Volume 12, Issue 4 (10-2024)
J. Pediatr. Rev 2024, 12(4): 377-384 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fallahinejad Ghajari M, Ansari G, Mohaveri M, Eghbali Zarch A, Razavi S. Sedative Effect of Nasal Midazolam-Ketamine at Different Doses on 3-6-year-old Uncooperative Dental Patients. J. Pediatr. Rev 2024; 12 (4) :377-384
URL: http://jpr.mazums.ac.ir/article-1-518-en.html
URL: http://jpr.mazums.ac.ir/article-1-518-en.html
Masoud Fallahinejad Ghajari1 

 , Ghassem Ansari *2
, Ghassem Ansari *2 

 , Mostafa Mohaveri1
, Mostafa Mohaveri1 
 , Ahmad Eghbali Zarch1
, Ahmad Eghbali Zarch1 

 , Sajjad Razavi3
, Sajjad Razavi3 




 , Ghassem Ansari *2
, Ghassem Ansari *2 

 , Mostafa Mohaveri1
, Mostafa Mohaveri1 
 , Ahmad Eghbali Zarch1
, Ahmad Eghbali Zarch1 

 , Sajjad Razavi3
, Sajjad Razavi3 


1- Department of Pediatric Dentistry, Dental Research Center, School of Dentistry, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Pediatric Dentistry, Dental Research Center, School of Dentistry, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,profgansari@gmail.com
3- Department of Anesthesiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Pediatric Dentistry, Dental Research Center, School of Dentistry, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
3- Department of Anesthesiology, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 393 kb]
(643 Downloads)
| Abstract (HTML) (1784 Views)
Full-Text: (769 Views)
Introduction
Pediatric dentistry training provides skills to tackle dental anxiety and pain in most instances; however, these problems may not be easily modified through behavior control techniques and the need for a pharmacological approach comes to light [1]. Conscious sedation alongside good local anesthesia could act as a suitable alternative to physical restraint on one side and general anesthesia on the other. An ideal sedation is expected to provide an immediate, stable state with quick recovery [2].
Oral and nasal routes of sedation are among the noninvasive methods that allow dentists to create a pleasant memory of treatment sessions for the child. Midazolam and ketamine, either alone or in combination, are safe and effective in intranasal application among dental sedation routes [3]. Intranasal sedation is increasingly utilized in emergency wards as well as outpatient clinics and out-of-hospital settings. This pain-free and needle-free method helps to overcome most of the fear in those individuals who otherwise would have to receive no treatment or go to the operating room [4]. The nasal mucosa serves as one of the best for drug absorption due to its considerable blood flow, which rapidly transfers receiving agents to the main bloodstream and subsequently to the cerebrospinal complex. On the other hand, since there is no passing through the digestive tract the intrahepatic metabolism will no longer be a concern resulting in a much higher capacity of drug delivery and effect compared to the oral route [5].
Several earlier investigations have suggested that the use of combinations of drugs can enhance the potential of action while reducing the side effects of single higher doses of drugs [6]. Today, midazolam is considered the drug of choice for sedation among restless children. This benzodiazepine agent can be used in combination with other sedatives to allow reducing the dose required to achieve accepted levels of sedation. The dose recommended for intranasal midazolam is 0.5 mg/kg, which can establish sufficient sedative effect for easy separation from parents with minimum to no side effects [7].
On the other hand, ketamine is known as one of the most effective anesthetic drugs used extensively in medicine for more than 50 years. The intranasal ketamine passes through a mucous path for direct absorption and travel to the brain [8]. The combined use of midazolam and ketamine has been referred to as a safe, effective and practical method for the management of children for minor dental interventions. It is strictly emphasized that employing such a technique requires advanced skills in airway management [9]. On the other hand, oral ketamine and midazolam have been reportedly effective equally in the sedation of children for venipuncture and mask acceptance [10] and child-parent separation and venipuncture [11]. The midazolam-ketamine combination looks to produce greater cooperation than alone with guided behavior in children under the age of 3 years [12, 13, 14].
Another investigation found that the extent of sedation and anxiety mitigation were much higher in ketamine/midazolam combination with ketamine dose of higher than 4 mg/kg, compared to midazolam alone without any side effects [15]. Elevated blood pressure and heart rate were reported following administration of Ketamine without any statistical significance [16]. Intranasal Ketamine provided higher sedation scores than oral Ketamine using the Houpt scale, with an elongated recovery time [17].
Accordingly, this investigation compares the efficacy of intranasal ketamine 3 mg/kg to midazolam 0.5 mg/kg and ketamine 4 mg/kg to midazolam 0.4 mg/kg on the sedation of 3-6-year-old uncooperative children for dental treatment.
Methods
This double-blind randomized clinical trial was conducted on a group of 23 uncooperative children aged 3-6-years who scored definitely negative in Frankel scale. Cases with at least two similar treatment sessions need were included in this investigation. The exclusion criteria included children with any sign of high temperature, recent cold or other respiratory infections, allergy to medication, American Society of Anesthesiologists II and above.
The parents were fully instructed before sedation to observe at least 6 h of fasting for the child before the sedative drug administration. Each child was randomly assigned into one of the groups of A or B using a computer-generated random coded list. Group A received a nasal combination of midazolam 0.4 mg/kg (5 mg/mL, Darupakhsh Co., Iran; maximum=10 mg) plus ketamine (4 mg/kg; 50 mg/mL; ROTEXMEDICA Co., Germany) in the first session, while group B received nasal combination of midazolam 0.5 mg/kg plus ketamine 3 mg/kg 15-20 min before initiating the treatment in the first session. Each child received the other combination dose in the second session to investigate the sequence effect.
The drugs were prepared and coded by the anesthesiologist in charge before administration by the operating pediatric dentist, who was blind to the drug composition. All vital sign changes were recorded including heart rate, respiratory rate, and peripheral oxygen saturation in a prepared data recording form. A continuous oxygen supply was established for each patient throughout the procedure through a tape-secured nasal cannula. Dental treatment was initiated following observation of the primary signs of sedation. A local anesthetic was administered using lidocaine 2% with epinephrine 1/100000 (Persocaine-E, Darupakhsh Co., Iran).
The sedation scores were recorded for each child at the time of anesthetic injection, and 10 min intervals using the Houpt scale. Measured criteria were the level of crying, sleepiness, and movement judged by the operating pediatric dentist, who was blind to the group. Recording data was based on the lowest judged score. Treatment sessions were limited to a maximum of 30 min.
Discharge was conducted based on the following criteria: Satisfactory and stable cardiovascular function; satisfactorily open airway tract; the patient responds to stimuli easily and the protective reactions are healthy; the patient talks and sits; the presence of the child’s guardian or parent.
Drug side effects were recorded during and after the completion of treatment up to 24 h post-operative and reported by parents by phone. The parent’s and operator’s satisfaction were also recorded at the end of each treatment session.
Statistical analysis
The two-way repeated measure of analysis of variance was used to compare the mean heart rate, peripheral oxygen saturation, and blood pressure systolic/diastolic between the two groups. Marginal models were used for analyzing sleepiness, crying, movement and overall behavior. Wilcoxon signed-rank test was used to compare Houpt scales between groups. The Mc-Nemar test was employed to compare the parents’ views on the effectiveness of different sedative drugs. Analyses were performed using the SPSS software, version 20.
Results
A total of 23 children aged 3-6-years were successfully included in this investigation, from which three were excluded (one did not return for the second session and two did not cooperate in the first session). Out of the 20 treated children (11 girls and 9 boys). The children’s mean age was 4.35±0.91 years and their mean weight was 16.36±2.56 kg. The mean duration of the treatment time was 21 min, whereby the minimum and maximum time of treatment were 10 and 35 min, respectively.
A total of 12 children did not report having any post-treatment nausea following any of the two methods. Two children in group B had nausea and 6 children in group A had reported to have experienced nausea to certain degrees. McNemar test did not show any significant differences between the two groups (P=0.289).
Meanwhile, 11 parents stated that both sedation sessions were effective while 5 parents stated that both were very effective. Two parents said drug combination I as effective and drug combination II as very effective; two parents regarded these drug combinations as vice versa. McNemar test did not show any significant difference between these two drug groups (P=1.000).
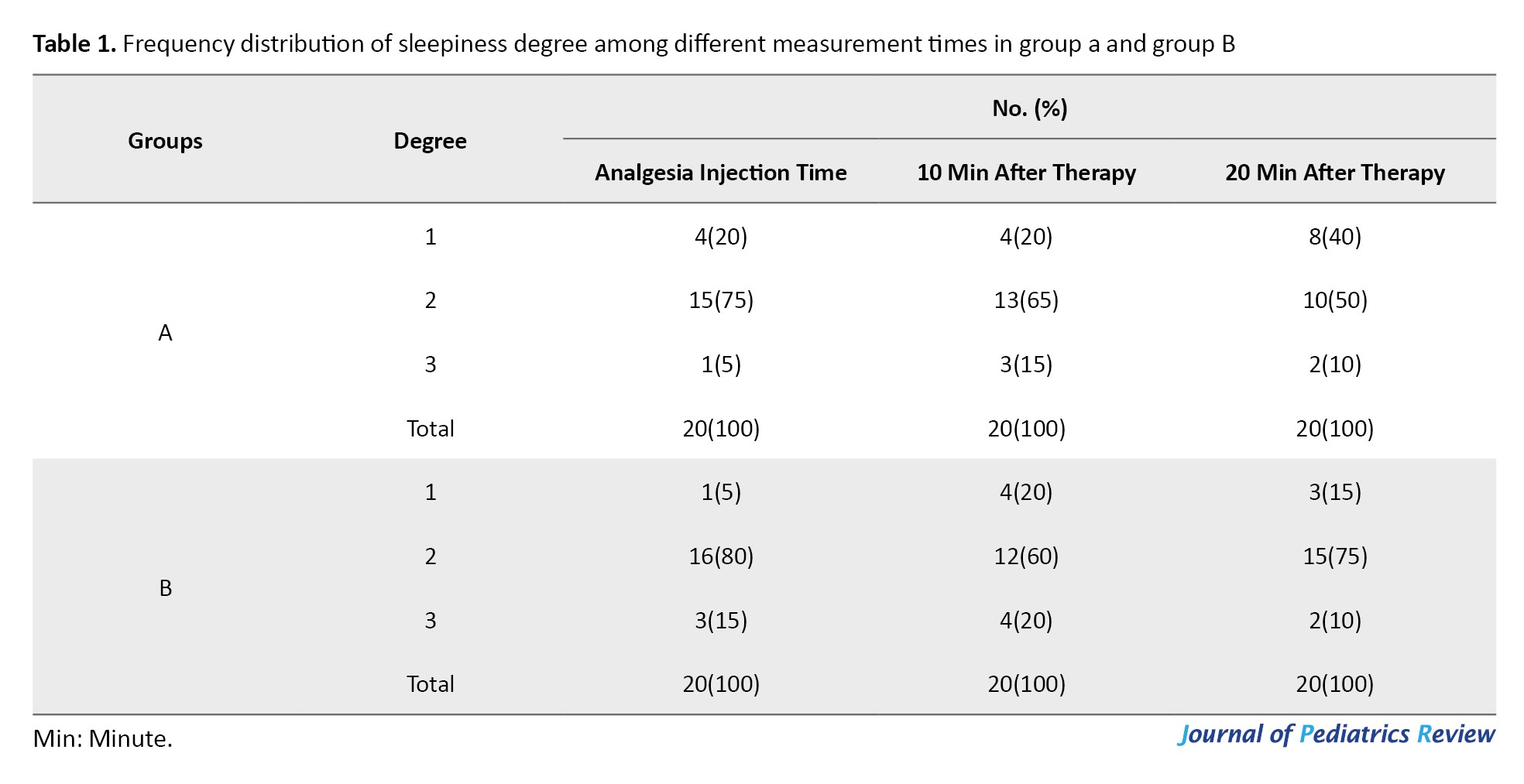
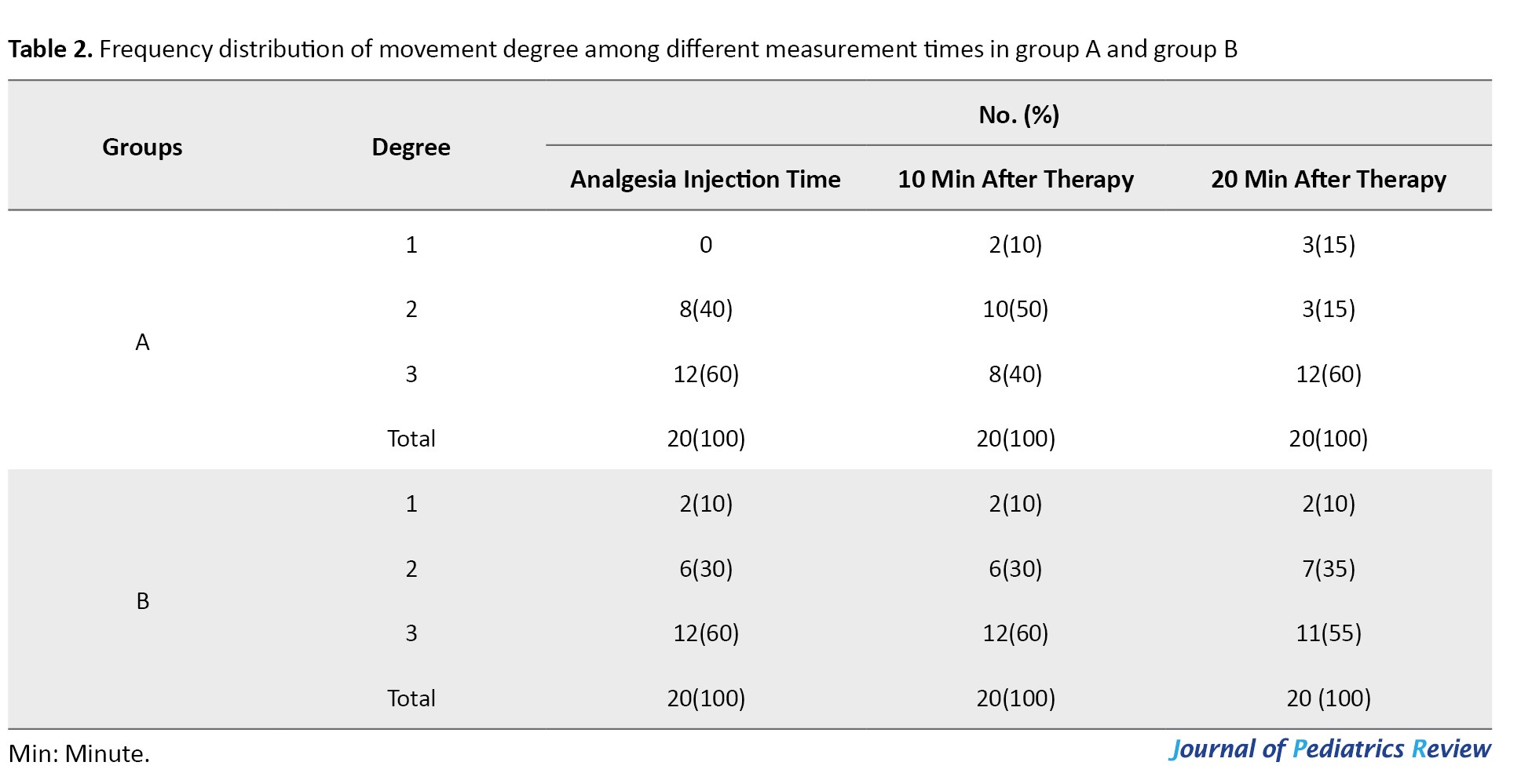
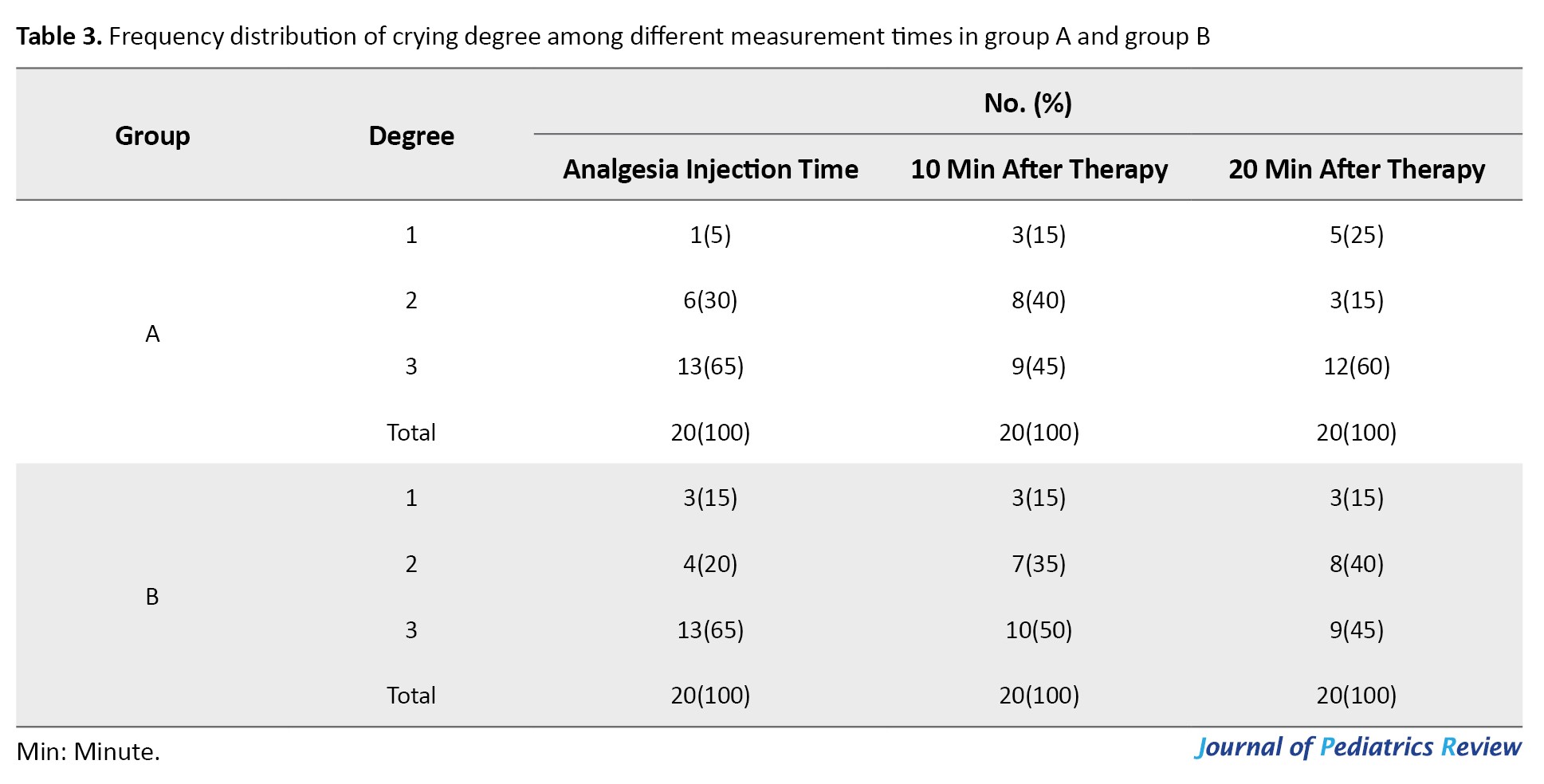
Recorded data revealed no statistically significant differences between the two groups on sleepiness, movement, and crying at three measured times using the generalized estimating equations method (P=0.143, P=0.593, P=0.281, respectively). However, as time passed a significant difference was noted between groups with signs of drowsiness diminishing (P=0.006) (Tables 1, 2 and 3).
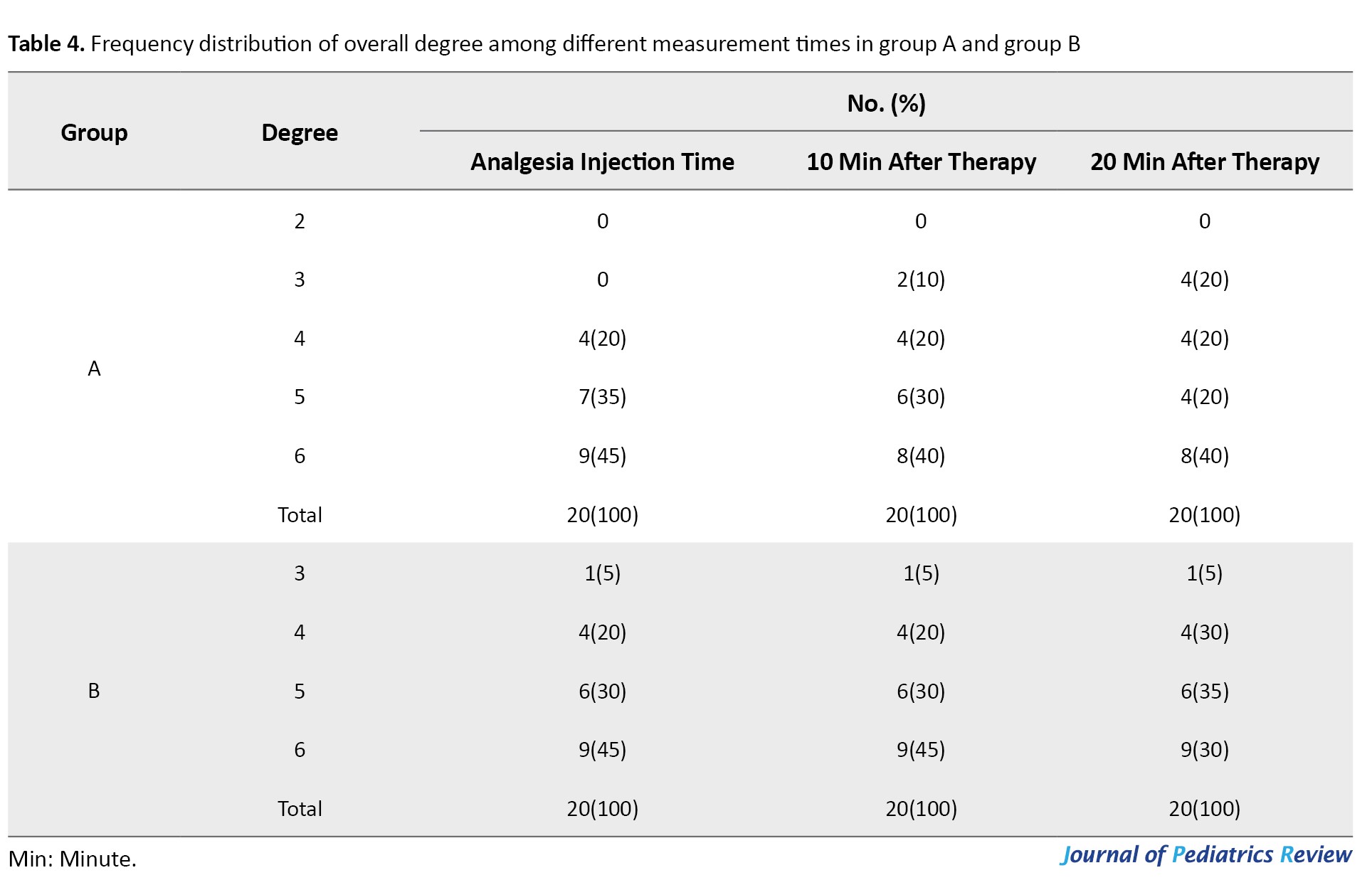
Looking at the overall behavior (O) data revealed that no statistically significant differences could be noticed at recorded steps in the two groups using the marginal model of longitudinal data analysis via the generalized estimating equations method (P=0.224; Table 4).
The Kolmogorov-Smirnov test revealed a normal distribution of data in measured vital signs of heart rate, peripheral oxygen saturation, and blood pressure at all measured times. A comparison of the drugs’ efficacy was performed using the parametric method of analysis of variance. The results indicated that the two groups did not differ significantly in heart rate (P=0.689), while a significant increase in heart rate was noted at the time of initiating the treatment in both groups (P=0.001), with no further significant differences afterward (P=0.347).
A similar analysis was performed on peripheral oxygen saturation and diastolic blood pressure changes in groups with no statistically significant differences between the two groups (P=0.930); however, the difference turned significant as soon as the treatment was started when compared to the baseline (P=0.001). The mean diastolic and systolic blood pressure were increased at the end of treatment with a statistically significant difference between the groups tested (P=0.018 and P=0.005, respectively),
Discussion
Dental appointments are associated with degrees of fear and anxiety, especially among younger-aged children. Such exaggerated levels of fear and anxiety can seriously affect the course of treatment [18]. An anxious child may be predisposed to potentially dangerous psychological and physiological risks. Various premedication methods and drugs have been tested for many years among which oral, nasal and rectal routes are seen as more readily available to dentists in practice while parenteral methods including intradermal and intravenous require high skills and more importantly license to practice. Among all routes, there are advantages and disadvantages to each depending on patient and treatment needs [19]. Among the routes, none is considered the ideal sedative method above others to have no undesirable side effects too. Sedative drugs are also chosen based on the patient’s age, therapeutic methods, health status and dentist and anesthesiologist desire [20].
Rapid reliable onset of an agent is desired to be able to remove pain during injections and be convenient in use. The intranasal route may cover most of the positive points referred to earlier which had made it popular among operators and patients [15]. Both oral and intranasal methods are less invasive when compared to other sedative methods. Among these two, the intranasal method seems to require less child cooperation while providing a faster onset of action. The intranasal method has been advocated as a good substitute for cases with respiratory monitoring [21].
These days, many parents seek office-based sedation for treating their child’s teeth and intranasal sedation is among the most desirable and selected methods in this regard. Intranasal sedative drug administration had been confirmed safe based on minimal to no changes in physiologic vital signs [22] yet significantly higher sedative effects were reported in intranasal compared to oral sedation, while longer recovery course. Higher levels of sedation were noted in the intranasal ketamine-midazolam group with higher satisfaction rates for shorter dental procedures (35 min) based on the Houpt scoring system [17].
Differences in sleepiness were not significant between the two groups at the time of injecting the anesthetic, as well as 10 and 20 min after the initiation of the treatment. Nevertheless, the effect of time was significant, suggesting that in both groups improved levels of sleepiness were observed over time.
The two groups did not differ significantly in terms of movement and crying as well as overall behavior at the tested times (P>0.05). In another investigation, no significant differences were reported between changes in blood oxygen saturation and respiration rate of the two similarly sedative cocktails tested [16] similar to the current findings in this investigation. Concurrent use of midazolam and ketamine, in addition to the positive synergism of the drugs, can subsequently reduce the side effects of each, separately [23].
It is suggested that 6-10 mg/kg oral and 5-6 mg/kg intranasal ketamine could be considered as a safe and effective dose spectrum for a desired level of sedation in children [23]. The use of other sedative drugs alongside ketamine could help in reducing the needed dose of ketamine, accelerating a quick recovery, while reducing the incidence of complications known as recovery room phenomenon [24]. In case of 5 min advance benzodiazepine administration or concurrent with ketamine, the drug side effects would be minimized [25].
The combination of ketamine and midazolam reduced the anxiety of preschoolers without an increase in the incidence of side effects resulting in relaxation and cooperation of children [15]. The depth of sedation and anxiety mitigation could be proved far better in the midazolam-ketamine combination when compared to that of midazolam alone with no side effects in any of the two groups15. In yet another study it is indicated that intranasal ketamine would provide significantly deeper sedation at the separation stage of a child from parents and intravenous line conduction [11].
Significantly higher sedation scores are reported along with lower anxiety, easier acceptance of intravenous injection and mask placement over the nose when ketamine-midazolam had been administered. No significant changes were reported in measures of vital signs during and after the operation. Nasal ketamine-midazolam combined induced rapid sedation while the depth was almost the same as the quality of sedation, analgesia, and comfort without any clear side effects [13].
The most common post-treatment complications include nausea, vomiting, sleepiness, and diminished activity within the first four hours of both oral and intranasal midazolam/ketamine sedation [17]. Such side effects were recorded in the current investigation as twelve with no post-treatment nausea following any of the sessions up to 2 h postoperatively while 8 reported degrees of nausea during the same period. Recorded data of the current investigation revealed that 11 parents expressed their satisfaction with the effectiveness of both doses while 5 disagreed. Statistical analysis did not show any significant differences between the two groups (P>0.05).
Conclusion
Differences in satisfaction from intranasal sedation with the two different doses were statistically significant based on operator and parents’ satisfaction scores (P<0.05). No significant differences were observed in vital signs and behavioral reaction changes of children at two different doses (P>0.05). Reduction of the provided ketamine dose for patients had a desirable effect on the course of treatment and the attitude of parents.
Ethical Considerations
Compliance with ethical guidelines
The ethical approval was obtained from the Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.DRC.REC.1398.212) and this study was registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20090506001882N9). Written informed consent was signed by the accompanying parents. Only children in the American Society of Anesthesiologists I guidelines were included as indicated for this type of office-based sedation.
Funding
The paper was extracted from the research project of Masoud fallahinejad Ghajari, approved by the Department of Pediatric Dentistry, Dental Faculty of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors contributions
Conceptualization and supervision: Masoud Fallahinejad Ghajari and Ghassem Ansari; Methodology and validation: Ahmad Eghbali Zarch and Sajad Razavi; Writing: Mostafa Mohaveri.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The Dental Research Center, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences is highly appreciated for the scientific and financial support provided. The authors would also like to express their appreciation for those who participated in this project.
References
Pediatric dentistry training provides skills to tackle dental anxiety and pain in most instances; however, these problems may not be easily modified through behavior control techniques and the need for a pharmacological approach comes to light [1]. Conscious sedation alongside good local anesthesia could act as a suitable alternative to physical restraint on one side and general anesthesia on the other. An ideal sedation is expected to provide an immediate, stable state with quick recovery [2].
Oral and nasal routes of sedation are among the noninvasive methods that allow dentists to create a pleasant memory of treatment sessions for the child. Midazolam and ketamine, either alone or in combination, are safe and effective in intranasal application among dental sedation routes [3]. Intranasal sedation is increasingly utilized in emergency wards as well as outpatient clinics and out-of-hospital settings. This pain-free and needle-free method helps to overcome most of the fear in those individuals who otherwise would have to receive no treatment or go to the operating room [4]. The nasal mucosa serves as one of the best for drug absorption due to its considerable blood flow, which rapidly transfers receiving agents to the main bloodstream and subsequently to the cerebrospinal complex. On the other hand, since there is no passing through the digestive tract the intrahepatic metabolism will no longer be a concern resulting in a much higher capacity of drug delivery and effect compared to the oral route [5].
Several earlier investigations have suggested that the use of combinations of drugs can enhance the potential of action while reducing the side effects of single higher doses of drugs [6]. Today, midazolam is considered the drug of choice for sedation among restless children. This benzodiazepine agent can be used in combination with other sedatives to allow reducing the dose required to achieve accepted levels of sedation. The dose recommended for intranasal midazolam is 0.5 mg/kg, which can establish sufficient sedative effect for easy separation from parents with minimum to no side effects [7].
On the other hand, ketamine is known as one of the most effective anesthetic drugs used extensively in medicine for more than 50 years. The intranasal ketamine passes through a mucous path for direct absorption and travel to the brain [8]. The combined use of midazolam and ketamine has been referred to as a safe, effective and practical method for the management of children for minor dental interventions. It is strictly emphasized that employing such a technique requires advanced skills in airway management [9]. On the other hand, oral ketamine and midazolam have been reportedly effective equally in the sedation of children for venipuncture and mask acceptance [10] and child-parent separation and venipuncture [11]. The midazolam-ketamine combination looks to produce greater cooperation than alone with guided behavior in children under the age of 3 years [12, 13, 14].
Another investigation found that the extent of sedation and anxiety mitigation were much higher in ketamine/midazolam combination with ketamine dose of higher than 4 mg/kg, compared to midazolam alone without any side effects [15]. Elevated blood pressure and heart rate were reported following administration of Ketamine without any statistical significance [16]. Intranasal Ketamine provided higher sedation scores than oral Ketamine using the Houpt scale, with an elongated recovery time [17].
Accordingly, this investigation compares the efficacy of intranasal ketamine 3 mg/kg to midazolam 0.5 mg/kg and ketamine 4 mg/kg to midazolam 0.4 mg/kg on the sedation of 3-6-year-old uncooperative children for dental treatment.
Methods
This double-blind randomized clinical trial was conducted on a group of 23 uncooperative children aged 3-6-years who scored definitely negative in Frankel scale. Cases with at least two similar treatment sessions need were included in this investigation. The exclusion criteria included children with any sign of high temperature, recent cold or other respiratory infections, allergy to medication, American Society of Anesthesiologists II and above.
The parents were fully instructed before sedation to observe at least 6 h of fasting for the child before the sedative drug administration. Each child was randomly assigned into one of the groups of A or B using a computer-generated random coded list. Group A received a nasal combination of midazolam 0.4 mg/kg (5 mg/mL, Darupakhsh Co., Iran; maximum=10 mg) plus ketamine (4 mg/kg; 50 mg/mL; ROTEXMEDICA Co., Germany) in the first session, while group B received nasal combination of midazolam 0.5 mg/kg plus ketamine 3 mg/kg 15-20 min before initiating the treatment in the first session. Each child received the other combination dose in the second session to investigate the sequence effect.
The drugs were prepared and coded by the anesthesiologist in charge before administration by the operating pediatric dentist, who was blind to the drug composition. All vital sign changes were recorded including heart rate, respiratory rate, and peripheral oxygen saturation in a prepared data recording form. A continuous oxygen supply was established for each patient throughout the procedure through a tape-secured nasal cannula. Dental treatment was initiated following observation of the primary signs of sedation. A local anesthetic was administered using lidocaine 2% with epinephrine 1/100000 (Persocaine-E, Darupakhsh Co., Iran).
The sedation scores were recorded for each child at the time of anesthetic injection, and 10 min intervals using the Houpt scale. Measured criteria were the level of crying, sleepiness, and movement judged by the operating pediatric dentist, who was blind to the group. Recording data was based on the lowest judged score. Treatment sessions were limited to a maximum of 30 min.
Discharge was conducted based on the following criteria: Satisfactory and stable cardiovascular function; satisfactorily open airway tract; the patient responds to stimuli easily and the protective reactions are healthy; the patient talks and sits; the presence of the child’s guardian or parent.
Drug side effects were recorded during and after the completion of treatment up to 24 h post-operative and reported by parents by phone. The parent’s and operator’s satisfaction were also recorded at the end of each treatment session.
Statistical analysis
The two-way repeated measure of analysis of variance was used to compare the mean heart rate, peripheral oxygen saturation, and blood pressure systolic/diastolic between the two groups. Marginal models were used for analyzing sleepiness, crying, movement and overall behavior. Wilcoxon signed-rank test was used to compare Houpt scales between groups. The Mc-Nemar test was employed to compare the parents’ views on the effectiveness of different sedative drugs. Analyses were performed using the SPSS software, version 20.
Results
A total of 23 children aged 3-6-years were successfully included in this investigation, from which three were excluded (one did not return for the second session and two did not cooperate in the first session). Out of the 20 treated children (11 girls and 9 boys). The children’s mean age was 4.35±0.91 years and their mean weight was 16.36±2.56 kg. The mean duration of the treatment time was 21 min, whereby the minimum and maximum time of treatment were 10 and 35 min, respectively.
A total of 12 children did not report having any post-treatment nausea following any of the two methods. Two children in group B had nausea and 6 children in group A had reported to have experienced nausea to certain degrees. McNemar test did not show any significant differences between the two groups (P=0.289).
Meanwhile, 11 parents stated that both sedation sessions were effective while 5 parents stated that both were very effective. Two parents said drug combination I as effective and drug combination II as very effective; two parents regarded these drug combinations as vice versa. McNemar test did not show any significant difference between these two drug groups (P=1.000).
Recorded data revealed no statistically significant differences between the two groups on sleepiness, movement, and crying at three measured times using the generalized estimating equations method (P=0.143, P=0.593, P=0.281, respectively). However, as time passed a significant difference was noted between groups with signs of drowsiness diminishing (P=0.006) (Tables 1, 2 and 3).



Looking at the overall behavior (O) data revealed that no statistically significant differences could be noticed at recorded steps in the two groups using the marginal model of longitudinal data analysis via the generalized estimating equations method (P=0.224; Table 4).

The Kolmogorov-Smirnov test revealed a normal distribution of data in measured vital signs of heart rate, peripheral oxygen saturation, and blood pressure at all measured times. A comparison of the drugs’ efficacy was performed using the parametric method of analysis of variance. The results indicated that the two groups did not differ significantly in heart rate (P=0.689), while a significant increase in heart rate was noted at the time of initiating the treatment in both groups (P=0.001), with no further significant differences afterward (P=0.347).
A similar analysis was performed on peripheral oxygen saturation and diastolic blood pressure changes in groups with no statistically significant differences between the two groups (P=0.930); however, the difference turned significant as soon as the treatment was started when compared to the baseline (P=0.001). The mean diastolic and systolic blood pressure were increased at the end of treatment with a statistically significant difference between the groups tested (P=0.018 and P=0.005, respectively),
Discussion
Dental appointments are associated with degrees of fear and anxiety, especially among younger-aged children. Such exaggerated levels of fear and anxiety can seriously affect the course of treatment [18]. An anxious child may be predisposed to potentially dangerous psychological and physiological risks. Various premedication methods and drugs have been tested for many years among which oral, nasal and rectal routes are seen as more readily available to dentists in practice while parenteral methods including intradermal and intravenous require high skills and more importantly license to practice. Among all routes, there are advantages and disadvantages to each depending on patient and treatment needs [19]. Among the routes, none is considered the ideal sedative method above others to have no undesirable side effects too. Sedative drugs are also chosen based on the patient’s age, therapeutic methods, health status and dentist and anesthesiologist desire [20].
Rapid reliable onset of an agent is desired to be able to remove pain during injections and be convenient in use. The intranasal route may cover most of the positive points referred to earlier which had made it popular among operators and patients [15]. Both oral and intranasal methods are less invasive when compared to other sedative methods. Among these two, the intranasal method seems to require less child cooperation while providing a faster onset of action. The intranasal method has been advocated as a good substitute for cases with respiratory monitoring [21].
These days, many parents seek office-based sedation for treating their child’s teeth and intranasal sedation is among the most desirable and selected methods in this regard. Intranasal sedative drug administration had been confirmed safe based on minimal to no changes in physiologic vital signs [22] yet significantly higher sedative effects were reported in intranasal compared to oral sedation, while longer recovery course. Higher levels of sedation were noted in the intranasal ketamine-midazolam group with higher satisfaction rates for shorter dental procedures (35 min) based on the Houpt scoring system [17].
Differences in sleepiness were not significant between the two groups at the time of injecting the anesthetic, as well as 10 and 20 min after the initiation of the treatment. Nevertheless, the effect of time was significant, suggesting that in both groups improved levels of sleepiness were observed over time.
The two groups did not differ significantly in terms of movement and crying as well as overall behavior at the tested times (P>0.05). In another investigation, no significant differences were reported between changes in blood oxygen saturation and respiration rate of the two similarly sedative cocktails tested [16] similar to the current findings in this investigation. Concurrent use of midazolam and ketamine, in addition to the positive synergism of the drugs, can subsequently reduce the side effects of each, separately [23].
It is suggested that 6-10 mg/kg oral and 5-6 mg/kg intranasal ketamine could be considered as a safe and effective dose spectrum for a desired level of sedation in children [23]. The use of other sedative drugs alongside ketamine could help in reducing the needed dose of ketamine, accelerating a quick recovery, while reducing the incidence of complications known as recovery room phenomenon [24]. In case of 5 min advance benzodiazepine administration or concurrent with ketamine, the drug side effects would be minimized [25].
The combination of ketamine and midazolam reduced the anxiety of preschoolers without an increase in the incidence of side effects resulting in relaxation and cooperation of children [15]. The depth of sedation and anxiety mitigation could be proved far better in the midazolam-ketamine combination when compared to that of midazolam alone with no side effects in any of the two groups15. In yet another study it is indicated that intranasal ketamine would provide significantly deeper sedation at the separation stage of a child from parents and intravenous line conduction [11].
Significantly higher sedation scores are reported along with lower anxiety, easier acceptance of intravenous injection and mask placement over the nose when ketamine-midazolam had been administered. No significant changes were reported in measures of vital signs during and after the operation. Nasal ketamine-midazolam combined induced rapid sedation while the depth was almost the same as the quality of sedation, analgesia, and comfort without any clear side effects [13].
The most common post-treatment complications include nausea, vomiting, sleepiness, and diminished activity within the first four hours of both oral and intranasal midazolam/ketamine sedation [17]. Such side effects were recorded in the current investigation as twelve with no post-treatment nausea following any of the sessions up to 2 h postoperatively while 8 reported degrees of nausea during the same period. Recorded data of the current investigation revealed that 11 parents expressed their satisfaction with the effectiveness of both doses while 5 disagreed. Statistical analysis did not show any significant differences between the two groups (P>0.05).
Conclusion
Differences in satisfaction from intranasal sedation with the two different doses were statistically significant based on operator and parents’ satisfaction scores (P<0.05). No significant differences were observed in vital signs and behavioral reaction changes of children at two different doses (P>0.05). Reduction of the provided ketamine dose for patients had a desirable effect on the course of treatment and the attitude of parents.
Ethical Considerations
Compliance with ethical guidelines
The ethical approval was obtained from the Ethics Committee at Shahid Beheshti University of Medical Sciences, Tehran, Iran (Code: IR.SBMU.DRC.REC.1398.212) and this study was registered by Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20090506001882N9). Written informed consent was signed by the accompanying parents. Only children in the American Society of Anesthesiologists I guidelines were included as indicated for this type of office-based sedation.
Funding
The paper was extracted from the research project of Masoud fallahinejad Ghajari, approved by the Department of Pediatric Dentistry, Dental Faculty of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
Authors contributions
Conceptualization and supervision: Masoud Fallahinejad Ghajari and Ghassem Ansari; Methodology and validation: Ahmad Eghbali Zarch and Sajad Razavi; Writing: Mostafa Mohaveri.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The Dental Research Center, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences is highly appreciated for the scientific and financial support provided. The authors would also like to express their appreciation for those who participated in this project.
References
- American Dental Association. Guideline for the use of sedation and general anesthesia by dentists. New York: American Dental Association; 2007. [Link]
- Parworth LP, Frost DE, Zuniga JR, Bennett T. Propofol and fentanyl compared with midazolam and fentanyl during third molar surgery. J Oral Maxillofac Surg. 1998; 56(4):447-53; discussion 453-4. [DOI:10.1016/S0278-2391(98)90710-8] [PMID]
- Bahetwar SK, Pandey RK, Saksena AK, Chandra G. A comparative evaluation of intranasal midazolam, ketamine and their combination for sedation of young uncooperative pediatric dental patients: A triple blind randomized crossover trial. J Clin Pediatr Dent. 2011; 35(4):415-20.[DOI:10.17796/jcpd.35.4.l43h3354705u2574] [PMID]
- Rech MA, Barbas B, Chaney W, Greenhalgh E, Turck C. When to Pick the Nose: Out of hospital and emergency department intranasal administration of medications. Ann Emerg Med. 2017; 70(2):203-11. [DOI:10.1016/j.annemergmed.2017.02.015] [PMID]
- Lee-Kim SJ, Fadavi S, Punwani I, Koerber A. Nasal versus oral midazolam sedation for pediatric dental patients. J Dent Child (Chic). 2004; 71(2):126-30. [PMID]
- Disma N, Astuto M, Rizzo G, Rosano G, Naso P, Aprile G. Propofol sedation with fentanyl or midazolam during esophagogastroduodenoscopy in children. Eur J Anaesthesiol. 2005; 22(11):848-52. [DOI:10.1017/S0265021505001432] [PMID]
- Chokshi AA, Patel VR, Chauhan P, Patel DJ, Chadha IA, Ramani MN. Evaluation of intranasal Midazolam spray as a sedative in pediatric patients for radiological imaging procedures. Anesth Essays Res. 2013; 7(2):189-93. [DOI:10.4103/0259-1162.118954] [PMID]
- Shimonovich S, Gigi R, Shapira A, Sarig-Meth T, Nadav D, Rozenek M, et al. Intranasal ketamine for acute traumatic pain in the Emergency Department: A prospective, randomized clinical trial of efficacy and safety. BMC Emerg Med. 2016; 16(1):43. [DOI:10.1186/s12873-016-0107-0] [PMID]
- Roelofse JA, Joubert JJ, Roelofse PG. A double-blind randomized comparison of midazolam alone and midazolam combined with ketamine for sedation of pediatric dental patients. J Oral Maxillofac Surg. 1996; 54(7):838-44; discussion 845-6. [DOI:10.1016/S0278-2391(96)90531-5] [PMID]
- Narendra PL, Naphade RW, Nallamilli S, Mohd S. A comparison of intranasal ketamine and intranasal midazolam for pediatric premedication. Anesth Essays Res. 2015; 9(2):213-8. [DOI:10.4103/0259-1162.154051] [PMID]
- Kumari M, Sood S, Taneja R, Dhir G. Comparative study of intranasal midazolam and ketamine for premedication in paediatric surgical patients. J Evid Based Med Healthc. 2018; 5(38):2731-4. [Link]
- Moreira TA, Costa PS, Costa LR, Jesus-França CM, Antunes DE, Gomes HS, et al. Combined oral midazolam-ketamine better than midazolam alone for sedation of young children: A randomized controlled trial. Int J Paediatr Dent. 2013; 23(3):207-15. [DOI:10.1111/j.1365-263X.2012.01246.x] [PMID]
- Khatavkar SS, Bakhshi RG. Comparison of nasal Midazolam with Ketamine versus nasal Midazolam as a premedication in children. Saudi J Anaesth. 2014; 8(1):17-21. [DOI:10.4103/1658-354X.125904] [PMID]
- Sado-Filho J, Viana KA, Corrêa-Faria P, Costa LR, Costa PS. Randomized clinical trial on the efficacy of intranasal or oral ketamine-midazolam combinations compared to oral midazolam for outpatient pediatric sedation. Plos One. 2019; 14(3):e0213074. [DOI:10.1371/journal.pone.0213074] [PMID]
- Pansini V, Curatola A, Gatto A, Lazzareschi I, Ruggiero A, Chiaretti A. Intranasal drugs for analgesia and sedation in children admitted to pediatric emergency department: A narrative review. Ann Transl Med. 2021; 9(2):189.[DOI:10.21037/atm-20-5177] [PMID]
- Preethy NA, Somasundaram S. Sedative and behavioral effects of intranasal midazolam in comparison with other administrative routes in children undergoing dental treatment - A systematic review. Contemp Clin Dent. 2021; 12(2):105-20. [DOI:10.4103/ccd.ccd_470_20] [PMID]
- Fallahinejad Ghajari M, Ansari G, Soleymani AA, Fotuhi Ardakani F. Comparison of oral and intranasal midazolam/ketamine sedation in 3-6 years-old uncooperative dental patients. J Dent Res Dent Clin Dent Prospects. 2015; 9(2):61-5. [DOI:10.15171/joddd.2015.013] [PMID]
- Mehran M, Ansari G, Vahid Golpayegani M, Shayeghi S, Shafiei L. Comparison of sedative effects of oral midazolam/chloral hydrate and midazolam/promethazine in pediatric dentistry. J Dent Res Dent Clin Dent Prospects. 2018; 12(3):221-6. [DOI:10.15171/joddd.2018.034] [PMID]
- Steward D. Psychological preparation and pre medication. In: Steward D, editor. Pediatric Anaesthesia. New York: Churchill Livingstone; 1989. [Link]
- Dal T, Sazak H, Tunç M, Sahin S, Yılmaz A. A comparison of ketamine-midazolam and ketamine-propofol combinations used for sedation in the endobronchial ultrasound-guided transbronchial needle aspiration: A prospective, single-blind, randomized study. J Thorac Dis. 2014; 6: 742-51. [DOI: 10.3978/j.issn.2072-1439.2014.04.10] [PMID]
- Malinovsky JM, Populaire C, Cozian A, Lepage JY, Lejus C, Pinaud M. Premedication with midazolam in children. Effect of intranasal, rectal and oral routes on plasma midazolam concentrations. Anaesthesia. 1995; 50(4):351-4.[DOI:10.1111/j.1365-2044.1995.tb04616.x] [PMID]
- AlSarheed MA. Intranasal sedatives in pediatric dentistry. Saudi Med J. 2016; 37(9):948-56. [DOI:10.15537/smj.2016.9.15003] [PMID]
- Pardo MC, Miller RD. Basics of anesthesia. Amsterdam: Elsevier: 2018. [Link]
- Malamed SJ. Sedation: A guide to patient management. St Louis; Mosby: 2009. [Link]
- Johnson PN, Miller JL, Hagemann TM. Sedation and analgesia in critically ill children. AACN Adv Crit Care. 2012; 23(4):415-34; quiz 435-6. [DOI:10.4037/NCI.0b013e31826b4dea] [PMID]
Type of Study: Original Article |
Subject:
Pediatrics
Received: 2024/01/3 | Accepted: 2024/03/18 | Published: 2024/10/1
Received: 2024/01/3 | Accepted: 2024/03/18 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






