnosed by maintaining a high index of suspicion. In some circumstances, the clinical picture may deviate greatly from the typical typhoid presentation [17].
Case 5 was a 13-year-old boy presented with complaints of low-grade fever for 1 week and vomiting for 3 days. Examinations revealed pallor and hepatosplenomegaly, and the child was also sick looking due to poor oral intake. After performing all relevant baseline investigations and with clinical suspicion of enteric fever, a blood culture was sent, and the child was started on empirical antibiotics of ceftriaxone. Fever episodes did not resolve after 48 hours of treatment, and the blood culture was reportedly sterile. As part of further evaluation, infective workup with scrub typhus, dengue serology, and malaria were normal. COVID-19 RT-PCR and H1N1 were performed, and the results were normal. The child continued to have high-grade intermittent fever episodes after 9 days of hospital stay. Later, viral studies and autoimmune workups were also done, which also turned normal. Even after extensive evaluation, the child had high-grade intermittent fever episodes 9 days after a hospital stay. Hence, we made the diagnosis of FUO.
Case 7 was a 13-year-old girl who presented with complaints of fever, headache, and myalgia for 1 month with no significant examination finding and no focus. Tuberculosis was initially suspected, because there was a history of weight loss over the last 1 month. The child was admitted for further evaluation, and fever patterns were recorded during the hospital stay. The chest x-ray was normal, and the Mantoux test was negative. Gene-Xpert was sent, which also turned out to be negative. Further investigations and blood cultures were sent, and the child was started on empirical ceftriaxone antibiotics. However, the child continued to have fever episodes even after 48 hours of antibiotic therapy. Blood culture reports showed no growth of organisms. As part of further evaluation, all relevant infective workups were also done and found to be negative. The child continued to have low-grade fever spikes after 1 week of hospital admission. Since the child had a fever for more than 3 weeks and no cause was found even after 1 week of evaluation, we diagnosed it as a fever of unknown origin. Cases 5 and 7 posed a diagnostic challenge in which the causes were not found after extensive evaluation, and the fever persisted even after 10 days of admission and management. They had spontaneous resolution of fever without the achievement of a specific diagnosis. Consequently, the diagnosis of FUO was given. The fever patterns and frequency can sometimes aid in diagnosis. Children with recorded temperatures of more than 38°C (100.4°F) by healthcare professionals and for whom the reason could not be determined after at least 8 days of assessment should be classified as having FUO. However, most illnesses resulting from FUO lack a typical fever pattern. In addition to a thorough history and physical examination, the evaluation of FUO calls for a few screening laboratory tests, additional laboratory work, and imaging work based on the history, abnormalities found during the examination, or preliminary screening tests. fevers with unexplained causes are usually uncommon manifestations of common illnesses. Since there are initially no associated or specific physical examination findings, and all laboratory results are negative or normal, the definitive diagnosis can only be made after prolonged observation in some cases. Even after extensive investigation, the reason for the fever is still unknown in up to 25% of children with a persistent fever [18].
Conclusion
• A thorough history right from antenatal period, immunization status of children, and detailed examination are essential in pediatric febrile illnesses.
• Common causes of prolonged fever in children comprise infections, connective tissue disorder, and malignancy.
• Epidemiological factors like seasonal variations and endemic areas of disease prevalence exposure to wild animals should be considered while evaluating infectious causes.
• If a patient is not clinically responding to treatment, it could be due to antibiotic resistance or suspected coinfections. Antimicrobial drugs should empirically not be used as they may obscure the diagnosis.
• We have found that there might be deviators during disease evolution. We should not stay with a single diagnosis until a good clinical response is achieved and also consider alternative diagnoses or coinfections.
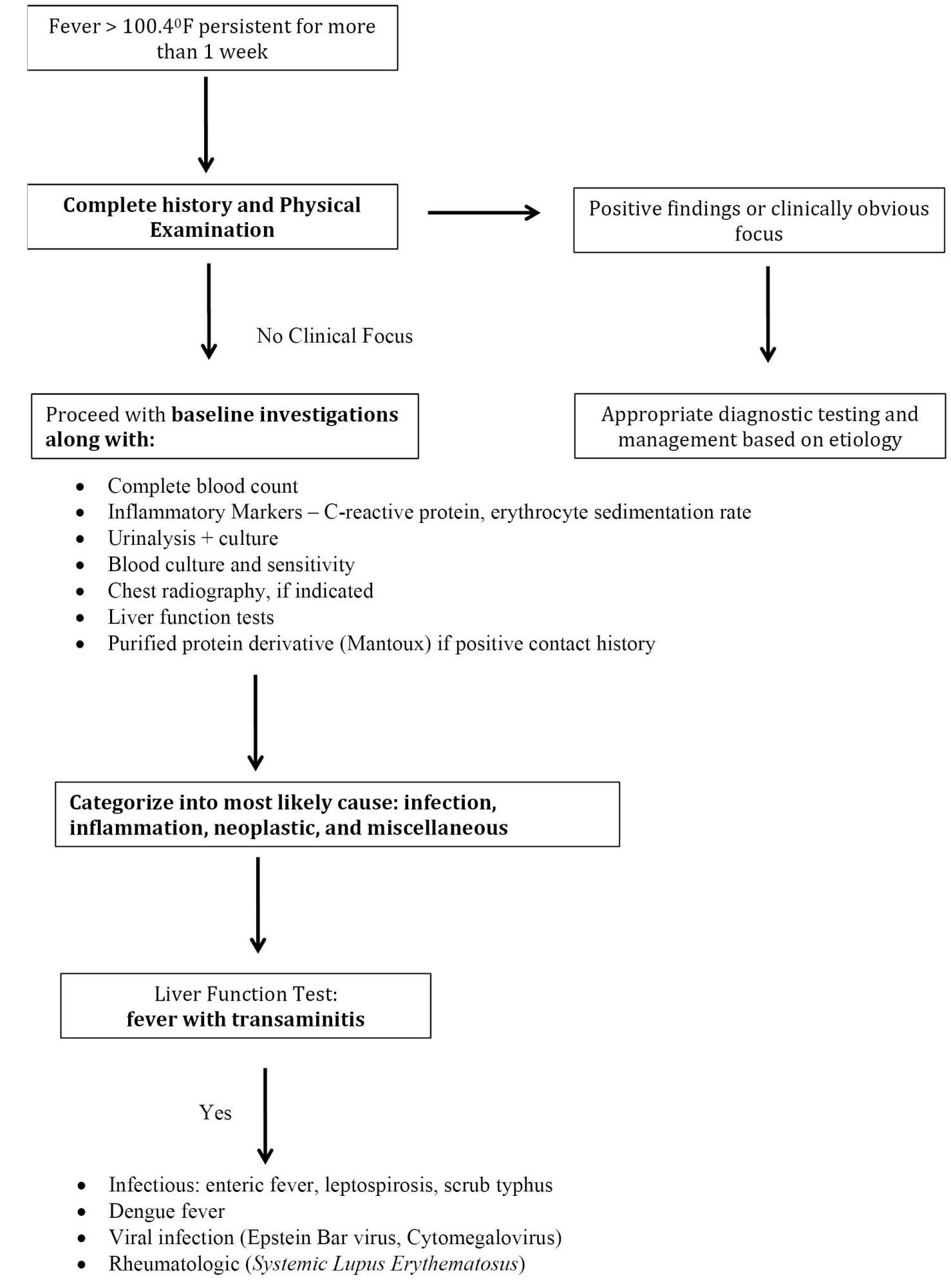
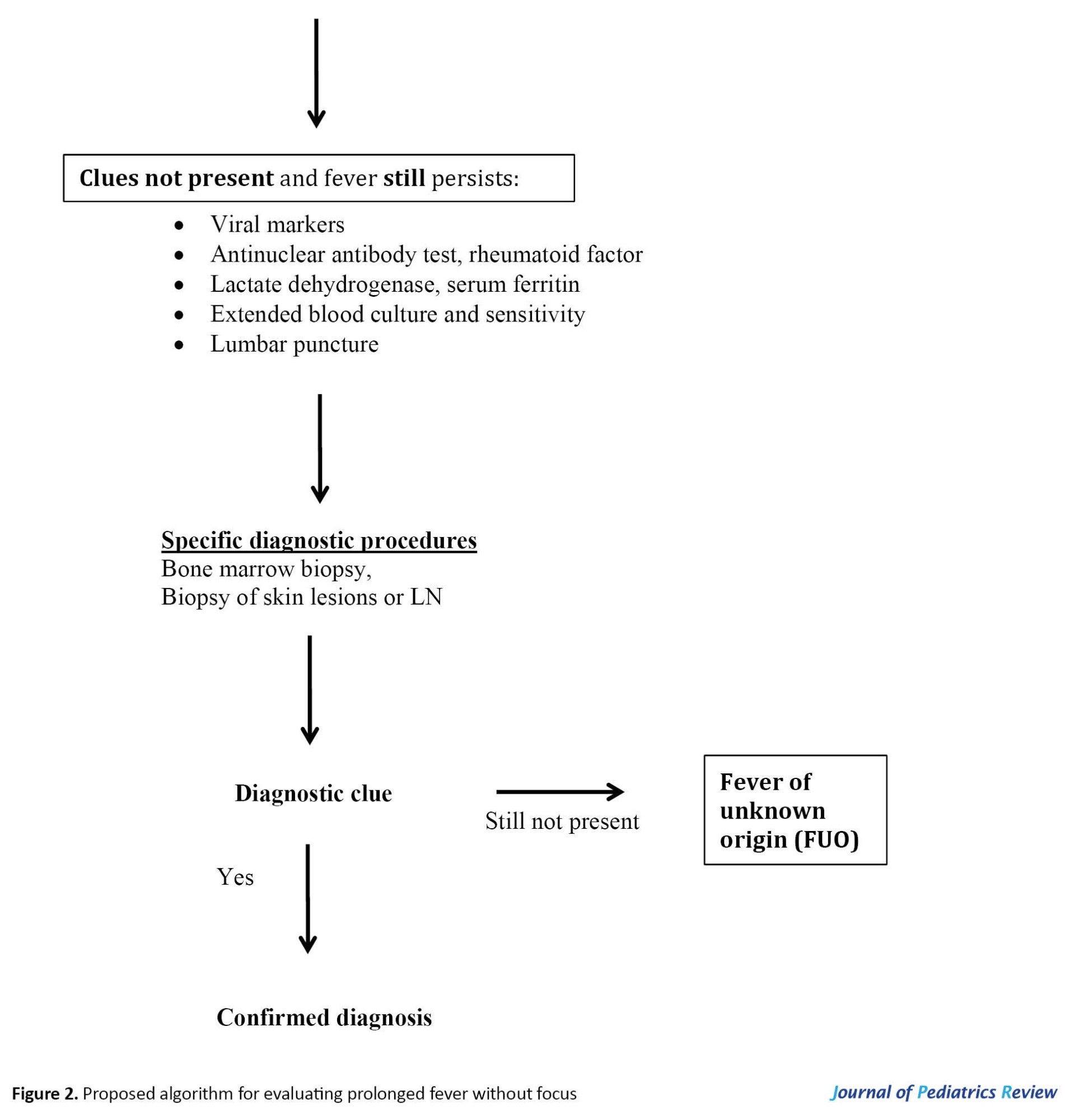
• A practical, systematic, and stepwise approach (Figure 2) can be helpful in assessing and managing prolonged fever in children.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and supervision: Jaishree Vasudevan; Data collection and data analysis: Noorul Aina and Indumathi Dhayalan; Investigation, writing the original draft, review & editing: All Authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge all patients of Pediatrics Department, Chettinad Hospital and Research Institute.
References
Fever, defined as a rectal temperature of 100.4oF or more, is a physiological response characterized by an elevation of body temperature above normal [1]. Fever is one of the common causes of medical consultation in children, responsible for 15%–25% of consultations in pediatrics practice [2]. Children with prolonged fever can worry parents and pediatricians alike. The causes of fever can be classified under 4 main categories: Infectious, inflammatory, neoplastic, and miscellaneous causes. The exact duration of prolonged fever is undefined. However, children with a temperature more than 38°C (100.4°F) recorded by a healthcare professional without any determined reason after at least 8 days of evaluation should be classified as having a “fever of unknown origin” (FUO). When the initial physical evaluation does not reveal any associated or distinctive findings, and all laboratory values are inconclusive or normal, the definitive diagnosis can be made only after extensive evaluation and observation. Most fevers with unknown causes usually are atypical presentations of common illnesses [3, 4, 5, 6]. Infections, connective tissue conditions, and neoplasms are the 3 most frequent causes of prolonged fever in children. The infectious diseases are usually salmonellosis, tuberculosis, malaria, rickettsia, and so on. The connective tissue disorder commonly linked to prolonged fever is juvenile rheumatoid arthritis. The third most common cause of prolonged fever in children is malignancy. The persistence of fever raises clinical queries towards diagnosis, especially in patients without identifiable cause. Here, we present a series of children with prolonged fever who presented as a diagnostic challenge.
Case Presentation
We reviewed the case records of 9 children admitted to our pediatric ward with prolonged fever episodes from December 2021 to October 2022 that were diagnostic challenges for clinicians. The demographic, clinical, laboratory parameters and examination findings are listed in Table 1.

Figure 1 displays a comprehensive overview and diagnosis of all our cases. Two children had scrub typhus, 1 acute lymphoblastic leukemia, and 1 multidrug-resistant typhoid fever. However, two cases remained as FUO. Three children were found to have coinfections and diagnosed with enteric fever with other coinfections like dengue, leptospirosis, and urinary tract infection.
Discussion
We described 9 children admitted to our pediatric ward with prolonged fever. All cases were evaluated primarily based on the clues given through history, physical examination, and routine investigations. First-line investigations included complete blood count, acute phase reactants (C-reactive protein), and urinalysis. In addition, imaging studies like abdominal ultrasound and chest radiographs were done as clinically indicated. Further specific microbiological or pathological investigations were done on case-to-case basis. We started on empirical antibiotics in patients suspected of bacterial infections after taking blood and urine cultures and sensitivity. Further screening for tuberculosis workup like chest x-ray, Mantoux test, and gene-xpert has also been done in suspected cases, especially those with a history of significant contact with tuberculosis. Infective workups for scrub typhus, enteric fever, leptospirosis, and malaria were also done in cases of clinical suspicion.
Case 1 was a 14-year-old boy who presented with complaints of high-grade fever, headache and abdominal pain, loose stools, and non-bilious vomiting for 10 days. In addition, there were complaints of headaches intermittently for 10 days. This case had a history of travel, outside food consumption, and a history of playing in rainwater. He was initially treated in another hospital with a course of oral antibiotics, but the fever did not seem to settle, and was referred to our hospital on day 10 of fever. Examination revealed right hypochondriac tenderness and hepatosplenomegaly. The child was started on empiric antibiotics of ceftriaxone and doxycycline. Initial investigations showed raised transaminase levels, reactive inflammatory markers (C-reactive protein [CRP] and erythrocyte sedimentation rate [ESR]), and an elevated Widal test titer. Hence, the child was continued on injection of ceftriaxone. An infective workup was also done, and Leptospira IgM was reactive (32.94 Panbio units). Although the initial blood culture and sensitivity showed no growth of organisms, extended blood culture and sensitivity showed significant growth of Salmonella typhi. Hence a diagnosis of enteric fever and leptospirosis was made. Workup for other infective etiologies was negative. There were still persistent high-grade fever spikes; hence on day 8 of admission, the child was started on oral azithromycin. Hence on day 10 of admission, antibiotics were escalated due to the persistence of fever spikes to IV ofloxacin according to sensitivity pattern. The fever subsided within 48 hours on day 12 of admission, and the child was discharged with oral ofloxacin to be continued for 5 more days.
Case 2 had a similar picture to case 1. She was a 13-year-old girl, referred with complaints of high-grade fever, vomiting, abdominal pain for 12 days, and occasional complaints of high-colored urine. On examination, right hypochondriac, and epigastric tenderness were present. The child was initially treated in an outside hospital with oral fluoroquinolones and continued with oral azithromycin. However, fever episodes persisted even after 3 days of initial management. Hence child was evaluated extensively, and investigations revealed raised transaminase levels, reactive inflammatory markers (CRP and ESR), and an elevated Widal test titer. An infective workup was also done, and Leptospira IGM was reactive. On day 6 of admission, Given persistent fever spikes, intravenous ceftriaxone was started. In addition to that, COVID-19 RT-PCR, real-time reverse transcription polymerase chain reaction (RT-PCR) was also reactive. Hence, she was diagnosed with leptospirosis and enteric fever with COVID-19 infection, and the child responded to the treatment and became afebrile from day 10 of admission.
Cases 1 and 2 had persistent fever episodes despite appropriate antibiotic therapy based on sensitivity patterns, which was a significant challenge. Apart from serological evidence, the modified Faine’s clinical criteria confirmed leptospirosis in both cases. Leptospirosis coinfection is more common with pathogenic agents causing dengue, malaria, and scrub typhus [7], and recently coinfection has also been observed with coronavirus [8]. However, coinfection with Leptospira and Salmonella is rare [9]. In a study conducted by Parker et al. to determine the prevalence of coinfections from 100 fever cases, there was only one coinfection with leptospirosis, typhoid, and malaria [10].
Cases 3 and 6 had initial diagnostic deviations due to their atypical presentations. Case 3 was a 10-year-old boy presented with 12 days of fever along with arthritis and left ankle swelling. The swelling was unilateral and associated with tenderness. Initially, a clinical suspicion of acute rheumatic fever and reactive arthritis was made. Autoimmune workup was also done and found to be negative. Inflammatory markers (ESR and CRP) were mildly elevated; ASO titers and ANA were non-reactive. Because of thrombocytopenia at admission, scrub typhus serology was done, which turned positive. The child was started on oral doxycycline, and his fever defervescence started on day 4 of admission, on day 16 of his illness. Reactive arthritis, as in numerous infectious diseases, is still a potential localization with scrub typhus and may be mistaken for septic arthritis. Monoarthritis is a rare presenting feature of scrub typhus, and to our knowledge, only one pediatric case has been previously reported in India [11].
Case 6 was a 9-year-old girl who presented with complaints of fever for 10 days and swelling behind the right postauricular region for 10 days. On examination, the child was febrile with an ulcer with a white base measuring 1x1 cm beneath the ear lobe. Usually, a typical eschar starts with a primary papular lesion, which eventually becomes a black ulcer with central necrosis. However, the appearance of this lesion was atypical, presenting an ulcer with a white base. Due to the site of the lesion and onset of eschar associated with fever, we suspected scrub typhus. Hence, scrub typhus serology was sent, which turned out to be reactive. The child responded well with oral doxycycline drug, and fever defervescence started on day 6 of admission. Eschar is the most helpful diagnostic evidence and is pathognomonic for Orientia tsutsugamushi. However, it is only present in fewer than 10% of patients in the Indian subcontinent. Clinical and laboratory characteristics of scrub typhus are non-specific [12]. Scrub typhus is commonly underdiagnosed in India due to the disease’s non-specific presentation, the lack of eschar, and the scarcity of confirming diagnostic testing [13]. Hence, it is important to recognize various clinical presentations of scrub typhus, which will aid in prompt diagnosis and treatment. Failure to do so can result in serious complications [14].
In case 4, a 12-year-old boy presented with a fever for 1 week and weight loss for 2 months. On examination, the child had hepatosplenomegaly, and blood investigations showed bicytopenia with normal peripheral smear, and CRP was elevated. Initial differential diagnoses included acute infectious illnesses (typhoid, malaria, and tropical diseases), acute viral illnesses, viral hepatitis, Epstein–Barr virus (EBV), and tuberculosis (TB). TB workup was negative, and the blood culture was sterile. Uric acid, phosphorous, and calcium were normal. Because of suspicion of hemophagocytic lymphohistiocytosis (HLH), further workup was done with serum ferritin and fibrinogen, which ruled out HLH. Viral panel studies were also done, which ruled out viral etiology. Given persistent bicytopenia, hematological malignancy was suspected. Bone marrow aspiration was done as a next step which showed evidence of blast cells. Flow cytometry confirmed the diagnosis of T-cell acute lymphoblastic leukemia. Bone marrow evaluation is important to peripheral smear blood analysis since some patients do not have circulating peripheral blast cells [15]. Children with leukemia frequently present with non-specific symptoms of illness, such as fever and fatigue. Fever is the most prevalent and obvious sign of infection. Acute lymphoblastic leukemia (ALL) comprised 77% of childhood leukemia cases and was the first widely prevalent malignancy to be proven to be treatable [16].
In case 8, a 12-year-old boy presented with high-grade fever, headache, and vomiting, which was non-bilious for 1 week, and there were no significant findings in the examination. Along with baseline investigations, a blood culture was sent, and the child was started on empirical antibiotics (injection of ceftriaxone). C-reactive protein was non-reactive, and another infective workup was also normal. During the initial treatment, the boy had no clinical response even with 5 days of empirical antibiotics administration, and the blood culture and sensitivity turned out to be sterile. The child persistently had a high-grade and intermittent fever, even 1 week after hospital management. Hence with clinical evidence and after suspecting multi-drug resistant typhoid, the child was further upgraded from antibiotics to intravenous azithromycin. The child demonstrated clinical response well, and the temperature gradually subsided. Fever defervescence started on day 6 of hospitalization.
Case 9 was a 3.5-year-old girl who had the longest hospital stay in our series. She initially presented with complaints of high-grade fever, vomiting with abdominal pain for 6 days, and examination findings revealed hepatomegaly. The child had an initial provisional diagnosis of Dengue fever with positive serology and was managed symptomatically. However, the child continued febrile with high-temperature spikes during the hospital stay. Although blood culture and sensitivity were sterile, urine culture showed significant growth of Klebsiella, and hence we upgraded antibiotics (amikacin and piperacillin+tazobactum) according to the sensitivity pattern. However, the repeated urine culture was sterile. Also, fever spikes continued to persist. Hence, we proceeded with an evaluation with bone marrow culture, which showed growth of Salmonella. The child was started ceftriaxone, based on the sensitivity pattern to which she responded by day 7. Fever episodes began to subside within 48 hours of antibiotic therapy, and fever defervescence started on the second week of the hospital stay.
When a child exhibits considerable pyrexia without localizing symptoms in endemic locations, typhoid fever should be considered a differential diagnosis. Typhoid fever should be promptly diagnosed by maintaining a high index of suspicion. In some circumstances, the clinical picture may deviate greatly from the typical typhoid presentation [17].
Case 5 was a 13-year-old boy presented with complaints of low-grade fever for 1 week and vomiting for 3 days. Examinations revealed pallor and hepatosplenomegaly, and the child was also sick looking due to poor oral intake. After performing all relevant baseline investigations and with clinical suspicion of enteric fever, a blood culture was sent, and the child was started on empirical antibiotics of ceftriaxone. Fever episodes did not resolve after 48 hours of treatment, and the blood culture was reportedly sterile. As part of further evaluation, infective workup with scrub typhus, dengue serology, and malaria were normal. COVID-19 RT-PCR and H1N1 were performed, and the results were normal. The child continued to have high-grade intermittent fever episodes after 9 days of hospital stay. Later, viral studies and autoimmune workups were also done, which also turned normal. Even after extensive evaluation, the child had high-grade intermittent fever episodes 9 days after a hospital stay. Hence, we made the diagnosis of FUO.
Case 7 was a 13-year-old girl who presented with complaints of fever, headache, and myalgia for 1 month with no significant examination finding and no focus. Tuberculosis was initially suspected, because there was a history of weight loss over the last 1 month. The child was admitted for further evaluation, and fever patterns were recorded during the hospital stay. The chest x-ray was normal, and the Mantoux test was negative. Gene-Xpert was sent, which also turned out to be negative. Further investigations and blood cultures were sent, and the child was started on empirical ceftriaxone antibiotics. However, the child continued to have fever episodes even after 48 hours of antibiotic therapy. Blood culture reports showed no growth of organisms. As part of further evaluation, all relevant infective workups were also done and found to be negative. The child continued to have low-grade fever spikes after 1 week of hospital admission. Since the child had a fever for more than 3 weeks and no cause was found even after 1 week of evaluation, we diagnosed it as a fever of unknown origin. Cases 5 and 7 posed a diagnostic challenge in which the causes were not found after extensive evaluation, and the fever persisted even after 10 days of admission and management. They had spontaneous resolution of fever without the achievement of a specific diagnosis. Consequently, the diagnosis of FUO was given. The fever patterns and frequency can sometimes aid in diagnosis. Children with recorded temperatures of more than 38°C (100.4°F) by healthcare professionals and for whom the reason could not be determined after at least 8 days of assessment should be classified as having FUO. However, most illnesses resulting from FUO lack a typical fever pattern. In addition to a thorough history and physical examination, the evaluation of FUO calls for a few screening laboratory tests, additional laboratory work, and imaging work based on the history, abnormalities found during the examination, or preliminary screening tests. fevers with unexplained causes are usually uncommon manifestations of common illnesses. Since there are initially no associated or specific physical examination findings, and all laboratory results are negative or normal, the definitive diagnosis can only be made after prolonged observation in some cases. Even after extensive investigation, the reason for the fever is still unknown in up to 25% of children with a persistent fever [18].
Conclusion
• A thorough history right from antenatal period, immunization status of children, and detailed examination are essential in pediatric febrile illnesses.
• Common causes of prolonged fever in children comprise infections, connective tissue disorder, and malignancy.
• Epidemiological factors like seasonal variations and endemic areas of disease prevalence exposure to wild animals should be considered while evaluating infectious causes.
• If a patient is not clinically responding to treatment, it could be due to antibiotic resistance or suspected coinfections. Antimicrobial drugs should empirically not be used as they may obscure the diagnosis.
• We have found that there might be deviators during disease evolution. We should not stay with a single diagnosis until a good clinical response is achieved and also consider alternative diagnoses or coinfections.
• A practical, systematic, and stepwise approach (Figure 2) can be helpful in assessing and managing prolonged fever in children.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and supervision: Jaishree Vasudevan; Data collection and data analysis: Noorul Aina and Indumathi Dhayalan; Investigation, writing the original draft, review & editing: All Authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We acknowledge all patients of Pediatrics Department, Chettinad Hospital and Research Institute.
References
- National Collaborating Centre for Women’s and Children’s Health . Feverish illness in children: Assessment and initial management in children younger than 5 years. National Collaborating Centre for Women’s and Children’s Health; London; 2013. [Link]
- Whitburn S, Costelloe C, Montgomery AA, Redmond NM, Fletcher M, Peters TJ, et al. The frequency distribution of presenting symptoms in children aged six months to six years to primary care. Prim Health Care Res Dev. 2011; 12(2):123-34. [DOI:10.1017/S146342361000040X] [PMID]
- De Bont EG, Lepot JM, Hendrix DA, Loonen N, Guldemond-Hecker Y, Dinant GJ, et al. Workload and management of childhood fever at general practice out-of-hours care: An observational cohort study. BMJ Open. 2015; 5(5):e007365. [PMID]
- Sands R, Shanmugavadivel D, Stephenson T, Wood D. Medical problems presenting to paediatric emergency departments: 10 years on. Emerg Med J. 2012; 29(5):379-82.[DOI:10.1136/emj.2010.106229] [PMID]
- Bhat D, Dhooria GS, Bains HS. Coinfection of hepatitis A and E with Salmonella infection; A case report. Iran J Pediatr. 2009; 19(1):79-81. [Link]
- Lohr JA, Hendley JO. Prolonged fever of unknown origin: A record of experiences with 54 childhood patients. Clin Pediatr (Phila). 1977; 16(9):768-73. [DOI:10.1177/000992287701600905] [PMID]
- Md-Lasim A, Mohd-Taib FS, Abdul-Halim M, Mohd-Ngesom AM, Nathan S, Md-Nor S. Leptospirosis and coinfection: Should we be concerned? Int J Environ Res Public Health. 2021; 18(17):9411. [DOI:10.3390/ijerph18179411] [PMID] [PMCID]
- Gupta N, Wilson W, Ravindra P, Raghu R, Saravu K. Coinfection of leptospirosis and coronavirus disease 2019: A retrospective case series from a coastal region in South India. J Med Virol. 2022; 94(9):4508-11. [DOI:10.1002/jmv.27816] [PMID] [PMCID]
- Negi A, Tejan N, Sahu C, Dhole T. Coinfection by salmonella and leptospira presenting as subacute intestinal obstruction with colitis: A diagnostic dilemma. J Clin Diagn Res. 218; 12(3):DD01 - 2. [DOI:10.7860/JCDR/2018/32611.11272]
- Parker TM, Murray CK, Richards AL, Samir A, Ismail T, Fadeel MA, et al. Concurrent infections in acute febrile illness patients in Egypt. Am J Trop Med Hyg. 2007; 77(2):390-2. [DOI:10.4269/ajtmh.2007.77.390] [PMID]
- Handattu K, Bhat Yellanthoor R, Konda KC, Kini S. Acute severe monarthritis: A rare manifestation of scrub typhus. BMJ Case Rep. 2018; 11(1):bcr2018227002. [DOI:10.1136/bcr-2018-227002] [PMID] [PMCID]
- Varghese GM, Abraham OC, Mathai D, Thomas K, Aaron R, Kavitha ML, et al. Scrub typhus among hospitalised patients with febrile illness in South India: Magnitude and clinical predictors. J Infect. 2006; 52(1):56-60. [DOI:10.1016/j.jinf.2005.02.001] [PMID]
- Isaac R, Varghese GM, Mathai E, J M, Joseph I. Scrub typhus: Prevalence and diagnostic issues in rural Southern India. Clin Infect Dis. 2004; 39(9):1395-6. [DOI:10.1086/424748] [PMID]
- Premaratna R, Chandrasena TG, Dassayake AS, Loftis AD, Dasch GA, de Silva HJ. Acute hearing loss due to scrub typhus: A forgotten complication of a reemerging disease. Clin Infect Dis. 2006; 42(1):e6-8. [DOI:10.1086/498747] [PMID]
- Percival ME, Lai C, Estey E, Hourigan CS. Bone marrow evaluation for diagnosis and monitoring of acute myeloid leukemia. Blood Rev. 2017; 31(4):185-192. [DOI:10.1016/j.blre.2017.01.003] [PMID] [PMCID]
- Nelson WE, Behrman RE, Kliegman R, W. SGJ, Tubergen DG, Bleyer, A, et al. The leukemias. In: Kliegman RM, Marcdante K, Behrman RE, Jenson HB, editors. Nelson textbook of pediatrics. Amsterdam: Elsevier; 2020. [Link]
- Wicks AC, Holmes GS, Davidson L. Endemic typhoid fever: A diagnostic pitfall. Q J Med. 1971; 40(159):341-54. [PMID]
- Nelson WE, Behrman RE, Kliegman R, W. SGJ, Steenhoff AP. Fever of unknown origin. In: Kliegman RM, Marcdante K, Behrman RE, Jenson HB, editors. Nelson textbook of pediatrics. Amsterdam: Elsevier; 2020. [Link]
Type of Study: Case & Review |
Received: 2022/11/25 | Accepted: 2023/07/3 | Published: 2023/07/1
Received: 2022/11/25 | Accepted: 2023/07/3 | Published: 2023/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |