Volume 12, Issue 1 (1-2024)
J. Pediatr. Rev 2024, 12(1): 15-26 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Shahraki Jazinaki M, Rashidmayvan M, Safarian M, Norouzy A. Investigating the Effects of Zinc Supplementation on Growth-Related Factors in Infants With Failure to Thrive: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Pediatr. Rev 2024; 12 (1) :15-26
URL: http://jpr.mazums.ac.ir/article-1-582-en.html
URL: http://jpr.mazums.ac.ir/article-1-582-en.html
1- Department of Nutrition, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- Department of Nutrition, Social Determinants of Health Research Center, School of Medicine, Gonabad University of Medical Science, Gonabad, Iran.
3- Department of Nutrition, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. ,Norouzya@mums.ac.ir
2- Department of Nutrition, Social Determinants of Health Research Center, School of Medicine, Gonabad University of Medical Science, Gonabad, Iran.
3- Department of Nutrition, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran. ,
Full-Text [PDF 1072 kb]
(4497 Downloads)
| Abstract (HTML) (3077 Views)
Full-Text: (4807 Views)
Introduction
In the early years of life, growth is considered a parameter indicating the suitable condition of the infant [1]. If the high energy requirements in infants are not met well, it leads to lower-than-expected weight growth based on specific growth charts for gender and age; this is a common definition for failure to thrive (FTT). If a child weighs less than the fifth percentile for his age and gender on the growth chart, that is considered FTT, according to more scientific definitions. Alternatively, the child’s weight growth percentile line recedes as much as the two lines or more [2]. Previous studies have reported the relationship between FTT and developmental delay, long-term deficits in height, weight, and academic performance [1, 3, 4]. Furthermore, FTT can increase the risk of being overweight and obese in later decades of life [5]. Often, children with FTT are treated on an outpatient basis by referring them to interdisciplinary clinics and based on recommendations from the American Academy of Pediatrics Committee on Nutrition [1, 6-8]. Chronic diseases and anemias, the lack of access to food, dysfunction of the digestive system, digestive symptoms, and lack of appetite are among the most critical causes of FTT [9]. The management of FTT includes providing energy and macronutrients, correcting nutrient deficiencies, financial support, family counseling, ongoing nutritional assessments, and long-term monitoring [9].
Zinc is an essential trace element in the structure of some proteins and molecules in the body [10]. It plays a role in cell division, cell differentiation, membrane integrity, enzyme, and antioxidant system function [11]. Also, zinc is vital for synthesizing nucleic acid, and proteins. Furthermore, it is necessary for maintaining lean body mass [12]. In addition, zinc deficiency in children is known as a growth-limiting factor [13]. In several past studies, a significant effect of zinc supplementation on the height and weight growth of children has been reported, but there is no consensus about it yet [11, 14]. This meta-analysis investigates the effect of zinc supplementation on growth-related factors in infants with FTT.
Methods
This systematic review was based on the preferred reporting items of systematic reviews and meta-analysis framework [15].
Search strategy
Medline, Scopus, and ISI databases were comprehensively searched up to June 2023 to find eligible trials based on the inclusion criteria. This search did not include any language or time restrictions. The applied search strategy consisted of the following medical subject headings and non-medical subject headings terms:
(“Zinc” OR “zinc supplementation” OR “zinc sulfate” OR “zinc gluconate”) AND (“FTT” OR “failure to thrive” OR “catch-up growth” OR “growth-retarded”) AND (“intervention” OR “randomized” OR “placebo” OR “clinical trials” OR “trial” OR “randomized controlled trial” OR “RCT” OR “cross-over” OR “parallel”).
All the reference lists of eligible studies were assessed, and Google Scholar was manually searched to avoid missing relevant articles.
Eligibility criteria
The studies obtained from the initial search were screened to find randomized controlled trials that investigated the effect of zinc supplementation on growth-related factors in children with FTT. Disagreements were discussed until a consensus was reached. Two researchers (Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan) performed the screening independently via the Endnote software using the titles and abstracts of papers. The inclusion and exclusion criteria were designed based on the participant, intervention, comparison, outcome, and studies framework as follows: Participant=FTT patients, intervention=zinc supplement, comparison=control group, outcome=growth-related outcomes, study=randomized controlled trials [16]. The inclusion criteria for meta-analysis comprised the following items: Human intervention studies, articles with randomized controlled trial design, intervention with zinc supplementation in the FTT population, and reporting the changes in growth-related outcomes during the intervention Mean±SD. Meanwhile, the exclusion criteria included animal studies, combined treatment, no control group, observational studies, review articles, and letters to the editor.
Data extraction
Two authors (Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan) extracted the related data from relevant articles obtained from screening independently. The desired information of this review, including the name of the first author, the year of publication, country, the characteristics of each of the study groups (number of people, mean age, and weight), type, and dose and duration of zinc supplementation, and Mean±SD of outcomes for both the intervention and control groups were extracted from the studies entered by two authors. Disagreements were discussed until a consensus was reached.
Quality assessment
The assessment of the risk of bias in the included studies was performed using the Cochran quality assessment tool (risk of bias 1) by two researchers (Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan), independently [17]. This tool assessed the risk of bias for each study in the following seven subclasses: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The risk of bias in each subclass was classified as high, unclear, and low.
The general risk of bias was considered high if the number of items with a high risk of bias in each study was ≥3; if it was 2, it was considered moderate and if it was ≤1, it was considered low general bias. Disagreements were resolved in consultation with Mohammad Safarian.
Data synthesis and statistical analysis
The pooled effect size was estimated using weighted mean differences (WMD) and the SD of measures from both intervention and control groups for different variables and by implementing the random effect model based on the DerSimonian and Laird method [18]. In the case of not reporting Mean±SD changes, mean change was calculated by subtracting variable values at the beginning of the intervention from the end. Also, change SD was estimated using the Equation 1 [19]:

Reported standard error, medians, interquartile ranges, and 95% CI were converted to SDs using the method of Hozo et al. [20]. The Cochran Q test evaluated the studies’ heterogeneity and reported them by I2 statistic [21]. Accordingly, I2>40% or P<0.05 was interpreted as significant heterogeneity. To investigate the effect of the quality and characteristics of each study on the overall size effect, a sensitivity analysis was performed [22]. In all the analyses that were performed using the STATA software, version 17 and P<0.05 were considered statistically significant.
Results
Study selection
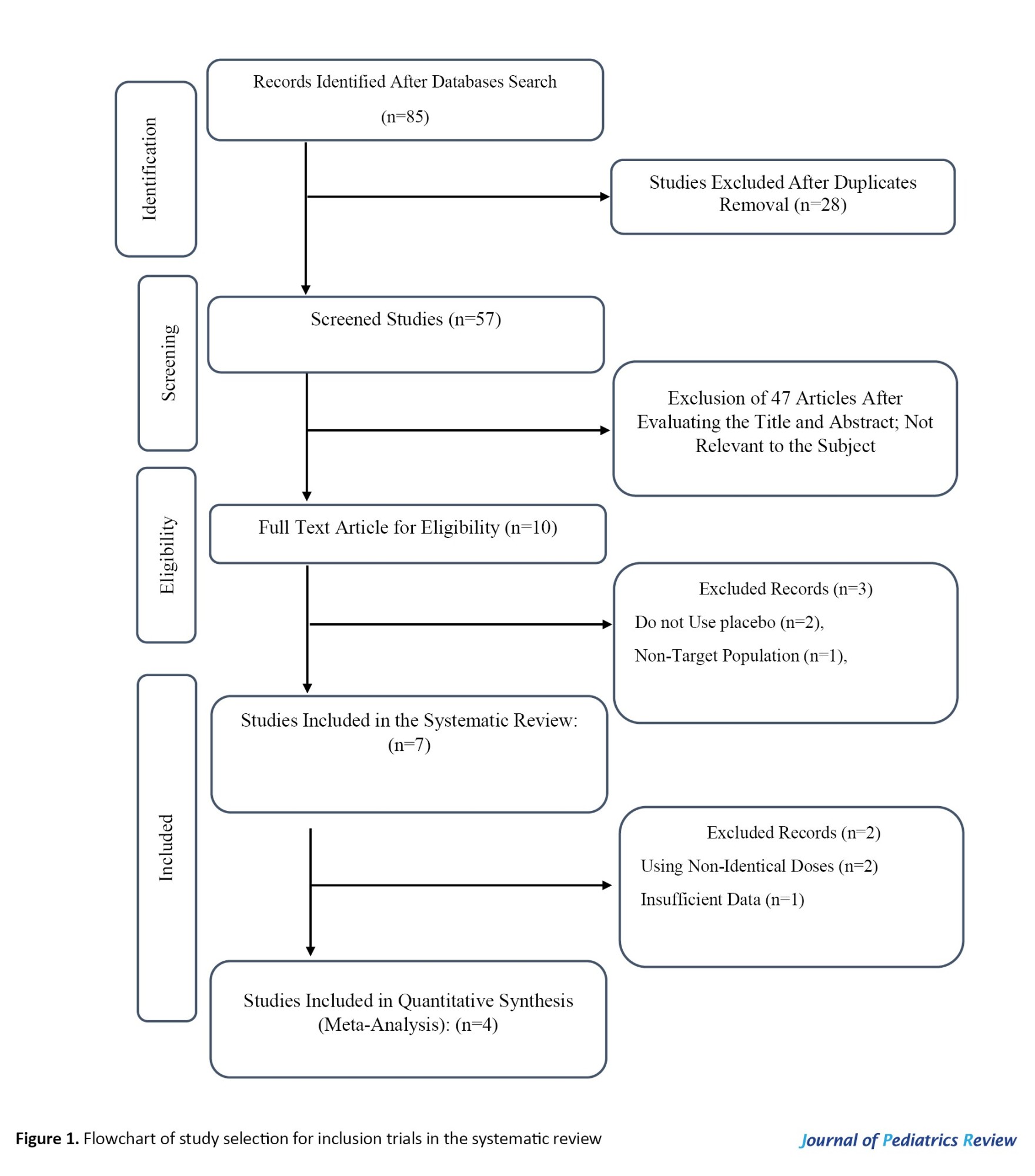
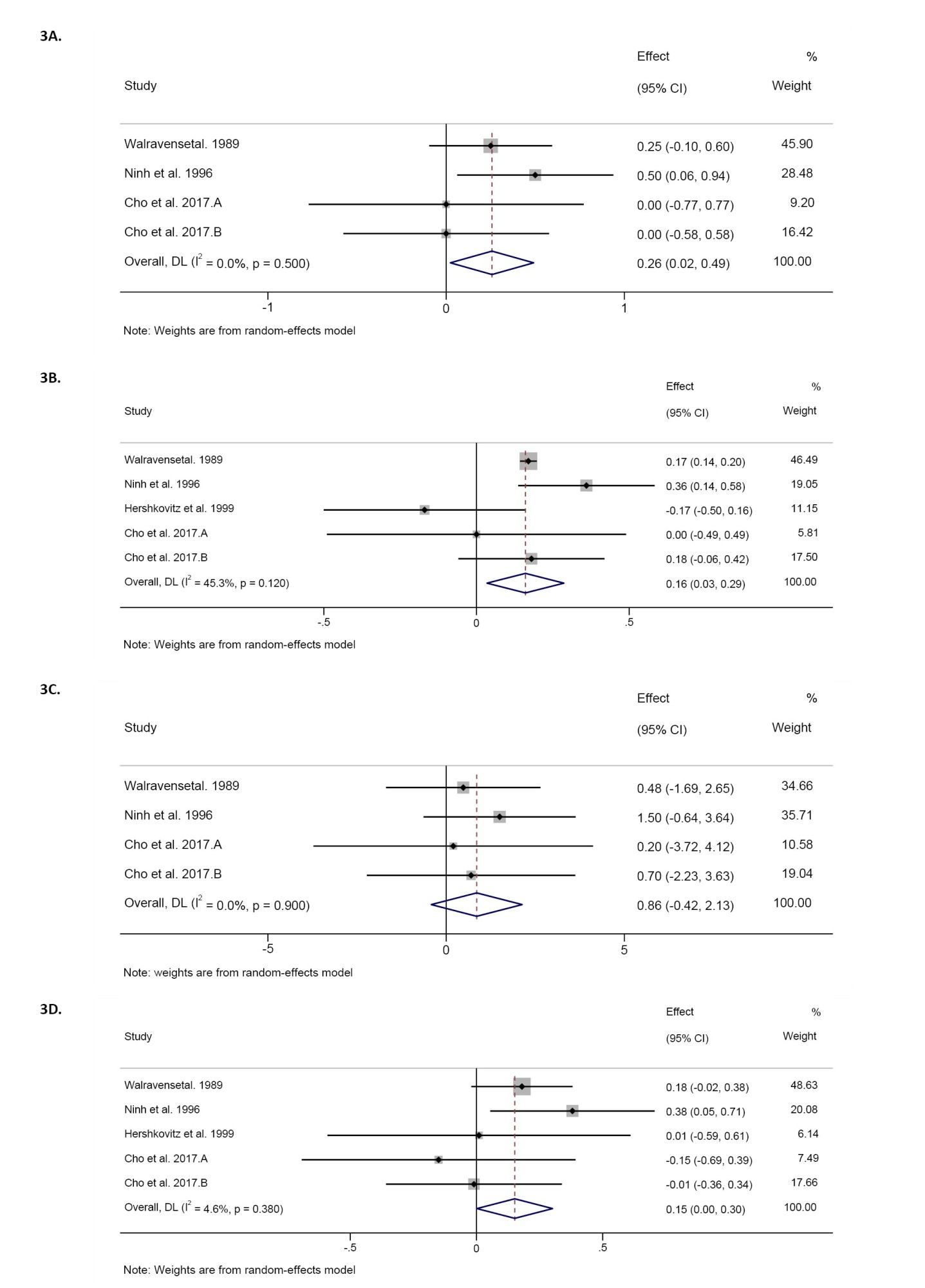
As a result of the comprehensive search, 94 studies were found in the databases. After removing 28 duplicate studies, 57 were screened based on their titles and abstracts. Next, the full text of 10 articles was read, 3 studies were excluded due to lack of randomized controlled trials, and 2 studies were excluded due to not reporting the desired data. Finally, 4 studies (effect sizes=5) with 222 participants were included in this systematic review (Figure 1) [23-26].
Study characteristics
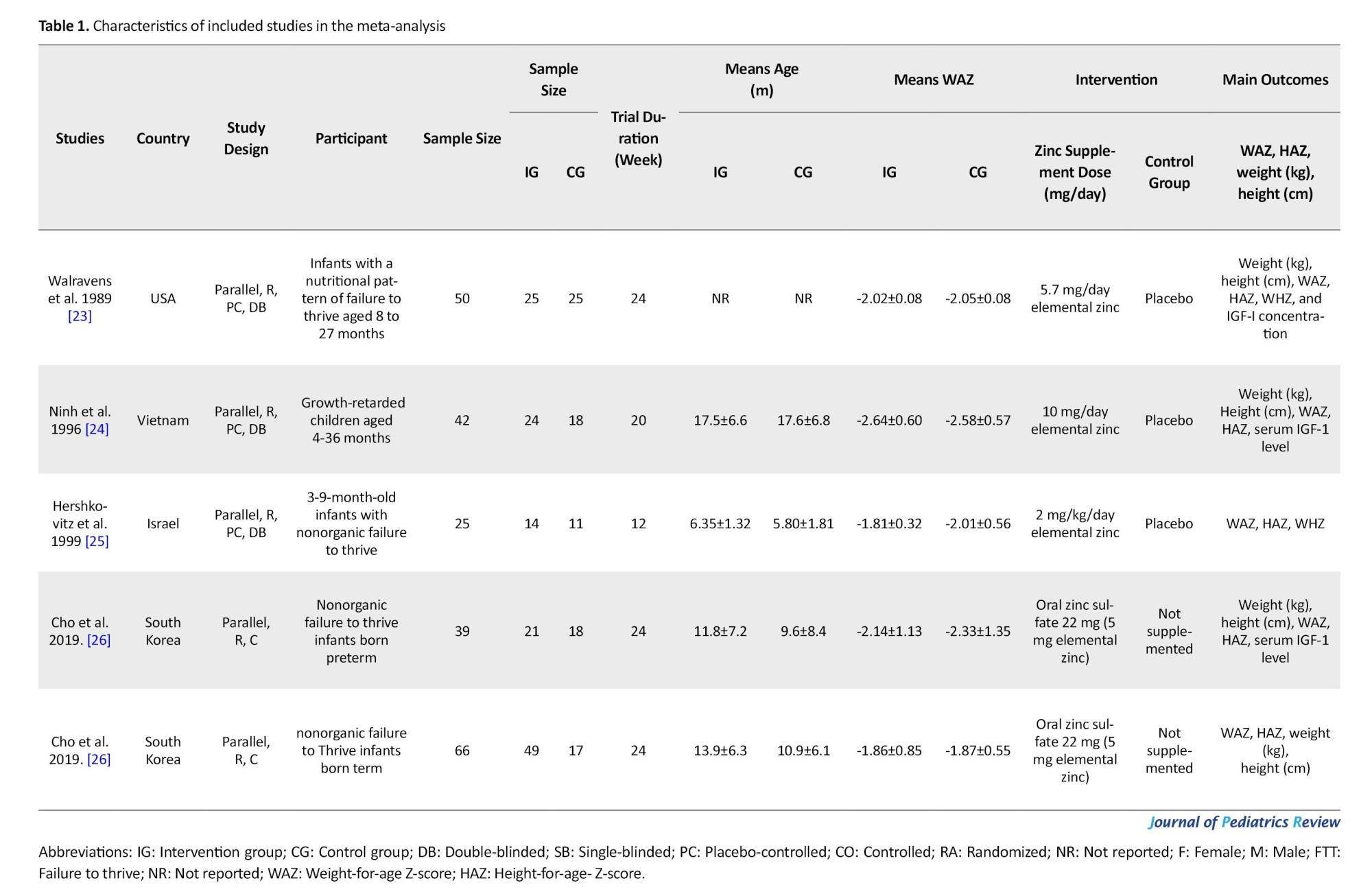
Eligible studies were published from 1989 [23] to 2019 [26]. The countries where the studies were conducted included the USA [23], Vietnam [24], Israel [25], and South Korea [26]. The sample size of the included arms varied from 25 [23] to 66 individuals [26]. The mean age of the participants was from 6.07 [25] to 17.55 months [24]. The control group of all included studies, except for Cho et al. [26], received a placebo. The duration of zinc supplementation in the eligible studies was between 12 [25] and 24 weeks [23, 26] and the dose of elemental zinc received almost varied from 5 [26] to 10 mg per day [24]. The characteristics of the included studies are summarized in Table 1.
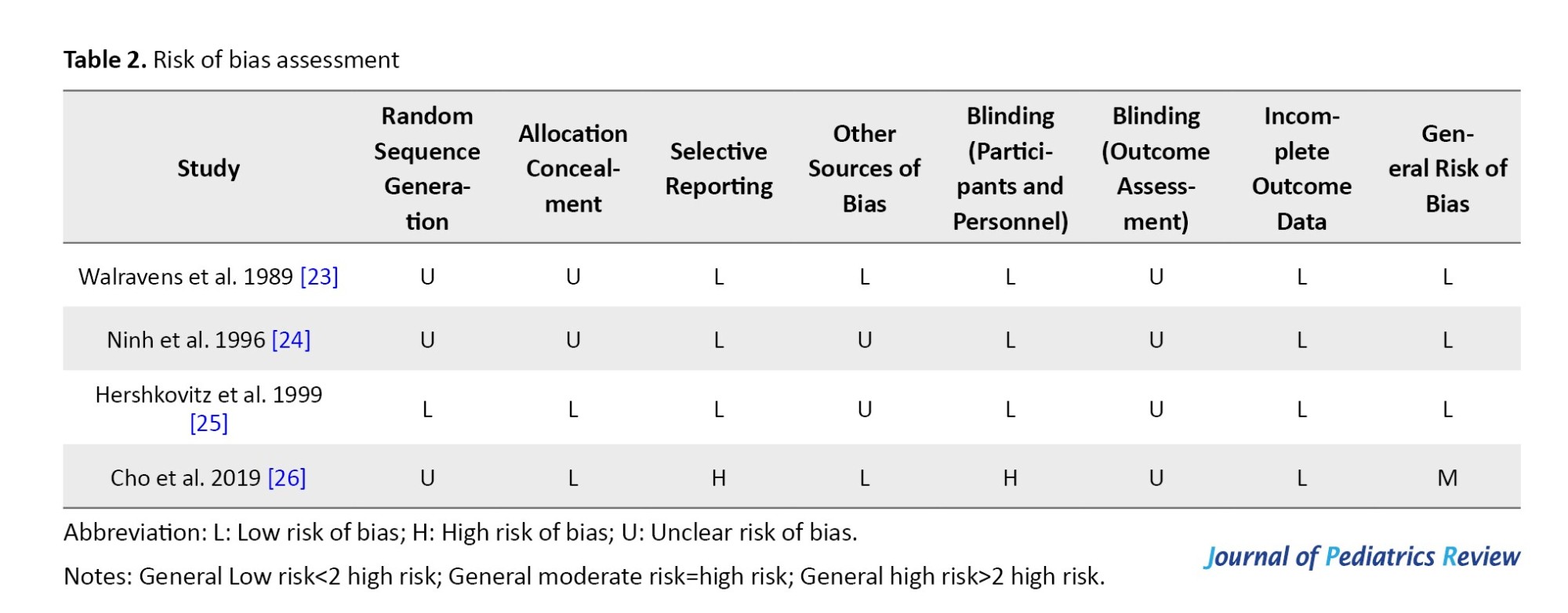
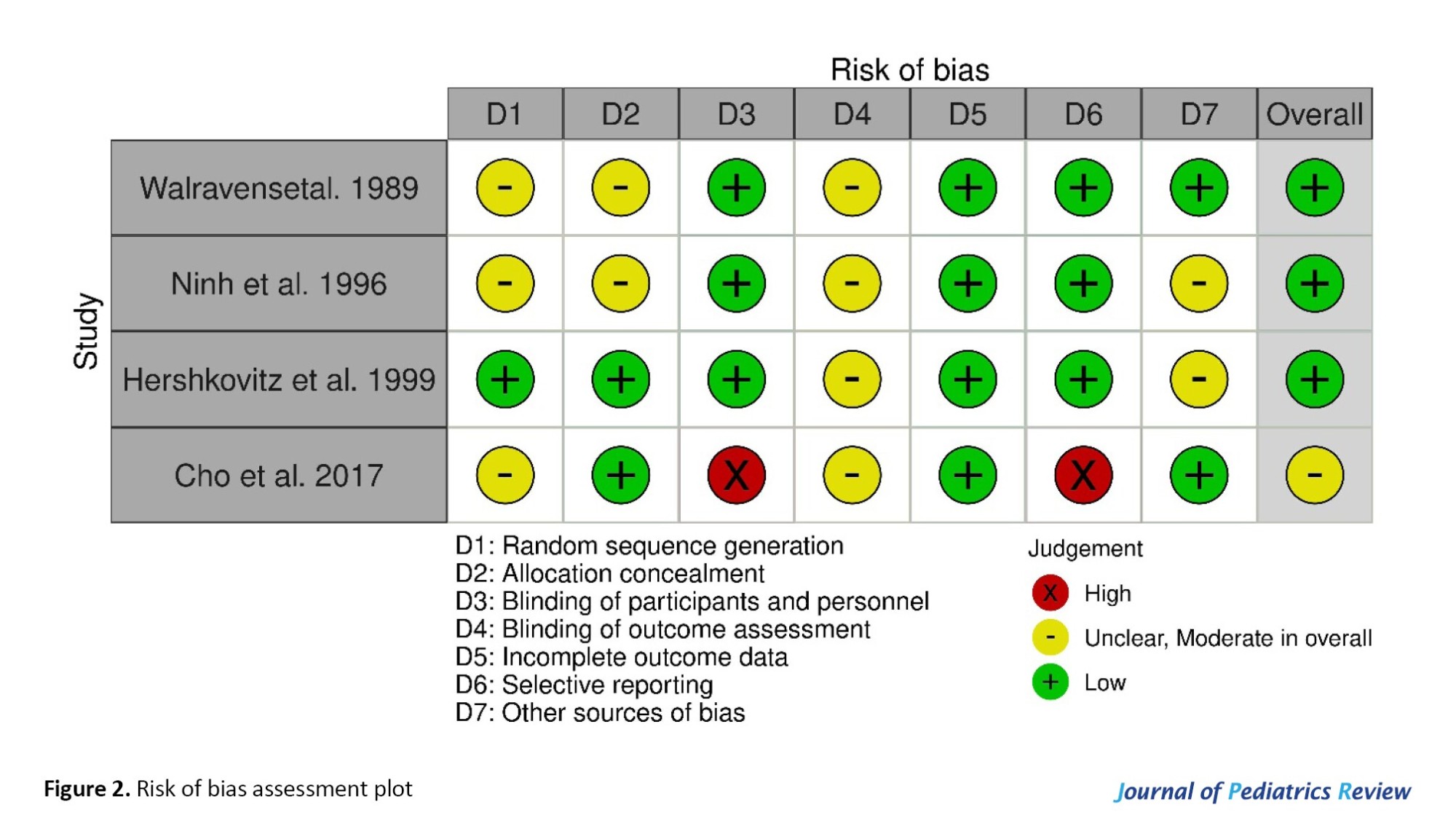
Risk of bias included studies were performed using the Cochrane collaboration risk of bias tool. The general risk of bias was low for 3 studies [23-25] and moderate for one [26]. Details of the risk of bias assessment are shown in Table 2 and Figure 2.
Meta‑analysis
Effects of zinc supplementation on weight
Pooling of 4 effect sizes showed that zinc supplementation led to a significant increase in weight compared to the control groups (WMD=-0.25 kg; 95% CI, 0.02%, 0.49%; P=0.03; n=197 participants). However, significant heterogeneity was not detected among the included studies (I2=0.0%, P=0.50) (Figure 3A).
Effects of zinc supplementation on height
The combination of 4 effect sizes demonstrated that zinc supplementation did not cause a significant change in height compared to the control groups (WMD=0.85 cm; 95% CI, -0.42%, 2.13%; P=0.18; n=197 participants). Also, no significant heterogeneity was observed among the included studies (I2=0.0%, P=0.90).
Effects of zinc supplementation on weight-for-age Z-score
The meta-analysis of 5 effect sizes showed that zinc supplementation led to a significant increase in weight-for-age Z-score compared to control groups (WMD=0.16; 95% CI, 0.03%, 0.28%; P=0.01; n=222 participants). However, there was no significant heterogeneity among the included studies (I2=45.3%, P=0.12) (Figure 3B).
Effects of zinc supplementation on height-for-age Z-score
The combination of 5 effect sizes showed a significant increasing effect of zinc supplementation compared to control groups on height-for-age Z-score (WMD=0.15; 95% CI, 0.001%, 0.30%; P=0.04; n=222 participants). Meanwhile, significant heterogeneity was observed among the included studies (I2=4.6%, P=0.38) (Figure 3D).
Effects of zinc supplementation on Insulin-like growth factor 1 (IGF-1) serum levels
The combination of 2 effect sizes revealed the non-significance of zinc supplementation on IGF-1 serum levels compared to the control groups (WMD=-7.01; 95% CI, -38.36%, 24.33%; P=0.66; n=105 participants). Meanwhile, high heterogeneity among included studies was mentioned (I2=81.6%, P=0.02) (Figure 3E).
Sensitivity analysis and publication bias
Sensitivity analysis of the significant difference in overall effect size of zinc supplementation effect on weight after omitting Walravens et al. (WMD=0.24 kg; 95% CI, -0.10%, 0.60%) [23], and Ninh et al. (WMD=0.16 kg; 95% CI, -0.11%, 0.43%) [24] was reported. Also pooled effect size for weight for age Z-score after excluding Walravens et al. (WMD=0.12; 95% CI, -0.10%, 0.36%) [23], Cochrane Handbook et al. (WMD=0.12; 95% CI, -0.00%, 0.24%) [24], and Cho et al. (WMD=0.14; 95% CI, -0.03%, 0.31%) [26], changed significantly. Furthermore, the overall effect size for height for age Z-score significantly changed after excluding Walravens et al. (WMD=0.10; 95% CI, -0.13%, 0.35%) [23], Ninh et al. (WMD=0.09; 95% CI, -0.06%, 0.25%) [24], and Hershkovitz et al. (WMD=0.15; 95% CI, -0.02%, 0.33%) [25]. The significance of the overall effect of zinc supplementation on height was not dependent on the presence of any of the pooled effect sizes.
Because the effect sizes included in this meta-analysis were <10, publication bias was not evaluated according to the Cochrane handbook [27]. Because 10 effect sizes or more are needed to draw accurate and interpretable conclusions from publication bias.
Discussion
According to the results of our meta-analysis, zinc supplementation significantly increased weight, weight-for-age Z-score, and height-for-age Z-score compared to control groups in FTT infants. However, we detected no significant effect of zinc supplementation on height. Additionally, the combination of 2 effect sizes revealed the non-significance of zinc supplementation on IGF-1 serum levels in FTT infants.
Effects of zinc supplementation on weight, height and growth
Prior reports have demonstrated that zinc supplementation improves growth parameters in infants. The weight-for-age Z-score and weight-for-length Z-score of an infant whose daily zinc intake is 10 mg are 4% greater than those of an infant whose daily zinc intake is 5 mg [28]. On the other hand, neither healthy infants in the Gambia nor healthy infants in the United States responded to supplementation with increased growth [29, 30]. A review of 25 studies on the effects of zinc supplements on the growth of children in developing countries found small but significant effects on growth, with effect sizes of +0.22 for height gains and +0.26 for weight gains [31]. This meta-analysis was updated by Brown et al. and used 33 randomized controlled trials. It showed a significant overall effect size of 0.350 (95% CI, 0.189%, 0.511%) for height, 0.309 (95% CI, 0.178%, 0.439%) for weight, and ≈0 for weight-for-length Z-score increments [32]. Thus, the effects of zinc supplementation on children’s development have been extensively studied in developing countries, but less is known in industrialized nations [32]. Therefore, the potential benefits of increased zinc intake for children in industrialized countries remain undetermined.
Previous meta-analyses estimated the dose-response relation between zinc intake and some growth parameters (weight-for-age Z-score and weight-for-length Z-score) in the infant population. These data can supplement evidence for supporting zinc reference values; however, extrapolation of results to other populations, especially developing populations should be treated with caution [28]. Other growth parameters in meta-analyses had no effect [28]. Zinc supplementation improved specific growth outcomes, including height, weight, and weight-for-age Z-score in infants and children but not in pregnant women, according to another systematic review and meta-analysis of randomized controlled trials. Furthermore, evidence was found suggesting that the effects on height and height-for-age Z-score may be more pronounced in children aged two years and older, as opposed to infants [14]. Other growth outcomes, like the risk of stunting, underweight, or wasting, were not found to be significantly influenced by zinc supplementation [14].
In line with our results, zinc supplementation may be more beneficial for height, height-for-age Z-score, and weight in children younger than 2 years of age. Infants may have a lower risk of zinc deficiency because their mothers are providing them with zinc through breastfeeding or because their zinc stores are already relatively full at birth [33]. According to the last meta-analysis, most trials did not check the zinc status of participants before they started; therefore, it was difficult to tell if the results would be different depending on the zinc levels at the start. Possible explanations for the smaller effect size during infancy are that measuring growth, especially weight-for-age Z-score, is more difficult in the field during infancy than it is during childhood [34].
According to Vakili’s research, zinc supplementation has a greater impact on body mass index and weight-for-age Z-score in women than in men. While zinc supplementation had a greater effect on height-for-age Z-score in males than placebo, this effect was not statistically significant in females. This supports the finding that zinc improves growth velocity in short-statured boys [35], but not in females [36]. Zinc improves growth in relatively healthy children [8, 10, 11] but some studies in developing countries have found no effect of zinc on growth, likely due to other growth-limiting factors [29, 37, 38]. Previous meta-analyses [32, 39, 40] that implemented trials of zinc supplementation throughout childhood found that it was associated with a small but significant increase in height and weight; in sub-analysis, these benefits persisted in groups aged 1-5 and 5-13 but not 6-12 months [40].
Effects of zinc supplementation on IGF-1 serum levels
The previous study showed that providing humans with zinc supplements can make their IGF-1 levels rise significantly. Furthermore, more substantial improvements were noted under the conditions of an 8-week intervention period and a daily zinc intake of 10 mg [41]. This is in contrast to the findings that zinc supplementation for >8 weeks significantly increased IGF-1. This could be because zinc-deficient patients need to take supplements for longer than 8 weeks to restore zinc deposits, which could be related to the baseline serum zinc concentrations of the subjects who were given zinc. The levels of IGF-1 are raised by zinc supplementation in both zinc-deficient and healthy individuals [42]. However, earlier research has suggested that zinc supplementation is more helpful for patients with zinc deficiency and abnormal serum zinc levels. In a study on zinc supplementation and IGF1 levels in children with FTT, Park et al. found no significant changes in serum IGF-1 levels after the study ended, likely because the study group had normal zinc and IGF-1 levels before the zinc intervention [43].
Due to its involvement in cell growth, immunity, tissue repair, protein and DNA synthesis, thyroid gland and optimal bone functioning, and more, zinc is often referred to as the metal of life [44, 45]. Similar to proteins, phosphorus, magnesium, sodium, and potassium, zinc inhibits linear growth when present in deficiency [46]. Infants who are small for their gestational age have lower levels of zinc in their placental proteins, iron stores, and hemoglobin compared to infants who are large for their gestational ages. This suggests that zinc is needed as early as fetal-placental development. Therefore, according to the study of Akram et al. zinc supplementation during pregnancy may reduce the risk of preterm birth and have a beneficial effect on the pregnancy’s outcome and the birth weight of the infant [47]. In murine models of zinc deficiency, increasing caloric intake or external administration does not reverse the growth retardation, despite increasing IGF-1 levels [48]. Consuming an excessive amount of zinc every day is a reasonable benefit, but zinc is an important part of raising IGF-1 levels. According to some studies, zinc supplementation does not affect IGF-1 levels. According to a study zinc supplementation (7 mg tablets or micronutrient powder consisting of 10 mg zinc+6 mg iron+13 other micronutrients) to 419 laotian children did not elevate IGF-1 levels. The recommended daily allowance for zinc in humans is 14–30 mg, but values between 2.8 and 40 mg/day can reportedly yield physiological zinc homeostasis, with excess zinc being primarily eliminated through the gastrointestinal tract [49].
Conclusion
The current meta-analysis revealed that zinc supplementation significantly increased the weight and Z-score of weight for age and height for age in infants with FTT. Furthermore, our review indicated that zinc supplementation did not lead to a significant change in the other growth parameters including height and IGF-1. Due to the limited number of included studies and the non-ideal quality of some of them, it is not possible to draw definitive conclusions and generalize the findings of this issue, so it is recommended that RCTs with larger sample sizes and higher sensitivity investigate this intervention on the growth-related parameters in the infants with FTT.
Study limitations
To the best of our knowledge, this is the first systematic review and meta-analysis investigating the effect of zinc supplementation on growth-related factors in infants with FTT. Despite its novelty, this meta-analysis had several limitations, including the limited number of included studies, insufficient sample size, and non-uniformity of the type of zinc supplement received.
Ethical Considerations
Compliance with ethical guidelines
The protocol for this systematic review and meta-analysis was registered at the PROSPERO database (Code: CRD42023477547).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and study design: Mostafa Shahraki Jazinaki and Mohammad Safarian; Searching strategy, data extraction: Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan; Data interpretation: Mostafa Shahraki Jazinaki; Manuscript preparation: Mohammad Safarian, Mohammad Rashidmayvan and Mostafa Shahraki Jazinaki; Review and editing: Mostafa Shahraki Jazinaki and Abdolreza Norouzy; Final approval: All authors.
Conflicts of interest
The author declared no conflict of interest.
Acknowledgements
The authors thank all the staff and employees of Mashhad University of Medical Sciences for their support of this study.
References
In the early years of life, growth is considered a parameter indicating the suitable condition of the infant [1]. If the high energy requirements in infants are not met well, it leads to lower-than-expected weight growth based on specific growth charts for gender and age; this is a common definition for failure to thrive (FTT). If a child weighs less than the fifth percentile for his age and gender on the growth chart, that is considered FTT, according to more scientific definitions. Alternatively, the child’s weight growth percentile line recedes as much as the two lines or more [2]. Previous studies have reported the relationship between FTT and developmental delay, long-term deficits in height, weight, and academic performance [1, 3, 4]. Furthermore, FTT can increase the risk of being overweight and obese in later decades of life [5]. Often, children with FTT are treated on an outpatient basis by referring them to interdisciplinary clinics and based on recommendations from the American Academy of Pediatrics Committee on Nutrition [1, 6-8]. Chronic diseases and anemias, the lack of access to food, dysfunction of the digestive system, digestive symptoms, and lack of appetite are among the most critical causes of FTT [9]. The management of FTT includes providing energy and macronutrients, correcting nutrient deficiencies, financial support, family counseling, ongoing nutritional assessments, and long-term monitoring [9].
Zinc is an essential trace element in the structure of some proteins and molecules in the body [10]. It plays a role in cell division, cell differentiation, membrane integrity, enzyme, and antioxidant system function [11]. Also, zinc is vital for synthesizing nucleic acid, and proteins. Furthermore, it is necessary for maintaining lean body mass [12]. In addition, zinc deficiency in children is known as a growth-limiting factor [13]. In several past studies, a significant effect of zinc supplementation on the height and weight growth of children has been reported, but there is no consensus about it yet [11, 14]. This meta-analysis investigates the effect of zinc supplementation on growth-related factors in infants with FTT.
Methods
This systematic review was based on the preferred reporting items of systematic reviews and meta-analysis framework [15].
Search strategy
Medline, Scopus, and ISI databases were comprehensively searched up to June 2023 to find eligible trials based on the inclusion criteria. This search did not include any language or time restrictions. The applied search strategy consisted of the following medical subject headings and non-medical subject headings terms:
(“Zinc” OR “zinc supplementation” OR “zinc sulfate” OR “zinc gluconate”) AND (“FTT” OR “failure to thrive” OR “catch-up growth” OR “growth-retarded”) AND (“intervention” OR “randomized” OR “placebo” OR “clinical trials” OR “trial” OR “randomized controlled trial” OR “RCT” OR “cross-over” OR “parallel”).
All the reference lists of eligible studies were assessed, and Google Scholar was manually searched to avoid missing relevant articles.
Eligibility criteria
The studies obtained from the initial search were screened to find randomized controlled trials that investigated the effect of zinc supplementation on growth-related factors in children with FTT. Disagreements were discussed until a consensus was reached. Two researchers (Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan) performed the screening independently via the Endnote software using the titles and abstracts of papers. The inclusion and exclusion criteria were designed based on the participant, intervention, comparison, outcome, and studies framework as follows: Participant=FTT patients, intervention=zinc supplement, comparison=control group, outcome=growth-related outcomes, study=randomized controlled trials [16]. The inclusion criteria for meta-analysis comprised the following items: Human intervention studies, articles with randomized controlled trial design, intervention with zinc supplementation in the FTT population, and reporting the changes in growth-related outcomes during the intervention Mean±SD. Meanwhile, the exclusion criteria included animal studies, combined treatment, no control group, observational studies, review articles, and letters to the editor.
Data extraction
Two authors (Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan) extracted the related data from relevant articles obtained from screening independently. The desired information of this review, including the name of the first author, the year of publication, country, the characteristics of each of the study groups (number of people, mean age, and weight), type, and dose and duration of zinc supplementation, and Mean±SD of outcomes for both the intervention and control groups were extracted from the studies entered by two authors. Disagreements were discussed until a consensus was reached.
Quality assessment
The assessment of the risk of bias in the included studies was performed using the Cochran quality assessment tool (risk of bias 1) by two researchers (Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan), independently [17]. This tool assessed the risk of bias for each study in the following seven subclasses: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. The risk of bias in each subclass was classified as high, unclear, and low.
The general risk of bias was considered high if the number of items with a high risk of bias in each study was ≥3; if it was 2, it was considered moderate and if it was ≤1, it was considered low general bias. Disagreements were resolved in consultation with Mohammad Safarian.
Data synthesis and statistical analysis
The pooled effect size was estimated using weighted mean differences (WMD) and the SD of measures from both intervention and control groups for different variables and by implementing the random effect model based on the DerSimonian and Laird method [18]. In the case of not reporting Mean±SD changes, mean change was calculated by subtracting variable values at the beginning of the intervention from the end. Also, change SD was estimated using the Equation 1 [19]:

Reported standard error, medians, interquartile ranges, and 95% CI were converted to SDs using the method of Hozo et al. [20]. The Cochran Q test evaluated the studies’ heterogeneity and reported them by I2 statistic [21]. Accordingly, I2>40% or P<0.05 was interpreted as significant heterogeneity. To investigate the effect of the quality and characteristics of each study on the overall size effect, a sensitivity analysis was performed [22]. In all the analyses that were performed using the STATA software, version 17 and P<0.05 were considered statistically significant.
Results
Study selection
As a result of the comprehensive search, 94 studies were found in the databases. After removing 28 duplicate studies, 57 were screened based on their titles and abstracts. Next, the full text of 10 articles was read, 3 studies were excluded due to lack of randomized controlled trials, and 2 studies were excluded due to not reporting the desired data. Finally, 4 studies (effect sizes=5) with 222 participants were included in this systematic review (Figure 1) [23-26].

Study characteristics
Eligible studies were published from 1989 [23] to 2019 [26]. The countries where the studies were conducted included the USA [23], Vietnam [24], Israel [25], and South Korea [26]. The sample size of the included arms varied from 25 [23] to 66 individuals [26]. The mean age of the participants was from 6.07 [25] to 17.55 months [24]. The control group of all included studies, except for Cho et al. [26], received a placebo. The duration of zinc supplementation in the eligible studies was between 12 [25] and 24 weeks [23, 26] and the dose of elemental zinc received almost varied from 5 [26] to 10 mg per day [24]. The characteristics of the included studies are summarized in Table 1.

Risk of bias included studies were performed using the Cochrane collaboration risk of bias tool. The general risk of bias was low for 3 studies [23-25] and moderate for one [26]. Details of the risk of bias assessment are shown in Table 2 and Figure 2.


Meta‑analysis
Effects of zinc supplementation on weight
Pooling of 4 effect sizes showed that zinc supplementation led to a significant increase in weight compared to the control groups (WMD=-0.25 kg; 95% CI, 0.02%, 0.49%; P=0.03; n=197 participants). However, significant heterogeneity was not detected among the included studies (I2=0.0%, P=0.50) (Figure 3A).

Effects of zinc supplementation on height
The combination of 4 effect sizes demonstrated that zinc supplementation did not cause a significant change in height compared to the control groups (WMD=0.85 cm; 95% CI, -0.42%, 2.13%; P=0.18; n=197 participants). Also, no significant heterogeneity was observed among the included studies (I2=0.0%, P=0.90).
Effects of zinc supplementation on weight-for-age Z-score
The meta-analysis of 5 effect sizes showed that zinc supplementation led to a significant increase in weight-for-age Z-score compared to control groups (WMD=0.16; 95% CI, 0.03%, 0.28%; P=0.01; n=222 participants). However, there was no significant heterogeneity among the included studies (I2=45.3%, P=0.12) (Figure 3B).
Effects of zinc supplementation on height-for-age Z-score
The combination of 5 effect sizes showed a significant increasing effect of zinc supplementation compared to control groups on height-for-age Z-score (WMD=0.15; 95% CI, 0.001%, 0.30%; P=0.04; n=222 participants). Meanwhile, significant heterogeneity was observed among the included studies (I2=4.6%, P=0.38) (Figure 3D).
Effects of zinc supplementation on Insulin-like growth factor 1 (IGF-1) serum levels
The combination of 2 effect sizes revealed the non-significance of zinc supplementation on IGF-1 serum levels compared to the control groups (WMD=-7.01; 95% CI, -38.36%, 24.33%; P=0.66; n=105 participants). Meanwhile, high heterogeneity among included studies was mentioned (I2=81.6%, P=0.02) (Figure 3E).
Sensitivity analysis and publication bias
Sensitivity analysis of the significant difference in overall effect size of zinc supplementation effect on weight after omitting Walravens et al. (WMD=0.24 kg; 95% CI, -0.10%, 0.60%) [23], and Ninh et al. (WMD=0.16 kg; 95% CI, -0.11%, 0.43%) [24] was reported. Also pooled effect size for weight for age Z-score after excluding Walravens et al. (WMD=0.12; 95% CI, -0.10%, 0.36%) [23], Cochrane Handbook et al. (WMD=0.12; 95% CI, -0.00%, 0.24%) [24], and Cho et al. (WMD=0.14; 95% CI, -0.03%, 0.31%) [26], changed significantly. Furthermore, the overall effect size for height for age Z-score significantly changed after excluding Walravens et al. (WMD=0.10; 95% CI, -0.13%, 0.35%) [23], Ninh et al. (WMD=0.09; 95% CI, -0.06%, 0.25%) [24], and Hershkovitz et al. (WMD=0.15; 95% CI, -0.02%, 0.33%) [25]. The significance of the overall effect of zinc supplementation on height was not dependent on the presence of any of the pooled effect sizes.
Because the effect sizes included in this meta-analysis were <10, publication bias was not evaluated according to the Cochrane handbook [27]. Because 10 effect sizes or more are needed to draw accurate and interpretable conclusions from publication bias.
Discussion
According to the results of our meta-analysis, zinc supplementation significantly increased weight, weight-for-age Z-score, and height-for-age Z-score compared to control groups in FTT infants. However, we detected no significant effect of zinc supplementation on height. Additionally, the combination of 2 effect sizes revealed the non-significance of zinc supplementation on IGF-1 serum levels in FTT infants.
Effects of zinc supplementation on weight, height and growth
Prior reports have demonstrated that zinc supplementation improves growth parameters in infants. The weight-for-age Z-score and weight-for-length Z-score of an infant whose daily zinc intake is 10 mg are 4% greater than those of an infant whose daily zinc intake is 5 mg [28]. On the other hand, neither healthy infants in the Gambia nor healthy infants in the United States responded to supplementation with increased growth [29, 30]. A review of 25 studies on the effects of zinc supplements on the growth of children in developing countries found small but significant effects on growth, with effect sizes of +0.22 for height gains and +0.26 for weight gains [31]. This meta-analysis was updated by Brown et al. and used 33 randomized controlled trials. It showed a significant overall effect size of 0.350 (95% CI, 0.189%, 0.511%) for height, 0.309 (95% CI, 0.178%, 0.439%) for weight, and ≈0 for weight-for-length Z-score increments [32]. Thus, the effects of zinc supplementation on children’s development have been extensively studied in developing countries, but less is known in industrialized nations [32]. Therefore, the potential benefits of increased zinc intake for children in industrialized countries remain undetermined.
Previous meta-analyses estimated the dose-response relation between zinc intake and some growth parameters (weight-for-age Z-score and weight-for-length Z-score) in the infant population. These data can supplement evidence for supporting zinc reference values; however, extrapolation of results to other populations, especially developing populations should be treated with caution [28]. Other growth parameters in meta-analyses had no effect [28]. Zinc supplementation improved specific growth outcomes, including height, weight, and weight-for-age Z-score in infants and children but not in pregnant women, according to another systematic review and meta-analysis of randomized controlled trials. Furthermore, evidence was found suggesting that the effects on height and height-for-age Z-score may be more pronounced in children aged two years and older, as opposed to infants [14]. Other growth outcomes, like the risk of stunting, underweight, or wasting, were not found to be significantly influenced by zinc supplementation [14].
In line with our results, zinc supplementation may be more beneficial for height, height-for-age Z-score, and weight in children younger than 2 years of age. Infants may have a lower risk of zinc deficiency because their mothers are providing them with zinc through breastfeeding or because their zinc stores are already relatively full at birth [33]. According to the last meta-analysis, most trials did not check the zinc status of participants before they started; therefore, it was difficult to tell if the results would be different depending on the zinc levels at the start. Possible explanations for the smaller effect size during infancy are that measuring growth, especially weight-for-age Z-score, is more difficult in the field during infancy than it is during childhood [34].
According to Vakili’s research, zinc supplementation has a greater impact on body mass index and weight-for-age Z-score in women than in men. While zinc supplementation had a greater effect on height-for-age Z-score in males than placebo, this effect was not statistically significant in females. This supports the finding that zinc improves growth velocity in short-statured boys [35], but not in females [36]. Zinc improves growth in relatively healthy children [8, 10, 11] but some studies in developing countries have found no effect of zinc on growth, likely due to other growth-limiting factors [29, 37, 38]. Previous meta-analyses [32, 39, 40] that implemented trials of zinc supplementation throughout childhood found that it was associated with a small but significant increase in height and weight; in sub-analysis, these benefits persisted in groups aged 1-5 and 5-13 but not 6-12 months [40].
Effects of zinc supplementation on IGF-1 serum levels
The previous study showed that providing humans with zinc supplements can make their IGF-1 levels rise significantly. Furthermore, more substantial improvements were noted under the conditions of an 8-week intervention period and a daily zinc intake of 10 mg [41]. This is in contrast to the findings that zinc supplementation for >8 weeks significantly increased IGF-1. This could be because zinc-deficient patients need to take supplements for longer than 8 weeks to restore zinc deposits, which could be related to the baseline serum zinc concentrations of the subjects who were given zinc. The levels of IGF-1 are raised by zinc supplementation in both zinc-deficient and healthy individuals [42]. However, earlier research has suggested that zinc supplementation is more helpful for patients with zinc deficiency and abnormal serum zinc levels. In a study on zinc supplementation and IGF1 levels in children with FTT, Park et al. found no significant changes in serum IGF-1 levels after the study ended, likely because the study group had normal zinc and IGF-1 levels before the zinc intervention [43].
Due to its involvement in cell growth, immunity, tissue repair, protein and DNA synthesis, thyroid gland and optimal bone functioning, and more, zinc is often referred to as the metal of life [44, 45]. Similar to proteins, phosphorus, magnesium, sodium, and potassium, zinc inhibits linear growth when present in deficiency [46]. Infants who are small for their gestational age have lower levels of zinc in their placental proteins, iron stores, and hemoglobin compared to infants who are large for their gestational ages. This suggests that zinc is needed as early as fetal-placental development. Therefore, according to the study of Akram et al. zinc supplementation during pregnancy may reduce the risk of preterm birth and have a beneficial effect on the pregnancy’s outcome and the birth weight of the infant [47]. In murine models of zinc deficiency, increasing caloric intake or external administration does not reverse the growth retardation, despite increasing IGF-1 levels [48]. Consuming an excessive amount of zinc every day is a reasonable benefit, but zinc is an important part of raising IGF-1 levels. According to some studies, zinc supplementation does not affect IGF-1 levels. According to a study zinc supplementation (7 mg tablets or micronutrient powder consisting of 10 mg zinc+6 mg iron+13 other micronutrients) to 419 laotian children did not elevate IGF-1 levels. The recommended daily allowance for zinc in humans is 14–30 mg, but values between 2.8 and 40 mg/day can reportedly yield physiological zinc homeostasis, with excess zinc being primarily eliminated through the gastrointestinal tract [49].
Conclusion
The current meta-analysis revealed that zinc supplementation significantly increased the weight and Z-score of weight for age and height for age in infants with FTT. Furthermore, our review indicated that zinc supplementation did not lead to a significant change in the other growth parameters including height and IGF-1. Due to the limited number of included studies and the non-ideal quality of some of them, it is not possible to draw definitive conclusions and generalize the findings of this issue, so it is recommended that RCTs with larger sample sizes and higher sensitivity investigate this intervention on the growth-related parameters in the infants with FTT.
Study limitations
To the best of our knowledge, this is the first systematic review and meta-analysis investigating the effect of zinc supplementation on growth-related factors in infants with FTT. Despite its novelty, this meta-analysis had several limitations, including the limited number of included studies, insufficient sample size, and non-uniformity of the type of zinc supplement received.
Ethical Considerations
Compliance with ethical guidelines
The protocol for this systematic review and meta-analysis was registered at the PROSPERO database (Code: CRD42023477547).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and study design: Mostafa Shahraki Jazinaki and Mohammad Safarian; Searching strategy, data extraction: Mostafa Shahraki Jazinaki and Mohammad Rashidmayvan; Data interpretation: Mostafa Shahraki Jazinaki; Manuscript preparation: Mohammad Safarian, Mohammad Rashidmayvan and Mostafa Shahraki Jazinaki; Review and editing: Mostafa Shahraki Jazinaki and Abdolreza Norouzy; Final approval: All authors.
Conflicts of interest
The author declared no conflict of interest.
Acknowledgements
The authors thank all the staff and employees of Mashhad University of Medical Sciences for their support of this study.
References
- Black MM, Dubowitz H, Krishnakumar A, Starr RH Jr. Early intervention and recovery among children with failure to thrive: Follow-up at age 8. Pediatrics. 2007; 120(1):59-69. [DOI:10.1542/peds.2006-1657] [PMID]
- Smith AE, Shah M, Badireddy M. Failure to Thrive. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PMID]
- Glaser HH, Heagarty MC, Bullard DM Jr, Pivchik EC. Physical and psychological development of children with early failure to thrive. J Pediatr. 1968; 73(5):690-8. [DOI:10.1016/S0022-3476(68)80174-X] [PMID]
- Bithoney WG, McJunkin J, Michalek J, Snyder J, Egan H, Epstein D. The effect of a multidisciplinary team approach on weight gain in nonorganic failure-to-thrive children. J Dev Behav Pediatr. 1991; 12(4):254-8. [DOI:10.1097/00004703-199108000-00007] [PMID]
- La Scola C, Rivetti G, Bertulli C, Di Sessa A, Guarino S, Pasini A, et al. Failure to thrive in children with tubulopathies increases the risk of overweight later in life. Int J Obes (Lond). 2024; 48(1):127-9. [DOI:10.1038/s41366-023-01386-2] [PMID]
- Kessler DB, Dawson P. Failure to thrive and pediatric undernutrition: A transdisciplinary approach. Baltimore: Brookes Publishing Co; 1999. [Link]
- Black MM, Dubowitz H, Hutcheson J, Berenson-Howard J, Starr RH Jr. A randomized clinical trial of home intervention for children with failure to thrive. Pediatrics. 1995; 95(6):807-14. [DOI:10.1542/peds.95.6.807] [PMID]
- Wright CM, Callum J, Birks E, Jarvis S. Effect of community based management in failure to thrive: Randomised controlled trial. BMJ. 1998; 317(7158):571-4. [DOI:10.1136/bmj.317.7158.571] [PMID]
- Jeong SJ. Nutritional approach to failure to thrive. Korean J Pediatr. 2011; 54(7):277-81. [DOI:10.3345/kjp.2011.54.7.277] [PMID]
- Maxfield L, Shukla S, Crane JS. Zinc Deficiency. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PMID]
- Vakili R, Yazdan Bakhsh M, Vahedian M, Mahmoudi M, Saeidi M, Vakili S. The effect of zinc supplementation on linear growth and growth factors in primary schoolchildren in the suburbs Mashhad, Iran. Int J Pediatr. 2015; 3(2.1):1-7. [DOI:10.22038/ijp.2015.3931]
- Cunha TA, Vermeulen-Serpa KM, Grilo EC, Leite-Lais L, Brandão-Neto J, Vale SHL. Association between zinc and body composition: An integrative review. J Trace Elem Med Biol. 2022; 71:126940. [DOI:10.1016/j.jtemb.2022.126940] [PMID]
- Michaelsen KF, Samuelson G, Graham TW, Lönnerdal B. Zinc intake, zinc status and growth in a longitudinal study of healthy Danish infants. Acta Paediatr. 1994; 83(11):1115-21. [DOI:10.1111/j.1651-2227.1994.tb18262.x] [PMID]
- Liu E, Pimpin L, Shulkin M, Kranz S, Duggan CP, Mozaffarian D, et al. Effect of zinc supplementation on growth outcomes in children under 5 years of age. Nutrients. 2018; 10(3):377. [DOI:10.3390/nu10030377] [PMID]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009; 151(4):264-9. [DOI:10.7326/0003-4819-151-4-200908180-00135] [PMID]
- Methley AM, Campbell S, Chew-Graham C, McNally R, Cheraghi-Sohi S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv Res. 2014; 14:579. [DOI:10.1186/s12913-014-0579-0] [PMID]
- Higgins J, Green S. Cochrane handbook for systematic reviews of interventions, version 5.0. 2 [internet] [updated Sept 2009]. Available at: [Link]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986; 7(3):177-88. [DOI:10.1016/0197-2456(86)90046-2] [PMID]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. New Jersey: John Wiley & Sons; 2021. [Link]
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005; 5:13. [DOI:10.1186/1471-2288-5-13] [PMID]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003; 327(7414):557-60. [DOI:10.1136/bmj.327.7414.557] [PMID]
- Tobias A. Assessing the influence of a single study in the meta-analysis estimate. Stata Tech Bull. 1999; 47:15-7. [Link]
- Walravens PA, Hambidge KM, Koepfer DM. Zinc supplementation in infants with a nutritional pattern of failure to thrive: A double-blind, controlled study. Pediatrics. 1989; 83(4):532-8. [DOI:10.1542/peds.83.4.532] [PMID]
- Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin-like growth factor I (IGF-I) in growth-retarded Vietnamese children. Am J Clin Nutr. 1996; 63(4):514-9. [DOI:10.1093/ajcn/63.4.514] [PMID]
- Hershkovitz E, Printzman L, Segev Y, Levy J, Phillip M. Zinc supplementation increases the level of serum insulin-like growth factor-I but does not promote growth in infants with nonorganic failure to thrive. Horm Res. 1999; 52(4):200-4. [DOI:10.1159/000023461] [PMID]
- Cho JM, Kim JY, Yang HR. Effects of oral zinc supplementation on zinc status and catch-up growth during the first 2 years of life in children with non-organic failure to thrive born preterm and at term. Pediatr Neonatol. 2019; 60(2):201-9. [DOI:10.1016/j.pedneo.2018.06.006] [PMID]
- Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: A new edition of the cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019; 10(10):ED000142. [DOI:10.1002/14651858.ED000142] [PMID]
- Nissensohn M, Sánchez-Villegas A, Fuentes Lugo D, Henríquez Sánchez P, Doreste Alonso J, Peña Quintana L, et al. Effect of zinc intake on growth in infants: A meta-analysis. Crit Rev Food Sci Nutr. 2016; 56(3):350-63. [DOI:10.1080/10408398.2013.802661] [PMID]
- Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop-Clewes CA, et al. A trial of zinc supplementation in young rural Gambian children. Br J Nutr. 1993; 69(1):243-55. [DOI:10.1079/BJN19930026] [PMID]
- Heinig MJ, Brown KH, Lönnerdal B, Dewey KG. Zinc supplementation does not affect growth, morbidity, or motor development of US term breastfed infants at 4-10 mo of age. Am J Clin Nutr. 2006; 84(3):594-601. [DOI:10.1093/ajcn/84.3.594] [PMID]
- Brown KH, Peerson JM, Allen LH. Effect of zinc supplementation on children's growth: A meta-analysis of intervention trials. Bibl Nutr Dieta. 1998; (54):76-83.[DOI:10.1159/000059448] [PMID]
- Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trials. Am J Clin Nutr. 2002; 75(6):1062-71. [DOI:10.1093/ajcn/75.6.1062] [PMID]
- Brown KH, Engle-Stone R, Krebs NF, Peerson JM. Dietary intervention strategies to enhance zinc nutrition: Promotion and support of breastfeeding for infants and young children. Food Nutr Bull. 2009; 30(1 Suppl):S144-71. [DOI:10.1177/15648265090301S108] [PMID]
- Mwangome MK, Berkley JA. The reliability of weight-for-length/height Z scores in children. Matern Child Nutr. 2014; 10(4):474-80. [DOI:10.1111/mcn.12124] [PMID]
- Nakamura T, Nishiyama S, Futagoishi-Suginohara Y, Matsuda I, Higashi A. Mild to moderate zinc deficiency in short children: Effect of zinc supplementation on linear growth velocity. J Pediatr. 1993; 123(1):65-9. [DOI:10.1016/S0022-3476(05)81538-0] [PMID]
- Garenne M, Becher H, Ye Y, Kouyate B, Müller O. Sex-specific responses to zinc supplementation in Nouna, Burkina Faso. Journal of pediatric gastroenterology and nutrition. 2007; 44(5):619-28. [DOI:10.1097/MPG.0b013e31802c695e] [PMID]
- Kikafunda JK, Walker AF, Allan EF, Tumwine JK. Effect of zinc supplementation on growth and body composition of Ugandan preschool children: A randomized, controlled, intervention trial. Am J Clin Nutr. 1998; 68(6):1261-6. [DOI:10.1093/ajcn/68.6.1261] [PMID]
- Saeidi M, Vakili R, Khakshour A, Taghizadeh Moghaddam H, Kiani M. Iron and multivitamin supplements in children and its association with growth rate. Int J Pediatr. 2014; 2(2.1):21. [DOI:10.22038/ijp.2014.2475]
- Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food Nutr Bull. 2009; 30(1 Suppl):S12-40. [DOI:10.1177/15648265090301S103] [PMID]
- Mayo-Wilson E, Junior JA, Imdad A, Dean S, Chan XHS, Chan ES, et al. Zinc supplementation for preventing mortality, morbidity, and growth failure in children aged 6 months to 12 years of age. Cochrane Database Syst Rev. 2014; 5:CD009384.[DOI:10.1002/14651858.CD009384.pub2]
- Guo J, Xie J, Zhou B, Găman MA, Kord-Varkaneh H, Clark CCT, et al. The influence of zinc supplementation on IGF-1 levels in humans: A systematic review and meta-analysis. J King Saud University-Science. 2020; 32(3):1824-30. [DOI:10.1016/j.jksus.2020.01.018]
- Rocha ÉD, de Brito NJ, Dantas MM, Silva Ade A, Almeida Md, Brandão-Neto J. Effect of zinc supplementation on GH, IGF1, IGFBP3, OCN, and ALP in non-zinc-deficient children. J Am Coll Nutr. 2015; 34(4):290-9. [DOI:10.1080/07315724.2014.929511] [PMID]
- Park SG, Choi HN, Yang HR, Yim JE. Effects of zinc supplementation on catch-up growth in children with failure to thrive. Nutr Res Pract. 2017; 11(6):487-91. [DOI:10.4162/nrp.2017.11.6.487] [PMID]
- Kaur K, Gupta R, Saraf SA, Saraf SK. Zinc: The metal of life. Compr Rev Food Sci Food Saf. 2014; 13(4):358-76. [DOI:10.1111/1541-4337.12067] [PMID]
- Maggio M, De Vita F, Lauretani F, Buttò V, Bondi G, Cattabiani C, et al. IGF-1, the cross road of the nutritional, inflammatory and hormonal pathways to frailty. Nutrients. 2013; 5(10):4184-205. [DOI:10.3390/nu5104184] [PMID]
- Millward DJ. Nutrition, infection and stunting: The roles of deficiencies of individual nutrients and foods, and of inflammation, as determinants of reduced linear growth of children. Nutr Res Rev. 2017; 30(1):50-72. [DOI:10.1017/S0954422416000238] [PMID]
- Akram SK, Carlsson-Skwirut C, Bhutta ZA, Söder O. Placental IGF-I, IGFBP-1, zinc, and iron, and maternal and infant anthropometry at birth. Acta Paediatr. 2011; 100(11):1504-9 [DOI:10.1111/j.1651-2227.2011.02336.x] [PMID]
- MacDonald RS. The role of zinc in growth and cell proliferation. J Nutr. 2000; 130(5S Suppl):1500S-8S. [DOI:10.1093/jn/130.5.1500S] [PMID]
- Roohani N, Hurrell R, Kelishadi R, Schulin R. Zinc and its importance for human health: An integrative review. J Res Med Sci. 2013; 18(2):144-57. [PMID]
Type of Study: Review Article |
Subject:
Pediatrics
Received: 2023/11/11 | Accepted: 2023/12/28 | Published: 2024/01/1
Received: 2023/11/11 | Accepted: 2023/12/28 | Published: 2024/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









