Volume 13, Issue 1 (1-2025)
J. Pediatr. Rev 2025, 13(1): 57-64 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Rajasegaran V P, Tamilarasan P, Thirunavukkarasu B K. Cord Blood Alkaline Phosphatase Level as a Predictor for Neonatal Jaundice in Healthy Term Newborns. J. Pediatr. Rev 2025; 13 (1) :57-64
URL: http://jpr.mazums.ac.ir/article-1-563-en.html
URL: http://jpr.mazums.ac.ir/article-1-563-en.html
1- Department of Pediatrics, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India.
2- Department of Pediatrics, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India. ,preethi.dr@gmail.com
2- Department of Pediatrics, Sri Manakula Vinayagar Medical College and Hospital, Puducherry, India. ,
Full-Text [PDF 448 kb]
(779 Downloads)
| Abstract (HTML) (1996 Views)
Full-Text: (654 Views)
Introduction
Neonatal hyperbilirubinemia is one of the most common benign conditions occurring in the first week of life, affecting both term and preterm infants [1]. It is characterized by yellow discoloration of the skin and sclera, which occurs due to excess bilirubin deposition in the skin and mucous membranes [2]. Jaundice becomes visible clinically when the total serum bilirubin (TSB) level exceeds 5 to 7 mg/dL [3]. The balance between bilirubin synthesis and excretion is the primary determinant of TSB level [4].
Physiological jaundice is the most common jaundice seen in neonates. This condition results from the immature hepatic systems that cannot handle the increased bilirubin load. It is usually seen between 24 and 72 hours after birth.
Pathological jaundice is defined as visible jaundice occurring within the initial 24 hours of life. It is characterized by yellowishness up to palms and soles, TSB levels increasing by more than 0.2 mg/dL/h or more than 5 mg/dL/d, or TSB concentration exceeding the 95th percentile according to the age-specific bilirubin nomogram [3]. High serum bilirubin levels are toxic to the brain and can lead to bilirubin-induced neurological dysfunction (BIND). Mostly, neonatal jaundice is physiological and self-resolving. However, 5%-10% of the babies develop clinically significant jaundice, which requires early therapy to prevent BIND.
Due to the early discharge of neonates from the hospital (caused by various reasons like social reasons, economic constraints, and prevention of nosocomial infections) and increased rate of readmission for hyperbilirubinemia, early diagnosis and timely initiation of treatment is necessary [5]. Many noninvasive, simple, and reliable markers for early prediction of neonatal jaundice have been studied, including cord blood bilirubin, albumin, hydrogen peroxide, and alpha-fetoprotein [6, 7, 8]. Only a few studies have shown whether cord blood alkaline phosphatase (ALP) can be an early indicator of severe neonatal hyperbilirubinemia [9]. Hence, this study was conducted to ascertain the role of ALP as an indicator of significant jaundice.
ALP is an intracellular hydrolase enzyme found in all body cells, especially the red blood cells. Hence, the enzyme gets released into the plasma during hemolysis and can be used as an early indicator of red blood cell hemolysis, which may eventually result in severe hyperbilirubinemia [10].
Study objectives
We compared the cord ALP levels between jaundiced and non-jaundiced term neonates without risk factors and determined the cut-off value. We intend to correlate the cord ALP values with serum bilirubin values in newborn jaundices.
Methods
Study setting
This study is hospital-based and was conducted in Sri Manakula Vinayagar Medical College and Hospital (SMVMCH), Puducherry, under the Department of Pediatrics. SMVMCH is a 950-bed tertiary care center in a semi-urban area of Puducherry, southern India.
Study design and participants
This research was a hospital-based prospective cohort study. All the healthy term newborns born at 37 and 42 weeks of gestation to healthy mothers satisfying the inclusion and exclusion criteria and consenting for the study were included. The study was conducted over one and a half years from the approval date of the Institutional Ethics Committee, February 2021 to August 2022.
Study procedure
By taking the prevalence of non-physiological jaundice of 19.1% among 560 newborns from a study done in Andhra Pradesh by Sahoo et al. [11], the sample size was calculated to be 124 using the Open Epi Software, version 3 for a finite estimated population of 1000, at 95% confidence interval and 6.5% absolute precision.
All the normal-term babies satisfying the inclusion criteria were included until continuous sampling reached the required sample size.
The inclusion criteria were as follows: Inborn neonate, healthy term, having an appearance, pulse, grimace, activity, and respiration (APGAR) score of more than 7 at the first and fifth minutes of life, lacking significant illness or major congenital anomaly, a healthy mother, and being appropriate for gestational age.
The exclusion criteria were as follows: Outborn neonates; neonates born to mothers with comorbidities like diabetes, bone, kidney, or liver diseases; eclampsia or preterm delivery; having an APGAR score below 7 at the first and fifth minutes of life; neonates with apparent significant congenital malformations, neonatal sepsis, and neonates with cephalhematoma or contusions or significant bleeding.
Data collection
A prospective cohort study was conducted in the Pediatrics Department, SMVMCH, a tertiary-care hospital in Puducherry after the approval of the Research Committee, the Institutional Ethics Committee, and conforming to the 1975 Declaration of Helsinki.
The study was performed on 126 healthy term newborns born between 37 and 42 weeks of gestation after obtaining informed and written consent from the parents. They were delivered vaginally, by instrumental delivery, or by cesarean section, with an APGAR score ≥7 from healthy mothers without comorbidities. Five milliliters of umbilical cord blood was obtained from all neonates enrolled in the study at birth to estimate ALP levels. All the neonates included in the study were further subjected to complete history taking with clinical examination and laboratory investigations (cord blood ALP).
After a complete history and clinical examination, all the neonates included in the study were followed up for 3 days after birth to assess the development of clinical jaundice based on the observation by the parents and or the physicians.
Neonates found to have clinical jaundice were further subjected to measurement of serum bilirubin level and other routine blood investigations and received the appropriate treatment as per the American Academy of Pediatrics (AAP) protocols (2004) [12].
The study population was classified into two groups: Neonates without clinical jaundice and neonates with clinical jaundice. The latter group was subdivided into neonates with clinical jaundice without phototherapy and neonates with clinical jaundice with phototherapy according to AAP protocols (2004) [12].
Neonates with clinical jaundice requiring phototherapy were subjected to clinical evaluation and laboratory investigations, including complete blood count with peripheral smear, C-reactive protein (if necessary), blood grouping and typing, and direct Coombs test (if necessary).
The above investigations are routinely performed for neonates with significant jaundice and were not done solely for this study. All the details will be entered in a pre-structured proforma.
Statistical analysis
The data were collected using the pre-structured proforma, entered into MS Excel, and analyzed using IBM SPSS statistic software, version 24 for Windows.
The distribution of study variables was presented as frequency and percentage for qualitative data and Mean±SD, median, and median for quantitative data.
Independent quantitative data were compared using the sample t-test. Comparison of categorical data was done by χ2 test. The receiver operating curve (ROC) was used to predict the cut-off value of ALP. P<0.05 is taken as significant.
Results
A total of 126 neonates meeting the inclusion and exclusion criteria were enrolled in the study. Out of them, 64 neonates were males, and 62 were females. All neonates were born at term gestation; their mean gestational age was 38 weeks + 4 days, and their mean birth weight was 2.92±0.422 g. Regarding the mode of delivery, most neonates in this study (66.7%) were delivered by lower (uterine) segment cesarean section.
Out of the 126 neonates, 97(77%) developed clinical jaundice, and 29(23%) had no jaundice. Among the 97 neonates with clinical jaundice, 24 required phototherapy. Non-jaundiced neonates did not require any intervention. Of the neonates with clinical jaundice, 31.7% had jaundice up to the abdomen, 27.8% had icterus up to the thighs, and only 7.9% had icterus up to the legs. All neonates with jaundice up to legs, palms, and soles significantly correlated with phototherapy requirements.
No statistically significant difference was seen between jaundiced and non-jaundiced neonates, comparing the sociodemographic features and obstetric characteristics like mode of delivery, APGAR score at birth, gender, and gestational age.
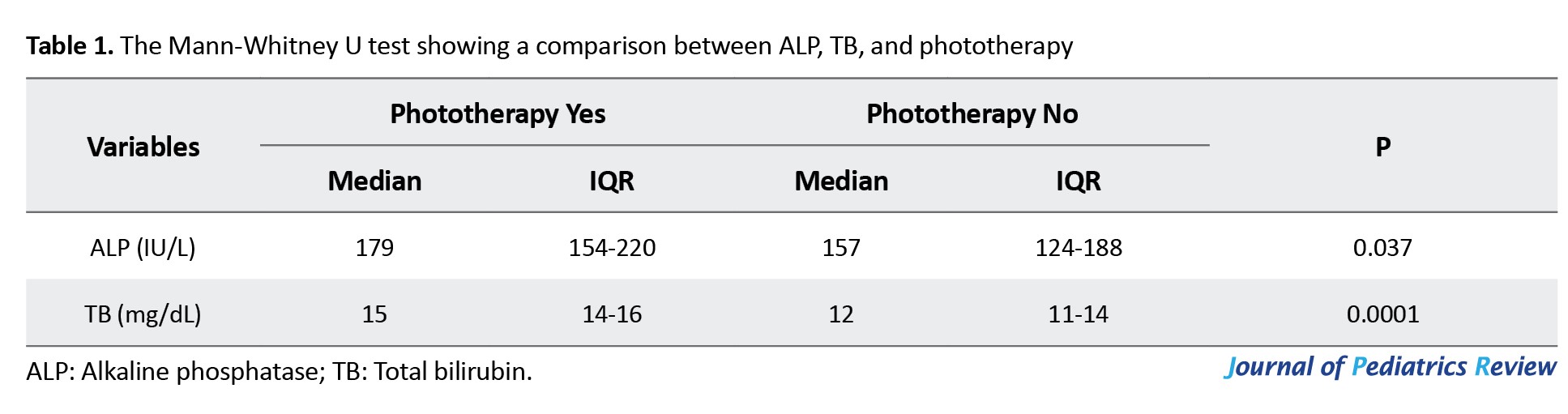
The median total bilirubin (TB) level among neonates who required phototherapy was 15 mg/dL, and among neonates who did not require phototherapy was 12 mg/dL, which was statistically significant.
Using the Mann-Whitney U test, the median ALP level was 179 IU/L among neonates who required phototherapy and 157 IU/L among neonates who did not, which was statistically significant (Table 1).
A statistically significant correlation was found between cord blood ALP level and clinical jaundice, with a mean of 174 IU/L and a median of 325 IU/L noted in the jaundice group. These values were higher than the non-jaundice group, with a mean of 154 IU/L and a median of 184 IU/L (Table 2).

This study shows ALP with an AUC value of 0.665, which is satisfactory, as shown in Figure 1. According to the ROC curve, a cut-off level of cord blood ALP above 147 IU/L was found to be the most appropriate cut-off level for predicting neonatal jaundice with sensitivity of 52%, specificity of 66%, positive predictive value of 60.47%, negative predictive value of 57.89% and diagnostic accuracy of 59%.
Discussion
Neonatal jaundice is a common and benign condition during the first week of life. Though most neonatal jaundice cases are physiological and resolve spontaneously, a fraction of newborns may remain unnoticed and go on to develop severe hyperbilirubinemia requiring treatment. This condition may occur due to the early discharge of newborns who were born by normal vaginal delivery, inadequate follow-up, limited resources, lack of knowledge about severe neonatal hyperbilirubinemia, and parental emotional attachment. The main aim of this prospective cohort study was to determine whether cord blood ALP can be used as a predictor of neonatal jaundice so that early treatment initiation can be made, thereby reducing the complications, morbidity, and mortality due to BIND.
Among the 126 healthy term neonates, there was no significant correlation seen between APGAR score at birth, mode of delivery, gender, and gestational age with the development of clinical jaundice. This outcome agrees with several studies, who concluded no significant relationship between gender and significant neonatal hyperbilirubinemia [13-16]. In contrast, some studies (Satrya et al., Maisels and Kring, and El-Gendy et al.) found a significant correlation between gender and neonatal hyperbilirubinemia [17-19]. In terms of the mode of delivery, no significant correlation was found between the mode of delivery and neonatal hyperbilirubinemia. However, Awasthi and Rehman determined that serum bilirubin levels were higher in neonates who were delivered by normal vaginal delivery [20].
Comparing the gestational age, this study agreed with a study by El-Amin et al., who also found no statistically significant correlation, but Watchko et al. concluded that neonatal hyperbilirubinemia is less common as gestational age increases [21].
Comparison of ALP between jaundiced and non-jaundiced neonates showed a significant correlation between cord blood ALP and the development of clinical jaundice. The mean cord blood ALP level was 174.062±61.55 IU/L in jaundiced neonates and 154.10±47.77 IU/L in non-jaundiced neonates, which is statistically significant (P=0.0001). Eid et al. concluded in their study that the cord blood ALP level was higher in neonates with pathological hyperbilirubinemia who required treatment. Their mean cord blood ALP level in the treatment group vs non-jaundice and the non-treatment group was 352.11±53.49 vs 204.89±34.5 and 217.10±49.51, respectively [5].
In this study, the Mann-Whitney U test showed a significant correlation between cord blood ALP level, TSB, and phototherapy requirement. The median ALP level was around 179 IU/L among neonates who required phototherapy and 157 IU/L among neonates who did not, which is statistically significant (P=0.037). The median TB level among neonates who required phototherapy was around 15 mg/dL, and among neonates who did not require phototherapy was around 12 mg/dL, which is statistically significant (P=0.0001). This finding was in agreement with some studies (Eid et al., Nalbantoglu et al., and Ahmadpour-Kacho et al.), who identified a significant correlation between cord blood ALP level and jaundiced neonates requiring phototherapy [5, 9, 22]. However, Saboute et al. found that although ALP level was higher in neonates with pathological jaundice than the neonates without pathological jaundice, there was no significant correlation between the ALP level and serum bilirubin level [23].
In this study, the AUC for ALP was 0.665, which is satisfactory. According to the ROC curve of this study, the cord blood ALP level above 147 IU/L was the most appropriate cut-off value for predicting jaundice, with a sensitivity of 52% and specificity of 66%. This finding was not consistent with the ROC curve analysis of El-Amin et al. They showed that a cord blood ALP level of more than 145 IU/L has a good predictive value for identifying significant hyperbilirubinemia with a sensitivity of 72% and specificity of 85.71% [13]. In a study by Ahmadpour-Kacho et al., a cord blood ALP level of more than 314 IU/L had a sensitivity of 80% and a specificity of 63% in determining the risk of neonatal hyperbilirubinemia requiring treatment [22]. In another study by Al Assal et al., a cord blood ALP level of more than 314 IU/L had a sensitivity of 84.21% and specificity of 84.48% in determining the risk of neonatal hyperbilirubinemia requiring treatment [24]. This difference might be due to the small sample size, the inclusion of only healthy term neonates by us, and the higher cut-off value in their studies, which are the limitations of our study.
Several predictors of neonatal jaundice have been studied to identify those neonates who are at increased risk of developing subsequent hyperbilirubinemia and also to shorten the hospital stay of healthy newborns born at term gestation.
Deghady et al. found that the cord blood bilirubin-albumin ratio was the most accurate, followed by cord blood bilirubin and cord blood albumin in predicting neonatal hyperbilirubinemia. The least accurate was cord blood hydrogen peroxide [6]. El Mashad et al. concluded that cord blood albumin levels below 2.8 g/dL and cord blood bilirubin level of 1.88 mg/dL can be used as an early helpful marker for predicting significant hyperbilirubinemia [25]. This finding was not in agreement with a study carried out by Carbonell et al., who found that a cord bilirubin level of 2.2 mg/dL showed a sensitivity of 22.2% and a negative predictive value of 97.4% in predicting subsequent neonatal jaundice [26]. Chou et al. studied cord blood hydrogen peroxide. They found that a signal level of 2500 counts/10 seconds was the most appropriate cut-off value for determining severe neonatal hyperbilirubinemia with a sensitivity of 76.2%, specificity of 54.8%, and negative predictive value of 93.3 % [7]. Zahedpasha et al. found that umbilical cord alpha-fetoprotein levels had no significant positive association with the development of subsequent indirect neonatal hyperbilirubinemia [8].
Conclusion
Despite low sensitivity and specificity in this study, possibly due to low sample size and low cut-off value, cord ALP showed a statistically significant correlation in predicting the risk of neonatal hyperbilirubinemia and the need for phototherapy. Hence, cord blood ALP can be taken as a reliable marker in healthy term neonates, such that even if they are discharged early from the hospital, neonates above the cut-off value can be followed up on an out-patient basis for timely detection of hyperbilirubinemia and initiation of treatment. This finding provides a cost-effective screening tool, which may also be combined with other predictors for accurately identifying at-risk newborns, especially in resource-restricted settings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Institutional Ethics Committee of Sri Manakula Vinayagar Medical College and Hospital (SMVMCH), Puducherry, India (Code: SMVMCH-ECO/AL/121/2021). The study was done with strict adherence to the 1975 Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, study design, data analysis and interpretation: All authors; Data acquisition: Vishnu Priyaa Rajasegaran; Statistical analysis and writing the original draft: Vishnu Priyaa Rajasegaran and Preethi Tamilarasan; Review and editing: Tamilarasan and Thirunavukkarasu; Supervision, project administration and technical, and material support: Bharath Kumar Thirunavukkarasu;
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Department of Community Medicine, Sri Manakula Vinayagar Medical College and Hospital (SMVMCH), Puducherry, India for their support with statistical analysis.
References
Neonatal hyperbilirubinemia is one of the most common benign conditions occurring in the first week of life, affecting both term and preterm infants [1]. It is characterized by yellow discoloration of the skin and sclera, which occurs due to excess bilirubin deposition in the skin and mucous membranes [2]. Jaundice becomes visible clinically when the total serum bilirubin (TSB) level exceeds 5 to 7 mg/dL [3]. The balance between bilirubin synthesis and excretion is the primary determinant of TSB level [4].
Physiological jaundice is the most common jaundice seen in neonates. This condition results from the immature hepatic systems that cannot handle the increased bilirubin load. It is usually seen between 24 and 72 hours after birth.
Pathological jaundice is defined as visible jaundice occurring within the initial 24 hours of life. It is characterized by yellowishness up to palms and soles, TSB levels increasing by more than 0.2 mg/dL/h or more than 5 mg/dL/d, or TSB concentration exceeding the 95th percentile according to the age-specific bilirubin nomogram [3]. High serum bilirubin levels are toxic to the brain and can lead to bilirubin-induced neurological dysfunction (BIND). Mostly, neonatal jaundice is physiological and self-resolving. However, 5%-10% of the babies develop clinically significant jaundice, which requires early therapy to prevent BIND.
Due to the early discharge of neonates from the hospital (caused by various reasons like social reasons, economic constraints, and prevention of nosocomial infections) and increased rate of readmission for hyperbilirubinemia, early diagnosis and timely initiation of treatment is necessary [5]. Many noninvasive, simple, and reliable markers for early prediction of neonatal jaundice have been studied, including cord blood bilirubin, albumin, hydrogen peroxide, and alpha-fetoprotein [6, 7, 8]. Only a few studies have shown whether cord blood alkaline phosphatase (ALP) can be an early indicator of severe neonatal hyperbilirubinemia [9]. Hence, this study was conducted to ascertain the role of ALP as an indicator of significant jaundice.
ALP is an intracellular hydrolase enzyme found in all body cells, especially the red blood cells. Hence, the enzyme gets released into the plasma during hemolysis and can be used as an early indicator of red blood cell hemolysis, which may eventually result in severe hyperbilirubinemia [10].
Study objectives
We compared the cord ALP levels between jaundiced and non-jaundiced term neonates without risk factors and determined the cut-off value. We intend to correlate the cord ALP values with serum bilirubin values in newborn jaundices.
Methods
Study setting
This study is hospital-based and was conducted in Sri Manakula Vinayagar Medical College and Hospital (SMVMCH), Puducherry, under the Department of Pediatrics. SMVMCH is a 950-bed tertiary care center in a semi-urban area of Puducherry, southern India.
Study design and participants
This research was a hospital-based prospective cohort study. All the healthy term newborns born at 37 and 42 weeks of gestation to healthy mothers satisfying the inclusion and exclusion criteria and consenting for the study were included. The study was conducted over one and a half years from the approval date of the Institutional Ethics Committee, February 2021 to August 2022.
Study procedure
By taking the prevalence of non-physiological jaundice of 19.1% among 560 newborns from a study done in Andhra Pradesh by Sahoo et al. [11], the sample size was calculated to be 124 using the Open Epi Software, version 3 for a finite estimated population of 1000, at 95% confidence interval and 6.5% absolute precision.
All the normal-term babies satisfying the inclusion criteria were included until continuous sampling reached the required sample size.
The inclusion criteria were as follows: Inborn neonate, healthy term, having an appearance, pulse, grimace, activity, and respiration (APGAR) score of more than 7 at the first and fifth minutes of life, lacking significant illness or major congenital anomaly, a healthy mother, and being appropriate for gestational age.
The exclusion criteria were as follows: Outborn neonates; neonates born to mothers with comorbidities like diabetes, bone, kidney, or liver diseases; eclampsia or preterm delivery; having an APGAR score below 7 at the first and fifth minutes of life; neonates with apparent significant congenital malformations, neonatal sepsis, and neonates with cephalhematoma or contusions or significant bleeding.
Data collection
A prospective cohort study was conducted in the Pediatrics Department, SMVMCH, a tertiary-care hospital in Puducherry after the approval of the Research Committee, the Institutional Ethics Committee, and conforming to the 1975 Declaration of Helsinki.
The study was performed on 126 healthy term newborns born between 37 and 42 weeks of gestation after obtaining informed and written consent from the parents. They were delivered vaginally, by instrumental delivery, or by cesarean section, with an APGAR score ≥7 from healthy mothers without comorbidities. Five milliliters of umbilical cord blood was obtained from all neonates enrolled in the study at birth to estimate ALP levels. All the neonates included in the study were further subjected to complete history taking with clinical examination and laboratory investigations (cord blood ALP).
After a complete history and clinical examination, all the neonates included in the study were followed up for 3 days after birth to assess the development of clinical jaundice based on the observation by the parents and or the physicians.
Neonates found to have clinical jaundice were further subjected to measurement of serum bilirubin level and other routine blood investigations and received the appropriate treatment as per the American Academy of Pediatrics (AAP) protocols (2004) [12].
The study population was classified into two groups: Neonates without clinical jaundice and neonates with clinical jaundice. The latter group was subdivided into neonates with clinical jaundice without phototherapy and neonates with clinical jaundice with phototherapy according to AAP protocols (2004) [12].
Neonates with clinical jaundice requiring phototherapy were subjected to clinical evaluation and laboratory investigations, including complete blood count with peripheral smear, C-reactive protein (if necessary), blood grouping and typing, and direct Coombs test (if necessary).
The above investigations are routinely performed for neonates with significant jaundice and were not done solely for this study. All the details will be entered in a pre-structured proforma.
Statistical analysis
The data were collected using the pre-structured proforma, entered into MS Excel, and analyzed using IBM SPSS statistic software, version 24 for Windows.
The distribution of study variables was presented as frequency and percentage for qualitative data and Mean±SD, median, and median for quantitative data.
Independent quantitative data were compared using the sample t-test. Comparison of categorical data was done by χ2 test. The receiver operating curve (ROC) was used to predict the cut-off value of ALP. P<0.05 is taken as significant.
Results
A total of 126 neonates meeting the inclusion and exclusion criteria were enrolled in the study. Out of them, 64 neonates were males, and 62 were females. All neonates were born at term gestation; their mean gestational age was 38 weeks + 4 days, and their mean birth weight was 2.92±0.422 g. Regarding the mode of delivery, most neonates in this study (66.7%) were delivered by lower (uterine) segment cesarean section.
Out of the 126 neonates, 97(77%) developed clinical jaundice, and 29(23%) had no jaundice. Among the 97 neonates with clinical jaundice, 24 required phototherapy. Non-jaundiced neonates did not require any intervention. Of the neonates with clinical jaundice, 31.7% had jaundice up to the abdomen, 27.8% had icterus up to the thighs, and only 7.9% had icterus up to the legs. All neonates with jaundice up to legs, palms, and soles significantly correlated with phototherapy requirements.
No statistically significant difference was seen between jaundiced and non-jaundiced neonates, comparing the sociodemographic features and obstetric characteristics like mode of delivery, APGAR score at birth, gender, and gestational age.
The median total bilirubin (TB) level among neonates who required phototherapy was 15 mg/dL, and among neonates who did not require phototherapy was 12 mg/dL, which was statistically significant.
Using the Mann-Whitney U test, the median ALP level was 179 IU/L among neonates who required phototherapy and 157 IU/L among neonates who did not, which was statistically significant (Table 1).

A statistically significant correlation was found between cord blood ALP level and clinical jaundice, with a mean of 174 IU/L and a median of 325 IU/L noted in the jaundice group. These values were higher than the non-jaundice group, with a mean of 154 IU/L and a median of 184 IU/L (Table 2).

This study shows ALP with an AUC value of 0.665, which is satisfactory, as shown in Figure 1. According to the ROC curve, a cut-off level of cord blood ALP above 147 IU/L was found to be the most appropriate cut-off level for predicting neonatal jaundice with sensitivity of 52%, specificity of 66%, positive predictive value of 60.47%, negative predictive value of 57.89% and diagnostic accuracy of 59%.
Discussion
Neonatal jaundice is a common and benign condition during the first week of life. Though most neonatal jaundice cases are physiological and resolve spontaneously, a fraction of newborns may remain unnoticed and go on to develop severe hyperbilirubinemia requiring treatment. This condition may occur due to the early discharge of newborns who were born by normal vaginal delivery, inadequate follow-up, limited resources, lack of knowledge about severe neonatal hyperbilirubinemia, and parental emotional attachment. The main aim of this prospective cohort study was to determine whether cord blood ALP can be used as a predictor of neonatal jaundice so that early treatment initiation can be made, thereby reducing the complications, morbidity, and mortality due to BIND.
Among the 126 healthy term neonates, there was no significant correlation seen between APGAR score at birth, mode of delivery, gender, and gestational age with the development of clinical jaundice. This outcome agrees with several studies, who concluded no significant relationship between gender and significant neonatal hyperbilirubinemia [13-16]. In contrast, some studies (Satrya et al., Maisels and Kring, and El-Gendy et al.) found a significant correlation between gender and neonatal hyperbilirubinemia [17-19]. In terms of the mode of delivery, no significant correlation was found between the mode of delivery and neonatal hyperbilirubinemia. However, Awasthi and Rehman determined that serum bilirubin levels were higher in neonates who were delivered by normal vaginal delivery [20].
Comparing the gestational age, this study agreed with a study by El-Amin et al., who also found no statistically significant correlation, but Watchko et al. concluded that neonatal hyperbilirubinemia is less common as gestational age increases [21].
Comparison of ALP between jaundiced and non-jaundiced neonates showed a significant correlation between cord blood ALP and the development of clinical jaundice. The mean cord blood ALP level was 174.062±61.55 IU/L in jaundiced neonates and 154.10±47.77 IU/L in non-jaundiced neonates, which is statistically significant (P=0.0001). Eid et al. concluded in their study that the cord blood ALP level was higher in neonates with pathological hyperbilirubinemia who required treatment. Their mean cord blood ALP level in the treatment group vs non-jaundice and the non-treatment group was 352.11±53.49 vs 204.89±34.5 and 217.10±49.51, respectively [5].
In this study, the Mann-Whitney U test showed a significant correlation between cord blood ALP level, TSB, and phototherapy requirement. The median ALP level was around 179 IU/L among neonates who required phototherapy and 157 IU/L among neonates who did not, which is statistically significant (P=0.037). The median TB level among neonates who required phototherapy was around 15 mg/dL, and among neonates who did not require phototherapy was around 12 mg/dL, which is statistically significant (P=0.0001). This finding was in agreement with some studies (Eid et al., Nalbantoglu et al., and Ahmadpour-Kacho et al.), who identified a significant correlation between cord blood ALP level and jaundiced neonates requiring phototherapy [5, 9, 22]. However, Saboute et al. found that although ALP level was higher in neonates with pathological jaundice than the neonates without pathological jaundice, there was no significant correlation between the ALP level and serum bilirubin level [23].
In this study, the AUC for ALP was 0.665, which is satisfactory. According to the ROC curve of this study, the cord blood ALP level above 147 IU/L was the most appropriate cut-off value for predicting jaundice, with a sensitivity of 52% and specificity of 66%. This finding was not consistent with the ROC curve analysis of El-Amin et al. They showed that a cord blood ALP level of more than 145 IU/L has a good predictive value for identifying significant hyperbilirubinemia with a sensitivity of 72% and specificity of 85.71% [13]. In a study by Ahmadpour-Kacho et al., a cord blood ALP level of more than 314 IU/L had a sensitivity of 80% and a specificity of 63% in determining the risk of neonatal hyperbilirubinemia requiring treatment [22]. In another study by Al Assal et al., a cord blood ALP level of more than 314 IU/L had a sensitivity of 84.21% and specificity of 84.48% in determining the risk of neonatal hyperbilirubinemia requiring treatment [24]. This difference might be due to the small sample size, the inclusion of only healthy term neonates by us, and the higher cut-off value in their studies, which are the limitations of our study.
Several predictors of neonatal jaundice have been studied to identify those neonates who are at increased risk of developing subsequent hyperbilirubinemia and also to shorten the hospital stay of healthy newborns born at term gestation.
Deghady et al. found that the cord blood bilirubin-albumin ratio was the most accurate, followed by cord blood bilirubin and cord blood albumin in predicting neonatal hyperbilirubinemia. The least accurate was cord blood hydrogen peroxide [6]. El Mashad et al. concluded that cord blood albumin levels below 2.8 g/dL and cord blood bilirubin level of 1.88 mg/dL can be used as an early helpful marker for predicting significant hyperbilirubinemia [25]. This finding was not in agreement with a study carried out by Carbonell et al., who found that a cord bilirubin level of 2.2 mg/dL showed a sensitivity of 22.2% and a negative predictive value of 97.4% in predicting subsequent neonatal jaundice [26]. Chou et al. studied cord blood hydrogen peroxide. They found that a signal level of 2500 counts/10 seconds was the most appropriate cut-off value for determining severe neonatal hyperbilirubinemia with a sensitivity of 76.2%, specificity of 54.8%, and negative predictive value of 93.3 % [7]. Zahedpasha et al. found that umbilical cord alpha-fetoprotein levels had no significant positive association with the development of subsequent indirect neonatal hyperbilirubinemia [8].
Conclusion
Despite low sensitivity and specificity in this study, possibly due to low sample size and low cut-off value, cord ALP showed a statistically significant correlation in predicting the risk of neonatal hyperbilirubinemia and the need for phototherapy. Hence, cord blood ALP can be taken as a reliable marker in healthy term neonates, such that even if they are discharged early from the hospital, neonates above the cut-off value can be followed up on an out-patient basis for timely detection of hyperbilirubinemia and initiation of treatment. This finding provides a cost-effective screening tool, which may also be combined with other predictors for accurately identifying at-risk newborns, especially in resource-restricted settings.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Institutional Ethics Committee of Sri Manakula Vinayagar Medical College and Hospital (SMVMCH), Puducherry, India (Code: SMVMCH-ECO/AL/121/2021). The study was done with strict adherence to the 1975 Declaration of Helsinki.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, study design, data analysis and interpretation: All authors; Data acquisition: Vishnu Priyaa Rajasegaran; Statistical analysis and writing the original draft: Vishnu Priyaa Rajasegaran and Preethi Tamilarasan; Review and editing: Tamilarasan and Thirunavukkarasu; Supervision, project administration and technical, and material support: Bharath Kumar Thirunavukkarasu;
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Department of Community Medicine, Sri Manakula Vinayagar Medical College and Hospital (SMVMCH), Puducherry, India for their support with statistical analysis.
References
- Rennie J, Burman-Roy S, Murphy MS; Guideline Development Group. Neonatal jaundice: Summary of NICE guidance. BMJ. 2010; 340:c2409. [DOI:10.1136/bmj.c2409] [PMID]
- Woodgate P, Jardine LA. Neonatal jaundice. BMJ Clin Evid. 2011; 2011:0319. [PMID]
- Agarwal R, Deorari A, Paul VK. AIIMS protocols in Neonatology. Delhi: Noble Vision Publishers; 2019. [Link]
- Eichenwald EC, Hansen AR. Cloherty and Stark’s Manual of Neonatal Care. Philadelphia: Lippincott Williams & Wilkins/Wolters Kluwer; 2021. [Link]
- Eid S, Mohamed AG, Okda HT, Salam HM. Evaluation of cord blood alkaline phosphatase as a predictor of hyperbilirubinemia in Egyptian neonates. J Arab Soc Med Res. 2020; 15(1):11-17. [DOI:10.4103/jasmr.jasmr_30_19]
- Deghady SA, Rowisha MA, Ibrahim AM, Kishk WA. Cord blood bilirubin, albumin and bilirubin/albumin ratio versus hydrogen peroxide as early predictors of significant neonatal hyperbilirubinemia. J Adv Med Med Res. 2020; 32(18):34-42. [DOI:10.9734/jammr/2020/v32i1830653]
- Chou HC, Chien CT, Tsao PN, Hsieh WS, Chen CY, Chang MH. Prediction of severe neonatal hyperbilirubinemia using cord blood hydrogen peroxide: A prospective study. Plos One. 2014; 9(1):e86797. [DOI:10.1371/journal.pone.0086797] [PMID]
- Zahedpasha Y, Ahmadpour-Kacho M, Khalafi J, Bijani A. Cord blood-fetoprotein as a predictive index for indirect hyperbilirubinemia in term neonates. Caspian J Intern Med. 2011; 2(4):326-30. [PMID]
- Nalbantoğlu A, Ovali F, Nalbantoğlu B. Alkaline phosphatase as an early marker of hemolysis in newborns. Pediatr Int. 2011; 53(6):936-8. [DOI:10.1111/j.1442-200X.2011.03491.x] [PMID]
- Tinnion RJ, Embleton ND. How to use… alkaline phosphatase in neonatology. Arch Dis Child Educ Pract Ed. 2012; 97(4):157-63. [DOI:10.1136/archdischild-2012-301633] [PMID]
- Sahoo M, Arigela V, Pramitha L, Sudarsini P, Rao KU. Study of neonatal jaundice in a tertiary care centre of South India. Int J Pediatr Res. 2016; 3(8):589-8. [DOI:10.17511/ijpr.2016.i08.07]
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of Hyperbilirubinemia in the Newborn Infant 35 or More Weeks of gestation. Pediatrics. 2004; 114(1):297-316. [DOI:10.1542/peds.114.1.297] [PMID]
- El-Amin DM, Ahmed YA, Hashim AM, Asmail AH. Evaluation of cord blood alkaline phosphatase levels as an indicator of neonatal jaundice. Al-Azhar Assiut Med J. 2020; 18(2):112-7. [DOI:10.4103/AZMJ.AZMJ_92_19]
- Rostami N, Mehrabi YE, Asadzadeh F. Identifying the newborns at risk for developing significant hyperbilirubinemia by measuring cord bilirubin levels. J Arab Neonatal Forum. 2005; 365-9. [Link]
- Taksande A, Vilhekar K, Jain M, Zade P, Atkari S, Verkey S. Prediction of the development of neonatal hyperbilirubinemia by increased umbilical cord blood bilirubin. Curr Pediatr. 2005; 9:5-9. [Link]
- Sahu S, Abraham R, John J, Mathew MA. Cord blood albumin as a predictor of neonatal jaundice. Int J Biol Med Res. 2011; 2(1):436-8. [Link]
- Satrya R, Hidayat Effendi S, Gurnida DA. Correlation between cord blood bilirubin level and incidence of hyperbilirubinemia in term newborns. Paediatr Indones. 2009; 49(6):349-54. [DOI:10.14238/pi49.6.2009.349-54]
- Maisels MJ, Kring E. Length of stay Jaundice and hospital readmission. Pediatrics. 1998; 101(6):995-8. [DOI:10.1542/peds.101.6.995] [PMID]
- Khattab AA, El-Lahony DM, El-Gendy Fady M, Hassane Fahima M, Ashour Noha M. Predictive ability of first-day serum bilirubin and haptoglobin for subsequent significant hyperbilirubinemia in healthy-term and near-term newborn. Menouf Med J. 2013; 26(2):127. [DOI:10.4103/1110-2098.126143]
- Awasthi S, Rehman H. Early prediction of neonatal hyperbilirubinemia. Indian J Pediatr. 1998; 65:131-9. [Link]
- Watchko JF. Hyperbilirubinemia and bilirubin toxicity in the late preterm infant. Clin Perinatol 2006; 33(4):839-52; abstract ix. [DOI:10.1016/j.clp.2006.09.002] [PMID]
- Ahmadpour-Kacho M, Zahed Pasha Y, Haghshenas M, Akbarian Rad Z, Firouzjahi A, Bijani A, et al. Cord blood alkaline phosphatase as an indicator of neonatal jaundice. Iran J Pediatr. 2015; 25(5):e718. [DOI:10.5812/ijp.718]
- Saboute M, Mahmoudian A, Khalesi N, Vahedi Z, Khosravi N, Allahqoli L. Correlation between Alkaline Phosphatase and Neonatal Jaundice. Med J Islam Repub Iran. 2022; 36:52. [DOI:10.47176/mjiri.36.52] [PMID]
- Al Assal HM, Hablas HR, Afia AA, Khedr MA, El Kzaz HM. Utility of cord blood alkaline phosphatase enzyme as a predictor of significant neonatal jaundice in well term infants. NY Sci J. 2017; 10(4):70-4. [Link]
- El Mashad GM, El Sayed HM, El Shafie WA. Cord blood albumin-bilirubin as a predictor for neonatal hyperbilirubinemia. Menoufia Med J. 2019; 32(3):1071. [DOI:10.4103/mmj.mmj_13_18]
- Carbonell X, Botet F, Figueras J, Riu-Godó A. Prediction of hyperbilirubinemia in the healthy term newborn. Acta Paediatr. 2001; 90(2):166-70. [PMID]
Type of Study: Original Article |
Subject:
Neonatology
Received: 2023/09/4 | Accepted: 2024/11/17 | Published: 2025/01/21
Received: 2023/09/4 | Accepted: 2024/11/17 | Published: 2025/01/21
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |










