Volume 12, Issue 4 (10-2024)
J. Pediatr. Rev 2024, 12(4): 385-396 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Varshoei F, Rezai M S, Farhadi R, Hajialibeig A, Mohammadi S, Moradi M et al . Clinical Characteristics of COVID-19 in Neonates and Infants Younger Than 3 Months. J. Pediatr. Rev 2024; 12 (4) :385-396
URL: http://jpr.mazums.ac.ir/article-1-693-en.html
URL: http://jpr.mazums.ac.ir/article-1-693-en.html
Fatemeh Varshoei1 

 , Mohammad Sadegh Rezai1
, Mohammad Sadegh Rezai1 

 , Roya Farhadi1
, Roya Farhadi1 

 , Azin Hajialibeig1
, Azin Hajialibeig1 

 , Saeid Mohammadi1
, Saeid Mohammadi1 

 , Masoud Moradi2
, Masoud Moradi2 

 , Fatemeh Hosseinzadeh *3
, Fatemeh Hosseinzadeh *3 




 , Mohammad Sadegh Rezai1
, Mohammad Sadegh Rezai1 

 , Roya Farhadi1
, Roya Farhadi1 

 , Azin Hajialibeig1
, Azin Hajialibeig1 

 , Saeid Mohammadi1
, Saeid Mohammadi1 

 , Masoud Moradi2
, Masoud Moradi2 

 , Fatemeh Hosseinzadeh *3
, Fatemeh Hosseinzadeh *3 


1- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,fatima.hzade@gmail.com
2- Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,
Keywords: Characteristic, COVID-19, Multisystem inflammatory system in children (MIS-C), Neonate, Infant
Full-Text [PDF 462 kb]
(825 Downloads)
| Abstract (HTML) (3114 Views)
Full-Text: (555 Views)
Introduction
SARS-CoV-2-associated infection known as COVID-19 (novel coronavirus disease 2019) is a disease appeared in China, in 2019 [1]. This pandemic has affected the world’s population, including children [2]. Clinical manifestations of COVID-19 in children range from asymptomatic infection to severe pneumonia and a multisystem inflammatory system in children (MIS-C) [3, 4]. Early infancy could be a risk factor for infectious disease due to inadequately developed immune system during the first months of life and more active innate immune responses among children [5]. The clinical picture in neonates and early infants with COVID-19 has been described in some reports, suggesting that they can develop a more severe form of the disease than older children [1, 6-13]. Newborn and young infants are usually asymptomatic or present with mild symptoms like older children [8], presumably because of their healthier respiratory tracts and fewer underlying diseases [12].
According to previous reports on newborns and infants [8, 14], fever was the most reported symptom in infants under 3 months, followed by rhinorrhea and cough. Unlike adults, infants are more likely to present with gastrointestinal symptoms, such as diarrhea and vomiting. Moderate to severe symptoms, such as poor feeding, lethargy, and respiratory distress, have been reported less frequently [13, 15]. In addition, MIS-C diagnosis is increasing in neonates, a condition that subsequently evolves as a post-infectious inflammatory condition associated with abnormal immune function, left ventricular cardiac dysfunction, coronary artery aneurysm, atrioventricular block, and clinical deterioration with multi-organ involvement. MIS-C has increased the importance of research in this age group [16-19].
During the initial waves of COVID-19, when the Alpha and Delta variants were predominant, few cases of neonates and young infants were reported in the United States, where children younger than one year old represented less than 1% of all cases [20]. During the fifth wave, when the Omicron variant was predominant, more cases were reported in children 0-4 years old. The hospitalization rate was five times higher than the previous peak of Delta variant predominance [21]. Most hospitalized children (63%) were healthy with no underlying medical conditions; infants under 6 months old accounted for 44% of hospitalizations [21, 12]. Over time, the diagnosis and treatment of COVID-19 in different age groups have improved, but our knowledge about the clinical manifestations, disease course, and outcomes in neonates and early infants is still limited [12]. However, some researchers have shown a potentially higher risk of severe disease [8, 22] and a higher rate of hospitalization [9] in neonates and young infants compared to older children. Accurately determining the number of neonates infected with COVID-19 is impossible. COVID-19-positive infants had a much higher hospitalization rate than other age groups in an American study [9]. The lack of pediatric cases and their unknown outcomes result in difficulty in making a clinical diagnosis in neonates [23].
Most studies conducted in this field have only considered data related to hospitalized infants and have only partially examined the full spectrum of the disease in this age group [19]. Therefore, given the existing scientific gaps and the absence of approved vaccines for infants, understanding the characteristics associated with hospitalization, severe illness due to COVID-19, prognosis, and outcome in this age group will help inform clinical management and public health interventions. Considering that no study has been conducted on hospitalized children under three months of age in Iran, this study aimed to determine the demographic data, clinical manifestations, and laboratory characteristics of hospitalized neonates and infants under three months of age with COVID-19 to manage preventive measures against COVID-19 in this age group.
Methods
Study design, participants, and settings
This retrospective study was performed at Bouali and Imam Khomeini tertiary hospitals in Sari City, Mazandaran Province, northern Iran, between March 2019 and March 2022. Patients diagnosed with COVID-19 infection were categorized into two groups based on age: younger than 30 days, referred to as the “neonate” group, and those 1-3 months old, referred to as the “infant” group. Neonates with the clinical signs of COVID-19 were also evaluated. Neonates born to mothers with positive real‐time reverse transcriptase‐polymerase chain reaction (RT-PCR) results were admitted following birth for observation. In infants, the diagnostic criteria were the diagnostic criteria for a positive RT-PCR result, clinical signs of COVID-19, and a history of close contact with a known COVID-19 case. The severity of COVID-19 was defined according to World Health Organization (WHO) guidelines [24] as follows: Mild with mild clinical symptoms with no radiographic findings compatible with pneumonia, moderate cases with fever, respiratory symptoms, and radiographic findings compatible with pneumonia with <30% pulmonary involvement, severe/MIS-C cases showing one of the following factors; respiratory distress (retraction, grunting, central cyanosis, etc.), tachypnea (respiratory rate ≥30/min), hypoxemia, requiring supplemental oxygen and oxygen saturation (SpO2) <94% in the air room, >50% pulmonary involvement.
Demographic data, such as age, sex, comorbidity, clinical characteristics and symptoms, administered therapies, and laboratory tests, including complete blood count (CBC)-diff, blood and urine culture, cerebrospinal fluid (CSF) analysis, and culture, were extracted from the patient’s medical records. In addition, the prognosis, duration of hospitalization, admission to the intensive care unit (neonate or pediatric), need for mechanical ventilation, and mortality were recorded. The clinical signs or symptoms of COVID-19 include fever, cough, poor feeding, dyspnea or respiratory distress, lethargy, diarrhea, inconsolability, rhinorrhea or nasal congestion, vomiting, sneezing, and hypotonia. Physical examination findings were recorded, such as tachypnea, retraction, fine crackles, wheezes, tachycardia, conjunctivitis, rash, and hypotension.
Statistical analysis
The results are presented as Mean±SD or median with interquartile range (IQR) for continuous variables and frequency with percentage for categorical variables in each group. According to the Kolmogorov-Smirnov test, the distribution of quantitative variables was not normal. Therefore, the Kruskal-Wallis test was used for intergroup comparisons. Univariate and multivariate logistic regression Cox proportional hazard models were used to determine the factors affecting disease severity during the hospitalization for COVID-19 inpatients. Statistical analysis was performed using SPSS software, version 22. P<0.05 were considered to be statistically significant.
Results
This study evaluated 206 children, including 88 neonates (42.71%) and 118 infants (57.28%). Sixty neonates were born to COVID-19-positive mothers and admitted following birth for observation. Three (5%) had a positive RT-PCR test result and respiratory distress syndrome, which required ICU admission and antibiotic therapy. All of them were born by cesarean section and did not present with fever or cough during hospitalization. Other 54 RT-PCR-negative neonates were excluded, and finally, 34 neonates (22.4%) and 118 infants (77.6%) with a median age of 49.5 [IQR: 33-66] days were included in the final analysis.
The frequencies of mild, moderate, and severe/MIS-C cases were 96(63.2%), 29(19.1%), and 27(17.8%), respectively (P=0.879), and 3 infants in the severe group had MIS-C. Overall, 90 patients (59.2%) were boys, and 62(40.8%) were girls, and there was no difference between the two groups (P=0.695). One hundred and thirteen children (74.3%) lived in urban areas.
The median gestational age was 38 [IQR: 36.5-39] weeks and did not differ between the groups (P=0.108). In addition, 111 patients (73%) were term (gestational age >37 weeks) and 33(21.7%) were preterm (gestational age <37 weeks) (P=1). The mode of delivery in 107(70.4%) children was cesarean section, and 36(23.7%) had a natural vaginal delivery that did not differ between the groups (P=0.488). Eighty-one children (53.3%) were breastfeeding, 19(12.5%) used formula feeding, and 52(34.2%) used both methods and did not differ between the groups (P=0.733).
COVID-19 RT-PCR test was performed in 125 children, and 65(42.8%) were detected positive for the virus, and infants were more likely to be RT-PCR positive (P=0.022). Ninety-six children (63.2%) had a history of COVID-19 in their families, significantly higher among infants (53.8% vs 20%, P=0.031). In total, 3 children (2%) expired, all infants (Table 1).
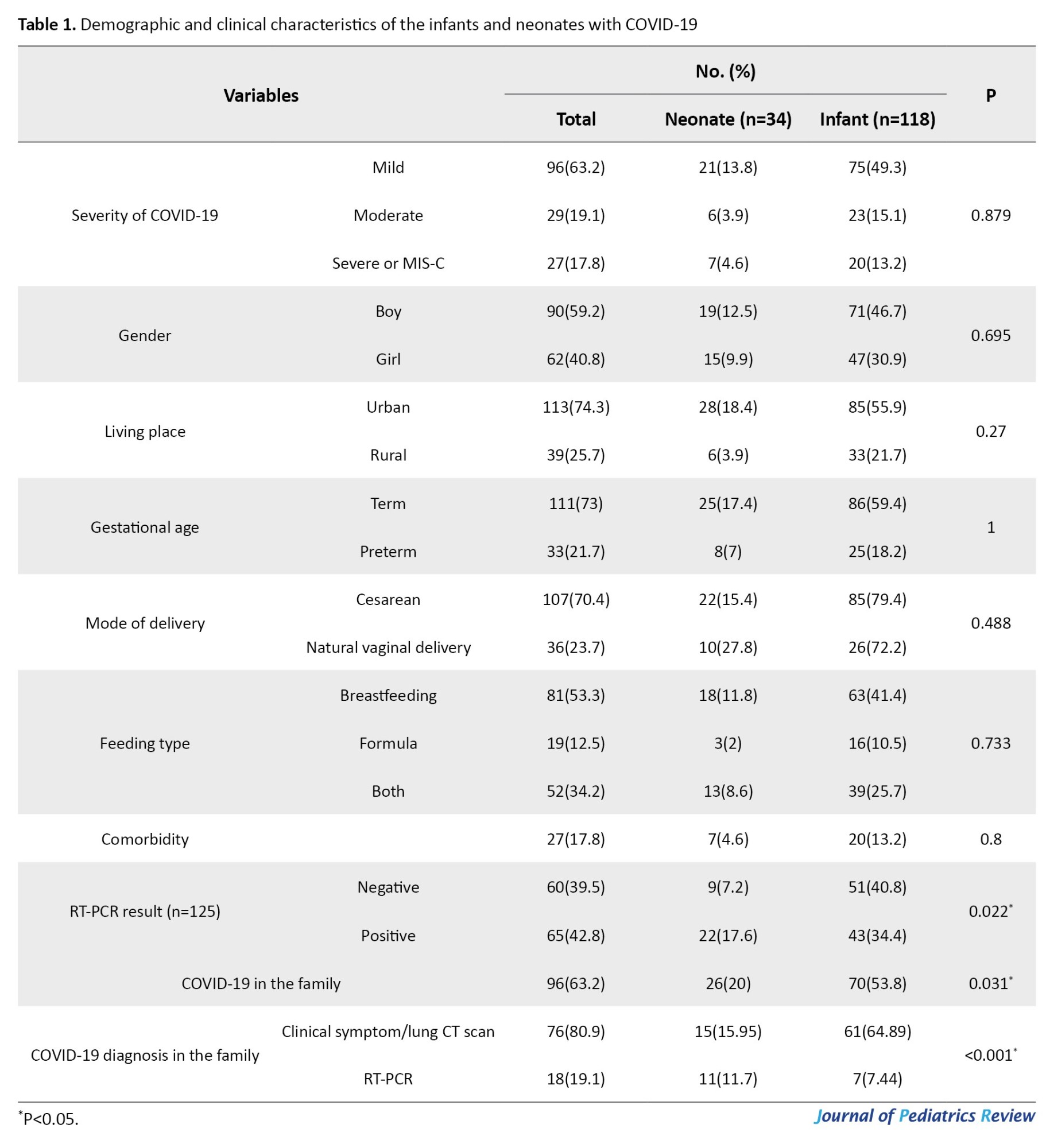
Fifty-five children (26.21%) had a history of hospitalization without a significant difference between the two groups (P=0.105). Overall, 27 children (17.8%) had comorbid diseases including G6PDd (14, 9.2%), congenital heart disease (9, 5.9%), seizures (3, 2%), congenital anomalies (2, 1.3%), and thyroid or renal diseases (2, 1.3%) which were not statistically different between the groups (P=0.8).
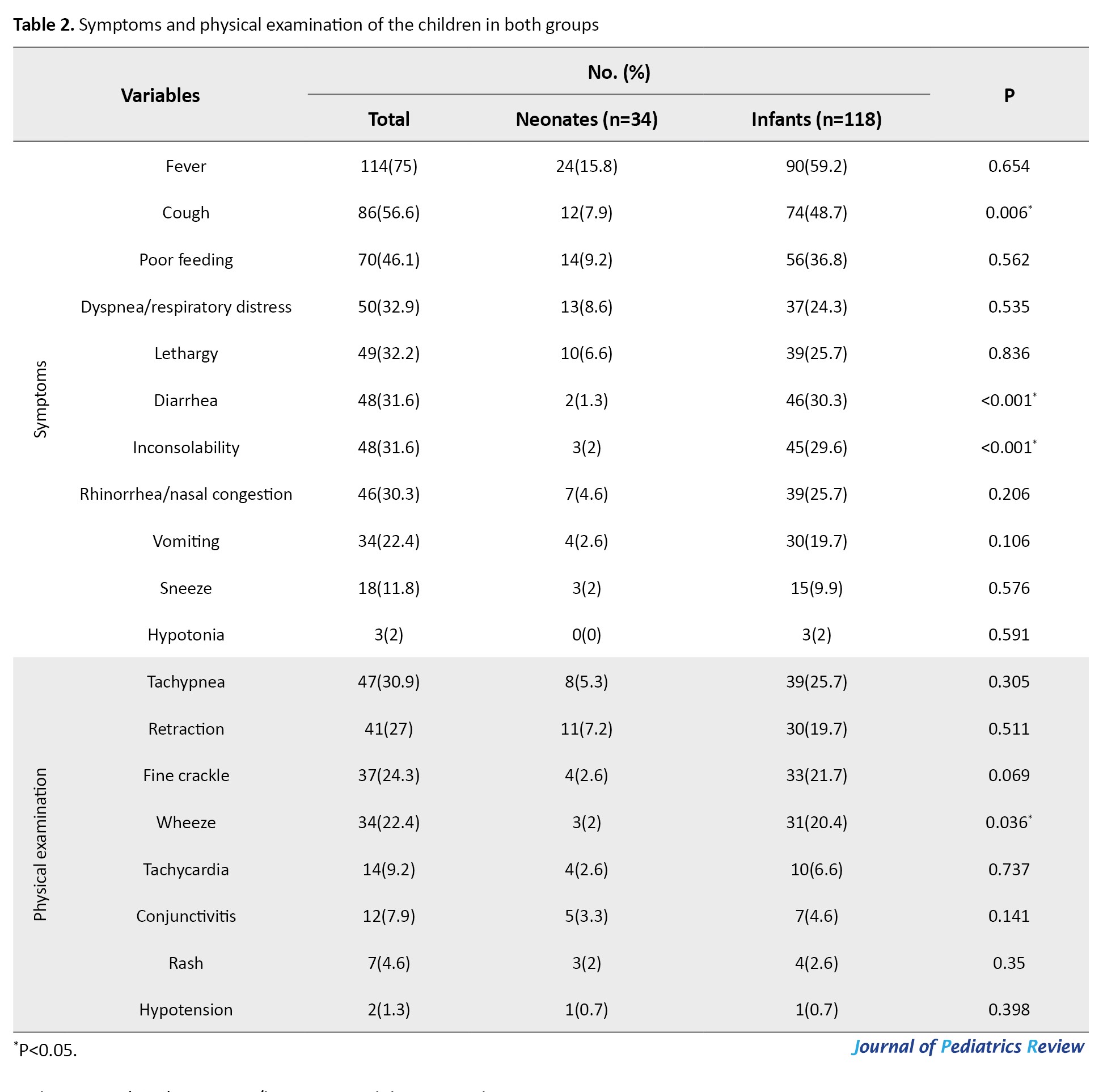
In terms of symptoms in children, fever was the most common symptom (75%), followed by cough (56.6%), poor feeding (46.1%), dyspnea/respiratory distress (32.9%), and lethargy (32.2%). The most common finding on physical examination was tachypnea (30.9%), followed by retraction (27%), fine crackles (24.3%), and wheezing (22.4%) (Table 2).
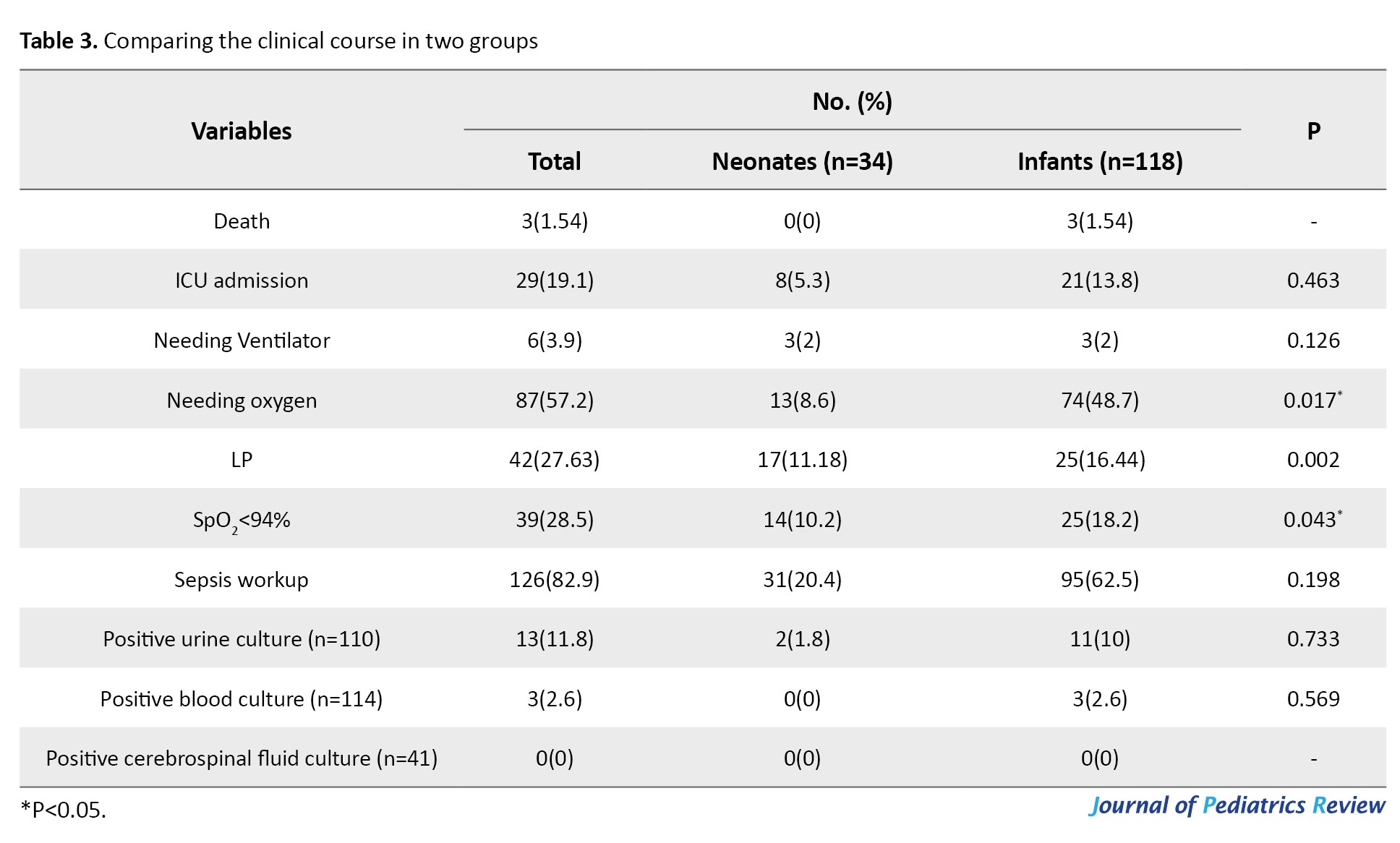
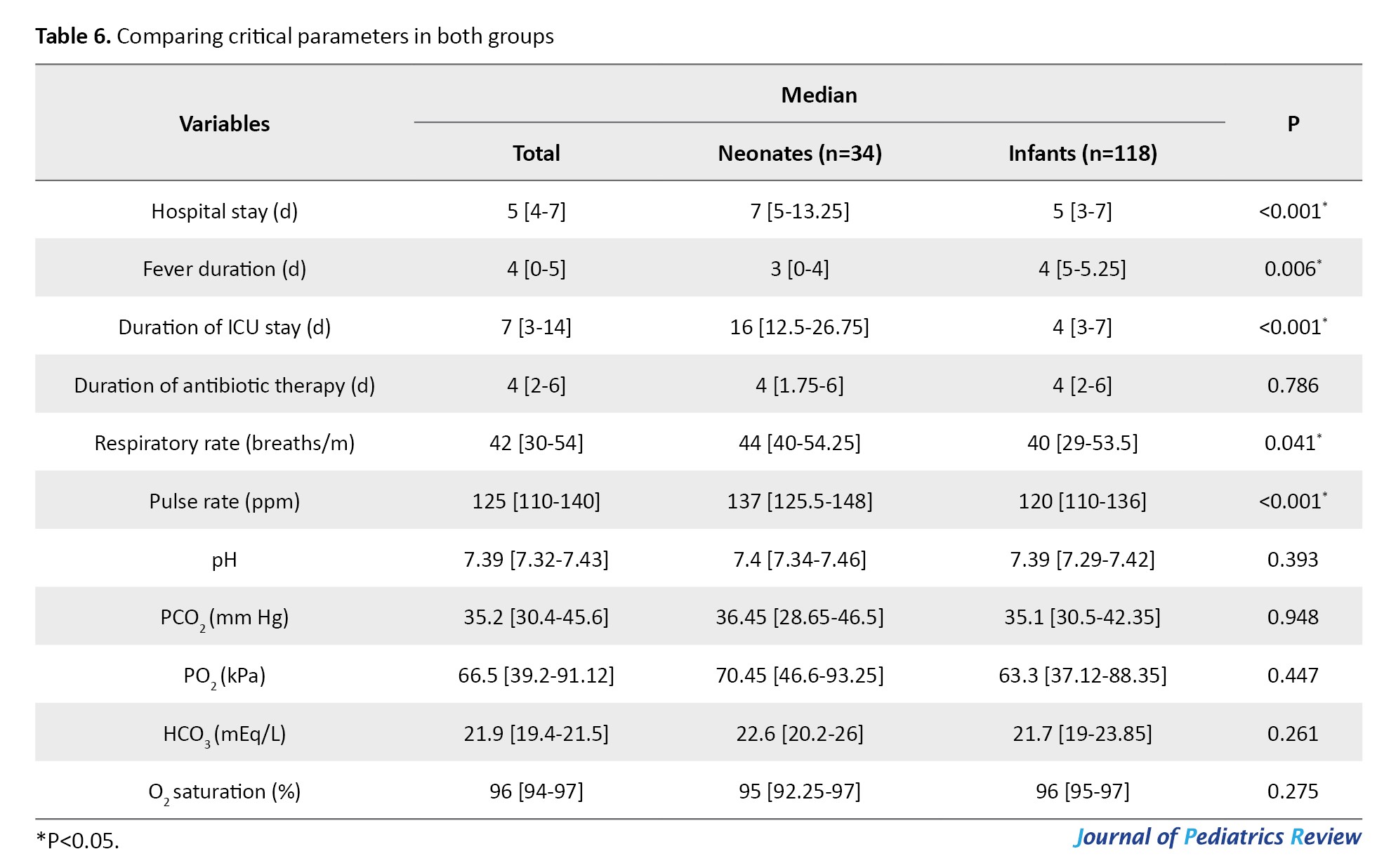
An oxygen saturation level <94% was observed in 39 patients (28.5%), and 87 children (57.2%) required oxygen therapy (P=0.043 and P=0.017, respectively). In addition, the duration of ICU stay was significantly higher in neonates (16 [IQR: 12.5-26.75] vs 4 [IQR: 3-7], P<0.001), 29 patients (19.1%) required ICU admission, and 6 patients (3.9%) underwent mechanical ventilation (P=0.463 and P=0.126, respectively) (Table 3).
The median duration of hospital stay was 5 days [IQR: 4-7 days], which was significantly longer in neonates (7 [IQR: 5-13.25] vs 5 [IQR: 3-7] days, P<0.001). The median fever duration was 4 [IQR: 0-5] days which was significantly longer in infants (4 [IQR: 5-5.25] vs 3 [IQR: 0-4] days, P=0.006).
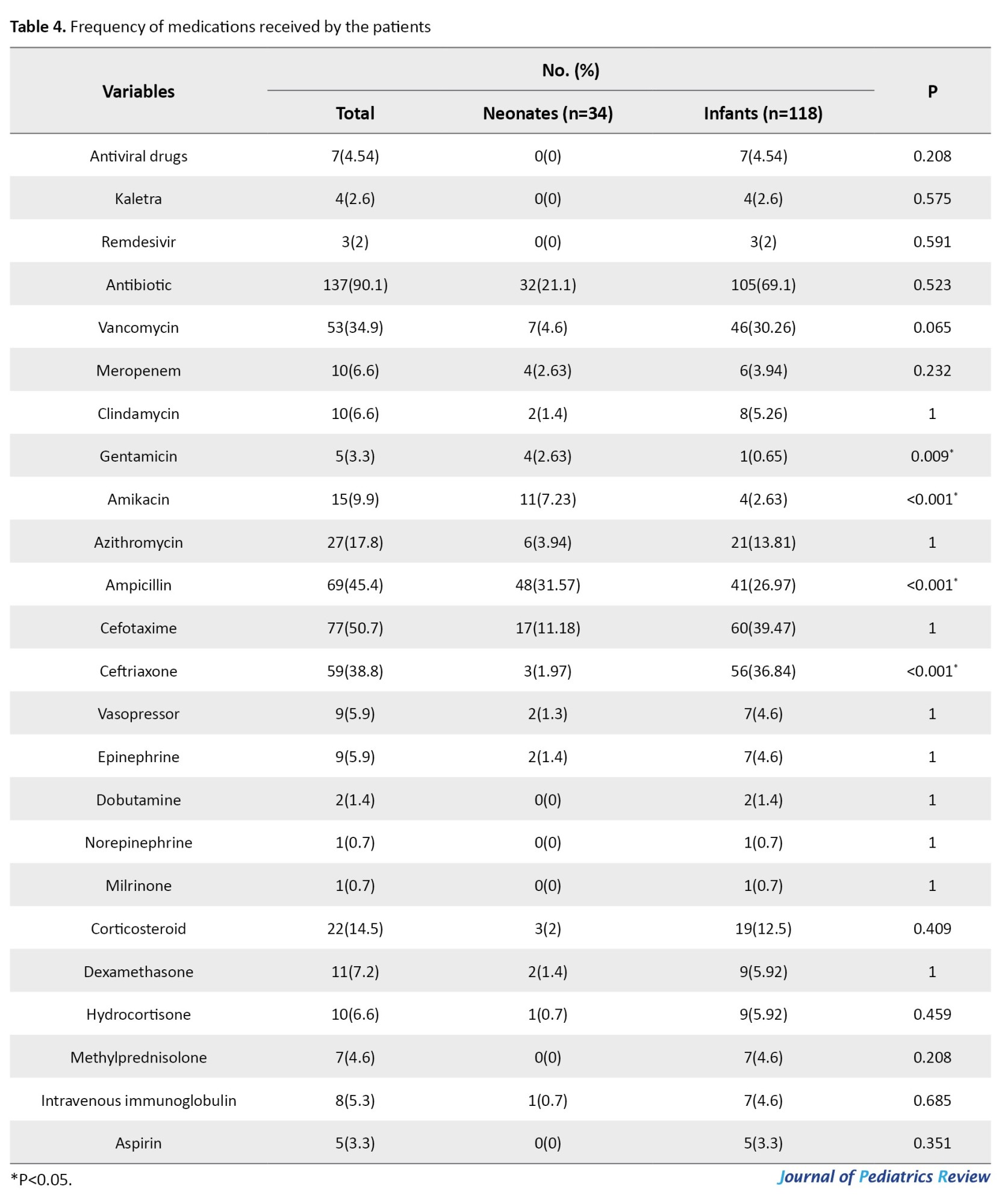
Approximately 137 patients (90.1%) received antibiotics, and 22(14.5%) received corticosteroids, both of which were insignificantly higher in infants (P>0.05). The median duration of antibiotic therapy did not differ between the groups (P=0.786). Eight children (5.3%) received antibiotics (P=0.685), and aspirin was administered to 5 infants (P=0.351). The other medications are shown in Table 4.
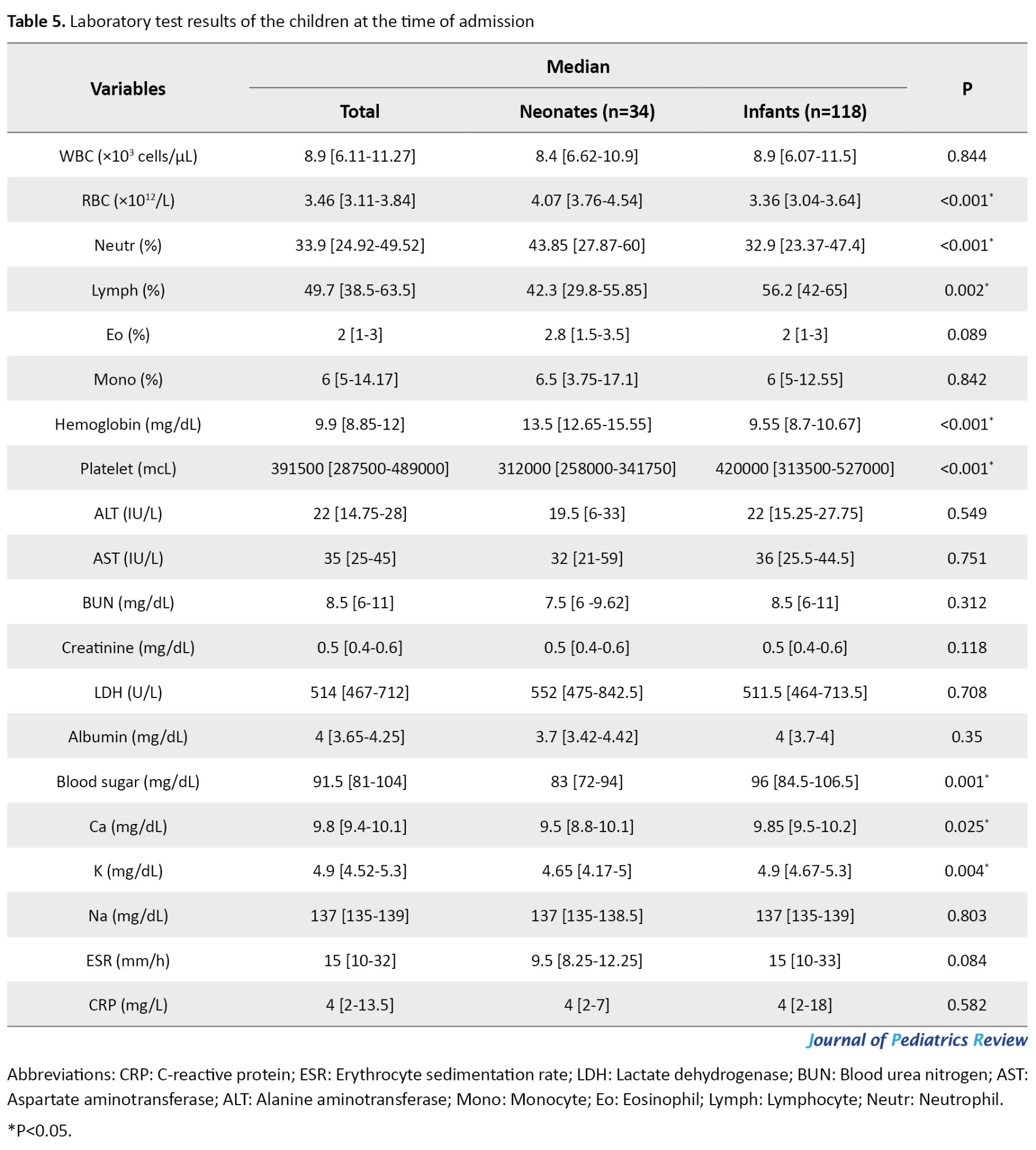
Neonates had significantly higher median red blood cell (RBC), neutrophil, and hemoglobin levels, and infants had significantly higher median lymphocyte and platelet levels (P<0.05). Leukocytosis (WBC >15.000) presented in 17(11.03%) and leukopenia (WBC <4.000) in 9 children (5.84%), and both were insignificantly higher in infants (P=0.765 and P=0.457, respectively). In addition, 39.61% had C-reactive protein (CRP) >5 mg/L, 24.02% had CRP>10 mg/L, and erythrocyte sedimentation rate (ESR)>30 mm/h presented in 32 patients (20.77%), and all of them were not different between the groups (P>0.05). Seventy-five children (48.7%) had hemoglobin levels of <10 mg/dL (P<0.001). Table 5 shows other laboratory test results.
Sepsis workup was performed in 82.9% of the patients, and lumbar puncture (LP) was performed in 42(27.63%), none of whom had a positive CSF culture. The analysis of symptoms and all variables based on gender was not statistically significant (P>0.05). Table 6 compares the critical parameters in both groups.
Discussion
The present study reports our experience with infants less than 3 months of age with COVID-19, and we found a 5% vertical transmission rate during delivery. The vertical transmission of COVID-19 in the third trimester is approximately 3.2%-4% [14, 25]. In a study by Hu et al. from seven infected pregnant mothers, one neonate was RT-PCR positive after birth [26], but no positive RT-PCR results were reported by Yang et al. on seven pregnant mothers [27]. Vertical transmission of COVID-19 is possible and seems to occur in a minority of cases of maternal infection in the third trimester [25]. Nonetheless, it remains unclear as none of these studies could persuasively claim mother-to-neonate transmission.
In this study, 42.8% had positive COVID-19 RT-PCR results, significantly higher in infants. In Spoulou et al.’s study, only 5.5% [20], and in the study by Lu et al., 12.3% were RT-PCR positive [28]. In a study in northern Iran, during the first three waves of the COVID-19 pandemic, 33.3% of neonates born to mothers with confirmed or probable COVID-19 infection had positive RT-PCR results [29]. Paret et al. reported a 15% SARS-CoV-2 infection rate in their study [30]. Most studies evaluated infants with laboratory-confirmed SARS-CoV-2 infection [8, 13]. Differences in results may be due to the availability of RT-PCR tests at different times, especially during the first months of the SARS-CoV-2 epidemic, when RT-PCR was not performed for all patients.
Sepsis workup and LP were performed in 82.9% and 27.63% of patients, respectively, and none had a positive CSF culture result. In addition, urine and blood cultures were positive in 11.8% and 2.6% of the patients, respectively. In a study by Paret et al., 3% of CSF cultures, 18% of urine cultures, and 15% of blood cultures were positive [30]. In Hassan et al.’s study, 3% of patients had a positive CSF culture, and no patient had a positive blood or urine culture [31]. Aronson et al. proposed eliminating the routine use of LP in managing fever in young infants who do not appear ill [32]. Based on our results, in case of a history of irritability, inconsolability, poor feeding, grunting breathing, seizure, poor urine output, and color changes such as pallor, mottling, or cyanosis in addition to hypothermia, performing LP is recommended in infants.
In this study, 63.2% had mild symptoms, 19.1% had moderate symptoms, and 17.8% had severe forms of the disease or MIS-C, and the severity of COVID-19 was not different between age groups. Similarly, in a study by Leibowitz et al., most COVID-19 infants had a mild course of infection [33]. In a study by Dong et al., most infections were mild (50.9%) or moderate (38.8%) [34]. In addition, severe/critical disease was present in 4.2% of Kanburoglu et al. [11] and 29.6% of Gale et al.’s study population [22]. In Dona, et al.’s study on infants <3 months, newborns were not at a higher risk of severe and critical infection compared with infants [8]. In a study by Parot et al., overall illness was mild to moderate [30]. All patients had mild illness with good outcomes and no mortality in the study by Hassan et al. [31].
According to our results, 63.2% of the children in their families had a history of COVID-19, which was significantly higher in infants. Dona et al. and Sobolewska-Pilarczyk et al. reported a 59% familial history of positive contact with COVID-19 [8, 13]. Among children aged <18 years with known exposure information, 91% were exposed to a COVID-19 patient in a house or community [6]. In Bellini et al.’s study, 92% had at least one parent who tested positive [1]. Therefore, household transmission of COVID-19 to children is a concern at the time of infection among family members [35].
We found fever and cough to be the most common symptoms, followed by poor feeding, respiratory distress, lethargy, diarrhea, inconsolability, and rhinorrhea, in which cough, diarrhea, and inconsolability were significantly higher in infants. Respiratory distress and wheezing occurred in 32.9% and 22.4% of the patients, respectively, and both were significantly higher in infants. Although other physical examination findings were more prevalent in infants, they were not statistically significant. In the Dona et al. study, the most common symptom was fever, followed by coryza, poor feeding, cough, and gastrointestinal manifestations; lethargy and respiratory distress were less frequently reported (3%-4%) [8]. Other studies have reported similar results [13, 28, 34, 36, 37]. Gastrointestinal symptoms were observed in 27% of the patients in the study by Sobolewska-Pilarczyk et al. [13].
In this study, approximately 90% of the patients received antibiotics for 4 [2-6] days. In infants, the use of ceftriaxone was significantly higher, whereas the use of gentamicin, amikacin, and ampicillin was more common in neonates. In Dona et al.’s study, among 15.27% of the children who needed medications, 84.84% received antibiotics [8]. Bhuiyan et al. reported that 71% of children were treated with antibiotics [14]. In a previous study, 42.8% of the infants received antibiotics [20]. In a study by Bellini et al., 15% of patients received antibiotic therapy [1]. In Hassan et al.’s study, 93.82% of the patients required antibiotics, cefotaxime and ampicillin were used empirically, and the duration of treatment was significantly shorter in the SARS-CoV-2-positive group (3.7 vs 7.5 days) [31]. While prophylactic treatment with antibiotics in infants with unknown sources of infection is routine in preventing bacteremia, urinary tract infection, or pneumonia, it still has limitations in populations with increased antimicrobial resistance [14].
In this study, 28.5% had SpO2 <94%, more than half of the patients needed oxygen therapy, and both were significantly higher in infants. In addition, 19.1% of the patients were admitted to the ICU, 3.9% underwent mechanical ventilation, and 1.54% expired. In the study by Dona et al., 4.6% required respiratory support, one preterm newborn needed ventilator support, 2.3% were admitted to the ICU, and they did not report any death [8]. In the study by Bhuiyan et al., 4.6% required respiratory support [14]. Only two out of 20 children required supplemental oxygen in the study by Leibowitz et al. [33]. Lu et al. reported 1.75% invasive mechanical ventilation and one death (0.58%) [28]. In a study by Bialek et al., 8.47% of patients were admitted to the ICU [6]. Sobolewska-Pilarczyk et al. reported one ICU admission without mechanical ventilation, and oxygen therapy was used in 2% of the cases [13]. The researchers did not include patients with severe COVID-19 in their study. Bialek et al. reported three deaths (0.002%) [6]. In the study by Paret et al., 22% needed respiratory support, 18% needed oxygen, 3% underwent mechanical ventilation, 33% were admitted to the ICU, and one child expired (1%) [30]. In Bellini et al.’s study on 39 infants under 6 months, only one child required ICU admission and underwent mechanical ventilation [1]. None of the patients in the study by Hassan et al. had SpO2 <94%, and 8.6% required ICU admission [31].
Our results showed abnormal leukocyte counts (leukocytosis and leukopenia) in 16.87% of the patients, and 24.02% had CRP levels >10 mg/L. Henry et al. reported 30.8% abnormal leukocyte counts and 13.6% elevated CRP [38]. Sobolewska-Pilarczyk et al. found elevated CRP levels in 18% of the patients [13]. Dhir et al. reported leukocytosis, lymphopenia, thrombocytopenia, and elevated inflammatory markers as the main laboratory evidence of COVID-19 infection in infants [7]. In the study by Hassan et al., 18.09% of the patients had CRP levels >5 mg/L [31]. Dona’ et al. reported 9.7% leukocytosis, 4.6% lymphopenia, and 15.7% elevated CRP [8]. Lymphopenia was present in 3.5% of the patients in Lu et al.’s study [28]. Dhir et al. reported leukocytosis, lymphopenia, thrombocytopenia, and elevated inflammatory markers as the main laboratory evidence of COVID-19 infection in infants [7]. The limited number of severe clinical COVID-19 cases may partly explain the low number of lymphopenia cases in our children.
Conclusion
Although this study showed a 5% vertical transmission rate, only delivery from a mother with COVID-19 or having a positive COVID-19 RT-PCR test result without symptoms is not a reason for a sepsis workup. Moreover, in infants under 3 months who are hospitalized due to confirmed COVID-19 and do not have sepsis symptoms, there is no indication for LP procedure.
Limitations
This study had several limitations; its retrospective design could introduce bias. First, the limited number of severe COVID-19 cases may have influenced the statistical power of our study. Our results highlight the need for a unique scoring system for COVID-19 in the pediatric population, especially for less than 3-month-old infants. Statistical comparisons could not be performed because of the high percentage of missing laboratory and imaging data. We could not evaluate any possible long-term sequelae in our study population. Therefore, further studies with long-term evaluation are needed to determine the complications of COVID-19.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.REC.1402.17714). All ethical principles of the Helsinki Declaration have been met, and informed consent has been obtained from the parents of all participants.
Funding
This study was extracted from the general medical doctorate thesis of Saeid Mohammadi, approved by Faculty of Medicine, Mazandaran University of Medical Sciences (Code: 22677). This study was supported by the Mazandaran University of Medical Science, Sari, Iran (Grant No.: 17714).
Authors contributions
Conceptualization: Mohammad Sadegh Rezai; Methodology: Mohammad Sadegh Rezai and Fatemeh Hosseinzadeh; Data curation: Mohammad Sadegh Rezai, Fatemeh Varshoei, Fatemeh Hosseinzadeh, and Saeid Mohammadi; Analysis: Masoud Moradi; Investigation: Fatemeh Varshoei, Fatemeh Hosseinzadeh, Roya Farhadi, and Saeid Mohammadi; Writing: Fatemeh Varshoei, Fatemeh Hosseinzadeh, Azin Hajialibeig, and Mohammad Sadegh Rezai; Supervision: Mohammad Sadegh Rezai and Fatemeh Varshoei; Fnal approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to thank all the medical, nursing, and supportive staff of the Buali and Imam Khomeini, Sari hospitals, Iran, for their dedication to caring for patients during this epidemic.
References
SARS-CoV-2-associated infection known as COVID-19 (novel coronavirus disease 2019) is a disease appeared in China, in 2019 [1]. This pandemic has affected the world’s population, including children [2]. Clinical manifestations of COVID-19 in children range from asymptomatic infection to severe pneumonia and a multisystem inflammatory system in children (MIS-C) [3, 4]. Early infancy could be a risk factor for infectious disease due to inadequately developed immune system during the first months of life and more active innate immune responses among children [5]. The clinical picture in neonates and early infants with COVID-19 has been described in some reports, suggesting that they can develop a more severe form of the disease than older children [1, 6-13]. Newborn and young infants are usually asymptomatic or present with mild symptoms like older children [8], presumably because of their healthier respiratory tracts and fewer underlying diseases [12].
According to previous reports on newborns and infants [8, 14], fever was the most reported symptom in infants under 3 months, followed by rhinorrhea and cough. Unlike adults, infants are more likely to present with gastrointestinal symptoms, such as diarrhea and vomiting. Moderate to severe symptoms, such as poor feeding, lethargy, and respiratory distress, have been reported less frequently [13, 15]. In addition, MIS-C diagnosis is increasing in neonates, a condition that subsequently evolves as a post-infectious inflammatory condition associated with abnormal immune function, left ventricular cardiac dysfunction, coronary artery aneurysm, atrioventricular block, and clinical deterioration with multi-organ involvement. MIS-C has increased the importance of research in this age group [16-19].
During the initial waves of COVID-19, when the Alpha and Delta variants were predominant, few cases of neonates and young infants were reported in the United States, where children younger than one year old represented less than 1% of all cases [20]. During the fifth wave, when the Omicron variant was predominant, more cases were reported in children 0-4 years old. The hospitalization rate was five times higher than the previous peak of Delta variant predominance [21]. Most hospitalized children (63%) were healthy with no underlying medical conditions; infants under 6 months old accounted for 44% of hospitalizations [21, 12]. Over time, the diagnosis and treatment of COVID-19 in different age groups have improved, but our knowledge about the clinical manifestations, disease course, and outcomes in neonates and early infants is still limited [12]. However, some researchers have shown a potentially higher risk of severe disease [8, 22] and a higher rate of hospitalization [9] in neonates and young infants compared to older children. Accurately determining the number of neonates infected with COVID-19 is impossible. COVID-19-positive infants had a much higher hospitalization rate than other age groups in an American study [9]. The lack of pediatric cases and their unknown outcomes result in difficulty in making a clinical diagnosis in neonates [23].
Most studies conducted in this field have only considered data related to hospitalized infants and have only partially examined the full spectrum of the disease in this age group [19]. Therefore, given the existing scientific gaps and the absence of approved vaccines for infants, understanding the characteristics associated with hospitalization, severe illness due to COVID-19, prognosis, and outcome in this age group will help inform clinical management and public health interventions. Considering that no study has been conducted on hospitalized children under three months of age in Iran, this study aimed to determine the demographic data, clinical manifestations, and laboratory characteristics of hospitalized neonates and infants under three months of age with COVID-19 to manage preventive measures against COVID-19 in this age group.
Methods
Study design, participants, and settings
This retrospective study was performed at Bouali and Imam Khomeini tertiary hospitals in Sari City, Mazandaran Province, northern Iran, between March 2019 and March 2022. Patients diagnosed with COVID-19 infection were categorized into two groups based on age: younger than 30 days, referred to as the “neonate” group, and those 1-3 months old, referred to as the “infant” group. Neonates with the clinical signs of COVID-19 were also evaluated. Neonates born to mothers with positive real‐time reverse transcriptase‐polymerase chain reaction (RT-PCR) results were admitted following birth for observation. In infants, the diagnostic criteria were the diagnostic criteria for a positive RT-PCR result, clinical signs of COVID-19, and a history of close contact with a known COVID-19 case. The severity of COVID-19 was defined according to World Health Organization (WHO) guidelines [24] as follows: Mild with mild clinical symptoms with no radiographic findings compatible with pneumonia, moderate cases with fever, respiratory symptoms, and radiographic findings compatible with pneumonia with <30% pulmonary involvement, severe/MIS-C cases showing one of the following factors; respiratory distress (retraction, grunting, central cyanosis, etc.), tachypnea (respiratory rate ≥30/min), hypoxemia, requiring supplemental oxygen and oxygen saturation (SpO2) <94% in the air room, >50% pulmonary involvement.
Demographic data, such as age, sex, comorbidity, clinical characteristics and symptoms, administered therapies, and laboratory tests, including complete blood count (CBC)-diff, blood and urine culture, cerebrospinal fluid (CSF) analysis, and culture, were extracted from the patient’s medical records. In addition, the prognosis, duration of hospitalization, admission to the intensive care unit (neonate or pediatric), need for mechanical ventilation, and mortality were recorded. The clinical signs or symptoms of COVID-19 include fever, cough, poor feeding, dyspnea or respiratory distress, lethargy, diarrhea, inconsolability, rhinorrhea or nasal congestion, vomiting, sneezing, and hypotonia. Physical examination findings were recorded, such as tachypnea, retraction, fine crackles, wheezes, tachycardia, conjunctivitis, rash, and hypotension.
Statistical analysis
The results are presented as Mean±SD or median with interquartile range (IQR) for continuous variables and frequency with percentage for categorical variables in each group. According to the Kolmogorov-Smirnov test, the distribution of quantitative variables was not normal. Therefore, the Kruskal-Wallis test was used for intergroup comparisons. Univariate and multivariate logistic regression Cox proportional hazard models were used to determine the factors affecting disease severity during the hospitalization for COVID-19 inpatients. Statistical analysis was performed using SPSS software, version 22. P<0.05 were considered to be statistically significant.
Results
This study evaluated 206 children, including 88 neonates (42.71%) and 118 infants (57.28%). Sixty neonates were born to COVID-19-positive mothers and admitted following birth for observation. Three (5%) had a positive RT-PCR test result and respiratory distress syndrome, which required ICU admission and antibiotic therapy. All of them were born by cesarean section and did not present with fever or cough during hospitalization. Other 54 RT-PCR-negative neonates were excluded, and finally, 34 neonates (22.4%) and 118 infants (77.6%) with a median age of 49.5 [IQR: 33-66] days were included in the final analysis.
The frequencies of mild, moderate, and severe/MIS-C cases were 96(63.2%), 29(19.1%), and 27(17.8%), respectively (P=0.879), and 3 infants in the severe group had MIS-C. Overall, 90 patients (59.2%) were boys, and 62(40.8%) were girls, and there was no difference between the two groups (P=0.695). One hundred and thirteen children (74.3%) lived in urban areas.
The median gestational age was 38 [IQR: 36.5-39] weeks and did not differ between the groups (P=0.108). In addition, 111 patients (73%) were term (gestational age >37 weeks) and 33(21.7%) were preterm (gestational age <37 weeks) (P=1). The mode of delivery in 107(70.4%) children was cesarean section, and 36(23.7%) had a natural vaginal delivery that did not differ between the groups (P=0.488). Eighty-one children (53.3%) were breastfeeding, 19(12.5%) used formula feeding, and 52(34.2%) used both methods and did not differ between the groups (P=0.733).
COVID-19 RT-PCR test was performed in 125 children, and 65(42.8%) were detected positive for the virus, and infants were more likely to be RT-PCR positive (P=0.022). Ninety-six children (63.2%) had a history of COVID-19 in their families, significantly higher among infants (53.8% vs 20%, P=0.031). In total, 3 children (2%) expired, all infants (Table 1).

Fifty-five children (26.21%) had a history of hospitalization without a significant difference between the two groups (P=0.105). Overall, 27 children (17.8%) had comorbid diseases including G6PDd (14, 9.2%), congenital heart disease (9, 5.9%), seizures (3, 2%), congenital anomalies (2, 1.3%), and thyroid or renal diseases (2, 1.3%) which were not statistically different between the groups (P=0.8).
In terms of symptoms in children, fever was the most common symptom (75%), followed by cough (56.6%), poor feeding (46.1%), dyspnea/respiratory distress (32.9%), and lethargy (32.2%). The most common finding on physical examination was tachypnea (30.9%), followed by retraction (27%), fine crackles (24.3%), and wheezing (22.4%) (Table 2).

An oxygen saturation level <94% was observed in 39 patients (28.5%), and 87 children (57.2%) required oxygen therapy (P=0.043 and P=0.017, respectively). In addition, the duration of ICU stay was significantly higher in neonates (16 [IQR: 12.5-26.75] vs 4 [IQR: 3-7], P<0.001), 29 patients (19.1%) required ICU admission, and 6 patients (3.9%) underwent mechanical ventilation (P=0.463 and P=0.126, respectively) (Table 3).

The median duration of hospital stay was 5 days [IQR: 4-7 days], which was significantly longer in neonates (7 [IQR: 5-13.25] vs 5 [IQR: 3-7] days, P<0.001). The median fever duration was 4 [IQR: 0-5] days which was significantly longer in infants (4 [IQR: 5-5.25] vs 3 [IQR: 0-4] days, P=0.006).
Approximately 137 patients (90.1%) received antibiotics, and 22(14.5%) received corticosteroids, both of which were insignificantly higher in infants (P>0.05). The median duration of antibiotic therapy did not differ between the groups (P=0.786). Eight children (5.3%) received antibiotics (P=0.685), and aspirin was administered to 5 infants (P=0.351). The other medications are shown in Table 4.

Neonates had significantly higher median red blood cell (RBC), neutrophil, and hemoglobin levels, and infants had significantly higher median lymphocyte and platelet levels (P<0.05). Leukocytosis (WBC >15.000) presented in 17(11.03%) and leukopenia (WBC <4.000) in 9 children (5.84%), and both were insignificantly higher in infants (P=0.765 and P=0.457, respectively). In addition, 39.61% had C-reactive protein (CRP) >5 mg/L, 24.02% had CRP>10 mg/L, and erythrocyte sedimentation rate (ESR)>30 mm/h presented in 32 patients (20.77%), and all of them were not different between the groups (P>0.05). Seventy-five children (48.7%) had hemoglobin levels of <10 mg/dL (P<0.001). Table 5 shows other laboratory test results.

Sepsis workup was performed in 82.9% of the patients, and lumbar puncture (LP) was performed in 42(27.63%), none of whom had a positive CSF culture. The analysis of symptoms and all variables based on gender was not statistically significant (P>0.05). Table 6 compares the critical parameters in both groups.

Discussion
The present study reports our experience with infants less than 3 months of age with COVID-19, and we found a 5% vertical transmission rate during delivery. The vertical transmission of COVID-19 in the third trimester is approximately 3.2%-4% [14, 25]. In a study by Hu et al. from seven infected pregnant mothers, one neonate was RT-PCR positive after birth [26], but no positive RT-PCR results were reported by Yang et al. on seven pregnant mothers [27]. Vertical transmission of COVID-19 is possible and seems to occur in a minority of cases of maternal infection in the third trimester [25]. Nonetheless, it remains unclear as none of these studies could persuasively claim mother-to-neonate transmission.
In this study, 42.8% had positive COVID-19 RT-PCR results, significantly higher in infants. In Spoulou et al.’s study, only 5.5% [20], and in the study by Lu et al., 12.3% were RT-PCR positive [28]. In a study in northern Iran, during the first three waves of the COVID-19 pandemic, 33.3% of neonates born to mothers with confirmed or probable COVID-19 infection had positive RT-PCR results [29]. Paret et al. reported a 15% SARS-CoV-2 infection rate in their study [30]. Most studies evaluated infants with laboratory-confirmed SARS-CoV-2 infection [8, 13]. Differences in results may be due to the availability of RT-PCR tests at different times, especially during the first months of the SARS-CoV-2 epidemic, when RT-PCR was not performed for all patients.
Sepsis workup and LP were performed in 82.9% and 27.63% of patients, respectively, and none had a positive CSF culture result. In addition, urine and blood cultures were positive in 11.8% and 2.6% of the patients, respectively. In a study by Paret et al., 3% of CSF cultures, 18% of urine cultures, and 15% of blood cultures were positive [30]. In Hassan et al.’s study, 3% of patients had a positive CSF culture, and no patient had a positive blood or urine culture [31]. Aronson et al. proposed eliminating the routine use of LP in managing fever in young infants who do not appear ill [32]. Based on our results, in case of a history of irritability, inconsolability, poor feeding, grunting breathing, seizure, poor urine output, and color changes such as pallor, mottling, or cyanosis in addition to hypothermia, performing LP is recommended in infants.
In this study, 63.2% had mild symptoms, 19.1% had moderate symptoms, and 17.8% had severe forms of the disease or MIS-C, and the severity of COVID-19 was not different between age groups. Similarly, in a study by Leibowitz et al., most COVID-19 infants had a mild course of infection [33]. In a study by Dong et al., most infections were mild (50.9%) or moderate (38.8%) [34]. In addition, severe/critical disease was present in 4.2% of Kanburoglu et al. [11] and 29.6% of Gale et al.’s study population [22]. In Dona, et al.’s study on infants <3 months, newborns were not at a higher risk of severe and critical infection compared with infants [8]. In a study by Parot et al., overall illness was mild to moderate [30]. All patients had mild illness with good outcomes and no mortality in the study by Hassan et al. [31].
According to our results, 63.2% of the children in their families had a history of COVID-19, which was significantly higher in infants. Dona et al. and Sobolewska-Pilarczyk et al. reported a 59% familial history of positive contact with COVID-19 [8, 13]. Among children aged <18 years with known exposure information, 91% were exposed to a COVID-19 patient in a house or community [6]. In Bellini et al.’s study, 92% had at least one parent who tested positive [1]. Therefore, household transmission of COVID-19 to children is a concern at the time of infection among family members [35].
We found fever and cough to be the most common symptoms, followed by poor feeding, respiratory distress, lethargy, diarrhea, inconsolability, and rhinorrhea, in which cough, diarrhea, and inconsolability were significantly higher in infants. Respiratory distress and wheezing occurred in 32.9% and 22.4% of the patients, respectively, and both were significantly higher in infants. Although other physical examination findings were more prevalent in infants, they were not statistically significant. In the Dona et al. study, the most common symptom was fever, followed by coryza, poor feeding, cough, and gastrointestinal manifestations; lethargy and respiratory distress were less frequently reported (3%-4%) [8]. Other studies have reported similar results [13, 28, 34, 36, 37]. Gastrointestinal symptoms were observed in 27% of the patients in the study by Sobolewska-Pilarczyk et al. [13].
In this study, approximately 90% of the patients received antibiotics for 4 [2-6] days. In infants, the use of ceftriaxone was significantly higher, whereas the use of gentamicin, amikacin, and ampicillin was more common in neonates. In Dona et al.’s study, among 15.27% of the children who needed medications, 84.84% received antibiotics [8]. Bhuiyan et al. reported that 71% of children were treated with antibiotics [14]. In a previous study, 42.8% of the infants received antibiotics [20]. In a study by Bellini et al., 15% of patients received antibiotic therapy [1]. In Hassan et al.’s study, 93.82% of the patients required antibiotics, cefotaxime and ampicillin were used empirically, and the duration of treatment was significantly shorter in the SARS-CoV-2-positive group (3.7 vs 7.5 days) [31]. While prophylactic treatment with antibiotics in infants with unknown sources of infection is routine in preventing bacteremia, urinary tract infection, or pneumonia, it still has limitations in populations with increased antimicrobial resistance [14].
In this study, 28.5% had SpO2 <94%, more than half of the patients needed oxygen therapy, and both were significantly higher in infants. In addition, 19.1% of the patients were admitted to the ICU, 3.9% underwent mechanical ventilation, and 1.54% expired. In the study by Dona et al., 4.6% required respiratory support, one preterm newborn needed ventilator support, 2.3% were admitted to the ICU, and they did not report any death [8]. In the study by Bhuiyan et al., 4.6% required respiratory support [14]. Only two out of 20 children required supplemental oxygen in the study by Leibowitz et al. [33]. Lu et al. reported 1.75% invasive mechanical ventilation and one death (0.58%) [28]. In a study by Bialek et al., 8.47% of patients were admitted to the ICU [6]. Sobolewska-Pilarczyk et al. reported one ICU admission without mechanical ventilation, and oxygen therapy was used in 2% of the cases [13]. The researchers did not include patients with severe COVID-19 in their study. Bialek et al. reported three deaths (0.002%) [6]. In the study by Paret et al., 22% needed respiratory support, 18% needed oxygen, 3% underwent mechanical ventilation, 33% were admitted to the ICU, and one child expired (1%) [30]. In Bellini et al.’s study on 39 infants under 6 months, only one child required ICU admission and underwent mechanical ventilation [1]. None of the patients in the study by Hassan et al. had SpO2 <94%, and 8.6% required ICU admission [31].
Our results showed abnormal leukocyte counts (leukocytosis and leukopenia) in 16.87% of the patients, and 24.02% had CRP levels >10 mg/L. Henry et al. reported 30.8% abnormal leukocyte counts and 13.6% elevated CRP [38]. Sobolewska-Pilarczyk et al. found elevated CRP levels in 18% of the patients [13]. Dhir et al. reported leukocytosis, lymphopenia, thrombocytopenia, and elevated inflammatory markers as the main laboratory evidence of COVID-19 infection in infants [7]. In the study by Hassan et al., 18.09% of the patients had CRP levels >5 mg/L [31]. Dona’ et al. reported 9.7% leukocytosis, 4.6% lymphopenia, and 15.7% elevated CRP [8]. Lymphopenia was present in 3.5% of the patients in Lu et al.’s study [28]. Dhir et al. reported leukocytosis, lymphopenia, thrombocytopenia, and elevated inflammatory markers as the main laboratory evidence of COVID-19 infection in infants [7]. The limited number of severe clinical COVID-19 cases may partly explain the low number of lymphopenia cases in our children.
Conclusion
Although this study showed a 5% vertical transmission rate, only delivery from a mother with COVID-19 or having a positive COVID-19 RT-PCR test result without symptoms is not a reason for a sepsis workup. Moreover, in infants under 3 months who are hospitalized due to confirmed COVID-19 and do not have sepsis symptoms, there is no indication for LP procedure.
Limitations
This study had several limitations; its retrospective design could introduce bias. First, the limited number of severe COVID-19 cases may have influenced the statistical power of our study. Our results highlight the need for a unique scoring system for COVID-19 in the pediatric population, especially for less than 3-month-old infants. Statistical comparisons could not be performed because of the high percentage of missing laboratory and imaging data. We could not evaluate any possible long-term sequelae in our study population. Therefore, further studies with long-term evaluation are needed to determine the complications of COVID-19.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.REC.1402.17714). All ethical principles of the Helsinki Declaration have been met, and informed consent has been obtained from the parents of all participants.
Funding
This study was extracted from the general medical doctorate thesis of Saeid Mohammadi, approved by Faculty of Medicine, Mazandaran University of Medical Sciences (Code: 22677). This study was supported by the Mazandaran University of Medical Science, Sari, Iran (Grant No.: 17714).
Authors contributions
Conceptualization: Mohammad Sadegh Rezai; Methodology: Mohammad Sadegh Rezai and Fatemeh Hosseinzadeh; Data curation: Mohammad Sadegh Rezai, Fatemeh Varshoei, Fatemeh Hosseinzadeh, and Saeid Mohammadi; Analysis: Masoud Moradi; Investigation: Fatemeh Varshoei, Fatemeh Hosseinzadeh, Roya Farhadi, and Saeid Mohammadi; Writing: Fatemeh Varshoei, Fatemeh Hosseinzadeh, Azin Hajialibeig, and Mohammad Sadegh Rezai; Supervision: Mohammad Sadegh Rezai and Fatemeh Varshoei; Fnal approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors want to thank all the medical, nursing, and supportive staff of the Buali and Imam Khomeini, Sari hospitals, Iran, for their dedication to caring for patients during this epidemic.
References
- Bellini T, Rotulo GA, Caruggi S, Carta S, Bonato I, Piccotti E. Characteristics of COVID-19 patients up to 6 months of age admitted to a paediatric emergency department. Acta Paediatr. 2022; 111(2):272-4. [DOI:10.1111/apa.16166] [PMID]
- Rezai MS, Ahangarkani F, Hill A, Ellis L, Mirchandani M, Davoudi A, et al. Non-effectiveness of ivermectin on inpatients and outpatients with covid-19; results of two randomized, double-blinded, placebo-controlled clinical trials. Front Med (Lausanne). 2022; 9:919708. [DOI:10.3389/fmed.2022.919708] [PMID]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1708-20. [DOI:10.1056/NEJMoa2002032] [PMID]
- Hajialibeig A, Navaeifar MR, Bordbari AH, Hosseinzadeh F, Rostami-Maskopaee F, Rezai MS. Clinical characteristics of COVID-19 in children: A large multicenter study from Iran. Front Pediatr. 2024; 12:1398106. [DOI:10.3389/fped.2024.1398106] [PMID]
- Navaeifar MR, Poudineh Ghazaghi M, Shahbaznejad L, Rouhanizadeh H, Abutalebi M, Ranjbar Varandi M, et al. Fever with rash is one of the first presentations of covid-19 in children: A case report. Int Med Case Rep J. 2020; 13:335-40. [DOI:10.2147/IMCRJ.S262935] [PMID]
- CDC COVID-19 Response Team. Coronavirus Disease 2019 in children - United States, February 12-April 2, 2020. MMWR Morb Mortal Wkly Rep. 2020; 69(14):422-6. [DOI:10.15585/mmwr.mm6914e4] [PMID]
- Dhir SK, Kumar J, Meena J, Kumar P. Clinical features and outcome of SARS-CoV-2 infection in Neonates: A systematic review. J Trop Pediatr. 2021; 67(3):fmaa059. [DOI:10.1093/tropej/fmaa059] [PMID]
- Dona' D, Montagnani C, Di Chiara C, Venturini E, Galli L, Lo Vecchio A, et al. COVID-19 in Infants Less than 3 Months: Severe or not severe disease? Viruses. 2022; 14(10):2256.[DOI:10.3390/v14102256] [PMID]
- Green J, Petty J, Bromley P, Walker K, Jones L. COVID-19 in babies: Knowledge for neonatal care. J Neonatal Nurs. 2020; 26(5):239-46. [DOI:10.1016/j.jnn.2020.06.005] [PMID]
- Iijima H, Funaki T, Kubota M. Clinical features and outcomes of coronavirus disease 2019 in early infants in Japan: A case series and literature review. J Infect Chemother. 2022; 28(4):582-6. [DOI:10.1016/j.jiac.2021.12.026] [PMID]
- Kanburoglu MK, Tayman C, Oncel MY, Akin IM, Can E, Demir N, et al. A multicentered study on epidemiologic and clinical characteristics of 37 neonates with community-acquired covid-19. Pediatr Infect Dis J. 2020; 39(10):e297-302. [DOI:10.1097/INF.0000000000002862] [PMID]
- Khoury L, Pillar G, Shehadeh S. COVID-19 in neonates and infants younger than 6 months - a mild viral illness. Eur J Pediatr. 2023; 182(7):3287-91. [DOI:10.1007/s00431-023-05016-x] [PMID]
- Sobolewska-Pilarczyk M, Pokorska-Śpiewak M, Stachowiak A, Marczyńska M, Talarek E, Ołdakowska A, et al. COVID-19 infections in infants. Sci Rep. 2022; 12(1):7765. [DOI:10.1038/s41598-022-11068-0] [PMID]
- Bhuiyan MU, Stiboy E, Hassan MZ, Chan M, Islam MS, Haider N, et al. Epidemiology of COVID-19 infection in young children under five years: A systematic review and meta-analysis. Vaccine. 2021; 39(4):667-77. [DOI:10.1016/j.vaccine.2020.11.078] [PMID]
- Sedighi I, Fahimzad A, Pak N, Khalili M, Shokrollahi MR, Heydari H, et al. A multicenter retrospective study of clinical features, laboratory characteristics, and outcomes of 166 hospitalized children with coronavirus disease 2019 (COVID-19): A preliminary report from Iranian Network for Research in Viral Diseases (INRVD). Pediatr Pulmonol. 2022; 57(2):498-507. [DOI:10.1002/ppul.25756] [PMID]
- More K, Aiyer S, Goti A, Parikh M, Sheikh S, Patel G, et al. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: A case series. Eur J Pediatr. 2022; 181(5):1883-98. [DOI:10.1007/s00431-022-04377-z] [PMID]
- Navaeifar MR, Shahbaznejad L, Sadeghi Lotfabadi A, Rezai MS. COVID-19-associated multisystem inflammatory syndrome complicated with giant coronary artery aneurysm. Case Rep Pediatr. 2021; 2021:8836403.[DOI:10.1155/2021/8836403] [PMID]
- Shahbaznejad L, Navaeifar MR, Abbaskhanian A, Hosseinzadeh F, Rahimzadeh G, Rezai MS. Clinical characteristics of 10 children with a pediatric inflammatory multisystem syndrome associated with COVID-19 in Iran. BMC Pediatr. 2020; 20(1):513. [DOI:10.1186/s12887-020-02415-z] [PMID]
- Shahbaznejad L, Rouhanizadeh H, Navaeifar MR, Hosseinzadeh F, Movahedi FS, Rezai MS. Clinical characteristics and outcomes of covid-19 in children in northern Iran. Int J Pediatr. 2021; 2021:5558287. [DOI:10.1155/2021/5558287] [PMID]
- Spoulou V, Noni M, Koukou D, Kossyvakis A, Michos A. Clinical characteristics of COVID-19 in neonates and young infants. Eur J Pediatr. 2021; 180(9):3041-5. [DOI:10.1007/s00431-021-04042-x] [PMID]
- Marks KJ, Whitaker M, Agathis NT, Anglin O, Milucky J, Patel K, et al. Hospitalization of infants and children aged 0-4 years with laboratory-confirmed COVID-19-COVID-NET, 14 states, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. 2022; 71(11):429-36. [DOI:10.15585/mmwr.mm7111e2] [PMID]
- Gale C, Quigley MA, Placzek A, Knight M, Ladhani S, Draper ES, et al. Characteristics and outcomes of neonatal SARS-CoV-2 infection in the UK: A prospective national cohort study using active surveillance. Lancet Child Adolesc Health. 2021; 5(2):113-21. [DOI:10.1016/S2352-4642(20)30342-4] [PMID]
- Li C, Luo F, Wu B. A 3-month-old child with COVID-19: A case report. Medicine (Baltimore). 2020; 99(23):e20661. [DOI:10.1097/MD.0000000000020661] [PMID]
- Rahimzadeh G, Ekrami Noghabi M, Kadkhodaei Elyaderani F, Navaeifar MR, Enayati AA, Manafi Anari A, et al. COVID-19 infection in Iranian children: A case series of 9 patients. J Pediatr Rev. 2020; 8(2):139-44. [DOI:10.32598/jpr.8.2.139]
- Kotlyar AM, Grechukhina O, Chen A, Popkhadze S, Grimshaw A, Tal O, et al. Vertical transmission of coronavirus disease 2019: A systematic review and meta-analysis. Am J Obstet Gynecol. 2021; 224(1):35-53.e3. [DOI:10.1016/j.ajog.2020.07.049] [PMID]
- Hu X, Gao J, Luo X, Feng L, Liu W, Chen J, et al. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission in neonates born to mothers with coronavirus disease 2019 (COVID-19) pneumonia. Obstet Gynecol. 2020; 136(1):65-67. [DOI:10.1097/AOG.0000000000003926] [PMID]
- Yang P, Wang X, Liu P, Wei C, He B, Zheng J, et al. Clinical characteristics and risk assessment of newborns born to mothers with COVID-19. J Clin Virol. 2020; 127:104356. [DOI:10.1016/j.jcv.2020.104356] [PMID]
- Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. SARS-CoV-2 infection in children. N Engl J Med. 2020; 382(17):1663-5. [DOI:10.1056/NEJMc2005073] [PMID]
- Farhadi R, Ghaffari V, Mehrpisheh S, Moosazadeh M, Haghshenas M, Ebadi A. Characteristics and outcome of infants born to mothers with SARS-CoV-2 infection during the first three waves of COVID-19 pandemic in northern Iran: A prospective cross-sectional study. Ann Med Surg (Lond). 2022; 78:103839. [DOI:10.1016/j.amsu.2022.103839] [PMID]
- Paret M, Lalani K, Hedari C, Jaffer A, Narayanan N, Noor A, et al. SARS-CoV-2 among infants< 90 days of age admitted for serious bacterial infection evaluation. Pediatrics. 2021; 148(4):e2020044685. [DOI:10.1542/peds.2020-044685] [PMID]
- Hassan M, Khalil A, Magboul S, Alomari O, Abdalla T, Alsliman H, et al. Neonates and young infants with COVID-19 presented with sepsis-like syndrome: A retrospective case controlled study. Front Pediatr. 2021; 9:634844.[DOI:10.3389/fped.2021.634844] [PMID]
- Aronson PL, Wang ME, Shapiro ED, Shah SS, DePorre AG, McCulloh RJ, et al. Risk stratification of febrile infants≤ 60 days old without routine lumbar puncture. Pediatrics. 2018; 142(6):e20181879. [DOI:10.1542/peds.2018-1879] [PMID]
- Leibowitz J, Krief W, Barone S, Williamson KA, Goenka PK, Rai S, et al. Comparison of clinical and epidemiologic characteristics of young febrile infants with and without severe acute respiratory syndrome coronavirus-2 infection. J Pediatr. 2021; 229:41-7.e1. [DOI:10.1016/j.jpeds.2020.10.002] [PMID]
- Dong Y, Mo X, Hu Y, Qi X, Jiang F, Jiang Z, et al. Epidemiology of covid-19 among children in China. Pediatrics. 2020; 145(6):e20200702. [DOI:10.1542/peds.2020-0702] [PMID]
- An P, Zhang M. Novel coronavirus SARS-CoV-2: Familial spread resulting in COVID-19 pneumonia in a pediatric patient. Diagn Interv Radiol. 2020; 26(3):262-3. [DOI:10.5152/dir.2020.20157] [PMID]
- Kainth MK, Goenka PK, Williamson KA, Fishbein JS, Subramony A, Barone S, et al. Early experience of covid-19 in a US children’s hospital. Pediatrics. 2020; 146(4):e2020003186. [DOI:10.1542/peds.2020-003186] [PMID]
- Jiehao C, Jin X, Daojiong L, Zhi Y, Lei X, Zhenghai Q, et al. A case series of children with 2019 novel coronavirus infection: Clinical and epidemiological features. Clin Infect Dis. 2020; 71(6):1547-51. [DOI:10.1093/cid/ciaa198] [PMID]
- Henry BM, Lippi G, Plebani M. Laboratory abnormalities in children with novel coronavirus disease 2019. Clin Chem Lab Med. 2020; 58(7):1135-8. [DOI:10.1515/cclm-2020-0272] [PMID]
Type of Study: Original Article |
Subject:
Pediatric Infectious Diseases
Received: 2024/11/4 | Accepted: 2024/11/16 | Published: 2024/10/1
Received: 2024/11/4 | Accepted: 2024/11/16 | Published: 2024/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






