Volume 11, Issue 3 (7-2023)
J. Pediatr. Rev 2023, 11(3): 261-266 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Pasha H, Yahyaei Shahandashti A, Haghshenas F, Bahari Bandari A. Aplasia Cutis Congenita in a Newborn of Diabetic Mother: A Case Report and Review of Literature. J. Pediatr. Rev 2023; 11 (3) :261-266
URL: http://jpr.mazums.ac.ir/article-1-522-en.html
URL: http://jpr.mazums.ac.ir/article-1-522-en.html
1- Social Determinants of Health Research Center, Health Research Institute, Babol University of Medical Sciences, Babol, Iran.
2- Neonate Department of Imam Ali (AS), Mzandaran University of Medical Sciences, Amol, Iran. ,arysh1970@yahoo.com
3- Neonate Department of Imam Ali (AS), Mzandaran University of Medical Sciences, Amol, Iran.
2- Neonate Department of Imam Ali (AS), Mzandaran University of Medical Sciences, Amol, Iran. ,
3- Neonate Department of Imam Ali (AS), Mzandaran University of Medical Sciences, Amol, Iran.
Full-Text [PDF 930 kb]
(1347 Downloads)
| Abstract (HTML) (3000 Views)
Full-Text: (1553 Views)
Background
Aplasia cutis congenita (ACC) is a rare and uncommon congenital abnormality involving various skin layers, mostly as a single lesion involving the midline vertex and, less commonly, the underlying bone and periosteum [1, 2]. The lesions can also be found in the limbs, abdomen, or chest. In 84% of cases, the defect is observed in the scalp; however, the lesion can occur anywhere in the body. Most lesions in the head area are isolated with a size of 0.5-10 cm, and sometimes multiple lesions may be seen. The lesions are round, oval, or jagged, but non-scalp lesions are usually bilaterally symmetric. Individual lesions are often benign but may be associated with other disorders and malformations. The form of the wound can vary from superficial erosion to a deep lesion, with the affected area covered with a transparent, thin membrane [2, 3]. ACC is usually a solitary clinical finding, but it may also happen in several genetic syndromes, including Bart syndrome, Adams-Oliver syndrome, Setleis syndrome, and Patau syndrome [4]. Aplasia cutis is 1 to 3 in 10000 live births [5]. Most presented cases are sporadic, with a few reports of familial occurrence in the form of autosomal dominant and autosomal recessive [6].
Histological evaluation of the wound reveals the lack of normal skin structures, such as sweat glands, sebaceous glands, or hair follicles, with the dermis devoid of collagen fibers [7]. The National Organization for Rare Disorders (NORD) has defined other names for ACC as a congenital lesion of the newborn, scalp defect congenita, and congenital defect of the scalp and skull [8]. Cordon, for the first time, described ACC in 1767. Neither the etiology nor pathogenesis of ACC has been explained; however, genetic, environmental, and exogenous causes have been signified as potential factors. These factors include vascular blood supply, poor blood supply to the skin, fetal and placental ischemia, vascular compromise, placental infarcts, intrauterine infections, adhesion of the amniotic membrane to fetal skin, amniogenesis, amniotic rupture sequence, syphilis, teratogenic substances, trauma, a sudden arrest of midline embryological development, failure in neural tube closure, ectodermal dysplasia, and maternal intrapartum drug use, such as methimazole, carbimazole, misoprostol, and valproic acid [9, 10, 11]. Studies show that the non-scalp ACC is usually large and can be associated with epidermolysis bullosa (EB) [3]. The association of ACC with EB could indicate other congenital anomalies such as renal abnormalities, pyloric or duodenal atresia, craniofacial abnormalities, ureteral stenosis, and nail dystrophy [12].
The diagnosis of ACC is based on clinical findings with a few reports on histopathology depending upon the depth of the defect, which shows a lack of epidermis, dermis, adnexa, and sometimes subcutaneous tissue. An increase in acetylcholinesterase and α-fetoprotein has also been reported in the amniotic fluid of mothers with ACC fetuses [13]. Regular ultrasonography during prenatal care sometimes contributes to the early diagnosis of ACC. The normal fetal skin generates strong echoes on ultrasound, while in ACC, such echoes are absent [14]. ACC can be associated with fetus papyraceous (FP) and occurs as an isolated defect or with other associated anomalies [2]. It has a bilaterally symmetrical pattern in buttocks, truncal, and thigh lesions, which is related to fetal death in the late first to early second trimester of pregnancy [15]. ACC associated with FP showing symmetrical circumferential scarring encircling the trunk has been less commonly described.
Differential diagnosis
ACC may be associated with congenital anomalies of the heart, gastrointestinal tract, urinary tract, genital tract (e.g. gastroschisis or omphalocele), and central nervous system (e.g. myelomeningocele or spinal dysraphism). Also, a small percentage of lesions will heal in utero, showing as an atrophic hairless scar, which can be mistaken for an epidermal nevus. Although ACC is often detectable with an exact clinical exam, lesions with similar presentations should be noticed and ruled out. Ultrasound and further workup with MRI can help to make this diagnosis [16].
Herein, we report an 8-hour-old girl with symmetrical circumferential scarring encircling the trunk associated with fetus papyraceus. In this study, one case of non-scalp ACC occurring in the trunk is reported, and a brief literature review is discussed.
Case Presentation
Anamnesis and status before treatment
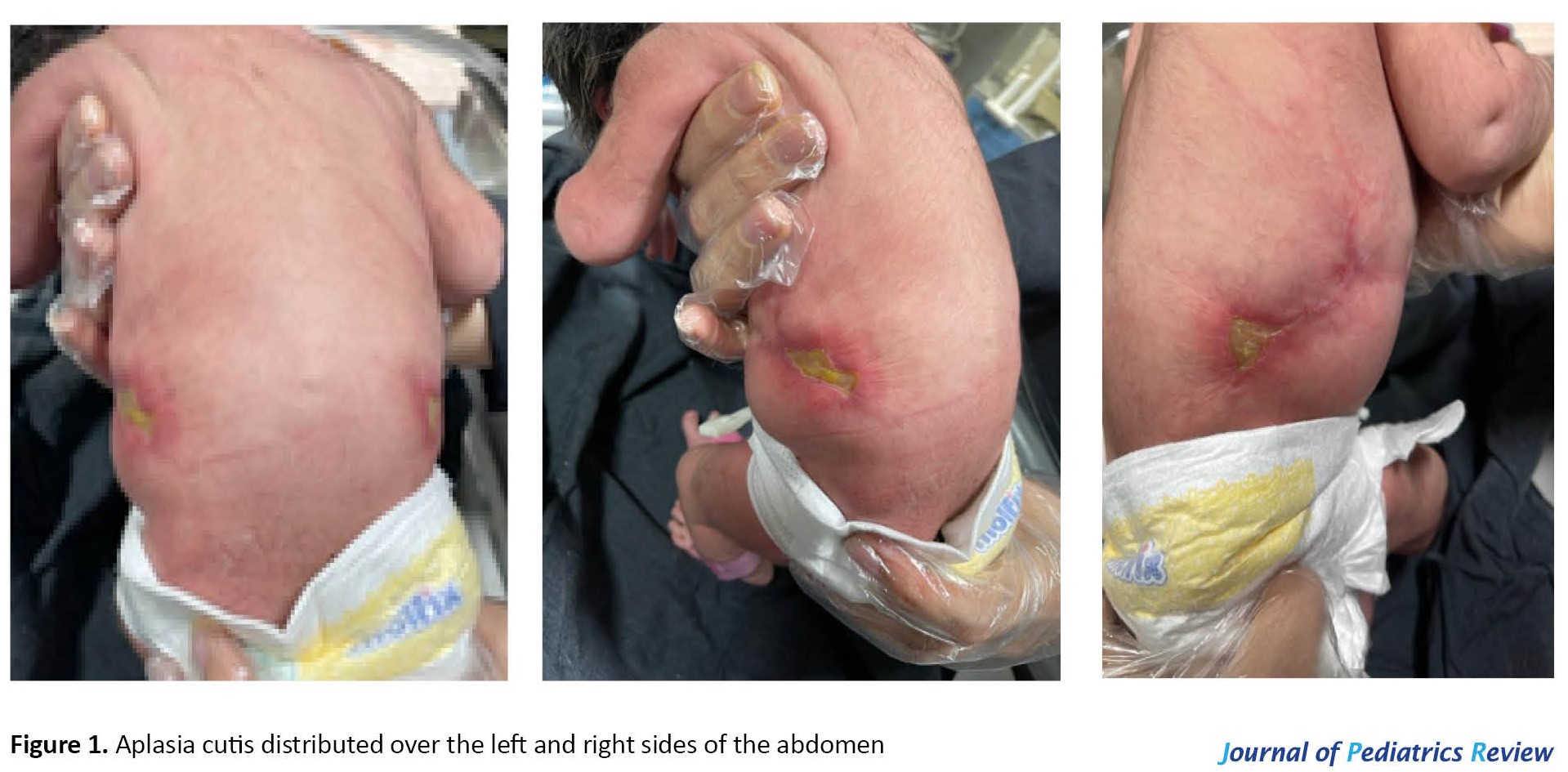
An 8-hour-old girl was admitted to the Neonate Unit of Imam Ali (AS) Hospital in Amol City, Iran. She had a skin lesion on her trunk (flanks), a bilaterally symmetrical, stellate type of truncal aplasia cutis congenita. A relatively broad lesion on the right and left sides of the baby with a width of 3x3 cm and depth of 2 to 3 mm and a clear margin was distinguished, which developed a secondary infection during hospitalization (Figure 1). The patient was born full term (38 wk+4 d) by cesarean section. The baby’s Apgar score increased from 9 at 1 minute to 10 after 5 minutes. The anthropometric parameters of the neonate were as follows: Weight, 3000 g; height, 52 cm; and head circumference, 35 cm. The baby was the family’s second child, and her parents were not next of kin. There was no positive family history of ACC.
The baby was the survivor of a twin as the other fetus died at a gestational age of 13 weeks and 3 days. The death of an identical twin complicated the pregnancy. This condition indicates congenital aplasia cutis with FP. On physical evaluation, the neonate was alert, well, and otherwise normal. Hemangiomata or other congenital defects were not observed in her body. The infant’s vital signs were normal (temperature 36.9°C, heart rate 128 beats per minute, and respiratory rate 48 breaths per minute). The rest of the examination was unremarkable. In particular, there was no evidence of other congenital abnormalities. All blood tests and urinalysis were normal. The blood group of the baby was A+. Culture and smearing of the wound site were performed, and the result was negative. Also, full abdominal and pelvis ultrasound (the liver, gallbladder, pancreas, bile ducts, spleen, kidneys, bladder, uterus, and abdominal aorta) showed normal results. Internal medicine and cardiac consultations were performed, and no abnormal results were reported. A skin consultation was performed.
The mother was 31 years old without a history of infectious diseases or trauma during pregnancy but with a history of drug intake (thyroid and diabetes drugs) during pregnancy. Regarding the baby’s mother’s history, she suffered from gestational diabetes and received insulin from 16 weeks of pregnancy. The weight and height of the mother, who was a housewife, were 92 kg and 164 cm, respectively.
Conservative treatment strategy
Antibiotics, infection control, and medical considerations
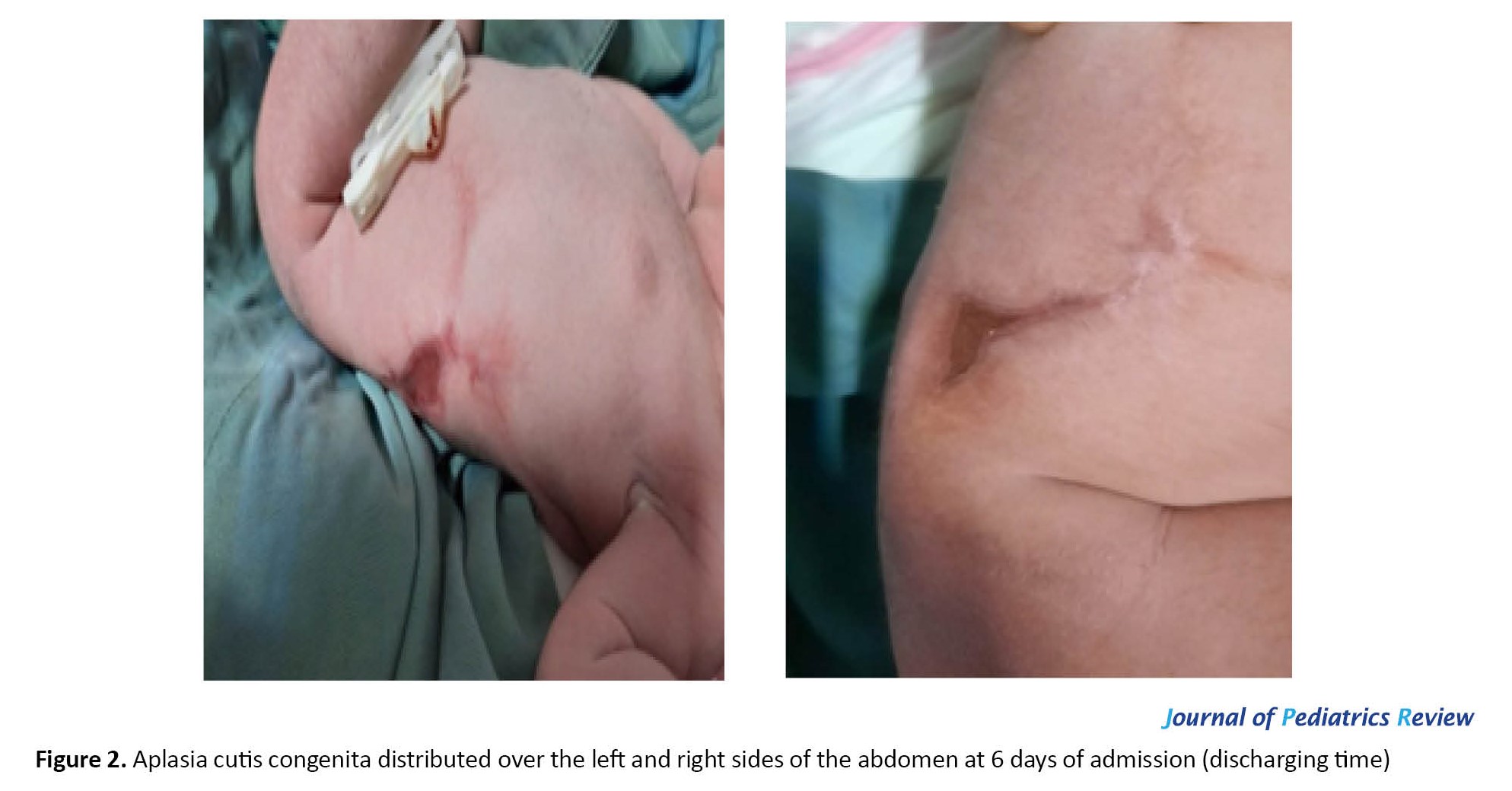
Conservative treatment was insufficient to ensure survival. The neonate received an intravenous infusion of vancomycin (30 mg in dextrose 10% in water), amikacin (30 mg) for 5 days, as well as 15 g of 2% mupirocin ointment topically two times per day (BID). The wound was washed with a normal saline solution, and the dressing was done with sterile gauze as BID. After 5 days, the skin lesion gradually decreased in size and dried up within 6 days of admission. On the other hand, it was relatively improved with systemic and local antibiotic treatment. Finally, she was discharged after healing the wound as an atrophic area with the following order: Daily wound washing with normal saline solution, topical ointment, dressing with sterile gauze, and consumption of cephalexin syrup (Figure 2).
Follow-up
Follow-up after 3 months indicated that the skin lesion completely healed, leaving a very small atrophic scar, and no further lesion management was required.
Discussion
ACC is a disease characterized by a complete or partial, localized, or widespread absence or scarcity of skin at birth. It can involve large areas of the face, buttocks, and trunk as an isolated condition or may be accompanied by other genetic syndromes [1]. The exact mechanism of ACC development is still poorly understood [2]. Mohaddes et al. reported that not all types of these lesions could be justified with one theory because they are phenotypes of different diseases, and more than one mechanism is involved in their occurrence [17].
ACC associated with FP has been reported in numerous studies. In the present case report, the death of a twin occurred at 13 wk+3 d of gestation. Tempark et al. reported that this rare condition is a congenital skin defect and intrauterine death of a fetus with or without a stillborn fetus pressed flat by the growing twin (fetus papyraceous) at birth. There was a reproducible and distinctive distribution pattern of bilateral symmetrical truncal, thigh, and buttocks lesions related to fetal death in the late first to early second trimester of pregnancy [15]. Although the mechanisms of ACC associated with FP are not clear, vascular ischemia is strongly suggested and could explain the skin’s bilateral and symmetrical congenital aplasia [18]. Hypotension and hypovolemia may lead to ischemia of the skin and other organs of the viable twin. Therefore, preventing and treating complications should be considered [19]. Therefore, attention should be paid to the prevention and treatment of complications.
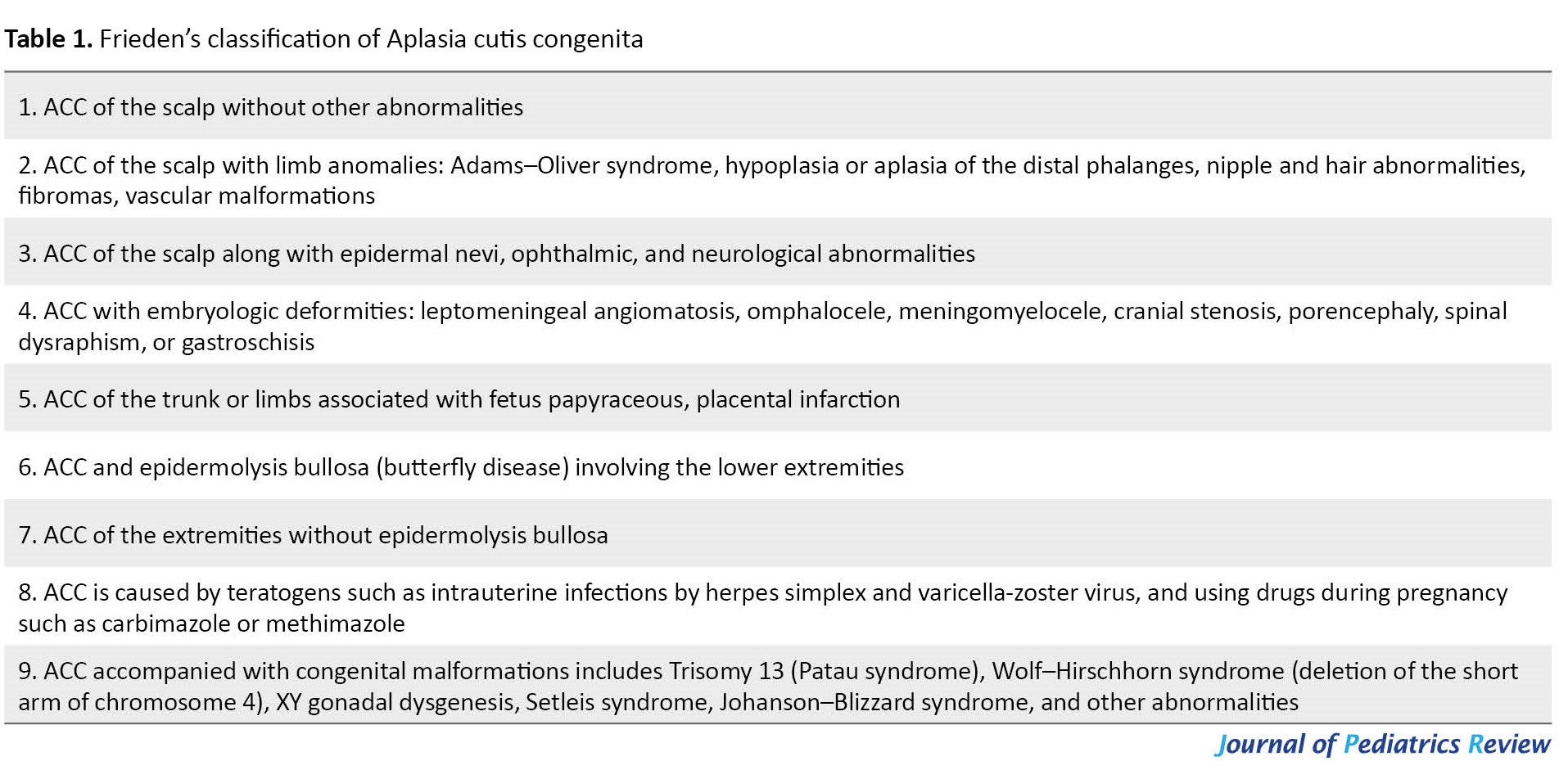
Aplasia lesions are classified into 9 groups according to location, lack of skin, and accompanying anomalies [17]. On the other hand, different classifications based on presentation and or etiologies have been proposed so far. Table 1 presents Frieden’s classification of aplasia cutis congenital [2].
According to Frieden’s classification of aplasia cutis congenital, the case reported in this research belongs to group 5.
ACC management is related to its pattern, underlying causes, location, and abnormalities [20]. However, according to the literature, most cases of FP-related ACC can be cured with conservative treatment [15, 21, 22]. The lesions can also heal in utero, with scars observed at birth [17]. Small localized lesions can be managed conservatively, while more extensive ones may require surgery [18]. Small areas of aplasia cutis usually heal over time, leaving a hairless scar. Gentle cleaning and special ointments can be used to prevent infection. Antibiotics can be useful in the event of infection, which was also observed in our case. Larger lesions or multiple scalp defects may need surgical repair, and sometimes a skin or bone graft may be needed; moreover, texture improvers could be used [23]. Therefore, ACC’s treatment mode can differ depending on the infant’s condition. Although the most popular regimen is conservative therapy, reports of surgical treatment of ACC rarely show the use of the scalp as the donor site [15]. Managing ACC with extensive skin defects is problematic because the best treatment has yet to be determined. Skin regeneration with and without grafts and conservative treatments have been performed with different outcomes [22, 24]. Duan et al. showed that using the scalp as the skin donor site for grafting is an effective therapy for large and deep ACC lesions that arise at sites other than the scalp [19]. Also, another study indicated two types of treatment: A conservative approach involving daily antiseptic dressings to allow for epithelialization of the scalp, improving conditions for secondary surgery, and closing the defect with local rotary flaps [25]. Abulezz et al. revealed that healing and recovery are spontaneous in most cases and that no special treatment is needed apart from keeping the wound clean [6]. Magliah et al., in their report of a 45-day-old baby with a flat scalp lesion on the anterior fontanel with a well-defined complex cyst, showed that intravenous infusion of vancomycin (67 mg in dextrose 5% in water) for 3 days did not improve scalp cysts. However, 15 g of 2% mupirocin ointment topically 3 times per day caused a gradual reduction in the size of the head cyst after 2 days, which dried up within 1 week [20]. A limitation of this study is the lack of genetic evaluation.
Conclusion
We describe an 8-hour-old girl with ACC encircling the trunk in a bilateral symmetrical form. The baby was the survivor of a twin pregnancy; the co-twin died 13 wk+3 d of gestation. To our knowledge, ACC associated with FP showing symmetrical circumferential scarring encircling the trunk has not been previously explained. Also, there is no consensus or guideline for the treatment strategy of ACC, although multiple therapeutic regimens are currently available. The decision between conservative and surgical management depends on the location and size of the lesion. The prompt and effective management of ACC is crucial for preventing fetal complications.
Key clinical message
ACC is a rare disease described by the absence of skin layers, mostly a lone lesion involving the midline vertex, but the whole body can be applied. We present aplasia of a baby born of a diabetic mother taking diabetes drugs (insulin).
Ethical Considerations
Compliance with ethical guidelines
This case study was approved by the Ethics Committee of Babol University of Medical Sciences (Code IR.MUBABOL.REC.1401.161) on February 7, 2023. Permission has been obtained from the neonate’s parents to publish the present case report and any photos of the child.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Neonatal clinical management: Alireza Yahyaei Shahandashti; Literature review and writing the original manuscript: Hajar Pasha and Fatemeh Haghshenas; Review and editing: Hajar Pasha, Alireza Yahyaei Shahandashti, and Amir Bahari Bandari; Final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
References
Aplasia cutis congenita (ACC) is a rare and uncommon congenital abnormality involving various skin layers, mostly as a single lesion involving the midline vertex and, less commonly, the underlying bone and periosteum [1, 2]. The lesions can also be found in the limbs, abdomen, or chest. In 84% of cases, the defect is observed in the scalp; however, the lesion can occur anywhere in the body. Most lesions in the head area are isolated with a size of 0.5-10 cm, and sometimes multiple lesions may be seen. The lesions are round, oval, or jagged, but non-scalp lesions are usually bilaterally symmetric. Individual lesions are often benign but may be associated with other disorders and malformations. The form of the wound can vary from superficial erosion to a deep lesion, with the affected area covered with a transparent, thin membrane [2, 3]. ACC is usually a solitary clinical finding, but it may also happen in several genetic syndromes, including Bart syndrome, Adams-Oliver syndrome, Setleis syndrome, and Patau syndrome [4]. Aplasia cutis is 1 to 3 in 10000 live births [5]. Most presented cases are sporadic, with a few reports of familial occurrence in the form of autosomal dominant and autosomal recessive [6].
Histological evaluation of the wound reveals the lack of normal skin structures, such as sweat glands, sebaceous glands, or hair follicles, with the dermis devoid of collagen fibers [7]. The National Organization for Rare Disorders (NORD) has defined other names for ACC as a congenital lesion of the newborn, scalp defect congenita, and congenital defect of the scalp and skull [8]. Cordon, for the first time, described ACC in 1767. Neither the etiology nor pathogenesis of ACC has been explained; however, genetic, environmental, and exogenous causes have been signified as potential factors. These factors include vascular blood supply, poor blood supply to the skin, fetal and placental ischemia, vascular compromise, placental infarcts, intrauterine infections, adhesion of the amniotic membrane to fetal skin, amniogenesis, amniotic rupture sequence, syphilis, teratogenic substances, trauma, a sudden arrest of midline embryological development, failure in neural tube closure, ectodermal dysplasia, and maternal intrapartum drug use, such as methimazole, carbimazole, misoprostol, and valproic acid [9, 10, 11]. Studies show that the non-scalp ACC is usually large and can be associated with epidermolysis bullosa (EB) [3]. The association of ACC with EB could indicate other congenital anomalies such as renal abnormalities, pyloric or duodenal atresia, craniofacial abnormalities, ureteral stenosis, and nail dystrophy [12].
The diagnosis of ACC is based on clinical findings with a few reports on histopathology depending upon the depth of the defect, which shows a lack of epidermis, dermis, adnexa, and sometimes subcutaneous tissue. An increase in acetylcholinesterase and α-fetoprotein has also been reported in the amniotic fluid of mothers with ACC fetuses [13]. Regular ultrasonography during prenatal care sometimes contributes to the early diagnosis of ACC. The normal fetal skin generates strong echoes on ultrasound, while in ACC, such echoes are absent [14]. ACC can be associated with fetus papyraceous (FP) and occurs as an isolated defect or with other associated anomalies [2]. It has a bilaterally symmetrical pattern in buttocks, truncal, and thigh lesions, which is related to fetal death in the late first to early second trimester of pregnancy [15]. ACC associated with FP showing symmetrical circumferential scarring encircling the trunk has been less commonly described.
Differential diagnosis
ACC may be associated with congenital anomalies of the heart, gastrointestinal tract, urinary tract, genital tract (e.g. gastroschisis or omphalocele), and central nervous system (e.g. myelomeningocele or spinal dysraphism). Also, a small percentage of lesions will heal in utero, showing as an atrophic hairless scar, which can be mistaken for an epidermal nevus. Although ACC is often detectable with an exact clinical exam, lesions with similar presentations should be noticed and ruled out. Ultrasound and further workup with MRI can help to make this diagnosis [16].
Herein, we report an 8-hour-old girl with symmetrical circumferential scarring encircling the trunk associated with fetus papyraceus. In this study, one case of non-scalp ACC occurring in the trunk is reported, and a brief literature review is discussed.
Case Presentation
Anamnesis and status before treatment
An 8-hour-old girl was admitted to the Neonate Unit of Imam Ali (AS) Hospital in Amol City, Iran. She had a skin lesion on her trunk (flanks), a bilaterally symmetrical, stellate type of truncal aplasia cutis congenita. A relatively broad lesion on the right and left sides of the baby with a width of 3x3 cm and depth of 2 to 3 mm and a clear margin was distinguished, which developed a secondary infection during hospitalization (Figure 1). The patient was born full term (38 wk+4 d) by cesarean section. The baby’s Apgar score increased from 9 at 1 minute to 10 after 5 minutes. The anthropometric parameters of the neonate were as follows: Weight, 3000 g; height, 52 cm; and head circumference, 35 cm. The baby was the family’s second child, and her parents were not next of kin. There was no positive family history of ACC.
The baby was the survivor of a twin as the other fetus died at a gestational age of 13 weeks and 3 days. The death of an identical twin complicated the pregnancy. This condition indicates congenital aplasia cutis with FP. On physical evaluation, the neonate was alert, well, and otherwise normal. Hemangiomata or other congenital defects were not observed in her body. The infant’s vital signs were normal (temperature 36.9°C, heart rate 128 beats per minute, and respiratory rate 48 breaths per minute). The rest of the examination was unremarkable. In particular, there was no evidence of other congenital abnormalities. All blood tests and urinalysis were normal. The blood group of the baby was A+. Culture and smearing of the wound site were performed, and the result was negative. Also, full abdominal and pelvis ultrasound (the liver, gallbladder, pancreas, bile ducts, spleen, kidneys, bladder, uterus, and abdominal aorta) showed normal results. Internal medicine and cardiac consultations were performed, and no abnormal results were reported. A skin consultation was performed.
The mother was 31 years old without a history of infectious diseases or trauma during pregnancy but with a history of drug intake (thyroid and diabetes drugs) during pregnancy. Regarding the baby’s mother’s history, she suffered from gestational diabetes and received insulin from 16 weeks of pregnancy. The weight and height of the mother, who was a housewife, were 92 kg and 164 cm, respectively.
Conservative treatment strategy
Antibiotics, infection control, and medical considerations
Conservative treatment was insufficient to ensure survival. The neonate received an intravenous infusion of vancomycin (30 mg in dextrose 10% in water), amikacin (30 mg) for 5 days, as well as 15 g of 2% mupirocin ointment topically two times per day (BID). The wound was washed with a normal saline solution, and the dressing was done with sterile gauze as BID. After 5 days, the skin lesion gradually decreased in size and dried up within 6 days of admission. On the other hand, it was relatively improved with systemic and local antibiotic treatment. Finally, she was discharged after healing the wound as an atrophic area with the following order: Daily wound washing with normal saline solution, topical ointment, dressing with sterile gauze, and consumption of cephalexin syrup (Figure 2).
Follow-up
Follow-up after 3 months indicated that the skin lesion completely healed, leaving a very small atrophic scar, and no further lesion management was required.
Discussion
ACC is a disease characterized by a complete or partial, localized, or widespread absence or scarcity of skin at birth. It can involve large areas of the face, buttocks, and trunk as an isolated condition or may be accompanied by other genetic syndromes [1]. The exact mechanism of ACC development is still poorly understood [2]. Mohaddes et al. reported that not all types of these lesions could be justified with one theory because they are phenotypes of different diseases, and more than one mechanism is involved in their occurrence [17].
ACC associated with FP has been reported in numerous studies. In the present case report, the death of a twin occurred at 13 wk+3 d of gestation. Tempark et al. reported that this rare condition is a congenital skin defect and intrauterine death of a fetus with or without a stillborn fetus pressed flat by the growing twin (fetus papyraceous) at birth. There was a reproducible and distinctive distribution pattern of bilateral symmetrical truncal, thigh, and buttocks lesions related to fetal death in the late first to early second trimester of pregnancy [15]. Although the mechanisms of ACC associated with FP are not clear, vascular ischemia is strongly suggested and could explain the skin’s bilateral and symmetrical congenital aplasia [18]. Hypotension and hypovolemia may lead to ischemia of the skin and other organs of the viable twin. Therefore, preventing and treating complications should be considered [19]. Therefore, attention should be paid to the prevention and treatment of complications.
Aplasia lesions are classified into 9 groups according to location, lack of skin, and accompanying anomalies [17]. On the other hand, different classifications based on presentation and or etiologies have been proposed so far. Table 1 presents Frieden’s classification of aplasia cutis congenital [2].

According to Frieden’s classification of aplasia cutis congenital, the case reported in this research belongs to group 5.
ACC management is related to its pattern, underlying causes, location, and abnormalities [20]. However, according to the literature, most cases of FP-related ACC can be cured with conservative treatment [15, 21, 22]. The lesions can also heal in utero, with scars observed at birth [17]. Small localized lesions can be managed conservatively, while more extensive ones may require surgery [18]. Small areas of aplasia cutis usually heal over time, leaving a hairless scar. Gentle cleaning and special ointments can be used to prevent infection. Antibiotics can be useful in the event of infection, which was also observed in our case. Larger lesions or multiple scalp defects may need surgical repair, and sometimes a skin or bone graft may be needed; moreover, texture improvers could be used [23]. Therefore, ACC’s treatment mode can differ depending on the infant’s condition. Although the most popular regimen is conservative therapy, reports of surgical treatment of ACC rarely show the use of the scalp as the donor site [15]. Managing ACC with extensive skin defects is problematic because the best treatment has yet to be determined. Skin regeneration with and without grafts and conservative treatments have been performed with different outcomes [22, 24]. Duan et al. showed that using the scalp as the skin donor site for grafting is an effective therapy for large and deep ACC lesions that arise at sites other than the scalp [19]. Also, another study indicated two types of treatment: A conservative approach involving daily antiseptic dressings to allow for epithelialization of the scalp, improving conditions for secondary surgery, and closing the defect with local rotary flaps [25]. Abulezz et al. revealed that healing and recovery are spontaneous in most cases and that no special treatment is needed apart from keeping the wound clean [6]. Magliah et al., in their report of a 45-day-old baby with a flat scalp lesion on the anterior fontanel with a well-defined complex cyst, showed that intravenous infusion of vancomycin (67 mg in dextrose 5% in water) for 3 days did not improve scalp cysts. However, 15 g of 2% mupirocin ointment topically 3 times per day caused a gradual reduction in the size of the head cyst after 2 days, which dried up within 1 week [20]. A limitation of this study is the lack of genetic evaluation.
Conclusion
We describe an 8-hour-old girl with ACC encircling the trunk in a bilateral symmetrical form. The baby was the survivor of a twin pregnancy; the co-twin died 13 wk+3 d of gestation. To our knowledge, ACC associated with FP showing symmetrical circumferential scarring encircling the trunk has not been previously explained. Also, there is no consensus or guideline for the treatment strategy of ACC, although multiple therapeutic regimens are currently available. The decision between conservative and surgical management depends on the location and size of the lesion. The prompt and effective management of ACC is crucial for preventing fetal complications.
Key clinical message
ACC is a rare disease described by the absence of skin layers, mostly a lone lesion involving the midline vertex, but the whole body can be applied. We present aplasia of a baby born of a diabetic mother taking diabetes drugs (insulin).
Ethical Considerations
Compliance with ethical guidelines
This case study was approved by the Ethics Committee of Babol University of Medical Sciences (Code IR.MUBABOL.REC.1401.161) on February 7, 2023. Permission has been obtained from the neonate’s parents to publish the present case report and any photos of the child.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Neonatal clinical management: Alireza Yahyaei Shahandashti; Literature review and writing the original manuscript: Hajar Pasha and Fatemeh Haghshenas; Review and editing: Hajar Pasha, Alireza Yahyaei Shahandashti, and Amir Bahari Bandari; Final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
References
- Coi A, Barisic I, Garne E, Pierini A, Addor MC, Aizpurua Atxega A, et al. Epidemiology of aplasia cutis congenita: A population-based study in Europe. J Eur Acad Dermatol Venereol. 2023; 37(3):581-9. [DOI:10.1111/jdv.18690] [PMID]
- Thadchanamoorthy V, Dayasiri K, Thirukumar M, Thamilvannan N, Chandraratne SH. Multiple aplasia cutis congenita type V and fetus papyraceous: A case report and review of the literature. J Med Case Rep. 2021; 15(1):110. [DOI:10.1186/s13256-021-02662-3] [PMID] [PMCID]
- Benvenuto C, Kraemer CK, Kruse RL, Cestari TF. Familial epidermolysis bullosa with aplasia cutis congenita: Bart's syndrome? Skinmed. 2003; 2(5):319-21.[DOI:10.1111/j.1540-9740.2003.02056.x] [PMID]
- Burkhead A, Poindexter G, Morrell DS. A case of extensive Aplasia Cutis Congenita with underlying skull defect and central nervous system malformation: Discussion of large skin defects, complications, treatment and outcome. J Perinatol. 2009; 29(8):582-4. [DOI:10.1038/jp.2008.250] [PMID]
- Chi Chi Kong P, Minh Nhat H, Ha Yen Chi L. Aplasia cutis congenita associated with the use of antithyroid medications during pregnancy: A case report. Iran J Neonatol. 2022; 13(2):125-9. [DOI:10.22038/IJN.2022.58139.2099]
- Abulezz TA, Shalkamy MA. Aplasia cutis congenita: Two cases of non-scalp lesions. Indian J Plast Surg. 2009; 42(2):261-4. [DOI:10.1055/s-0039-1699359] [PMID] [PMCID]
- Taifour Suliman M, Quazi A. Aplasia cutis congenita of the trunk in a Saudi newborn. Br J Plast Surg. 2004; 57(6):582-4. [DOI:10.1016/j.bjps.2003.12.026] [PMID]
- National Organization for Rare Disorders (NORD). Rare disease database. Quincy; 2020. [Link]
- Mukhtar-Yola M, Mshelia L, Mairami AB, Otuneye AT, Yawe ET, Igoche P, et al. Aplasia cutis congenita: A report of two cases from National Hospital Abuja, Nigeria and review of the literature. Pan Afr Med J. 2020; 36(1):1-9. [DOI:10.11604/pamj.2020.36.291.24523]
- Adigun CG. Aplasia cutis congenita [Internet]. 2018. [Updated August 2023]. Available from: [Link]
- Blionas A, Giakoumettis D, Antoniades E, Drosos E, Mitsios A, Plakas S, et al. Aplasia cutis congenita: Two case reports and discussion of the literature. Surg Neurol Int. 2017; 8:273. [DOI:10.4103/sni.sni_188_17] [PMID] [PMCID]
- Al-Salem AH. Congenital pyloric atresia and associated anomalies. Pediatr Surg Int. 2007; 23(6):559-63. [DOI:10.1007/s00383-007-1903-0] [PMID]
- Moss C, Shahidulla H. Naevi and other developmental defects. In: Burns T, Breathnach S, Cox N, Griffiths C, editors. Rook’s textbook of dermatology. Hoboken: WileyBlackwell Publication; 2010. [Link]
- Liu F, Chen X, Tu R, Liu S. Prenatal diagnosis of aplasia cutis congenita of the trunk. Int J Dermatol. 2014; 53(10):1269-71. [DOI:10.1111/j.1365-4632.2012.05732.x] [PMID]
- Tempark T, Shwayder TA. Aplasia cutis congenita with fetus papyraceus: report and review of the literature. Int J Dermatol. 2012; 51(12):1419-26. [DOI:10.1111/j.1365-4632.2012.05545.x] [PMID]
- Gao Z, Massimi L, Rogerio S, Raybaud C, Di Rocco C. Vertex cephaloceles: A review. Childs Nerv Syst. 2014; 30(1):65-72. [DOI:10.1007/s00381-013-2249-7] [PMID]
- Mohaddes G, Ameli H. [Congenital skin deficiency (Persian)]. Urmia Med J. 2010; 19(1):72-6. [Link]
- Louise L, Annabel M, Hubert L, Isabelle G, Gerard L. Fetus papyraceus: Congenital pulmonary anomalies associated with congenital aplasia cutis on the surviving twin. Pediatr Dermatol. 2013; 30(6):e143-5. [DOI:10.1111/pde.12096] [PMID]
- Duan X, Yang GE, Yu D, Yu C, Wang B, Guo Y. Aplasia cutis congenita: A case report and literature review. Exp Ther Med. 2015; 10(5):1893-5. [DOI:10.3892/etm.2015.2737] [PMID] [PMCID]
- Magliah T, Alghamdi F. Aplasia cutis congenita: A case report. Case Rep Dermatol. 2018; 10(2):182-6.[DOI:10.1159/000490786] [PMID] [PMCID]
- Blouin MM, Bernard J, Caron F, Auger I. Aplasia cutis congenita of the trunk and scalp associated with fetus papyraceus. Int J Dermatol. 2011; 50(6):733-5. [DOI:10.1111/j.1365-4632.2010.04619.x] [PMID]
- Gupta D, Levy ML, Corona R. Aplasia cutis congenital [Internet]. 2023 [Updated 2023 March 21]. Available from: [Link]
- Lei GF, Zhang JP, Wang XB, You XL, Gao JY, Li XM, et al. Treating aplasia cutis congenita in a newborn with the combination of ionic silver dressing and moist exposed burn ointment: A case report. World J Clin Cases. 2019; 7(17):2611-6. [DOI:10.12998/wjcc.v7.i17.2611] [PMID]
- Ahcan U, Janezic T. Management of aplasia cutis congenita in a non-scalp location. Br J Plast Surg. 2002; 55(6):530-2. [DOI:10.1054/bjps.2002.3915] [PMID]
- Moros Peña M, Labay Matías M, Valle Sánchez F, Valero Adán T, Martín-Calama Valero J, Muñoz Albillos M. [Aplasia cutis congenita in a newborn: Etiopathogenic review and diagnostic approach (Spanish)]. An Esp Pediatr. 2000; 52(5):453-6. [DOI:10.1016/S1695-4033(00)77379-6] [PMID]
Type of Study: Case & Review |
Subject:
Dermatology
Received: 2023/03/20 | Accepted: 2023/06/14 | Published: 2023/07/1
Received: 2023/03/20 | Accepted: 2023/06/14 | Published: 2023/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |