Volume 11, Issue 3 (7-2023)
J. Pediatr. Rev 2023, 11(3): 231-244 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rezai M S, Rostami-Maskopaee F, Navaeifar M R, Hajialibeig A, Gooran M, Haghighi Aski B, et al . Multisystem Inflammatory Syndrome Mortality Following COVID-19 in Iranian Children: A Case Series and Literature Review. J. Pediatr. Rev 2023; 11 (3) :231-244
URL: http://jpr.mazums.ac.ir/article-1-535-en.html
URL: http://jpr.mazums.ac.ir/article-1-535-en.html
Mohammad Sadegh Rezai1 

 , Fereshteh Rostami-Maskopaee *2
, Fereshteh Rostami-Maskopaee *2 

 , Mohammad Reza Navaeifar1
, Mohammad Reza Navaeifar1 

 , Azin Hajialibeig1
, Azin Hajialibeig1 

 , Maedeh Gooran1
, Maedeh Gooran1 

 , Behzad Haghighi Aski3
, Behzad Haghighi Aski3 

 , Ali Manafi Anari3
, Ali Manafi Anari3 

 , Eslam Shorafa4
, Eslam Shorafa4 

 , Seyedeh Narjes Abootalebi4
, Seyedeh Narjes Abootalebi4 




 , Fereshteh Rostami-Maskopaee *2
, Fereshteh Rostami-Maskopaee *2 

 , Mohammad Reza Navaeifar1
, Mohammad Reza Navaeifar1 

 , Azin Hajialibeig1
, Azin Hajialibeig1 

 , Maedeh Gooran1
, Maedeh Gooran1 

 , Behzad Haghighi Aski3
, Behzad Haghighi Aski3 

 , Ali Manafi Anari3
, Ali Manafi Anari3 

 , Eslam Shorafa4
, Eslam Shorafa4 

 , Seyedeh Narjes Abootalebi4
, Seyedeh Narjes Abootalebi4 


1- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran. ,rostamiraha5@gmail.com
3- Department of Pediatrics, Ali Asghar Children’s Hospital, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Pediatrics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
2- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran. ,
3- Department of Pediatrics, Ali Asghar Children’s Hospital, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Pediatrics, School of Medicine, Shiraz University of Medical Sciences, Shiraz, Iran.
Full-Text [PDF 571 kb]
(1190 Downloads)
| Abstract (HTML) (2760 Views)
Full-Text: (691 Views)
Introduction
COVID-19 causes morbidity and mortality in all age groups [1]. The symptoms and signs of the disease are milder in children than in adults [2, 3]. The most common clinical presentations of COVID-19 in children are fever and cough, but some children become critically ill and require intensive care [4-7]. As of May 2020, physicians announced the appearance of a new presentation of SARS-CoV-2 infection, known as multisystem inflammatory syndrome-children (MIS-C), which behaves more aggressively, causing mortality in the pediatric population [5, 8]. MIS-C is characterized by fever, laboratory evidence of inflammation, multisystem organ involvement, severe illness, and SARS-CoV-2 infection or exposure [9, 10].
Our knowledge about MIS-C patients’ characteristics associated with intensive care unit (ICU) admission and mortality is still limited [11]. Hence, we performed this case series study to present the clinical manifestations and treatment of MIS-C patients following COVID-19 who died in different geographical regions of Iran (Tehran, Fars, and Mazandaran provinces) from March 2020 to September 2021.
Case Presentation
Case 1
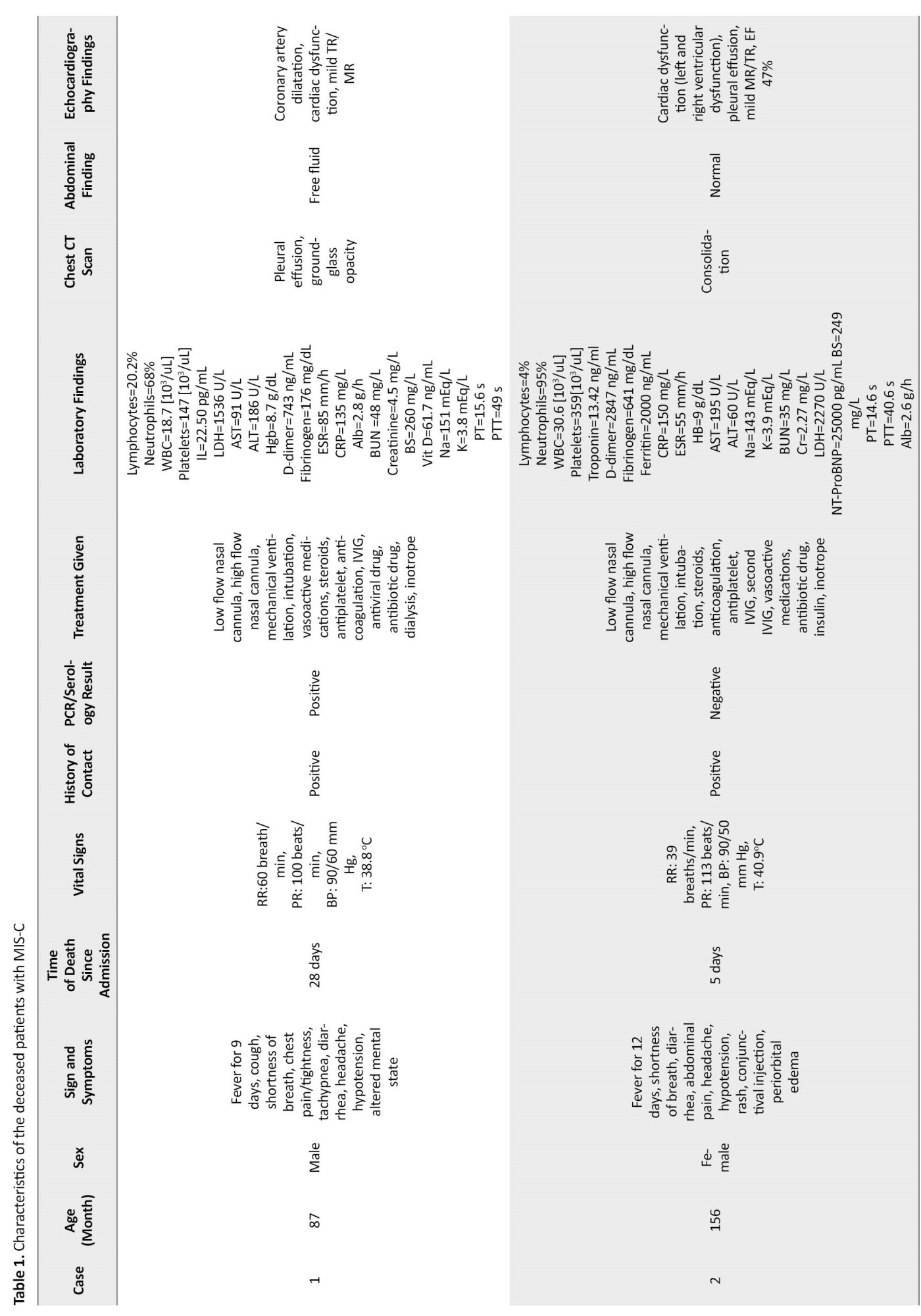
A 7-year-old boy with a history of fever in the past 8 days and cough, headache, and diarrhea was referred to the hospital emergency department (ED). On admission, he had respiratory distress with a toxic appearance. His oxygen saturation (SPO2) was 85%, which increased to 92% with oxygen through the mask and reservoir bag. The patient was transferred to PICU with an initial diagnosis of critical COVID-19. His weight was 43 kg, which was above the 97th percentile. The urine and blood culture results were negative. COVID-19 reverse transcription polymerase chain reaction (RT-PCR) test result was positive. Due to nosocomial infections, he received vancomycin, meropenem, enoxaparin, and dexamethasone on the first day of admission. Intravenous immunoglobulin (IVIG) and aspirin were administered. According to the lung CT scan and positive COVID-19 RT-PCR results, remdesivir was ordered. Intravenous inotropic agents were administered on the fourth day due to gallop and myocarditis in echocardiography results. On the fifth day, the patient was intubated because of severe hypoxia (SPO2 decline to 70%) and respiratory failure. Due to hyperglycemia (blood sugar [BS]=348 mg/dL), insulin was ordered. He underwent dialysis because of a glomerular filtration rate (GFR) <30 mL/min. Remdesivir was discontinued, and medications were adjusted based on GFR and dialysis. The patient’s condition was unstable, and he underwent dialysis several times. His general condition deteriorated, and he died after 28 days of hospitalization due to recurrent pulmonary hemorrhage and cardiac arrest.
Case 2
A 13-year-old girl was admitted with a history of high-grade fever in the last 4 days and periorbital (orbital) edema. She had shortness of breath 2 days before admission and was visited by her family physician. With supportive care, the patient’s condition worsened, and referred to the ED with fever, conjunctivitis, nausea, vomiting, diarrhea, dyspnea, and skin rash. At the initial examination, the patient had tachycardia and tachypnea. The RT-PCR test for COVID-19 was negative, but she had a history of COVID-19 in her family. Her SPO2 was 85%, which increased to 98% with oxygen through the mask and reservoir bag. The patient received vancomycin, IVIG, and pulse methylprednisolone on the first day of admission. On the second day, meropenem was ordered. Due to hyperglycemia (BS=249 mg/dL), insulin was prescribed to correct hypokalemia. The patient was intubated on the third day because of a severe decline in SPO2 and respiratory failure (respiratory rate [RR]=22/min). On the fourth day, intravenous inotropic agents were ordered due to gallop in echocardiography results. Still, she did not respond to the treatment and died on the 5th day following hospitalization due to heart failure and shock.
Case 3
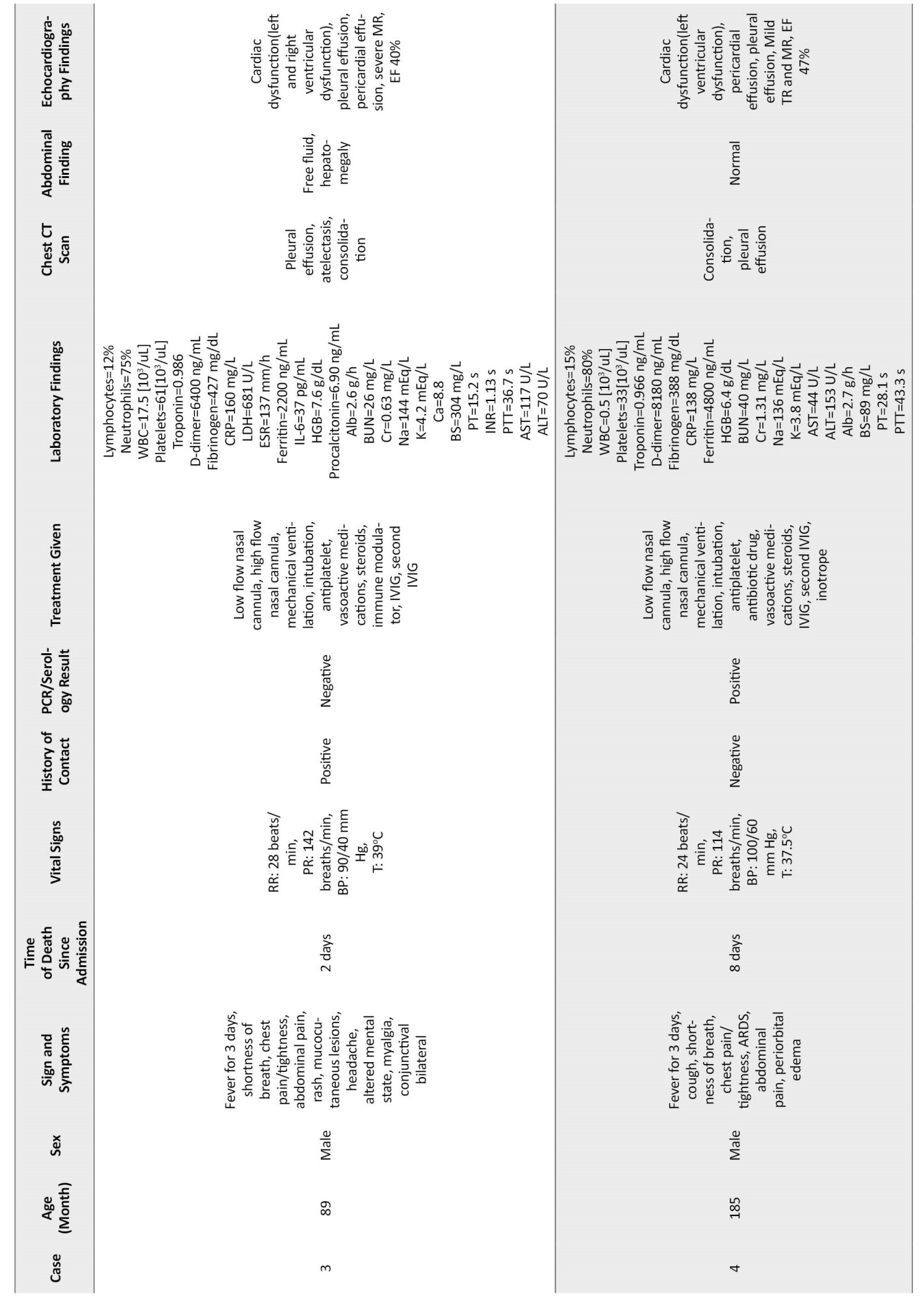
A 7-year-old boy was referred for fever and chills in the past 3 days, abdominal pain 2 days before admission, shortness of breath, lethargy, vomiting, and cough. He was hospitalized for 1 day. Despite supportive care, the patient’s condition worsened, and he was transferred to another the hospital’s ED. On physical examination, he had a fever, bilateral conjunctivitis, and skin rash. The patient had tachycardia and tachypnea. His weight was 35 kg, which was above the 97th percentile. The history of COVID-19 in family members was positive (father), and the COVID-19 RT-PCR test was negative. His SPO2 was 88%, which increased to 98% after using the mask and reservoir bag. The patient immediately became intubated. On the first day of admission, he received vancomycin, clindamycin, pulse methylprednisolone (350 mg), and IVIG (1 vial). The patient had fresh blood-stained secretion of the endotracheal tube (ETT). On the second day, insulin was injected due to hyperglycemia (BS=304 mg/dL). Because of the gallop in echocardiography, intravenous inotropic agents were ordered. The pulse methylprednisolone dose (700 mg) was increased, and the second dose of IVIG was injected. Tocilizumab was given to the patient. Hypoxemia (SPO2=80%) occurred on the last day, and cyanosis was evident in the extremities. He died after two days of hospitalization due to bradycardia (40-43 beats/min), ARDS (acute respiratory distress syndrome), and DIC (disseminated intravascular coagulation).
Case 4
A 15-year-old boy undergoing chemotherapy and granulocyte colony-stimulating factor due to acute lymphocytic leukemia (ALL) was referred to the ED for vomiting, sore throat, and diarrhea. The patient was tachypneic and pale with an ill and toxic appearance. Rale was heard in the pulmonary auscultation. He had orthostatic hypotension. COVID-19 RT-PCR test result was positive. His weight was 70 kg which was above the 97th percentile. Because of ALL, he was under chemotherapy for 2 years before admission. His SPO2 was 70%, which increased to 97% with oxygen through the mask and reservoir bag. The patient had fresh blood-stained secretion of ETT. He was intubated on the first day of admission and received vancomycin, meropenem, co-trimoxazole, amikacin, methylprednisolone (60 mg every 8 hours), and IVIG (50 g). According to the echocardiography results, intravenous inotropic agents were ordered. After 4 days of hospitalization, his ejection fraction (EF) declined from 78% to 47%, asystole and arrhythmia developed, and he died eventually.
Case 5
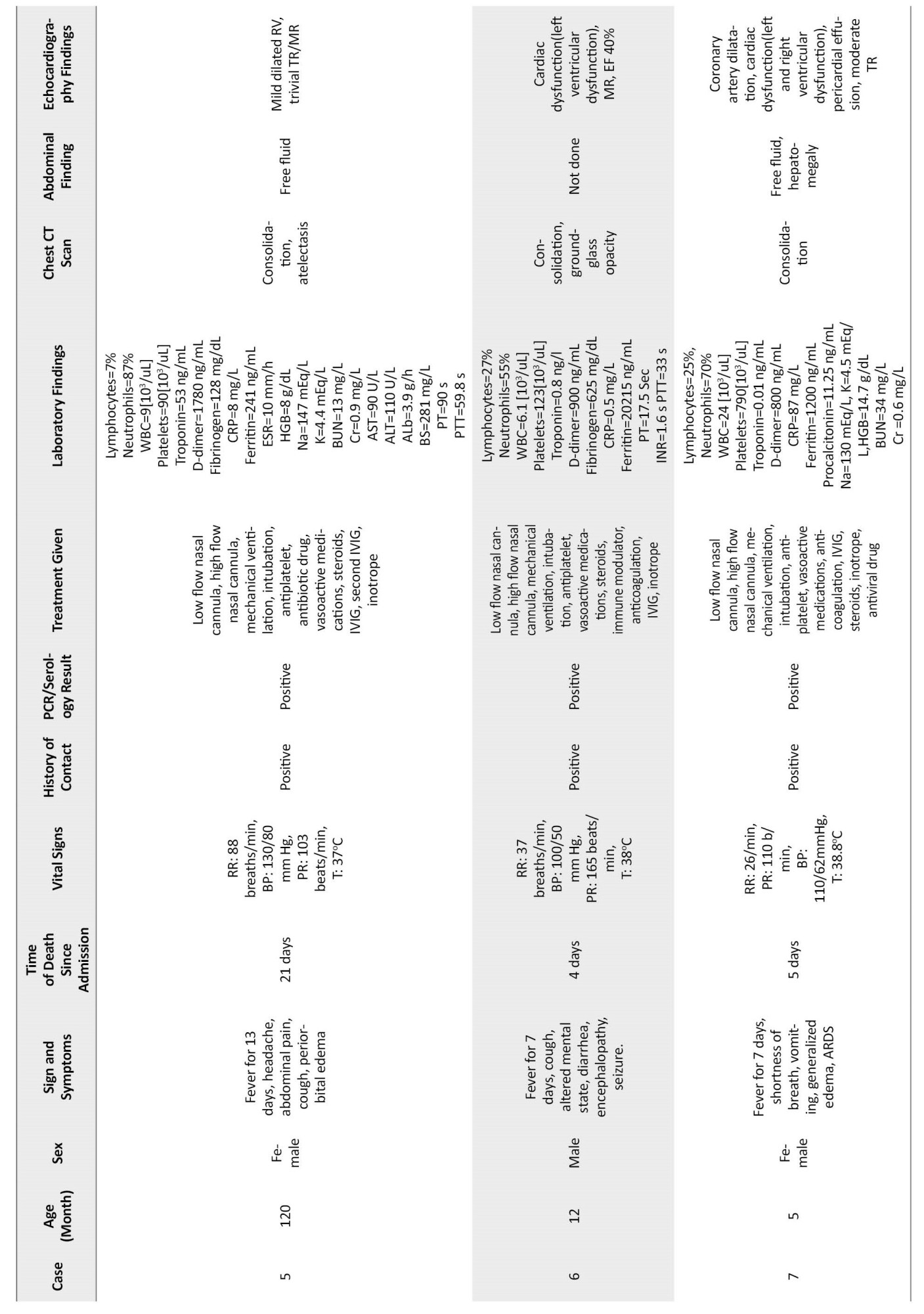
A 10-year-old girl was referred with a history of high fever for the last 7 days, abdominal pain, and cough before admission. She had lower extremity edema for 10 days. At hospitalization, the patient’s liver enzymes increased (hepatic encephalopathy). Due to reduced consciousness and drowsiness, transferring her to an equipped liver transplant center was necessary. Due to liver and brain involvement and a positive COVID-19 RT-PCR test, she was admitted to the pediatric intensive care unit (PICU) with an MIS-C diagnosis. At the time of admission, the patient was intubated. Her SPO2 was 80%, which increased to 95% with oxygen through the nasal cannula. Her brain MRI showed cerebral involvement following COVID-19. Due to a history of partial seizures, she received carbamazepine, vancomycin, cefotaxime, meropenem, pulse methylprednisolone (for 3 days), and IVIG were started for the patient. He had several seizures, so diazepam was ordered. According to echocardiography results, intravenous inotropic agents were added to her drugs. During the patient’s management, SPO2 decreased (69%). A second dose of IVIG was injected. The patient became hypotensive and bradycardic. Cardiopulmonary resuscitation (CPR) was performed, but she died on the 21st day of hospitalization due to hepatic encephalopathy, pulmonary edema, and cardiac arrest.
Case 6
A 1-year-old boy was referred to the ED with a fever in the 4 days ago, in addition to cough, diarrhea, and seizure. The night before hospitalization, he had a tonic attack without cyanosis (about ten minutes), which was controlled with diazepam and repeated after 3 to 4 minutes. He was transferred to the PICU with a status epilepsy diagnosis. He had a toxic appearance at admission time and was not conscious. COVID-19 RT-PCR test result was positive. His weight was 10 kg, which was above the 97th percentile. His SPO2 was 75%, which increased to 100% with oxygen through the nasal cannula. According to the MIS-C diagnosis, ciprofloxacin, meropenem, IVIG, pulse methyl prednisone, and enoxaparin were initiated. Seizures were poorly controlled on the second day of his stay, and midazolam and phenytoin were loaded. Remdesivir was added to the patient’s medication. Due to hyperglycemia (BS=300 mg/dL), insulin was ordered. On the third day of hospitalization, the patient’s consciousness level decreased, and he was intubated. Due to unstable hemodynamics, intravenous inotropic agents were administered. After 4 days of hospitalization, the EF of the patient reduced to 40%. Bradycardia and a severe decline in SPO2 occurred, and he died eventually.
Case 7
A 5-month-old female infant was referred to the ED with dyspnea, restlessness, and vomiting. The patient had a toxic appearance. On physical examination, she had generalized edema, respiratory distress, and hepatomegaly. COVID-19 RT-PCR test result was positive. Her SPO2 was 96% at room air. The patient’s blood culture result was positive. She was transferred to the PICU due to respiratory distress. On the first day of admission, she received vancomycin and cefotaxime. On the third day, meropenem, IVIG, remdesivir, and dexamethasone were ordered. With initial treatment, the patient’s condition improved somewhat, and she was transferred to the ward but returned to the PICU with a worsening of acidosis. On the fifth day of hospitalization, she was intubated due to severe respiratory distress and exacerbation of acidosis. Due to hyperglycemia (BS=240 mg/dL), insulin was administered. Heart failure occurred due to oliguria and exacerbation of acidosis, and intravenous inotropic agents were ordered. The patient’s SPO2 level declined suddenly, and she died after 5 days of hospitalization due to bradycardia and cardiac arrest.
Case 8
A 4-month-old infant with respiratory distress was referred to the ED from another hospital. On initial evaluation, the patient had a pale appearance. He had tachypnea and intercostal retraction. During the physical examination, palpable hepatosplenomegaly and hepatomegaly were detected. His SPO2 was 92%, which increased to 100% with oxygen through an oxygen hood. COVID-19 RT-PCR result was positive. The patient had anemia (Hb=7.9 mg/dL) and thrombocytopenia. Urine and stool cultures were positive (Klebsiella), and he had hematuria. He was under treatment for hemophagocytic lymphohistiocytosis (HLH) from 3 months of age. On the first day of admission, he received ciprofloxacin, amikacin, vancomycin, and meropenem. The patient was transferred to the PICU due to respiratory distress and became intubated on the second day. Epinephrine, IVIG, dexamethasone, and enoxaparin were ordered. He received remdesivir for 5 days. After improvement, the patient was transferred to the hematology department, but due to sepsis and respiratory distress, he returned to the PICU again. Due to tachypnea and a barely palpable pulse, intravenous inotropic agents (milrinone) were added to his medication. The patient’s fever persisted and could not be controlled (recurrent fever), and ferritin increased from 27000 to 73000. A second dose of IVIG was injected. Due to respiratory distress, he was re-intubated. Following bradycardia and hypoxia, he died after 71 days of hospitalization due to cardiac arrest.
Case 9
A 14-year-old girl with a background of thalassemia intermediate (hematologic anemia) and glucose-6-phosphate dehydrogenase (G6PD) deficiency was refered to the ED with a history of weakness, lethargy, loss of appetite, and dry cough for 6 days, in addition to high-grade fever since 10 days ago. During the physical examination, she was icteric, and rales were heard in the lung auscultation. She had dyspnea with inter-costal retraction. Her SPO2 was 70%, which increased to 99% with oxygen through the mask and reservoir bag. COVID-19 RT-PCR test result was positive. Her weight was 53 kg, which was above the 97th percentile. On the first day, he received vancomycin, clindamycin, meropenem, dexamethasone, and enoxaparin. Due to ground-glass opacity observed in the portable chest x-ray, remdesivir was ordered. According to echocardiography results, intravenous inotropic agents were administered. Methylprednisolone (three doses, 52 g every 6-12 hours) and IVIG (52 g every 12 hours) were ordered as the patient’s problems persisted. Due to hyperglycemia (BS=282 mg/dL), insulin was prescribed. Tocilizumab was given to the patient, too. The general condition deteriorated on day 8 of her stay, and she was intubated for 10 days. She died on the 17th day of hospitalization due to multiple organ involvement and, finally, ARDS.
Additional clinical characteristics, laboratory, and radiologic findings of dead patients are shown in Table 1.
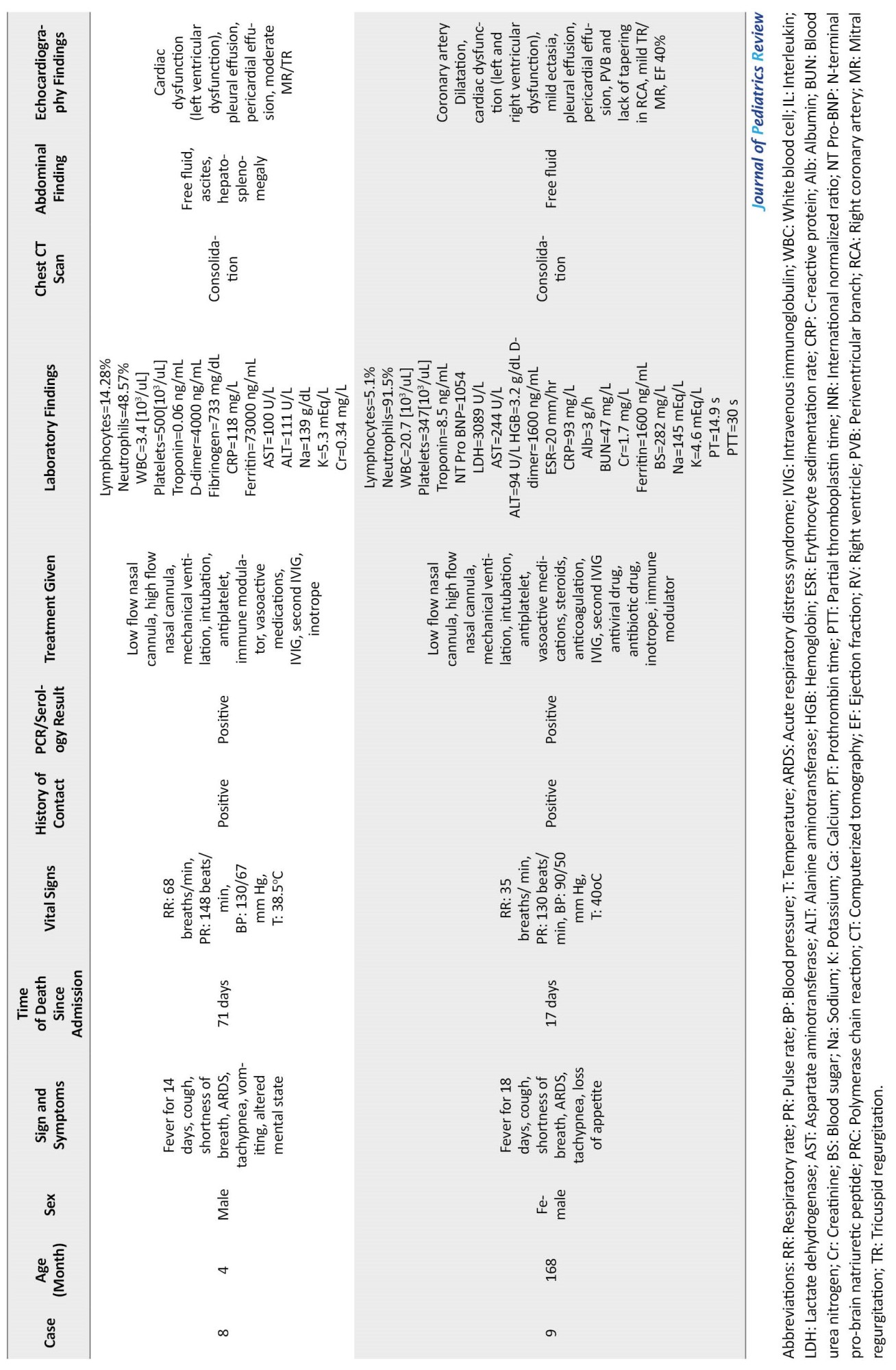
Discussion and literature review
The novel coronavirus disease-2019 (COVID-19) was identified in December 2019 in China, and at that time, the World Health Organization (WHO) declared its outbreak [1]. This disease causes morbidity and mortality in all age groups [4]. As of May 2020, physicians worldwide announced hospitalizations of children who developed fever and MIS-C [5, 8]. The Centers for Disease Control and Prevention (CDC) and WHO provided case definitions of MIS-C.
Criteria of MIS-C
Based on the CDC case definition, the following criteria were considered to define MIS-C patients: 1) Age <21 years; 2) Fever ≥38.0°C (100, 4°F) for >24 hours or report of subjective fever lasting >24 hours; 3) A history of current SARS-CoV-2 infection by positive RT-PCR, serology, or antigen test or contact with a suspected or confirmed COVID-19 case within 4 weeks before the onset of symptoms; 3) Signs and symptoms of multisystem (≥2) organ involvement, including cardiovascular (e.g. shock, elevated troponin), renal, gastrointestinal (e.g. abdominal pain, vomiting), hematologic (e.g. coagulopathy), neurologic (e.g. seizure, meningitis) and respiratory (e.g. pneumonia, pulmonary embolism); 4) Positive inflammatory markers, including elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), D-dimers, ferritin, interleukin-6 (IL-6), elevated neutrophils, low albumin; and 5) No other reasonable diagnosis.
Prevalence and mortality of MIS-C patients
The patients who meet CDC criteria and fulfill complete or partial criteria for Kawasaki disease (KD) should be considered MIS-C and reported. Importantly, MIS-C should be regarded in any pediatric death with evidence of SARS-CoV-2 infection [12]. As of November 2022, 9139 cases of MIS-C, including 74 deaths, were registered in the United States [13].
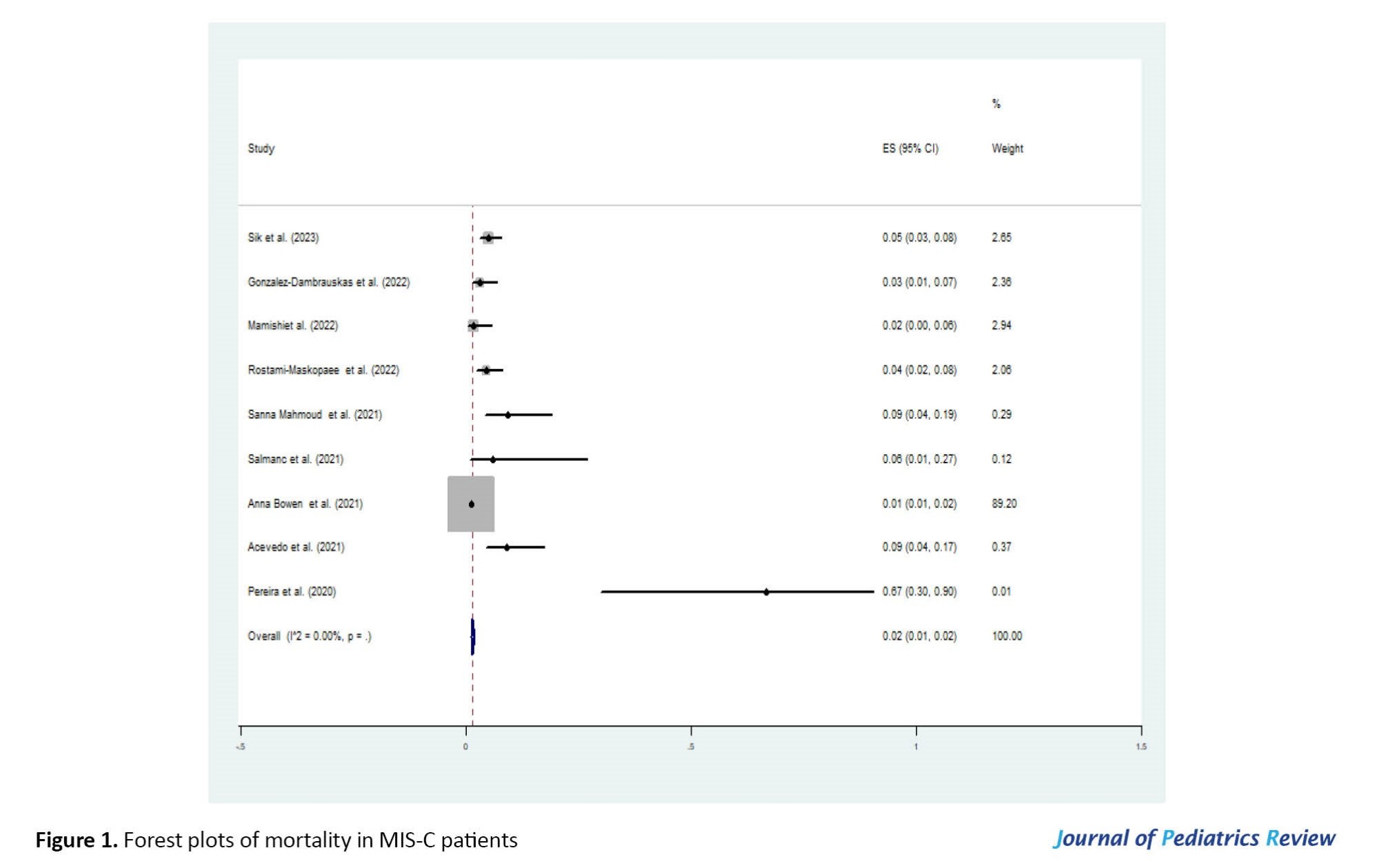
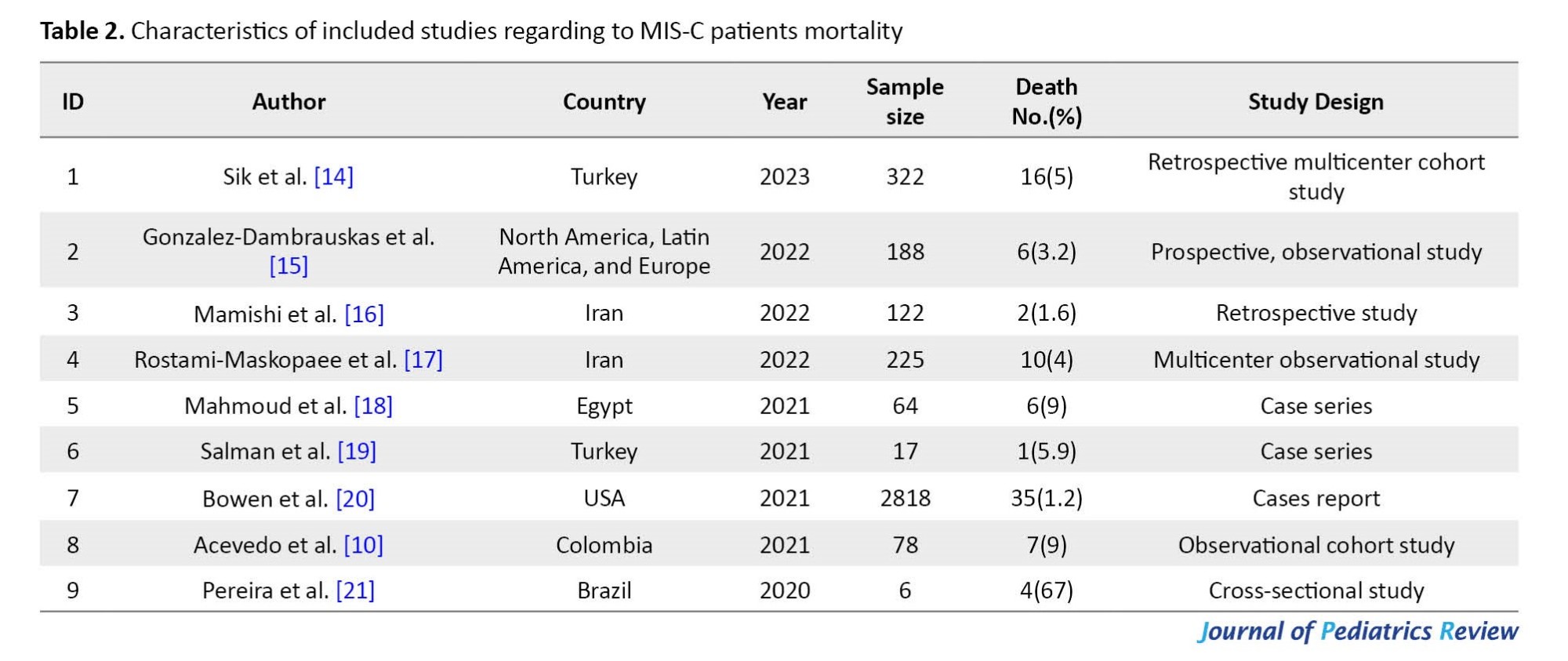
According to our literature review, numerous studies have been published internationally on MIS-C. Still, a few studies evaluate patients with severe diseases admitted to the PICU and died [10, 14-21]. The pooled mortality estimate in included studies was 0.02 (95% CI, 0.01%-0.02%) (Table 2, Figure 1).
At the time of this study, reliable evidence of cases of MIS-C and mortality is not published in Iran.
The risk factors of mortality
Several studies have found that diverse factors, including age, gender, number of organ involvement, comorbidities, the latency of diagnosis or treatment, and lack of drugs, may potentially affect the prognosis of MIS-C [17, 20].
Treatment of MIS-C
Up to now, evidence-based guideline for treating MIS-C patients is lacking. There are different treatment protocols for these patients, including vasoactive medications, steroids, immune modulators, antiplatelet, anticoagulation, and IVIG, which is usually best practice by the clinician based on the severity of the disease and the type of clinical manifestations that the patient presents.
Antibiotics are given to all patients due to nosocomial infections and duration of hospitalization. Antibiotics are discontinued when the diagnosis of MIS-C is made. In the era of COVID-19, there is a challenge for clinicians who must decide whether to start, continue, or stop antimicrobial therapy. Still, because MIS-C symptoms overlap with other life-threatening conditions, antimicrobial drugs are the primary therapy [22].
Discussion
In this case series study, we describe the clinical manifestations and treatment of MIS-C patients following COVID-19 who died in selected referral hospitals. We used the CDC checklist to identify children under 21 years who met the criteria of MIS-C. Of 225 MIS-C patients admitted to the hospital, 9 died (Table 3).
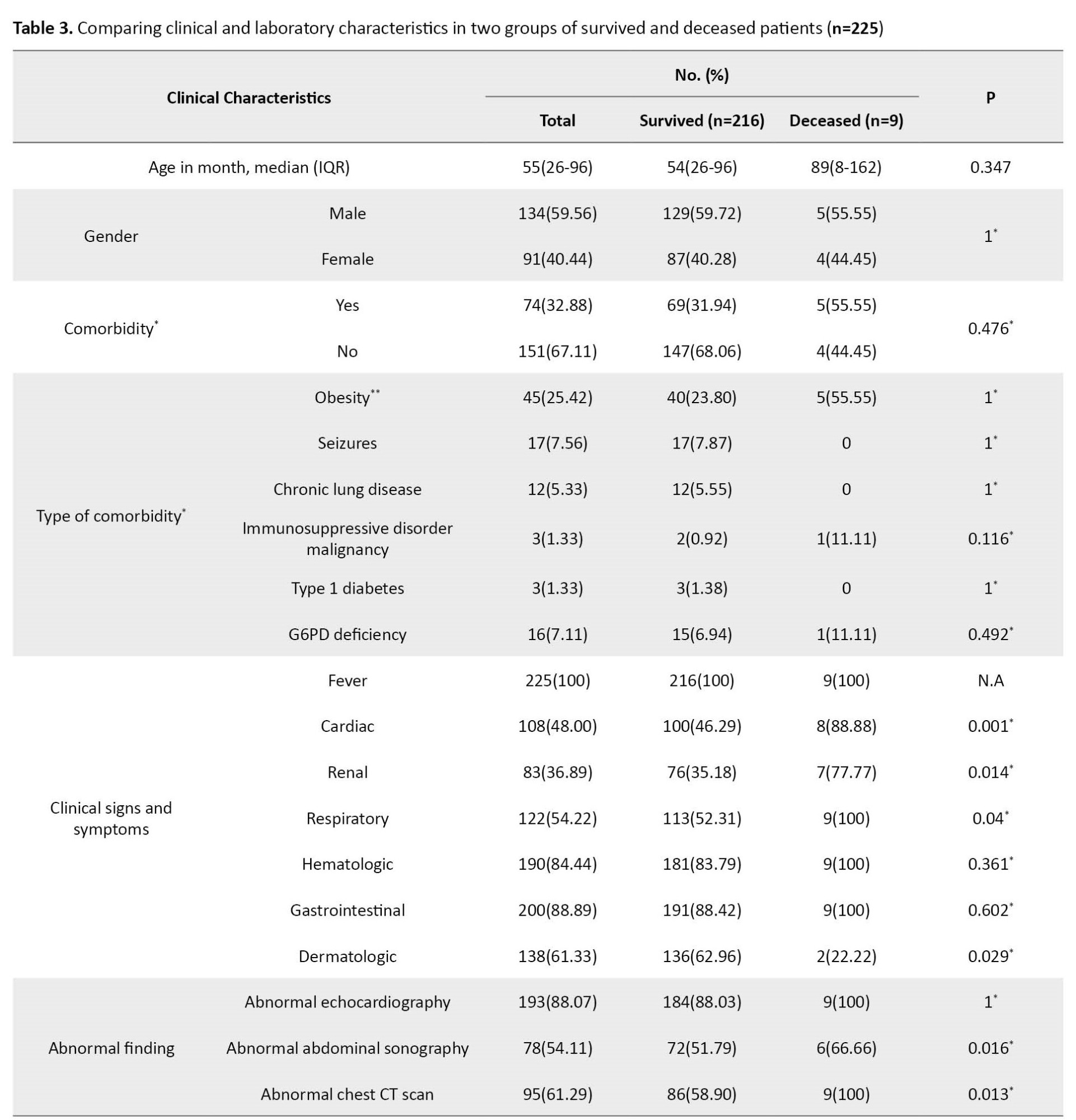
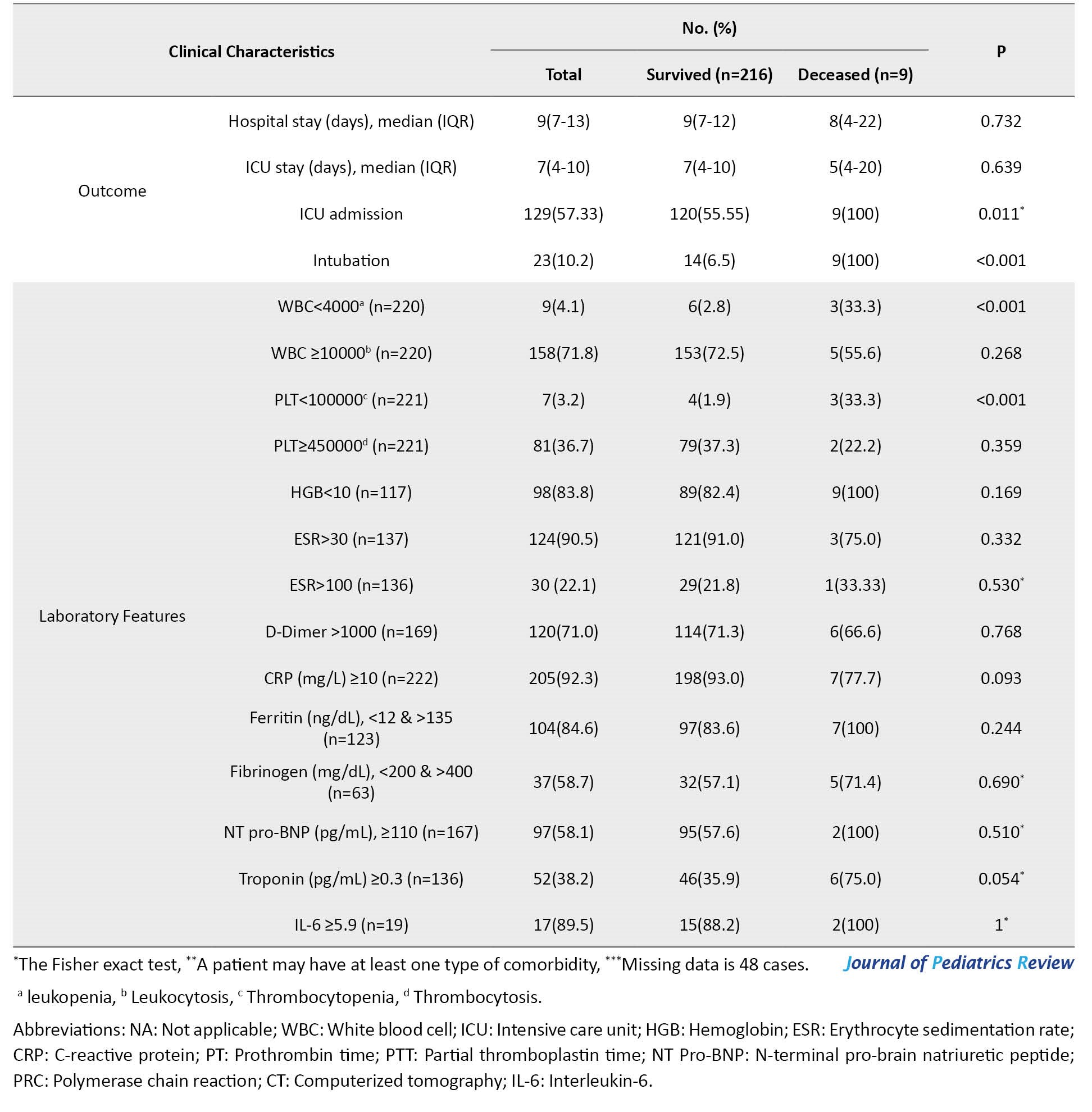
Persistent fever with respiratory, cardiac, and gastrointestinal symptoms was present in most children with MIS-C. This finding is consistent with other studies [10, 23, 24]. However, the dermatologic manifestation was observed in two patients.
Fever was present in all patients, and the mean fever duration before hospitalization was 6 days, similar to Pereira’s study [21]. In the study by Acevedo [10], the number of febrile days before diagnosis was 5 days, which is less than our study. In the COVID-19 pandemic, where the disease presents with different symptoms at each peak, children’s symptoms, especially fever, should be considered. Although many studies have confirmed that fever is a common symptom of COVID-19, unfortunately, decedent patients had a fever for a long time and were referred late. We mentioned that the late presentation of patients likely contributed to severe complications and ineffective treatment.
The cardiac, respiratory, renal, and liver were our study’s most common organ failures. Still, the most frequent cause of death in our cases was cardiac failure, and it is consistent with other studies [20, 25]. All patients had acute kidney injury, and 4 patients had renal failure during treatment.
Six patients were transferred directly to the PICU, and the rest were transferred to the PICU due to worsening symptoms during treatment. Of them, 5 patients were referred from another hospital or center. Initially, supportive care was taken considering the different clinical manifestations of the patients. In addition, all patients were treated with antibiotics for hospital-acquired infection on the first day of hospitalization. The antibiotic was discontinued as soon as the MIS-C diagnosis was established. IVIG and steroids were administered to all patients. Previous studies have reported that most patients receive IVIG and corticosteroids as the main treatment [10, 26-28]. Also, two children received tocilizumab.
In the current case series, 5 patients were male, similar to the Santos and Brizuela studies; MIS-C was mostly reported in males [23, 29]. As cases of MIS-C were emerging, the different clinical effects of COVID-19 on gender were still unclear. The gender difference in the incidence, severity, and mortality of patients with MIS-C should be clarified by large studies.
By calculating the obesity index according to the WHO criteria, 5 patients were obese. Consistent with other studies, obesity was the most prevalent comorbidity [10, 23, 30]. A systematic and meta-analysis study found that obese individuals were more likely to be hospitalized, 74% to be admitted to the ICU, and 48% to die [31]. Since obesity affects health status through various mechanisms, it should be considered a significant factor for early hospitalization and intensive care during the COVID-19 pandemic. Of 9 patients, 3 had other comorbidities, including ALL, HLH, and thalassemia intermediate.
Like other studies, our patients had abnormal echocardiographic results [25, 28]. Cardiac dysfunction and coronary dilatation were observed in 8 and 4 children, respectively.
Regarding biochemistry findings, significant increases in CRP, ESR, creatine kinase-MB (CK-MB), IL-6, D-dimer, and lactate dehydrogenase (LDH) levels were observed in our patients, similar to other studies [27, 32].
Conclusions
Cardiac, respiratory, liver, and renal involvements were the most common organ failure in our cases, but cardiopulmonary failure was the main cause of death. Early diagnosis and management of MIS-C are necessary to prevent severe complications and death.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Mazandaran University of Medical Sciences approved the study protocol (Code: IR.MAZUMS.REC.1401.145). Informed consent for publication was obtained from their parents or guardians.
Funding
This study was funded by the Deputy of Research and Technology of Mazandaran University of Medical Sciences (Code: 14126), and the Vice-Chancellor of Research at Mazandaran University of Medical Sciences supported data collection for this study
Authors contributions
Conceptualization, study design, supervision, review and editing: Mohammad Sadegh Rezai; Data curation, manuscript preparation, and editing of the manuscript: Fereshteh Rostami-Maskopaee; Data curation: Mohammad Reza Navaeifar, Azin Hajialibeig, Maedeh Gooran, Behzad Haghighi Aski, Ali Manafi Anari, Eslam Shorafa, and Seyedeh Narjes Abootalebi.
Final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank all patient parents or guardians for providing the case details and the Student Research Committee of Mazandaran University of Medical Sciences.
References
COVID-19 causes morbidity and mortality in all age groups [1]. The symptoms and signs of the disease are milder in children than in adults [2, 3]. The most common clinical presentations of COVID-19 in children are fever and cough, but some children become critically ill and require intensive care [4-7]. As of May 2020, physicians announced the appearance of a new presentation of SARS-CoV-2 infection, known as multisystem inflammatory syndrome-children (MIS-C), which behaves more aggressively, causing mortality in the pediatric population [5, 8]. MIS-C is characterized by fever, laboratory evidence of inflammation, multisystem organ involvement, severe illness, and SARS-CoV-2 infection or exposure [9, 10].
Our knowledge about MIS-C patients’ characteristics associated with intensive care unit (ICU) admission and mortality is still limited [11]. Hence, we performed this case series study to present the clinical manifestations and treatment of MIS-C patients following COVID-19 who died in different geographical regions of Iran (Tehran, Fars, and Mazandaran provinces) from March 2020 to September 2021.
Case Presentation
Case 1
A 7-year-old boy with a history of fever in the past 8 days and cough, headache, and diarrhea was referred to the hospital emergency department (ED). On admission, he had respiratory distress with a toxic appearance. His oxygen saturation (SPO2) was 85%, which increased to 92% with oxygen through the mask and reservoir bag. The patient was transferred to PICU with an initial diagnosis of critical COVID-19. His weight was 43 kg, which was above the 97th percentile. The urine and blood culture results were negative. COVID-19 reverse transcription polymerase chain reaction (RT-PCR) test result was positive. Due to nosocomial infections, he received vancomycin, meropenem, enoxaparin, and dexamethasone on the first day of admission. Intravenous immunoglobulin (IVIG) and aspirin were administered. According to the lung CT scan and positive COVID-19 RT-PCR results, remdesivir was ordered. Intravenous inotropic agents were administered on the fourth day due to gallop and myocarditis in echocardiography results. On the fifth day, the patient was intubated because of severe hypoxia (SPO2 decline to 70%) and respiratory failure. Due to hyperglycemia (blood sugar [BS]=348 mg/dL), insulin was ordered. He underwent dialysis because of a glomerular filtration rate (GFR) <30 mL/min. Remdesivir was discontinued, and medications were adjusted based on GFR and dialysis. The patient’s condition was unstable, and he underwent dialysis several times. His general condition deteriorated, and he died after 28 days of hospitalization due to recurrent pulmonary hemorrhage and cardiac arrest.
Case 2
A 13-year-old girl was admitted with a history of high-grade fever in the last 4 days and periorbital (orbital) edema. She had shortness of breath 2 days before admission and was visited by her family physician. With supportive care, the patient’s condition worsened, and referred to the ED with fever, conjunctivitis, nausea, vomiting, diarrhea, dyspnea, and skin rash. At the initial examination, the patient had tachycardia and tachypnea. The RT-PCR test for COVID-19 was negative, but she had a history of COVID-19 in her family. Her SPO2 was 85%, which increased to 98% with oxygen through the mask and reservoir bag. The patient received vancomycin, IVIG, and pulse methylprednisolone on the first day of admission. On the second day, meropenem was ordered. Due to hyperglycemia (BS=249 mg/dL), insulin was prescribed to correct hypokalemia. The patient was intubated on the third day because of a severe decline in SPO2 and respiratory failure (respiratory rate [RR]=22/min). On the fourth day, intravenous inotropic agents were ordered due to gallop in echocardiography results. Still, she did not respond to the treatment and died on the 5th day following hospitalization due to heart failure and shock.
Case 3
A 7-year-old boy was referred for fever and chills in the past 3 days, abdominal pain 2 days before admission, shortness of breath, lethargy, vomiting, and cough. He was hospitalized for 1 day. Despite supportive care, the patient’s condition worsened, and he was transferred to another the hospital’s ED. On physical examination, he had a fever, bilateral conjunctivitis, and skin rash. The patient had tachycardia and tachypnea. His weight was 35 kg, which was above the 97th percentile. The history of COVID-19 in family members was positive (father), and the COVID-19 RT-PCR test was negative. His SPO2 was 88%, which increased to 98% after using the mask and reservoir bag. The patient immediately became intubated. On the first day of admission, he received vancomycin, clindamycin, pulse methylprednisolone (350 mg), and IVIG (1 vial). The patient had fresh blood-stained secretion of the endotracheal tube (ETT). On the second day, insulin was injected due to hyperglycemia (BS=304 mg/dL). Because of the gallop in echocardiography, intravenous inotropic agents were ordered. The pulse methylprednisolone dose (700 mg) was increased, and the second dose of IVIG was injected. Tocilizumab was given to the patient. Hypoxemia (SPO2=80%) occurred on the last day, and cyanosis was evident in the extremities. He died after two days of hospitalization due to bradycardia (40-43 beats/min), ARDS (acute respiratory distress syndrome), and DIC (disseminated intravascular coagulation).
Case 4
A 15-year-old boy undergoing chemotherapy and granulocyte colony-stimulating factor due to acute lymphocytic leukemia (ALL) was referred to the ED for vomiting, sore throat, and diarrhea. The patient was tachypneic and pale with an ill and toxic appearance. Rale was heard in the pulmonary auscultation. He had orthostatic hypotension. COVID-19 RT-PCR test result was positive. His weight was 70 kg which was above the 97th percentile. Because of ALL, he was under chemotherapy for 2 years before admission. His SPO2 was 70%, which increased to 97% with oxygen through the mask and reservoir bag. The patient had fresh blood-stained secretion of ETT. He was intubated on the first day of admission and received vancomycin, meropenem, co-trimoxazole, amikacin, methylprednisolone (60 mg every 8 hours), and IVIG (50 g). According to the echocardiography results, intravenous inotropic agents were ordered. After 4 days of hospitalization, his ejection fraction (EF) declined from 78% to 47%, asystole and arrhythmia developed, and he died eventually.
Case 5
A 10-year-old girl was referred with a history of high fever for the last 7 days, abdominal pain, and cough before admission. She had lower extremity edema for 10 days. At hospitalization, the patient’s liver enzymes increased (hepatic encephalopathy). Due to reduced consciousness and drowsiness, transferring her to an equipped liver transplant center was necessary. Due to liver and brain involvement and a positive COVID-19 RT-PCR test, she was admitted to the pediatric intensive care unit (PICU) with an MIS-C diagnosis. At the time of admission, the patient was intubated. Her SPO2 was 80%, which increased to 95% with oxygen through the nasal cannula. Her brain MRI showed cerebral involvement following COVID-19. Due to a history of partial seizures, she received carbamazepine, vancomycin, cefotaxime, meropenem, pulse methylprednisolone (for 3 days), and IVIG were started for the patient. He had several seizures, so diazepam was ordered. According to echocardiography results, intravenous inotropic agents were added to her drugs. During the patient’s management, SPO2 decreased (69%). A second dose of IVIG was injected. The patient became hypotensive and bradycardic. Cardiopulmonary resuscitation (CPR) was performed, but she died on the 21st day of hospitalization due to hepatic encephalopathy, pulmonary edema, and cardiac arrest.
Case 6
A 1-year-old boy was referred to the ED with a fever in the 4 days ago, in addition to cough, diarrhea, and seizure. The night before hospitalization, he had a tonic attack without cyanosis (about ten minutes), which was controlled with diazepam and repeated after 3 to 4 minutes. He was transferred to the PICU with a status epilepsy diagnosis. He had a toxic appearance at admission time and was not conscious. COVID-19 RT-PCR test result was positive. His weight was 10 kg, which was above the 97th percentile. His SPO2 was 75%, which increased to 100% with oxygen through the nasal cannula. According to the MIS-C diagnosis, ciprofloxacin, meropenem, IVIG, pulse methyl prednisone, and enoxaparin were initiated. Seizures were poorly controlled on the second day of his stay, and midazolam and phenytoin were loaded. Remdesivir was added to the patient’s medication. Due to hyperglycemia (BS=300 mg/dL), insulin was ordered. On the third day of hospitalization, the patient’s consciousness level decreased, and he was intubated. Due to unstable hemodynamics, intravenous inotropic agents were administered. After 4 days of hospitalization, the EF of the patient reduced to 40%. Bradycardia and a severe decline in SPO2 occurred, and he died eventually.
Case 7
A 5-month-old female infant was referred to the ED with dyspnea, restlessness, and vomiting. The patient had a toxic appearance. On physical examination, she had generalized edema, respiratory distress, and hepatomegaly. COVID-19 RT-PCR test result was positive. Her SPO2 was 96% at room air. The patient’s blood culture result was positive. She was transferred to the PICU due to respiratory distress. On the first day of admission, she received vancomycin and cefotaxime. On the third day, meropenem, IVIG, remdesivir, and dexamethasone were ordered. With initial treatment, the patient’s condition improved somewhat, and she was transferred to the ward but returned to the PICU with a worsening of acidosis. On the fifth day of hospitalization, she was intubated due to severe respiratory distress and exacerbation of acidosis. Due to hyperglycemia (BS=240 mg/dL), insulin was administered. Heart failure occurred due to oliguria and exacerbation of acidosis, and intravenous inotropic agents were ordered. The patient’s SPO2 level declined suddenly, and she died after 5 days of hospitalization due to bradycardia and cardiac arrest.
Case 8
A 4-month-old infant with respiratory distress was referred to the ED from another hospital. On initial evaluation, the patient had a pale appearance. He had tachypnea and intercostal retraction. During the physical examination, palpable hepatosplenomegaly and hepatomegaly were detected. His SPO2 was 92%, which increased to 100% with oxygen through an oxygen hood. COVID-19 RT-PCR result was positive. The patient had anemia (Hb=7.9 mg/dL) and thrombocytopenia. Urine and stool cultures were positive (Klebsiella), and he had hematuria. He was under treatment for hemophagocytic lymphohistiocytosis (HLH) from 3 months of age. On the first day of admission, he received ciprofloxacin, amikacin, vancomycin, and meropenem. The patient was transferred to the PICU due to respiratory distress and became intubated on the second day. Epinephrine, IVIG, dexamethasone, and enoxaparin were ordered. He received remdesivir for 5 days. After improvement, the patient was transferred to the hematology department, but due to sepsis and respiratory distress, he returned to the PICU again. Due to tachypnea and a barely palpable pulse, intravenous inotropic agents (milrinone) were added to his medication. The patient’s fever persisted and could not be controlled (recurrent fever), and ferritin increased from 27000 to 73000. A second dose of IVIG was injected. Due to respiratory distress, he was re-intubated. Following bradycardia and hypoxia, he died after 71 days of hospitalization due to cardiac arrest.
Case 9
A 14-year-old girl with a background of thalassemia intermediate (hematologic anemia) and glucose-6-phosphate dehydrogenase (G6PD) deficiency was refered to the ED with a history of weakness, lethargy, loss of appetite, and dry cough for 6 days, in addition to high-grade fever since 10 days ago. During the physical examination, she was icteric, and rales were heard in the lung auscultation. She had dyspnea with inter-costal retraction. Her SPO2 was 70%, which increased to 99% with oxygen through the mask and reservoir bag. COVID-19 RT-PCR test result was positive. Her weight was 53 kg, which was above the 97th percentile. On the first day, he received vancomycin, clindamycin, meropenem, dexamethasone, and enoxaparin. Due to ground-glass opacity observed in the portable chest x-ray, remdesivir was ordered. According to echocardiography results, intravenous inotropic agents were administered. Methylprednisolone (three doses, 52 g every 6-12 hours) and IVIG (52 g every 12 hours) were ordered as the patient’s problems persisted. Due to hyperglycemia (BS=282 mg/dL), insulin was prescribed. Tocilizumab was given to the patient, too. The general condition deteriorated on day 8 of her stay, and she was intubated for 10 days. She died on the 17th day of hospitalization due to multiple organ involvement and, finally, ARDS.
Additional clinical characteristics, laboratory, and radiologic findings of dead patients are shown in Table 1.




Discussion and literature review
The novel coronavirus disease-2019 (COVID-19) was identified in December 2019 in China, and at that time, the World Health Organization (WHO) declared its outbreak [1]. This disease causes morbidity and mortality in all age groups [4]. As of May 2020, physicians worldwide announced hospitalizations of children who developed fever and MIS-C [5, 8]. The Centers for Disease Control and Prevention (CDC) and WHO provided case definitions of MIS-C.
Criteria of MIS-C
Based on the CDC case definition, the following criteria were considered to define MIS-C patients: 1) Age <21 years; 2) Fever ≥38.0°C (100, 4°F) for >24 hours or report of subjective fever lasting >24 hours; 3) A history of current SARS-CoV-2 infection by positive RT-PCR, serology, or antigen test or contact with a suspected or confirmed COVID-19 case within 4 weeks before the onset of symptoms; 3) Signs and symptoms of multisystem (≥2) organ involvement, including cardiovascular (e.g. shock, elevated troponin), renal, gastrointestinal (e.g. abdominal pain, vomiting), hematologic (e.g. coagulopathy), neurologic (e.g. seizure, meningitis) and respiratory (e.g. pneumonia, pulmonary embolism); 4) Positive inflammatory markers, including elevated C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), D-dimers, ferritin, interleukin-6 (IL-6), elevated neutrophils, low albumin; and 5) No other reasonable diagnosis.
Prevalence and mortality of MIS-C patients
The patients who meet CDC criteria and fulfill complete or partial criteria for Kawasaki disease (KD) should be considered MIS-C and reported. Importantly, MIS-C should be regarded in any pediatric death with evidence of SARS-CoV-2 infection [12]. As of November 2022, 9139 cases of MIS-C, including 74 deaths, were registered in the United States [13].
According to our literature review, numerous studies have been published internationally on MIS-C. Still, a few studies evaluate patients with severe diseases admitted to the PICU and died [10, 14-21]. The pooled mortality estimate in included studies was 0.02 (95% CI, 0.01%-0.02%) (Table 2, Figure 1).

At the time of this study, reliable evidence of cases of MIS-C and mortality is not published in Iran.
The risk factors of mortality
Several studies have found that diverse factors, including age, gender, number of organ involvement, comorbidities, the latency of diagnosis or treatment, and lack of drugs, may potentially affect the prognosis of MIS-C [17, 20].
Treatment of MIS-C
Up to now, evidence-based guideline for treating MIS-C patients is lacking. There are different treatment protocols for these patients, including vasoactive medications, steroids, immune modulators, antiplatelet, anticoagulation, and IVIG, which is usually best practice by the clinician based on the severity of the disease and the type of clinical manifestations that the patient presents.
Antibiotics are given to all patients due to nosocomial infections and duration of hospitalization. Antibiotics are discontinued when the diagnosis of MIS-C is made. In the era of COVID-19, there is a challenge for clinicians who must decide whether to start, continue, or stop antimicrobial therapy. Still, because MIS-C symptoms overlap with other life-threatening conditions, antimicrobial drugs are the primary therapy [22].
Discussion
In this case series study, we describe the clinical manifestations and treatment of MIS-C patients following COVID-19 who died in selected referral hospitals. We used the CDC checklist to identify children under 21 years who met the criteria of MIS-C. Of 225 MIS-C patients admitted to the hospital, 9 died (Table 3).


Persistent fever with respiratory, cardiac, and gastrointestinal symptoms was present in most children with MIS-C. This finding is consistent with other studies [10, 23, 24]. However, the dermatologic manifestation was observed in two patients.
Fever was present in all patients, and the mean fever duration before hospitalization was 6 days, similar to Pereira’s study [21]. In the study by Acevedo [10], the number of febrile days before diagnosis was 5 days, which is less than our study. In the COVID-19 pandemic, where the disease presents with different symptoms at each peak, children’s symptoms, especially fever, should be considered. Although many studies have confirmed that fever is a common symptom of COVID-19, unfortunately, decedent patients had a fever for a long time and were referred late. We mentioned that the late presentation of patients likely contributed to severe complications and ineffective treatment.
The cardiac, respiratory, renal, and liver were our study’s most common organ failures. Still, the most frequent cause of death in our cases was cardiac failure, and it is consistent with other studies [20, 25]. All patients had acute kidney injury, and 4 patients had renal failure during treatment.
Six patients were transferred directly to the PICU, and the rest were transferred to the PICU due to worsening symptoms during treatment. Of them, 5 patients were referred from another hospital or center. Initially, supportive care was taken considering the different clinical manifestations of the patients. In addition, all patients were treated with antibiotics for hospital-acquired infection on the first day of hospitalization. The antibiotic was discontinued as soon as the MIS-C diagnosis was established. IVIG and steroids were administered to all patients. Previous studies have reported that most patients receive IVIG and corticosteroids as the main treatment [10, 26-28]. Also, two children received tocilizumab.
In the current case series, 5 patients were male, similar to the Santos and Brizuela studies; MIS-C was mostly reported in males [23, 29]. As cases of MIS-C were emerging, the different clinical effects of COVID-19 on gender were still unclear. The gender difference in the incidence, severity, and mortality of patients with MIS-C should be clarified by large studies.
By calculating the obesity index according to the WHO criteria, 5 patients were obese. Consistent with other studies, obesity was the most prevalent comorbidity [10, 23, 30]. A systematic and meta-analysis study found that obese individuals were more likely to be hospitalized, 74% to be admitted to the ICU, and 48% to die [31]. Since obesity affects health status through various mechanisms, it should be considered a significant factor for early hospitalization and intensive care during the COVID-19 pandemic. Of 9 patients, 3 had other comorbidities, including ALL, HLH, and thalassemia intermediate.
Like other studies, our patients had abnormal echocardiographic results [25, 28]. Cardiac dysfunction and coronary dilatation were observed in 8 and 4 children, respectively.
Regarding biochemistry findings, significant increases in CRP, ESR, creatine kinase-MB (CK-MB), IL-6, D-dimer, and lactate dehydrogenase (LDH) levels were observed in our patients, similar to other studies [27, 32].
Conclusions
Cardiac, respiratory, liver, and renal involvements were the most common organ failure in our cases, but cardiopulmonary failure was the main cause of death. Early diagnosis and management of MIS-C are necessary to prevent severe complications and death.
Ethical Considerations
Compliance with ethical guidelines
The Ethics Committee of Mazandaran University of Medical Sciences approved the study protocol (Code: IR.MAZUMS.REC.1401.145). Informed consent for publication was obtained from their parents or guardians.
Funding
This study was funded by the Deputy of Research and Technology of Mazandaran University of Medical Sciences (Code: 14126), and the Vice-Chancellor of Research at Mazandaran University of Medical Sciences supported data collection for this study
Authors contributions
Conceptualization, study design, supervision, review and editing: Mohammad Sadegh Rezai; Data curation, manuscript preparation, and editing of the manuscript: Fereshteh Rostami-Maskopaee; Data curation: Mohammad Reza Navaeifar, Azin Hajialibeig, Maedeh Gooran, Behzad Haghighi Aski, Ali Manafi Anari, Eslam Shorafa, and Seyedeh Narjes Abootalebi.
Final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank all patient parents or guardians for providing the case details and the Student Research Committee of Mazandaran University of Medical Sciences.
References
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020; 91(1):157-60. [PMID]
- Shahbaznejad L, Navaeifar MR, Abbaskhanian A, Hosseinzadeh F, Rahimzadeh G, Rezai MS. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. 2020; 20(1):513. [DOI:10.1186/s12887-020-02415-z] [PMID] [PMCID]
- Christophers B, Gallo Marin B, Oliva R, Powell WT, Savage TJ, Michelow IC. Trends in clinical presentation of children with COVID-19: A systematic review of individual participant data. Pediatr Res. 2022; 91(3):494-501. [DOI:10.1038/s41390-020-01161-3] [PMID] [PMCID]
- Rafferty MS, Burrows H, Joseph JP, Leveille J, Nihtianova S, Amirian ES. Multisystem inflammatory syndrome in children (MIS-C) and the coronavirus pandemic: Current knowledge and implications for public health. J Infect Public Health. 2021; 14(4):484-94. [DOI:10.1016/j.jiph.2021.01.008] [PMID] [PMCID]
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020; 145(6):e20200702. [DOI:10.1542/peds.2020-0702] [PMID]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708-20. [DOI:10.1056/NEJMoa2002032] [PMID] [PMCID]
- Karimi A, Tabatabaei SR, Rajabnejad M, Pourmoghaddas Z, Rahimi H, Armin S, et al. An algorithmic approach to diagnosis and treatment of coronavirus disease 2019 (COVID-19) in children: Iranian expert’s consensus statement.Arch Pediatr Infect Dis. 2020; 8(2):e102400. [DOI:10.5812/pedinfect.102400]
- Jain S, Kashyap A, Singh A. Spectrum of Multisystem Inflammatory Syndrome in Children (MIS-C)-a report of three cases. SN Compr Clin Med. 2021; 3(12):2635-9. [DOI:10.1007/s42399-021-01057-1] [PMID] [PMCID]
- Whittaker E, Bamford A, Kenny J, Kaforou M, Jones CE, Shah P, et al. Clinical characteristics of 58 children with a pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2. JAMA. 2020; 324(3):259-69. [DOI:10.1001/jama.2020.10369] [PMID] [PMCID]
- Acevedo L, Piñeres-Olave BE, Niño-Serna LF, Vega LM, Gomez IJA, Chacón S, et al. Mortality and clinical characteristics of multisystem inflammatory syndrome in children (MIS-C) associated with covid-19 in critically ill patients: An observational multicenter study (MISCO study). BMC Pediatr. 2021; 21(1):516. [DOI:10.1186/s12887-021-02974-9] [PMID] [PMCID]
- Leoni MLG, Lombardelli L, Colombi D, Bignami EG, Pergolotti B, Repetti F, et al. Prediction of 28-day mortality in critically ill patients with COVID-19: Development and internal validation of a clinical prediction model. PLoS One. 2021; 16(7):e0254550. [DOI:10.1371/journal.pone.0254550] [PMID] [PMCID]
- Center for Disease Control and PREVENTION. Multisystem inflammatory syndrome (MIS). Atlanta: National Center for Immunization and Respiratory Diseases; 2020. [Link]
- Bean M. MIS-C cases by state [Internet]. 2021 [Updated 16 November 2021]. Available from: [Link].
- Sık G, Inamlık A, Akçay N, Kesici S, Aygun F, Kendırlı T, et al. Mortality risk factors among critically ill children with MIS-C in PICUs: A multicenter study. Pediatr Res. 2023; 94(2):730-7. [PMID]
- Gonzalez-Dambrauskas S, Vasquez-Hoyos P, Camporesi A, Cantillano EM, Dallefeld S, Dominguez-Rojas J, et al. Paediatric critical COVID-19 and mortality in a multinational prospective cohort. Lancet Reg Health Am. 2022; 12:100272. [DOI:10.1101/2021.08.20.21262122]
- Mamishi S, Olfat M, Pourakbari B, Eshaghi H, Abdolsalehi MR, Shahbabaie MA, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in children: Update and new insights from the second report of an Iranian referral hospital. Epidemiol Infect. 2022; 150:e179. [DOI:10.1017/S0950268822001522] [PMID] [PMCID]
- Rostami-Maskopaee F, Ladomenou F, Razavi-Amoli SK, Navaeifar MR, Hajialibeig A, Shahbaznejad L, et al. Clinical characteristics and outcomes of the multisystem inflammatory syndrome in children (MIS-C) following COVID-19 infection in Iran: A multicenter study. PLoS One. 2022; 17(9):e0274104. [DOI:10.1371/journal.pone.0274104] [PMID] [PMCID]
- Mahmoud S, Fouda EM, Kotby A, Ibrahim HM, Gamal M, El Gendy YG, et al. The “Golden Hours” algorithm for the management of the multisystem inflammatory syndrome in children (MIS-C). Glob Pediatr Health. 2021; 8:2333794X21990339. [DOI:10.1177/2333794X21990339] [PMID] [PMCID]
- Salman H, Aslan N, Akçam M, Arslan M, Akkuzu E, Yılmaz Keskin E, et al. COVID-19-associated multisystem inflammatory syndrome in children: Experiences of three centres in Turkey. Mod Rheumatol. 2022; 32(2):460-6. [DOI:10.1093/mr/roab042] [PMID] [PMCID]
- Bowen A, Miller AD, Zambrano LD, Wu MJ, Oster ME, Godfred-Cato S, et al. Demographic and clinical factors associated with death among persons< 21 years old with multisystem inflammatory syndrome in children-United States, February 2020-March 2021. Open Forum Infect Dis. 2021; 8(8):ofab388. [DOI:10.1093/ofid/ofab388] [PMID] [PMCID]
- Pereira MFB, Litvinov N, Farhat SCL, Eisencraft AP, Gibelli MABC, Carvalho WB, et al. Severe clinical spectrum with high mortality in pediatric patients with COVID-19 and multisystem inflammatory syndrome. Clinics. 2020; 75:e2209.[DOI:10.6061/clinics/2020/e2209] [PMID] [PMCID]
- Toczyłowski K, Łasecka-Zadrożna J, Pałyga-Bysiecka I, Ludwikowska KM, Okarska-Napierała M, Dudek N, et al. Use of broad-spectrum antibiotics in children diagnosed with multisystem inflammatory syndrome temporarily associated with SARS-CoV-2 infection in Poland: The MOIS-CoR study. Int J Infect Dis. 2022; 122:703-9. [DOI:10.1016/j.ijid.2022.07.021] [PMID] [PMCID]
- Santos MO, Gonçalves LC, Silva PAN, Moreira ALE, Ito CRM, Peixoto FAO, et al. Multisystem inflammatory syndrome (MIS-C): A systematic review and meta-analysis of clinical characteristics, treatment, and outcomes. J Pediatr. 2022; 98(4):338-49. [DOI:10.1016/j.jped.2021.08.006] [PMID] [PMCID]
- Lima-Setta F, Magalhães-Barbosa MC, Rodrigues-Santos G, Figueiredo EADN, Jacques ML, Zeitel RS, et al. Multisystem inflammatory syndrome in children (MIS-C) during SARS-CoV-2 pandemic in Brazil: A multicenter, prospective cohort study. J Pediatr. 2021; 97(3):354-61. [DOI:10.1016/j.jped.2020.10.008] [PMID] [PMCID]
- Blumfield E, Levin TL, Kurian J, Lee EY, Liszewski MC. Imaging findings in multisystem inflammatory syndrome in children (MIS-C) associated with coronavirus disease (COVID-19). AJR Am J Roentgenol. 2021; 216(2):507-17. [DOI:10.2214/AJR.20.24032] [PMID]
- Chiotos K, Bassiri H, Behrens EM, Blatz AM, Chang J, Diorio C, et al. Multisystem inflammatory syndrome in children during the coronavirus 2019 pandemic: A case series. J Pediatric Infect Dis Soc. 2020; 9(3):393-8. [DOI:10.1093/jpids/piaa069] [PMID] [PMCID]
- Kaushik A, Gupta S, Sood M, Sharma S, Verma S. A systematic review of multisystem inflammatory syndrome in children associated with SARS-CoV-2 infection. Pediatr Infect Dis J. 2020; 39(11):e340-6. [DOI:10.1097/INF.0000000000002888] [PMID]
- Mamishi S, Movahedi Z, Mohammadi M, Ziaee V, Khodabandeh M, Abdolsalehi MR, et al. Multisystem inflammatory syndrome associated with SARS-CoV-2 infection in 45 children: A first report from Iran. Epidemiol Infect. 2020; 148:e196. [DOI:10.1017/S095026882000196X] [PMID] [PMCID]
- Brizuela M, Lenzi J, Ulloa-Gutiérrez R, Antúnez-Montes OY, Aida JAR, del Aguila O, et al. Influence of sex on disease severity in children with multisystem inflammatory syndrome and covid-19 in Latin America. Ital J Gend-Specif Med. 2021; 7(3):128-33. [DOI:10.1101/2021.02.07.21251212]
- Cai Q, Chen F, Wang T, Luo F, Liu X, Wu Q, et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care. 2020; 43(7):1392-8. [DOI:10.2337/dc20-0576] [PMID]
- Popkin BM, Du S, Green WD, Beck MA, Algaith T, Herbst CH, et al. Individuals with obesity and COVID-19: A global perspective on the epidemiology and biological relationships. Obes Rev. 2020; 21(11):e13128. [DOI:10.1111/obr.13128] [PMID] [PMCID]
- Yao Y, Cao J, Wang Q, Shi Q, Liu K, Luo Z, et al. D-dimer as a biomarker for disease severity and mortality in COVID-19 patients: A case control study. J Intensive Care. 2020; 8:49. [DOI:10.1186/s40560-020-00466-z] [PMID] [PMCID]
Type of Study: Case & Review |
Subject:
Pediatric infection disease
Received: 2023/04/30 | Accepted: 2023/06/12 | Published: 2023/07/1
Received: 2023/04/30 | Accepted: 2023/06/12 | Published: 2023/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |