Volume 12, Issue 3 (7-2024)
J. Pediatr. Rev 2024, 12(3): 223-232 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemi M M, Dadras A, Toloui A, Kiah M, Bazargani B, Ataei N, et al . Urinary Calprotectin for Early Detection of Pediatric Acute Kidney Injury: A Systematic Review and Meta-analysis. J. Pediatr. Rev 2024; 12 (3) :223-232
URL: http://jpr.mazums.ac.ir/article-1-602-en.html
URL: http://jpr.mazums.ac.ir/article-1-602-en.html
Mohammad Mehdi Hashemi1 

 , Ayda Dadras1
, Ayda Dadras1 
 , Amirmohammad Toloui1
, Amirmohammad Toloui1 
 , Mohammad Kiah1
, Mohammad Kiah1 
 , Behnaz Bazargani2
, Behnaz Bazargani2 
 , Neamatollah Ataei2
, Neamatollah Ataei2 
 , Hamzah Adel Ramawad3
, Hamzah Adel Ramawad3 
 , Mahmoud Yousefifard *4
, Mahmoud Yousefifard *4 

 , Mostafa Hosseini5
, Mostafa Hosseini5 




 , Ayda Dadras1
, Ayda Dadras1 
 , Amirmohammad Toloui1
, Amirmohammad Toloui1 
 , Mohammad Kiah1
, Mohammad Kiah1 
 , Behnaz Bazargani2
, Behnaz Bazargani2 
 , Neamatollah Ataei2
, Neamatollah Ataei2 
 , Hamzah Adel Ramawad3
, Hamzah Adel Ramawad3 
 , Mahmoud Yousefifard *4
, Mahmoud Yousefifard *4 

 , Mostafa Hosseini5
, Mostafa Hosseini5 


1- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Pediatrics Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Emergency Medicine, NYC Health & Hospitals, New York, United States.
4- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran. ,yousefifard20@gmail.com
5- Department of Pediatrics, Valiasr Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
2- Pediatrics Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Emergency Medicine, NYC Health & Hospitals, New York, United States.
4- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran. ,
5- Department of Pediatrics, Valiasr Hospital, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
Full-Text [PDF 1363 kb]
(841 Downloads)
| Abstract (HTML) (2275 Views)
Full-Text: (638 Views)
Introduction
Acute kidney injury (AKI) is commonly defined as a sudden decrease in renal function. AKI occurs within hours or days, clinically manifesting as elevated blood creatinine, electrolyte imbalance, and a significant decrease in urinary output [1]. It is suggested that even a minor decline or loss of renal function can serve as a good predictive factor for adverse clinical outcomes [2]. AKI is present on admission in one in every three children, usually due to a reduction in blood flow to the renal tissue [3]. The accompanying metabolic acidosis, increased blood potassium levels, and fluid overload associated with AKI are linked to chronic kidney disease and high morbidity and mortality rates. Therefore, early diagnosis is crucial for effective disease management [4].
Standard laboratory tests, such as serum creatinine and blood urea nitrogen (BUN), are useful for evaluating and monitoring kidney function. However, these markers have low sensitivity and specificity for the early diagnosis of AKI [5]. Recent studies have identified other diagnostic biomarkers for AKI, such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), interleukin 8 (IL-8), and human liver-type fatty acid-binding protein (L-FABP). As the utility of these biomarkers continues to emerge, further investigations are needed to assess their efficacy [6-8].
Measurement of urinary calprotectin, a protein found in the cytoplasm of neutrophils, has been demonstrated to be a promising biomarker for the early diagnosis of AKI. Calprotectin is also released by the renal tubular epithelium in response to inflammation and injury [6, 9]. Early studies have suggested that urinary calprotectin is significantly elevated in patients with intrinsic AKI and may be even used as a biomarker to distinguish between intrinsic or prerenal AKI [10]. However, there is still disagreement in the current evidence on the discriminatory yield of urinary calprotectin in pediatric AKI. Therefore, the aim of this present meta-analysis was to determine the diagnostic value of urinary calprotectin in identifying AKI in the pediatric population.
Methods
Study design
To conduct the present study, the meta-analysis of observational studies in epidemiology (MOOSE) guideline was used. The patients, index test, and target condition (PIT) concept was defined as the pediatric population with suspected AKI (P), urinary calprotectin (I) and the presence of AKI, distinguishing between intrinsic and prerenal AKI (T), respectively.
Search strategy
All keywords and synonyms related to AKI and urinary calprotectin were collected, and a separate search query was utilized for each database using appropriate tags and Boolean operators. The keywords were chosen according to the expert opinion, the titles of related articles in a preliminary search and controlled vocabularies, including MeSH and Emtree databases. Electronic databases, including Medline, Embase, Scopus and Web of Science, were searched until April 27, 2024. Also, gray literature was explored by screening potentially related records in Google and Google Scholar. In addition, we performed reference tracking and citation tracking to find additional articles.
Selection criteria
Diagnostic studies conducted on the value of urinary calprotectin in AKI were included. Exclusion criteria included a lack of reporting on the method of determining the level of urinary calprotectin, measurement of urinary calprotectin more than 72 hours after patient admission, studies involving the adult population, duplicate reports, review studies, lack of required data and preclinical studies.
Data extraction
Two independent reviewers assessed the search records and any disagreements were resolved through discussion with a third researcher. The articles were summarized based on a checklist designed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. The summarized data included study design, patients’ characteristics (mean age, gender distribution, cause of AKI), sample size, definition of AKI, type of AKI (prerenal and intrinsic), type of control group, mean and standard deviation of urinary calprotectin levels and its diagnostic performance. The authors were contacted in cases where the data could not be extracted from the articles.
Risk of bias assessment
The risk of bias was assessed by two reviewers using the quality assessment of diagnostic accuracy studies version 2 (QUADAS-2) guidelines [11], independently by two reviewers. Any disagreement was resolved through discussion with a third researcher.
Statistical analysis
wResults
Characteristics of the included studies
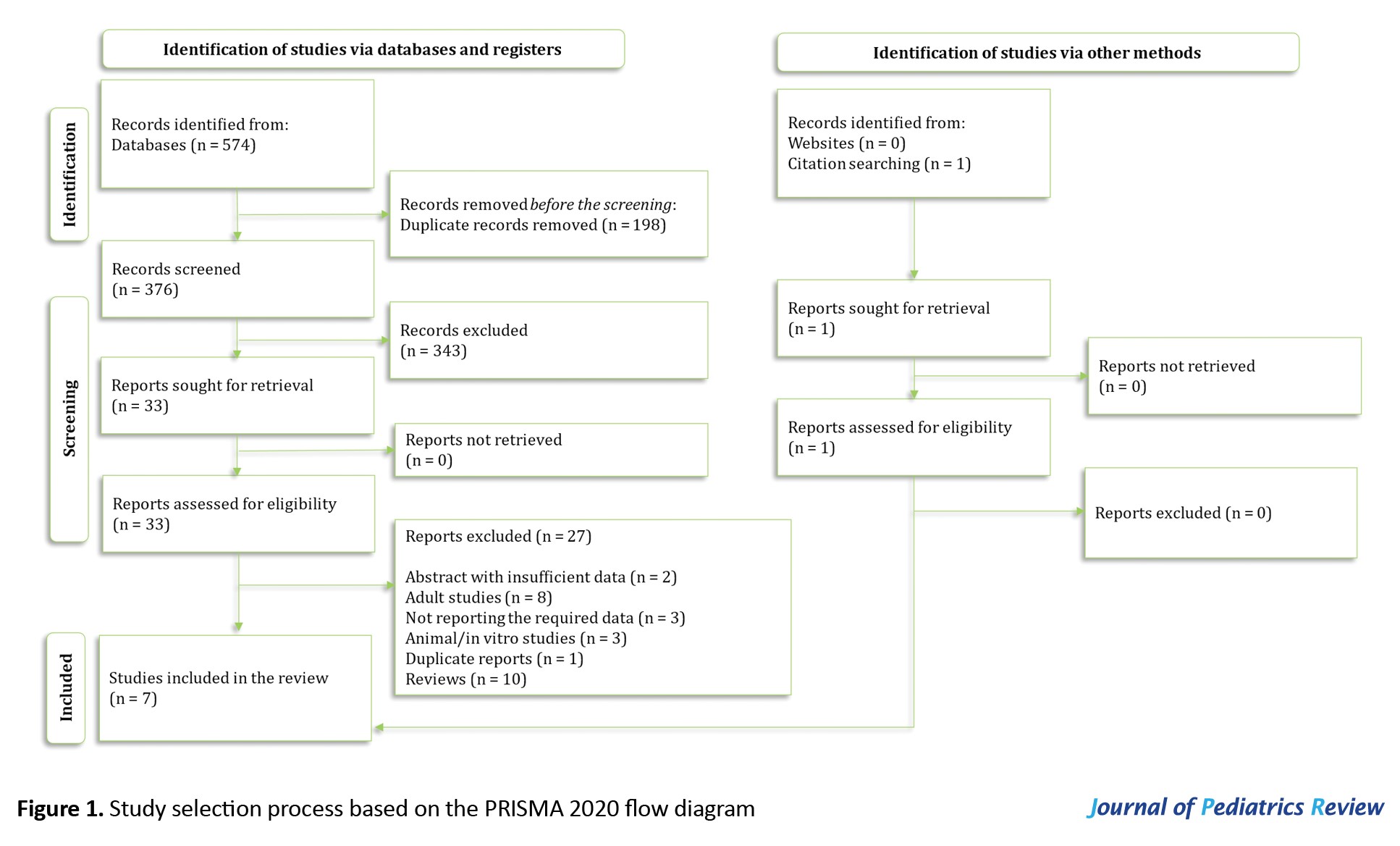
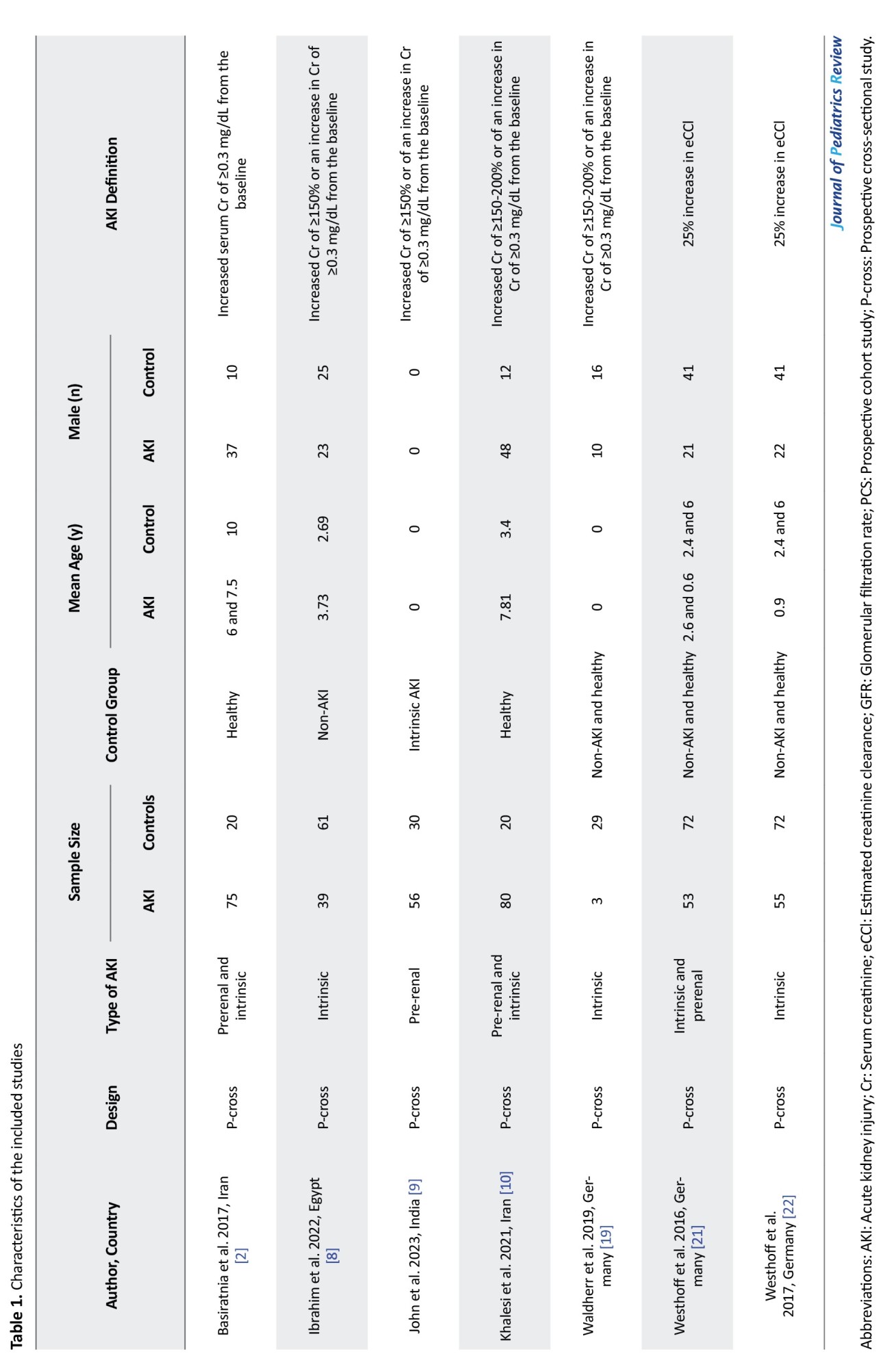
In the systematic search, 574 articles were found, and after the removal of duplicates, 33 full texts were reviewed. Ultimately, data from seven articles were included [13-18]. Ten review articles, three preclinical studies, eight papers on the adult population and one duplicate report were excluded. Moreover, five studies were excluded due to a lack of required data or abstracts with insufficient information after contacting the authors (Figure 1). Out of the seven studies included, five were prospective cross-sectional studies, while the other two were prospective cohort studies. These studies included data from 352 AKI patients and 274 individuals in the non-AKI group. In three studies, the type of AKI was exclusively intrinsic AKI, while four studies aimed to compare the value of calprotectin in distinguishing intrinsic causes of AKI from prerenal causes. Table 1 shows a summary of the findings of the present study.
The role of calprotectin levels in identifying AKI
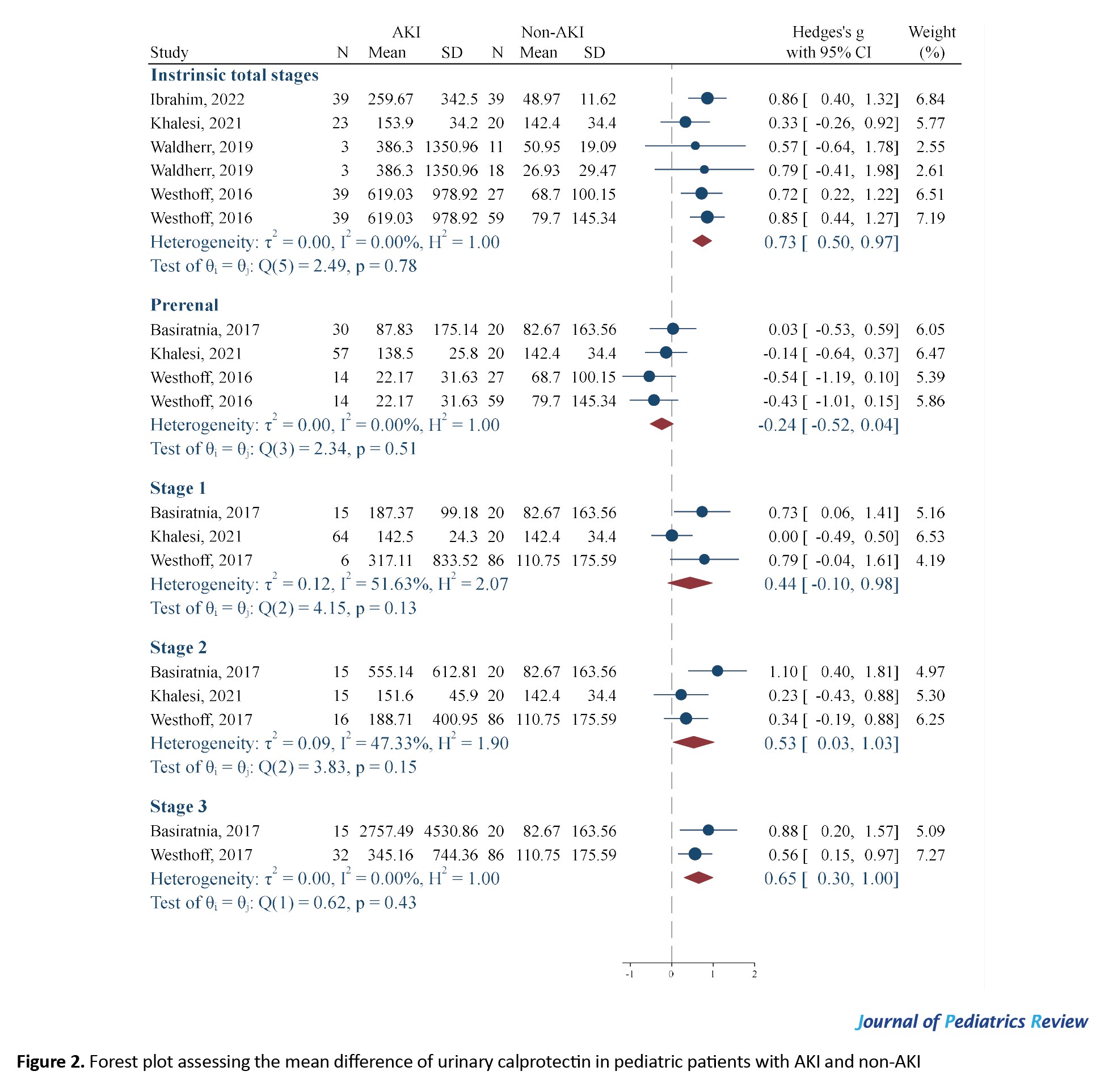
The mean urinary levels of calprotectin in intrinsic AKI children were significantly higher than in non-AKI children (SMD=0.73; 95% CI, 0.50%, 0.97%; I2=0%). However, the urinary levels of calprotectin in children with prerenal AKI did not demonstrate a significant difference from the non-AKI group (SMD=-0.24; 95% CI, -0.52%, 0.04%; I2=0%). As noted, no evidence of statistical heterogeneity was observed in this section (Figure 2). The SMD obtained to compare the average urinary levels of calprotectin in AKI and non-AKI children was relatively weak. Therefore, as a subgroup analysis, the value of this biomarker was investigated in different stages of intrinsic AKI. When the analyses were separated based on the stage of AKI, it was found that the urinary level of calprotectin in stage 2 (SMD=0.53; 95% CI, 0.03%, 1.03%; I²=47.33%) and stage 3 (SMD=0.65; 95% CI, 0.3 %, 1.00%; I²=0.00%) of AKI increased significantly compared to non-AKI individuals, while the level of this biomarker in stage 1 (SMD=0.44; 95% CI, -0.10%, 0.98%; I²=51.63%) did not differ from that of the non-AKI group (Figure 2).
The value of urinary calprotectin levels in differentiating prerenal AKI from intrinsic AKI
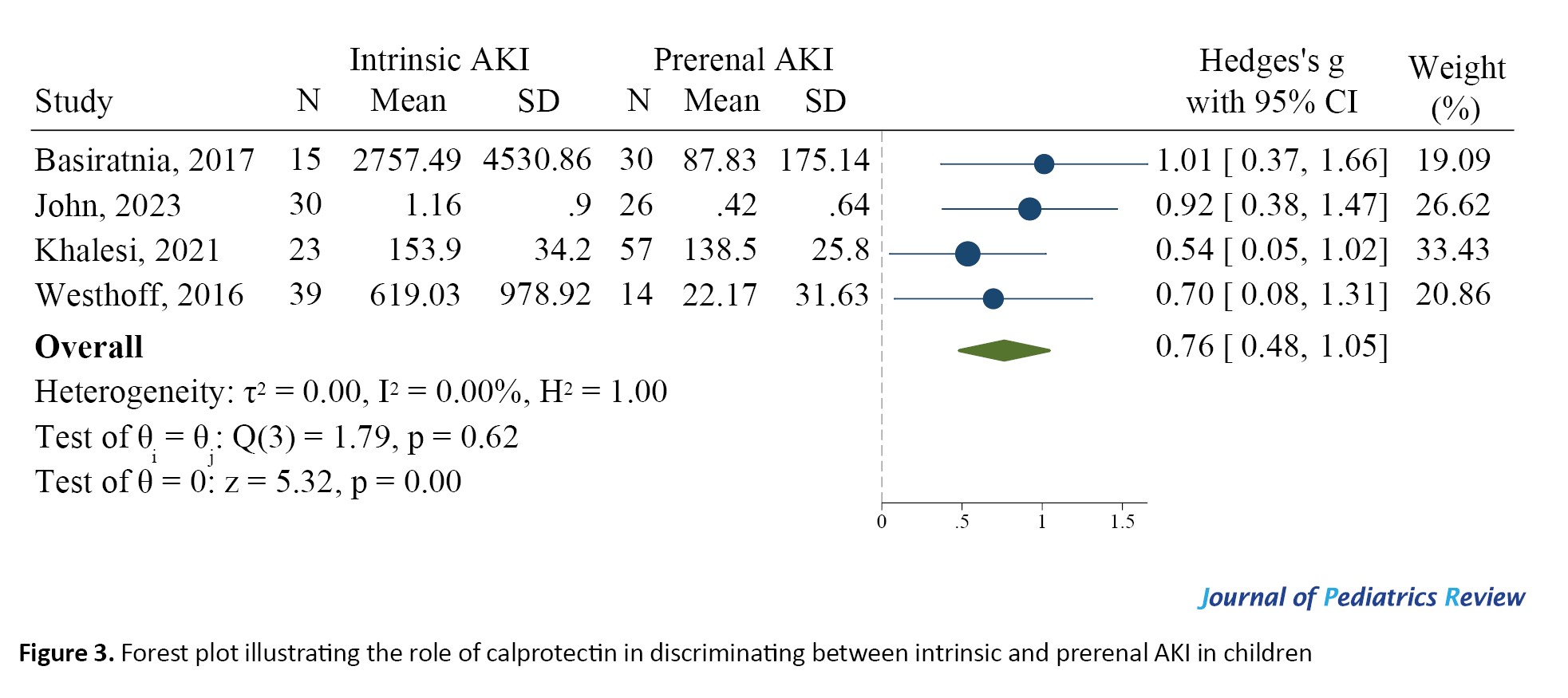
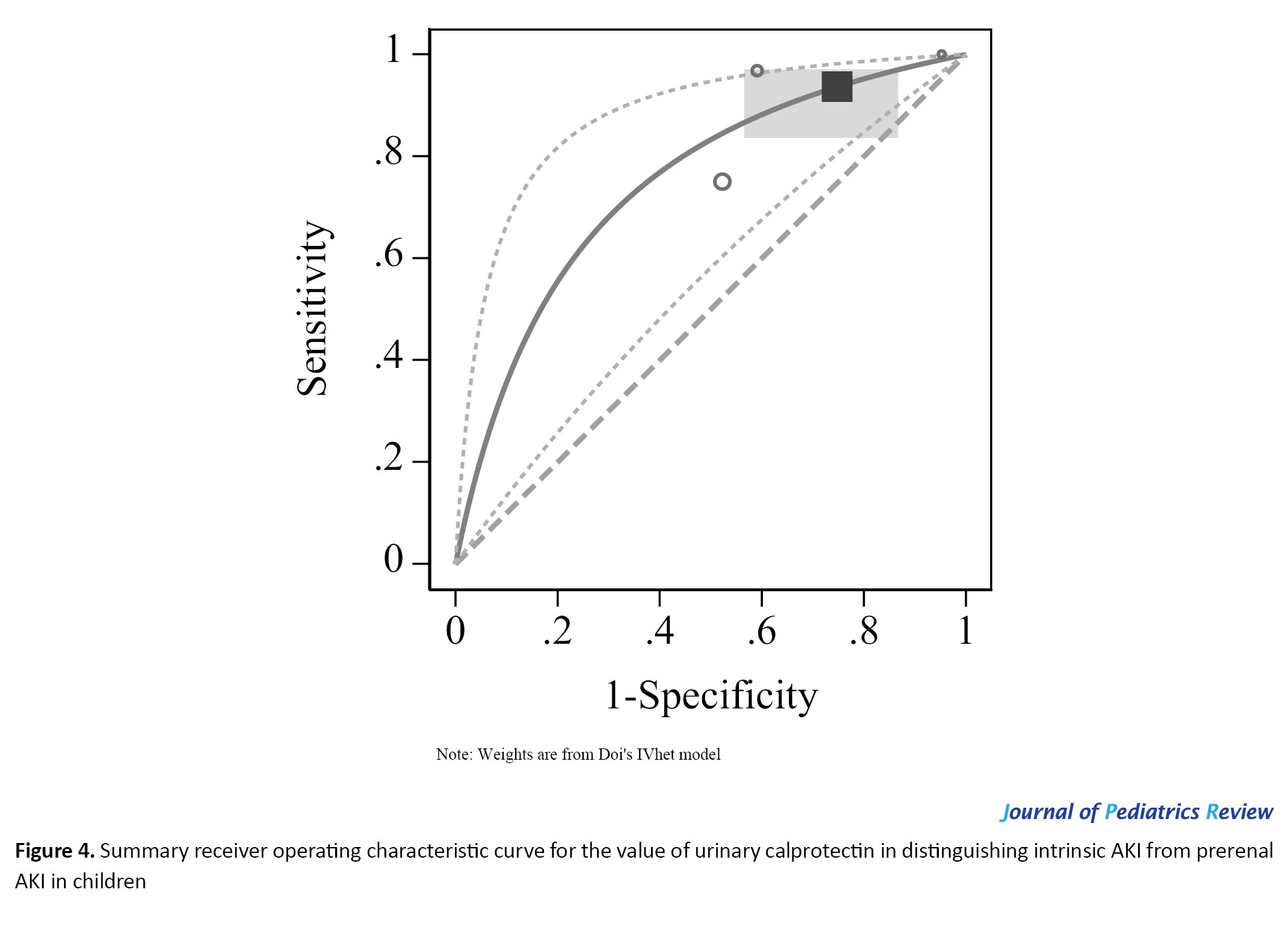
In this section, data from four studies were included. The urinary levels of calprotectin in children with intrinsic AKI were significantly higher than those in children with prerenal AKI (SMD=0.76; 95% CI, 0.48%, 1.05%; 95% CI, 0%) (Figure 3). In existing studies, the suggested cut-off points for calprotectin in differentiating between intrinsic and prerenal AKI were reported to be between 76 and 230 ng/ml. At these cut points, the AUC of urinary calprotectin for differentiating intrinsic AKI from prerenal AKI was equal to 0.691 (95% CI, 0.541%, 0.809%). The sensitivity and specificity of this biomarker were 0.937 (95% CI, 0.829%, 0.978%) and 0.252 (95% CI, 0.126%, 0.442%), respectively (Figure 4). Also, the diagnostic odds ratio of calprotectin was 4.982, indicating the high value of this biomarker in differentiating intrinsic AKI from prerenal AKI.
Risk of bias assessment and publication bias
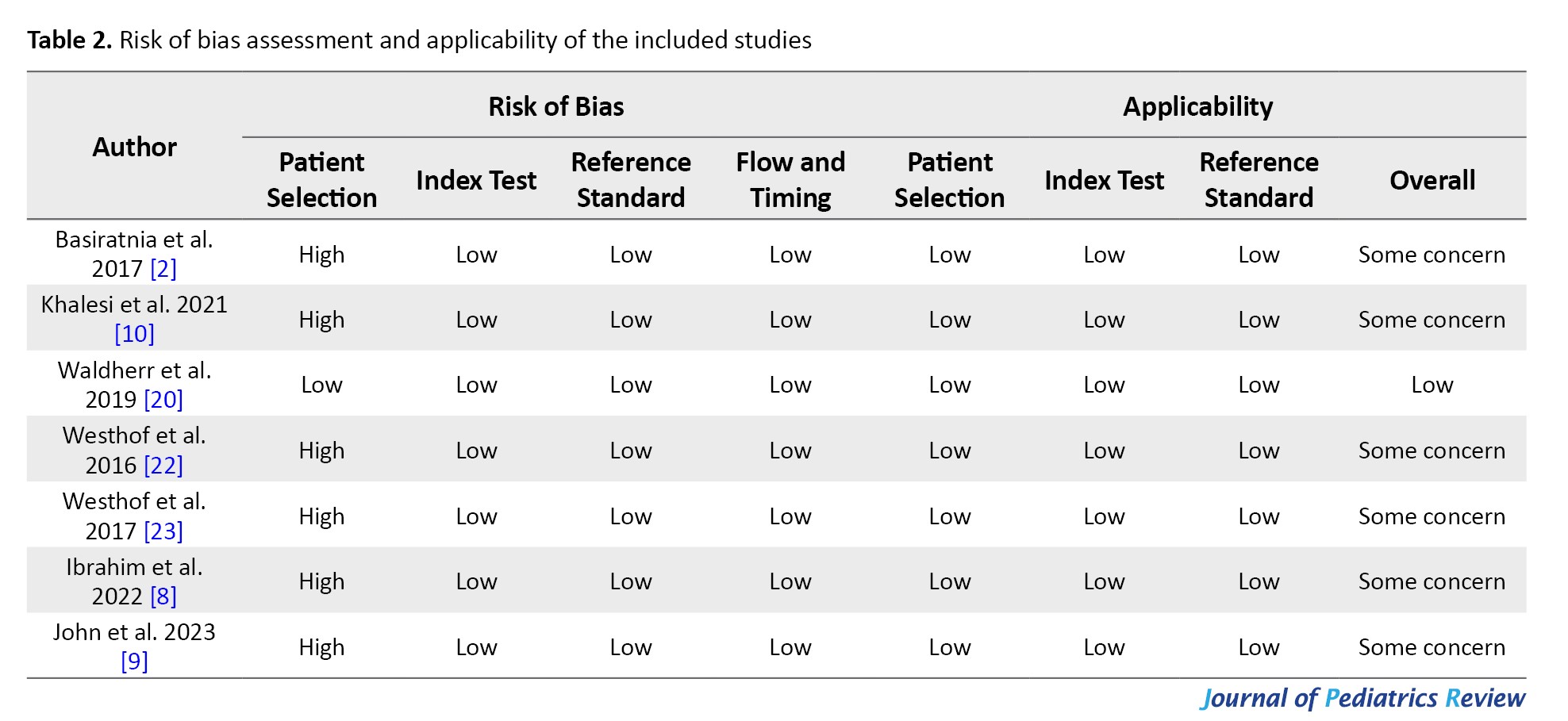
In the assessment of risk of bias for the included studies, six studies were considered to be at high risk in the domain of patient selection. This was due to the inclusion of a group of healthy individuals who were clearly not suspected of having AKI at the beginning of the study. For all other domains, all articles were judged to be at low risk of bias. Overall, the risk of bias was deemed to be a concern in six studies and low in one study (Table 2).
Publication bias assessment was not applicable due to data scarcity.
Discussion
In our analysis, we found that urinary calprotectin levels in children with AKI were significantly higher than those in the non-AKI group. In contrast, the urinary calprotectin levels in patients with prerenal AKI showed no considerable difference compared to healthy subjects. Additionally, we found that urinary calprotectin can identify stages 2 and 3 of intrinsic AKI in the pediatric population, but it cannot discern a difference between children with stage 1 intrinsic AKI and healthy children. These findings support the conclusion that urinary calprotectin may be a useful biomarker for differentiating intrinsic AKI from prerenal AKI. The sensitivity and specificity of urinary calprotectin for this differentiation were 0.937 and 0.252, respectively.
The presence of calprotectin in the urine is an indicator of inflammation in the kidney or damage to renal collecting duct epithelium cells [19]. It has been demonstrated that urinary calprotectin levels are increased in renal diseases, such as glomerulonephritis and nephrotic syndrome [20]. As a result, urinary calprotectin is a helpful indicator for distinguishing between prerenal and intrinsic AKI. Our findings emphasize that calprotectin may not be able to solely identify AKI, but it can be useful for differentiating intrinsic AKI from prerenal AKI. This finding is following the evidence provided by Chen et al. in their meta-analysis [10].
Our findings demonstrated that urinary calprotectin could not discriminate between stage 1 AKI and the non-AKI group. In other words, calprotectin is not able to identify the earliest stages of AKI. A possible explanation is that the degree of renal damage in the early stages of AKI is not severe enough to cause a significant increase in the levels of urinary calprotectin.
Despite its utility as a biomarker to identify intrinsic AKI, calprotectin levels also increase in other diseases, such as rheumatoid arthritis, inflammatory bowel disease, myocardial infarction, and some cancers [19, 21-24]. Therefore, the presence of such comorbidities reduces the diagnostic value of urinary calprotectin in identifying AKI.
The reported cutoff point for urinary calprotectin to distinguish prerenal causes of AKI from intrinsic AKI has varied between 76 and 230 ng/mL. At this time, an absolute cutoff point for calprotectin cannot be established. Therefore, it is necessary to assess the optimum cutoff point in future studies. In this regard, diagnostic value studies with large sample sizes are recommended.
Of the seven included studies, the risk of bias was classified as “some concern” in six studies and “low risk” in one study. The main reason for the high risk of bias in six studies was the inclusion of healthy individuals and the researcher’s awareness of the absence of AKI from the beginning of the study. Therefore, the evidence reported in these quantitative studies should be interpreted with caution. As a suggestion for future studies, it would be better to include only people suspected of having AKI to avoid bias in patient selection.
Conclusion
The urinary calprotectin levels in the pediatric population with intrinsic AKI are significantly higher than those in non-AKI individuals, while the urinary levels of this biomarker in prerenal AKI showed no difference compared to the non-AKI group. It was also found that the urinary calprotectin levels can identify stages 2 and 3 of intrinsic AKI in the pediatric population but cannot differentiate between stage 1 AKI and non-AKI groups. Urinary calprotectin has fair screening performance characteristics for differentiating intrinsic AKI from prerenal AKI in children. However, the low specificity calls for additional diagnostic testing in cases with positive results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.CHMC.REC.1399.151).
Funding
The study was financially supported by Tehran University of Medical Sciences (Grant No.: 99-2-231-50040).
Authors contributions
Study design: Mahmoud Yousefifard, Mohammad Mehdi Hashemi, and Neamatollah Ataei; Data collection: Mohammad Mehdi Hashemi, Amirmohammad Toloui, Behnaz Bazargani, Ayda Dadras, Mohammad Kiah; Data analysis: Mahmoud Yousefifard, Mostafa Hosseini; Writing the original draft: Mohammad Mehdi Hashemi, Mahmoud Yousefifard, Hamzah Adel Ramawad; Data interpretation, review and editing: All authors.
Conflicts of interest
The authors declared no conflict of interest.
References
Acute kidney injury (AKI) is commonly defined as a sudden decrease in renal function. AKI occurs within hours or days, clinically manifesting as elevated blood creatinine, electrolyte imbalance, and a significant decrease in urinary output [1]. It is suggested that even a minor decline or loss of renal function can serve as a good predictive factor for adverse clinical outcomes [2]. AKI is present on admission in one in every three children, usually due to a reduction in blood flow to the renal tissue [3]. The accompanying metabolic acidosis, increased blood potassium levels, and fluid overload associated with AKI are linked to chronic kidney disease and high morbidity and mortality rates. Therefore, early diagnosis is crucial for effective disease management [4].
Standard laboratory tests, such as serum creatinine and blood urea nitrogen (BUN), are useful for evaluating and monitoring kidney function. However, these markers have low sensitivity and specificity for the early diagnosis of AKI [5]. Recent studies have identified other diagnostic biomarkers for AKI, such as cystatin C, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule-1 (KIM-1), interleukin 8 (IL-8), and human liver-type fatty acid-binding protein (L-FABP). As the utility of these biomarkers continues to emerge, further investigations are needed to assess their efficacy [6-8].
Measurement of urinary calprotectin, a protein found in the cytoplasm of neutrophils, has been demonstrated to be a promising biomarker for the early diagnosis of AKI. Calprotectin is also released by the renal tubular epithelium in response to inflammation and injury [6, 9]. Early studies have suggested that urinary calprotectin is significantly elevated in patients with intrinsic AKI and may be even used as a biomarker to distinguish between intrinsic or prerenal AKI [10]. However, there is still disagreement in the current evidence on the discriminatory yield of urinary calprotectin in pediatric AKI. Therefore, the aim of this present meta-analysis was to determine the diagnostic value of urinary calprotectin in identifying AKI in the pediatric population.
Methods
Study design
To conduct the present study, the meta-analysis of observational studies in epidemiology (MOOSE) guideline was used. The patients, index test, and target condition (PIT) concept was defined as the pediatric population with suspected AKI (P), urinary calprotectin (I) and the presence of AKI, distinguishing between intrinsic and prerenal AKI (T), respectively.
Search strategy
All keywords and synonyms related to AKI and urinary calprotectin were collected, and a separate search query was utilized for each database using appropriate tags and Boolean operators. The keywords were chosen according to the expert opinion, the titles of related articles in a preliminary search and controlled vocabularies, including MeSH and Emtree databases. Electronic databases, including Medline, Embase, Scopus and Web of Science, were searched until April 27, 2024. Also, gray literature was explored by screening potentially related records in Google and Google Scholar. In addition, we performed reference tracking and citation tracking to find additional articles.
Selection criteria
Diagnostic studies conducted on the value of urinary calprotectin in AKI were included. Exclusion criteria included a lack of reporting on the method of determining the level of urinary calprotectin, measurement of urinary calprotectin more than 72 hours after patient admission, studies involving the adult population, duplicate reports, review studies, lack of required data and preclinical studies.
Data extraction
Two independent reviewers assessed the search records and any disagreements were resolved through discussion with a third researcher. The articles were summarized based on a checklist designed according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement. The summarized data included study design, patients’ characteristics (mean age, gender distribution, cause of AKI), sample size, definition of AKI, type of AKI (prerenal and intrinsic), type of control group, mean and standard deviation of urinary calprotectin levels and its diagnostic performance. The authors were contacted in cases where the data could not be extracted from the articles.
Risk of bias assessment
The risk of bias was assessed by two reviewers using the quality assessment of diagnostic accuracy studies version 2 (QUADAS-2) guidelines [11], independently by two reviewers. Any disagreement was resolved through discussion with a third researcher.
Statistical analysis
wResults
Characteristics of the included studies
In the systematic search, 574 articles were found, and after the removal of duplicates, 33 full texts were reviewed. Ultimately, data from seven articles were included [13-18]. Ten review articles, three preclinical studies, eight papers on the adult population and one duplicate report were excluded. Moreover, five studies were excluded due to a lack of required data or abstracts with insufficient information after contacting the authors (Figure 1). Out of the seven studies included, five were prospective cross-sectional studies, while the other two were prospective cohort studies. These studies included data from 352 AKI patients and 274 individuals in the non-AKI group. In three studies, the type of AKI was exclusively intrinsic AKI, while four studies aimed to compare the value of calprotectin in distinguishing intrinsic causes of AKI from prerenal causes. Table 1 shows a summary of the findings of the present study.

The role of calprotectin levels in identifying AKI
The mean urinary levels of calprotectin in intrinsic AKI children were significantly higher than in non-AKI children (SMD=0.73; 95% CI, 0.50%, 0.97%; I2=0%). However, the urinary levels of calprotectin in children with prerenal AKI did not demonstrate a significant difference from the non-AKI group (SMD=-0.24; 95% CI, -0.52%, 0.04%; I2=0%). As noted, no evidence of statistical heterogeneity was observed in this section (Figure 2). The SMD obtained to compare the average urinary levels of calprotectin in AKI and non-AKI children was relatively weak. Therefore, as a subgroup analysis, the value of this biomarker was investigated in different stages of intrinsic AKI. When the analyses were separated based on the stage of AKI, it was found that the urinary level of calprotectin in stage 2 (SMD=0.53; 95% CI, 0.03%, 1.03%; I²=47.33%) and stage 3 (SMD=0.65; 95% CI, 0.3 %, 1.00%; I²=0.00%) of AKI increased significantly compared to non-AKI individuals, while the level of this biomarker in stage 1 (SMD=0.44; 95% CI, -0.10%, 0.98%; I²=51.63%) did not differ from that of the non-AKI group (Figure 2).
The value of urinary calprotectin levels in differentiating prerenal AKI from intrinsic AKI
In this section, data from four studies were included. The urinary levels of calprotectin in children with intrinsic AKI were significantly higher than those in children with prerenal AKI (SMD=0.76; 95% CI, 0.48%, 1.05%; 95% CI, 0%) (Figure 3). In existing studies, the suggested cut-off points for calprotectin in differentiating between intrinsic and prerenal AKI were reported to be between 76 and 230 ng/ml. At these cut points, the AUC of urinary calprotectin for differentiating intrinsic AKI from prerenal AKI was equal to 0.691 (95% CI, 0.541%, 0.809%). The sensitivity and specificity of this biomarker were 0.937 (95% CI, 0.829%, 0.978%) and 0.252 (95% CI, 0.126%, 0.442%), respectively (Figure 4). Also, the diagnostic odds ratio of calprotectin was 4.982, indicating the high value of this biomarker in differentiating intrinsic AKI from prerenal AKI.
Risk of bias assessment and publication bias
In the assessment of risk of bias for the included studies, six studies were considered to be at high risk in the domain of patient selection. This was due to the inclusion of a group of healthy individuals who were clearly not suspected of having AKI at the beginning of the study. For all other domains, all articles were judged to be at low risk of bias. Overall, the risk of bias was deemed to be a concern in six studies and low in one study (Table 2).

Publication bias assessment was not applicable due to data scarcity.
Discussion
In our analysis, we found that urinary calprotectin levels in children with AKI were significantly higher than those in the non-AKI group. In contrast, the urinary calprotectin levels in patients with prerenal AKI showed no considerable difference compared to healthy subjects. Additionally, we found that urinary calprotectin can identify stages 2 and 3 of intrinsic AKI in the pediatric population, but it cannot discern a difference between children with stage 1 intrinsic AKI and healthy children. These findings support the conclusion that urinary calprotectin may be a useful biomarker for differentiating intrinsic AKI from prerenal AKI. The sensitivity and specificity of urinary calprotectin for this differentiation were 0.937 and 0.252, respectively.
The presence of calprotectin in the urine is an indicator of inflammation in the kidney or damage to renal collecting duct epithelium cells [19]. It has been demonstrated that urinary calprotectin levels are increased in renal diseases, such as glomerulonephritis and nephrotic syndrome [20]. As a result, urinary calprotectin is a helpful indicator for distinguishing between prerenal and intrinsic AKI. Our findings emphasize that calprotectin may not be able to solely identify AKI, but it can be useful for differentiating intrinsic AKI from prerenal AKI. This finding is following the evidence provided by Chen et al. in their meta-analysis [10].
Our findings demonstrated that urinary calprotectin could not discriminate between stage 1 AKI and the non-AKI group. In other words, calprotectin is not able to identify the earliest stages of AKI. A possible explanation is that the degree of renal damage in the early stages of AKI is not severe enough to cause a significant increase in the levels of urinary calprotectin.
Despite its utility as a biomarker to identify intrinsic AKI, calprotectin levels also increase in other diseases, such as rheumatoid arthritis, inflammatory bowel disease, myocardial infarction, and some cancers [19, 21-24]. Therefore, the presence of such comorbidities reduces the diagnostic value of urinary calprotectin in identifying AKI.
The reported cutoff point for urinary calprotectin to distinguish prerenal causes of AKI from intrinsic AKI has varied between 76 and 230 ng/mL. At this time, an absolute cutoff point for calprotectin cannot be established. Therefore, it is necessary to assess the optimum cutoff point in future studies. In this regard, diagnostic value studies with large sample sizes are recommended.
Of the seven included studies, the risk of bias was classified as “some concern” in six studies and “low risk” in one study. The main reason for the high risk of bias in six studies was the inclusion of healthy individuals and the researcher’s awareness of the absence of AKI from the beginning of the study. Therefore, the evidence reported in these quantitative studies should be interpreted with caution. As a suggestion for future studies, it would be better to include only people suspected of having AKI to avoid bias in patient selection.
Conclusion
The urinary calprotectin levels in the pediatric population with intrinsic AKI are significantly higher than those in non-AKI individuals, while the urinary levels of this biomarker in prerenal AKI showed no difference compared to the non-AKI group. It was also found that the urinary calprotectin levels can identify stages 2 and 3 of intrinsic AKI in the pediatric population but cannot differentiate between stage 1 AKI and non-AKI groups. Urinary calprotectin has fair screening performance characteristics for differentiating intrinsic AKI from prerenal AKI in children. However, the low specificity calls for additional diagnostic testing in cases with positive results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.CHMC.REC.1399.151).
Funding
The study was financially supported by Tehran University of Medical Sciences (Grant No.: 99-2-231-50040).
Authors contributions
Study design: Mahmoud Yousefifard, Mohammad Mehdi Hashemi, and Neamatollah Ataei; Data collection: Mohammad Mehdi Hashemi, Amirmohammad Toloui, Behnaz Bazargani, Ayda Dadras, Mohammad Kiah; Data analysis: Mahmoud Yousefifard, Mostafa Hosseini; Writing the original draft: Mohammad Mehdi Hashemi, Mahmoud Yousefifard, Hamzah Adel Ramawad; Data interpretation, review and editing: All authors.
Conflicts of interest
The authors declared no conflict of interest.
References
- Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019; 394(10212):1949-64. [DOI:10.1016/S0140-6736(19)32563-2] [PMID]
- Basiratnia M, Kosimov M, Farhadi P, Azimi A, Hooman N. Urinary calprotectin as a marker to distinguish functional and structural acute kidney injury in pediatric population. Iran J Pediatr. 2017; 27(5):e9727. [DOI:10.5812/ijp.9727]
- Brun JG, Madland TM, Gran JT, Myklebust G. A longitudinal study of calprotectin in patients with polymyalgia rheumatica or temporal arteritis: Relation to disease activity. Scand J Rheumatol. 2005; 34(2):125-8. [DOI:10.1080/03009740410009931]
- Chen JJ, Fan PC, Kou G, Chang SW, Chen YT, Lee CC, et al. Meta-analysis: Urinary calprotectin for discrimination of intrinsic and prerenal acute kidney injury. J Clin Med. 2019; 8(1):74. [DOI:10.3390/jcm8010074]
- Deeks JJ, Bossuyt PM, Leeflang MM, Takwoingi Y. Cochrane handbook for systematic reviews of diagnostic test accuracy. Hoboken: John Wiley & Sons; 2023. [DOI:10.1002/9781119756194]
- Ebbing J, Mathia S, Seibert FS, Pagonas N, Bauer F, Erber B, et al. Urinary calprotectin: A new diagnostic marker in urothelial carcinoma of the bladder. World J Urol. 2014; 32:1485-92. [DOI:10.1007/s00345-013-1227-8]
- Fazel M, Sarveazad A, Mohamed Ali K, Yousefifard M, Hosseini M. Accuracy of urine kidney injury molecule-1 in predicting acute kidney injury in children; A systematic review and meta-analysis. Arch Acad Emerg Med. 2020; 8(1):e44. [PMID]
- Ibrahim HE, Khalifa NA, Afifi RA, Mowafy AH. Assessment of urinary calprotectin in early diagnosis of intrinsic acute kidney injury in critically Ill children at Zagazig University Hospitals. Egypt J Hosp Med. 2022; 89(1):5586-90. [DOI:10.21608/ejhm.2022.265293]
- John JS, Deepthi RV, Rebekah G, Prabhu SB, Ajitkumar P, Mathew G, et al. Usefulness of urinary calprotectin as a novel marker differentiating functional from structural acute kidney injury in the critical care setting. J Nephrol. 2023; 36(3):695-704. [DOI:10.1007/s40620-022-01534-3]
- Khalesi N, Mohammadian S, Hooman N, Khodadost M, Allahqoli L. Accuracy of urine calprotectin in the diagnosis of acute kidney injury in neonates: A cross-sectional study. Iran J Neonatol. 2021; 12(4):7-13. [Link]
- Azramezani Kopi T, Shahrokh S, Mirzaei S, Asadzadeh Aghdaei H, Amini Kadijani A. The role of serum calprotectin as a novel biomarker in inflammatory bowel diseases: A review study. Gastroenterol Hepatol Bed Bench. 2019; 12(3):183-9. [PMID]
- Libório AB, Leite TT, de Oliveira Neves FM, Teles F, de Melo Bezerra CT. AKI complications in critically ill patients: Association with mortality rates and RRT. Clin J Am Soc Nephrol. 2015; 10(1):21-8. [DOI:10.2215/CJN.04750514]
- Makris K, Spanou L. Acute Kidney Injury: Definition, Pathophysiology and Clinical Phenotypes. Clin Biochem Rev. 2016; 37(2):85-98. [PMID]
- Montagnana M, Danese E, Lippi G. Calprotectin and cardiovascular events. A narrative review. Clin Biochemistry. 2014; 47(12):996-1001. [DOI:10.1016/j.clinbiochem.2014.02.021]
- Rizvi MS, Kashani KB. Biomarkers for early detection of acute kidney injury. J Appl Lab Med. 2017; 2(3):386-99. [DOI:10.1373/jalm.2017.023325]
- Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury–pathophysiological basis and clinical performance. Acta Physiol. 2017; 219(3):556-74. [DOI:10.1111/apha.12764]
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al. World incidence of AKI: A meta-analysis. Clin J Am Soc Nephrol. 2013; 8(9):1482-93. [DOI:10.2215/CJN.00710113]
- Vaidya VS, Ferguson MA, Bonventre JV. Biomarkers of acute kidney injury. Annu Rev Pharmacol Toxicol. 2008; 48(1):463-93. [DOI:10.1146/annurev.pharmtox.48.113006.094615]
- Waldherr S, Fichtner A, Beedgen B, Bruckner T, Schaefer F, Tönshoff B, et al. Urinary acute kidney injury biomarkers in very low-birth-weight infants on indomethacin for patent ductus arteriosus. Pediatr Res. 2019; 85(5):678-86. [DOI:10.1038/s41390-019-0332-9] [PMID]
- Walsham NE, Sherwood RA. Fecal calprotectin in inflammatory bowel disease. Clin Experiment Gastroenterol. 2016; 9:21-9. [DOI:10.2147/CEG.S51902]
- Westhoff JH, Fichtner A, Waldherr S, Pagonas N, Seibert FS, Babel N, et al. Urinary biomarkers for the differentiation of prerenal and intrinsic pediatric acute kidney injury. Pediatr Nephrol. 2016; 31:2353-63. [DOI:10.1007/s00467-016-3418-1]
- Westhoff JH, Seibert FS, Waldherr S, Bauer F, Tönshoff B, Fichtner A, et al. Urinary calprotectin, kidney injury molecule-1, and neutrophil gelatinase-associated lipocalin for the prediction of adverse outcome in pediatric acute kidney injury. Eurn J Pediatr. 2017; 176:745-55. [DOI:10.1007/s00431-017-2907-y]
- Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011; 155(8):529-36. [DOI:10.7326/0003-4819-155-8-201110180-00009]
- Wolfs TG, Buurman WA, van Schadewijk A, de Vries B, Daemen MA, Hiemstra PS, et al. In vivo expression of Toll-like receptor 2 and 4 by renal epithelial cells: IFN-γ and TNF-α mediated up-regulation during inflammation. J Immunol. 2002; 168(3):1286-93. [DOI:10.4049/jimmunol.168.3.1286]
Type of Study: Meta-analysis Review |
Subject:
Nephrology
Received: 2024/02/4 | Accepted: 2024/09/1 | Published: 2024/07/1
Received: 2024/02/4 | Accepted: 2024/09/1 | Published: 2024/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |