Volume 13, Issue 3 (7-2025)
J. Pediatr. Rev 2025, 13(3): 193-208 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehrabi H, Ghasemikhah R. Clinical Features, Diagnosis, and Treatment Plans of Balamuthia mandrillaris Encephalitis in Pediatrics: A Systematic Review. J. Pediatr. Rev 2025; 13 (3) :193-208
URL: http://jpr.mazums.ac.ir/article-1-689-en.html
URL: http://jpr.mazums.ac.ir/article-1-689-en.html
1- Student Research Committee, School of Medicine, Arak University of Medical Sciences, Arak, Iran.
2- Infectious Disease Research Center (IDRC), School of Medicine, Arak University of Medical Sciences, Arak, Iran. & Department of Parasitology and Mycology, School of Medicine, Arak University of Medical Sciences, Arak, Iran. ,ghasemikhah@gmail.com
2- Infectious Disease Research Center (IDRC), School of Medicine, Arak University of Medical Sciences, Arak, Iran. & Department of Parasitology and Mycology, School of Medicine, Arak University of Medical Sciences, Arak, Iran. ,
Full-Text [PDF 1140 kb]
(598 Downloads)
| Abstract (HTML) (828 Views)
Full-Text: (473 Views)
Introduction
Encephalitis is an acute and progressive infection of the brain parenchyma, which can lead to neurological deficits and even death. This infection occurs in both adults and children, and a wide range of etiologies cause encephalitis, including viral, bacterial, autoimmune agents, and parasites. Encephalitis usually manifests as altered consciousness, abnormal behaviors, seizures, fever, and headaches. Diagnosis is typically made by neuroimaging, laboratory analysis of cerebrospinal fluid (CSF) (protein levels, glucose levels, blood cell counts), and electroencephalography (EEG). Treatment differs in each case based on the etiology of encephalitis. Antiviral agents, antibiotics, and corticosteroid therapy are mostly used [1].
Balamuthia mandrillaris is a free-living amoeba and an opportunistic protozoan pathogen. This pathogen can lead to a severe encephalitis with an extremely high mortality rate (>98%) [2]. The organism’s life cycle includes dormant cysts and vegetative trophic stages. Its trophozoites measure 15 to 50 μm and have round nuclei with a dense nucleolus and a cytoplasm containing empty vacuoles [3].
Some studies have shown that B. mandrillaris can be isolated from soil and water; however, this amoeba is very difficult to isolate and culture [3-5]. Studies have suggested that contact with contaminated soil is a major risk factor for Balamuthia amoebic encephalitis (BAE) [5]. Visvesvara et al. reported the first human B. mandrillaris infection in 1993 [6], and to date, the number of BAE reported cases is more than 300 globally, which also includes pediatrics [7, 8]. It does not matter whether the host is immunocompromised or immunocompetent; this amoeba can infect both groups [9]. Pediatric patients may present differently compared to adults, and also their immune response and treatment tolerability can be significantly different, which highlights the need for a child-specific analysis. Based on case reports, only less than ten infected children have survived worldwide. Diagnosis is also challenging due to insufficient awareness and inefficient health systems [10]. Despite the severity of this infection, especially in pediatrics, there is no comprehensive summary of published case reports in the literature. Therefore, it is important to be aware of manifestations, paraclinical findings, and possible treatments for BAE, especially in early stages. This systematic review evaluated pediatric patients (0–18 years) diagnosed with BAE, examining clinical presentations, diagnostic methods (including polymerase chain reaction (PCR), imaging, and CSF analysis), and pharmacological treatments (miltefosine, pentamidine, fluconazole). Only case reports and cross-sectional studies in English were included.
Methods
Search strategy and data sources
This study is structured as a systematic review that followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist. The review protocol was registered in the PROSPERO database under registration number 1034394. On July 23 and 24, 2023, PubMed, Web of Science, Scopus, and ScienceDirect databases were searched using keywords “Balamuthia mandrillaris”, “encephalitis”, “Balamuthia mandrillaris encephalitis”, “pediatrics”, “neonatal”, “infant”, and “children”. To find more articles, Google Scholar was also searched, and the first 250 results were screened. The search in all databases was finally updated on July 26, 2023. A systematic search in PubMed was conducted using a combination of Medical Subject Headings (MeSH) terms and free-text keywords related to Balamuthia encephalitis in pediatric patients.
The search terms used included (“Balamuthia”[MeSH Terms] OR “Balamuthia”[All Fields]) AND (“encephalitis”[MeSH Terms] OR “encephalitis”[All Fields]) AND (“child”[MeSH Terms] OR “pediatrics”[MeSH Terms] OR “child”[All Fields] OR “pediatric”[All Fields]).
Only peer-reviewed, published papers were included in this review. Grey literature, preprints, and unpublished data were not included in this study.
Data extraction
All the retrieved articles were entered into EndNote software, version 20.2.1 and screening of all articles based on title and abstract was carried out considering the inclusion and exclusion criteria. The remaining studies were then evaluated on a full-text basis. Additionally, reference lists of the included literature were also reviewed, and if they met the inclusion criteria, they were included in our study. The first author (Hoda Mehrabi) independently was responsible for checking search terms and strategy, and it was confirmed and supervised by the second author (Reza Ghasemikhah).
Inclusion and exclusion criteria
Inclusion criteria consisted of English-language case reports and cross-sectional articles reporting patients with encephalitis due to B. mandrillaris in the age range of neonates up to 18 years. There were no restrictions regarding nationality, race, ethnicity, number of individuals, or gender.
Exclusion criteria included irrelevant and duplicate reports, quasi-experimental articles, articles whose full text was not available or published, and articles in which cases were older than 18 years.
Quality assessment
The quality of the included case reports and case series was independently assessed by two authors using the Joanna Briggs Institute (JBI) critical appraisal checklists. Discrepancies were resolved through discussion or consultation with another reviewer.
General considerations
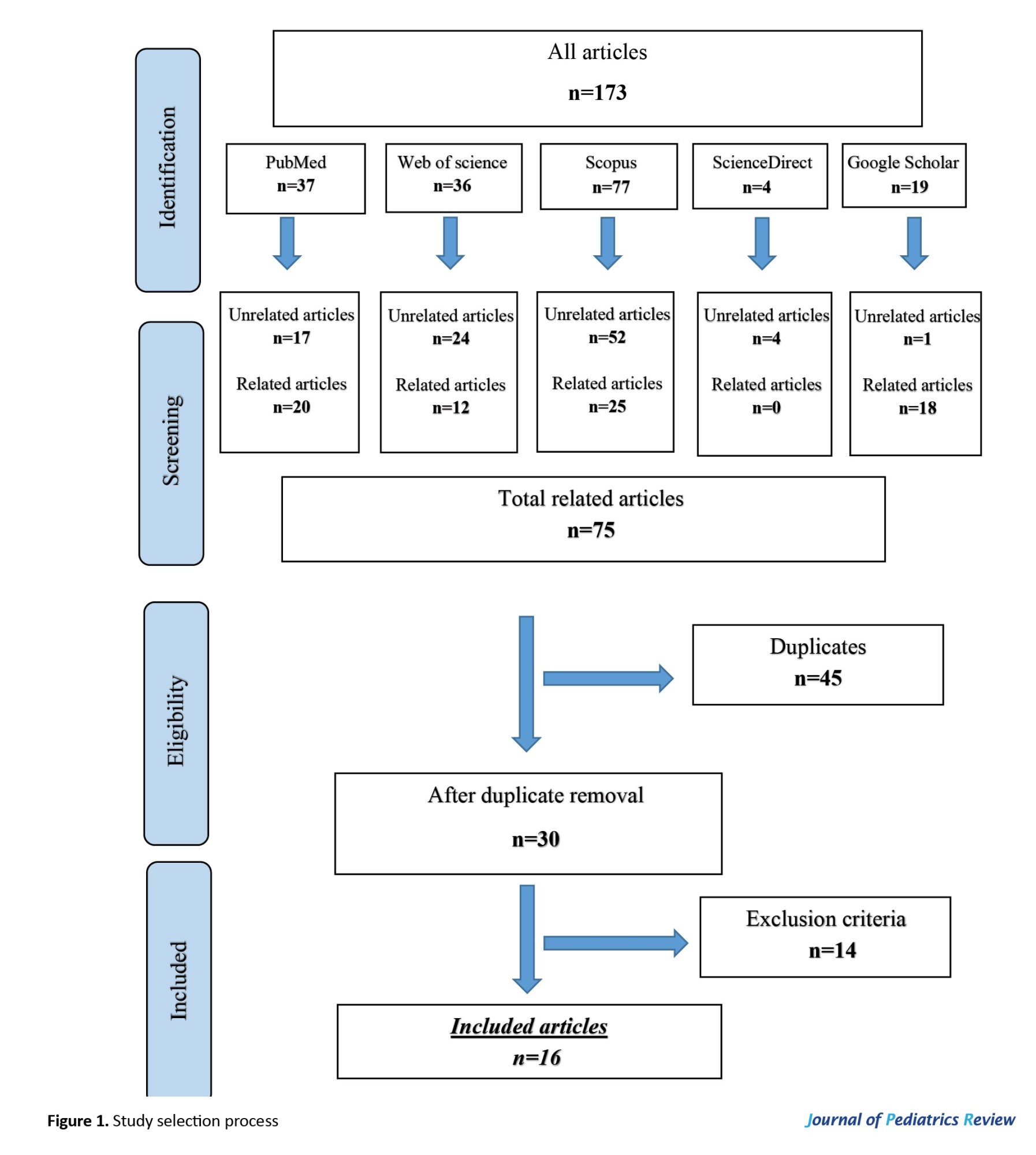
Based on the paper selection stages (Figure 1), 880 articles were identified after searching Scopus, Web of Science, PubMed, ScienceDirect, and Google Scholar. Some unrelated papers (717) and related articles (163) were identified, among which 111 were duplicates. In the end, 34 papers out of 52 articles met the inclusion criteria and entered into our study.
Assessment of publication bias
Due to the descriptive nature of the included articles (case reports and case series) and the absence of quantitative data, which were suitable for meta-analysis, formal assessment of publication bias was not feasible.
Data synthesis
Because of the heterogeneity of the included articles in terms of patient characteristics, clinical presentations, diagnostic methods, and treatment plans, no meta-analysis was conducted. Instead, a narrative synthesis was performed. Relevant data were summarized in tables, which highlight clinical presentations, diagnostic findings, treatment approaches, and outcomes across pediatric cases.
Results
Study selection
A total of 163 records were identified through database search, and after removing 111 duplicates, 52 articles remained for screening of title and abstract, and then full text. Of these, 34 studies were included in this systematic review. The study selection process is illustrated in Figure 1.
Due to methodological and clinical heterogeneity across the included case reports, a meta-analysis was not feasible. Instead, a narrative descriptive synthesis was evaluated, summarizing the demographic, clinical, diagnostic, and therapeutic data across articles. Because of the limited number of included studies and variability in reported data, no pooled effect estimates or confidence intervals were calculated.
Risk of bias assessment
The methodological quality of the included case reports and case series was evaluated using the JBI critical appraisal tool. Overall, the studies demonstrated high methodological rigor. All included literature met the minimum quality standards required for inclusion in this systematic review.
Demographic information
A total of 57 pediatric patients were included in this study, with 5 missing data points regarding the sex of the patients. In the 52 cases with known sex, 36 patients were male (69%) and 16 patients were female (32%) (Table 1).
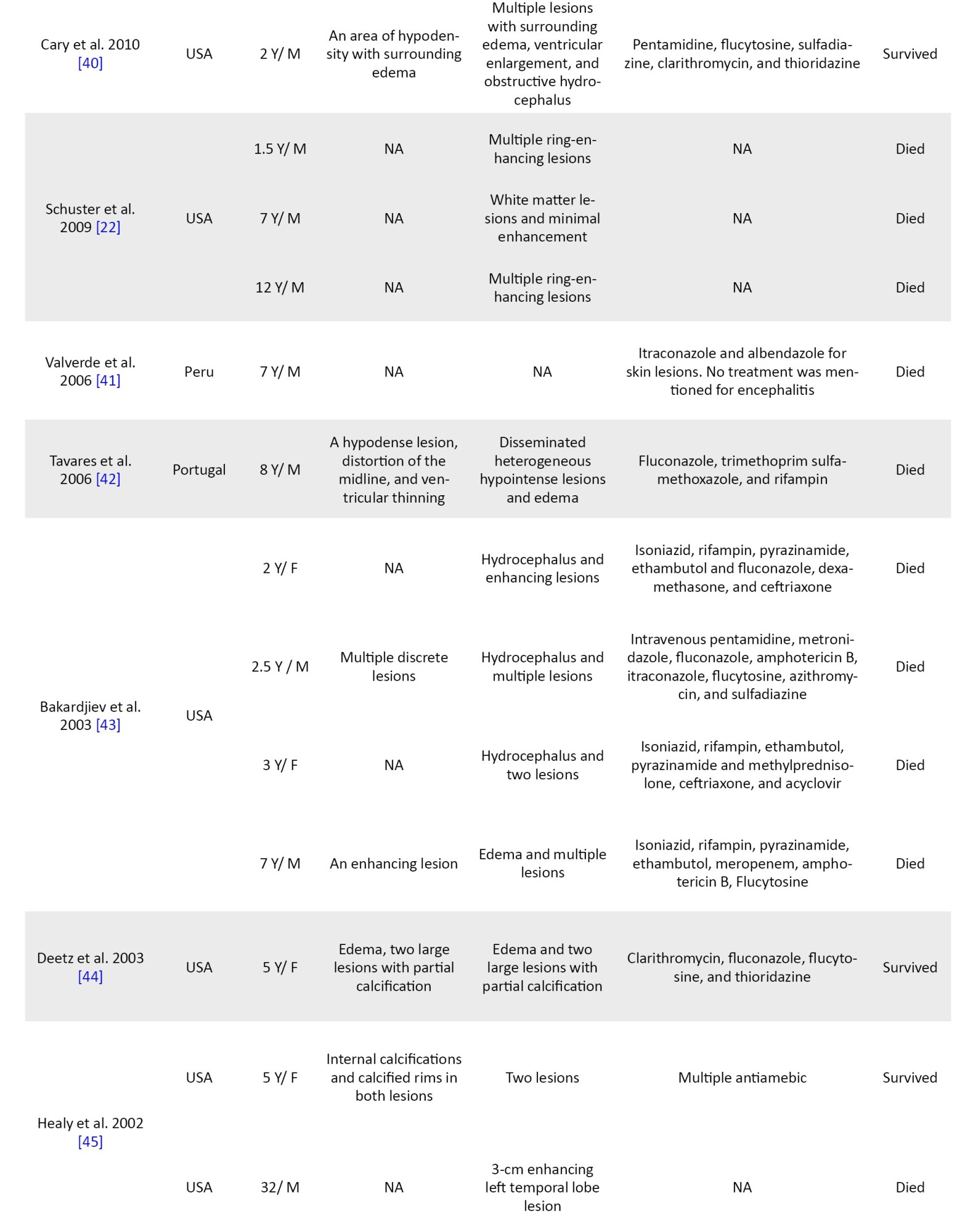
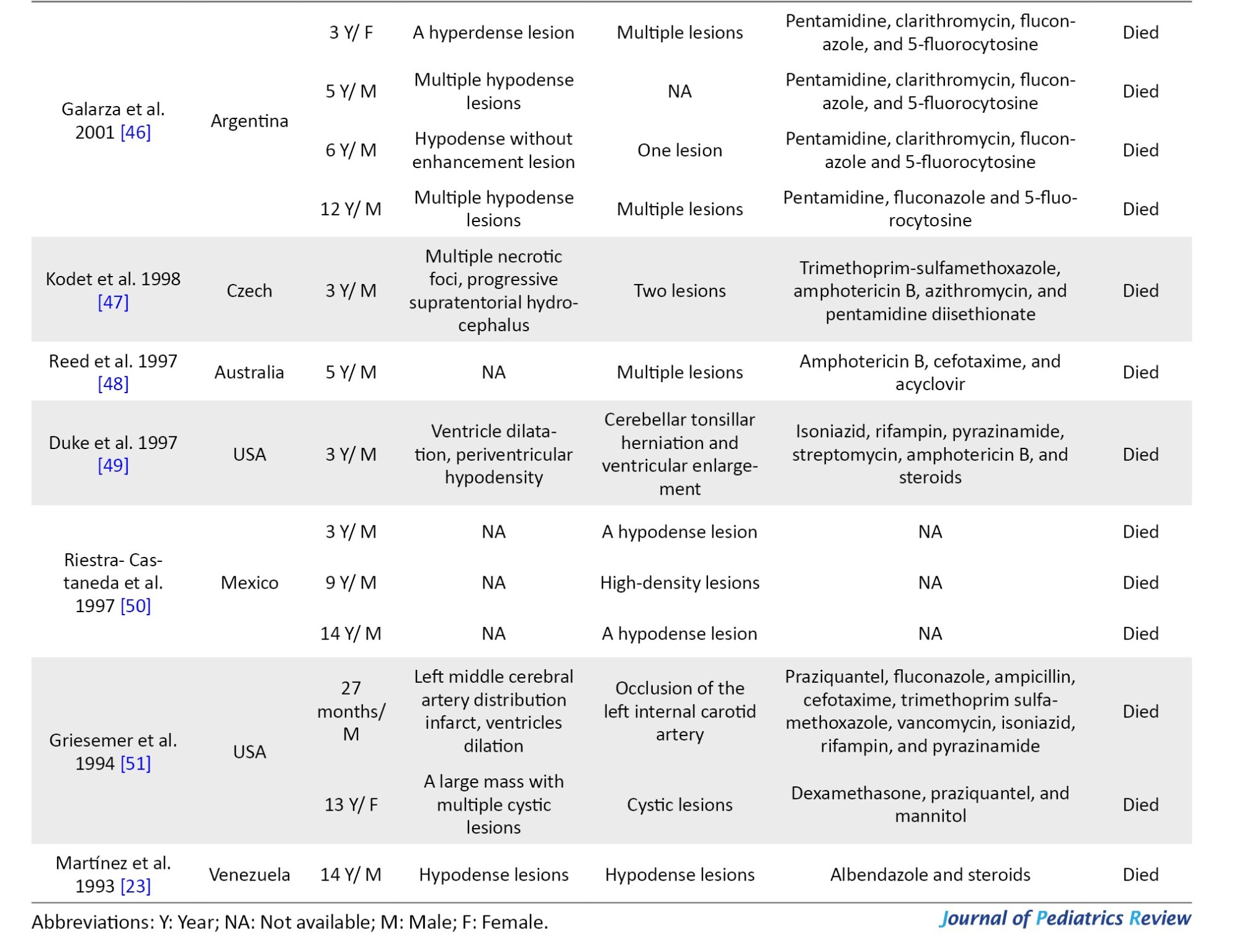
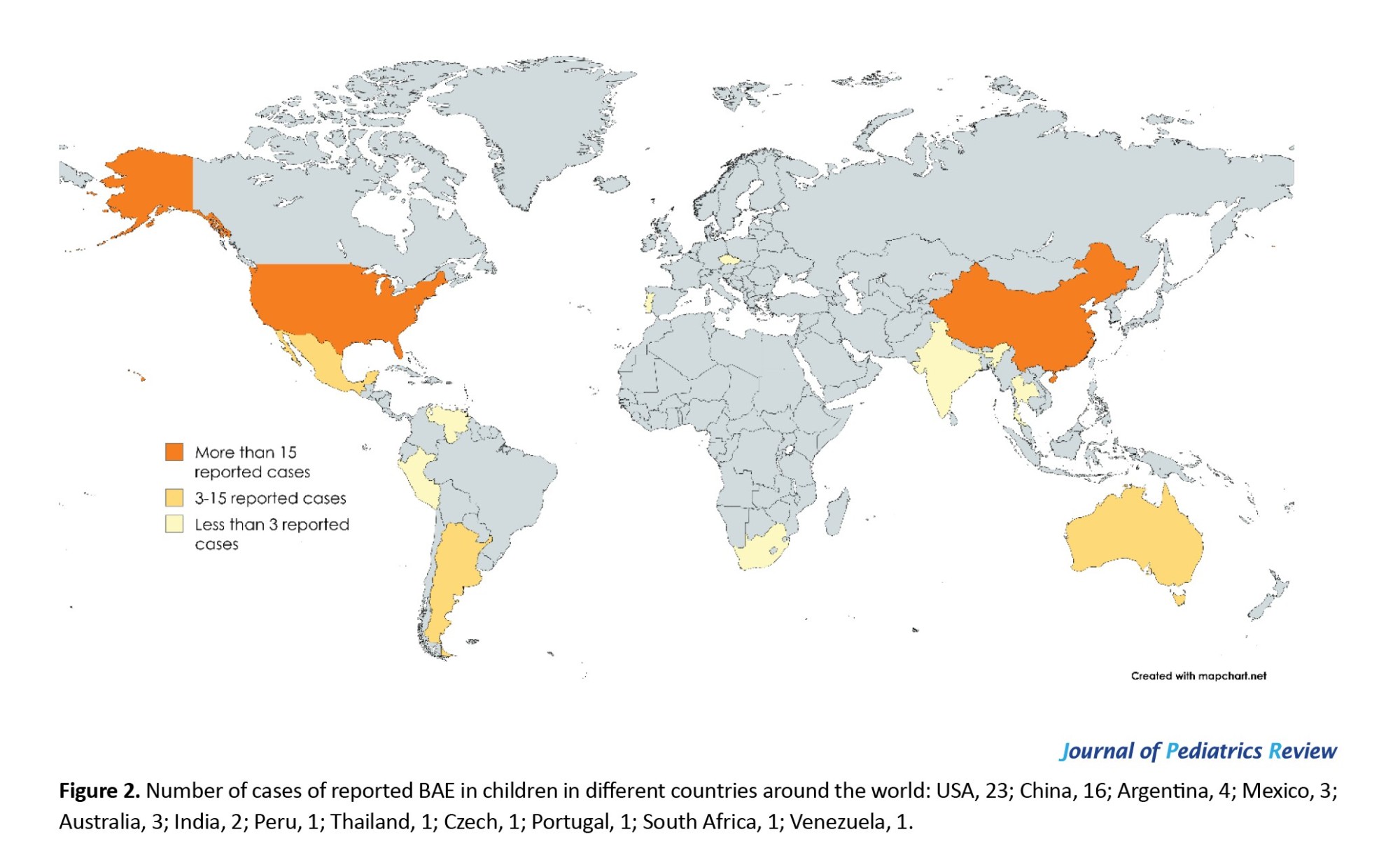
The mean age of the patients was 7.8 years (pediatric age range: 0–18 years). There is a variety of countries represented, and this infection has been reported on all continents (Figure 2).
Manifestations
Articles have mentioned different manifestations in infected children, with the most common ones reported as follows:
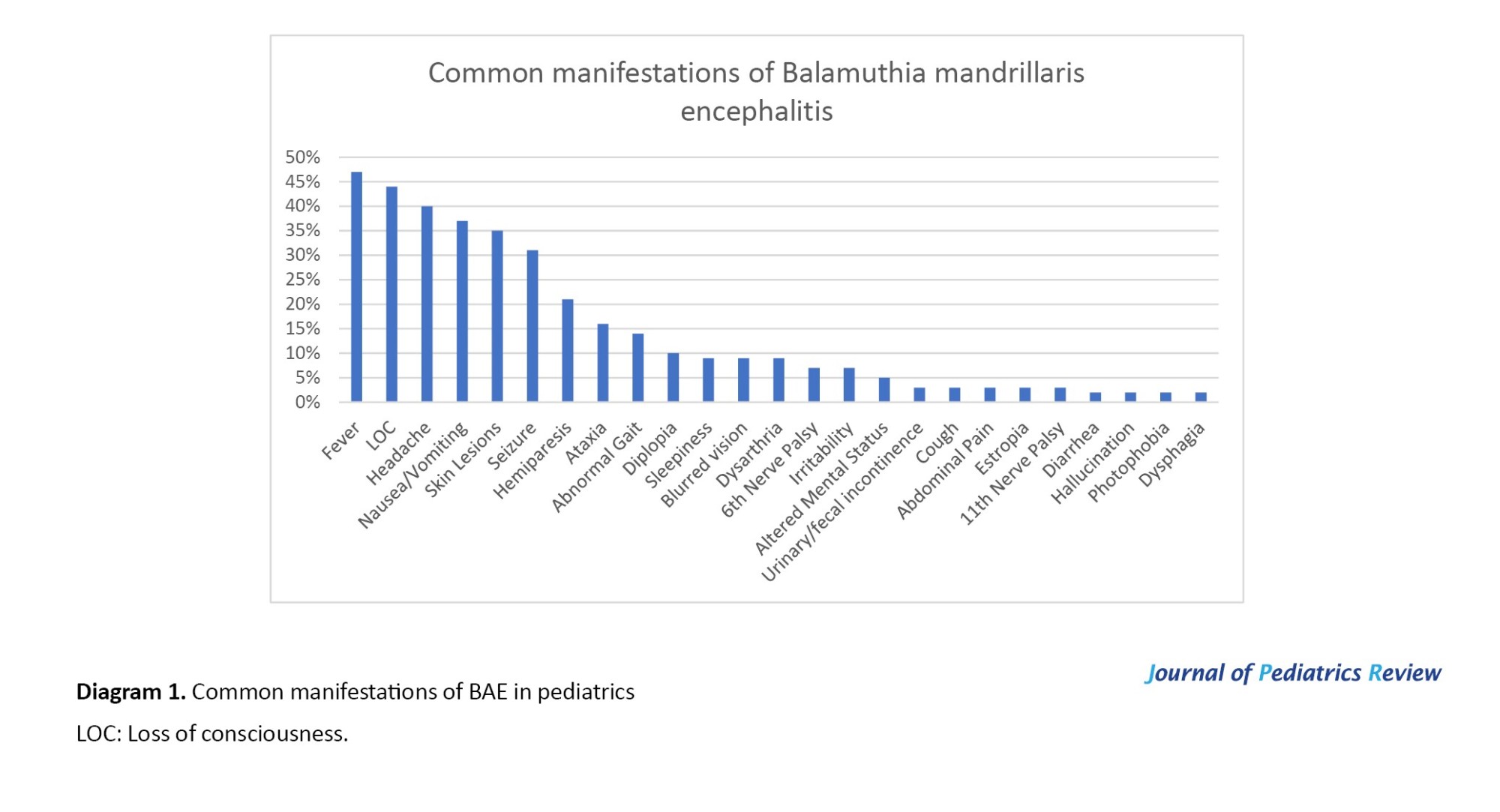
Fever (in 27 cases), headache (in 23 cases), loss of consciousness (in 25 cases), seizures (in 18 cases), hemiparesis (in 12 cases), and others (Figure 1). These symptoms are usually due to increased intracranial pressure (ICP), which is caused by B. mandrillaris infection in the central nervous system.
Research has suggested two patterns of manifestations for this encephalitis. In the first pattern, patients developed specific skin lesions mainly on their faces months or years before the onset of encephalitis; this type has been reported mostly in China and Peru [10, 11]. In our study, we observed that 35% of cases experienced skin lesions before or during the occurrence of encephalitis. In the second pattern, patients presented with encephalitis without any signs of cutaneous lesions, which has usually been reported in the USA [12].
Diagnosis
Confirmatory method
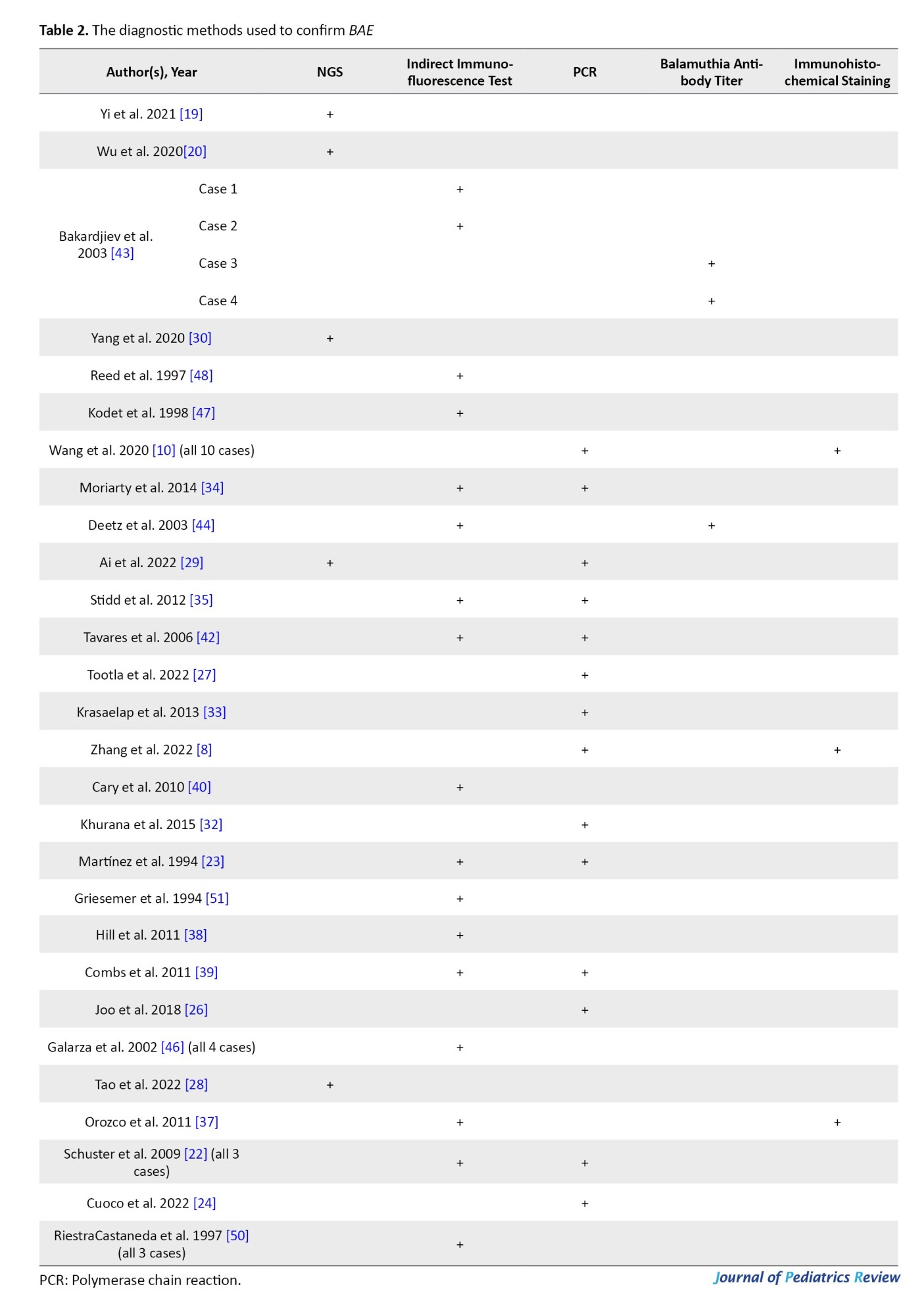
Depending on the date of each case report, various diagnostic methods have been used to confirm the infection. Confirmatory techniques are reported as follows: next-generation sequencing (NGS) on CSF (in 5 cases), indirect immunofluorescence test on brain tissue (in 24 cases), serum or CSF antibody titer (in 3 cases), PCR (on CSF, brain tissue, or skin lesion) (in 25 cases), and immunohistochemical staining (in 3 cases). It is noteworthy that in some reports, more than one confirmatory test has been performed (Table 2).
CSF analysis
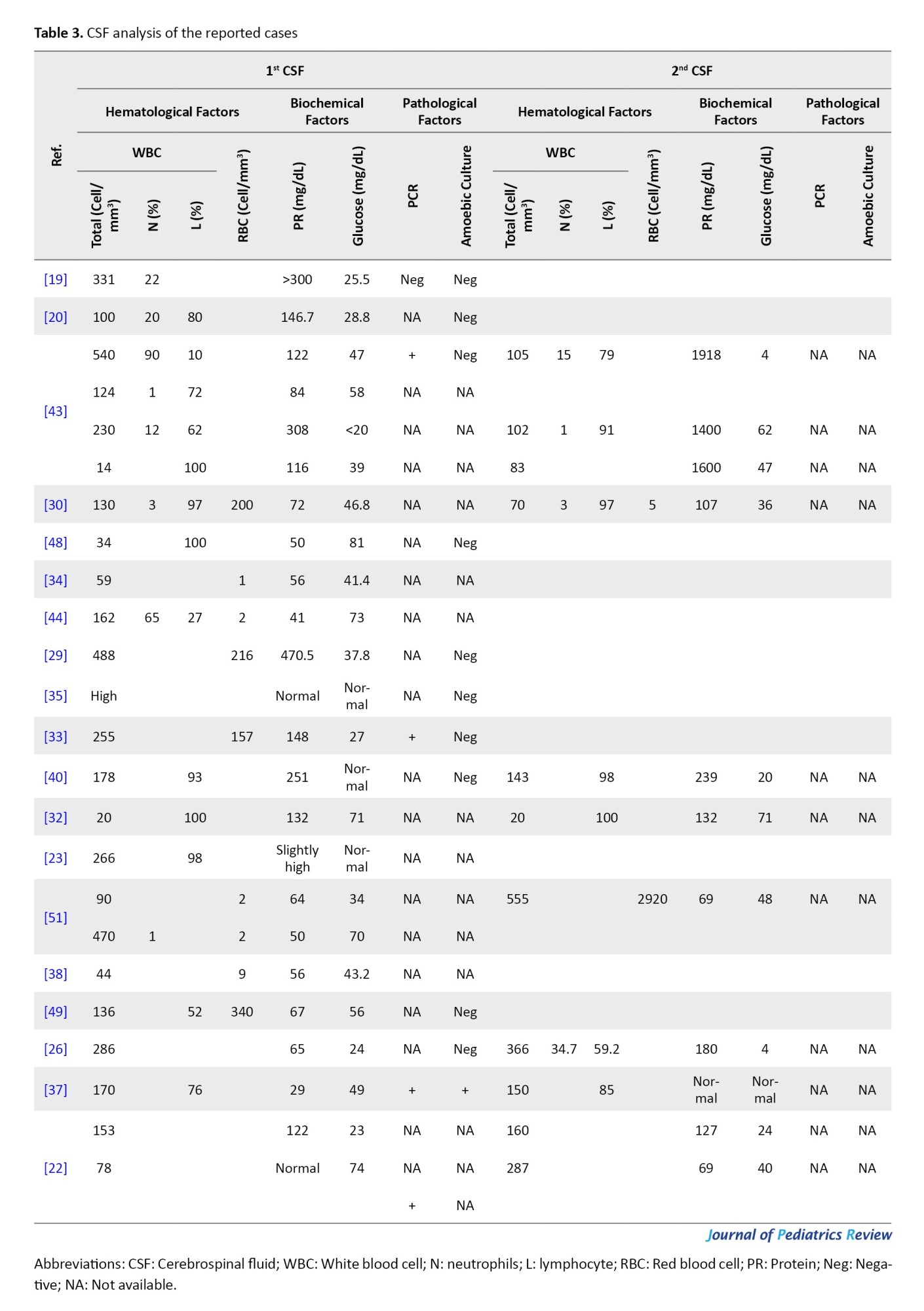
CSF analysis was reported in detail for 25 cases. Leukocytosis was observed in all cases. The maximum and minimum counts of CSF leukocytes were 555 and 14 cells/mm³, which belonged to a 27-month-old boy and a 2.5-year-old boy, respectively. Lymphocyte dominance was detected in almost all the tests performed. The CSF protein level was high (more than 45 mg/dL) in all patients except for four, who had normal protein levels. The glucose level was generally low, except in four tests in which normal levels were reported (Table 3).
Computed tomography (CT) scan and magnetic resonance imaging (MRI) findings
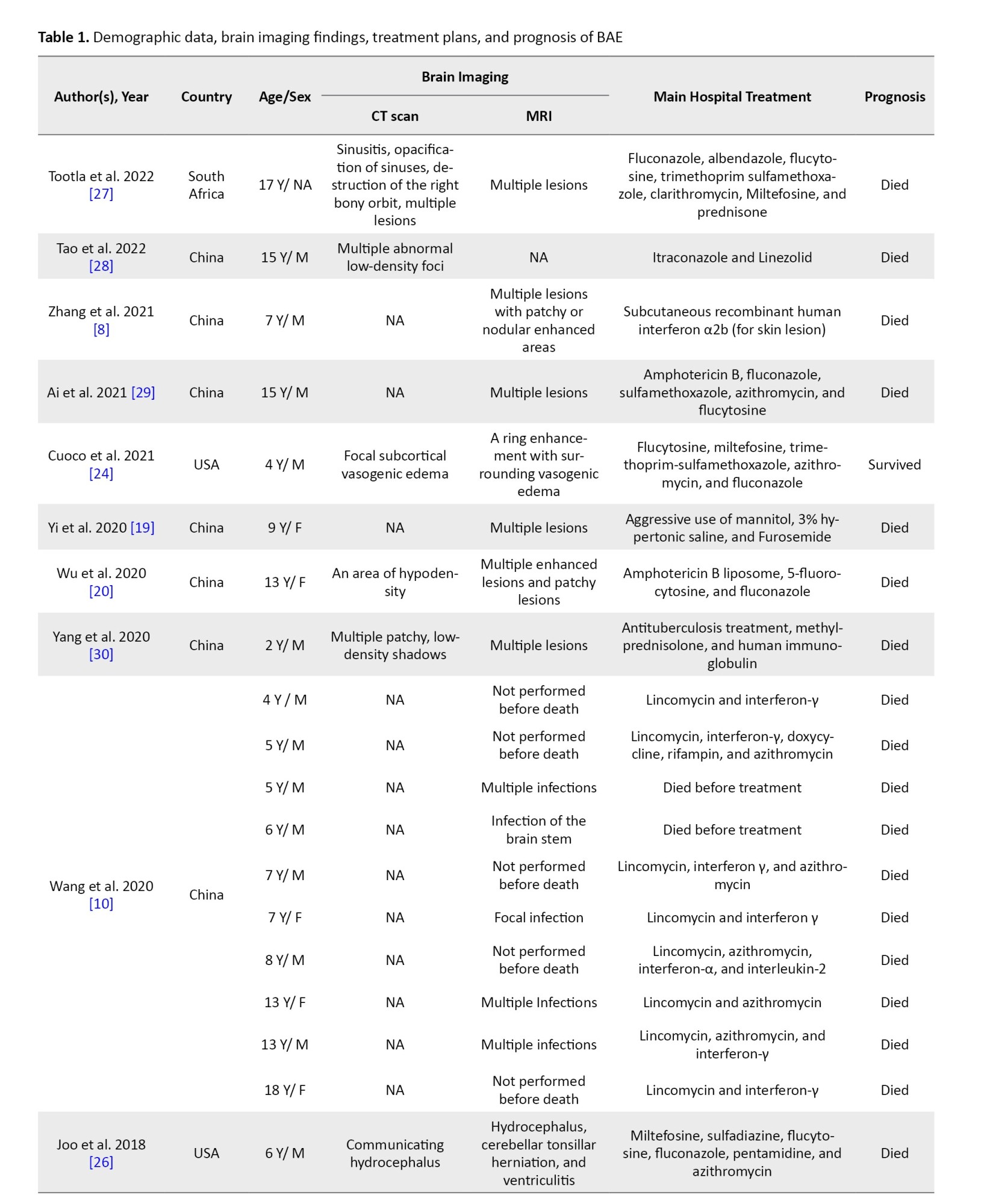
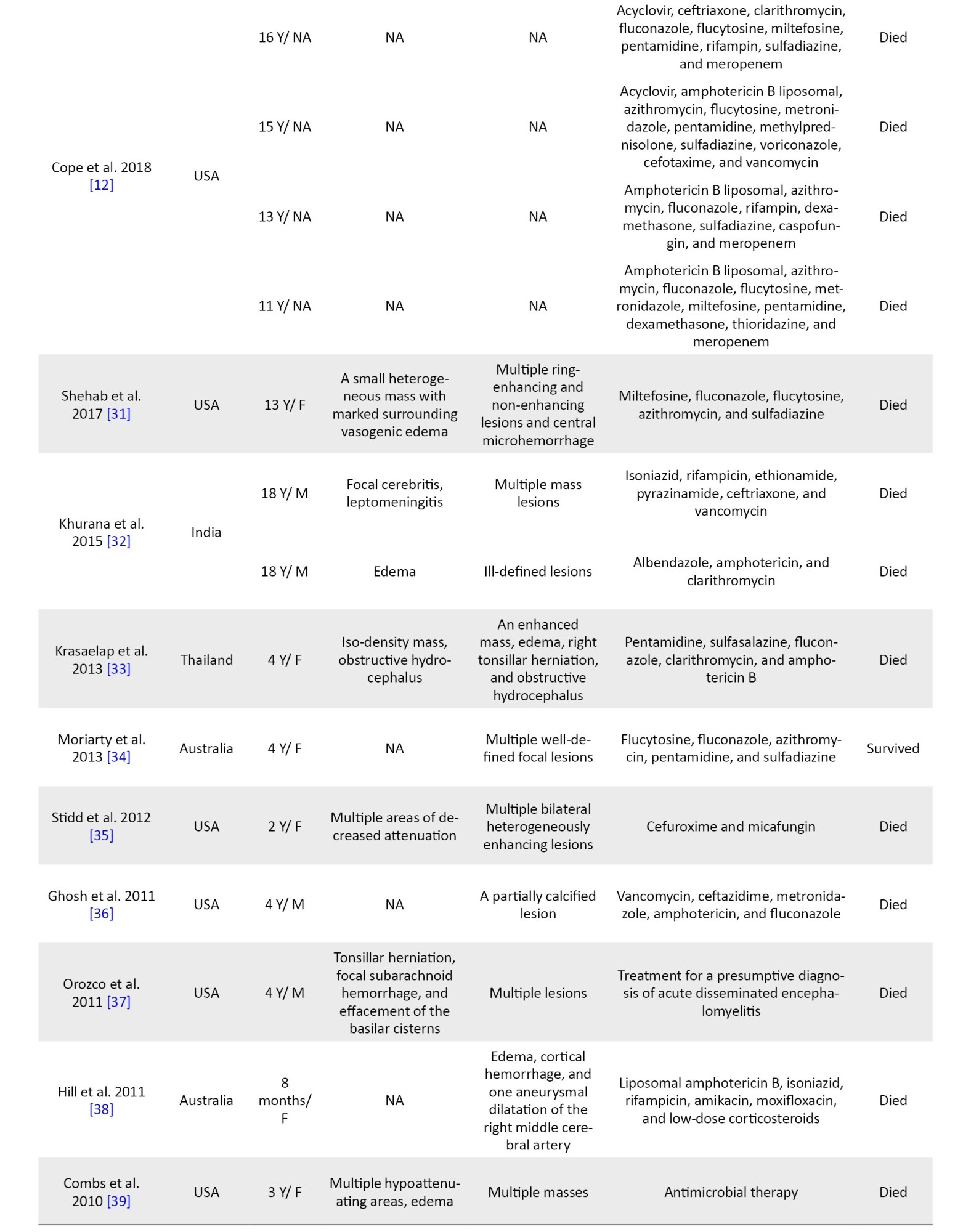
In 27 cases, CT scan findings were mentioned. The most common findings were hyperdense or hypodense lesions (s) (13 cases), brain edema (6 cases), and hydrocephalus (3 cases). In 45 patients, the MRI findings are mentioned. The most common MRI finding was lesions (in 34 patients) (Table 1).
Therapy
Treatment details were available for 49 cases (Table 1). Among the surviving patients, the treatment included multiple drugs, such as flucytosine, fluconazole, pentamidine, sulfadiazine, clarithromycin, thioridazine, miltefosine, trimethoprim-sulfamethoxazole, and azithromycin.
In other cases, based on an unknown diagnosis in early stages, different empirical treatments were administered. For instance, anti-tuberculosis therapy was used in 7 cases, but none of them led to survival. Amphotericin B was also used in 12 patients, all of whom experienced fatal outcomes (Table 1). Due to the limited number and variability of cases, statistical comparison of treatment results could not be conducted, and a descriptive overview of the therapies and their associated outcomes is provided.
Discussion
Balamuthia mandrillaris is a free-living amoeba responsible for a rare but fatal encephalitis in healthy and immunosuppressed hosts. Its cysts exist in soil and water, and this amoeba can infect humans via the skin, olfactory nerve, or pulmonary system [13, 14].
Encephalitis manifestations caused by B. mandrillaris are similar to other causes, and fever, headache, and seizures may be observed. These similar symptoms may lead specialists to misdiagnosis, and they might consider this infection tuberculoid, viral, or bacterial encephalitis.
We compared the demographic data, clinical symptoms, diagnostic tools, and treatment plans of pediatric patients with encephalitis caused by B. mandrillaris. This is the first systematic review globally intended to explore BAE in children. The age range of reported cases was from an 8-month-old female infant to an 18-year-old female teenager. The majority of cases were male children. The infection was mostly reported in the USA and China, and Africa had the fewest reported cases. It seems that the less-diagnosed cases in every area are related to the lack of awareness and inappropriate diagnostic tools. It is known that B. mandrillaris spreads and transmits via inhalation or injection from injured skin. It can reach the patient’s CNS through blood flow. Another theory for the transmission of this amoeba is the inhalation of airborne cysts [15]. In this study, probable risk factors were reported in 22 children, of which the most common are traumas leading to skin injury (12 cases) and contact with infected water (3 cases – swimming or flood).
Diagnosing this amoeba is still challenging for healthcare systems because of the lack of knowledge about this infection. Due to this condition, there was a delay and misdiagnosis in most cases, and some patients presented with severe manifestations.
Different diagnostic methods, such as NGS, PCR, serological tests, biological tests, immunohistochemical assays, biopsy, and special staining methods for brain or skin lesions, have been used to confirm BAE. However, many of these tests do not need a specialty and are unavailable in some areas [8].
In our study, the most common confirming diagnostic tool was PCR on the involved body tissues. PCR on CSF, brain tissue, or skin lesions is a rapid and sensitive diagnostic tool to detect B. mandrillaris. It can be useful in clinical diagnosis and has been developed recently [16].
ELISA and indirect immunofluorescence assay are two serological methods that detect B. mandrillaris. Based on some studies, positive serology is a good way to diagnose BAE, but it cannot always be a certain tool to indicate a diagnosis [17].
Another agent for detecting this amoeba is indirect immunofluorescence using rabbit anti-Balamuthia spp. serum or a rabbit antiserum-based immunohistochemical assay on brain or skin tissue samples [18].
NGS is a new, noninvasive, and rapid method for diagnosing BAE (used in 5 patients) [19]. Wu et al. could diagnose BAE in a 13-year-old Chinese girl via NGS of CSF [20]. Yi et al. confirmed the presence of this amoeba using metagenomic NGS of CSF [19].
Neuroimaging studies, such as CT scans and MRIs, have shown some abnormalities, such as multiple hypodense and hyperdense lesions and edema. This is consistent with findings from Piper et al. [21].
In the CSF findings, lymphocytic pleocytosis, elevated protein levels, and low glucose have been predominantly reported. Overall, no significant signs in CSF analysis distinguish BAE from other encephalitis causes [8].
Until now, a definitive treatment method for this infection has not been established. Based on a study, the average survival time for patients admitted to the hospital is about 16 days, which indicates the poor prognosis of BAE [22]. Combination therapy is suggested, and in surviving cases, it includes multiple drugs, such as flucytosine, fluconazole, pentamidine, sulfadiazine, Clarithromycin, thioridazine, miltefosine, trimethoprim-sulfamethoxazole, and azithromycin. Miltefosine is a drug approved by the US Food and Drug Administration (FDA) for leishmaniasis treatment in 2013. It has been found useful against B. mandrillaris in vitro in combination with other drugs [23]. In this study, we found that milfetosin (in combination with other drugs) was used in 6 cases, but only one patient survived (Table 1) [24].
More investigation is needed to establish the appropriate and certain guidelines for this disease and its combination therapy. It should be noted that intracranial pressure should decrease as soon as possible in cases of high intracranial pressure. It has been found that steroids are not indicated because they can trigger both skin and CNS lesions [25].
In conclusion, further investigation is necessary to improve diagnosis and develop more effective therapies. Although it is rare and fatal, early detection and appropriate treatment can provide a chance of survival [26].
Conclusion
The manifestations of BAE may resemble those of any other encephalitis, which could lead to a loss of critical time and misdiagnosis, potentially resulting in the death of patients. Thus, it is recommended that every relevant specialist gain more knowledge and awareness of this lethal infection to facilitate early diagnosis and the best treatment plan. Further investigations into this infection and the development of more effective treatments are recommended to improve the survival chances of these patients.
Limitations
This systematic review has several limitations. First, all the included literature consisted of case reports and case series, which carry a risk of bias due to selective reporting and also lack of a control group. Second, the heterogeneity across articles in terms of patient demographics, diagnostic tools, and treatment regimens limited the ability to conduct a meta-analysis. Additionally, the small sample size in most articles restricted the generalizability of the results.
Despite these limitations, our review highlights the need for early diagnosis and more efficient treatment plans for this encephalitis.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or nonprofit sectors.
Authors contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflicts of interest
The authors declared no conflict of interest.
References
Encephalitis is an acute and progressive infection of the brain parenchyma, which can lead to neurological deficits and even death. This infection occurs in both adults and children, and a wide range of etiologies cause encephalitis, including viral, bacterial, autoimmune agents, and parasites. Encephalitis usually manifests as altered consciousness, abnormal behaviors, seizures, fever, and headaches. Diagnosis is typically made by neuroimaging, laboratory analysis of cerebrospinal fluid (CSF) (protein levels, glucose levels, blood cell counts), and electroencephalography (EEG). Treatment differs in each case based on the etiology of encephalitis. Antiviral agents, antibiotics, and corticosteroid therapy are mostly used [1].
Balamuthia mandrillaris is a free-living amoeba and an opportunistic protozoan pathogen. This pathogen can lead to a severe encephalitis with an extremely high mortality rate (>98%) [2]. The organism’s life cycle includes dormant cysts and vegetative trophic stages. Its trophozoites measure 15 to 50 μm and have round nuclei with a dense nucleolus and a cytoplasm containing empty vacuoles [3].
Some studies have shown that B. mandrillaris can be isolated from soil and water; however, this amoeba is very difficult to isolate and culture [3-5]. Studies have suggested that contact with contaminated soil is a major risk factor for Balamuthia amoebic encephalitis (BAE) [5]. Visvesvara et al. reported the first human B. mandrillaris infection in 1993 [6], and to date, the number of BAE reported cases is more than 300 globally, which also includes pediatrics [7, 8]. It does not matter whether the host is immunocompromised or immunocompetent; this amoeba can infect both groups [9]. Pediatric patients may present differently compared to adults, and also their immune response and treatment tolerability can be significantly different, which highlights the need for a child-specific analysis. Based on case reports, only less than ten infected children have survived worldwide. Diagnosis is also challenging due to insufficient awareness and inefficient health systems [10]. Despite the severity of this infection, especially in pediatrics, there is no comprehensive summary of published case reports in the literature. Therefore, it is important to be aware of manifestations, paraclinical findings, and possible treatments for BAE, especially in early stages. This systematic review evaluated pediatric patients (0–18 years) diagnosed with BAE, examining clinical presentations, diagnostic methods (including polymerase chain reaction (PCR), imaging, and CSF analysis), and pharmacological treatments (miltefosine, pentamidine, fluconazole). Only case reports and cross-sectional studies in English were included.
Methods
Search strategy and data sources
This study is structured as a systematic review that followed the preferred reporting items for systematic reviews and meta-analysis (PRISMA) checklist. The review protocol was registered in the PROSPERO database under registration number 1034394. On July 23 and 24, 2023, PubMed, Web of Science, Scopus, and ScienceDirect databases were searched using keywords “Balamuthia mandrillaris”, “encephalitis”, “Balamuthia mandrillaris encephalitis”, “pediatrics”, “neonatal”, “infant”, and “children”. To find more articles, Google Scholar was also searched, and the first 250 results were screened. The search in all databases was finally updated on July 26, 2023. A systematic search in PubMed was conducted using a combination of Medical Subject Headings (MeSH) terms and free-text keywords related to Balamuthia encephalitis in pediatric patients.
The search terms used included (“Balamuthia”[MeSH Terms] OR “Balamuthia”[All Fields]) AND (“encephalitis”[MeSH Terms] OR “encephalitis”[All Fields]) AND (“child”[MeSH Terms] OR “pediatrics”[MeSH Terms] OR “child”[All Fields] OR “pediatric”[All Fields]).
Only peer-reviewed, published papers were included in this review. Grey literature, preprints, and unpublished data were not included in this study.
Data extraction
All the retrieved articles were entered into EndNote software, version 20.2.1 and screening of all articles based on title and abstract was carried out considering the inclusion and exclusion criteria. The remaining studies were then evaluated on a full-text basis. Additionally, reference lists of the included literature were also reviewed, and if they met the inclusion criteria, they were included in our study. The first author (Hoda Mehrabi) independently was responsible for checking search terms and strategy, and it was confirmed and supervised by the second author (Reza Ghasemikhah).
Inclusion and exclusion criteria
Inclusion criteria consisted of English-language case reports and cross-sectional articles reporting patients with encephalitis due to B. mandrillaris in the age range of neonates up to 18 years. There were no restrictions regarding nationality, race, ethnicity, number of individuals, or gender.
Exclusion criteria included irrelevant and duplicate reports, quasi-experimental articles, articles whose full text was not available or published, and articles in which cases were older than 18 years.
Quality assessment
The quality of the included case reports and case series was independently assessed by two authors using the Joanna Briggs Institute (JBI) critical appraisal checklists. Discrepancies were resolved through discussion or consultation with another reviewer.

General considerations
Based on the paper selection stages (Figure 1), 880 articles were identified after searching Scopus, Web of Science, PubMed, ScienceDirect, and Google Scholar. Some unrelated papers (717) and related articles (163) were identified, among which 111 were duplicates. In the end, 34 papers out of 52 articles met the inclusion criteria and entered into our study.

Assessment of publication bias
Due to the descriptive nature of the included articles (case reports and case series) and the absence of quantitative data, which were suitable for meta-analysis, formal assessment of publication bias was not feasible.
Data synthesis
Because of the heterogeneity of the included articles in terms of patient characteristics, clinical presentations, diagnostic methods, and treatment plans, no meta-analysis was conducted. Instead, a narrative synthesis was performed. Relevant data were summarized in tables, which highlight clinical presentations, diagnostic findings, treatment approaches, and outcomes across pediatric cases.
Results
Study selection
A total of 163 records were identified through database search, and after removing 111 duplicates, 52 articles remained for screening of title and abstract, and then full text. Of these, 34 studies were included in this systematic review. The study selection process is illustrated in Figure 1.
Due to methodological and clinical heterogeneity across the included case reports, a meta-analysis was not feasible. Instead, a narrative descriptive synthesis was evaluated, summarizing the demographic, clinical, diagnostic, and therapeutic data across articles. Because of the limited number of included studies and variability in reported data, no pooled effect estimates or confidence intervals were calculated.
Risk of bias assessment
The methodological quality of the included case reports and case series was evaluated using the JBI critical appraisal tool. Overall, the studies demonstrated high methodological rigor. All included literature met the minimum quality standards required for inclusion in this systematic review.
Demographic information
A total of 57 pediatric patients were included in this study, with 5 missing data points regarding the sex of the patients. In the 52 cases with known sex, 36 patients were male (69%) and 16 patients were female (32%) (Table 1).




The mean age of the patients was 7.8 years (pediatric age range: 0–18 years). There is a variety of countries represented, and this infection has been reported on all continents (Figure 2).

Manifestations
Articles have mentioned different manifestations in infected children, with the most common ones reported as follows:
Fever (in 27 cases), headache (in 23 cases), loss of consciousness (in 25 cases), seizures (in 18 cases), hemiparesis (in 12 cases), and others (Figure 1). These symptoms are usually due to increased intracranial pressure (ICP), which is caused by B. mandrillaris infection in the central nervous system.
Research has suggested two patterns of manifestations for this encephalitis. In the first pattern, patients developed specific skin lesions mainly on their faces months or years before the onset of encephalitis; this type has been reported mostly in China and Peru [10, 11]. In our study, we observed that 35% of cases experienced skin lesions before or during the occurrence of encephalitis. In the second pattern, patients presented with encephalitis without any signs of cutaneous lesions, which has usually been reported in the USA [12].
Diagnosis
Confirmatory method
Depending on the date of each case report, various diagnostic methods have been used to confirm the infection. Confirmatory techniques are reported as follows: next-generation sequencing (NGS) on CSF (in 5 cases), indirect immunofluorescence test on brain tissue (in 24 cases), serum or CSF antibody titer (in 3 cases), PCR (on CSF, brain tissue, or skin lesion) (in 25 cases), and immunohistochemical staining (in 3 cases). It is noteworthy that in some reports, more than one confirmatory test has been performed (Table 2).

CSF analysis
CSF analysis was reported in detail for 25 cases. Leukocytosis was observed in all cases. The maximum and minimum counts of CSF leukocytes were 555 and 14 cells/mm³, which belonged to a 27-month-old boy and a 2.5-year-old boy, respectively. Lymphocyte dominance was detected in almost all the tests performed. The CSF protein level was high (more than 45 mg/dL) in all patients except for four, who had normal protein levels. The glucose level was generally low, except in four tests in which normal levels were reported (Table 3).

Computed tomography (CT) scan and magnetic resonance imaging (MRI) findings
In 27 cases, CT scan findings were mentioned. The most common findings were hyperdense or hypodense lesions (s) (13 cases), brain edema (6 cases), and hydrocephalus (3 cases). In 45 patients, the MRI findings are mentioned. The most common MRI finding was lesions (in 34 patients) (Table 1).
Therapy
Treatment details were available for 49 cases (Table 1). Among the surviving patients, the treatment included multiple drugs, such as flucytosine, fluconazole, pentamidine, sulfadiazine, clarithromycin, thioridazine, miltefosine, trimethoprim-sulfamethoxazole, and azithromycin.
In other cases, based on an unknown diagnosis in early stages, different empirical treatments were administered. For instance, anti-tuberculosis therapy was used in 7 cases, but none of them led to survival. Amphotericin B was also used in 12 patients, all of whom experienced fatal outcomes (Table 1). Due to the limited number and variability of cases, statistical comparison of treatment results could not be conducted, and a descriptive overview of the therapies and their associated outcomes is provided.
Discussion
Balamuthia mandrillaris is a free-living amoeba responsible for a rare but fatal encephalitis in healthy and immunosuppressed hosts. Its cysts exist in soil and water, and this amoeba can infect humans via the skin, olfactory nerve, or pulmonary system [13, 14].
Encephalitis manifestations caused by B. mandrillaris are similar to other causes, and fever, headache, and seizures may be observed. These similar symptoms may lead specialists to misdiagnosis, and they might consider this infection tuberculoid, viral, or bacterial encephalitis.
We compared the demographic data, clinical symptoms, diagnostic tools, and treatment plans of pediatric patients with encephalitis caused by B. mandrillaris. This is the first systematic review globally intended to explore BAE in children. The age range of reported cases was from an 8-month-old female infant to an 18-year-old female teenager. The majority of cases were male children. The infection was mostly reported in the USA and China, and Africa had the fewest reported cases. It seems that the less-diagnosed cases in every area are related to the lack of awareness and inappropriate diagnostic tools. It is known that B. mandrillaris spreads and transmits via inhalation or injection from injured skin. It can reach the patient’s CNS through blood flow. Another theory for the transmission of this amoeba is the inhalation of airborne cysts [15]. In this study, probable risk factors were reported in 22 children, of which the most common are traumas leading to skin injury (12 cases) and contact with infected water (3 cases – swimming or flood).
Diagnosing this amoeba is still challenging for healthcare systems because of the lack of knowledge about this infection. Due to this condition, there was a delay and misdiagnosis in most cases, and some patients presented with severe manifestations.
Different diagnostic methods, such as NGS, PCR, serological tests, biological tests, immunohistochemical assays, biopsy, and special staining methods for brain or skin lesions, have been used to confirm BAE. However, many of these tests do not need a specialty and are unavailable in some areas [8].
In our study, the most common confirming diagnostic tool was PCR on the involved body tissues. PCR on CSF, brain tissue, or skin lesions is a rapid and sensitive diagnostic tool to detect B. mandrillaris. It can be useful in clinical diagnosis and has been developed recently [16].
ELISA and indirect immunofluorescence assay are two serological methods that detect B. mandrillaris. Based on some studies, positive serology is a good way to diagnose BAE, but it cannot always be a certain tool to indicate a diagnosis [17].
Another agent for detecting this amoeba is indirect immunofluorescence using rabbit anti-Balamuthia spp. serum or a rabbit antiserum-based immunohistochemical assay on brain or skin tissue samples [18].
NGS is a new, noninvasive, and rapid method for diagnosing BAE (used in 5 patients) [19]. Wu et al. could diagnose BAE in a 13-year-old Chinese girl via NGS of CSF [20]. Yi et al. confirmed the presence of this amoeba using metagenomic NGS of CSF [19].
Neuroimaging studies, such as CT scans and MRIs, have shown some abnormalities, such as multiple hypodense and hyperdense lesions and edema. This is consistent with findings from Piper et al. [21].
In the CSF findings, lymphocytic pleocytosis, elevated protein levels, and low glucose have been predominantly reported. Overall, no significant signs in CSF analysis distinguish BAE from other encephalitis causes [8].
Until now, a definitive treatment method for this infection has not been established. Based on a study, the average survival time for patients admitted to the hospital is about 16 days, which indicates the poor prognosis of BAE [22]. Combination therapy is suggested, and in surviving cases, it includes multiple drugs, such as flucytosine, fluconazole, pentamidine, sulfadiazine, Clarithromycin, thioridazine, miltefosine, trimethoprim-sulfamethoxazole, and azithromycin. Miltefosine is a drug approved by the US Food and Drug Administration (FDA) for leishmaniasis treatment in 2013. It has been found useful against B. mandrillaris in vitro in combination with other drugs [23]. In this study, we found that milfetosin (in combination with other drugs) was used in 6 cases, but only one patient survived (Table 1) [24].
More investigation is needed to establish the appropriate and certain guidelines for this disease and its combination therapy. It should be noted that intracranial pressure should decrease as soon as possible in cases of high intracranial pressure. It has been found that steroids are not indicated because they can trigger both skin and CNS lesions [25].
In conclusion, further investigation is necessary to improve diagnosis and develop more effective therapies. Although it is rare and fatal, early detection and appropriate treatment can provide a chance of survival [26].
Conclusion
The manifestations of BAE may resemble those of any other encephalitis, which could lead to a loss of critical time and misdiagnosis, potentially resulting in the death of patients. Thus, it is recommended that every relevant specialist gain more knowledge and awareness of this lethal infection to facilitate early diagnosis and the best treatment plan. Further investigations into this infection and the development of more effective treatments are recommended to improve the survival chances of these patients.
Limitations
This systematic review has several limitations. First, all the included literature consisted of case reports and case series, which carry a risk of bias due to selective reporting and also lack of a control group. Second, the heterogeneity across articles in terms of patient demographics, diagnostic tools, and treatment regimens limited the ability to conduct a meta-analysis. Additionally, the small sample size in most articles restricted the generalizability of the results.
Despite these limitations, our review highlights the need for early diagnosis and more efficient treatment plans for this encephalitis.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or nonprofit sectors.
Authors contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflicts of interest
The authors declared no conflict of interest.
References
- Roos KL. Encephalitis. Neurol Clin. 1999; 17(4):813-33. [DOI:10.1016/S0733-8619(05)70168-7] [PMID]
- Martinez AJ, Visvesvara GS. Laboratory diagnosis of pathogenic free-living amoebas: Naegleria, acanthamoeba, and leptomyxid. Clin Lab Med. 1991; 11(4):861-72. [DOI:10.1016/S0272-2712(18)30524-9]
- Schuster FL. Cultivation of pathogenic and opportunistic free-living amebas. Clin Microbiol Rev. 2002; 15(3):342-54. [DOI:10.1128/CMR.15.3.342-354.2002] [PMID] [PMCID]
- Dunnebacke TH, Schuster FL, Yagi S, Booton GC. Balamuthia mandrillaris from soil samples. Microbiology. 2004; 150(Pt 9):2837-42. [DOI:10.1099/mic.0.27218-0] [PMID]
- Schuster FL, Dunnebacke TH, Booton GC, Yagi S, Kohlmeier CK, Glaser C, et al. Environmental isolation of Balamuthia mandrillaris associated with a case of amebic encephalitis. J Clin Microbiol. 2003; 41(7):3175-80. [DOI:10.1128/JCM.41.7.3175-3180.2003] [PMID] [PMCID]
- Visvesvara GS, Schuster FL, Martinez AJ. Balamuthia mandrillaris, N. G., N. Sp., agent of amebic meningoencephalitis in humans and other animals. J Eukaryot Microbiol. 1993; 40(4):504-14. [DOI:10.1111/j.1550-7408.1993.tb04943.x] [PMID]
- Visvesvara GS, Moura H, Schuster FL. Pathogenic and opportunistic free-living amoebae: Acanthamoeba spp., Balamuthia mandrillaris, Naegleria fowleri, and Sappinia diploidea. FEMS Immunol Med Microbiol. 2007; 50(1):1-26. [DOI:10.1111/j.1574-695X.2007.00232.x] [PMID]
- Zhang Z, Liang J, Wei R, Feng X, Wang L, Wang L, et al. Facial Balamuthia mandrillaris infection with neurological involvement in an immunocompetent child. Lancet Infect Dis. 2022; 22(3):e93-100. [DOI:10.1016/S1473-3099(21)00334-0] [PMID]
- Visvesvara GS, Martinez AJ, Schuster FL, Leitch GJ, Wallace SV, Sawyer TK, et al. Leptomyxid ameba, a new agent of amebic meningoencephalitis in humans and animals. J Clin Microbiol. 1990; 28(12):2750-6. [DOI:10.1128/jcm.28.12.2750-2756.1990] [PMID] [PMCID]
- Wang L, Cheng W, Li B, Jian Z, Qi X, Sun D, et al. Balamuthia mandrillaris infection in China: A retrospective report of 28 cases. Emerg Microbes Infect. 2020; 9(1):2348-57. [DOI:10.1080/22221751.2020.1835447] [PMID] [PMCID]
- Cabello-Vílchez AM, Rodríguez-Zaragoza S, Piñero J, Valladares B, Lorenzo-Morales J. Balamuthia mandrillaris in South America: An emerging potential hidden pathogen in Perú. Exp Parasitol. 2014; 145Suppl:S10-9. [DOI:10.1016/j.exppara.2014.05.007] [PMID]
- Cope JR, Landa J, Nethercut H, Collier SA, Glaser C, Moser M, et al. The epidemiology and clinical features of balamuthia mandrillaris disease in the United States, 1974-2016. Clin Infect Dis. 2019; 68(11):1815-22. [DOI:10.1093/cid/ciy813] [PMID] [PMCID]
- Bravo F, Sanchez MR. New and re-emerging cutaneous infectious diseases in Latin America and other geographic areas. Dermatol Clin. 2003; 21(4):655-68, viii. [DOI:10.1016/S0733-8635(03)00090-1] [PMID]
- Kiderlen AF, Laube U. Balamuthia mandrillaris, an opportunistic agent of granulomatous amebic encephalitis, infects the brain via the olfactory nerve pathway. Parasitol Res. 2004; 94(1):49-52. [DOI:10.1007/s00436-004-1163-z] [PMID]
- Mungroo MR, Khan NA, Siddiqui R. Balamuthia mandrillaris: Pathogenesis, diagnosis, and treatment. Expert Opinion on Orphan Drugs. 2020; 8(4):111-9. [DOI:10.1080/21678707.2020.1758061]
- Booton GC, Carmichael JR, Visvesvara GS, Byers TJ, Fuerst PA. Genotyping of balamuthia mandrillaris based on nuclear 18S and mitochondrial 16S rRNA genes. Am J Trop Med Hyg. 2003; 68(1):65-9. [DOI:10.4269/ajtmh.2003.68.65] [PMID]
- Schuster FL, Yagi S, Wilkins PP, Gavali S, Visvesvara GS, Glaser CA. Balamuthia mandrillaris, agent of amebic encephalitis: detection of serum antibodies and antigenic similarity of isolates by enzyme immunoassay. J Eukaryot Microbiol. 2008; 55(4):313-20. [DOI:10.1111/j.1550-7408.2008.00333.x] [PMID]
- Guarner J, Bartlett J, Shieh WJ, Paddock CD, Visvesvara GS, Zaki SR. Histopathologic spectrum and immunohistochemical diagnosis of amebic meningoencephalitis. Mod Pathol. 2007; 20(12):1230-7. [DOI:10.1038/modpathol.3800973] [PMID]
- Yi Z, Zhong J, Wu H, Li X, Chen Y, Chen H,et al. Balamuthia mandrillaris encephalitis in a child: case report and literature review. Diagn Microbiol Infect Dis. 2021; 100(4):115180. [DOI:10.1016/j.diagmicrobio.2020.115180] [PMID]
- Wu X, Yan G, Han S, Ye Y, Cheng X, Gong H, et al. Diagnosing Balamuthia mandrillaris encephalitis via next-generation sequencing in a 13-year-old girl. Emerg Microbes Infect. 2020; 9(1):1379-87. [DOI:10.1080/22221751.2020.1775130] [PMID] [PMCID]
- Piper KJ, Foster H, Susanto D, Maree CL, Thornton SD, Cobbs CS. Fatal Balamuthia mandrillaris brain infection associated with improper nasal lavage. Int J Infect Dis. 2018; 77:18-22. [DOI:10.1016/j.ijid.2018.09.013] [PMID]
- Schuster FL, Yagi S, Gavali S, Michelson D, Raghavan R, Blomquist I, et al. Under the radar: Balamuthia amebic encephalitis. Clin Infect Dis. 2009; 48(7):879-87. [DOI:10.1086/597260] [PMID]
- Martínez AJ, Guerra AE, García-Tamayo J, Céspedes G, González-Alfonzo JE, Visvesvara GS. Granulomatous amebic encephalitis: A review and report of a spontaneous case from Venezuela. Acta Neuropathol. 1994; 87(4):430-4. [DOI:10.1007/BF00313614] [PMID]
- Cuoco JA, Klein BJ, LeBel DP, Faulhaber J, Apfel LS, Witcher MR. Successful treatment of a balamuthia mandrillaris cerebral abscess in a pediatric patient with complete surgical resection and antimicrobial therapy. Pediatr Infect Dis J. 2022; 41(2):e54-7. [DOI:10.1097/INF.0000000000003418] [PMID]
- Doyle JS, Campbell E, Fuller A, Spelman DW, Cameron R, Malham G, et al. Balamuthia mandrillaris brain abscess successfully treated with complete surgical excision and prolonged combination antimicrobial therapy. J Neurosurg. 2011; 114(2):458-62. [DOI:10.3171/2010.10.JNS10677] [PMID]
- Joo SJ, Thompson AB, Philipsborn R, Emrath E, Camacho-Gonzalez AF, Chahroudi A, et al. An unusual cause of fever and headache in a school-aged male. Clin Pediatr. 2018; 57(11):1359-62. [DOI:10.1177/0009922818772056] [PMID] [PMCID]
- Tootla HD, Eley BS, Enslin JMN, Frean JA, Hlela C, Kilborn TN, et al. Balamuthia mandrillaris granulomatous amoebic encephalitis: The first African experience. J Pediatric Infect Dis Soc. 2022; 11(12):578-81. [DOI:10.1093/jpids/piac096] [PMID]
- Tao K, Wang T, Zhang L, Yang XC, Zhai ZF. Fatal balamuthia mandrillaris infection with red plaques on the nasal dorsum as the first presentation. An Bras Dermatol. 2022; 97(4):498-500. [DOI:10.1016/j.abd.2021.12.001] [PMID] [PMCID]
- Ai J, Zhang H, Yu S, Li J, Chen S, Zhang W, Mao R. A case of fatal amoebic encephalitis caused by Balamuthia mandrillaris, China. Infect Genet Evol. 2022; 97:105190. [DOI:10.1016/j.meegid.2021.105190] [PMID]
- Yang Y, Hu X, Min L, Dong X, Guan Y. Balamuthia mandrillaris-related primary amoebic encephalitis in china diagnosed by next generation sequencing and a review of the literature. Lab Med. 2020; 51(2):e20-6. [DOI:10.1093/labmed/lmz079] [PMID]
- Shehab KW, Aboul-Nasr K, Elliott SP. Balamuthia mandrillaris granulomatous amebic encephalitis with renal dissemination in a previously healthy child: Case report and review of the pediatric literature. J Pediatric Infect Dis Soc. 2018; 7(3):e163-8. [DOI:10.1093/jpids/pix089] [PMID]
- Khurana S, Hallur V, Goyal MK, Sehgal R, Radotra BD. Emergence of balamuthia mandrillaris meningoencephalitis in India. Indian J Med Microbiol. 2015; 33(2):298-300. [DOI:10.4103/0255-0857.154887] [PMID]
- Krasaelap A, Prechawit S, Chansaenroj J, Punyahotra P, Puthanakit T, Chomtho K, et al. Fatal balamuthia amebic encephalitis in a healthy child: A case report with review of survival cases. Korean J Parasitol. 2013; 51(3):335-41. [DOI:10.3347/kjp.2013.51.3.335] [PMID] [PMCID]
- Moriarty P, Burke C, McCrossin D, Campbell R, Cherian S, Shahab MS, et al. Balamuthia mandrillaris encephalitis: Survival of a child with severe meningoencephalitis and review of the literature. J Pediatric Infect Dis Soc. 2014; 3(1):e4-9. [DOI:10.1093/jpids/pit033] [PMID]
- Stidd DA, Root B, Weinand ME, Anton R. Granulomatous amoebic encephalitis caused by Balamuthia mandrillaris in an immunocompetent girl. World Neurosurg. 2012; 78(6):715.e7-12. [DOI:10.1016/j.wneu.2011.10.040] [PMID]
- Ghosh PS, Ghosh D, Loddenkemper T, Prayson RA, Tekautz T, Sriram CS, et al. Necrotizing granulomatous meningoencephalitis due to Balamuthia in an immunocompetent child. Neurology. 2011; 77(8):801-2. [DOI:10.1212/WNL.0b013e31822b0100] [PMID]
- Orozco L, Hanigan W, Khan M, Fratkin J, Lee M. Neurosurgical intervention in the diagnosis and treatment of Balamuthia mandrillaris encephalitis. J Neurosurg. 2011; 115(3):636-40. [DOI:10.3171/2011.4.JNS102057] [PMID]
- Hill CP, Damodaran O, Walsh P, Jevon GP, Blyth CC. Balamuthia amebic meningoencephalitis and mycotic aneurysms in an infant. Pediatr Neurol. 2011; 45(1):45-8. [DOI:10.1016/j.pediatrneurol.2011.05.003] [PMID]
- Combs FJ Jr, Erly WK, Valentino CM, Rance NE. Best cases from the AFIP: Balamuthia mandrillaris amebic meningoencephalitis. Radiographics. 2011; 31(1):31-5. [DOI:10.1148/rg.311105067] [PMID]
- Cary LC, Maul E, Potter C, Wong P, Nelson PT, Given C 2nd, et al. Balamuthia mandrillaris meningoencephalitis: Survival of a pediatric patient. Pediatrics. 2010; 125(3):e699-703. [DOI:10.1542/peds.2009-1797] [PMID]
- Valverde J, Arrese JE, Piérard GE. Granulomatous cutaneous centrofacial and meningocerebral amebiasis. Am J Clin Dermatol. 2006; 7(4):267-9. [DOI:10.2165/00128071-200607040-00009] [PMID]
- Tavares M, Correia da Costa JM, Carpenter SS, Santos LA, Afonso C, Aguiar A, et al. Diagnosis of first case of Balamuthia amoebic encephalitis in Portugal by immunofluorescence and PCR. J Clin Microbiol. 2006; 44(7):2660-3. [DOI:10.1128/JCM.00479-06] [PMID] [PMCID]
- Bakardjiev A, Azimi PH, Ashouri N, Ascher DP, Janner D, Schuster FL, et al. Amebic encephalitis caused by Balamuthia mandrillaris: Report of four cases. Pediatr Infect Dis J. 2003; 22(5):447-53. [DOI:10.1097/00006454-200305000-00013] [PMID]
- Deetz TR, Sawyer MH, Billman G, Schuster FL, Visvesvara GS. Successful treatment of Balamuthia amoebic encephalitis: Presentation of 2 cases. Clin Infect Dis. 2003; 37(10):1304-12. [DOI:10.1086/379020] [PMID]
- Healy JF. Balamuthia amebic encephalitis: Radiographic and pathologic findings. AJNR. 2002; 23(3):486. [Link]
- Galarza M, Cuccia V, Sosa FP, Monges JA. Pediatric granulomatous cerebral amebiasis: A delayed diagnosis. Pediatr Neurol. 2002; 26(2):153-6. [DOI:10.1016/S0887-8994(01)00360-5] [PMID]
- Kodet R, Nohýnková E, Tichý M, Soukup J, Visvesvara GS. Amebic encephalitis caused by Balamuthia mandrillaris in a Czech child: Description of the first case from Europe. Pathol Res Pract. 1998; 194(6):423-9. [DOI:10.1016/S0344-0338(98)80033-2] [PMID]
- Reed RP, Cooke-Yarborough CM, Jaquiery AL, Grimwood K, Kemp AS, Su JC, et al. Fatal granulomatous amoebic encephalitis caused by Balamuthia mandrillaris. Med J Aust. 1997; 167(2):82-4. [DOI:10.5694/j.1326-5377.1997.tb138785.x] [PMID]
- Duke BJ, Tyson RW, DeBiasi R, Freeman JE, Winston KR. Balamuthia mandrillaris meningoencephalitis presenting with acute hydrocephalus. Pediatr Neurosurg. 1997; 26(2):107-11. [DOI:10.1159/000121172] [PMID]
- Riestra-Castaneda JM, Riestra-Castaneda R, Gonzalez-Garrido AA, Pena Moreno P, Martinez AJ, Visvesvara GS, et al. Granulomatous amebic encephalitis due to Balamuthia mandrillaris (Leptomyxiidae): Report of four cases from Mexico. Am J Trop Med Hyg. 1997; 56(6):603-7. [DOI:10.4269/ajtmh.1997.56.603] [PMID]
- Griesemer DA, Barton LL, Reese CM, Johnson PC, Gabrielsen JA, Talwar D, et al. Amebic meningoencephalitis caused by Balamuthia mandrillaris. Pediatr Neurol. 1994; 10(3):249-54. [DOI:10.1016/0887-8994(94)90034-5] [PMID]
Type of Study: Systematic Review |
Subject:
Pediatric infection disease
Received: 2024/10/12 | Accepted: 2025/06/21 | Published: 2025/07/19
Received: 2024/10/12 | Accepted: 2025/06/21 | Published: 2025/07/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









