Volume 12, Issue 1 (1-2024)
J. Pediatr. Rev 2024, 12(1): 53-64 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Harsha N, Kotha R, Madireddy A. Investing Neonatal Septic Arthritis: A Systematic Review. J. Pediatr. Rev 2024; 12 (1) :53-64
URL: http://jpr.mazums.ac.ir/article-1-580-en.html
URL: http://jpr.mazums.ac.ir/article-1-580-en.html
1- Department of Neonatology, Niloufer Hospital, Hyderabad, India.
2- Department of Neonatology, Niloufer Hospital, Hyderabad, India. ,dr.rakeshkotha@gmail.com
2- Department of Neonatology, Niloufer Hospital, Hyderabad, India. ,
Full-Text [PDF 571 kb]
(1337 Downloads)
| Abstract (HTML) (2671 Views)
Full-Text: (1411 Views)
Introduction
Septic arthritis is the inflammation of the joints, secondary to an infectious aetiology that most commonly includes bacterial but can also be fungal, viral, or have other pathogens [1]. It is an uncommon and critical condition in neonates mainly because babies can become permanent handicaps if not diagnosed and treated early [2]. The important reason for the delayed diagnosis is the paucity of symptoms and signs in neonates [3]. Osteoarticular infections in newborns are usually blood-borne infections [4, 5]. Neonates requiring neonatal intensive care unit admission for prematurity, ventilator therapy, and invasive procedures are known to be predisposing actors, which are related to the risk of bacteremia [6].
The incidence of septic arthritis varies in different regions, such as 0.12/1000 live births in Singapore [5] and 1 in 1500 in India [7]. Such a low rate of incidence indicates the difficulty of earlier diagnosis. Also, attention has been paid to neonatal septic arthritis of the lower limbs, especially the hip [8–13], but there are reports of involvement of the shoulder joint [14].
Staphylococcus aureus is the most prevalent organism involved in osteoarticular infections as the risk of this bacteremia rises with invasive procedures. However, there is an increasing trend of cases due to Klebsiella that have been reported [9–11, 15], a possible reason being contaminated equipment, which should alert the physicians for further cases. Other reported bacterial pathogens include Candida [16], Pseudomonas, Staphylococcus epidermidis, Streptococcus spp., Haemophilus influenza, Proteus, and so on.
Neonatal septic arthritis is an uncommon condition and less reported; accordingly, this systematic review summarizes the epidemiology, presenting symptoms, laboratory values, radiological inputs, microbiology, and treatment results of neonatal septic arthritis and demonstrates the changing trends.
Methods
Search strategy
The literature was screened by the authors using the following searches: Medical subject heading (MeSH) term=population, search fields=neonate; MeSH term=diagnosis, search fields=septic arthritis. PubMed, Scopus, Embase, and Ovid/Medical Literature Analysis and Retrieval System Online (MEDLINE) databases were used. Search fields were alternated until no new articles were revealed. At that point, the search was considered complete.
During neonatal periods and infancy, there is an anastomosis between joints and bone arteries that facilitates the transmission of bacterial infections from bone to joint and vice versa. Therefore, during neonatal periods and early infancy, in most cases, these infections are associated with osteoarthritis. Therefore, we included these two diseases.
Study screening and selection
The search yielded a total of 253 studies (Figure 1).
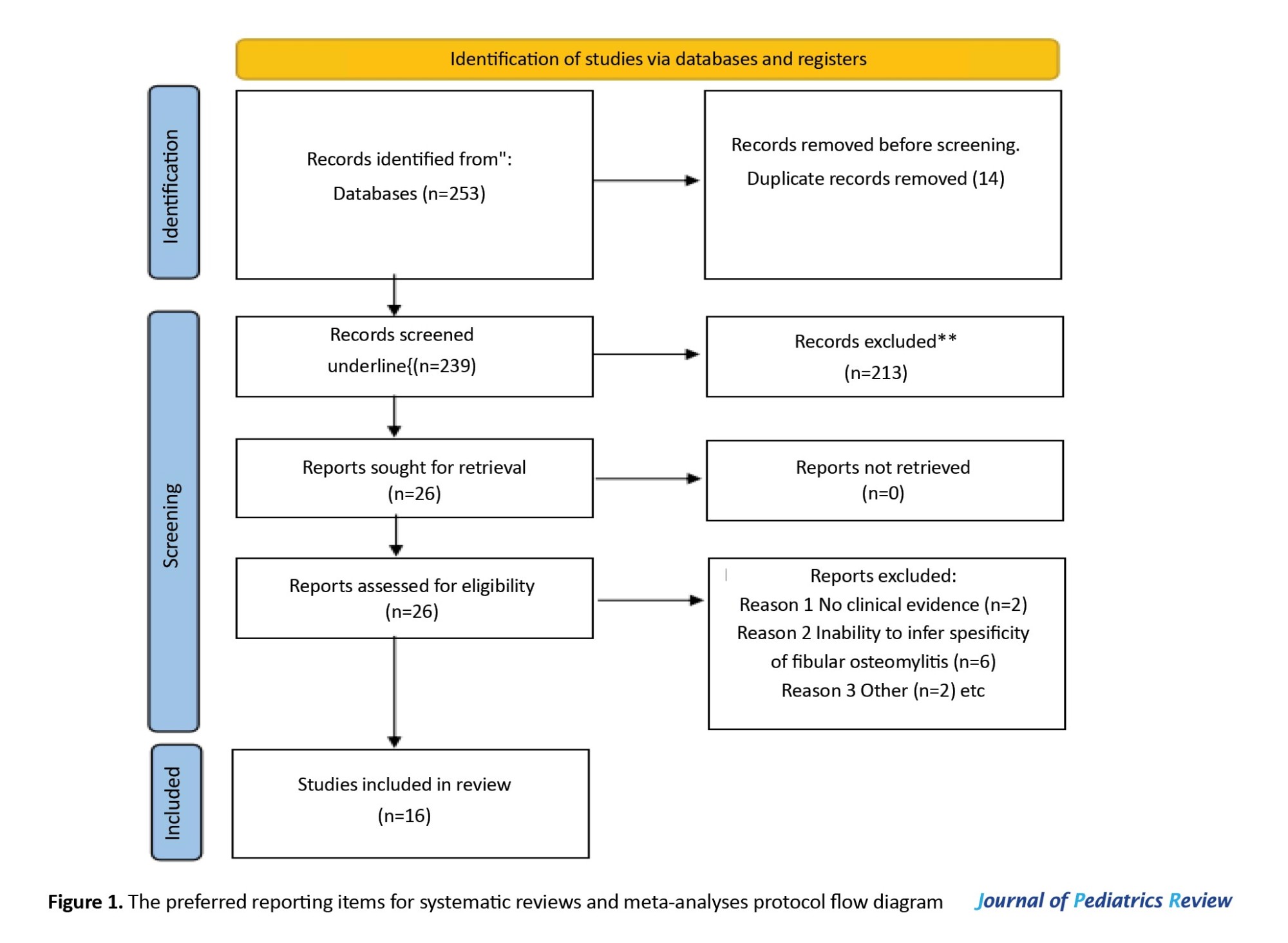
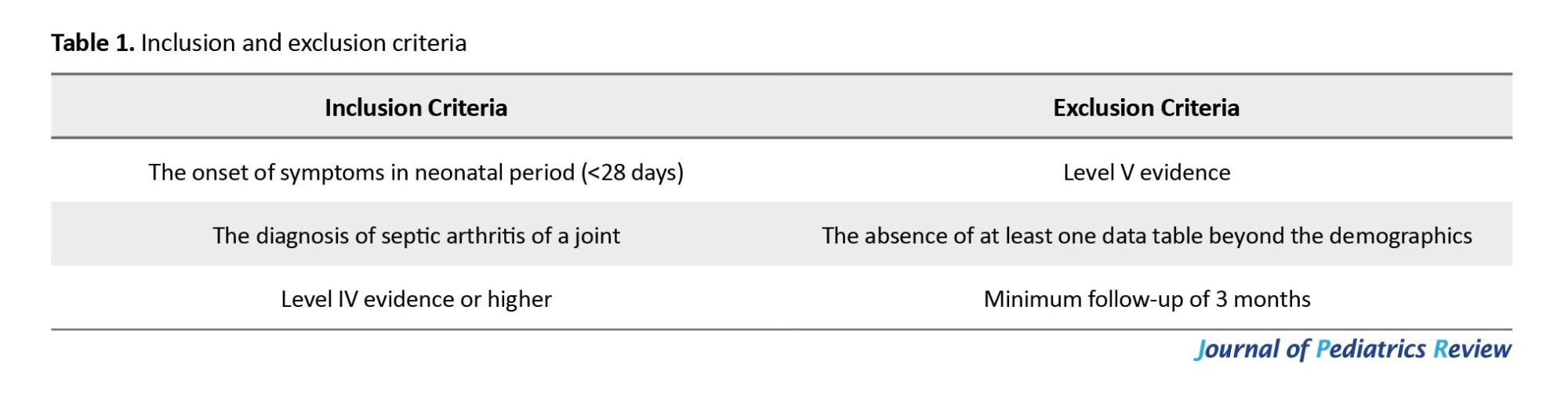
After the removal of duplicate articles, 239 studies remained. Based on the title and abstract review performed by all four authors, a total of 26 articles were included in the qualitative review. The bibliographies of all the reviewed articles were thoroughly evaluated to determine if any other relevant articles had been unintentionally overlooked. The independent full-text screenings of the articles were performed by all four authors. Of the 26 screened articles, 10 studies did not meet our inclusion/exclusion criteria (Table 1). The resulting 16 articles underwent quantitative analysis. We incorporated all studies, regardless of their timeframe. We specifically included articles that were in the English language.
Data collection
The categories for data gathering included population demographics, gestational age, age of presentation, average birth weight, presence of risk factors, such as prematurity, presenting features, number of joints involved, probable causative factors, laboratory values, imaging modalities, microbiologic diagnosis, medical management, surgical management, and complications. The variables under demographic features were gestational age, emphasizing prematurity, age of presentation, and gender, with a mainly male predominance. The presenting features were recorded as binary variables. The C-reactive protein (CRP), leukocyte count, and initial temperature were recorded. The use and positive results of ultrasound, computed tomography, and magnetic resonance imaging were recorded. Joint involvement, aspirate, surgical, and blood cultures as well as the disease course and hospitalization details were all documented. The duration of treatment, including intravenous or oral antibiotics was noted. Meanwhile, open versus ultrasound/computed tomography-guided percutaneous drainage operations performed along with type/number are also included in records. After follow-up completion, any secondary sequelae detected were accounted for accordingly.
Quality assessment
To assess the included studies, which were predominantly case series, the guidelines from the National Institutes of Health (NIH) were used [17]. Since all the published literature is limited to a level of clinical evidence of IV, a quality assessment tool for case series under NIH was used. This tool has been modified to appropriately evaluate the methodologic quality of case series in light of their most common pitfalls. It includes nine questions. According to the NIH quality assessment tool for case series studies, as described by Uthraraj et al. (2022) and Rubin et al [8, 13]. (2020), it was determined that these two studies are in the poor-quality category (6/9). On the other hand, Salamon [19], Li et al. [20], Khriesat et al. [10] were classified as good-quality studies (9/9). The remaining studies were classified as of fair quality (8/9) [19, 20].
Data abstraction and statistical analysis
Due to heterogeneous data and a smaller sample size, we also included an extended time frame, which is why a meta-analysis could not be performed.
All data were summarized descriptively according to the recommendations of Dhawan et al. [18]. Frequencies and proportions were calculated for categorical variables. Mean±SD were calculated for continuous variables. Data abstraction and figure creation were performed via Microsoft Excel software, version 16.42.
Results
Demographic characteristics
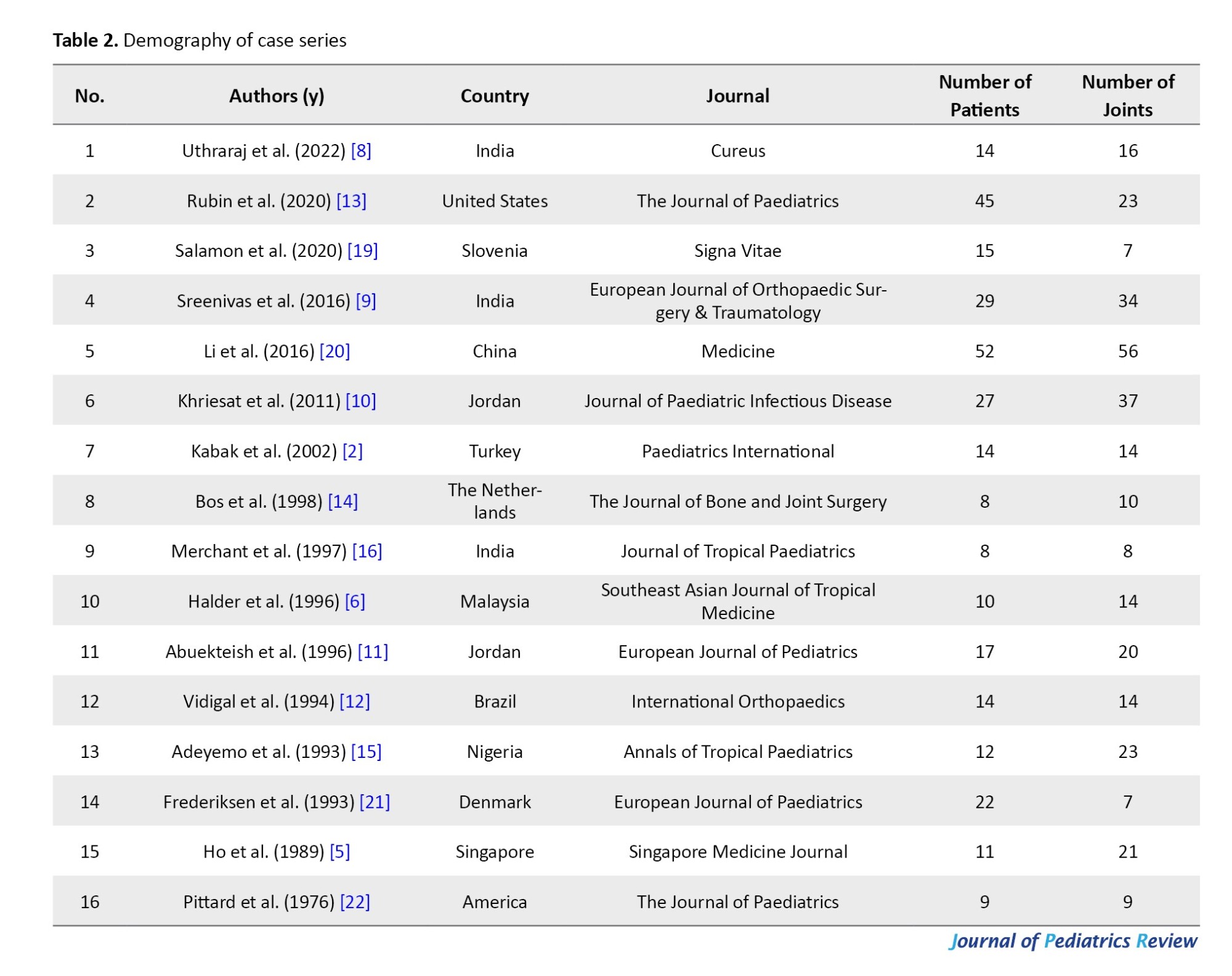
This review included 16 articles for a total of 307 neonates with involvement in 313 joints (Table 2). This review included neonates worldwide from 1976 to 2022.
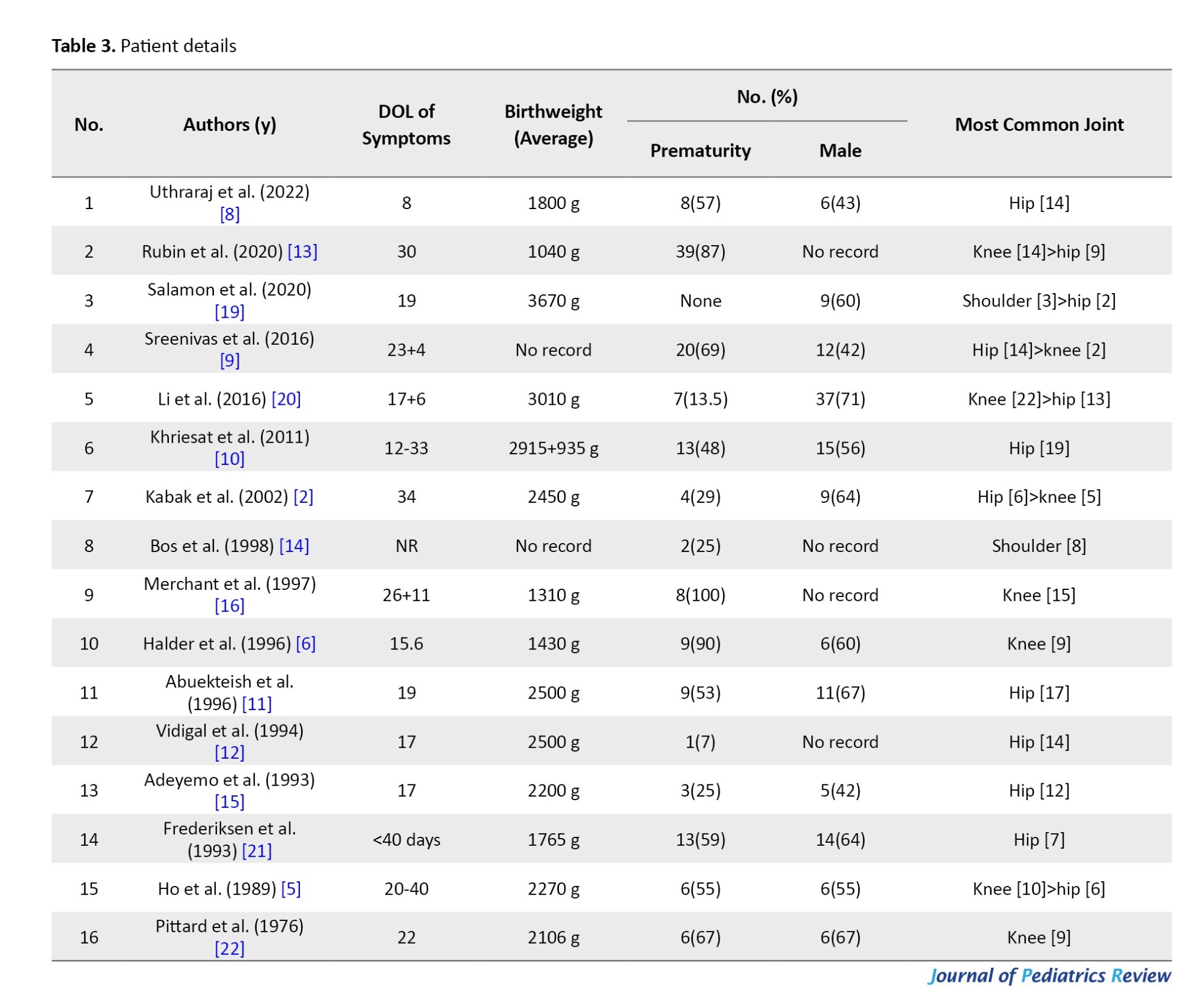
This study included neonates with a prematurity (<37 weeks) proportion of 66% (7%-100%). A total of 12 case series mentioned the gender proportion, of which males comprised 136/228 cases (60%), and females comprised 92/228 cases (40%; Table 3) The age of onset of symptoms was predominantly in the third week, at an average of 20 days (8–40 days).
Involved joints
The most common site of involvement was the hip joint (42%), followed by the knee joint (27%).
Presenting symptoms
The paucity of signs and symptoms is one of the important reasons for the delayed diagnosis of septic arthritis. Similarly, the presence of initial symptoms was vague and less reported, which included fever and irritability. However, most of the neonates presented with joint swelling, tenderness, erythema, and a reduced range of movements of the affected joint, with or without pseudo-paralysis, in variable order (Table 4).
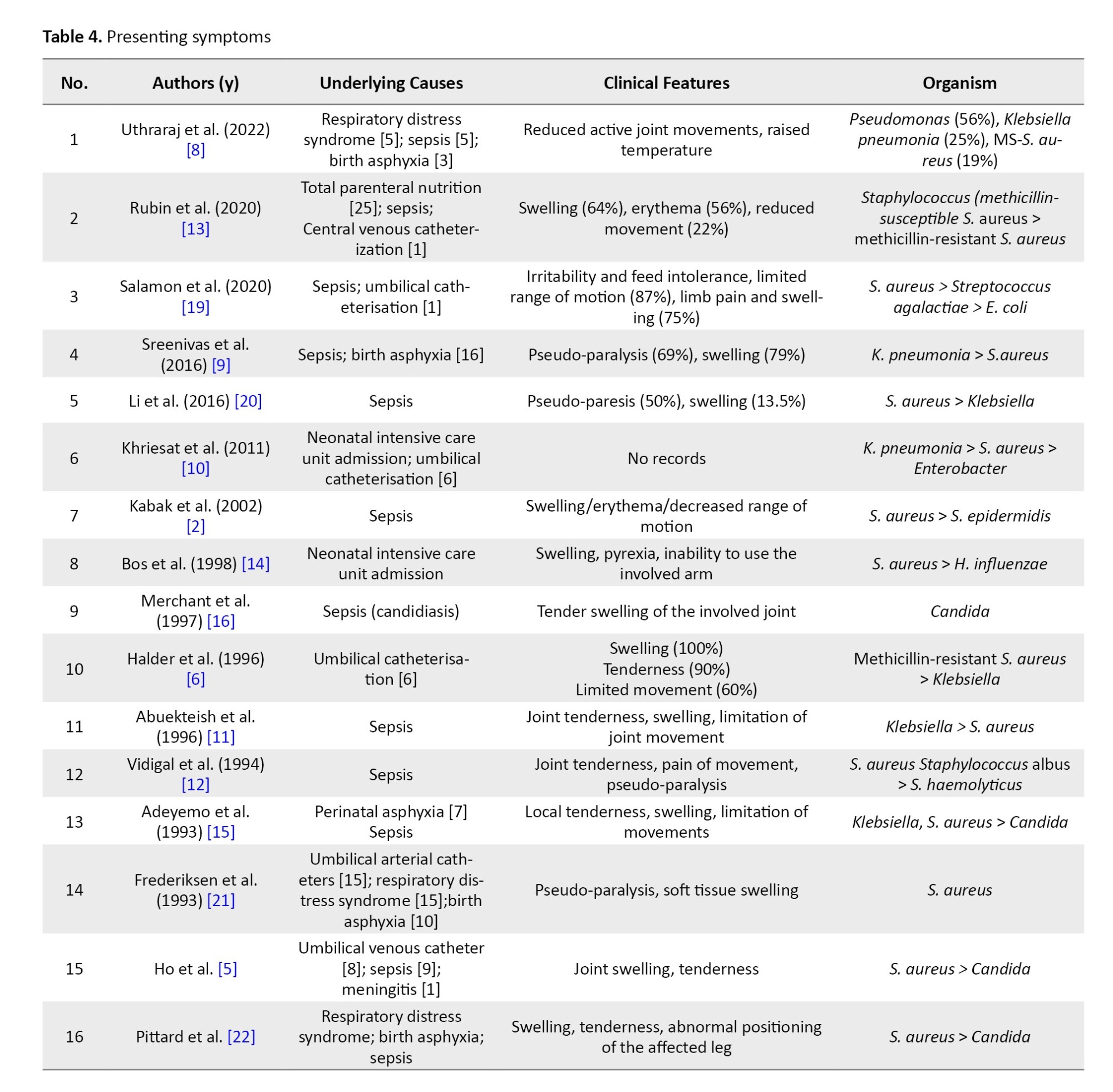
Underlying causes
The most important cause documented is neonatal intensive care unit admission, with the reasons being respiratory distress syndrome, sepsis, and birth asphyxia (Table 4). Umbilical catheterization has been a documented risk factor in 36 cases in 4 case series.
Microbiology
S. aureus (40%) was the most commonly cultured pathogen, followed by Klebsiella (18%). One case series included eight exclusively reported cases of Candida. Other microorganisms reported were Pseudomonas, Streptococcus, H. influenzae, Staphylococcus haemolyticus, Escherichia coli, Proteus, and so on.
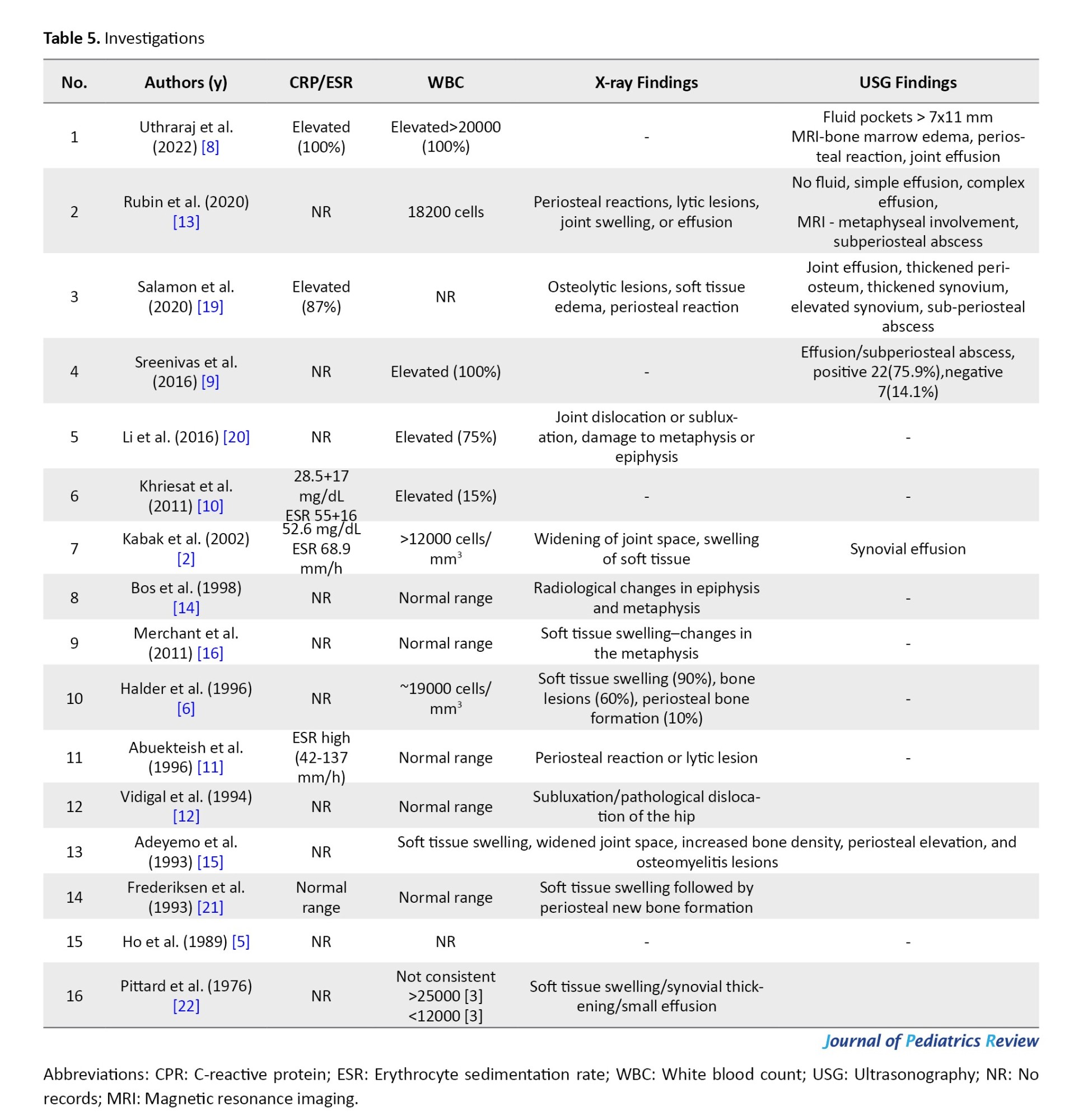
Laboratory assessment
The blood parameters usually assessed in cases of osteoarticular infections are white blood cell count, erythrocyte sedimentation rate, and CRP (Table 5). In the reported case series, CRP and erythrocyte sedimentation are elevated. The white blood cell counts also showed variability, with five case series showing a normal range and seven case series showing elevated levels. As the literature says, elevated inflammatory markers are not always necessary in cases of septic arthritis.
Imaging
Earlier case series studies in this review mainly used radiographs as the modality of imaging (Table 5). The important findings were radiological changes in epiphysis and metaphysis, soft tissue swelling, bone lesions, periosteal bone formation, joint dislocation, or subluxation, in variable order and depending on the day of diagnosis. Subsequently, ultrasonography has become the imaging modality, where findings include joint effusion, thickened periosteum, thickened synovium, elevated synovium, and subperiosteal abscess. Magnetic resonance imaging was done in two case series, where findings included bone marrow edema, periosteal reaction, and joint effusion.
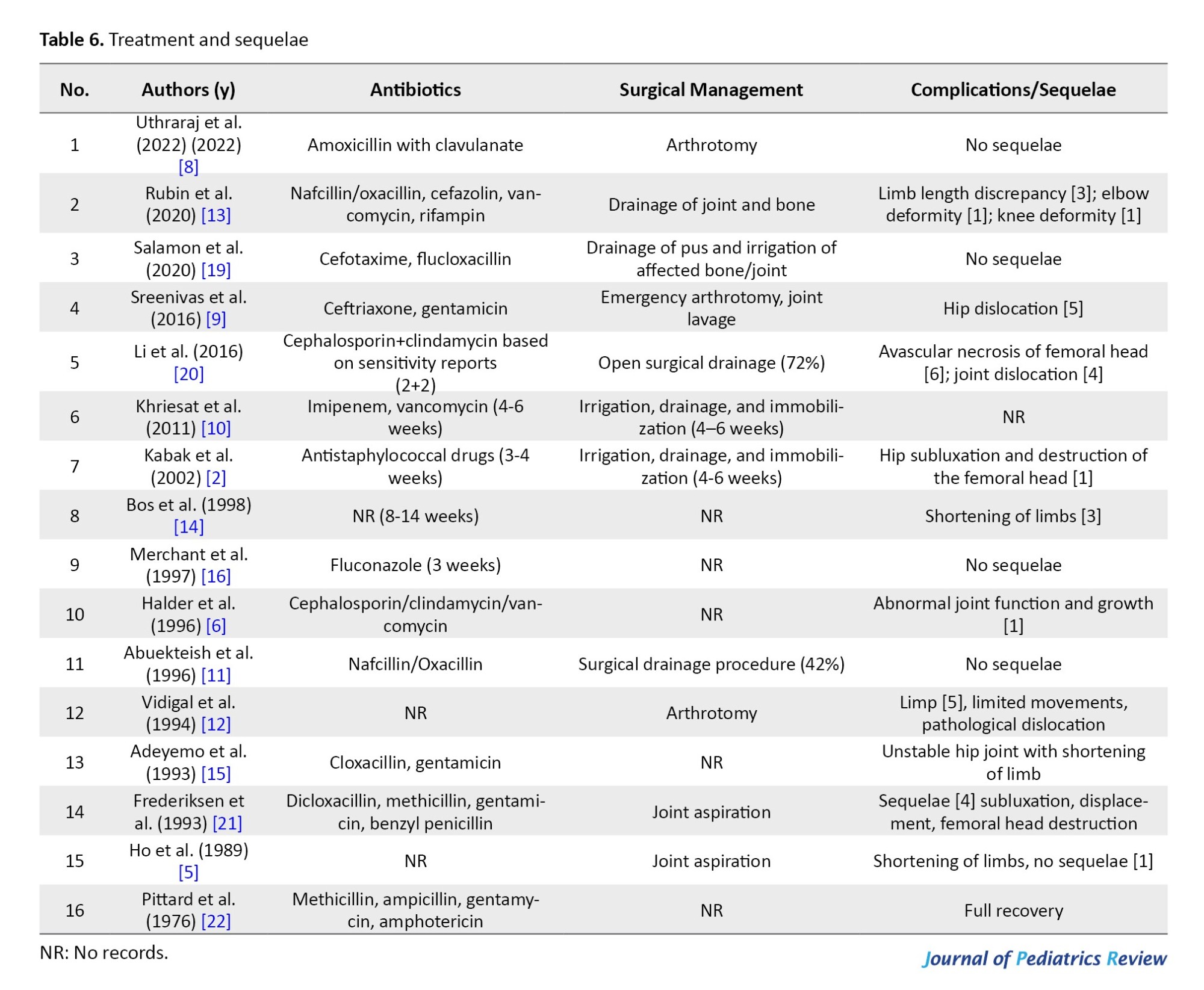
Medical management
The mean duration of intravenous therapy followed by oral antibiotics was 2–6 weeks. Antibiotics alone were reported in only 12% (38 babies) of the cases.
Surgical management
Open surgery i.e. a throtomy was performed on 16% of the 49 babies. The rest of the babies responded to joint aspiration and irrigation, with or without immobilization (Table 6).
Complications or sequelae
Five case series were documented to have no sequelae on follow-up (Table 6), which cited early diagnosis and prompt management as the reasons for this result. The rest of the case series included limb length discrepancy as the most common complication, a possible reason being a joint subluxation, dislocation, or avascular necrosis of the femoral head (Table 6).
Discussion
Our review included 16 articles for a total of 307 neonates with the involvement of 313 joints. It included neonates with a prematurity (<37 weeks) proportion of 66% (7%-100%). These are in line with Kleinman et al., who demonstrated that preterm and low birth weight infants are 3-10 times more likely to develop septic arthritis 12 case series mentioned the gender proportion of which males comprised 136/228 cases (60%), and females comprised 92/228 (40%), immunological differences in gender may explain above findings. The age of onset of symptoms was predominantly in the third week, at an average of 20 days (8-40 days). The delayed presentation explained by low immunity in the preterm neonate to cause inflammation and late-onset sepsis catheterization and hospital admissions were the major risk factors [23].
The presence of initial symptoms was vague and less reported, which included fever and irritability. Fever, even though considered common in septic arthritis, is difficult to document in neonates because of physiological neonatal hypothermia [24]. This review emphasizes the importance of susceptibility to septic arthritis with the above signs. However, most of the neonates presented with joint swelling, tenderness, erythema, and a reduced range of movements of the affected joint, with or without pseudo-paralysis, in variable order. The most important cause documented is neonatal intensive care unit admission, with the reasons being respiratory distress syndrome, sepsis, and birth asphyxia.
Umbilical catheterization has been a documented risk factor in 36 cases in 4 case series [2, 5, 6, 19]. Accordingly, diagnosis is difficult [25]. Kabak et al. mentioned that intravenous canulation (3/14) is a risk factor for septic arthritis [2]. Bergdahl and Woong. also reported that intravenous canulation is a major risk factor. In our study, intravenous canulation-induced septic arthritis was not uncommon [25, 26].
In the reported case series, CRP and erythrocyte sedimentation rates are elevated. The white blood cell count also showed variability, with five cases showing a normal range and seven cases showing elevated levels. Thus, measuring erythrocyte sedimentation rate and CRP may slightly improve sensitivity and negative predictive value for diagnosis; however, further investigation is necessary to establish a certain diagnostic role in septic arthritis. In our review, most of the cases were not reported. In some cases, they were also normal.
The important findings in radiographs were changes in epiphysis and metaphysis, soft tissue swelling, bone lesions, periosteal bone formation, joint dislocation, or subluxation, in variable order and depending on the day of diagnosis. Subsequently, ultrasonography has become the imaging modality, where findings include joint effusion, thickened periosteum, thickened synovium, elevated synovium, and subperiosteal abscess. Magnetic resonance imaging was done in seven patients in two case series [13, 19], where findings included bone marrow edema, periosteal reaction, and joint effusion. This is to emphasize that imaging plays a crucial role in establishing early and timely diagnosis, which is subsequently helpful in management, thus decreasing the incidence of long-term sequelae [19].
S. aureus (40%) was the most commonly cultured pathogen, followed by Klebsiella (18%). One case series, which included 8 cases, exclusively reported the cases of Candida [16]. Other microorganisms reported were Pseudomonas [8], Streptococcus, and S. hemolyticus, H. influenzae, E. coli, Proteus, and so on. This is in concordance with the published literature [27]. An increase in methicillin-resistant S. aureus has been reported in the pediatric population and should be considered when deciding on empiric antimicrobial treatment. Four of the reviewed case series [9–11, 15] highlighted the increasing incidence of Klebsiella-related septic arthritis, citing the possibility of using contaminated equipment [28], which is an important reason for nosocomial infection. Another reason noted was a severe shortage of water supply [15], which, when corrected, led to no recurrence of this infection. Pseudomonas reported in Uthraraj et al. [8] was important because of its nosocomial nature. The main cause of this outbreak was inadequate infection control by staff members. Thus, guidelines formulated to mitigate the risk led to no further outbreak [8]. H. influenzae was cultured in only one case [14], which is consistent with the drastic decrease in the cases of osteomyelitis secondary to H. influenzae after the introduction of large-scale vaccinations [27]. The culture and identification of the offending pathogen allow for definitive therapy with a narrow spectrum representing the cost-effective and responsible use of antibiotic resources [29].
Administration of antibiotics alone was reported in only 12% (38 babies) of the cases. Initial choices were mainly anti-staphylococcal drugs with a combination of aminoglycosides, followed by cephalosporins. Also, for fungal arthritis, fluconazole was effective [16]; however, amphotericin was helpful in another case series [22]. Open surgery was performed in 16% (49 babies). The rest of the babies responded to joint aspiration and irrigation, with or without immobilization.
There is still no consensus as regards the management of septic arthritis, let alone neonatal arthritis. Some advocate arthrotomy [8, 9, 11, 12, 20], while others prefer aspiration of infected joints [2, 10, 13, 19, 21, 22]. Conservative therapy was elected in small premature infants, while a surgical approach was done in newborns with larger joints [22]. Another discussion to decide on the choice of therapy based on the site of pus detection. If the pus is superficial and swelling is easily visible and monitored, repeated careful aspirations are the preferred method for decompression, and when it is deep-seated, especially in hip joints, an arthrotomy is the preferred method [5].
The importance of this review lies in the fact that an early diagnosis and appropriate management would decrease the incidence of sequelae in newborn babies with septic arthritis. 5 case series are documented to have no sequelae on follow-up [8, 11, 16, 19, 22]. The rest of the case series included limb length discrepancy as the most common complication, a possible reason being a joint subluxation, dislocation, or avascular necrosis of the femoral head. This is also seen in septic arthritis of the shoulder joint [14]. According to Diana et al. infants with lupus may have weakened immunity, making them more vulnerable to septic arthritis [30]. Parmar et al. reported a case of a 5-week-old baby presenting with septic arthritis in the knee and temporomandibular joint believed to be caused by omphalitis [31].
Conclusion
Unexplained fever and irritability in a newborn with a significant neonatal intensive care unit course should raise suspicion of neonatal septic arthritis, which, on examination, if associated with any joint swelling, tenderness, or decreased range of movements, should be advised for further radiographic evaluation. Although laboratory screening tests may be normal, radiographs can help reduce the delay in diagnosis and improve outcomes. For focused treatment, blood and tissue cultures are to be performed. A prolonged stay is a major concern as it may involve invasive procedures like simple intravenous cannula insertion. Improvements in our microbiological diagnosis have the potential to improve antibiotic selection. Local methicillin-resistant S. aureus prevalence must be taken into consideration when starting empiric antibiotic treatment. Follow-up is a must as complications are common in delayed cases.
Study limitations
This study is not without its limitations. Due to the rarity of neonatal septic arthritis, this systematic review was limited to case series and is thus of level IV evidence. Due to heterogeneous data and variable follow-up, we are limited in the interpretation of our data about presenting symptoms, choice of treatment, and complications, so we are unable to do a meta-analysis. The follow-up period was very short in comparison. Some studies were in single numbers. The time frame of the studies was very wide. Only three studies were of good quality.
Ethical Considerations
Compliance with ethical guidelines
The preferred reporting items for systematic reviews and meta-analyses protocol guidelines were strictly followed. Additionally, the review was registered by International Prospective Register of Systematic Reviews (PROSPERO) (Code: CRD42021281293).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
References
Septic arthritis is the inflammation of the joints, secondary to an infectious aetiology that most commonly includes bacterial but can also be fungal, viral, or have other pathogens [1]. It is an uncommon and critical condition in neonates mainly because babies can become permanent handicaps if not diagnosed and treated early [2]. The important reason for the delayed diagnosis is the paucity of symptoms and signs in neonates [3]. Osteoarticular infections in newborns are usually blood-borne infections [4, 5]. Neonates requiring neonatal intensive care unit admission for prematurity, ventilator therapy, and invasive procedures are known to be predisposing actors, which are related to the risk of bacteremia [6].
The incidence of septic arthritis varies in different regions, such as 0.12/1000 live births in Singapore [5] and 1 in 1500 in India [7]. Such a low rate of incidence indicates the difficulty of earlier diagnosis. Also, attention has been paid to neonatal septic arthritis of the lower limbs, especially the hip [8–13], but there are reports of involvement of the shoulder joint [14].
Staphylococcus aureus is the most prevalent organism involved in osteoarticular infections as the risk of this bacteremia rises with invasive procedures. However, there is an increasing trend of cases due to Klebsiella that have been reported [9–11, 15], a possible reason being contaminated equipment, which should alert the physicians for further cases. Other reported bacterial pathogens include Candida [16], Pseudomonas, Staphylococcus epidermidis, Streptococcus spp., Haemophilus influenza, Proteus, and so on.
Neonatal septic arthritis is an uncommon condition and less reported; accordingly, this systematic review summarizes the epidemiology, presenting symptoms, laboratory values, radiological inputs, microbiology, and treatment results of neonatal septic arthritis and demonstrates the changing trends.
Methods
Search strategy
The literature was screened by the authors using the following searches: Medical subject heading (MeSH) term=population, search fields=neonate; MeSH term=diagnosis, search fields=septic arthritis. PubMed, Scopus, Embase, and Ovid/Medical Literature Analysis and Retrieval System Online (MEDLINE) databases were used. Search fields were alternated until no new articles were revealed. At that point, the search was considered complete.
During neonatal periods and infancy, there is an anastomosis between joints and bone arteries that facilitates the transmission of bacterial infections from bone to joint and vice versa. Therefore, during neonatal periods and early infancy, in most cases, these infections are associated with osteoarthritis. Therefore, we included these two diseases.
Study screening and selection
The search yielded a total of 253 studies (Figure 1).

After the removal of duplicate articles, 239 studies remained. Based on the title and abstract review performed by all four authors, a total of 26 articles were included in the qualitative review. The bibliographies of all the reviewed articles were thoroughly evaluated to determine if any other relevant articles had been unintentionally overlooked. The independent full-text screenings of the articles were performed by all four authors. Of the 26 screened articles, 10 studies did not meet our inclusion/exclusion criteria (Table 1). The resulting 16 articles underwent quantitative analysis. We incorporated all studies, regardless of their timeframe. We specifically included articles that were in the English language.

Data collection
The categories for data gathering included population demographics, gestational age, age of presentation, average birth weight, presence of risk factors, such as prematurity, presenting features, number of joints involved, probable causative factors, laboratory values, imaging modalities, microbiologic diagnosis, medical management, surgical management, and complications. The variables under demographic features were gestational age, emphasizing prematurity, age of presentation, and gender, with a mainly male predominance. The presenting features were recorded as binary variables. The C-reactive protein (CRP), leukocyte count, and initial temperature were recorded. The use and positive results of ultrasound, computed tomography, and magnetic resonance imaging were recorded. Joint involvement, aspirate, surgical, and blood cultures as well as the disease course and hospitalization details were all documented. The duration of treatment, including intravenous or oral antibiotics was noted. Meanwhile, open versus ultrasound/computed tomography-guided percutaneous drainage operations performed along with type/number are also included in records. After follow-up completion, any secondary sequelae detected were accounted for accordingly.
Quality assessment
To assess the included studies, which were predominantly case series, the guidelines from the National Institutes of Health (NIH) were used [17]. Since all the published literature is limited to a level of clinical evidence of IV, a quality assessment tool for case series under NIH was used. This tool has been modified to appropriately evaluate the methodologic quality of case series in light of their most common pitfalls. It includes nine questions. According to the NIH quality assessment tool for case series studies, as described by Uthraraj et al. (2022) and Rubin et al [8, 13]. (2020), it was determined that these two studies are in the poor-quality category (6/9). On the other hand, Salamon [19], Li et al. [20], Khriesat et al. [10] were classified as good-quality studies (9/9). The remaining studies were classified as of fair quality (8/9) [19, 20].
Data abstraction and statistical analysis
Due to heterogeneous data and a smaller sample size, we also included an extended time frame, which is why a meta-analysis could not be performed.
All data were summarized descriptively according to the recommendations of Dhawan et al. [18]. Frequencies and proportions were calculated for categorical variables. Mean±SD were calculated for continuous variables. Data abstraction and figure creation were performed via Microsoft Excel software, version 16.42.
Results
Demographic characteristics
This review included 16 articles for a total of 307 neonates with involvement in 313 joints (Table 2). This review included neonates worldwide from 1976 to 2022.

This study included neonates with a prematurity (<37 weeks) proportion of 66% (7%-100%). A total of 12 case series mentioned the gender proportion, of which males comprised 136/228 cases (60%), and females comprised 92/228 cases (40%; Table 3) The age of onset of symptoms was predominantly in the third week, at an average of 20 days (8–40 days).

Involved joints
The most common site of involvement was the hip joint (42%), followed by the knee joint (27%).
Presenting symptoms
The paucity of signs and symptoms is one of the important reasons for the delayed diagnosis of septic arthritis. Similarly, the presence of initial symptoms was vague and less reported, which included fever and irritability. However, most of the neonates presented with joint swelling, tenderness, erythema, and a reduced range of movements of the affected joint, with or without pseudo-paralysis, in variable order (Table 4).

Underlying causes
The most important cause documented is neonatal intensive care unit admission, with the reasons being respiratory distress syndrome, sepsis, and birth asphyxia (Table 4). Umbilical catheterization has been a documented risk factor in 36 cases in 4 case series.
Microbiology
S. aureus (40%) was the most commonly cultured pathogen, followed by Klebsiella (18%). One case series included eight exclusively reported cases of Candida. Other microorganisms reported were Pseudomonas, Streptococcus, H. influenzae, Staphylococcus haemolyticus, Escherichia coli, Proteus, and so on.
Laboratory assessment
The blood parameters usually assessed in cases of osteoarticular infections are white blood cell count, erythrocyte sedimentation rate, and CRP (Table 5). In the reported case series, CRP and erythrocyte sedimentation are elevated. The white blood cell counts also showed variability, with five case series showing a normal range and seven case series showing elevated levels. As the literature says, elevated inflammatory markers are not always necessary in cases of septic arthritis.

Imaging
Earlier case series studies in this review mainly used radiographs as the modality of imaging (Table 5). The important findings were radiological changes in epiphysis and metaphysis, soft tissue swelling, bone lesions, periosteal bone formation, joint dislocation, or subluxation, in variable order and depending on the day of diagnosis. Subsequently, ultrasonography has become the imaging modality, where findings include joint effusion, thickened periosteum, thickened synovium, elevated synovium, and subperiosteal abscess. Magnetic resonance imaging was done in two case series, where findings included bone marrow edema, periosteal reaction, and joint effusion.
Medical management
The mean duration of intravenous therapy followed by oral antibiotics was 2–6 weeks. Antibiotics alone were reported in only 12% (38 babies) of the cases.
Surgical management
Open surgery i.e. a throtomy was performed on 16% of the 49 babies. The rest of the babies responded to joint aspiration and irrigation, with or without immobilization (Table 6).

Complications or sequelae
Five case series were documented to have no sequelae on follow-up (Table 6), which cited early diagnosis and prompt management as the reasons for this result. The rest of the case series included limb length discrepancy as the most common complication, a possible reason being a joint subluxation, dislocation, or avascular necrosis of the femoral head (Table 6).
Discussion
Our review included 16 articles for a total of 307 neonates with the involvement of 313 joints. It included neonates with a prematurity (<37 weeks) proportion of 66% (7%-100%). These are in line with Kleinman et al., who demonstrated that preterm and low birth weight infants are 3-10 times more likely to develop septic arthritis 12 case series mentioned the gender proportion of which males comprised 136/228 cases (60%), and females comprised 92/228 (40%), immunological differences in gender may explain above findings. The age of onset of symptoms was predominantly in the third week, at an average of 20 days (8-40 days). The delayed presentation explained by low immunity in the preterm neonate to cause inflammation and late-onset sepsis catheterization and hospital admissions were the major risk factors [23].
The presence of initial symptoms was vague and less reported, which included fever and irritability. Fever, even though considered common in septic arthritis, is difficult to document in neonates because of physiological neonatal hypothermia [24]. This review emphasizes the importance of susceptibility to septic arthritis with the above signs. However, most of the neonates presented with joint swelling, tenderness, erythema, and a reduced range of movements of the affected joint, with or without pseudo-paralysis, in variable order. The most important cause documented is neonatal intensive care unit admission, with the reasons being respiratory distress syndrome, sepsis, and birth asphyxia.
Umbilical catheterization has been a documented risk factor in 36 cases in 4 case series [2, 5, 6, 19]. Accordingly, diagnosis is difficult [25]. Kabak et al. mentioned that intravenous canulation (3/14) is a risk factor for septic arthritis [2]. Bergdahl and Woong. also reported that intravenous canulation is a major risk factor. In our study, intravenous canulation-induced septic arthritis was not uncommon [25, 26].
In the reported case series, CRP and erythrocyte sedimentation rates are elevated. The white blood cell count also showed variability, with five cases showing a normal range and seven cases showing elevated levels. Thus, measuring erythrocyte sedimentation rate and CRP may slightly improve sensitivity and negative predictive value for diagnosis; however, further investigation is necessary to establish a certain diagnostic role in septic arthritis. In our review, most of the cases were not reported. In some cases, they were also normal.
The important findings in radiographs were changes in epiphysis and metaphysis, soft tissue swelling, bone lesions, periosteal bone formation, joint dislocation, or subluxation, in variable order and depending on the day of diagnosis. Subsequently, ultrasonography has become the imaging modality, where findings include joint effusion, thickened periosteum, thickened synovium, elevated synovium, and subperiosteal abscess. Magnetic resonance imaging was done in seven patients in two case series [13, 19], where findings included bone marrow edema, periosteal reaction, and joint effusion. This is to emphasize that imaging plays a crucial role in establishing early and timely diagnosis, which is subsequently helpful in management, thus decreasing the incidence of long-term sequelae [19].
S. aureus (40%) was the most commonly cultured pathogen, followed by Klebsiella (18%). One case series, which included 8 cases, exclusively reported the cases of Candida [16]. Other microorganisms reported were Pseudomonas [8], Streptococcus, and S. hemolyticus, H. influenzae, E. coli, Proteus, and so on. This is in concordance with the published literature [27]. An increase in methicillin-resistant S. aureus has been reported in the pediatric population and should be considered when deciding on empiric antimicrobial treatment. Four of the reviewed case series [9–11, 15] highlighted the increasing incidence of Klebsiella-related septic arthritis, citing the possibility of using contaminated equipment [28], which is an important reason for nosocomial infection. Another reason noted was a severe shortage of water supply [15], which, when corrected, led to no recurrence of this infection. Pseudomonas reported in Uthraraj et al. [8] was important because of its nosocomial nature. The main cause of this outbreak was inadequate infection control by staff members. Thus, guidelines formulated to mitigate the risk led to no further outbreak [8]. H. influenzae was cultured in only one case [14], which is consistent with the drastic decrease in the cases of osteomyelitis secondary to H. influenzae after the introduction of large-scale vaccinations [27]. The culture and identification of the offending pathogen allow for definitive therapy with a narrow spectrum representing the cost-effective and responsible use of antibiotic resources [29].
Administration of antibiotics alone was reported in only 12% (38 babies) of the cases. Initial choices were mainly anti-staphylococcal drugs with a combination of aminoglycosides, followed by cephalosporins. Also, for fungal arthritis, fluconazole was effective [16]; however, amphotericin was helpful in another case series [22]. Open surgery was performed in 16% (49 babies). The rest of the babies responded to joint aspiration and irrigation, with or without immobilization.
There is still no consensus as regards the management of septic arthritis, let alone neonatal arthritis. Some advocate arthrotomy [8, 9, 11, 12, 20], while others prefer aspiration of infected joints [2, 10, 13, 19, 21, 22]. Conservative therapy was elected in small premature infants, while a surgical approach was done in newborns with larger joints [22]. Another discussion to decide on the choice of therapy based on the site of pus detection. If the pus is superficial and swelling is easily visible and monitored, repeated careful aspirations are the preferred method for decompression, and when it is deep-seated, especially in hip joints, an arthrotomy is the preferred method [5].
The importance of this review lies in the fact that an early diagnosis and appropriate management would decrease the incidence of sequelae in newborn babies with septic arthritis. 5 case series are documented to have no sequelae on follow-up [8, 11, 16, 19, 22]. The rest of the case series included limb length discrepancy as the most common complication, a possible reason being a joint subluxation, dislocation, or avascular necrosis of the femoral head. This is also seen in septic arthritis of the shoulder joint [14]. According to Diana et al. infants with lupus may have weakened immunity, making them more vulnerable to septic arthritis [30]. Parmar et al. reported a case of a 5-week-old baby presenting with septic arthritis in the knee and temporomandibular joint believed to be caused by omphalitis [31].
Conclusion
Unexplained fever and irritability in a newborn with a significant neonatal intensive care unit course should raise suspicion of neonatal septic arthritis, which, on examination, if associated with any joint swelling, tenderness, or decreased range of movements, should be advised for further radiographic evaluation. Although laboratory screening tests may be normal, radiographs can help reduce the delay in diagnosis and improve outcomes. For focused treatment, blood and tissue cultures are to be performed. A prolonged stay is a major concern as it may involve invasive procedures like simple intravenous cannula insertion. Improvements in our microbiological diagnosis have the potential to improve antibiotic selection. Local methicillin-resistant S. aureus prevalence must be taken into consideration when starting empiric antibiotic treatment. Follow-up is a must as complications are common in delayed cases.
Study limitations
This study is not without its limitations. Due to the rarity of neonatal septic arthritis, this systematic review was limited to case series and is thus of level IV evidence. Due to heterogeneous data and variable follow-up, we are limited in the interpretation of our data about presenting symptoms, choice of treatment, and complications, so we are unable to do a meta-analysis. The follow-up period was very short in comparison. Some studies were in single numbers. The time frame of the studies was very wide. Only three studies were of good quality.
Ethical Considerations
Compliance with ethical guidelines
The preferred reporting items for systematic reviews and meta-analyses protocol guidelines were strictly followed. Additionally, the review was registered by International Prospective Register of Systematic Reviews (PROSPERO) (Code: CRD42021281293).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
References
- Momodu II, Savaliya V. Septic Arthritis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023; [Link]
- Kabak S, Halici M, Akcakus M, Cetin N, Narin N. Septic Arthritis in patients followed-up in neonatal intensive care unit. Pediatr Int. 2002; 44(6):652-7. [DOI:10.1046/j.1442-200X.2002.01649.x] [PMID]
- Nade S. Acute septic Arthritis in infancy and childhood. J Bone Joint Surg Br. 1983; 65(3):234-41. [DOI:10.1302/0301-620X.65B3.6841388] [PMID]
- Williamson JB, Galasko CS, Robinson MJ. Outcome after acute osteomyelitis in preterm infants. Arch Dis Child. 1990; 65(10 Spec No):1060-2. [DOI:10.1136/adc.65.10_Spec_No.1060] [PMID]
- Ho NK, Low YP, See HF. Septic Arthritis in the newborn--a 17 years' clinical experience. Singapore Med J. 1989; 30(4):356-8. [PMID]
- Halder D, Seng QB, Malik AS, Choo KE. Neonatal septic Arthritis. Southeast Asian J Trop Med Public Health. 1996; 27(3):600-5. [Link]
- Narang A, Mukhopadhyay K, Kumar P, Bhakoo ON. Bone and joint infection in neonates. Indian J Pediatr. 1998; 65(3):461-4. [DOI:10.1007/BF02761144] [PMID]
- Uthraraj N, Sahukar S, Hiriyur Prakash M, Sriraam LM, Virani S, Guruprasad G, et al. septic Arthritis of neonates: Descriptive study of a neonatal intensive care unit nosocomial outbreak. Cureus. 2022; 14(4):e24543. [DOI:10.7759/cureus.24543]
- Sreenivas T, Nataraj AR, Kumar A, Menon J. Neonatal septic Arthritis in a tertiary care hospital: A descriptive study. Eur J Orthop Surg Traumatol. 2016; 26(5):477-81. [DOI:10.1007/s00590-016-1776-9] [PMID]
- Khriesat W, Al-Zoubi S, Makhloof F, Altaa’ni D, Lataifeh I. Neonatal septic Arthritis in the North of Jordan. J Pediatric Infect Dis. 2011; 6(2):117-120. [DOI:10.3233/JPI-2011-0300]
- Abuekteish F, Daoud AS, Mesmar M, Obeidat A. Nosocomial neonatal septic Arthritis. Eur J Pediatr. 1996; 155(2):102-5. [DOI:10.1007/BF02075760] [PMID]
- Vidigal EC, Jácomo AD. Early diagnosis of septic Arthritis of the hip in neonates. Int Orthop. 1994; 18(3):189-92. [DOI:10.1007/BF00192478] [PMID]
- Rubin LG, Shin J, Kaur I, Scheuerman O, Levy I, Long SS. Frequency of multifocal disease and pyogenic Arthritis of the hip in infants with osteoarticular infection in three neonatal intensive care units. J Pediatr. 2020; 227:157-62. [DOI:10.1016/j.jpeds.2020.07.055] [PMID]
- Bos CF, Mol LJ, Obermann WR, Tjin a Ton ER. Late sequelae of neonatal septic Arthritis of the shoulder. J Bone Joint Surg Br. 1998; 80(4):645-50. [DOI:10.1302/0301-620X.80B4.8596] [PMID]
- Adeyemo AA, Akindele JA, Omokhodion SI. Klebsiella septicaemia, osteomyelitis and septic Arthritis in neonates in Ibadan, Nigeria. Ann Trop Paediatr. 1993; 13(3):285-9. [DOI:10.1080/02724936.1993.11747661] [PMID]
- Merchant RH, Sanghvi KP, Sridhar N, Sonigara S, Mehta KP, Joshi NC. Nursery outbreak of neonatal fungal Arthritis treated with fluconazole. J Trop Pediatr. 1997; 43(2):106-8. [DOI:10.1093/tropej/43.2.106] [PMID]
- National Heart, Lung, and Blood Institute (NHLBI). Study quality assessment tools [Internet]. 2023 [updated 2023 October 22]. NHLBI. Available from: [Link]
- Dhawan A, Brand JC, Provencher MT, Rossi MJ, Lubowitz JH. Research pearls: The significance of statistics and perils of pooling. Arthroscopy. 2017; 33(6):1099-101. [DOI:10.1016/j.arthro.2017.03.013] [PMID]
- Salamon AS, Capuder S, Kljucevsek D, Schara K, Paro-Panjan D. Osteoarticular infections in newborns: Prognostic factors and outcome. Signa Vitae. 2020; 16(1):5-11. [DOI:10.22514/sv.2020.16.0002]
- Li Y, Zhou Q, Liu Y, Chen W, Li J, Yuan Z, et al. Delayed treatment of septic Arthritis in the neonate: A review of 52 cases. Medicine (Baltimore). 2016; 95(51):e5682. [DOI:10.1097/MD.0000000000005682] [PMID]
- Frederiksen B, Christiansen P, Knudsen FU. Acute osteomyelitis and septic Arthritis in the neonate, risk factors and outcome. Eur J Pediatr. 1993; 152(7):577-80. [DOI:10.1007/BF01954084] [PMID]
- Pittard WB, Thullen JD, Fanaroff AA. Neonatal septic Arthritis. J Pediatr. 1976; 88(4 Pt 1):621-4. [DOI:10.1016/S0022-3476(76)80022-4] [PMID]
- Kliegman RM, St Geme III JW. Nelson textbook of pediatrics e-book. Amsterdam: Elsevier Health; 2019. [Link]
- Gatto A, Lazzareschi I, Onesimo R, Iannotta R, Rigante D, Capossela L, et al. Short therapy in a septic Arthritis of the neonatal hip. Pediatr Rep. 2019; 11(3):8161. [PMID]
- Bergdahl S, Ekengren K, Eriksson M. Neonatal hematogenous osteomyelitis: Risk factors for long-term sequelae. J Pediatr Orthop. 1985; 5(5):564-8. [Link]
- Wong M, Isaacs D, Howman-Giles R, Uren R. Clinical and diagnostic features of osteomyelitis occurring in the first three months of life. Pediatr Infect Dis J. 1995; 14(12):1047-53. [Link]
- Castellazzi L, Mantero M, Esposito S. Update on the management of pediatric acute osteomyelitis and septic Arthritis. Int J Mol Sci. 2016; 17(6):855. [DOI:10.3390/ijms17060855] [PMID]
- Montgomerie JZ. Epidemiology of klebsiella and hospital-associated infections. Rev Infect Dis. 1979; 1(5):736-53. [DOI:10.1093/clinids/1.5.736] [PMID]
- Woods CR, Bradley JS, Chatterjee A, Copley LA, Robinson J, Kronman MP, et al. Clinical practice guideline by the pediatric infectious diseases society and the Infectious Diseases Society of America: 2021 guideline on diagnosis and management of acute hematogenous osteomyelitis in pediatrics. J Pediatr Infect Dis Soc. 2021; 10(8):801-44. [DOI:10.1093/jpids/piab027] [PMID]
- Diana V, Etika R, Utomo MT, Handayani KD, Savitri QM. Osteomyelitis and septic Arthritis in neonatal lupus erythematosus patients. J Pediatr Surg Case Rep. 2022; 76:102095. [DOI:10.1016/j.epsc.2021.102095]
- Parmar J. Case report: Septic arthritis of the temporomandibular joint in a neonate. Br J Oral Maxillofac Surg. 2008; 46(6):505-6. [PMID]
Type of Study: Review Article |
Subject:
Pediatrics
Received: 2023/10/23 | Accepted: 2023/12/13 | Published: 2024/01/1
Received: 2023/10/23 | Accepted: 2023/12/13 | Published: 2024/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









