Volume 11, Issue 3 (7-2023)
J. Pediatr. Rev 2023, 11(3): 193-208 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Taksande A, Jameel P Z, Rao R, Taksande B, Damke S. Prevalence of Cardiac Anomalies in Fetuses Diagnosed With Intracardiac Echogenic Foci: A Systematic Review and Meta-analysis. J. Pediatr. Rev 2023; 11 (3) :193-208
URL: http://jpr.mazums.ac.ir/article-1-536-en.html
URL: http://jpr.mazums.ac.ir/article-1-536-en.html
1- Department of Paediatrics, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), India. , amar.taksande@gmail.com
2- Department of Paediatrics, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), India.
3- Department of Medicine, Mahatma Gandhi Institute of Medical Sciences (MGIMS), Sewagram, India.
2- Department of Paediatrics, Jawaharlal Nehru Medical College, Datta Meghe Institute of Medical Sciences, Sawangi (Meghe), India.
3- Department of Medicine, Mahatma Gandhi Institute of Medical Sciences (MGIMS), Sewagram, India.
Full-Text [PDF 1352 kb]
(2342 Downloads)
| Abstract (HTML) (3340 Views)
Full-Text: (1997 Views)
Introduction
Intracardiac echogenic focus (ICEF) is demonstrated by ultrasound inside the fetal heart with a brightness comparable to that of the bone. It was first described by Schechter et al. [1] in 1987 in the left ventricle of a fetal heart, which they attributed to a thickening of the chordae. Usually, the focus has no acoustic shadow and is located near or within the papillary muscles. It moves in synchronization with the atrioventricular valves. It can be visualized in a 4 chambers view when performing a basic echocardiogram [2]. ICEF is most frequently visualized in the left ventricle and less commonly on the right side or both ventricles. While a single ICEF in the left ventricle is the most frequent finding, multiple foci may be seen often. These foci vary in size but are usually less than 6 mm [1, 2]. Echogenic foci suggest micro-calcification of the chordae and papillary muscles. Echogenic foci are increasingly associated with cardiac structural anomalies and chromosomal abnormalities.
When diagnosed, echogenic foci bring a clinical conundrum as their origin, and definitive significance are not yet completely understood. However, echogenic foci are increasingly considered markers for chromosomal abnormality and underlying structural cardiac defect in the fetus, especially in high-risk women [3, 4]. Therefore, the detection of ICEF warrants further investigations, such as fetal echocardiography. Fetal echocardiography has proven to be an invaluable tool for the early and accurate detection of fetal structural heart defects. Despite its challenges, fetal echocardiography helps in the early detection of ICEF. The importance of detecting ICEF can be highlighted by the fact that some clinicians recommend performing fetal echocardiography in all cases of ICEF [3-5].
The exact prevalence of ICEF is difficult to ascertain because of the different populations and methodologies used across various studies. In addition, several studies have included both high- and low-risk populations, while others have reported retrospective and prospective studies including a wide range of gestational ages [6]. The prevalence of ICEF varies between 0.17% and 20% according to the populations studied, gestational age, fetal position, and equipment quality. The highest prevalence is among Asian, Middle-Eastern, and African-American populations [5, 7]. There are few systematic reviews and meta-analyses on the diagnostic performance of the presence of echogenic cardiac foci for detecting chromosomal anomalies. Still, there is a lack of strong evidence about the prevalence of cardiac anomalies in ICEF. This study aims to conduct a systematic review and meta-analysis of published studies on detecting cardiac anomalies in fetuses with ICEF in clinical practice.
Methods
This systematic review followed the recommendations of the meta-analyses in observational studies (MOOSE) guidance statement [8].
Search strategy for identifying relevant studies
The search strategy was implemented in two stages.
Bibliographic database search
Electronic databases (Cochrane Library, PubMed, EMBASE, Scopus, and Web of Science) were used as data sources. The search was restricted to English language publications involving human subjects but not limited by date or publication type. Studies with insufficient data, only abstracts, and duplicate publications were excluded. Two reviewers (PZJ and RR) independently performed data extraction and quality control. A third reviewer (AT) was involved in any conflict that occurred. The following search keywords were used: “Intracardiac”[All Fields] AND (“echogeneity”[All Fields] OR “echogenic”[All Fields] OR “echogenicities” [All Fields] OR “echogenicity”[All Fields] OR “echogenity”[All Fields]) AND “foci” [All Fields] AND (“fetus”[MeSH Terms] OR “fetus” [All Fields] OR “fetuses” [All Fields] OR “fetus s” [All Fields] OR “foetu”[All Fields] OR “fetus” [All Fields]) AND ((“cardiacs” [All Fields] OR “heart”[MeSH Terms] OR “heart” [All Fields] OR “cardiac” [All Fields]) AND (“abnormalities” [MeSH Subheading] OR “abnormalities”[All Fields] OR “malformations”[All Fields] OR “congenital abnormalities” [MeSH Terms] OR (“congenital”[All Fields] AND “abnormalities” [All Fields]) OR “congenital abnormalities” [All Fields] OR “malformation” [All Fields] OR “malformational” [All Fields] OR “malformative” [All Fields] OR “malformed” [All Fields])). The last electronic search was carried out on May 30, 2021.
Searching other sources
We conducted a manual search, scanning the reference lists of eligible papers, other relevant review articles, and specialist journals. Reference lists of included articles and relevant reviews were searched for additional articles. All studies were imported to the literature management software Endnote X7 to eliminate duplicate records. Two authors (AT and PZJ) independently conducted a preliminary screening of studies by reading titles and abstracts. After screening titles and abstracts, the full texts of potentially relevant articles were downloaded. Additionally, we conducted a second round of screening by reading full texts. Studies were selected if they met the inclusion criteria. Methods were adapted as per PRISMA (preferred reporting items for systematic reviews and meta-analyses) guideline for meta-analysis [9].
Eligibility criteria for studies
Studies considered in this meta-analysis were observational studies reporting the prevalence of cardiac anomalies associated with ICEF seen in fetuses. These studies had to provide the total number of patients with ICEF and the number of children with cardiac anomalies occurring in the cohort of ICEF in the fetuses.
The inclusion criteria were all cross-sectional, case-control, or cohort studies reporting the prevalence of ICEF and detecting structural cardiac defects later on in the fetuses and published from January 1, 1980, to June 30, 2020. The exclusion criteria were studies not performed on human participants, case reports, reviews, letters, commentaries, and editorials, studies with insufficient data, abstracts, and duplicate publications, and studies whose key data were not accessible even after a request from authors.
Selection of studies for inclusion in the review
Two investigators (PZJ and RR) independently identified articles and sequentially screened their titles and abstracts for eligibility. Full texts of articles deemed potentially eligible were acquired. These investigators further independently assessed the full text of each study for eligibility and consensually retained studies to be included. Disagreements were resolved by a third author (AT). We used a screening guide to ensure all review authors reliably applied the selection criteria. The agreement was measured using the kappa (κ) statistic [10].
Data extraction and management
A standard data extraction form was used to extract relevant information and data from each study included in the analysis. Two review authors (PZJ and RR) participated in data extraction independently. PZJ and RR extracted data with general information (authors, year, and country), study design, ultrasonography operator, number of ICEF in the fetus, and cardiac anomalies. Studies with only primary data (sample size and number of outcomes) were used to calculate the prevalence estimates. Data were extracted using a preconceived and standardized data abstraction form. Studies with un-interpretable data were excluded from the analysis. The agreement was measured using the κ statistic [10].
Appraisal of the quality of included studies
Two investigators (PZJ and RR) evaluated all the included studies for methodological quality and risk of bias using an adapted version of the Risk of Bias Tool for Prevalence Studies developed by Hoy and associates [11]. Furthermore, the reporting quality of each study was assessed using the STROBE checklist [12]. Two authors performed the reporting of observational studies in epidemiology (STROBE), scoring from 0 to 22, with 22 reflecting the highest quality. The STROBE statement is a checklist of 22 items. These items refer to the article’s title and abstract (item 1), the introduction (items 2 and 3), methods (items 4–12), results (items 13–17), discussion sections (items 18–21), and other information (item 22 on funding). The agreement was measured using the κ statistic [10].
Statistical analysis
In each study, the prevalence of cardiac anomalies associated with ICEF in fetuses was considered the probability of binomial distribution. Forest plots were drawn to visualize the combined prevalence and extent of heterogeneity between studies. Owing to the differences across patients in the studies, a random-effects meta-analysis was used to pool prevalence data [13, 14]. To evaluate the heterogeneity of the studies, Cochran's Q test and I2 index were used [15]. There are three categories for the I2 index: Heterogeneity lower than 25%, heterogeneity between 25% and 75%, and heterogeneity more than 75%. Considering the heterogeneity of the studies, a random effects model was used to combine cardiac anomaly prevalence. Sensitivity analysis was performed to identify the influence of a single study on the combined result prevalence. To determine the cause of heterogeneity of cardiac anomaly prevalence, sub-group analysis of cardiac anomaly in the fetuses with ICEF was carried out based on geographical region, etiology, and quality of studies. The meta-regression model (method of moments) was carried out based on the year of studies [16]. Subgroup analysis was conducted by geographical distribution, maternal age, gestational age, year of publication, and ultrasonography operator. Egger and Begg's tests were used to identify publication bias. Data analysis was performed using comprehensive meta-analysis software version 2, and the significance level in the test was considered lower than 0.05 [17].
Results
Characteristics of included studies
Initially, 531 articles were identified (Figure 1). After eliminating duplicates, screening titles, and abstracts, 385 papers were found completely irrelevant and excluded. Agreement between investigators on abstract selection was high (κ=0.90, P<0.001). Full texts of the remaining 43 studies were scrutinized for eligibility, among which 11 studies were excluded. There was no disagreement between investigators for full-text selection. Overall, 32 studies were found eligible and included in the meta-analysis (Figure 1).
All 32 studies reported the number of cardiac anomalies in fetuses with ICEF without any detailed analysis. The included studies were published from 1987 to 2021. While 13 studies retrospectively collected data, the remaining 19 studies collected the data prospectively. Characteristics of these studies are summarized in Table 1 and Table 2.
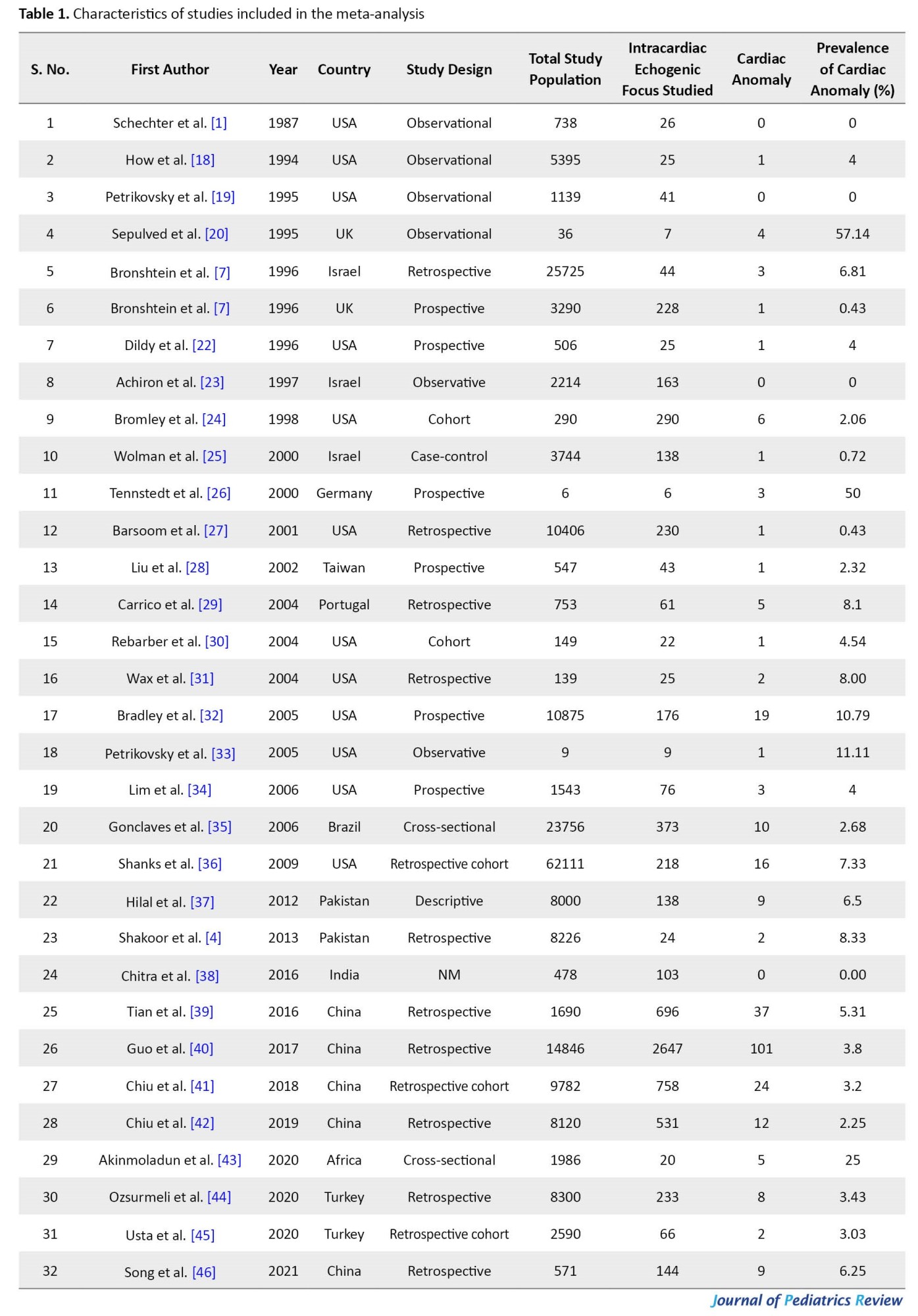


The studies varied in sample size between 6 to 2647 subjects, with a total sample size of 7568 inclusive of all the studies.
Quality of studies
The quality assessment results are presented in Table 3.

None of the studies met all the criteria of the quality assessment score. Based on the criteria enlisted in the STROBE checklist, studies varied in their quality score from 10 to 16. A score of <14 was considered low quality, and >14 was considered good/fair quality. The reporting quality was low for 13 studies while good/fair for the remaining 19 studies. Of the 22 items from the STROBE assessment, the most common problems were a failure to estimate the required sample size and the poor generalizability of the results.
Risk of bias and heterogeneity
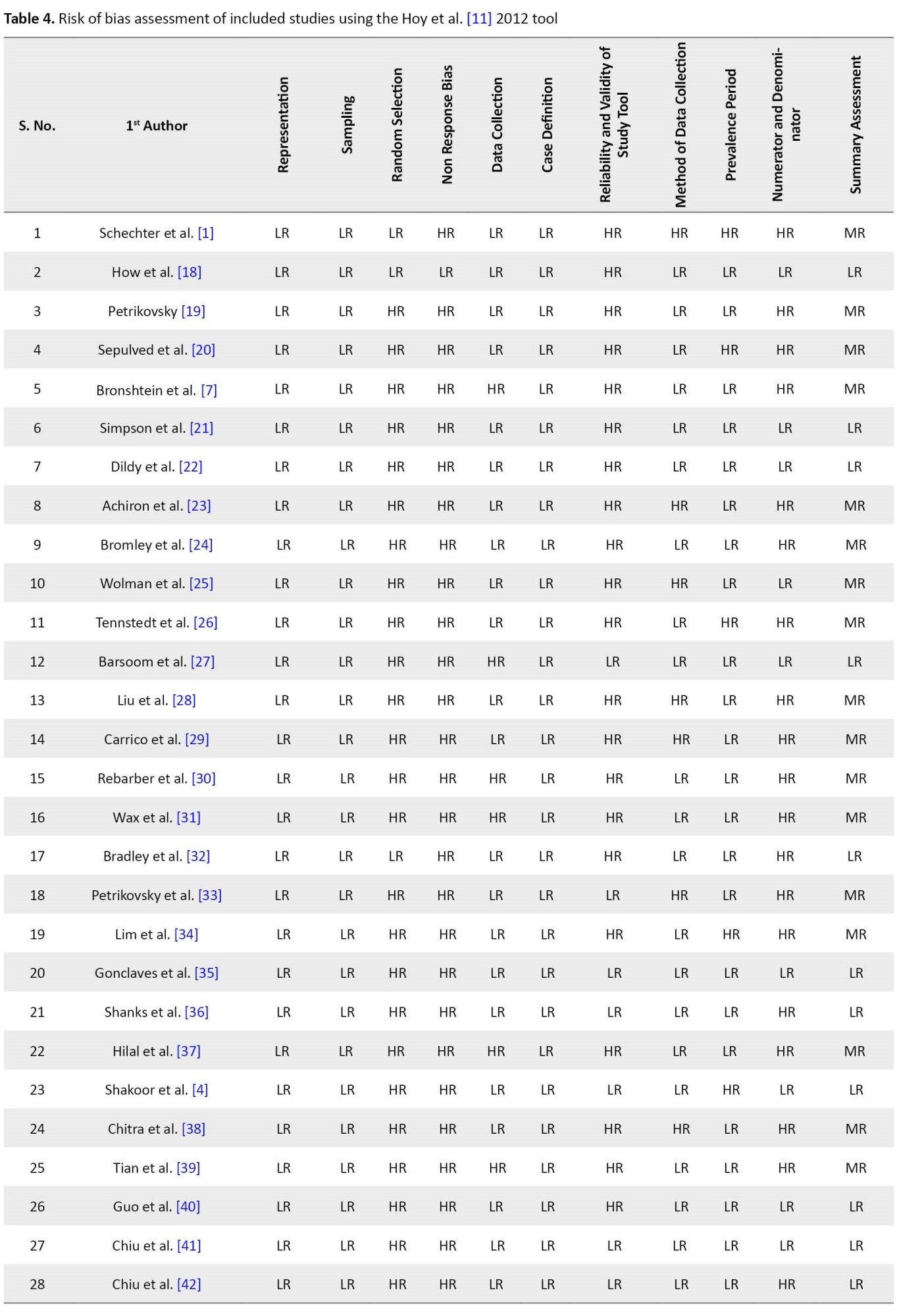
Quality assessment was also conducted for each study in 10 items using the risk of bias assessment tool11we required a tool to assess the risk of study bias. Our objectives were to (1. Of the 32 included studies, our summary assessment (Table 4) showed a low risk of bias for 14 studies (43.75%), a moderate risk of bias for 18 studies (56.25%), and no studies with a high risk of bias.

Agreement between investigators on the quality assessment of studies was high (κ=0.90, P<0.001). The included studies exhibited high heterogeneity according to the Cochrane Q test (Q test P=0.00001) and I2 test (73.50%), which indicates using the random-effects model.
Prevalence of cardiac anomalies associated with ICEF in fetuses
Prior studies have estimated a large variation, ranging from 0% to 57%, in reporting the prevalence of cardiac anomalies in fetuses diagnosed with ICEF. However, definitive data from large population sizes are lacking. According to the Der Simonian-Laird random-effects model, the overall prevalence of the meta-analysis of 32 studies revealed that the pooled prevalence of cardiac anomalies in the fetuses with ICEF was 4.8% (95% CI, 3.6%-6.4%). The forest plot is shown in Figure 2. There was a wide variation in cardiac anomalies prevalence in the fetus with ICEF. The heterogeneity was high (I2=73.50%, P<0.000).
Sensitivity analysis
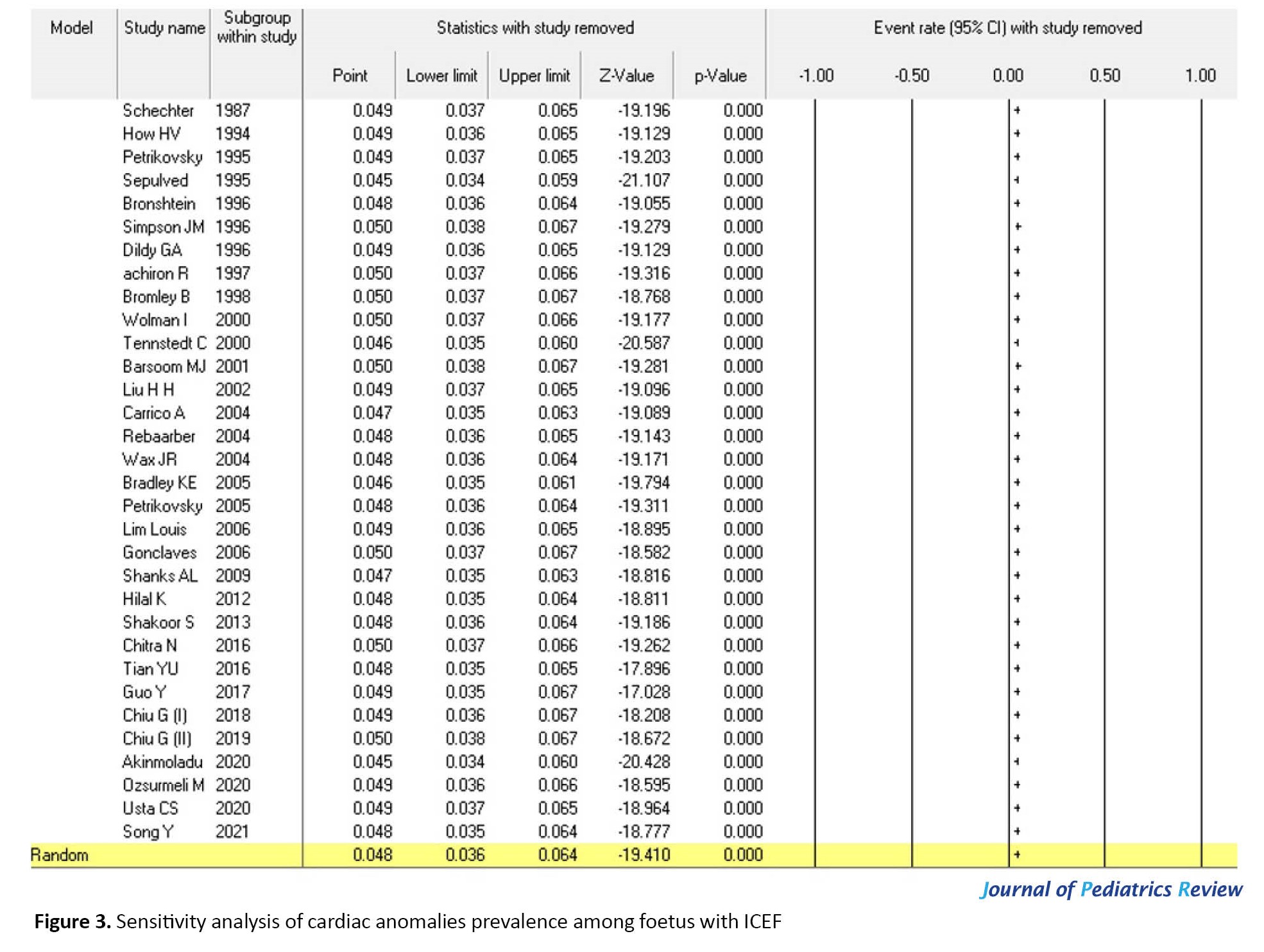
A sensitivity analysis (Figure 3) was performed to assess the stability of the meta-analysis. The results remained largely unchanged. The statistically similar results indicated the stability of this meta-analysis. However, sensitivity analysis did not identify any factors that substantially influenced the heterogeneity of the results.
Subgroup analysis
To reduce the heterogeneity, subgroup analysis was performed. The pooled estimates of the prevalence in different subgroups are shown in Table 5.

There were significant differences for subgroups of geographical regions, maternal age, gestational age, study publication year, risk of bias, and ultrasonography operator (P<0.05 for all).
Region
The prevalence of cardiac anomalies among fetuses with ICEF from the Africa continent (25%; 95% CI, 0.108%-0.478%) was higher than in other continents. The European continent’s prevalence of cardiac anomalies among fetuses with ICEF was 4.3% (95% CI, 0.018%-0.579%), followed by the American continent and then Asia.
Maternal age
According to the maternal age group, 21 studies were divided into two categories: Studies conducted in maternal age less than 30 years (15 studies) and those conducted in maternal age more than 30 years (6 studies). The prevalence of cardiac anomalies among fetuses with ICEF in maternal age of >30 years groups (5.81%; 95% CI, 0.03%-0.10%) was higher than studies conducted in maternal age <30 year groups (3.57%; 95% CI, 0.03%-0.04%).
Gestational age
According to the gestational age group, 24 included studies were divided into two categories: Studies conducted in the gestational age group <20 weeks (10 studies) and studies conducted gestational age group >20 weeks (14 studies). The prevalence of cardiac anomalies among fetuses with ICEF in gestational age group <20 weeks groups (3.97%; 95% CI, 0.02%-0.07%) was higher than studies conducted in gestational age group >20 weeks group (3.76%; 95% CI, 0.02%-0.05%).
Published studies
The prevalence of cardiac anomalies among fetuses with ICEF was lower among the published studies before 2000 (1.88%; 95% CI, 0.01%-0.09%) than those published after 2000 (4.03%; 95% CI, 0.03%-0.06%).
Risk of bias
Subgroup analyses showed the prevalence of cardiac anomalies among fetuses with ICEF in moderate risk studies (3.91%; 95% CI, 0.03%-0.06%) was higher than in studies with low risk of bias (3.11%; 95% CI, 0.028%-0.061%).
Operator
According to the operator performing fetal echocardiography or sonography to detect cardiac anomalies, 13 studies were divided into 4 categories: pediatric cardiologist, fetal cardiography, fetal medicine specialist, and ultrasonographer. The higher prevalence of cardiac anomalies among fetuses with ICEF was detected by a fetal medicine specialist (8.73%; 95% CI, 0.02%-0.18%) and fetal cardiography (8.19%; 95% CI, 0.03%-0.18%) versus the pediatric cardiologist and ultrasonographer.
Publication bias
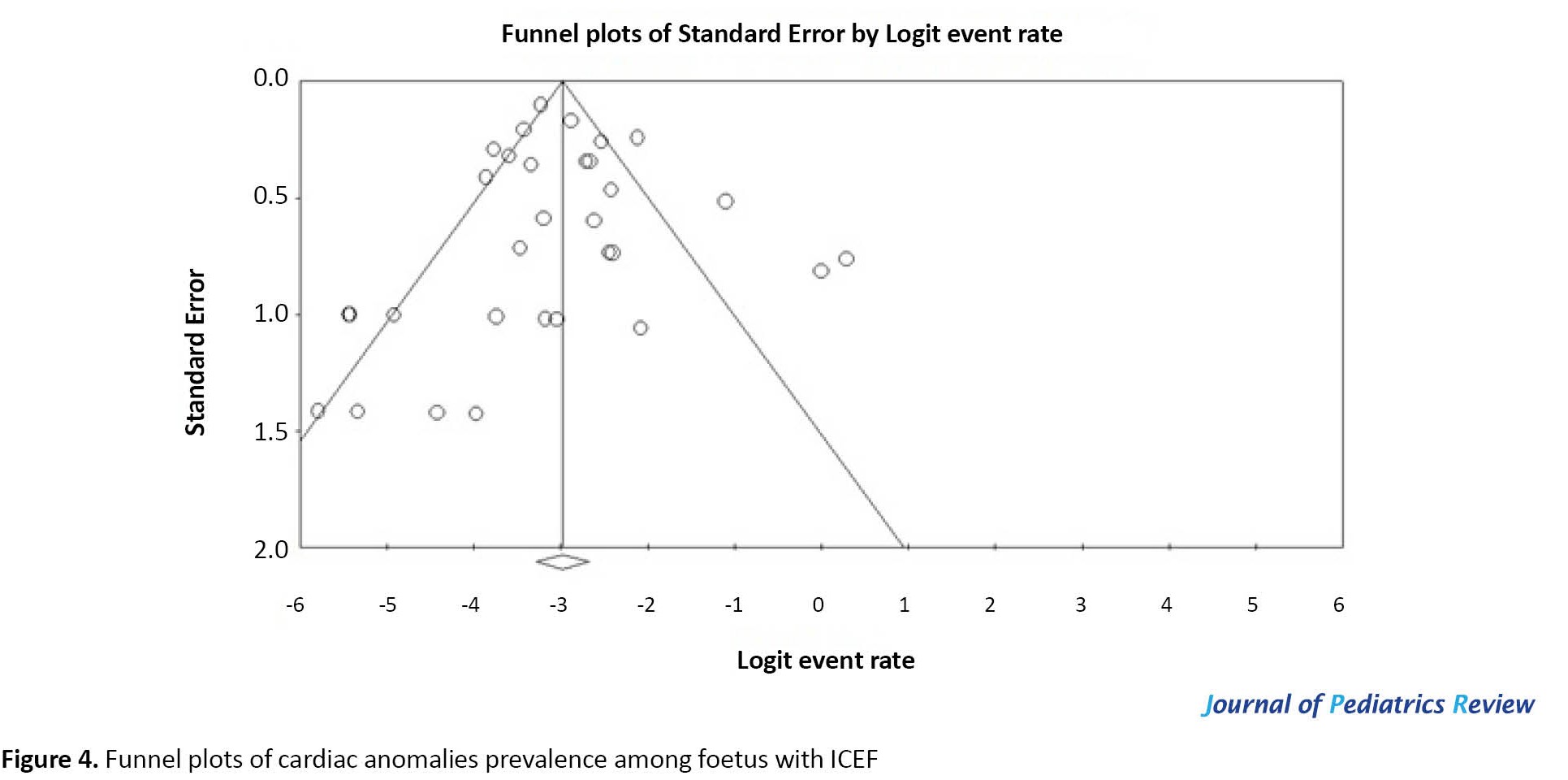
The Egger weighted regression statistics (P=0.873) and Begg rank correlation statistics (P=0.57) indicated no evidence of publication bias. There was no publication bias or asymmetry in the funnel plot (Figure 4). Meta-regression model in Figure 5 shows that the prevalence of cardiac anomalies among fetuses with ICEF is increasing according to the year of study. However, this relationship is not statistically significant (meta-regression coefficient: 0.0022, 95% CI, -0.0319% to 0.0362%, P=0.900).
Discussion
Diagnosis of echogenic foci is increasingly associated with an underlying structural cardiac defect and chromosomal abnormalities. Despite various previous studies, the relationship of ICEF with the cardiac anomaly is unclear [29, 47, 48]. Most studies show that the presence of ICEF should be interpreted as a possible risk factor for congenital heart defects. While some studies have found that fetal echogenic foci were not associated with underlying congenital heart disease, structural heart defects, or extra-cardiac anomalies [1, 23, 33, 38]. Sotiriadis et al. [49] conducted the first meta-analysis associating intracardiac echogenic foci to trisomy 21 and observed a 5-7 times higher risk in patients diagnosed with echogenic foci. Another meta-analysis by Lorente et al. [50] confirmed the association of echogenic foci with chromosomal anomalies. The identification rate of trisomy 21 in children diagnosed with echogenic foci was low (21.8%), with a low false positive rate (4.1%). In addition, the determined likelihood ratios in their study showed that echogenic foci have an important role in confirmation rather than ruling out trisomy 21 [50]. This meta-analysis assessed the prevalence of cardiac defects in fetuses diagnosed with echogenic foci. The present study found the overall prevalence to be 4.8% (95% CI, 3.6%-6.4%) from a pool of 32 studies that met the inclusion criteria. In addition to this analysis, sensitivity analysis was performed to assess the stability of our meta-analysis showed largely unchanged results suggestive of stable and widely applicable results.
In the meta-analysis, significant heterogeneity among studies was reported, and we tried to address heterogeneity with a sensitivity and sub-group analysis that needs to be considered when interpreting the results of this review. The large heterogeneity found in all types of prevalence indicates the existence of characteristics of the studies causing this variability. Furthermore, subgroup analysis was done according to regions, age of the mothers, year in which the study was published, risk of bias, and individual performing ultrasonography to reduce heterogeneity. As per the geographic distribution, the European population (4.3%) was found to have the highest prevalence, followed by the Asian (3.8%) and American people (3.6%). In the included studies in our meta-analysis, no analysis by ethnicity was possible because these articles did not assess this population’s characteristics concerning cardiac anomaly and echogenic foci. Therefore, further studies on this aspect would be advisable. In addition, subgroup analysis also showed a higher prevalence of cardiac anomalies associated with ICEF when diagnosed in mothers over the age of 30 years (5.8% vs 3.5%) and at less than 20 weeks of gestational age (3.9% vs 3.7%). In evaluating the studies on gestational age, those studying fetuses during later gestational ages are more sensitive and specific. This outcome could be attributed to increased heart sizes, possible enlargement of the focus with gestational age, and the persistence of EIF display during pregnancy. In the literature, the persistence of EIF in ultrasound scans ranges from 25% to 92.3% [48, 51]. The publication year influenced the lifetime prevalence rates, with the higher prevalence rates reported in the most recent studies. This result seems to be very solid, as in the multiple meta-regression models, publication year was one of the two predictors that achieved a statistically significant relationship with the lifetime prevalence once controlled by the methodological quality of the studies. A higher prevalence was also observed in studies published after 2000, probably due to improved imaging modalities and increased personnel experience. The same subgroup analysis also shows that prevalence is highest when a fetal medicine specialist performs ultrasonography; thus, showing operator experience is vital in identifying ICEF. The echogenic foci detection sensitivity is higher in medium-/high-quality studies, although not statistically significant; the false positive ratio is also higher. This outcome may be attributable to a greater population selection and ultrasound studies being performed by more trained professionals.
Strengths and weaknesses of the study
This study’s strength is its use of multiple databases to avoid missing any eligible research. Data extraction was also done reproducibly using a pre-set and pre-tested checklist to minimize errors that could affect the estimate. This systematic review and meta-analysis also included studies from different geographical regions worldwide. However, the study is not free from potential limitations, as it is restricted to articles published in English. Also, the articles included in this review are weak to establish a causal relationship between the associated factors and the outcome because they are cross-sectional. As a result, this meta-analysis is helpful if interpreted considering both the inherent limitations of the original studies and the current meta-analysis. There was significant clinical and statistical heterogeneity. The heterogeneity was mainly related to the gestational age at assessment, maternal age, different operators, and the sample size (which ranged from 6 to 2647). Because heterogeneity was anticipated, we used a random-effects model for the meta-analysis. The high statistical heterogeneity was due to the varied prevalence of cardiac anomalies in the fetuses with ICEF in the included studies. Our results enable us to make recommendations for future research in this field.
Conclusion
The current study represents the first and only meta-analysis concerning the prevalence of cardiac anomaly in the fetus diagnosed with ICEF. Most recent studies seem to show higher prevalence rates than the older ones, and studies with a better methodology tend to show higher lifetime prevalence rates than methodologically poor ones. This study supports a definitive relationship between ICEF and underlying congenital heart disease and chromosomal anomalies such as trisomy 21. We recommend increased training of individuals performing this ultrasonography to improve early detection, ultimately enhancing the care given to infants immediately after delivery. In addition, further longitudinal studies with long follow-ups are necessary to better explore the determinants of cardiac anomaly in the study subjects in resource-limited settings for successful interventions.
Ethical Considerations
Compliance with ethical guidelines
The protocol for this systematic review and meta-analysis was registered at the International Prospective Register of Systematic Reviews PROSPERO (Code: #CRD42021253664).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The author declared no conflict of interest.
References
Intracardiac echogenic focus (ICEF) is demonstrated by ultrasound inside the fetal heart with a brightness comparable to that of the bone. It was first described by Schechter et al. [1] in 1987 in the left ventricle of a fetal heart, which they attributed to a thickening of the chordae. Usually, the focus has no acoustic shadow and is located near or within the papillary muscles. It moves in synchronization with the atrioventricular valves. It can be visualized in a 4 chambers view when performing a basic echocardiogram [2]. ICEF is most frequently visualized in the left ventricle and less commonly on the right side or both ventricles. While a single ICEF in the left ventricle is the most frequent finding, multiple foci may be seen often. These foci vary in size but are usually less than 6 mm [1, 2]. Echogenic foci suggest micro-calcification of the chordae and papillary muscles. Echogenic foci are increasingly associated with cardiac structural anomalies and chromosomal abnormalities.
When diagnosed, echogenic foci bring a clinical conundrum as their origin, and definitive significance are not yet completely understood. However, echogenic foci are increasingly considered markers for chromosomal abnormality and underlying structural cardiac defect in the fetus, especially in high-risk women [3, 4]. Therefore, the detection of ICEF warrants further investigations, such as fetal echocardiography. Fetal echocardiography has proven to be an invaluable tool for the early and accurate detection of fetal structural heart defects. Despite its challenges, fetal echocardiography helps in the early detection of ICEF. The importance of detecting ICEF can be highlighted by the fact that some clinicians recommend performing fetal echocardiography in all cases of ICEF [3-5].
The exact prevalence of ICEF is difficult to ascertain because of the different populations and methodologies used across various studies. In addition, several studies have included both high- and low-risk populations, while others have reported retrospective and prospective studies including a wide range of gestational ages [6]. The prevalence of ICEF varies between 0.17% and 20% according to the populations studied, gestational age, fetal position, and equipment quality. The highest prevalence is among Asian, Middle-Eastern, and African-American populations [5, 7]. There are few systematic reviews and meta-analyses on the diagnostic performance of the presence of echogenic cardiac foci for detecting chromosomal anomalies. Still, there is a lack of strong evidence about the prevalence of cardiac anomalies in ICEF. This study aims to conduct a systematic review and meta-analysis of published studies on detecting cardiac anomalies in fetuses with ICEF in clinical practice.
Methods
This systematic review followed the recommendations of the meta-analyses in observational studies (MOOSE) guidance statement [8].
Search strategy for identifying relevant studies
The search strategy was implemented in two stages.
Bibliographic database search
Electronic databases (Cochrane Library, PubMed, EMBASE, Scopus, and Web of Science) were used as data sources. The search was restricted to English language publications involving human subjects but not limited by date or publication type. Studies with insufficient data, only abstracts, and duplicate publications were excluded. Two reviewers (PZJ and RR) independently performed data extraction and quality control. A third reviewer (AT) was involved in any conflict that occurred. The following search keywords were used: “Intracardiac”[All Fields] AND (“echogeneity”[All Fields] OR “echogenic”[All Fields] OR “echogenicities” [All Fields] OR “echogenicity”[All Fields] OR “echogenity”[All Fields]) AND “foci” [All Fields] AND (“fetus”[MeSH Terms] OR “fetus” [All Fields] OR “fetuses” [All Fields] OR “fetus s” [All Fields] OR “foetu”[All Fields] OR “fetus” [All Fields]) AND ((“cardiacs” [All Fields] OR “heart”[MeSH Terms] OR “heart” [All Fields] OR “cardiac” [All Fields]) AND (“abnormalities” [MeSH Subheading] OR “abnormalities”[All Fields] OR “malformations”[All Fields] OR “congenital abnormalities” [MeSH Terms] OR (“congenital”[All Fields] AND “abnormalities” [All Fields]) OR “congenital abnormalities” [All Fields] OR “malformation” [All Fields] OR “malformational” [All Fields] OR “malformative” [All Fields] OR “malformed” [All Fields])). The last electronic search was carried out on May 30, 2021.
Searching other sources
We conducted a manual search, scanning the reference lists of eligible papers, other relevant review articles, and specialist journals. Reference lists of included articles and relevant reviews were searched for additional articles. All studies were imported to the literature management software Endnote X7 to eliminate duplicate records. Two authors (AT and PZJ) independently conducted a preliminary screening of studies by reading titles and abstracts. After screening titles and abstracts, the full texts of potentially relevant articles were downloaded. Additionally, we conducted a second round of screening by reading full texts. Studies were selected if they met the inclusion criteria. Methods were adapted as per PRISMA (preferred reporting items for systematic reviews and meta-analyses) guideline for meta-analysis [9].
Eligibility criteria for studies
Studies considered in this meta-analysis were observational studies reporting the prevalence of cardiac anomalies associated with ICEF seen in fetuses. These studies had to provide the total number of patients with ICEF and the number of children with cardiac anomalies occurring in the cohort of ICEF in the fetuses.
The inclusion criteria were all cross-sectional, case-control, or cohort studies reporting the prevalence of ICEF and detecting structural cardiac defects later on in the fetuses and published from January 1, 1980, to June 30, 2020. The exclusion criteria were studies not performed on human participants, case reports, reviews, letters, commentaries, and editorials, studies with insufficient data, abstracts, and duplicate publications, and studies whose key data were not accessible even after a request from authors.
Selection of studies for inclusion in the review
Two investigators (PZJ and RR) independently identified articles and sequentially screened their titles and abstracts for eligibility. Full texts of articles deemed potentially eligible were acquired. These investigators further independently assessed the full text of each study for eligibility and consensually retained studies to be included. Disagreements were resolved by a third author (AT). We used a screening guide to ensure all review authors reliably applied the selection criteria. The agreement was measured using the kappa (κ) statistic [10].
Data extraction and management
A standard data extraction form was used to extract relevant information and data from each study included in the analysis. Two review authors (PZJ and RR) participated in data extraction independently. PZJ and RR extracted data with general information (authors, year, and country), study design, ultrasonography operator, number of ICEF in the fetus, and cardiac anomalies. Studies with only primary data (sample size and number of outcomes) were used to calculate the prevalence estimates. Data were extracted using a preconceived and standardized data abstraction form. Studies with un-interpretable data were excluded from the analysis. The agreement was measured using the κ statistic [10].
Appraisal of the quality of included studies
Two investigators (PZJ and RR) evaluated all the included studies for methodological quality and risk of bias using an adapted version of the Risk of Bias Tool for Prevalence Studies developed by Hoy and associates [11]. Furthermore, the reporting quality of each study was assessed using the STROBE checklist [12]. Two authors performed the reporting of observational studies in epidemiology (STROBE), scoring from 0 to 22, with 22 reflecting the highest quality. The STROBE statement is a checklist of 22 items. These items refer to the article’s title and abstract (item 1), the introduction (items 2 and 3), methods (items 4–12), results (items 13–17), discussion sections (items 18–21), and other information (item 22 on funding). The agreement was measured using the κ statistic [10].
Statistical analysis
In each study, the prevalence of cardiac anomalies associated with ICEF in fetuses was considered the probability of binomial distribution. Forest plots were drawn to visualize the combined prevalence and extent of heterogeneity between studies. Owing to the differences across patients in the studies, a random-effects meta-analysis was used to pool prevalence data [13, 14]. To evaluate the heterogeneity of the studies, Cochran's Q test and I2 index were used [15]. There are three categories for the I2 index: Heterogeneity lower than 25%, heterogeneity between 25% and 75%, and heterogeneity more than 75%. Considering the heterogeneity of the studies, a random effects model was used to combine cardiac anomaly prevalence. Sensitivity analysis was performed to identify the influence of a single study on the combined result prevalence. To determine the cause of heterogeneity of cardiac anomaly prevalence, sub-group analysis of cardiac anomaly in the fetuses with ICEF was carried out based on geographical region, etiology, and quality of studies. The meta-regression model (method of moments) was carried out based on the year of studies [16]. Subgroup analysis was conducted by geographical distribution, maternal age, gestational age, year of publication, and ultrasonography operator. Egger and Begg's tests were used to identify publication bias. Data analysis was performed using comprehensive meta-analysis software version 2, and the significance level in the test was considered lower than 0.05 [17].
Results
Characteristics of included studies
Initially, 531 articles were identified (Figure 1). After eliminating duplicates, screening titles, and abstracts, 385 papers were found completely irrelevant and excluded. Agreement between investigators on abstract selection was high (κ=0.90, P<0.001). Full texts of the remaining 43 studies were scrutinized for eligibility, among which 11 studies were excluded. There was no disagreement between investigators for full-text selection. Overall, 32 studies were found eligible and included in the meta-analysis (Figure 1).
All 32 studies reported the number of cardiac anomalies in fetuses with ICEF without any detailed analysis. The included studies were published from 1987 to 2021. While 13 studies retrospectively collected data, the remaining 19 studies collected the data prospectively. Characteristics of these studies are summarized in Table 1 and Table 2.



The studies varied in sample size between 6 to 2647 subjects, with a total sample size of 7568 inclusive of all the studies.
Quality of studies
The quality assessment results are presented in Table 3.

None of the studies met all the criteria of the quality assessment score. Based on the criteria enlisted in the STROBE checklist, studies varied in their quality score from 10 to 16. A score of <14 was considered low quality, and >14 was considered good/fair quality. The reporting quality was low for 13 studies while good/fair for the remaining 19 studies. Of the 22 items from the STROBE assessment, the most common problems were a failure to estimate the required sample size and the poor generalizability of the results.
Risk of bias and heterogeneity
Quality assessment was also conducted for each study in 10 items using the risk of bias assessment tool11we required a tool to assess the risk of study bias. Our objectives were to (1. Of the 32 included studies, our summary assessment (Table 4) showed a low risk of bias for 14 studies (43.75%), a moderate risk of bias for 18 studies (56.25%), and no studies with a high risk of bias.


Agreement between investigators on the quality assessment of studies was high (κ=0.90, P<0.001). The included studies exhibited high heterogeneity according to the Cochrane Q test (Q test P=0.00001) and I2 test (73.50%), which indicates using the random-effects model.
Prevalence of cardiac anomalies associated with ICEF in fetuses
Prior studies have estimated a large variation, ranging from 0% to 57%, in reporting the prevalence of cardiac anomalies in fetuses diagnosed with ICEF. However, definitive data from large population sizes are lacking. According to the Der Simonian-Laird random-effects model, the overall prevalence of the meta-analysis of 32 studies revealed that the pooled prevalence of cardiac anomalies in the fetuses with ICEF was 4.8% (95% CI, 3.6%-6.4%). The forest plot is shown in Figure 2. There was a wide variation in cardiac anomalies prevalence in the fetus with ICEF. The heterogeneity was high (I2=73.50%, P<0.000).
Sensitivity analysis
A sensitivity analysis (Figure 3) was performed to assess the stability of the meta-analysis. The results remained largely unchanged. The statistically similar results indicated the stability of this meta-analysis. However, sensitivity analysis did not identify any factors that substantially influenced the heterogeneity of the results.
Subgroup analysis
To reduce the heterogeneity, subgroup analysis was performed. The pooled estimates of the prevalence in different subgroups are shown in Table 5.

There were significant differences for subgroups of geographical regions, maternal age, gestational age, study publication year, risk of bias, and ultrasonography operator (P<0.05 for all).
Region
The prevalence of cardiac anomalies among fetuses with ICEF from the Africa continent (25%; 95% CI, 0.108%-0.478%) was higher than in other continents. The European continent’s prevalence of cardiac anomalies among fetuses with ICEF was 4.3% (95% CI, 0.018%-0.579%), followed by the American continent and then Asia.
Maternal age
According to the maternal age group, 21 studies were divided into two categories: Studies conducted in maternal age less than 30 years (15 studies) and those conducted in maternal age more than 30 years (6 studies). The prevalence of cardiac anomalies among fetuses with ICEF in maternal age of >30 years groups (5.81%; 95% CI, 0.03%-0.10%) was higher than studies conducted in maternal age <30 year groups (3.57%; 95% CI, 0.03%-0.04%).
Gestational age
According to the gestational age group, 24 included studies were divided into two categories: Studies conducted in the gestational age group <20 weeks (10 studies) and studies conducted gestational age group >20 weeks (14 studies). The prevalence of cardiac anomalies among fetuses with ICEF in gestational age group <20 weeks groups (3.97%; 95% CI, 0.02%-0.07%) was higher than studies conducted in gestational age group >20 weeks group (3.76%; 95% CI, 0.02%-0.05%).
Published studies
The prevalence of cardiac anomalies among fetuses with ICEF was lower among the published studies before 2000 (1.88%; 95% CI, 0.01%-0.09%) than those published after 2000 (4.03%; 95% CI, 0.03%-0.06%).
Risk of bias
Subgroup analyses showed the prevalence of cardiac anomalies among fetuses with ICEF in moderate risk studies (3.91%; 95% CI, 0.03%-0.06%) was higher than in studies with low risk of bias (3.11%; 95% CI, 0.028%-0.061%).
Operator
According to the operator performing fetal echocardiography or sonography to detect cardiac anomalies, 13 studies were divided into 4 categories: pediatric cardiologist, fetal cardiography, fetal medicine specialist, and ultrasonographer. The higher prevalence of cardiac anomalies among fetuses with ICEF was detected by a fetal medicine specialist (8.73%; 95% CI, 0.02%-0.18%) and fetal cardiography (8.19%; 95% CI, 0.03%-0.18%) versus the pediatric cardiologist and ultrasonographer.
Publication bias
The Egger weighted regression statistics (P=0.873) and Begg rank correlation statistics (P=0.57) indicated no evidence of publication bias. There was no publication bias or asymmetry in the funnel plot (Figure 4). Meta-regression model in Figure 5 shows that the prevalence of cardiac anomalies among fetuses with ICEF is increasing according to the year of study. However, this relationship is not statistically significant (meta-regression coefficient: 0.0022, 95% CI, -0.0319% to 0.0362%, P=0.900).
Discussion
Diagnosis of echogenic foci is increasingly associated with an underlying structural cardiac defect and chromosomal abnormalities. Despite various previous studies, the relationship of ICEF with the cardiac anomaly is unclear [29, 47, 48]. Most studies show that the presence of ICEF should be interpreted as a possible risk factor for congenital heart defects. While some studies have found that fetal echogenic foci were not associated with underlying congenital heart disease, structural heart defects, or extra-cardiac anomalies [1, 23, 33, 38]. Sotiriadis et al. [49] conducted the first meta-analysis associating intracardiac echogenic foci to trisomy 21 and observed a 5-7 times higher risk in patients diagnosed with echogenic foci. Another meta-analysis by Lorente et al. [50] confirmed the association of echogenic foci with chromosomal anomalies. The identification rate of trisomy 21 in children diagnosed with echogenic foci was low (21.8%), with a low false positive rate (4.1%). In addition, the determined likelihood ratios in their study showed that echogenic foci have an important role in confirmation rather than ruling out trisomy 21 [50]. This meta-analysis assessed the prevalence of cardiac defects in fetuses diagnosed with echogenic foci. The present study found the overall prevalence to be 4.8% (95% CI, 3.6%-6.4%) from a pool of 32 studies that met the inclusion criteria. In addition to this analysis, sensitivity analysis was performed to assess the stability of our meta-analysis showed largely unchanged results suggestive of stable and widely applicable results.
In the meta-analysis, significant heterogeneity among studies was reported, and we tried to address heterogeneity with a sensitivity and sub-group analysis that needs to be considered when interpreting the results of this review. The large heterogeneity found in all types of prevalence indicates the existence of characteristics of the studies causing this variability. Furthermore, subgroup analysis was done according to regions, age of the mothers, year in which the study was published, risk of bias, and individual performing ultrasonography to reduce heterogeneity. As per the geographic distribution, the European population (4.3%) was found to have the highest prevalence, followed by the Asian (3.8%) and American people (3.6%). In the included studies in our meta-analysis, no analysis by ethnicity was possible because these articles did not assess this population’s characteristics concerning cardiac anomaly and echogenic foci. Therefore, further studies on this aspect would be advisable. In addition, subgroup analysis also showed a higher prevalence of cardiac anomalies associated with ICEF when diagnosed in mothers over the age of 30 years (5.8% vs 3.5%) and at less than 20 weeks of gestational age (3.9% vs 3.7%). In evaluating the studies on gestational age, those studying fetuses during later gestational ages are more sensitive and specific. This outcome could be attributed to increased heart sizes, possible enlargement of the focus with gestational age, and the persistence of EIF display during pregnancy. In the literature, the persistence of EIF in ultrasound scans ranges from 25% to 92.3% [48, 51]. The publication year influenced the lifetime prevalence rates, with the higher prevalence rates reported in the most recent studies. This result seems to be very solid, as in the multiple meta-regression models, publication year was one of the two predictors that achieved a statistically significant relationship with the lifetime prevalence once controlled by the methodological quality of the studies. A higher prevalence was also observed in studies published after 2000, probably due to improved imaging modalities and increased personnel experience. The same subgroup analysis also shows that prevalence is highest when a fetal medicine specialist performs ultrasonography; thus, showing operator experience is vital in identifying ICEF. The echogenic foci detection sensitivity is higher in medium-/high-quality studies, although not statistically significant; the false positive ratio is also higher. This outcome may be attributable to a greater population selection and ultrasound studies being performed by more trained professionals.
Strengths and weaknesses of the study
This study’s strength is its use of multiple databases to avoid missing any eligible research. Data extraction was also done reproducibly using a pre-set and pre-tested checklist to minimize errors that could affect the estimate. This systematic review and meta-analysis also included studies from different geographical regions worldwide. However, the study is not free from potential limitations, as it is restricted to articles published in English. Also, the articles included in this review are weak to establish a causal relationship between the associated factors and the outcome because they are cross-sectional. As a result, this meta-analysis is helpful if interpreted considering both the inherent limitations of the original studies and the current meta-analysis. There was significant clinical and statistical heterogeneity. The heterogeneity was mainly related to the gestational age at assessment, maternal age, different operators, and the sample size (which ranged from 6 to 2647). Because heterogeneity was anticipated, we used a random-effects model for the meta-analysis. The high statistical heterogeneity was due to the varied prevalence of cardiac anomalies in the fetuses with ICEF in the included studies. Our results enable us to make recommendations for future research in this field.
Conclusion
The current study represents the first and only meta-analysis concerning the prevalence of cardiac anomaly in the fetus diagnosed with ICEF. Most recent studies seem to show higher prevalence rates than the older ones, and studies with a better methodology tend to show higher lifetime prevalence rates than methodologically poor ones. This study supports a definitive relationship between ICEF and underlying congenital heart disease and chromosomal anomalies such as trisomy 21. We recommend increased training of individuals performing this ultrasonography to improve early detection, ultimately enhancing the care given to infants immediately after delivery. In addition, further longitudinal studies with long follow-ups are necessary to better explore the determinants of cardiac anomaly in the study subjects in resource-limited settings for successful interventions.
Ethical Considerations
Compliance with ethical guidelines
The protocol for this systematic review and meta-analysis was registered at the International Prospective Register of Systematic Reviews PROSPERO (Code: #CRD42021253664).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The author declared no conflict of interest.
References
- Schechter AG, Fakhry J, Shapiro LR, Gewitz MH. In utero thickening of the chordae tendinae. A cause of intracardiac echogenic foci. J Ultrasound Med. 1987; 6(12):691-5.[DOI:10.7863/jum.1987.6.12.691] [PMID]
- Rodriguez R, Herrero B, Bartha JL. The continuing enigma of the fetal echogenic intracardiac focus in prenatal ultrasound. Curr Opin Obstet Gynecol. 2013; 25(2):145-51.[DOI:10.1097/GCO.0b013e32835e14eb] [PMID]
- Tran SH, Caughey AB, Norton ME. Ethnic variation in the prevalence of echogenic intracardiac foci and the association with Down syndrome. Ultrasound Obstet Gynecol. 2005; 26(2):158-61. [DOI:10.1002/uog.1935] [PMID]
- Shakoor S, Ismail H, Munim S. Intracardiac echogenic focus and fetal outcome - review of cases from a tertiary care centre in Karachi, Pakistan. J Matern Fetal Neonatal Med. 2013; 26(1):2-4. [DOI:10.3109/14767058.2012.703724] [PMID]
- Levy DW, Mintz MC. The left ventricular echogenic focus: A normal finding. AJR Am J Roentgenol. 1988; 150(1):85-6. [DOI:10.2214/ajr.150.1.85] [PMID]
- Wessels MW, Los FJ, Frohn-Mulder IM, Niermeijer MF, Willems PJ, Wladimiroff JW. Poor outcome in down syndrome fetuses with cardiac anomalies or growth retardation. Am J Med Genet A. 2003; 116A(2):147-51. [DOI:10.1002/ajmg.a.10823] [PMID]
- Bronshtein M, Jakobi P, Ofir C. Multiple fetal intracardiac echogenic foci: Not always a benign sonographic finding. Prenat Diagn. 1996; 16(2):131-5. [DOI:10.1002/(SICI)1097-0223(199602)16:23.0.CO;2-Q] [PMID]
- Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000; 283(15):2008-12. [DOI:10.1001/jama.283.15.2008] [PMID]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. BMJ. 2009; 339:b2535. [DOI:10.1136/bmj.b2535] [PMID] [PMCID]
- Viera A, Garrett JM. Understanding interobserver agreement: The kappa statistic. Fam Med. 2005; 37(5):360-3. [Link]
- Hoy D, Brooks P, Woolf A, Blyth F, March L, Bain C, et al. Assessing risk of bias in prevalence studies: Modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012; 65(9):934-9. [DOI:10.1016/j.jclinepi.2011.11.014] [PMID]
- Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the reporting of observational studies in epidemiology (STROBE): Explanation and elaboration. Int J Surg. 2014; 12(12):1500-24. [DOI:10.1016/j.ijsu.2014.07.014] [PMID]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557-60. [DOI:10.1136/bmj.327.7414.557] [PMID] [PMCID]
- Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013; 67(11):974-8. [DOI:10.1136/jech-2013-203104] [PMID]
- Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken: Wiley; 2008. [Link]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Hoboken: John Wiley & Sons; 2009. [DOI:10.1002/9780470743386]
- Borenstein M, Rothstein H. Comprehensive meta-analysis: A computer program for research synthesis computer program. Englewood Cliffs: Biostat Inc; 1999. [Link]
- How HY, Villafane J, Parihus RR, Spinnato JA 2nd. Small hyperechoic ventricle: a benign foci of the fetal cardiac sonographic finding? Ultrasound Obstet Gynecol. 1994; 4(3):205-7. [DOI:10.1046/j.1469-0705.1994.04030205.x] [PMID]
- Petrikovsky BM, Challenger M, Wyse LJ. Natural history of echogenic foci within ventricles of the fetal heart. Ultrasound Obstet Gynecol. 1995; 5(2):92-4. [DOI:10.1046/j.1469-0705.1995.05020092.x] [PMID]
- Sepulveda W, Cullen S, Nicolaidis P, Hollingsworth J, Fisk NM. Echogenic foci in the fetal heart: A marker of chromosomal abnormality. Br J Obstet Gynaecol. 1995; 102(6):490-2. [DOI:10.1111/j.1471-0528.1995.tb11325.x] [PMID]
- Simpson JM, Cook A, Sharland G. The significance of echogenic foci in the fetal heart: A prospective study of 228 cases. Ultrasound Obstet Gynecol. 1996; 8(4):225-8.[DOI:10.1046/j.1469-0705.1996.08040225.x] [PMID]
- Dildy GA, Judd VE, Clark SL. Prospective evaluation of the antenatal incidence and postnatal significance of the fetal echogenic cardiac focus: A case-control study. Am J Obstet Gynecol. 1996; 175(4 Pt 1):1008-12. [DOI:10.1016/S0002-9378(96)80043-3] [PMID]
- Achiron R, Lipitz S, Gabbay U, Yagel S. Prenatal ultrasonographic diagnosis of fetal heart echogenic foci: No correlation with down syndrome. Obstet Gynecol. 1997; 89(6):945-8. [DOI:10.1016/S0029-7844(97)00131-2] [PMID]
- Bromley B, Lieberman E, Shipp TD, Richardson M, Benacerraf BR. Significance of an echogenic intracardiac focus in fetuses at high and low risk for aneuploidy. J Ultrasound Med. 1998; 17(2):127-31. [DOI:10.7863/jum.1998.17.2.127] [PMID]
- Wolman I, Jaffa A, Geva E, Diamant S, Strauss S, Lessing JB, et al. Intracardiac echogenic focus: No apparent association with structural cardiac abnormality. Fetal Diagn Ther. 2000; 15(4):216-8. [DOI:10.1159/000021009] [PMID]
- Tennstedt C, Chaoui R, Vogel M, Göldner B, Dietel M. Pathologic correlation of sonographic echogenic foci in the fetal heart. Prenat Diagn. 2000; 20(4):287-92. [DOI:10.1002/(SICI)1097-0223(200004)20:43.0.CO;2-K] [PMID]
- Barsoom MJ, Feldman DM, Borgida AF, Esters D, Diana D, Egan JF. Is an isolated fetal cardiac echogenic focus an indication for fetal echocardiography? J Ultrasound Med. 2001; 20(10):1043-6. [DOI:10.7863/jum.2001.20.10.1043] [PMID]
- Liu HH, Lin MT, Chang CC, Wang JK, Wu MH, Shyu MK, et al. Postnatal outcome of fetal cardiac echogenic foci. J Formos Med Assoc Taiwan Yi Zhi. 200; 101(5):329-36. [Link]
- Carriço A, Matias A, Areias JC. How important is a cardiac echogenic focus in a routine fetal examination? Rev Port Cardiol. 2004; 23(3):459-61. [PMID]
- Rebarber A, Levey KA, Funai E, Monda S, Paidas M. An ethnic predilection for fetal echogenic intracardiac focus identified during targeted midtrimester ultrasound examination: A retrospective review. BMC Pregnancy Childbirth. 2004; 4(1):12. [DOI:10.1186/1471-2393-4-12] [PMID] [PMCID]
- Wax JR, Cartin A, Pinette MG, Blackstone J. Are intracardiac echogenic foci markers of congenital heart disease in the fetus with chromosomal abnormalities? J Ultrasound Med. 2004; 23(7):895-8. [DOI:10.7863/jum.2004.23.7.895] [PMID]
- Bradley KE, Santulli TS, Gregory KD, Herbert W, Carlson DE, Platt LD. An isolated intracardiac echogenic focus as a marker for aneuploidy. Am J Obstet Gynecol. 2005; 192(6):2021-6. [DOI:10.1016/j.ajog.2005.03.033] [PMID]
- Petrikovsky BM, Challanger M, Ansari AH. Multiple isolated intracardiac echogenic foci. Are they significant? Ultrasound Obstet Gynecol. 2005; 26(7):795-6. [DOI:10.1002/uog.2626] [PMID]
- Lim L, Aptekar L, Bombard A, Juliard K, Meenakshi B, Weiner Z. Ethnicity and other factors that may affect the prevalence of echogenic intracardiac foci in the fetus. J Clin Ultrasound. 2006; 34(7):327-9. [DOI:10.1002/jcu.20240] [PMID]
- Gonçalves TR, Zamith MM, Murta CG, Bussamra LC, Torloni MR, Moron AF. Chromosomal and cardiac anomalies in fetuses with intracardiac echogenic foci. Int J Gynaecol Obstet. 2006; 95(2):132-7. [DOI:10.1016/j.ijgo.2006.06.020] [PMID]
- Shanks AL, Odibo AO, Gray DL. Echogenic intracardiac foci: Associated with increased risk for fetal trisomy 21 or not? J Ultrasound Med. 2009; 28(12):1639-43. [DOI:10.7863/jum.2009.28.12.1639] [PMID]
- Hilal K, Masroor I, Hussain Z, Mobin Z. Association of fetal intracardiac echogenic focus with fetal out come. 048-Scientific Exhibit. Paper presented at: European Congress of Radiology-RANZCR-AOCR 2012. 5 March 2012; Vienna, Austria. [Link]
- Chitra N, Vijayalakshmi IB. Fetal echocardiography for early detection of congenital heart diseases. J Echocardiogr. 2017; 15(1):13-7. [DOI:10.1007/s12574-016-0308-2] [PMID]
- Tian Y, Chen X, Wang H, Liu H. A re-investigation of the correlation between echogenic intracardiac focus and chromosomal abnormality. Int J Clin Exp Pathol. 2016; 9(11):11923-7. [Link]
- Guo Y, He Y, Gu X, Zhang Y, Sun L, Liu X, et al. Echogenic intracardiac foci and fetal cardiac anomalies: A review of cases from a tertiary care center in China. J Clin Ultrasound. 2018; 46(2):103-7. [DOI:10.1002/jcu.22533] [PMID]
- Chiu G, Zhao A, Zhang B, Zhang T. Intracardiac echogenic focus and its location: Association with congenital heart defects. J Matern Fetal Neonatal Med. 2019; 32(18):3074-8. [DOI:10.1080/14767058.2018.1558200] [PMID]
- Chiu G, Zhao A, Zhang T. Clinical value of isolated intracardiac echogenic focus in the fetal heart: A retrospective study in Chinese women. Clin Exp Obstet Gynecol. 2019; 46(6):972-6. [DOI:10.12891/ceog5066.2019]
- Akinmoladun JA, Adebayo B, Adekanmi AJ. Intracardiac echogenic focus: Its importance during routine prenatal ultrasound screening in a black African population. West Afr J Radiol. 2020; 27(1):1-6. [DOI:10.4103/wajr.wajr_25_18]
- Özsürmeli M, Susu M, Arslan E, Büyükkurt S. Perinatal outcome of fetuses with echogenic intracardiac focus. Clin Exp Obstet Gynecol. 2020; 47(3):372-5. [DOI:10.31083/j.ceog.2020.03.5121]
- Usta CS, Bulbul CB. Prevalence of echogenic intracardiac focus and its association with fetal aneuploidy and adverse perinatal outcomes in Turkish pregnancies. Ann Med Res. 2020; 27(10):2540-5. [DOI:10.5455/annalsmedres.2020.06.652]
- Song Y, Xu J, Li H, Gao J, Wu L, He G, et al. Application of copy number variation detection to fetal diagnosis of echogenic intracardiac focus during pregnancy. Front Genet. 2021; 12:626044. [DOI:10.3389/fgene.2021.626044] [PMID] [PMCID]
- Wax JR, Royer D, Mather J, Chen C, Aponte-García A, Steinfeld JD, et al. A preliminary study of sonographic grading of fetal intracardiac echogenic foci: Feasibility, reliability and association with aneuploidy. Ultrasound Obstet Gynecol. 2000; 16(2):123-7. [DOI:10.1046/j.1469-0705.2000.00206.x] [PMID]
- Arda S, Sayin NC, Varol FG, Süt N. Isolated fetal intracardiac hyperechogenic focus associated with neonatal outcome and triple test results. Arch Gynecol Obstet. 2007; 276(5):481-5. [DOI:10.1007/s00404-007-0366-9] [PMID]
- Sotiriadis A, Makrydimas G, Ioannidis JP. Diagnostic performance of intracardiac echogenic foci for Down syndrome: A meta-analysis. Obstet Gynecol. 2003; 101(5 Pt 1):1009-16. [DOI:10.1016/s0029-7844(03)00168-6] [PMID]
- Lorente AMR, Moreno-Cid M, Rodríguez MJ, Bueno G, Tenías JM, Román C, et al. Meta-analysis of validity of echogenic intracardiac foci for calculating the risk of Down syndrome in the second trimester of pregnancy. Taiwan J Obstet Gynecol. 2017; 56(1):16-22. [DOI:10.1016/j.tjog.2016.11.002] [PMID]
- Huang SY, Shaw SW, Cheuh HY, Cheng PJ. Intracardiac echogenic focus and trisomy 21 in a population previously evaluated by first-trimester combined screening. Acta Obstet Gynecol Scand. 2010; 89(8):1017-23. [DOI:10.3109/00016349.2010.485631] [PMID]
Type of Study: Review Article |
Subject:
Pediatric Cardiology
Received: 2023/05/2 | Accepted: 2023/07/3 | Published: 2023/07/1
Received: 2023/05/2 | Accepted: 2023/07/3 | Published: 2023/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |