Volume 13, Issue 1 (1-2025)
J. Pediatr. Rev 2025, 13(1): 73-86 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Salehifar E, Abed Khojasteh O, Hosseinimehr S J, Karami H, Naderi Sorki M, Darvish-Khezri H et al . 6-mercaptopurine Metabolites as Predictors of Neutropenia and Hepatotoxicity in Pediatric Acute Lymphoblastic Leukemia. J. Pediatr. Rev 2025; 13 (1) :73-86
URL: http://jpr.mazums.ac.ir/article-1-683-en.html
URL: http://jpr.mazums.ac.ir/article-1-683-en.html
Ebrahim Salehifar1 

 , Omid Abed Khojasteh *2
, Omid Abed Khojasteh *2 

 , Seyed Jalal Hosseinimehr3
, Seyed Jalal Hosseinimehr3 

 , Hossein Karami4
, Hossein Karami4 
 , Mohammad Naderi Sorki4
, Mohammad Naderi Sorki4 

 , Hadi Darvish-Khezri4
, Hadi Darvish-Khezri4 

 , Kimia Karami4
, Kimia Karami4 



 , Omid Abed Khojasteh *2
, Omid Abed Khojasteh *2 

 , Seyed Jalal Hosseinimehr3
, Seyed Jalal Hosseinimehr3 

 , Hossein Karami4
, Hossein Karami4 
 , Mohammad Naderi Sorki4
, Mohammad Naderi Sorki4 

 , Hadi Darvish-Khezri4
, Hadi Darvish-Khezri4 

 , Kimia Karami4
, Kimia Karami4 

1- Department of Clinical Pharmacy, Pharmaceutical Sciences Research Center, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Clinical Pharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran. ,omid.a.khojasteh.93@gmail.com
3- Department of Radiopharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, Semmelweis University, Budapest.
2- Department of Clinical Pharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran. ,
3- Department of Radiopharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Pharmaceutics, Faculty of Pharmaceutical Sciences, Semmelweis University, Budapest.
Full-Text [PDF 1177 kb]
(691 Downloads)
| Abstract (HTML) (1489 Views)
Full-Text: (804 Views)
Introduction
Acute lymphoblastic leukemia (ALL) is the most prevalent malignancy in children, accounting for approximately 80% of pediatric leukemias and 30% of all childhood malignancies. ALL is characterized by the rapid proliferation of immature white blood cells (WBCs), known as lymphoblasts [1, 2]. The typical symptoms of the disease include fatigue, fever, easy bruising, and bone pain [3]. Over the past 30 years, the prognosis of ALL significantly improved, achieving a 90% survival rate over 5 years [4]. Management of drug-related toxicity will be essential to prevent interruptions in chemotherapy to minimize disease relapses, thereby further increasing the survival rate [5]. To reduce drug-related toxicity and enhance the survival rate of vulnerable patients, it is essential to tailor treatment to the individual needs of each patient [6]. A portion of the treatment’s success can be attributed to the 18 to 24 months of adequate maintenance therapy necessary to prolong the remission achieved during the early stages of treatment [7].
The foundation of maintenance therapy for ALL is 6-mercaptopurine (6-MP) [8]. As a purine analog, 6-MP exerts its anti-leukemic effects by inhibiting de novo purine synthesis (DNPS), which is essential for leukemic cell proliferation [9, 10]. 6-MP is a prodrug metabolized through multiple enzymatic steps in the purine salvage pathway [11]. The anabolic pathway involving hypoxanthine-guanine phosphoribosyl transferase (HPRT) results in the production of 6-thioinosine monophosphate, which is subsequently converted into 6-thioguanine (6-TG) nucleotides [12]. Then, 6-TG is phosphorylated to form 6-thioinosine triphosphate or incorporated into nucleic acids [13]. The reversal of this process can be mediated by the enzyme inosine triphosphate pyrophosphatase [14]. Catabolic pathways inactivate 6-MP through xanthine oxidase, which converts it to 6-thiouric acid and thiopurine S-methyltransferase (TPMT), which metabolizes 6-MP to 6-methylmercaptopurine (6-MMP). Both of these catabolic metabolites inhibit DNPS [15].
The balance between 6-TG and 6-MMP is known to vary significantly and is mainly influenced by TPMT genetic polymorphisms [16]. The effectiveness and side effects of 6-MP in patients are known to be influenced by inter-individual variability in the concentrations of 6-TG and 6-MMP in the red blood cell (RBC), particularly in homozygous TPMT-deficient patients, who face a significant risk of myelosuppression [17, 18]. 6-TG metabolites are responsible for the anti-leukemic effects of 6-MP, while 6-MMP and its metabolites are associated with hepatotoxicity [19]. While 6-MP is generally well tolerated, side effects such as nausea, skin rashes, changes in appetite, bone marrow suppression, and hepatotoxicity may still occur [20, 21]. Maintaining an optimal balance between 6-MP metabolic pathways, including the production of 6-TG and 6-MMP, is crucial to achieving maximal anti-leukemic efficacy while minimizing the risk of hepatotoxicity or severe myelosuppression. In the current study, we measured the serum concentrations of 6-MP metabolites and assessed their relationship with the occurrence of 6-MP side effects, particularly hepatotoxicity, and neutropenia, in ALL patients receiving 6-MP during maintenance therapy.
Methods
Statistical population
Children with ALL in the maintenance phase of chemotherapy with 6-MP, who were referred to the Pediatric Oncology Department of Bou Ali Sina Hospital in Sari City, Iran, were included in this study. A confirmation of ALL is met when blast cells of lymphoid origin constitute ≥20% of marrow nucleated cells or ≥20% of non-erythroid cells when the erythroid component is >50%. The diagnosis of ALL in children was confirmed through complete blood count tests, peripheral blood smear observations by a hematopathologist, lymphoblast count, and, if possible, lymphoblast flow cytometry studies, cytogenetic studies, and finally, bone marrow aspiration.
Inclusion criteria
We included the patients under 18 years of age diagnosed with ALL who have been in the maintenance therapy phase for at least 2 months and are currently in remission and receiving a consistent daily dose of 6-MP and other medication, including corticosteroids and methotrexate for at least the past month, with no dose adjustments during this period were included.
Also, the patients should have not undergone packed RBC transfusions within the last 6 weeks.
Exclusion criteria
We excluded patients with previously known hypersensitivity to 6-MP or thioguanine, patients without consent to participate or continue participation in the study, and patients treated with allopurinol during the previous month.
Data collection
In this study, patient characteristics (gender, age, body mass index, MP dose, and number of dose reductions) were gathered at the time points (November 27, 2023 -March 12, 2024). Clinical data for each child were also collected at the time of blood sampling, including hemoglobin levels, RBC count, leukocyte count, liver function tests (e.g. alanine transaminase [ALT], aspartate transaminase [AST], lactate dehydrogenase [LDH]), bilirubin levels, and levels of urea, creatinine [Cr], and creatinine clearance [CrCl]. Based on the available clinical records, two groups of patients were examined.
Case and control groups definition
The case group consisted of patients with ALL who experienced hepatotoxicity (ALT more than 2.5 times the upper limit of normal) or neutropenia (absolute neutrophil count [ANC] less than 1500×109/L) during 6-MP treatment.
The control group included patients with ALL who did not experience hepatotoxicity or neutropenia while undergoing treatment with 6-MP.
Blood sampling
To prepare erythrocytes, 3-5 mL of venous blood samples were collected from each child and poured into EDTA-containing tubes. The samples were stored at 4 °C and processed within 24 hours. Subsequently, they were centrifuged at 2500 rpm for 10 minutes at 4 °C. The plasma and buffy coat were removed, and the remaining RBC volume was measured and recorded in the falcon tube. The cells were then washed with approximately two volumes of normal saline, vortexed at low speed (setting 1) for 15 seconds, and centrifuged at 2500 rpm for 10 minutes at 4 °C. After discarding the supernatant, this washing step was repeated. The sample was centrifuged at approximately 2500 rpm for 10 minutes at 4 °C. Following removing the supernatant, the cells were resuspended to an equal volume of normal saline. The RBC count was determined using a Beckman Coulter™ Counter (USA). Finally, the samples were stored in 1.5 mL tubes at -80 °C until further analysis.
High-performance liquid chromatography method (HPLC)
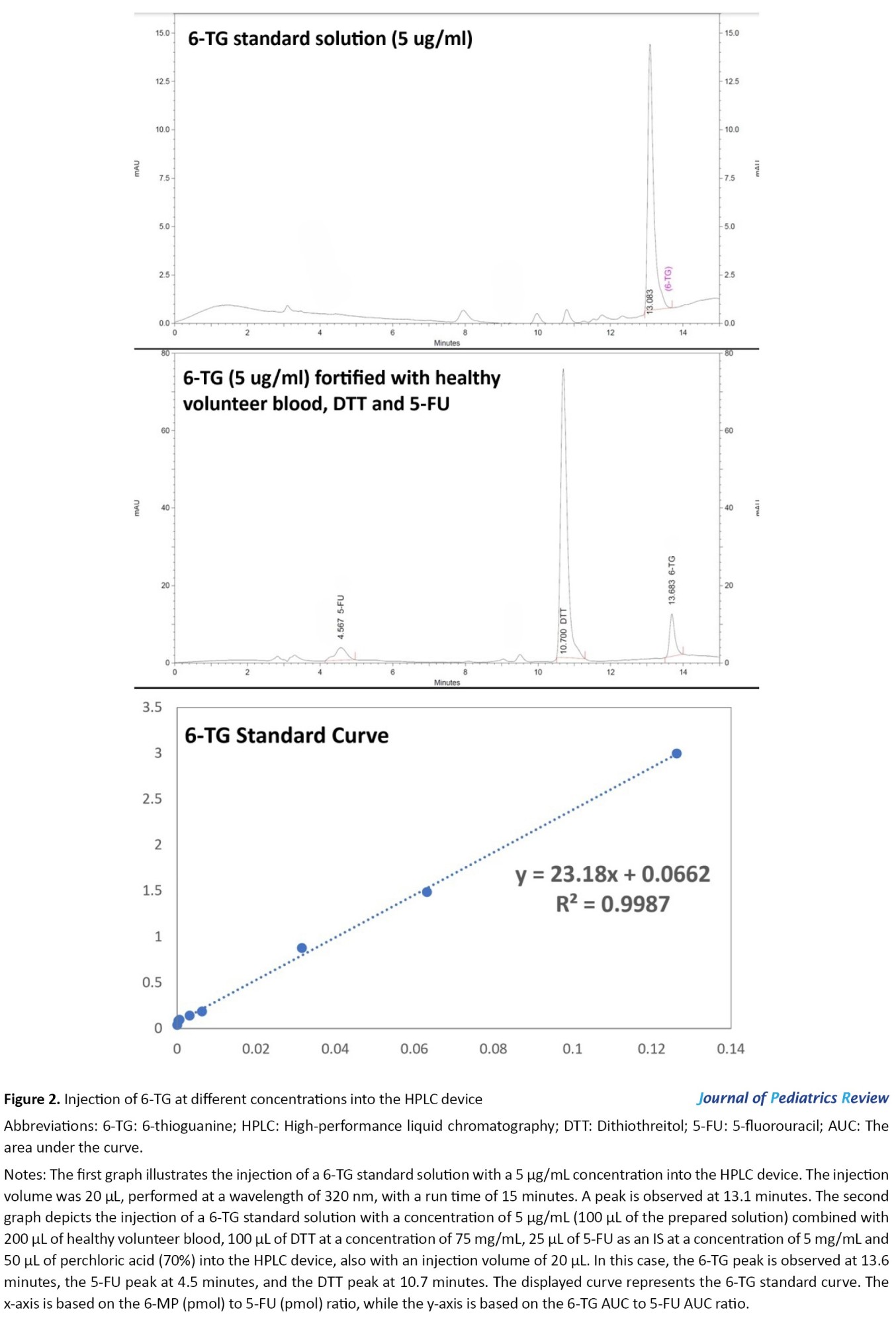
The HPLC system consists of a K-1001 solvent delivery system equipped with a Rheodyne injection valve (20 µL sample loop inserted) and a UV-Vis spectrophotometer detector model K-2600 set at 320 nm (all from Knauer company, Germany). Analysis was performed using an ODS-C18 column (150×4.6 mm i.d., 5-µm particle size) and the corresponding guard column. All solvents were filtered and degassed before entering the column. The optimum HPLC conditions included a mobile phase aqueous system consisting of 0.025 M phosphate buffer (pH 2.6), with the concentration of the organic modifier, methanol, in the elution solvent varied from 5% to 90% using a linear gradient profile. The total run time, including the equilibration time for the subsequent run, was 15 minutes. The flow rate was set at 1 mL/min, the temperature at 25 °C, and the detection wavelength at 320 nm for all analytes. In our study, 5-fluorouracil (5-FU) with a concentration of 5 mg/mL was used as the internal standard (IS) in the HPLC method [22].
6-MP metabolite assay
The extraction and determination of 6-MP metabolites (6-TG and 6-MMP) were performed using HPLC on RBCs. Briefly, A total of 200 µL of suspended erythrocytes was mixed with 100 µL of dithiothreitol (DTT) at a concentration of 75 mg/mL (resulting in a final concentration of 120 mM), along with 100 µL of water, 25 µL of 5-FU and 50 µL of perchloric acid (70%). This mixture was vortexed for 30 seconds in a 1.5-mL Eppendorf tube (Sarstedt, Germany) and centrifuged for 15 minutes at 13000 × g at room temperature. To hydrolyze thiopurine nucleotides into their corresponding bases, 300 µL of the supernatant was transferred to a new Eppendorf tube and heated in a compact thermomixer (Hamburg, Germany) for 45 minutes at 100 °C. During hydrolysis, 6-MMP is transformed into 4-amino-5-(methylthio)carbonyl imidazole. This product could easily be quantified using the same chromatographic conditions applied to the other bases, corresponding to the final product detected by the described method. About 20 µL aliquot of the cooled solution was injected into the column [22].
Statistical analysis
All statistical processes were operated using Stata software, version 14 (StataCorp, College Station, TX, USA). Data have been presented as Mean±SD or number (%). We deployed the Mann-Whitney U or student t-test and Fisher exact tests to compare the variables between two groups, case, and control. The normality was checked for continuous data using a histogram and the Shapiro-Wilk test. Data transformation was applied on the 6-MP metabolites as Ln-transformed. Then, β coefficients from linear regression models were estimated. A probability value of less than 0.05 was considered statistically significant.
Results
Patient characteristics and biochemical parameters
This study included 32 children with ALL, comprising 19 patients in the control group (7 boys and 12 girls) and 13 in the case group (6 boys and 7 girls). Their mean ages were 8.07±4.01 years for the control group and 8.38±3.96 years for the case group. The mean body mass index, the dose of mercaptopurine, and the number of dose reductions in the control and case groups are presented in Table 1.
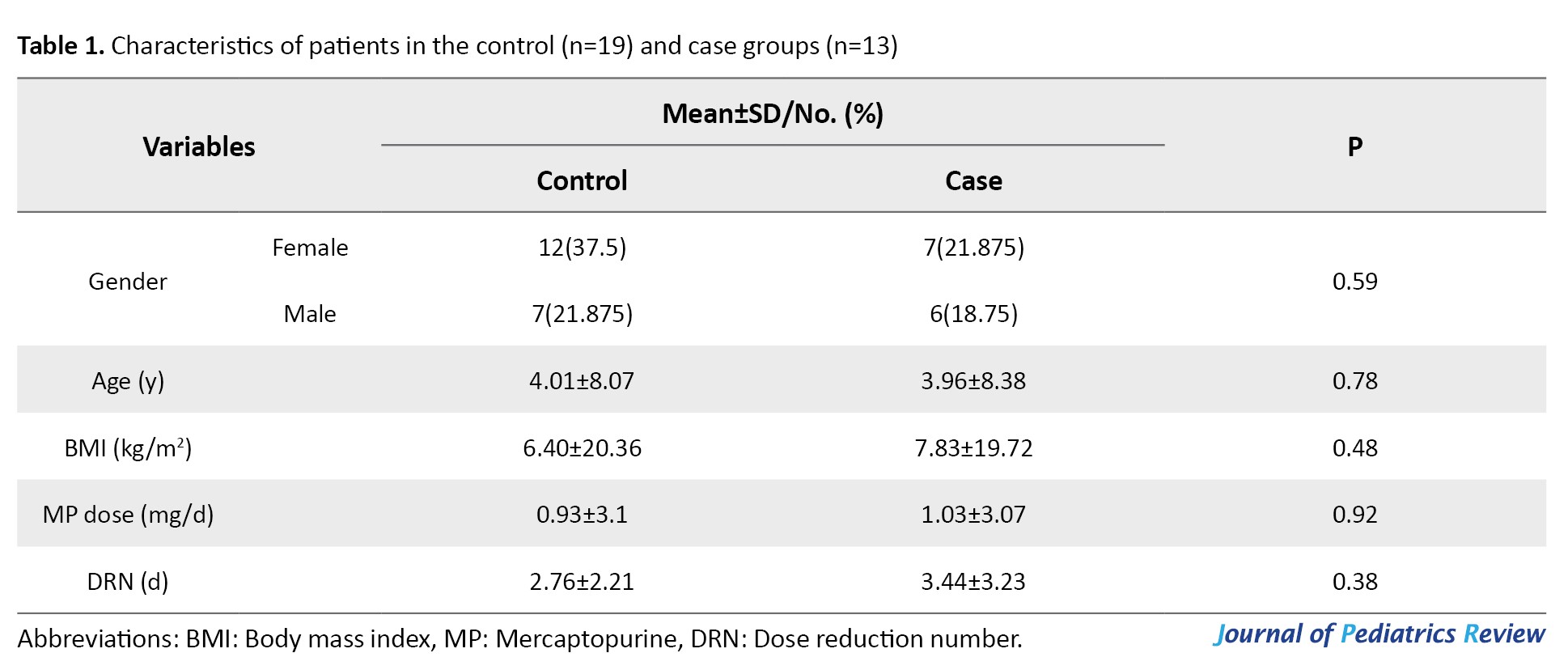
No significant differences were observed between the case and control groups regarding demographic characteristics (Table 1).
Table 2 lists the mean values of the biochemical parameters (WBC, HB, PLT, ALT, AST, HDL, total bilirubin, direct bilirubin, urea, Cr, and CrCl) among patients.
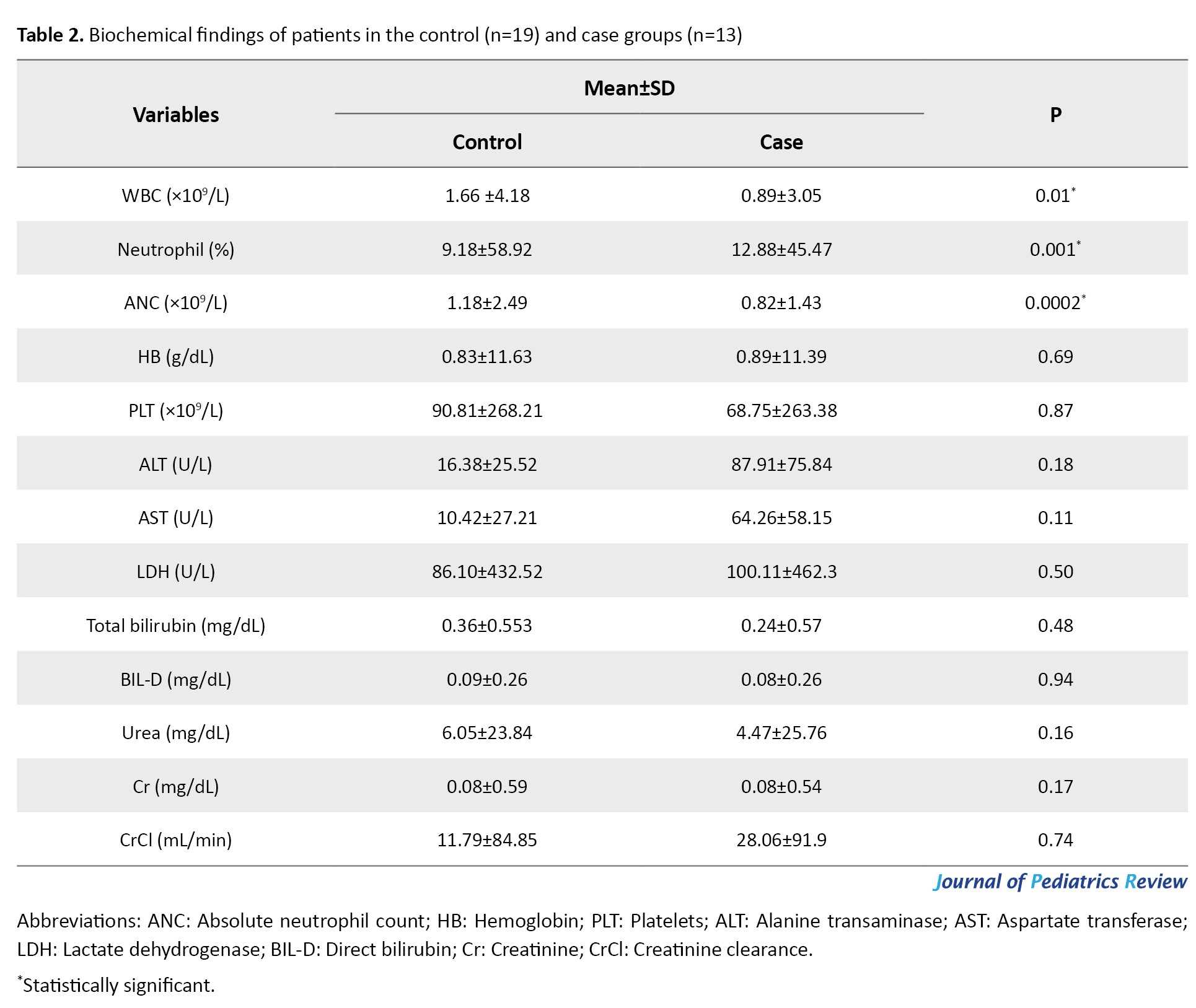
The Mean±SD values for WBC in the control and case groups were 4.18±1.66 and 3.05±0.89 ×109/L, respectively. Also, the mean values for neutrophils and absolute neutrophil count (ANC) in the control group were 58.92±9.18% and 2.49±1.18 ×109/L, respectively, while the case group had the values 45.47±12.88% and 1.43±0.82 ×109/L. Furthermore, there is a significant relationship between the case and control groups regarding WBC (P=0.01), neutrophil (P=0.001), and ANC (P=0.0002). Additionally, no significant changes were observed between the case and control groups in other biochemical parameters.
Mercaptopurine metabolites levels and ANC
Concentrations of 6-TG, 6-MP, and 6-MMP were measured using HPLC in RBCs of patients (Figures 1, 2 and 3). The Mean±SD Ln 6-TG concentration in the control and case groups were 8.9±1.44 pmol/8×108 RBC [IQR, 5.11-10.89] and 7.9±1.6) pmol/8×108 RBC [IQR, 5.86-9.98], respectively. The Mean±SD Ln 6-MP concentrations were 8.69±1.09 pmol/8×108 RBC [IQR, 6.71-10.37] for the control group and 9.03 (0.89) pmol/8×108 RBC [IQR, 7.84-10.2] for the case group. Also, Ln 6-MMP concentrations were 13.2±1.29 pmol/8×108 RBC [IQR, 10.53-16.02] in the control group and 12.85±1.31 pmol/8×108 RBC [IQR, 11.04-15.17] in the case group (Table 3).
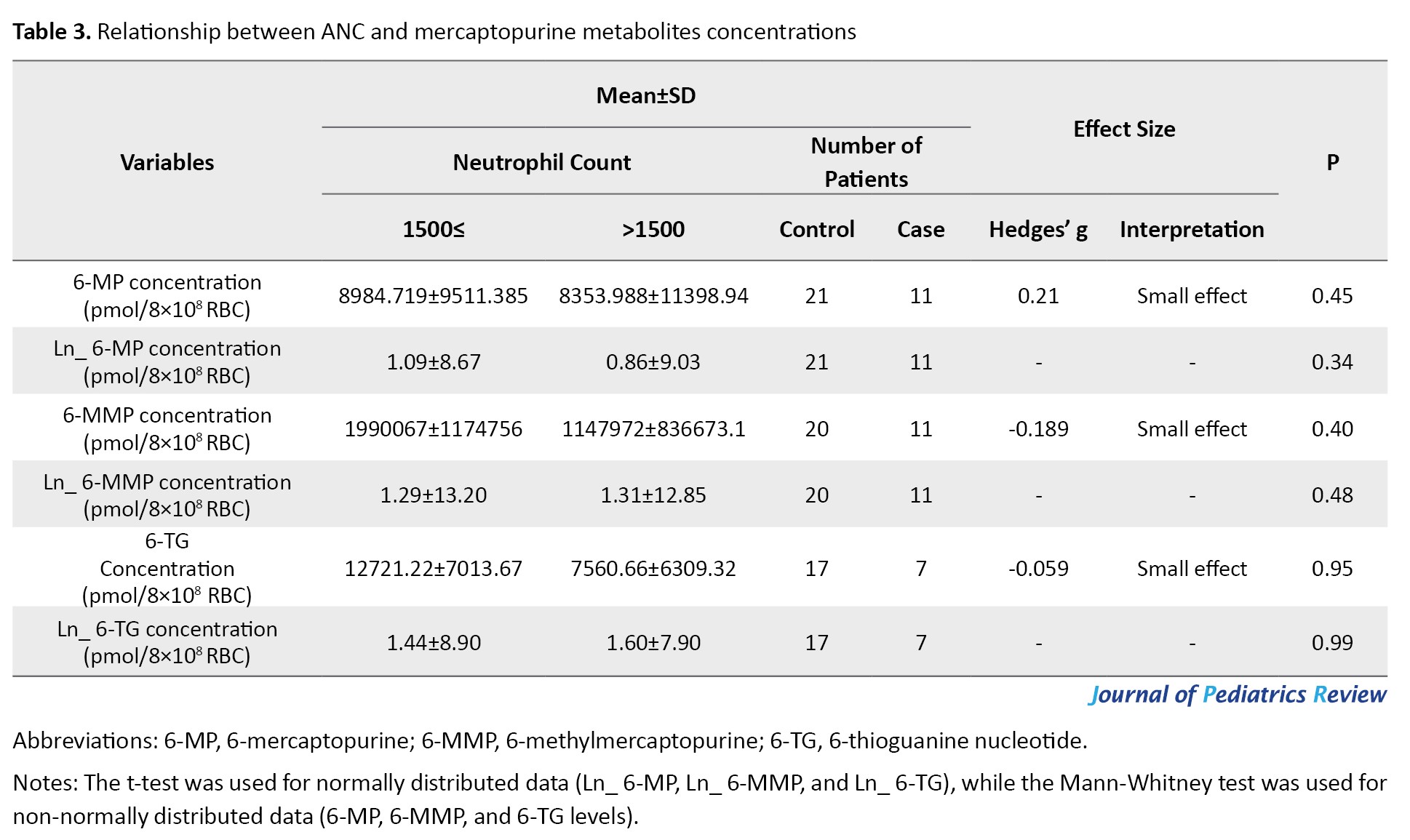
Association between mercaptopurine metabolites levels and ANC
The concentrations of 6-TG (Hedges’ g=-0.059; 95% CI, -0.908%, 0.792%; P=0.95) and 6-MMP (Hedges’ g=-0.189; 95% CI, -0.905%, 0.531%; P=0.40) were negatively related to ANC, while a positive connection was observed between 6-MP (Hedges’ g=0.215; 95% CI, 0.505%, 0.921%, P=0.45) and ANC. However, no significant differences exist between the case and control groups (Table 3).
Mercaptopurine metabolites levels and hepatotoxicity
In cases with hepatotoxicity, the mean concentrations of Ln 6-TG were 7.80 [IQR, 5.11-9.44] pmol/8×108 RBC in the control group and 8.07 [IQR, 5.86-10.89] in the case group. While the mean concentrations of Ln 6-MMP were reported to be 13.08 [IQR, 10.53-14.65] pmol/8×108 RBC in the control group and 13.06 [IQR, 11.04-16.02] pmol/8×108 RBC in the case group. Also, the mean concentrations of Ln 6-MP were 8.67 [IQR, 6.71-10.37] pmol/8×108 RBC in the control group and 8.97 [IQR, 7.84-10.2] pmol/8×108 RBC in case group. 6-MMP was slightly higher in patients with hepatotoxicity compared with patients without hepatotoxicity; however, this difference was not statistically significant (Table 4).
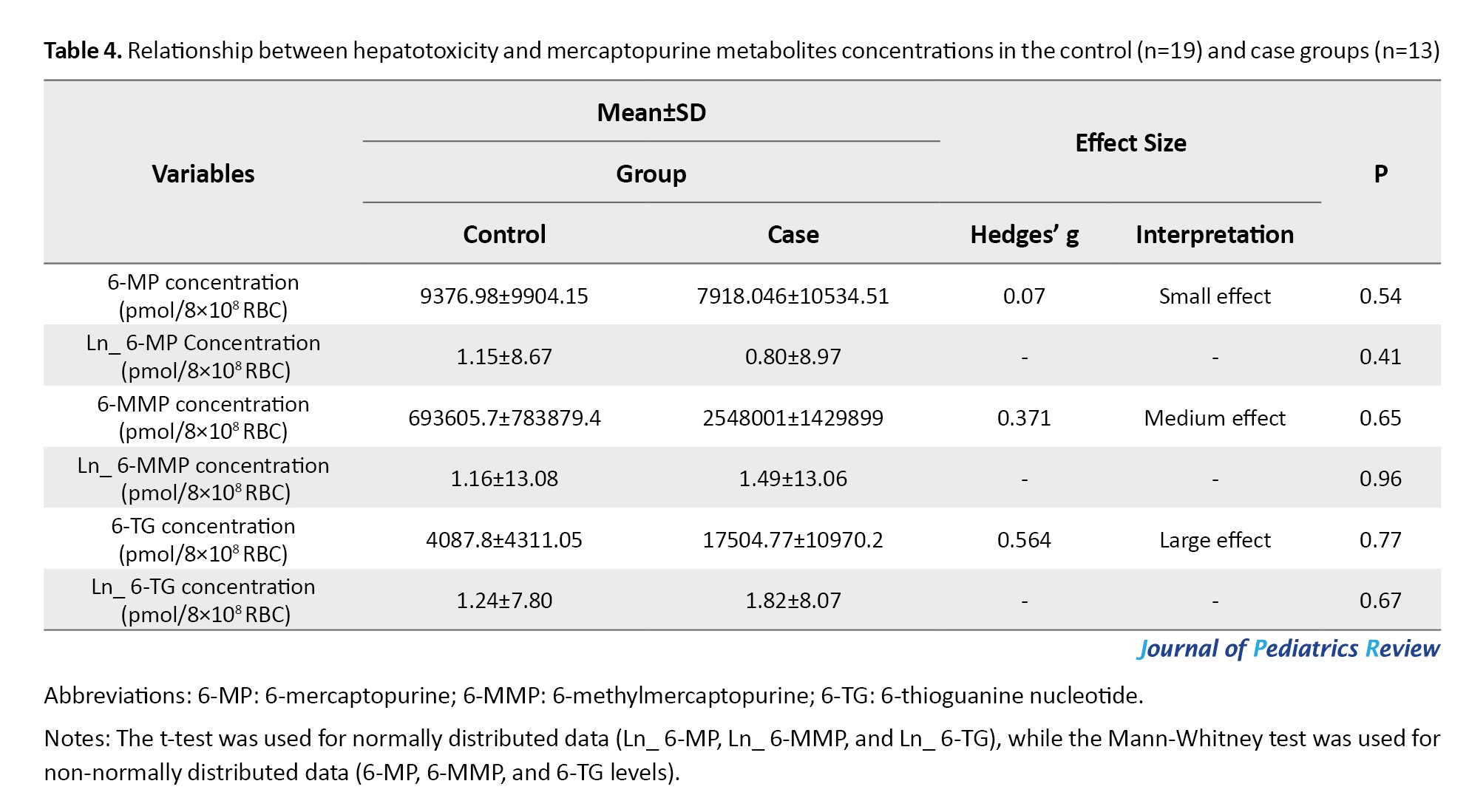
Association between mercaptopurine metabolites levels and hepatotoxicity
There is a positive association between the concentrations of 6-TG (Hedges’ g=0.56; 95% CI, -0.14%, 1.26%) and hepatotoxicity. Also, the concentrations of 6-MMP (Hedges’ g=0.371; 95% CI, -0.326%, 1.06%) and 6-MP (Hedges’ g=0.07, 95% CI, -0.61%, 0.75%) exhibited a positive relationship with hepatotoxicity (Table 4). However, no significant differences were observed between the groups.
Correlation of patient’s characteristics, liver function, and blood counts (including WBC, ANC, and neutrophil %) with mercaptopurine metabolites using the Pearson coefficient model.
The correlations between the patient’s characteristics, liver function, blood counts, and mercaptopurine metabolites were examined using the Pearson correlation coefficient. The result indicated that 6-TG concertation in RBC was inversely correlated with ANC (r=0.41, P=0.04), WBC (r=-0.32), and neutrophil (r=-0.44, P=0.03). These correlations indicate significant differences between 6-TG concentration and ANC and neutrophil levels. Additionally, concentrations of 6-MP and 6-MMP were negatively correlated with ANC, WBC, and neutrophil. ALT levels were slightly positively correlated with 6-MP concentration in RBC. In contrast, ALT levels showed an inverse correlation with both 6-TG and 6-MMP concentrations in RBC. These results are shown in Figure 4.
Relationships between ALT and neutropenia with mercaptopurine metabolites using regression model
The relationships between hepatotoxicity and neutropenia with mercaptopurine metabolites, as analyzed using the regression model, are presented in Table 5.
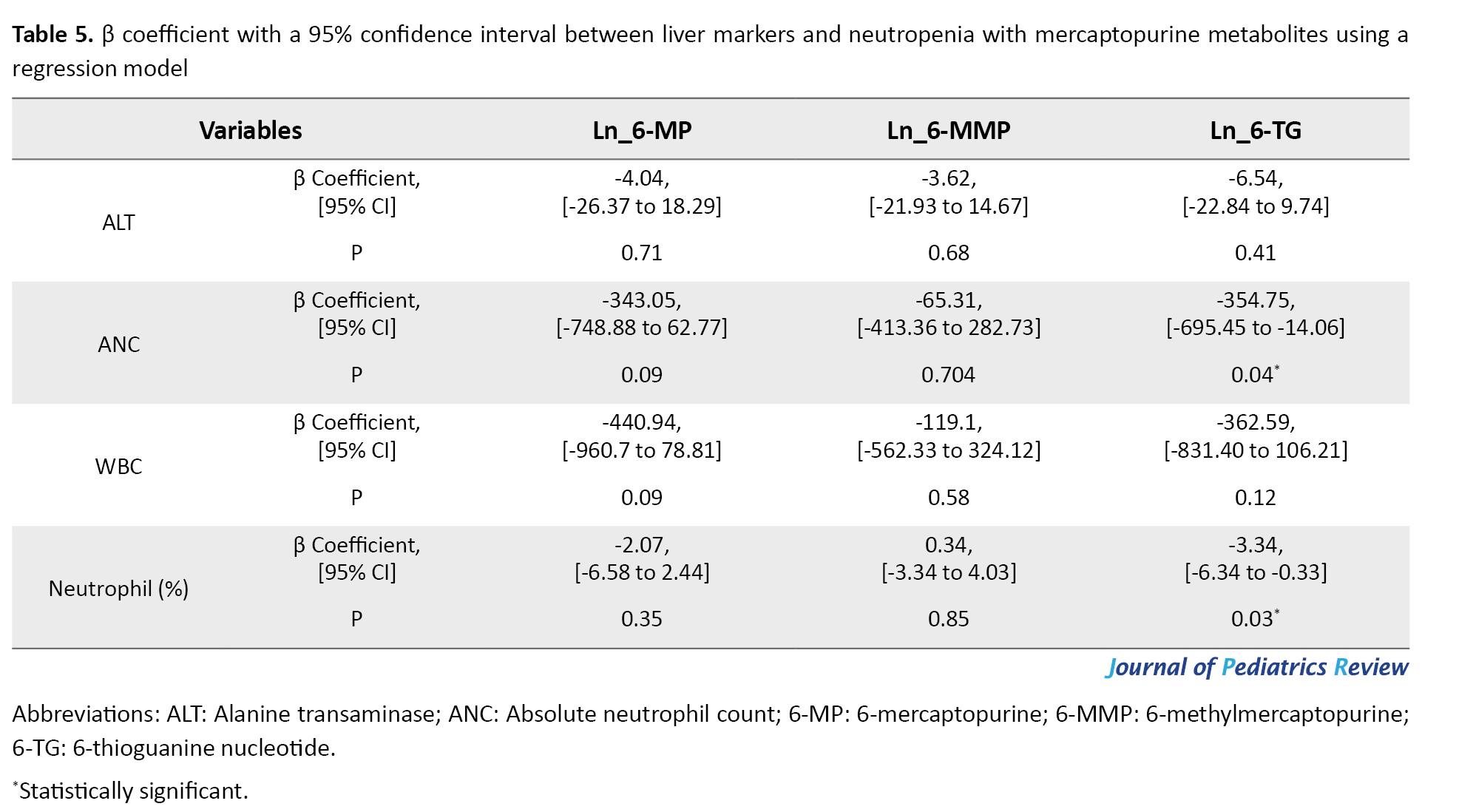
A negative association was observed between ALT levels, a liver marker, and the natural logarithm of the concentrations of 6-TG (β coefficient=-6.54; 95% CI, -22.84%, 9.74%; P=0.41), 6-MMP (β coefficient=-3.62; 95% CI, -21.93%, 14.67%; P=0.68), and 6-MP (β coefficient=-4.04; 95% CI, -26.37%, 18.29%; P=0.71), which was not statistically significant.
There was a negative association of ANC values with the natural logarithm of 6-TG concentrations (β coefficient=-354.75; 95% CI, -695.45%, -14.06%; P=0.04), 6-MP concentrations (β coefficient=-343.05; 95% CI, -748.88%, 62.77%; P=0.09) and a negative association with the natural logarithm of 6-MMP concentrations (β coefficient=-65.31; 95% CI, -413.36%, 282.73%; P=0.70). However, there was only a significant difference between ANC and 6-TG.
The percentage of neutrophils exhibited a negative association with the natural logarithm of 6-TG concentrations (β coefficient=-3.34; 95% CI, -6.34%, -0.33%; P=0.03) and 6-MP concentrations (β coefficient=-2.07; 95% CI, -6.58%, 2.44%; P=0.36) and a positive association with the natural logarithm of 6-MMP concentrations (β coefficient=0.34; 95% CI, -3.34%, 4.03%; P=0.85). However, there was only a significant difference between the percentage of neutrophils and 6-TG.
Moreover, it negatively associated with WBC count and the natural logarithm of 6-TG concentrations (β coefficient=-362.59; 95% CI, -831.40%, -106.21%; P=0.12), 6-MP concentrations (β coefficient=-440.94; 95% CI, -960.7%, 78.81%; P=0.09), and 6-MMP concentrations (β coefficient=-119.1; 95% CI, -562.23%, 324.12%; P=0.58). However, this association was not statistically significant.
Discussion
This study evaluated the concentrations of the metabolites 6-TG and 6-MMP in RBCs using HPLC during 6-MP therapy and their relationship with neutropenia and hepatotoxicity in the maintenance treatment of childhood ALL. Our results indicate that the WBC, neutrophils, and ANC levels among patients were significantly different between the study groups. High concentrations of 6-TG and 6-MMP were associated with neutropenia among patients. Mercaptopurine is an antineoplastic and immunosuppressive agent predominantly used to treat autoimmune diseases and certain neoplasms, such as leukemia [23]. The majority of its adverse effects are caused by metabolites, which may be responsible for serious complications like neutropenia and even hepatotoxicity [24]. Mercaptopurine is metabolized in the body into several compounds; the most significant are 6-MMP and 6-TG [25].
6-TG is an active metabolite of mercaptopurine that functions as an anticancer agent. However, excessive accumulation of 6-TG in the body can result in neutropenia. By affecting the bone marrow, this metabolite reduces the production of WBC [26]. Genetic variability in mercaptopurine-metabolizing enzymes, TPMT, and nudix hydrolase 15 (NUDT15) may dramatically impact the occurrence of neutropenia [27, 28]. Genetic mutations in the TPMT and NUDT15 are also associated with an increased risk of neutropenia. These mutations can lead to the accumulation of the active metabolite 6-TG, increasing the risk of developing neutropenia [28, 29]. In this study, 6-TG concentrations showed an inverse correlation with ANC, WBC, and neutrophil count. The concentrations of 6-TG and 6-MMP were positively correlated with ALT. These results indicate a relationship between myelosuppression to high levels of 6-TG and hepatotoxicity with high levels of 6-TG and 6-MMP [30, 31].
Confirmed and previously published [32], there is an association between ANC and neutropenia with elevated 6-TG levels. In line with the findings of our study, Wong et al. reported a similar relationship between 6-TG levels and neutropenia [33]. In contrast, another study has not found a relationship between concentrations of 6-TG and 6-MMP metabolites and myelotoxicity or hepatotoxicity [34]. Abdelsayed et al. confirmed the inverse correlation between WBC, neutrophils count, and ANC with 6-TG concentration, further establishing the association of 6-TG with myelotoxicity [35]. High levels of 6-TG are associated with reduced production of WBCs and neutrophils, leading to neutropenia [30]. Indeed, this relationship is supported by various studies that show that thiopurine metabolites can be incorporated into DNA [36]. This event may be mediated by incorporating 6 TG into DNA and RNA, which interferes with cellular processes, ultimately leading to decreased hematopoiesis in the bone marrow [37, 38]. A previous study identified a strong relationship between the metabolites of 6-MP and the incidence of neutropenia and hepatotoxicity. Elevated levels of 6-MMP and 6-TG are significantly associated with hepatotoxicity and neutropenia, respectively [39]. Moreover, an inverse correlation has been reported between the lymphocyte count and the level of 6-TG in erythrocytes of Crohn’s disease patients [40].
A previous study has also provided valuable insights into the concentrations of 6-TG and 6-MMP in pediatric patients with inflammatory bowel disease, which are linked to various biochemical abnormalities, including a reduction in WBC and neutrophil counts, as well as an increase in ALT levels, respectively [41].
In the present study, we observed that reduction of WBC and ANC were also associated with 6-MP and 6-MMP concentrations. Meijer et al. attributed thiopurine-induced myelotoxicity to increased levels of 6-MMP in patients [31]. Previous studies also report high levels of 6-MMP are associated with hepatotoxicity [42]. While 6-MMP is known to contribute to neutropenia, its association with hepatotoxicity has garnered more attention [43]. Elevated levels of 6-MMP can result in adverse effects, particularly in patients with deficiencies in metabolizing enzymes [32]. Another study conducted among pediatric patients in the remission phase of inflammatory bowel disease found that patients receiving 6-MP who experienced hepatotoxicity had not shown 6-MMP concentrations exceeding the toxic reference value [44]. The lack of a correlation between 6-MP and 6-MMP concentrations with neutropenia and hepatotoxicity may be influenced by the small number of patients experiencing these events. A retrospective study showed that patients who did not respond to azathioprine or 6-MP had high 6-MMP concentrations or an increased 6-MMP/6-TG ratio. It suggests that excessive production of 6-MMP in the hepatotoxic metabolite could result in hepatic damage and therapeutic failure [45]. The hepatotoxicity associated with 6-MMP is probably related to its accumulation due to altered metabolism of 6-MP [46]. However, we observed a weak correlation between high 6-MMP concentrations and hepatotoxicity in the present study. Supandi et al. reported that concentrations of 6-MMP, an inactive metabolite, ranged from 28 to 499 pmol/8×108 erythrocytes, lower than the hepatotoxic range [47]. In some reports, patients with hepatotoxicity did not constantly show high concentrations of 6-MMP [48]. While elevated 6-MMP levels (>5700 pmol/8×108 RBC) have been correlated with hepatotoxicity, [32], it is notable that other factors may contribute to hepatotoxicity, and elevated 6-MMP levels alone might not serve as a definitive agent.
This study had limitations, such as the small sample size and the inability to coordinate with all patients to take their last night’s dose at a specific and fixed time. It was also challenging to maintain uniform temperature conditions throughout the process, from sampling to injection into the HPLC device. This consistency was not achievable for all samples.
Maintaining a constant sample storage temperature is recommended. Additionally, if feasible, it would be preferred that patients who receive 6-MP from the same brand should be included in the study.
Conclusion
Our results show a strong negative association between high concentrations of 6-TG and WBC counts, neutrophil counts, and ANC. Additionally, a positive relationship was observed between the levels of metabolites of 6-TG and 6-MMP and hepatotoxicity. The results presented in this study support the proposed importance of monitoring the concentrations of 6-TG and 6-MMP metabolites in RBC during maintenance therapy. Monitoring the concentrations of active metabolites of 6-MP in RBC enables early identification of patients at increased risk of disease relapse due to inadequate levels of both metabolites. Confirming this conclusion requires further studies involving a larger group of children from various medical centers.
Ethical Considerations
Compliance with ethical guidelines
Approval for conducting the research was obtained from the Ethics Committee of Mazandaran University of Medical Sciences, Sari City, in Iran (Ethical Code: IR.MAZUMS.REC.1402.17134). Sufficient explanations regarding the purpose and procedure of the research were provided to the patients’ parents. After obtaining written informed consent from the parents, the patients were enrolled in the study. The method of obtaining consent varied among different age groups. For children under 7, informed consent was acquired from the parents. In children aged 7 to 15, informed consent was obtained from the legal guardian while ensuring that the child’s assent to participate in the study was obtained. However, for children aged 15 to 18, informed consent was obtained from both the child and their legal guardian. Thus, besides providing complete explanations to the child and the legal guardian, signatures from both parties were included in the informed consent form. Parents were assured that their children’s participation in the research was voluntary and that they could withdraw at any time if they wished to discontinue. It was emphasized that participation or non-participation in the study would not affect the level of care provided to the patient. Furthermore, participants were assured that their identities and information would remain confidential throughout the research process and in the publication of findings.
Funding
This research was conducted as a PhD dissertation of Omid Abed Khojasteh, approved by the Department of Clinical Pharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran. This study was supported by a grant from Mazandaran University of Medical Sciences, Sari, Iran (Grant No.: 9399).
Authors contributions
Project administration: Ebrahim Salehifar; Supervision and visualization Ebrahim Salehifar, Seyed Jalal Hosseinimehr, Hossein Karami, Mohammad Naderi Sorki; Conceptualization: Ebrahim Salehifar, Seyed Jalal Hosseinimehr, Hossein Karami, Mohammad Naderi Sorki, and Hadi Darvish-Khezri; Methodology: Ebrahim Salehifar, Omid Abed Khojasteh, Seyed Jalal Hosseinimehr, Hossein Karami, Mohammad Naderi Sorki; Software: Omid Abed Khojasteh, Seyed Jalal Hosseinimehr, and Hadi Darvish-Khezri; Validation: Seyed Jalal Hosseinimehr; Formal analysis: Omid Abed Khojasteh, Seyed Jalal Hosseinimehr, and Hadi Darvish-Khezri; Data curation: Omid Abed Khojasteh, and Hadi Darvish-Khezri; Investigation and writing the original draft: Omid Abed Khojasteh; Resources: Hossein Karami, and Mohammad Naderi Sorki; Review and editing: Omid Abed Khojasteh, and Kimia Karami.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express their gratitude to the children and their families for taking part in this research project. The authors also appreciate the medical and nursing professionals whose efforts made this work achievable.
References
Acute lymphoblastic leukemia (ALL) is the most prevalent malignancy in children, accounting for approximately 80% of pediatric leukemias and 30% of all childhood malignancies. ALL is characterized by the rapid proliferation of immature white blood cells (WBCs), known as lymphoblasts [1, 2]. The typical symptoms of the disease include fatigue, fever, easy bruising, and bone pain [3]. Over the past 30 years, the prognosis of ALL significantly improved, achieving a 90% survival rate over 5 years [4]. Management of drug-related toxicity will be essential to prevent interruptions in chemotherapy to minimize disease relapses, thereby further increasing the survival rate [5]. To reduce drug-related toxicity and enhance the survival rate of vulnerable patients, it is essential to tailor treatment to the individual needs of each patient [6]. A portion of the treatment’s success can be attributed to the 18 to 24 months of adequate maintenance therapy necessary to prolong the remission achieved during the early stages of treatment [7].
The foundation of maintenance therapy for ALL is 6-mercaptopurine (6-MP) [8]. As a purine analog, 6-MP exerts its anti-leukemic effects by inhibiting de novo purine synthesis (DNPS), which is essential for leukemic cell proliferation [9, 10]. 6-MP is a prodrug metabolized through multiple enzymatic steps in the purine salvage pathway [11]. The anabolic pathway involving hypoxanthine-guanine phosphoribosyl transferase (HPRT) results in the production of 6-thioinosine monophosphate, which is subsequently converted into 6-thioguanine (6-TG) nucleotides [12]. Then, 6-TG is phosphorylated to form 6-thioinosine triphosphate or incorporated into nucleic acids [13]. The reversal of this process can be mediated by the enzyme inosine triphosphate pyrophosphatase [14]. Catabolic pathways inactivate 6-MP through xanthine oxidase, which converts it to 6-thiouric acid and thiopurine S-methyltransferase (TPMT), which metabolizes 6-MP to 6-methylmercaptopurine (6-MMP). Both of these catabolic metabolites inhibit DNPS [15].
The balance between 6-TG and 6-MMP is known to vary significantly and is mainly influenced by TPMT genetic polymorphisms [16]. The effectiveness and side effects of 6-MP in patients are known to be influenced by inter-individual variability in the concentrations of 6-TG and 6-MMP in the red blood cell (RBC), particularly in homozygous TPMT-deficient patients, who face a significant risk of myelosuppression [17, 18]. 6-TG metabolites are responsible for the anti-leukemic effects of 6-MP, while 6-MMP and its metabolites are associated with hepatotoxicity [19]. While 6-MP is generally well tolerated, side effects such as nausea, skin rashes, changes in appetite, bone marrow suppression, and hepatotoxicity may still occur [20, 21]. Maintaining an optimal balance between 6-MP metabolic pathways, including the production of 6-TG and 6-MMP, is crucial to achieving maximal anti-leukemic efficacy while minimizing the risk of hepatotoxicity or severe myelosuppression. In the current study, we measured the serum concentrations of 6-MP metabolites and assessed their relationship with the occurrence of 6-MP side effects, particularly hepatotoxicity, and neutropenia, in ALL patients receiving 6-MP during maintenance therapy.
Methods
Statistical population
Children with ALL in the maintenance phase of chemotherapy with 6-MP, who were referred to the Pediatric Oncology Department of Bou Ali Sina Hospital in Sari City, Iran, were included in this study. A confirmation of ALL is met when blast cells of lymphoid origin constitute ≥20% of marrow nucleated cells or ≥20% of non-erythroid cells when the erythroid component is >50%. The diagnosis of ALL in children was confirmed through complete blood count tests, peripheral blood smear observations by a hematopathologist, lymphoblast count, and, if possible, lymphoblast flow cytometry studies, cytogenetic studies, and finally, bone marrow aspiration.
Inclusion criteria
We included the patients under 18 years of age diagnosed with ALL who have been in the maintenance therapy phase for at least 2 months and are currently in remission and receiving a consistent daily dose of 6-MP and other medication, including corticosteroids and methotrexate for at least the past month, with no dose adjustments during this period were included.
Also, the patients should have not undergone packed RBC transfusions within the last 6 weeks.
Exclusion criteria
We excluded patients with previously known hypersensitivity to 6-MP or thioguanine, patients without consent to participate or continue participation in the study, and patients treated with allopurinol during the previous month.
Data collection
In this study, patient characteristics (gender, age, body mass index, MP dose, and number of dose reductions) were gathered at the time points (November 27, 2023 -March 12, 2024). Clinical data for each child were also collected at the time of blood sampling, including hemoglobin levels, RBC count, leukocyte count, liver function tests (e.g. alanine transaminase [ALT], aspartate transaminase [AST], lactate dehydrogenase [LDH]), bilirubin levels, and levels of urea, creatinine [Cr], and creatinine clearance [CrCl]. Based on the available clinical records, two groups of patients were examined.
Case and control groups definition
The case group consisted of patients with ALL who experienced hepatotoxicity (ALT more than 2.5 times the upper limit of normal) or neutropenia (absolute neutrophil count [ANC] less than 1500×109/L) during 6-MP treatment.
The control group included patients with ALL who did not experience hepatotoxicity or neutropenia while undergoing treatment with 6-MP.
Blood sampling
To prepare erythrocytes, 3-5 mL of venous blood samples were collected from each child and poured into EDTA-containing tubes. The samples were stored at 4 °C and processed within 24 hours. Subsequently, they were centrifuged at 2500 rpm for 10 minutes at 4 °C. The plasma and buffy coat were removed, and the remaining RBC volume was measured and recorded in the falcon tube. The cells were then washed with approximately two volumes of normal saline, vortexed at low speed (setting 1) for 15 seconds, and centrifuged at 2500 rpm for 10 minutes at 4 °C. After discarding the supernatant, this washing step was repeated. The sample was centrifuged at approximately 2500 rpm for 10 minutes at 4 °C. Following removing the supernatant, the cells were resuspended to an equal volume of normal saline. The RBC count was determined using a Beckman Coulter™ Counter (USA). Finally, the samples were stored in 1.5 mL tubes at -80 °C until further analysis.
High-performance liquid chromatography method (HPLC)
The HPLC system consists of a K-1001 solvent delivery system equipped with a Rheodyne injection valve (20 µL sample loop inserted) and a UV-Vis spectrophotometer detector model K-2600 set at 320 nm (all from Knauer company, Germany). Analysis was performed using an ODS-C18 column (150×4.6 mm i.d., 5-µm particle size) and the corresponding guard column. All solvents were filtered and degassed before entering the column. The optimum HPLC conditions included a mobile phase aqueous system consisting of 0.025 M phosphate buffer (pH 2.6), with the concentration of the organic modifier, methanol, in the elution solvent varied from 5% to 90% using a linear gradient profile. The total run time, including the equilibration time for the subsequent run, was 15 minutes. The flow rate was set at 1 mL/min, the temperature at 25 °C, and the detection wavelength at 320 nm for all analytes. In our study, 5-fluorouracil (5-FU) with a concentration of 5 mg/mL was used as the internal standard (IS) in the HPLC method [22].
6-MP metabolite assay
The extraction and determination of 6-MP metabolites (6-TG and 6-MMP) were performed using HPLC on RBCs. Briefly, A total of 200 µL of suspended erythrocytes was mixed with 100 µL of dithiothreitol (DTT) at a concentration of 75 mg/mL (resulting in a final concentration of 120 mM), along with 100 µL of water, 25 µL of 5-FU and 50 µL of perchloric acid (70%). This mixture was vortexed for 30 seconds in a 1.5-mL Eppendorf tube (Sarstedt, Germany) and centrifuged for 15 minutes at 13000 × g at room temperature. To hydrolyze thiopurine nucleotides into their corresponding bases, 300 µL of the supernatant was transferred to a new Eppendorf tube and heated in a compact thermomixer (Hamburg, Germany) for 45 minutes at 100 °C. During hydrolysis, 6-MMP is transformed into 4-amino-5-(methylthio)carbonyl imidazole. This product could easily be quantified using the same chromatographic conditions applied to the other bases, corresponding to the final product detected by the described method. About 20 µL aliquot of the cooled solution was injected into the column [22].
Statistical analysis
All statistical processes were operated using Stata software, version 14 (StataCorp, College Station, TX, USA). Data have been presented as Mean±SD or number (%). We deployed the Mann-Whitney U or student t-test and Fisher exact tests to compare the variables between two groups, case, and control. The normality was checked for continuous data using a histogram and the Shapiro-Wilk test. Data transformation was applied on the 6-MP metabolites as Ln-transformed. Then, β coefficients from linear regression models were estimated. A probability value of less than 0.05 was considered statistically significant.
Results
Patient characteristics and biochemical parameters
This study included 32 children with ALL, comprising 19 patients in the control group (7 boys and 12 girls) and 13 in the case group (6 boys and 7 girls). Their mean ages were 8.07±4.01 years for the control group and 8.38±3.96 years for the case group. The mean body mass index, the dose of mercaptopurine, and the number of dose reductions in the control and case groups are presented in Table 1.

No significant differences were observed between the case and control groups regarding demographic characteristics (Table 1).
Table 2 lists the mean values of the biochemical parameters (WBC, HB, PLT, ALT, AST, HDL, total bilirubin, direct bilirubin, urea, Cr, and CrCl) among patients.

The Mean±SD values for WBC in the control and case groups were 4.18±1.66 and 3.05±0.89 ×109/L, respectively. Also, the mean values for neutrophils and absolute neutrophil count (ANC) in the control group were 58.92±9.18% and 2.49±1.18 ×109/L, respectively, while the case group had the values 45.47±12.88% and 1.43±0.82 ×109/L. Furthermore, there is a significant relationship between the case and control groups regarding WBC (P=0.01), neutrophil (P=0.001), and ANC (P=0.0002). Additionally, no significant changes were observed between the case and control groups in other biochemical parameters.
Mercaptopurine metabolites levels and ANC
Concentrations of 6-TG, 6-MP, and 6-MMP were measured using HPLC in RBCs of patients (Figures 1, 2 and 3). The Mean±SD Ln 6-TG concentration in the control and case groups were 8.9±1.44 pmol/8×108 RBC [IQR, 5.11-10.89] and 7.9±1.6) pmol/8×108 RBC [IQR, 5.86-9.98], respectively. The Mean±SD Ln 6-MP concentrations were 8.69±1.09 pmol/8×108 RBC [IQR, 6.71-10.37] for the control group and 9.03 (0.89) pmol/8×108 RBC [IQR, 7.84-10.2] for the case group. Also, Ln 6-MMP concentrations were 13.2±1.29 pmol/8×108 RBC [IQR, 10.53-16.02] in the control group and 12.85±1.31 pmol/8×108 RBC [IQR, 11.04-15.17] in the case group (Table 3).

Association between mercaptopurine metabolites levels and ANC
The concentrations of 6-TG (Hedges’ g=-0.059; 95% CI, -0.908%, 0.792%; P=0.95) and 6-MMP (Hedges’ g=-0.189; 95% CI, -0.905%, 0.531%; P=0.40) were negatively related to ANC, while a positive connection was observed between 6-MP (Hedges’ g=0.215; 95% CI, 0.505%, 0.921%, P=0.45) and ANC. However, no significant differences exist between the case and control groups (Table 3).
Mercaptopurine metabolites levels and hepatotoxicity
In cases with hepatotoxicity, the mean concentrations of Ln 6-TG were 7.80 [IQR, 5.11-9.44] pmol/8×108 RBC in the control group and 8.07 [IQR, 5.86-10.89] in the case group. While the mean concentrations of Ln 6-MMP were reported to be 13.08 [IQR, 10.53-14.65] pmol/8×108 RBC in the control group and 13.06 [IQR, 11.04-16.02] pmol/8×108 RBC in the case group. Also, the mean concentrations of Ln 6-MP were 8.67 [IQR, 6.71-10.37] pmol/8×108 RBC in the control group and 8.97 [IQR, 7.84-10.2] pmol/8×108 RBC in case group. 6-MMP was slightly higher in patients with hepatotoxicity compared with patients without hepatotoxicity; however, this difference was not statistically significant (Table 4).

Association between mercaptopurine metabolites levels and hepatotoxicity
There is a positive association between the concentrations of 6-TG (Hedges’ g=0.56; 95% CI, -0.14%, 1.26%) and hepatotoxicity. Also, the concentrations of 6-MMP (Hedges’ g=0.371; 95% CI, -0.326%, 1.06%) and 6-MP (Hedges’ g=0.07, 95% CI, -0.61%, 0.75%) exhibited a positive relationship with hepatotoxicity (Table 4). However, no significant differences were observed between the groups.
Correlation of patient’s characteristics, liver function, and blood counts (including WBC, ANC, and neutrophil %) with mercaptopurine metabolites using the Pearson coefficient model.
The correlations between the patient’s characteristics, liver function, blood counts, and mercaptopurine metabolites were examined using the Pearson correlation coefficient. The result indicated that 6-TG concertation in RBC was inversely correlated with ANC (r=0.41, P=0.04), WBC (r=-0.32), and neutrophil (r=-0.44, P=0.03). These correlations indicate significant differences between 6-TG concentration and ANC and neutrophil levels. Additionally, concentrations of 6-MP and 6-MMP were negatively correlated with ANC, WBC, and neutrophil. ALT levels were slightly positively correlated with 6-MP concentration in RBC. In contrast, ALT levels showed an inverse correlation with both 6-TG and 6-MMP concentrations in RBC. These results are shown in Figure 4.
Relationships between ALT and neutropenia with mercaptopurine metabolites using regression model
The relationships between hepatotoxicity and neutropenia with mercaptopurine metabolites, as analyzed using the regression model, are presented in Table 5.

A negative association was observed between ALT levels, a liver marker, and the natural logarithm of the concentrations of 6-TG (β coefficient=-6.54; 95% CI, -22.84%, 9.74%; P=0.41), 6-MMP (β coefficient=-3.62; 95% CI, -21.93%, 14.67%; P=0.68), and 6-MP (β coefficient=-4.04; 95% CI, -26.37%, 18.29%; P=0.71), which was not statistically significant.
There was a negative association of ANC values with the natural logarithm of 6-TG concentrations (β coefficient=-354.75; 95% CI, -695.45%, -14.06%; P=0.04), 6-MP concentrations (β coefficient=-343.05; 95% CI, -748.88%, 62.77%; P=0.09) and a negative association with the natural logarithm of 6-MMP concentrations (β coefficient=-65.31; 95% CI, -413.36%, 282.73%; P=0.70). However, there was only a significant difference between ANC and 6-TG.
The percentage of neutrophils exhibited a negative association with the natural logarithm of 6-TG concentrations (β coefficient=-3.34; 95% CI, -6.34%, -0.33%; P=0.03) and 6-MP concentrations (β coefficient=-2.07; 95% CI, -6.58%, 2.44%; P=0.36) and a positive association with the natural logarithm of 6-MMP concentrations (β coefficient=0.34; 95% CI, -3.34%, 4.03%; P=0.85). However, there was only a significant difference between the percentage of neutrophils and 6-TG.
Moreover, it negatively associated with WBC count and the natural logarithm of 6-TG concentrations (β coefficient=-362.59; 95% CI, -831.40%, -106.21%; P=0.12), 6-MP concentrations (β coefficient=-440.94; 95% CI, -960.7%, 78.81%; P=0.09), and 6-MMP concentrations (β coefficient=-119.1; 95% CI, -562.23%, 324.12%; P=0.58). However, this association was not statistically significant.
Discussion
This study evaluated the concentrations of the metabolites 6-TG and 6-MMP in RBCs using HPLC during 6-MP therapy and their relationship with neutropenia and hepatotoxicity in the maintenance treatment of childhood ALL. Our results indicate that the WBC, neutrophils, and ANC levels among patients were significantly different between the study groups. High concentrations of 6-TG and 6-MMP were associated with neutropenia among patients. Mercaptopurine is an antineoplastic and immunosuppressive agent predominantly used to treat autoimmune diseases and certain neoplasms, such as leukemia [23]. The majority of its adverse effects are caused by metabolites, which may be responsible for serious complications like neutropenia and even hepatotoxicity [24]. Mercaptopurine is metabolized in the body into several compounds; the most significant are 6-MMP and 6-TG [25].
6-TG is an active metabolite of mercaptopurine that functions as an anticancer agent. However, excessive accumulation of 6-TG in the body can result in neutropenia. By affecting the bone marrow, this metabolite reduces the production of WBC [26]. Genetic variability in mercaptopurine-metabolizing enzymes, TPMT, and nudix hydrolase 15 (NUDT15) may dramatically impact the occurrence of neutropenia [27, 28]. Genetic mutations in the TPMT and NUDT15 are also associated with an increased risk of neutropenia. These mutations can lead to the accumulation of the active metabolite 6-TG, increasing the risk of developing neutropenia [28, 29]. In this study, 6-TG concentrations showed an inverse correlation with ANC, WBC, and neutrophil count. The concentrations of 6-TG and 6-MMP were positively correlated with ALT. These results indicate a relationship between myelosuppression to high levels of 6-TG and hepatotoxicity with high levels of 6-TG and 6-MMP [30, 31].
Confirmed and previously published [32], there is an association between ANC and neutropenia with elevated 6-TG levels. In line with the findings of our study, Wong et al. reported a similar relationship between 6-TG levels and neutropenia [33]. In contrast, another study has not found a relationship between concentrations of 6-TG and 6-MMP metabolites and myelotoxicity or hepatotoxicity [34]. Abdelsayed et al. confirmed the inverse correlation between WBC, neutrophils count, and ANC with 6-TG concentration, further establishing the association of 6-TG with myelotoxicity [35]. High levels of 6-TG are associated with reduced production of WBCs and neutrophils, leading to neutropenia [30]. Indeed, this relationship is supported by various studies that show that thiopurine metabolites can be incorporated into DNA [36]. This event may be mediated by incorporating 6 TG into DNA and RNA, which interferes with cellular processes, ultimately leading to decreased hematopoiesis in the bone marrow [37, 38]. A previous study identified a strong relationship between the metabolites of 6-MP and the incidence of neutropenia and hepatotoxicity. Elevated levels of 6-MMP and 6-TG are significantly associated with hepatotoxicity and neutropenia, respectively [39]. Moreover, an inverse correlation has been reported between the lymphocyte count and the level of 6-TG in erythrocytes of Crohn’s disease patients [40].
A previous study has also provided valuable insights into the concentrations of 6-TG and 6-MMP in pediatric patients with inflammatory bowel disease, which are linked to various biochemical abnormalities, including a reduction in WBC and neutrophil counts, as well as an increase in ALT levels, respectively [41].
In the present study, we observed that reduction of WBC and ANC were also associated with 6-MP and 6-MMP concentrations. Meijer et al. attributed thiopurine-induced myelotoxicity to increased levels of 6-MMP in patients [31]. Previous studies also report high levels of 6-MMP are associated with hepatotoxicity [42]. While 6-MMP is known to contribute to neutropenia, its association with hepatotoxicity has garnered more attention [43]. Elevated levels of 6-MMP can result in adverse effects, particularly in patients with deficiencies in metabolizing enzymes [32]. Another study conducted among pediatric patients in the remission phase of inflammatory bowel disease found that patients receiving 6-MP who experienced hepatotoxicity had not shown 6-MMP concentrations exceeding the toxic reference value [44]. The lack of a correlation between 6-MP and 6-MMP concentrations with neutropenia and hepatotoxicity may be influenced by the small number of patients experiencing these events. A retrospective study showed that patients who did not respond to azathioprine or 6-MP had high 6-MMP concentrations or an increased 6-MMP/6-TG ratio. It suggests that excessive production of 6-MMP in the hepatotoxic metabolite could result in hepatic damage and therapeutic failure [45]. The hepatotoxicity associated with 6-MMP is probably related to its accumulation due to altered metabolism of 6-MP [46]. However, we observed a weak correlation between high 6-MMP concentrations and hepatotoxicity in the present study. Supandi et al. reported that concentrations of 6-MMP, an inactive metabolite, ranged from 28 to 499 pmol/8×108 erythrocytes, lower than the hepatotoxic range [47]. In some reports, patients with hepatotoxicity did not constantly show high concentrations of 6-MMP [48]. While elevated 6-MMP levels (>5700 pmol/8×108 RBC) have been correlated with hepatotoxicity, [32], it is notable that other factors may contribute to hepatotoxicity, and elevated 6-MMP levels alone might not serve as a definitive agent.
This study had limitations, such as the small sample size and the inability to coordinate with all patients to take their last night’s dose at a specific and fixed time. It was also challenging to maintain uniform temperature conditions throughout the process, from sampling to injection into the HPLC device. This consistency was not achievable for all samples.
Maintaining a constant sample storage temperature is recommended. Additionally, if feasible, it would be preferred that patients who receive 6-MP from the same brand should be included in the study.
Conclusion
Our results show a strong negative association between high concentrations of 6-TG and WBC counts, neutrophil counts, and ANC. Additionally, a positive relationship was observed between the levels of metabolites of 6-TG and 6-MMP and hepatotoxicity. The results presented in this study support the proposed importance of monitoring the concentrations of 6-TG and 6-MMP metabolites in RBC during maintenance therapy. Monitoring the concentrations of active metabolites of 6-MP in RBC enables early identification of patients at increased risk of disease relapse due to inadequate levels of both metabolites. Confirming this conclusion requires further studies involving a larger group of children from various medical centers.
Ethical Considerations
Compliance with ethical guidelines
Approval for conducting the research was obtained from the Ethics Committee of Mazandaran University of Medical Sciences, Sari City, in Iran (Ethical Code: IR.MAZUMS.REC.1402.17134). Sufficient explanations regarding the purpose and procedure of the research were provided to the patients’ parents. After obtaining written informed consent from the parents, the patients were enrolled in the study. The method of obtaining consent varied among different age groups. For children under 7, informed consent was acquired from the parents. In children aged 7 to 15, informed consent was obtained from the legal guardian while ensuring that the child’s assent to participate in the study was obtained. However, for children aged 15 to 18, informed consent was obtained from both the child and their legal guardian. Thus, besides providing complete explanations to the child and the legal guardian, signatures from both parties were included in the informed consent form. Parents were assured that their children’s participation in the research was voluntary and that they could withdraw at any time if they wished to discontinue. It was emphasized that participation or non-participation in the study would not affect the level of care provided to the patient. Furthermore, participants were assured that their identities and information would remain confidential throughout the research process and in the publication of findings.
Funding
This research was conducted as a PhD dissertation of Omid Abed Khojasteh, approved by the Department of Clinical Pharmacy, Faculty of Pharmacy, Mazandaran University of Medical Sciences, Sari, Iran. This study was supported by a grant from Mazandaran University of Medical Sciences, Sari, Iran (Grant No.: 9399).
Authors contributions
Project administration: Ebrahim Salehifar; Supervision and visualization Ebrahim Salehifar, Seyed Jalal Hosseinimehr, Hossein Karami, Mohammad Naderi Sorki; Conceptualization: Ebrahim Salehifar, Seyed Jalal Hosseinimehr, Hossein Karami, Mohammad Naderi Sorki, and Hadi Darvish-Khezri; Methodology: Ebrahim Salehifar, Omid Abed Khojasteh, Seyed Jalal Hosseinimehr, Hossein Karami, Mohammad Naderi Sorki; Software: Omid Abed Khojasteh, Seyed Jalal Hosseinimehr, and Hadi Darvish-Khezri; Validation: Seyed Jalal Hosseinimehr; Formal analysis: Omid Abed Khojasteh, Seyed Jalal Hosseinimehr, and Hadi Darvish-Khezri; Data curation: Omid Abed Khojasteh, and Hadi Darvish-Khezri; Investigation and writing the original draft: Omid Abed Khojasteh; Resources: Hossein Karami, and Mohammad Naderi Sorki; Review and editing: Omid Abed Khojasteh, and Kimia Karami.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors express their gratitude to the children and their families for taking part in this research project. The authors also appreciate the medical and nursing professionals whose efforts made this work achievable.
References
- Ekpa QL, Akahara PC, Anderson AM, Adekoya OO, Ajayi OO, Alabi PO, et al. A Review of Acute Lymphocytic Leukemia (ALL) in the pediatric population: Evaluating Current trends and changes in guidelines in the past decade. Cureus. 2023; 15(12):e49930. [DOI:10.7759/cureus.49930]
- Kakaje A, Alhalabi MM, Ghareeb A, Karam B, Mansour B, Zahra B, et al. Rates and trends of childhood acute lymphoblastic leukaemia: An epidemiology study. Sci Rep. 2020; 10(1):6756. [DOI:10.1038/s41598-020-63528-0] [PMID]
- Desmond LN, Fletcher MB, Warrier RP. Fever and Leg Pain: Consider ALL the Diagnoses. Ochsner J. 2019; 19(3):260-3. [DOI:10.31486/toj.18.0122] [PMID]
- Crespo-Solis E, Espinosa-Bautista K, Alvarado-Ibarra M, Rozen-Fuller E, Pérez-Rocha F, Nava-Gómez C, et al. Survival analysis of adult patients with ALL in Mexico City: First report from the Acute Leukemia Workgroup (ALWG)(GTLA). Cancer Med. 2018; 7(6):2423-33. [DOI:10.1002/cam4.1513] [PMID]
- Mittra I, Pal K, Pancholi N, Shaikh A, Rane B, Tidke P, et al. Prevention of chemotherapy toxicity by agents that neutralize or degrade cell-free chromatin. Ann Oncol. 2017; 28(9):2119-27. [DOI:10.1093/annonc/mdx318] [PMID]
- García-Carro C, Draibe J, Soler MJ. Onconephrology: Update in anticancer drug-related nephrotoxicity. Nephron. 2023; 147(2):65-77. [DOI:10.1159/000525029] [PMID]
- Vincenzi B, Armento G, Spalato Ceruso M, Catania G, Leakos M, Santini D, et al. Drug-induced hepatotoxicity in cancer patients-implication for treatment. Expert Opin Drug Saf. 2016; 15(9):1219-38. [PMID]
- Lu X, Xie Y, Wang F. Application and analysis of 6-mercaptopurine nanomedicine in the treatment of leukemia. J Nanosci Nanotechnol. 2021; 21(2):1001-7. [DOI:10.1166/jnn.2021.18695] [PMID]
- Chabner BA, Longo DL. Cancer Chemotherapy, Immunotherapy and Biotherapy. Alphen aan den Rijn: Wolters Kluwer; 2015. [Link]
- Toksvang LN, Lee SHR, Yang JJ, Schmiegelow K. Maintenance therapy for acute lymphoblastic leukemia: Basic science and clinical translations. Leukemia. 2022; 36(7):1749-58. [DOI:10.1038/s41375-022-01591-4] [PMID]
- Fernández-Ramos AA, Marchetti-Laurent C, Poindessous V, Antonio S, Laurent-Puig P, Bortoli S, et al. 6-mercaptopurine promotes energetic failure in proliferating T cells. Oncotarget. 2017; 8(26):43048-60. [DOI:10.18632/oncotarget.17889] [PMID]
- Roberts RL, Wallace MC, Seinen ML, van Bodegraven AA, Krishnaprasad K, Jones GT, et al. Nonsynonymous polymorphism in guanine monophosphate synthetase is a risk factor for unfavorable thiopurine metabolite ratios in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2018; 24(12):2606-12. [DOI:10.1093/ibd/izy163] [PMID]
- Rios-Usuga C, Martinez-Gutierrez M, Ruiz-Saenz J. Antiviral potential of azathioprine and its derivative 6-mercaptopurine: A narrative literature review. Pharmaceuticals (Basel). 2024; 17(2):174. [DOI:10.3390/ph17020174] [PMID]
- Zamzami MA. Inosine triphosphate pyrophosphatase (ITPase): Functions, mutations, polymorphisms and its impact on cancer therapies. Cells. 2022; 11(3):384. [DOI:10.3390/cells11030384] [PMID]
- Nadhum SA, Kamoon RA, Mohammed MH. 6-mercaptopurine derivatives: Maintenance Therapy Of Acute Lymphoblastic Leukemia: A review. Biochem Cell Arch. 2020; 20(1):2091-9. [Link]
- Zhou Y, Wang L, Zhai XY, Wen L, Tang F, Yang F, et al. Precision therapy of 6-mercaptopurine in Chinese children with acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2020; 86(8):1519-27. [DOI:10.1111/bcp.14258] [PMID]
- Tiphaine Ade B, Hjalgrim LL, Nersting J, Breitkreutz J, Nelken B, Schrappe M, et al. Evaluation of a pediatric liquid formulation to improve 6-mercaptopurine therapy in children. Eur J Pharm Sci. 2016; 83:1-7. [DOI:10.1016/j.ejps.2015.12.002] [PMID]
- Adam de Beaumais T, Medard Y, Amblard O, Goldwirt L, Simonin M, Martinez Vinson C, et al. Improved HPLC quantification of 6-mercaptopurine metabolites in red blood cells: Monitoring data and literature analysis. Int J Mol Sci. 2022; 23(19):11885. [DOI:10.3390/ijms231911885] [PMID]
- Adam de Beaumais T, Jacqz-Aigrain E. Pharmacogenetics: Applications to pediatric patients. Adv Pharmacol. 2018; 83:191-215. [DOI:10.1016/bs.apha.2018.04.006] [PMID]
- Zhou Y, Wang L, Sun LR, Zhang L, Wang HM, Liu XT, et al. Individualized Use of 6-Mercaptopurine in Chinese Children with ALL: A multicenter randomized controlled trial. Clin Pharmacol Ther. 2024; 115(2):213-20. [DOI:10.1002/cpt.3061] [PMID]
- Vasta LM, Zanetti RC, Parekh DS, Warwick AB, Lieuw K. A retrospective review of mercaptopurine metabolism reveals high rate of patients with suboptimal metabolites successfully corrected with allopurinol. J Pediatr Hematol Oncol. 2021; 43(7):e1003-9. [DOI:10.1097/MPH.0000000000001939] [PMID]
- Abdelsayed ME, Maksoud AS, Sidhom I, Gad ZM, Hanafi SR. HPLC determination of the levels of 6-mercaptopurine metabolites suitable for the clinical risk assessment of its toxicity among Egyptian children with acute lymphocytic leukemia. J Anal Bioanal Tech. 2017; 8:358.[DOI:10.4172/2155-9872.1000358]
- Kamojjala R, Bostrom B. Allopurinol to prevent mercaptopurine adverse effects in children and young adults with acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2021; 43(3):95-100. [DOI:10.1097/MPH.0000000000002117] [PMID]
- Cohen G, Cooper S, Sison EA, Annesley C, Bhuiyan M, Brown P. Allopurinol use during pediatric acute lymphoblastic leukemia maintenance therapy safely corrects skewed 6-mercaptopurine metabolism, improving inadequate myelosuppression and reducing gastrointestinal toxicity. Pediatr Blood Cancer. 2020; 67(11):e28360. [DOI:10.1002/pbc.28360] [PMID]
- Han J, Mei S, Xu J, Zhang D, Jin S, Zhao Z, et al. Simultaneous UPLC-MS/MS Determination of 6-mercaptopurine, 6-methylmercaptopurine and 6-thioguanine in plasma: application to the pharmacokinetic evaluation of novel dosage forms in beagle dogs. Curr Pharm Des. 2020; 26(46):6013-20. [DOI:10.2174/1381612826999200820161343] [PMID]
- Zochowska D, Zegarska J, Hryniewiecka E, Samborowska E, Jazwiec R, Tszyrsznic W, et al. Determination of concentrations of azathioprine metabolites 6-thioguanine and 6-methylmercaptopurine in whole blood with the use of liquid chromatography combined with mass spectrometry. Transplant Proc. 2016; 48(5):1836-9. [DOI:10.1016/j.transproceed.2016.01.084] [PMID]
- Bayoumy AB, Ansari AR, Mulder CJJ, Schmiegelow K, Florin T, De Boer NKH. Innovating thiopurine therapeutic drug monitoring: A systematic review and meta-analysis on DNA-Thioguanine Nucleotides (DNA-TG) as an Inclusive Biomarker in Thiopurine Therapy. Clin Pharmacokinet. 2024; 63(8):1089-109. [DOI:10.1007/s40262-024-01393-0] [PMID]
- Du S, Huang X, He X, Mao M, Chen M, Zhang R, et al. Association of NUDT15 gene polymorphism with adverse reaction, treatment efficacy, and dose of 6-mercaptopurine in patients with acute lymphoblastic leukemia: A systematic review and meta-analysis. Haematologica. 2024; 109(4):1053-68. [DOI:10.3324/haematol.2023.282761] [PMID]
- Wang S, Qin Y, Wen Q, Xia Q, Gu R, Wang S, et al. Intestinal microbiota-mediated biotransformations alter the pharmacokinetics of the major metabolites of azathioprine in rats after oral administration. Drug Metab Pharmacokinet. 2022; 45:100458. [DOI:10.1016/j.dmpk.2022.100458] [PMID]
- Wong DR, Coenen MJ, Vermeulen SH, Derijks LJ, van Marrewijk CJ, Klungel OH, et al. Early assessment of thiopurine metabolites identifies patients at risk of thiopurine-induced leukopenia in inflammatory bowel disease. J Crohns Colitis. 2017; 11(2):175-84. [DOI:10.1093/ecco-jcc/jjw130] [PMID]
- Meijer B, Kreijne JE, van Moorsel SAW, Derijks LJJ, Bouma G, Mulder CJJ, et al. 6-methylmercaptopurine-induced leukocytopenia during thiopurine therapy in inflammatory bowel disease patients. J Gastroenterol Hepatol. 2017; 32(6):1183-90. [DOI:10.1111/jgh.13656] [PMID]
- Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Théorêt Y, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000; 118(4):705-13.[DOI:10.1016/S0016-5085(00)70140-5] [PMID]
- Chang JY, Cheon JH. Thiopurine therapy in patients with inflammatory bowel disease: A focus on metabolism and pharmacogenetics. Dig Dis Sci. 2019; 64:2395-403. [Link]
- Lampič K, Trontelj J, Prosen H, Drobne D, Šmid A, Vovk T. Determination of 6-thioguanine and 6-methylmercaptopurine in dried blood spots using liquid chromatography-tandem mass spectrometry: Method development, validation and clinical application. Clin Chim Acta. 2019; 499:24-33. [DOI:10.1016/j.cca.2019.08.024]
- Wojtuszkiewicz A, Barcelos A, Dubbelman B, De Abreu R, Brouwer C, Bökkerink JP, et al. Assessment of mercaptopurine (6MP) metabolites and 6MP metabolic key-enzymes in childhood acute lymphoblastic leukemia. Nucleosides Nucleotides Nucleic Acids. 2014; 33(4-6):422-33. [PMID]
- Sousa P, Estevinho MM, Dias CC, Ministro P, Kopylov U, Danese S, et al. Thiopurines’ metabolites and drug toxicity: A meta-analysis. J Clin Med. 2020; 9(7):2216. [DOI:10.3390/jcm9072216] [PMID]
- Brem R, Karran P. Oxidation-Mediated DNA cross-linking contributes to the toxicity of 6-thioguanine in human cells. Cancer Res. 2012; 72(18):4787-95. [DOI:10.1158/0008-5472.CAN-12-1278] [PMID]
- Zaza G, Cheok M, Yang W, Panetta JC, Pui CH, Relling MV, et al. Gene expression and thioguanine nucleotide disposition in acute lymphoblastic leukemia after in vivo mercaptopurine treatment. Blood. 2005; 106(5):1778-85. [DOI:10.1182/blood-2005-01-0143] [PMID]
- Abdelsayed E, Maksoud S, Sidhom I, Gad M, Hanafi R. HPLC Determination of the levels of 6-mercaptopurine metabolites suitable for the clinical risk assessment of its toxicity among Egyptian children with acute lymphocytic leukemia. J Anal Bioanal Tech. 2017; 8:2. [Link]
- Ribeiro AC, Barroso LHF, Mourao-Junior CA, Chebli JMF, Nascimento JWL. Simultaneous Monitoring of azathioprine metabolites in erythrocytes of crohn’s disease patients by HPLC-UV. J Chromatogr Sci. 2022; 60(6):518-24. [DOI:10.1093/chromsci/bmab084] [PMID]
- Jagt JZ, Pothof CD, Buiter HJC, van Limbergen JE, van Wijk MP, Benninga MA, et al. Adverse Events of thiopurine therapy in pediatric inflammatory bowel disease and correlations with metabolites: A cohort study. Dig Dis Sci. 2022; 67(1):241-51. [DOI:10.1007/s10620-021-06836-3] [PMID]
- Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K, et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011; 71(4):575-84. [DOI:10.1111/j.1365-2125.2010.03867.x] [PMID]
- Khokhar OS, Lewis JH. Hepatotoxicity of agents used in the management of inflammatory bowel disease. Dig Dis. 2010; 28(3):508-18. [DOI:10.1159/000320410] [PMID]
- Ooi CY, Bohane TD, Lee D, Naidoo D, Day AS. Thiopurine metabolite monitoring in paediatric inflammatory bowel disease. Aliment Pharmacol Ther. 2007; 25(8):941-7. [DOI:10.1111/j.1365-2036.2007.03278.x] [PMID]
- Bradford K, Shih DQ. Optimizing 6-mercaptopurine and azathioprine therapy in the management of inflammatory bowel disease. World J Gastroenterol. 2011; 17(37):4166-73. [DOI:10.3748/wjg.v17.i37.4166] [PMID]
- Czaja AJ. Review article: Opportunities to improve and expand thiopurine therapy for autoimmune hepatitis. Aliment Pharmacol Ther. 2020; 51(12):1286-304. [DOI:10.1111/apt.15743] [PMID]
- Supandi S, Harahap Y, Harmita H, Andalusia R. Quantification of 6-mercaptopurine and its metabolites in patients with acute lympoblastic leukemia using dried blood spots and UPLC-MS/MS. Sci Pharm. 2018; 86(2):18. [DOI:10.3390/scipharm86020018] [PMID]
- Abreu MT, Shaye O, Simon K, Poordad FF, Martin P, Vierling J, et al. Relationship of 6-Mp/Aza metabolite levels with hepatotoxicity in adult patients with Ibd: 357. Am J Gastroenterol. 2004; 99:S115. [Link]
Type of Study: Original Article |
Subject:
Clinical Pharmacy
Received: 2024/09/25 | Accepted: 2024/10/14 | Published: 2025/01/21
Received: 2024/09/25 | Accepted: 2024/10/14 | Published: 2025/01/21
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |