Volume 12, Issue 2 (4-2024)
J. Pediatr. Rev 2024, 12(2): 191-198 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Motamedi N, Zamanfar D, Rostamian Motlagh F, Yazdani Charati J. Investigating Diabetes-associated Autoantibodies and Their Relationship to Clinical Characteristics in Children Diagnosed With Type 1 Diabetes. J. Pediatr. Rev 2024; 12 (2) :191-198
URL: http://jpr.mazums.ac.ir/article-1-577-en.html
URL: http://jpr.mazums.ac.ir/article-1-577-en.html
Niloofaralsadat Motamedi1 

 , Daniel Zamanfar *2
, Daniel Zamanfar *2 

 , Fatemeh Rostamian Motlagh3
, Fatemeh Rostamian Motlagh3 

 , Jamshid Yazdani Charati4
, Jamshid Yazdani Charati4 




 , Daniel Zamanfar *2
, Daniel Zamanfar *2 

 , Fatemeh Rostamian Motlagh3
, Fatemeh Rostamian Motlagh3 

 , Jamshid Yazdani Charati4
, Jamshid Yazdani Charati4 


1- Department of Pediatrics, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Diabetes Research Center, Mazandaran University of Medical Sciences, Sari, Iran. ,danielzamanfar@yahoo.com
3- Department of Medicine, Faculty of Medical Sciences, Sari Branch, Islamic Azad University, Sari, Iran.
4- Department of Biostatistics, Health Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Diabetes Research Center, Mazandaran University of Medical Sciences, Sari, Iran. ,
3- Department of Medicine, Faculty of Medical Sciences, Sari Branch, Islamic Azad University, Sari, Iran.
4- Department of Biostatistics, Health Sciences Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 390 kb]
(1100 Downloads)
| Abstract (HTML) (3184 Views)
Full-Text: (1288 Views)
Introduction
In type 1 diabetes mellitus (T1DM), genetic predisposition and environmental factors cause pancreatic βcell autoimmunity, ultimately leading to loss of function and destruction [1]. T1DM is characterized as either autoimmune of autoantibody-positive (iAb+) or idiopathic (iAb-). Autoantibody-positive diabetes is the most common T1DM, in which islet autoantibodies (iAb) destroy pancreatic cells [2]. The cause of β cell autoimmunity is unknown. Once β cell autoimmune is developed, the path to clinical T1DM may be divided into three stages: 1) Asymptomatic β cell autoimmunity with normoglycemia; 2) Asymptomatic β cell autoimmunity with dysglycemia; and 3) symptomatic T1DM [3]. Glutamic acid decarboxylase (GADA), islet cell cytoplasmic (ICA), insulinoma-associated-2/tyrosine phosphatase (IA-2A), insulin autoantibody (IAA), and zinc transporter-8 autoantibodies are the five types of T1DM iAb [4]. The number of identified autoantibodies is connected to the likelihood of clinical onset, with the presence of two or more autoantibodies associated with the greatest increase in risk [5]. As a result, the disease process begins with a single autoantibody, followed by intermolecular epitope spreading to multiple autoantibodies, loss of insulin secretory capability due to a combination of beta cell destruction, and function inhibition, leading to metabolic changes, and finally diabetes [6]. The detection of islet autoantibodies in young children peaks between 9 months and 2 years of age, with no seroconversion occurring at 3 or 6 months of age in children born to a mother or father with T1DM [7]. The incidence rate of T1DM is 15 per 100000 individuals, and its prevalence is 5.9 per 10000 people worldwide [1]. In Iran, the incidence rate is 11 per 100000 individuals, and its prevalence is 388.9 per 100000 people [2]. T1DM is often identified at stage 3 when the disease has advanced to diabetic ketoacidosis, a potentially fatal condition. As a result, it is critical to employ early screening and diagnostic methods to detect autoimmunity already present in the earliest years of life and limit the risk of catastrophic problems [1]. Determining the patterns and trends of iAb incidence in T1DM could advance knowledge of the population of children worldwide at risk of developing the disease and help explain observed variations in incidence, prevalence, and health outcomes of T1DM in children and adolescents between and within countries. Also, deeper comprehension of the prevalence of iAbs worldwide could aid in the early diagnosis and treatment of T1DM and lay the groundwork for future research into the severity of the corresponding autoantibody profiles, which would lower preventable morbidity and mortality among children and adolescents [8]. Accordingly, this study determines the frequency of GADA ICA, IA-2A, and IAA in the North of Iran.
Methods
This cross-sectional study was conducted from March 2019 to December 2020 at Bu Ali Sina Hospital in Sari City, Iran.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Children between 3 months to 18 years of age; diagnosis of T1DM by pediatric endocrinologist based on American Diabetes Association (ADA) criteria [9]; consent to participate in the study. Meanwhile, the exclusion criteria were syndromic disease, familial dyslipidemia, chronic cardiac, brain, liver, renal, or co‐infectious disorders, non-type one diabetes mellitus, or lack of informed consent.
Four types of typical autoantibodies (GADA, ICA, IAA, and IA-2A) were examined to confirm the autoimmune diabetes etiology. We used a commercially available enzyme-linked immunosorbent assay to evaluate diabetes-related autoantibodies. The GADA, IA-2A, IAA, and ICA levels were evaluated using a DiaMetra ELISA kit (DiaMetra Co, Milan, Italy) based on the international standard substance National Institute for Biological Standards and Control (NIBSC), which passed the 2015 international diabetes-related antibody standardized test verification (IASP 2015). The upper limit of the normal range was 5 IU/mL for GADA, 2.4 IU/mL for IAA, 1 IU/mL for ICA, and 7.5 IU/mL for IA-2A autoantibodies. Values greater than this cutoff value were considered positive. Also, the HbA1c level was measured using boronate affinity chromatography with the NYCOCARD HbA1c kit and NYCOCARD Reader II, both made by the Norwegian Company Axis-Shield.
The data collection tool was a checklist completed by the project implementers while reviewing the patients’ files. Data extracted from patients’ files included demographic information (age and sex), history profile, and clinical examination (age of onset and initial disease onset, positive family history of T1DM, the simultaneous presence of other autoimmune diseases, weight, and height of patients according to age and sex percentages at the time of diagnosis, etc. and laboratory information of patients (HbA1c, GADA, ICA, IA-2A, IAA) at the time of diagnosis. Ocular complications and microalbuminuria (increased urinary albumin secretion up to 30-300 mg in 24 h or 20-200 mg/min) were evaluated at the five-year mark after the onset of the disease or earlier if the patient had reached the age of 10 or puberty. Also, glycemic control was categorized as HbA1c < 58 mmol/mol (<7.5%), 58–74 mmol/mol (7.5–8.9%), and ≥75 mmol/mol (≥9%) [10]. Hyperglycemia was defined as BS >180 mg/dL [11].
Statistical analysis
The statistical analyses were performed using the SPSS software, version 24. Relationships between variables were tested using the chi-squared test and student t-test. To evaluate the correlation, the Spearman test was used.
The data were described using Mean±SD, and a confidence interval of 95% for numerical variables. Using correlation analysis and linear regression, the effects of each population variable and the identified categories on HbA1c, which indicates long-term blood sugar, were measured.
Results
Overall, 190 patients with T1DM (80 males and 110 females) with a mean age of 13.14±0.36 years were included. There were no significant differences regarding gender between the presence of autoantibodies type (P>0.05). Patients with ICA were significantly older (mean difference=2.02 [95% confidence interval (CI), 0.38%, 3.65%]) than ICA-negative cases (13.80±0.48 vs 11.79±0.67; P=0.01). However, other autoantibodies had similar age distribution in positive and negative cases (P>0.05).
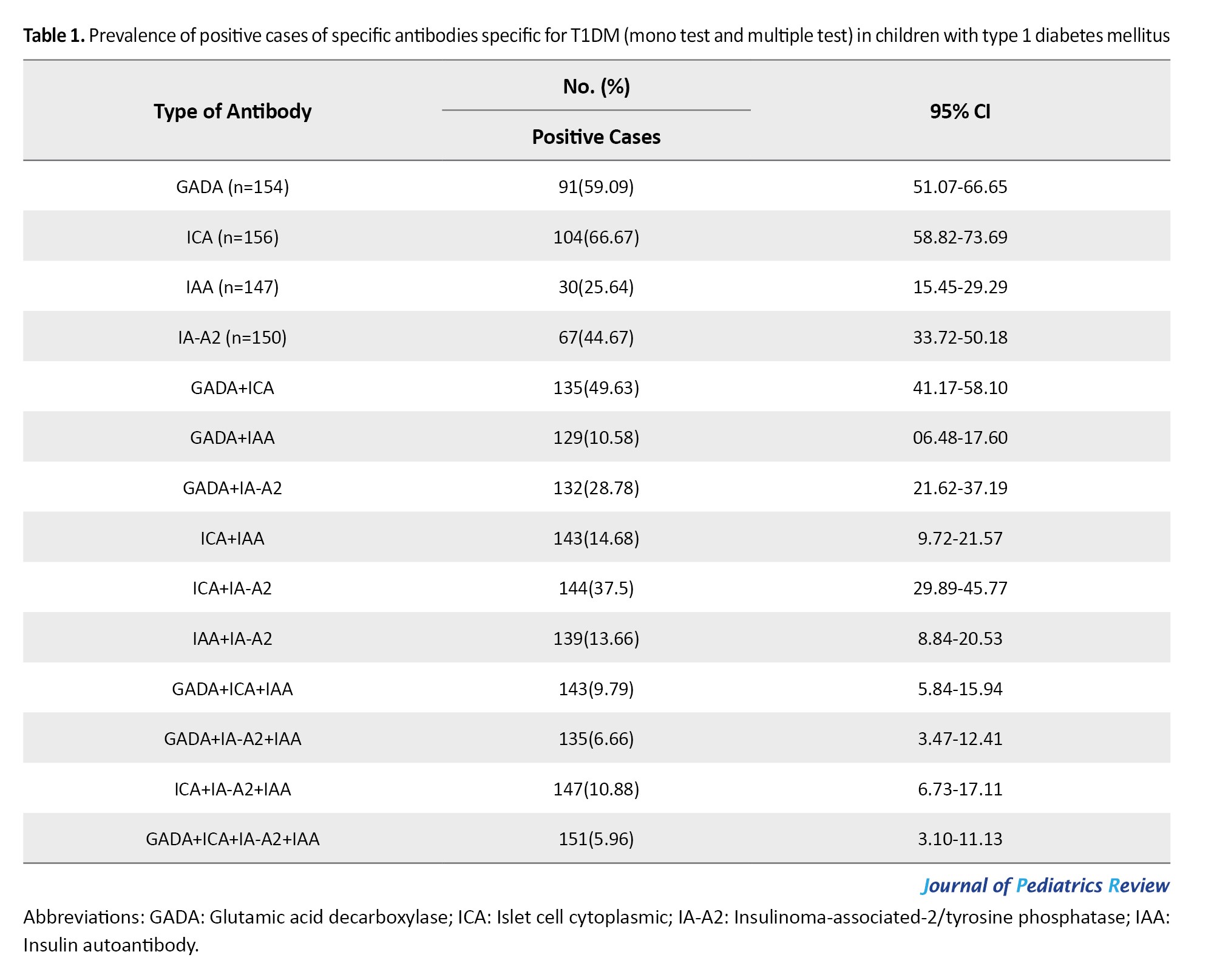
Based on the mono test, the highest prevalence was seen in ICA (104 [66.67%]), and the lowest prevalence was in IAA (30 [25.64%]). Also, based on multiple tests, ICA + GADA, and GADA + ICA + IA-A2 + IAA had the highest and lowest prevalence (135 [49.63%] and 151 [5.96%], respectively) (Table 1).
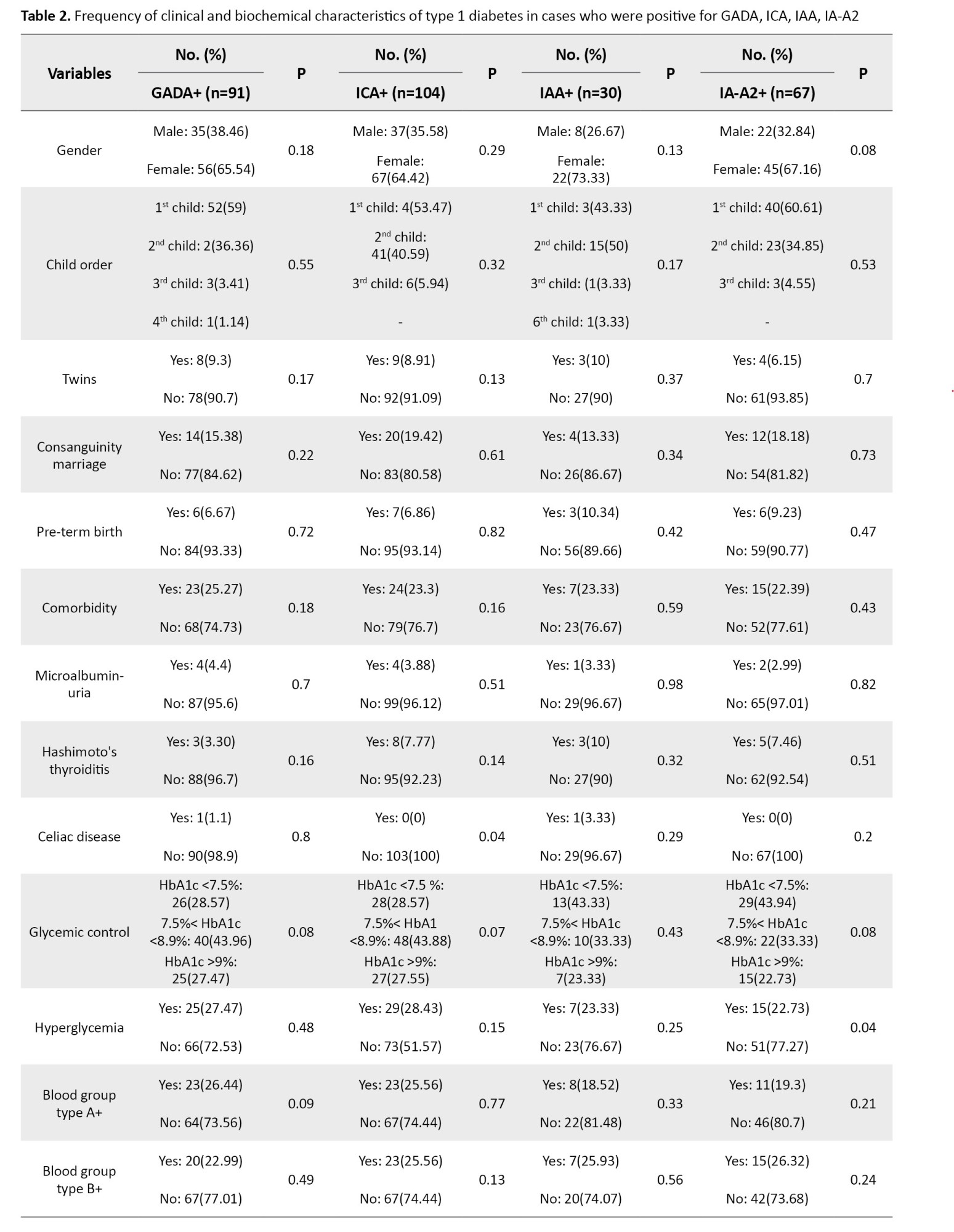
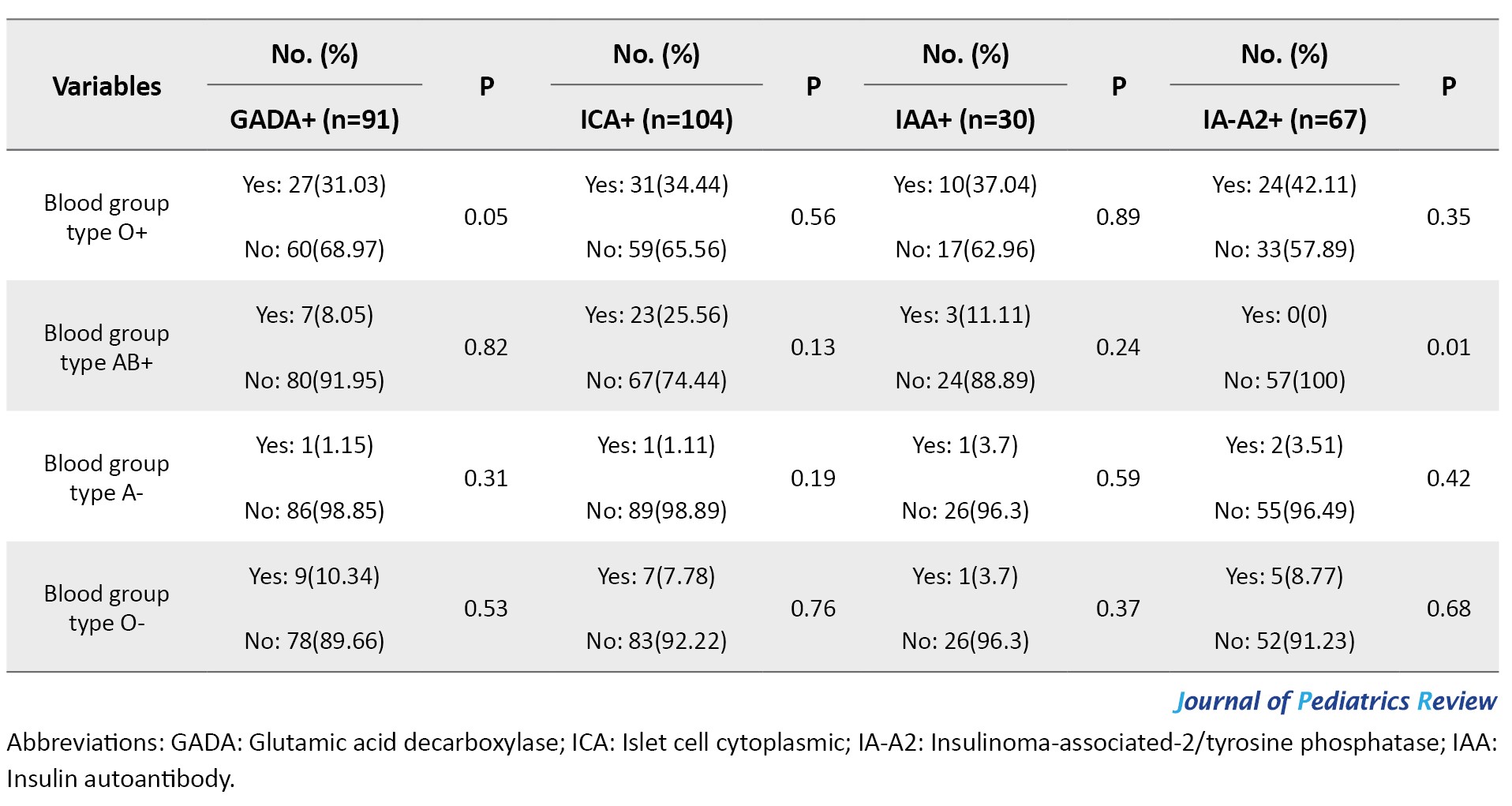
There was no significant difference between the frequency of the child’s rank, Hashimoto’s hypothyroidism and Celiac autoimmune disorders (CD), twinning, consanguineous marriage, birth weight, term or pre-term birth, comorbidities, blood group, the presence of microalbuminuria, and last average of HbA1C with the positive or negative GADA, ICA, IA-2A, IAA (P>0.05) (Table 2).
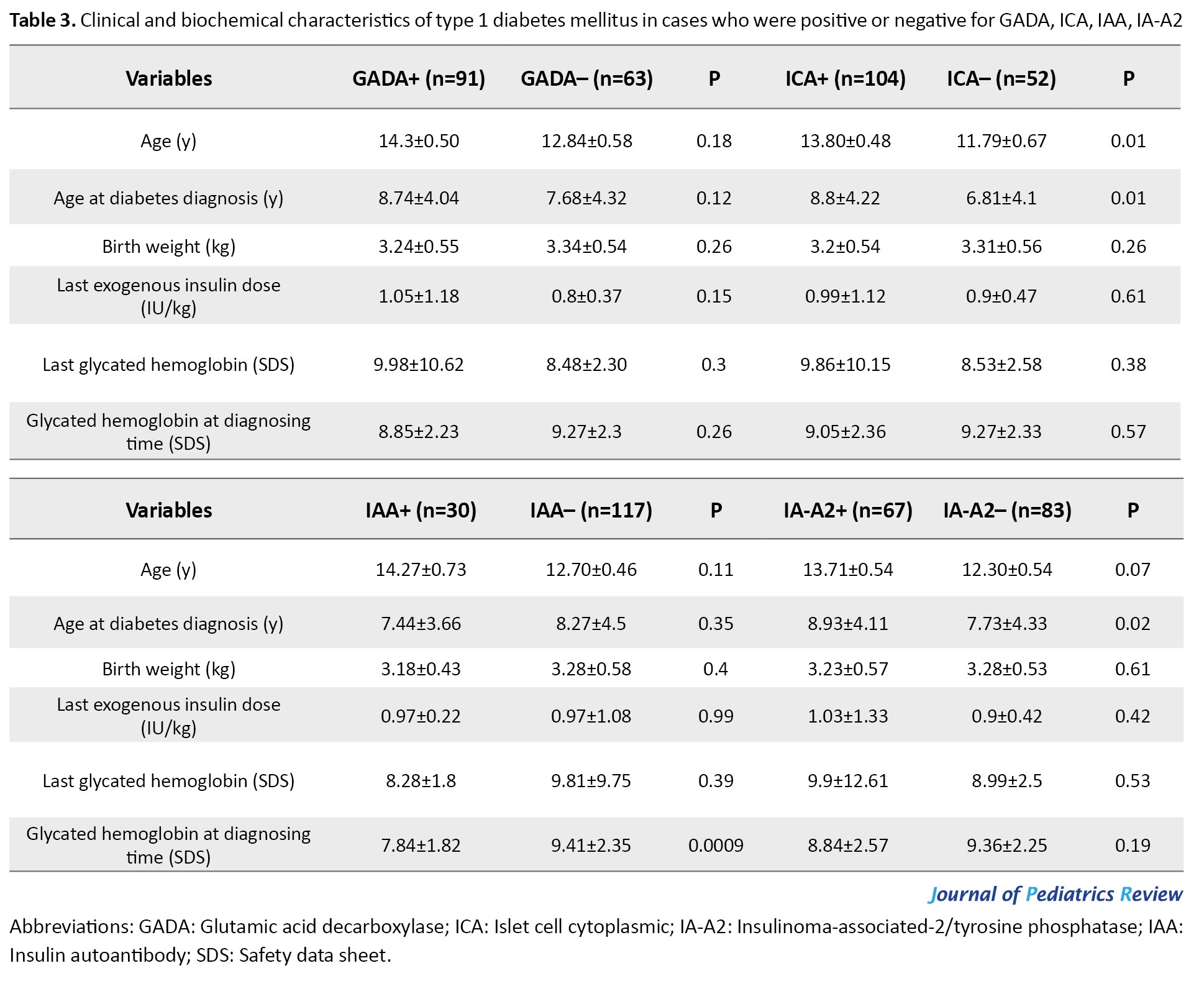
Patients with positive IA-A2 and ICA compared to negative IA-A2 and ICA had higher age at diabetes onset (8.93±4.11 vs 7.73±4.33, P=0.02; 8.8±4.22 vs 6.81±4.1, P=0.01), receptively. There was no significant difference between the presence of GADA and IAA and age at diabetes onset (P>0.05). Moreover, the HbA1c level at T1DM onset in patients with positive IAA was lower than negative IAA (7.84±1.82 vs 9.41±2.35, P=0.0009). Still, the HbA1c level had a similar distribution in patients with positive and negative GADA, IA-A2, and ICA (P>0.05). There was a significant difference in hyperglycemia with positive and negative IA-A2, in which the chance of positive IA-A2 was 54% lower in hyperglycemia than in euglycemia (odd ratio [OR]=0.46 [95% CI, 0.22%, 0.96%], P=0.04). However, there were no significant differences between unfavorable blood sugar (BS) and positive autoantibodies (P>0.05) (Table 3).
Discussion
According to the results, the highest prevalence of T1DM autoantibodies was seen in ICA. Patients with positive IA-A2 and ICA compared to negative IA-A2 and ICA had higher age at the T1DM diagnosing time, the HbA1c level at T1DM diagnosing time in patients with positive IAA was lower than negative IAA, and the chance of positive IA-A2 was 54% lower in unfavorable BS compared to favorable BS. Also, this study found no significant association between positive GADA, ICA, IA-2A, IAA, and the presence of Hashimoto hypothyroidism and CD.
Several studies have linked autoimmune thyroid disorders followed by celiac disease to T1DM [12-14]. Therefore, we examined Hashimoto thyroiditis and CD in our population. We found no significant difference between the relative frequency of patients with Hashimoto thyroiditis and positive or negative results of autoantibodies. In a previous study, anti-thyroid peroxidase was positive in 11% of GADA-positive, 16% of ICA-positive, and 11.6% of IAA-positive T1DM patients [15]. Thyroid autoimmunity was shown to be more common in T1DM patients with positive autoantibodies [16]. A study on the prevalence of gliadin immunoglobulin G/immunoglobulin A and transglutaminase immunoglobulin A was significantly higher in recent-onset T1DM patients with positive GADA, IAA, IA-A2, and ICA [17]. In a study on the co-occurrence of T1DM and CD. In terms of autoimmunity, it was found that islet autoantibodies typically appeared before tissue transglutaminase autoantibodies (tTGAs). Islet autoantibodies preceding tTGAs were linked with an increased probability of tTGAs (hazard ratio [HR]: 1.48; 95% CI, 1.15%, 1.91%) [18]. Also, based on a systematic review and meta-analysis on screening for CD in T1DM patients, since the majority of CD are discovered within five years after T1D diagnosis, screening should be considered at the time of T1D diagnosis and every 2 to 5 years afterward [19]. Accordingly, the non-significant association could be due to the late occurrence of CD after T1DM.
There are conflicting results regarding the most common autoantibodies in patients with T1DM. In our study, the highest prevalence was related to GADA and ICA in children with T1DM, and the lowest prevalence was seen in the combination of GADA+ ICA+ IA-A2+ IAA. In a study on T1DM autoantibodies in the Finland study group, the highest prevalence was reported for GADA and IA-A2 [20]. Some studies on Iranian and Saudi patients identified ICA and anti-IAA as the most prevalent autoantibodies in T1DM patients [21, 22]. These differences could be due to the effect of geographic regions [23].
Conclusion
Understanding the regional prevalence of iAb in T1DM children and adolescents could aid in the earlier identification of those at risk of developing T1DM and inform clinical practice, health policies, resource allocation, and targeted healthcare interventions to better screen, diagnose, and manage T1DM children and adolescents.
Ethical Considerations
Compliance with ethical guidelines
The Local Human Research Ethics Committee of Mazandaran University of Medical Sciences, approved the study protocol (Code: IR.MAZUMS.RIB.REC.1400.022). The study was conducted per the principles of the Declaration of Helsinki. After thoroughly explaining the study, all parents of the children enrolled in the study completed informed consent forms. All patients received adequate insulin replacement therapy.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, methodology, investigation and writing: All authors; Formal analysis: Jamshid Yazdani Charati.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Health Department of Mazandaran University of Medical Sciences and the Bu Ali Sina Hospital staff.
References
In type 1 diabetes mellitus (T1DM), genetic predisposition and environmental factors cause pancreatic βcell autoimmunity, ultimately leading to loss of function and destruction [1]. T1DM is characterized as either autoimmune of autoantibody-positive (iAb+) or idiopathic (iAb-). Autoantibody-positive diabetes is the most common T1DM, in which islet autoantibodies (iAb) destroy pancreatic cells [2]. The cause of β cell autoimmunity is unknown. Once β cell autoimmune is developed, the path to clinical T1DM may be divided into three stages: 1) Asymptomatic β cell autoimmunity with normoglycemia; 2) Asymptomatic β cell autoimmunity with dysglycemia; and 3) symptomatic T1DM [3]. Glutamic acid decarboxylase (GADA), islet cell cytoplasmic (ICA), insulinoma-associated-2/tyrosine phosphatase (IA-2A), insulin autoantibody (IAA), and zinc transporter-8 autoantibodies are the five types of T1DM iAb [4]. The number of identified autoantibodies is connected to the likelihood of clinical onset, with the presence of two or more autoantibodies associated with the greatest increase in risk [5]. As a result, the disease process begins with a single autoantibody, followed by intermolecular epitope spreading to multiple autoantibodies, loss of insulin secretory capability due to a combination of beta cell destruction, and function inhibition, leading to metabolic changes, and finally diabetes [6]. The detection of islet autoantibodies in young children peaks between 9 months and 2 years of age, with no seroconversion occurring at 3 or 6 months of age in children born to a mother or father with T1DM [7]. The incidence rate of T1DM is 15 per 100000 individuals, and its prevalence is 5.9 per 10000 people worldwide [1]. In Iran, the incidence rate is 11 per 100000 individuals, and its prevalence is 388.9 per 100000 people [2]. T1DM is often identified at stage 3 when the disease has advanced to diabetic ketoacidosis, a potentially fatal condition. As a result, it is critical to employ early screening and diagnostic methods to detect autoimmunity already present in the earliest years of life and limit the risk of catastrophic problems [1]. Determining the patterns and trends of iAb incidence in T1DM could advance knowledge of the population of children worldwide at risk of developing the disease and help explain observed variations in incidence, prevalence, and health outcomes of T1DM in children and adolescents between and within countries. Also, deeper comprehension of the prevalence of iAbs worldwide could aid in the early diagnosis and treatment of T1DM and lay the groundwork for future research into the severity of the corresponding autoantibody profiles, which would lower preventable morbidity and mortality among children and adolescents [8]. Accordingly, this study determines the frequency of GADA ICA, IA-2A, and IAA in the North of Iran.
Methods
This cross-sectional study was conducted from March 2019 to December 2020 at Bu Ali Sina Hospital in Sari City, Iran.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Children between 3 months to 18 years of age; diagnosis of T1DM by pediatric endocrinologist based on American Diabetes Association (ADA) criteria [9]; consent to participate in the study. Meanwhile, the exclusion criteria were syndromic disease, familial dyslipidemia, chronic cardiac, brain, liver, renal, or co‐infectious disorders, non-type one diabetes mellitus, or lack of informed consent.
Four types of typical autoantibodies (GADA, ICA, IAA, and IA-2A) were examined to confirm the autoimmune diabetes etiology. We used a commercially available enzyme-linked immunosorbent assay to evaluate diabetes-related autoantibodies. The GADA, IA-2A, IAA, and ICA levels were evaluated using a DiaMetra ELISA kit (DiaMetra Co, Milan, Italy) based on the international standard substance National Institute for Biological Standards and Control (NIBSC), which passed the 2015 international diabetes-related antibody standardized test verification (IASP 2015). The upper limit of the normal range was 5 IU/mL for GADA, 2.4 IU/mL for IAA, 1 IU/mL for ICA, and 7.5 IU/mL for IA-2A autoantibodies. Values greater than this cutoff value were considered positive. Also, the HbA1c level was measured using boronate affinity chromatography with the NYCOCARD HbA1c kit and NYCOCARD Reader II, both made by the Norwegian Company Axis-Shield.
The data collection tool was a checklist completed by the project implementers while reviewing the patients’ files. Data extracted from patients’ files included demographic information (age and sex), history profile, and clinical examination (age of onset and initial disease onset, positive family history of T1DM, the simultaneous presence of other autoimmune diseases, weight, and height of patients according to age and sex percentages at the time of diagnosis, etc. and laboratory information of patients (HbA1c, GADA, ICA, IA-2A, IAA) at the time of diagnosis. Ocular complications and microalbuminuria (increased urinary albumin secretion up to 30-300 mg in 24 h or 20-200 mg/min) were evaluated at the five-year mark after the onset of the disease or earlier if the patient had reached the age of 10 or puberty. Also, glycemic control was categorized as HbA1c < 58 mmol/mol (<7.5%), 58–74 mmol/mol (7.5–8.9%), and ≥75 mmol/mol (≥9%) [10]. Hyperglycemia was defined as BS >180 mg/dL [11].
Statistical analysis
The statistical analyses were performed using the SPSS software, version 24. Relationships between variables were tested using the chi-squared test and student t-test. To evaluate the correlation, the Spearman test was used.
The data were described using Mean±SD, and a confidence interval of 95% for numerical variables. Using correlation analysis and linear regression, the effects of each population variable and the identified categories on HbA1c, which indicates long-term blood sugar, were measured.
Results
Overall, 190 patients with T1DM (80 males and 110 females) with a mean age of 13.14±0.36 years were included. There were no significant differences regarding gender between the presence of autoantibodies type (P>0.05). Patients with ICA were significantly older (mean difference=2.02 [95% confidence interval (CI), 0.38%, 3.65%]) than ICA-negative cases (13.80±0.48 vs 11.79±0.67; P=0.01). However, other autoantibodies had similar age distribution in positive and negative cases (P>0.05).
Based on the mono test, the highest prevalence was seen in ICA (104 [66.67%]), and the lowest prevalence was in IAA (30 [25.64%]). Also, based on multiple tests, ICA + GADA, and GADA + ICA + IA-A2 + IAA had the highest and lowest prevalence (135 [49.63%] and 151 [5.96%], respectively) (Table 1).

There was no significant difference between the frequency of the child’s rank, Hashimoto’s hypothyroidism and Celiac autoimmune disorders (CD), twinning, consanguineous marriage, birth weight, term or pre-term birth, comorbidities, blood group, the presence of microalbuminuria, and last average of HbA1C with the positive or negative GADA, ICA, IA-2A, IAA (P>0.05) (Table 2).


Patients with positive IA-A2 and ICA compared to negative IA-A2 and ICA had higher age at diabetes onset (8.93±4.11 vs 7.73±4.33, P=0.02; 8.8±4.22 vs 6.81±4.1, P=0.01), receptively. There was no significant difference between the presence of GADA and IAA and age at diabetes onset (P>0.05). Moreover, the HbA1c level at T1DM onset in patients with positive IAA was lower than negative IAA (7.84±1.82 vs 9.41±2.35, P=0.0009). Still, the HbA1c level had a similar distribution in patients with positive and negative GADA, IA-A2, and ICA (P>0.05). There was a significant difference in hyperglycemia with positive and negative IA-A2, in which the chance of positive IA-A2 was 54% lower in hyperglycemia than in euglycemia (odd ratio [OR]=0.46 [95% CI, 0.22%, 0.96%], P=0.04). However, there were no significant differences between unfavorable blood sugar (BS) and positive autoantibodies (P>0.05) (Table 3).

Discussion
According to the results, the highest prevalence of T1DM autoantibodies was seen in ICA. Patients with positive IA-A2 and ICA compared to negative IA-A2 and ICA had higher age at the T1DM diagnosing time, the HbA1c level at T1DM diagnosing time in patients with positive IAA was lower than negative IAA, and the chance of positive IA-A2 was 54% lower in unfavorable BS compared to favorable BS. Also, this study found no significant association between positive GADA, ICA, IA-2A, IAA, and the presence of Hashimoto hypothyroidism and CD.
Several studies have linked autoimmune thyroid disorders followed by celiac disease to T1DM [12-14]. Therefore, we examined Hashimoto thyroiditis and CD in our population. We found no significant difference between the relative frequency of patients with Hashimoto thyroiditis and positive or negative results of autoantibodies. In a previous study, anti-thyroid peroxidase was positive in 11% of GADA-positive, 16% of ICA-positive, and 11.6% of IAA-positive T1DM patients [15]. Thyroid autoimmunity was shown to be more common in T1DM patients with positive autoantibodies [16]. A study on the prevalence of gliadin immunoglobulin G/immunoglobulin A and transglutaminase immunoglobulin A was significantly higher in recent-onset T1DM patients with positive GADA, IAA, IA-A2, and ICA [17]. In a study on the co-occurrence of T1DM and CD. In terms of autoimmunity, it was found that islet autoantibodies typically appeared before tissue transglutaminase autoantibodies (tTGAs). Islet autoantibodies preceding tTGAs were linked with an increased probability of tTGAs (hazard ratio [HR]: 1.48; 95% CI, 1.15%, 1.91%) [18]. Also, based on a systematic review and meta-analysis on screening for CD in T1DM patients, since the majority of CD are discovered within five years after T1D diagnosis, screening should be considered at the time of T1D diagnosis and every 2 to 5 years afterward [19]. Accordingly, the non-significant association could be due to the late occurrence of CD after T1DM.
There are conflicting results regarding the most common autoantibodies in patients with T1DM. In our study, the highest prevalence was related to GADA and ICA in children with T1DM, and the lowest prevalence was seen in the combination of GADA+ ICA+ IA-A2+ IAA. In a study on T1DM autoantibodies in the Finland study group, the highest prevalence was reported for GADA and IA-A2 [20]. Some studies on Iranian and Saudi patients identified ICA and anti-IAA as the most prevalent autoantibodies in T1DM patients [21, 22]. These differences could be due to the effect of geographic regions [23].
Conclusion
Understanding the regional prevalence of iAb in T1DM children and adolescents could aid in the earlier identification of those at risk of developing T1DM and inform clinical practice, health policies, resource allocation, and targeted healthcare interventions to better screen, diagnose, and manage T1DM children and adolescents.
Ethical Considerations
Compliance with ethical guidelines
The Local Human Research Ethics Committee of Mazandaran University of Medical Sciences, approved the study protocol (Code: IR.MAZUMS.RIB.REC.1400.022). The study was conducted per the principles of the Declaration of Helsinki. After thoroughly explaining the study, all parents of the children enrolled in the study completed informed consent forms. All patients received adequate insulin replacement therapy.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization, methodology, investigation and writing: All authors; Formal analysis: Jamshid Yazdani Charati.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Health Department of Mazandaran University of Medical Sciences and the Bu Ali Sina Hospital staff.
References
- Katsarou A, Gudbjörnsdottir S, Rawshani A, Dabelea D, Bonifacio E, Anderson BJ, et al. Type 1 diabetes mellitus. Nat Rev Dis Primers. 2017; 3:17016. [DOI:10.1038/nrdp.2017.16] [PMID]
- Atkinson MA, Eisenbarth GS, Michels AW. Type 1 diabetes. Lancet. 2014; 383(9911):69-82. [DOI:10.1016/S0140-6736(13)60591-7] [PMID]
- Insel RA, Dunne JL, Atkinson MA, Chiang JL, Dabelea D, Gottlieb PA, et al. Staging presymptomatic type 1 diabetes: A scientific statement of JDRF, the Endocrine Society, and the American Diabetes Association. Diabetes Care. 2015; 38(10):1964-74. [DOI:10.2337/dc15-1419] [PMID]
- Taplin CE, Barker JM. Autoantibodies in type 1 diabetes. Autoimmunity. 2008; 41(1):11-8. [DOI:10.1080/08916930701619169] [PMID]
- Krischer JP, Liu X, Lernmark Å, Hagopian WA, Rewers MJ, She JX, et al. Predictors of the Initiation of Islet Autoimmunity and Progression to Multiple Autoantibodies and Clinical Diabetes: The TEDDY Study. Diabetes Care. 2022; 45(10):2271-81. [DOI:10.2337/dc21-2612] [PMID]
- Ziegler AG, Rewers M, Simell O, Simell T, Lempainen J, Steck A, et al. Seroconversion to multiple islet autoantibodies and risk of progression to diabetes in children. JAMA. 2013; 309(23):2473-9. [DOI:10.1001/jama.2013.6285] [PMID]
- Ilonen J, Hammais A, Laine AP, Lempainen J, Vaarala O, Veijola R, et al. Patterns of β-cell autoantibody appearance and genetic associations during the first years of life. Diabetes. 2013; 62(10):3636-40. [DOI:10.2337/db13-0300] [PMID]
- Ross C, Ward ZJ, Gomber A, Owais M, Yeh JM, Reddy CL, et al. The prevalence of islet autoantibodies in children and adolescents with type 1 diabetes mellitus: A global scoping review. Front Endocrinol (Lausanne). 2022; 13:815703. [DOI:10.3389/fendo.2022.815703] [PMID]
- American Diabetes Association. (2) Classification and diagnosis of diabetes. Diabetes Care. 2015; 38(Suppl):S8-16. [DOI:10.2337/dc15-S005] [PMID]
- McKnight JA, Wild SH, Lamb MJ, Cooper MN, Jones TW, Davis EA, et al. Glycaemic control of Type 1 diabetes in clinical practice early in the 21st century: An international comparison. Diabet Med. 2015; 32(8):1036-50. [DOI:10.1111/dme.12676] [PMID]
- Agiostratidou G, Anhalt H, Ball D, Blonde L, Gourgari E, Harriman KN, et al. Standardizing clinically meaningful outcome measures beyond hba1c for type 1 diabetes: A consensus report of the American Association of Clinical Endocrinologists, the American Association of Diabetes Educators, the American Diabetes Association, the Endocrine Society, JDRF International, The Leona M. and Harry B. Helmsley Charitable Trust, the Pediatric Endocrine Society, and the T1D Exchange. Diabetes Care. 2017; 40(12):1622-30.[DOI:10.2337/dc17-1624] [PMID]
- Kahaly GJ, Hansen MP. Type 1 diabetes associated autoimmunity. Autoimmun Rev. 2016; 15(7):644-8. [DOI:10.1016/j.autrev.2016.02.017] [PMID]
- Camarca ME, Mozzillo E, Nugnes R, Zito E, Falco M, Fattorusso V, et al. Celiac disease in type 1 diabetes mellitus. Ital J Pediatr. 2012; 38:10. [DOI:10.1186/1824-7288-38-10] [PMID]
- Tomer Y, Dolan LM, Kahaly G, Divers J, D'Agostino RB Jr, Imperatore G, et al. Genome wide identification of new genes and pathways in patients with both autoimmune thyroiditis and type 1 diabetes. J Autoimmun. 2015; 60:32-9. [DOI:10.1016/j.jaut.2015.03.006] [PMID]
- Verkauskiene R, Danyte E, Dobrovolskiene R, Stankute I, Simoniene D, Razanskaite-Virbickiene D, et al. The course of diabetes in children, adolescents and young adults: Does the autoimmunity status matter? BMC Endocr Disord. 2016; 16(1):61. [DOI:10.1186/s12902-016-0145-3] [PMID]
- Shun CB, Donaghue KC, Phelan H, Twigg SM, Craig ME. Thyroid autoimmunity in Type 1 diabetes: Systematic review and meta-analysis. Diabet Med. 2014; 31(2):126-35. [DOI:10.1111/dme.12318] [PMID]
- Jaeger C, Hatziagelaki E, Petzoldt R, Bretzel RG. Comparative analysis of organ-specific autoantibodies and celiac disease-associated antibodies in type 1 diabetic patients, their first-degree relatives, and healthy control subjects. Diabetes Care. 2001; 24(1):27-32. [DOI:10.2337/diacare.24.1.27] [PMID]
- Hagopian W, Lee HS, Liu E, Rewers M, She JX, Ziegler AG, et al. Co-occurrence of type 1 diabetes and celiac disease autoimmunity. Pediatrics. 2017; 140(5):e20171305. [DOI:10.1542/peds.2017-1305] [PMID]
- Pham-Short A, Donaghue KC, Ambler G, Phelan H, Twigg S, Craig ME. Screening for celiac disease in type 1 diabetes: A systematic review. Pediatrics. 2015; 136(1):e170-6. [DOI:10.1542/peds.2014-2883] [PMID]
- Sabbah E, Savola K, Kulmala P, Veijola R, Vähäsalo P, Karjalainen J, et al. Diabetes-associated autoantibodies in relation to clinical characteristics and natural course in children with newly diagnosed type 1 diabetes. The Childhood Diabetes In Finland Study Group. J Clin Endocrinol Metab. 1999; 84(5):1534-9. [DOI:10.1210/jcem.84.5.5669] [PMID]
- Zamanfar D, Aarabi M, Amini M, Monajati M. Prevalence of autoantibodies in type 1 diabetes mellitus pediatrics in Mazandaran, North of Iran. J Pediatr Endocrinol Metab. 2020; 33(10):1299-305. [DOI:10.1515/jpem-2019-0396] [PMID]
- Aljabri K, Bokhari S, Alqurashi K. The prevalence of autoantibodies in Saudis patients with Type 1 Diabetes Mellitus. Open J Endocr Metab Dis. 2013; 3(2):132-6. [DOI:10.4236/ojemd.2013.32020]
- Kong YH, Kim MS, Lee DY. Comparison of the prevalence of islet autoantibodies according to age and disease duration in patients with type 1 diabetes mellitus. Ann Pediatr Endocrinol Metab. 2013; 18(2):65-70. [PMID]
Type of Study: Research Article |
Subject:
Endocrinology
Received: 2023/10/17 | Accepted: 2024/04/17 | Published: 2024/04/1
Received: 2023/10/17 | Accepted: 2024/04/17 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






