Volume 13, Issue 2 (4-2025)
J. Pediatr. Rev 2025, 13(2): 151-160 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Naseri M, Tafazoli N, Nikrou A, Tafazoli N. Examining the Recurrence of Urinary Tract Infections in Children With a History of Acute Pyelonephritis. J. Pediatr. Rev 2025; 13 (2) :151-160
URL: http://jpr.mazums.ac.ir/article-1-698-en.html
URL: http://jpr.mazums.ac.ir/article-1-698-en.html
1- Department of Pediatric Nephrology, Dr Sheikh Children Hospital, Mashhad University of Medical Sciences, Mashhad, Iran. , naserim@mums.ac.ir
2- School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
2- School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
Full-Text [PDF 625 kb]
(758 Downloads)
| Abstract (HTML) (1787 Views)
Full-Text: (311 Views)
Introduction
By the age of 16 years, at least one episode of urinary tract infection (UTI) will happen in up to 11% of girls and 7% of boys. Recurrence of infection is common, 1/2 to 1/3 of cases will have at least one episode of recurrent UTI within 1 to 2 years. A renal scar develops in 3% to 15% of patients following febrile UTI [1-3]. Acute pyelonephritis (APN) potentially can result in renal scarring [4, 5]. Uncircumcised conditions in boys, bladder bowel dysfunction (BBD), constipation, and urological abnormalities are among the factors that increase the incidence of recurrent UTIs [6, 7].
The incidence of UTI in male children after circumcision was reduced by 90% [8, 9]. Non-neurogenic neurogenic bladder results in ineffective bladder emptying and UTI [10]. Establishing a suitable approach and identifying children at risk of renal parenchymal damage is not a simple task [11]. The First outcome of the current study is to define the rate of recurrent urinary tract infections and renal scarring in specific groups of children presented by APN. Secondly, this study determines the risk factors for these two conditions.
Methods
Study design and population
A cross-sectional study was performed from October 2003 to 2016 to evaluate the frequency of recurrent UTIs following APN in children ≤18 years old. The census method determined the sample size. The eligible cases were children with a confirmed diagnosis of APN. Ultrasound (US) examination and voiding cystourethrogram (VCUG) were recommended for all cases with a diagnosis of APN. The inclusion criteria were a normal kidney US and normal VCUG. Meanwhile, the exclusion criteria were abnormal VCUG, including presence of vesicoureteral reflux (VUR) or posterior urethral valve (PUV); abnormal kidney US, including presence of dilated renal pelvis (hydronephrosis), dilated ureter (hydroureter), decreased kidney size, decreased renal cortical thickness, or presence of renal scar.
Patients who underwent a technetium-99m dimercaptosuccinic acid scan (Tc99m-DMSA) scan were evaluated for renal scarring. The roles of confounders in recurrent UTI and renal scarring were assessed. They included age at the presentation of APN (median, ≤ one year vs > one year, ≤5 compared to >5 years), gender, abnormal bladder US, increased bladder wall thickness (BWT), bladder trabeculation, and chronic constipation.
The study was done in the nephrology clinic of a tertiary academic children’s hospital. Sampling of urine was by urinary bags and midstream methods in non- toilet-trained and toilet-trained, respectively. The growth of a single organism with a colony-forming unit/mL ≥105 was considered a positive urine culture (UTI). Leukocyturia was defined as a white blood cell count ≥5 in the high-power field of urinary sediment in a centrifuged urine sample. APN was defined as a UTI accompanied by body temperature ≥38.5 °C.
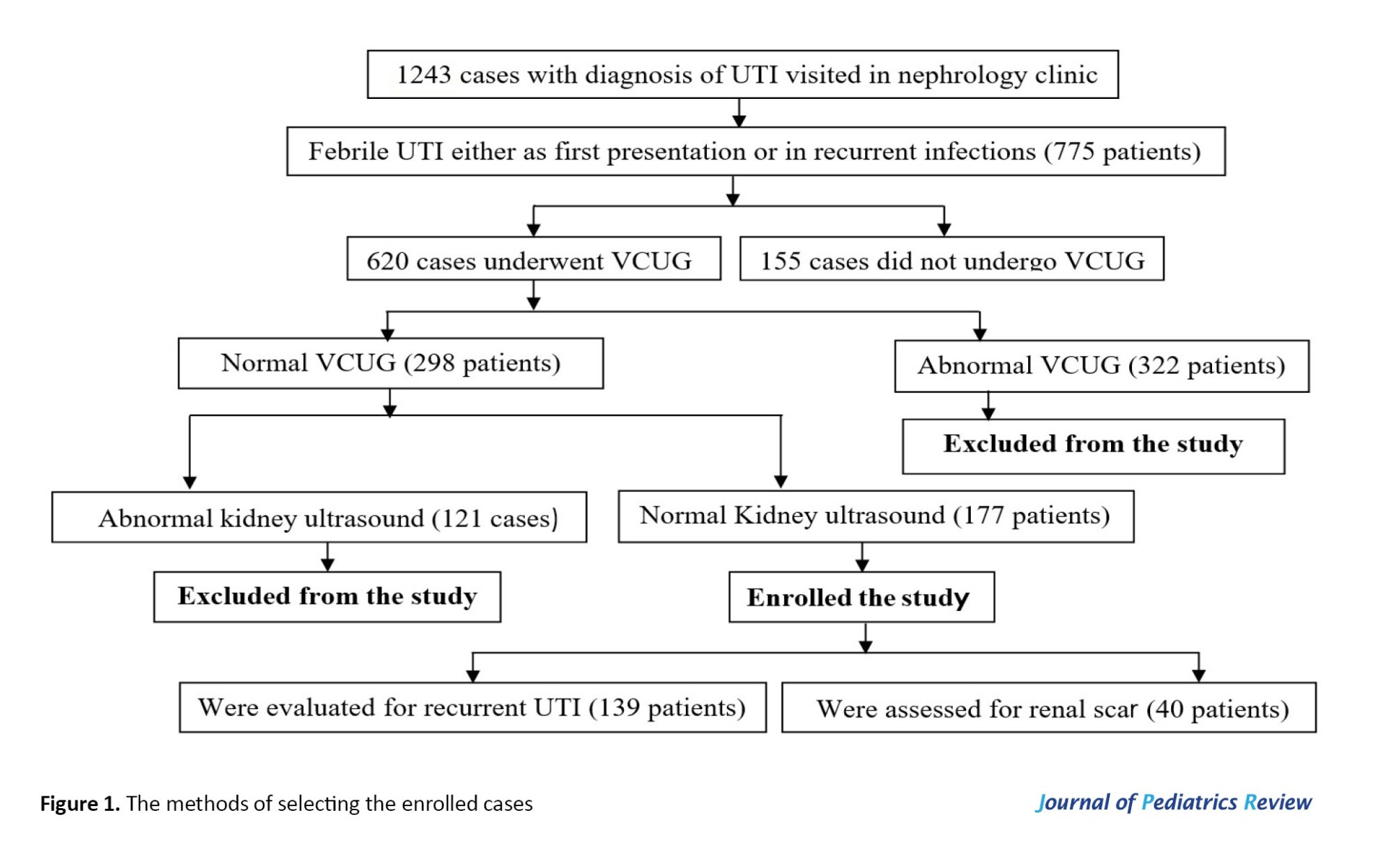
In patients whose urine samples were obtained by urine bags (non-toilet-trained patients), APN was confirmed in the presence of the following criteria: The presence of fever (body temperature ≥38.5 °C) + leukocyturia and positive urine culture. Figure 1 illustrates the methods of selecting the enrolled cases. Meanwhile, patients with a history of APN were included.
We recommended VCUG for any case of APN without considering age at presentation. Kidney-bladder US was done in the first days of diagnosis of UTI. Six months or later after APN, patients performed a Tc-99m DMSA scan to detect renal scarring. In addition, the following definitions were used in the study: Normal bladder US (Full BWT ≤3 mm and post-void urinary residual volume ≤5 mL); normal kidney US (absence of hydronephrosis [anterior-posterior diameter of renal pelvis ≥5 mm], hydro-ureter [ureteral diameter ≥4 mm], renal scarring, decreased renal cortical thickness, and decreased renal size); normal VCUG (absence of VUR and PUV); recurrence of UTI (at least one episode of UTI at follow-up, either cystitis or APN); renal scar (renal cortical uptake defect on Tc-99m-DMSA scan); grading of renal scar (no more than two scarred areas [grade I], more than two scars with some areas of normal parenchyma between them [grade II], generalized damage to the whole kidney [grade III]); shrunken kidney with little or no uptake of Tc-99M-DMSA (i.e. <10% of the overall function [grade IV]). Meanwhile, renal scars grades III and IV were considered severe scars [12].
Follow-up of the patients
Hospital admission was recommended for all children ≤2 years following APN. Outpatient management was considered in children >2 years. The second group was admitted to the hospital in the presence of high fever (body temperature ≥39 °C), vomiting, lack of response to oral antibiotic treatment (persistent fever for ≥48 h after starting the treatment), or unreliable parents. Antibiotic treatment was recommended for two weeks.
Patients were recommended to perform urine analysis (U/A) and culture one week after completing the course of treatment, then monthly for 3 months, and afterward in intervals of 2-3 months. Follow-up was stopped if there was no episode of recurrent UTIs for one year. In the case of recurrent APNs, prophylactic antibiotics were recommended for at least six months after the last UTI. Urodynamic studies were recommended in patients suspected of voiding dysfunction or neurogenic bladder. Presence of lower UTI (LUTs) in the absence of UTI was emphasized, and was considered as voiding dysfunction. These symptoms included enuresis, daytime wetting, urgency, urge incontinence, giggle incontinence, decreased and increased voiding frequency, and holding maneuver.
Statistical analysis
Descriptive statistics were expressed as Mean±SD or median and interquartile ranges (IQRs) and proportions. One-sample Kolmogorov-Smirnov test was used to define the normality of variables. Comparison of groups based on chi-square, Fisher exact, Mann-Whitney, and Post hoc Bonferroni tests. Meanwhile, P<0.05 was considered significant. The logistic regression analysis test was used to define risk factors. Also, a P<0.05 associated with an odds ratio >1 was considered a risk factor.
Results
Of 775 cases presented by APNs, 177 patients (22.8%) had normal kidney US and normal VCUG (Figure 1). The first episodes of UTIs were as APN in 154 patients (87%). In 23 cases (13%), the first presentation was as cystitis, and APN occurred during follow-up. A total of 38 cases (21.5%) lost follow-up. The recurrent rates of UTIs were evaluated in 139 patients (Figure 1).
Of 139 cases, 48.9% (n=68) were referred to the nephrology clinic following the second APNs. Their median age was 26 months (IQRs=10.6-55.5 months), and 94.2% (n=131) were girls. The median follow-up was 8 months (3-24 months). The etiologies of UTIs were Escherichia coli and non- E-coli organisms in 76(54.6%) and 14(10.1%) cases, respectively. The etiologies were not recorded in the medical files of 49 cases (35.3%). The recurrent UTIs occurred in 75 patients (54%).
The median follow-up in patients with recurrent UTIs was 12 months (IQR of 6-31 months). Those with no recurrent UTI followed up for a median of 4.75 months (IQR of 2-19 months; P=0.002). In total, 29.7% (n=19) of patients without and 52% (n=39) of the cases with recurrent UTIs had a follow-up of ≥1 year.
According to the duration of the follow-up, patients were divided into three groups (Table 1).
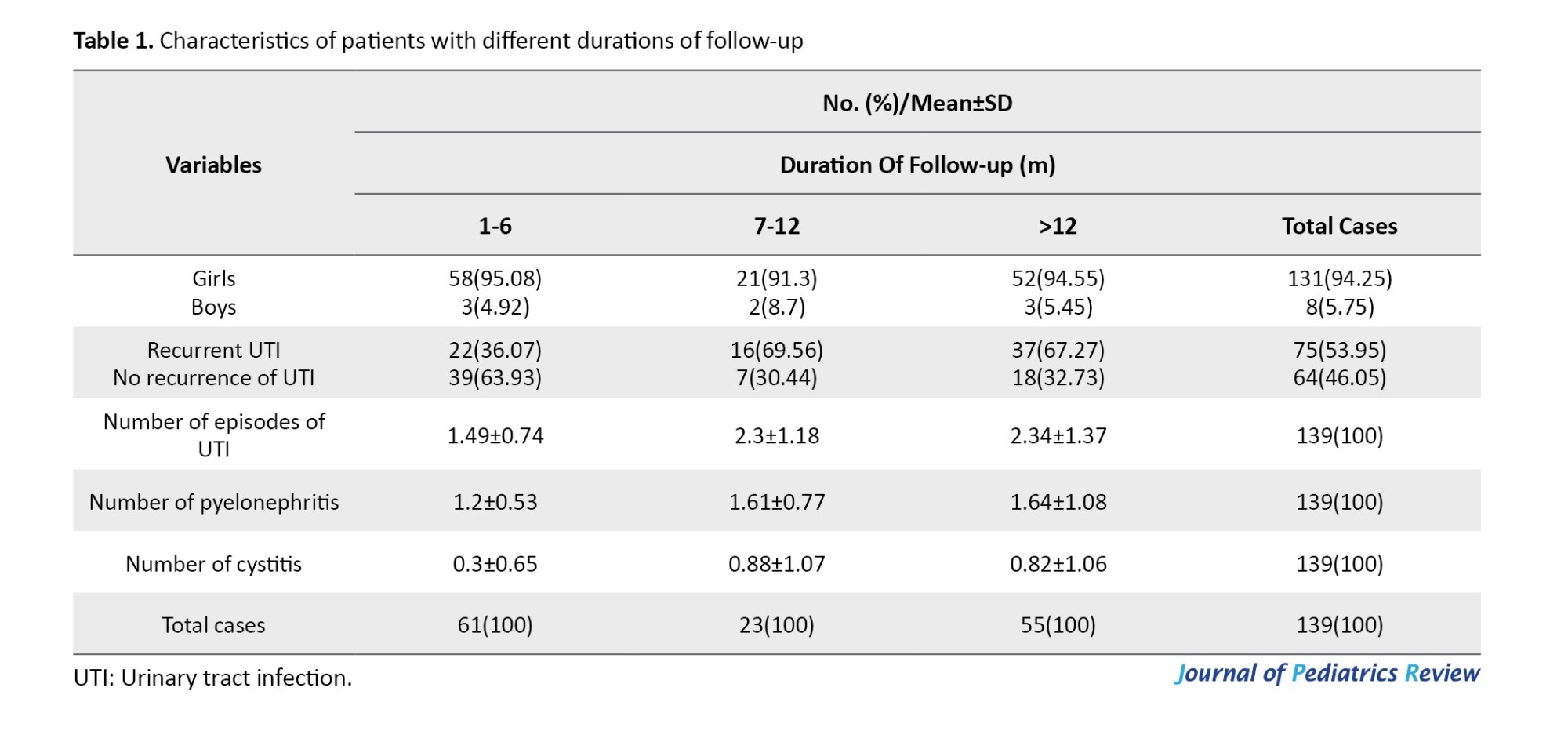
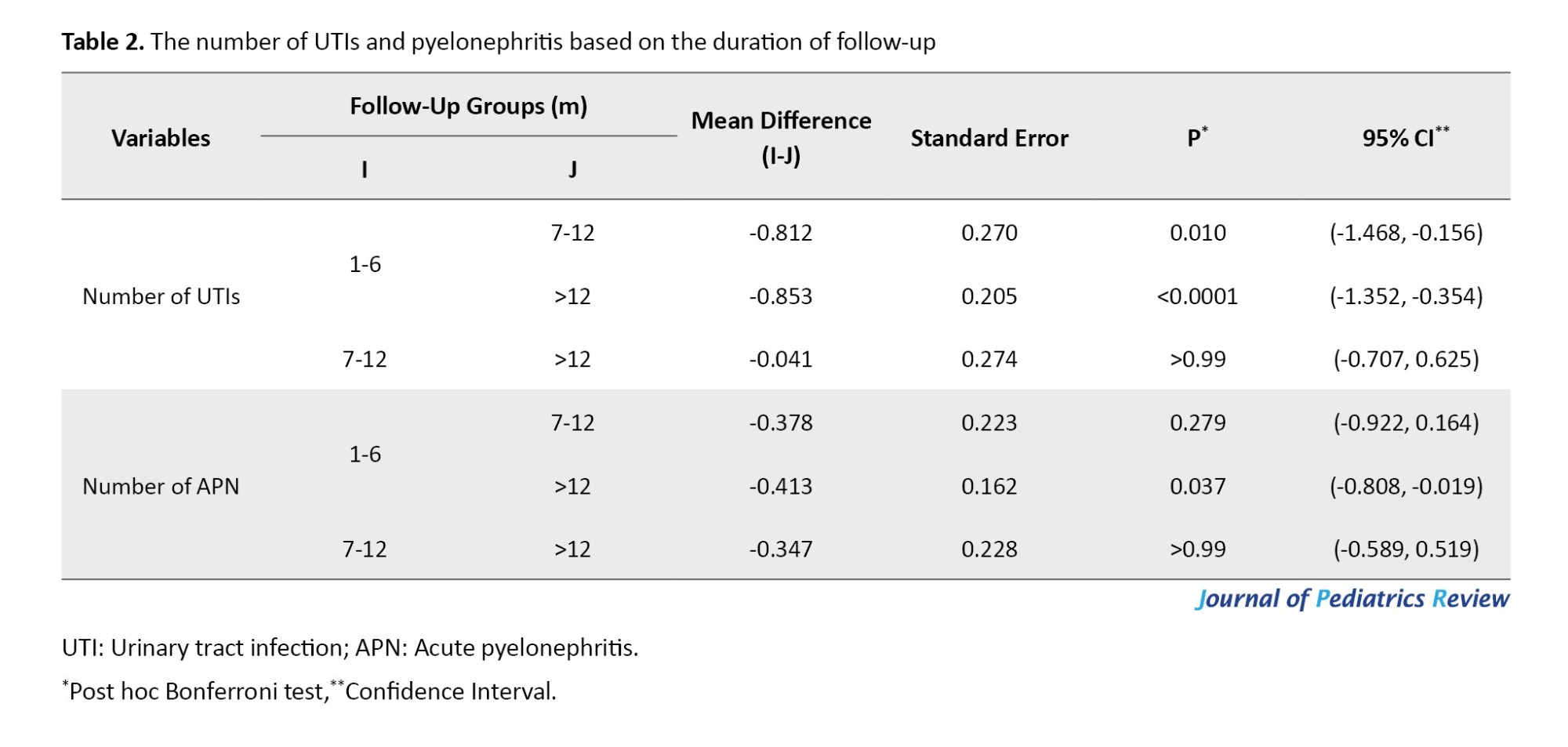
As Table 1 shows, recurrent UTIs were reported in 36%, 69.5%, and 67.3% of patients with follow-ups of 1-6, 7-12, and >12 months, respectively. Of 75 cases with recurrent UTIs, 49.3% (n=37) had a recurrence of infections in a follow-up of >1 year. The number of episodes of UTIs was significantly higher in patients with a 7-12-month vs 1-6-month follow-up (P=0.01) and in follow-up >12 months vs 1-6 months (P<0.0001; Table 2).
Patients with recurrent UTIs
Of 75 cases with recurrent UTIs, five cases (6.7%) were patients with a diagnosis of neurogenic bladder due to myelomeningocele (n=4) or cerebral palsy (n=1). Nine patients (12%) had LUTS, and urodynamic evaluations suggested voiding dysfunction as the etiology of APN and recurrent UTIs. Chronic constipation affected 21 cases (28%), including seven cases with either voiding dysfunction or neurogenic bladder. Two cases were uncircumcised boys (2.7%), and one patient had a horseshoe kidney. We found a possible factor for recurrent UTIs in 31 cases (41.3%).
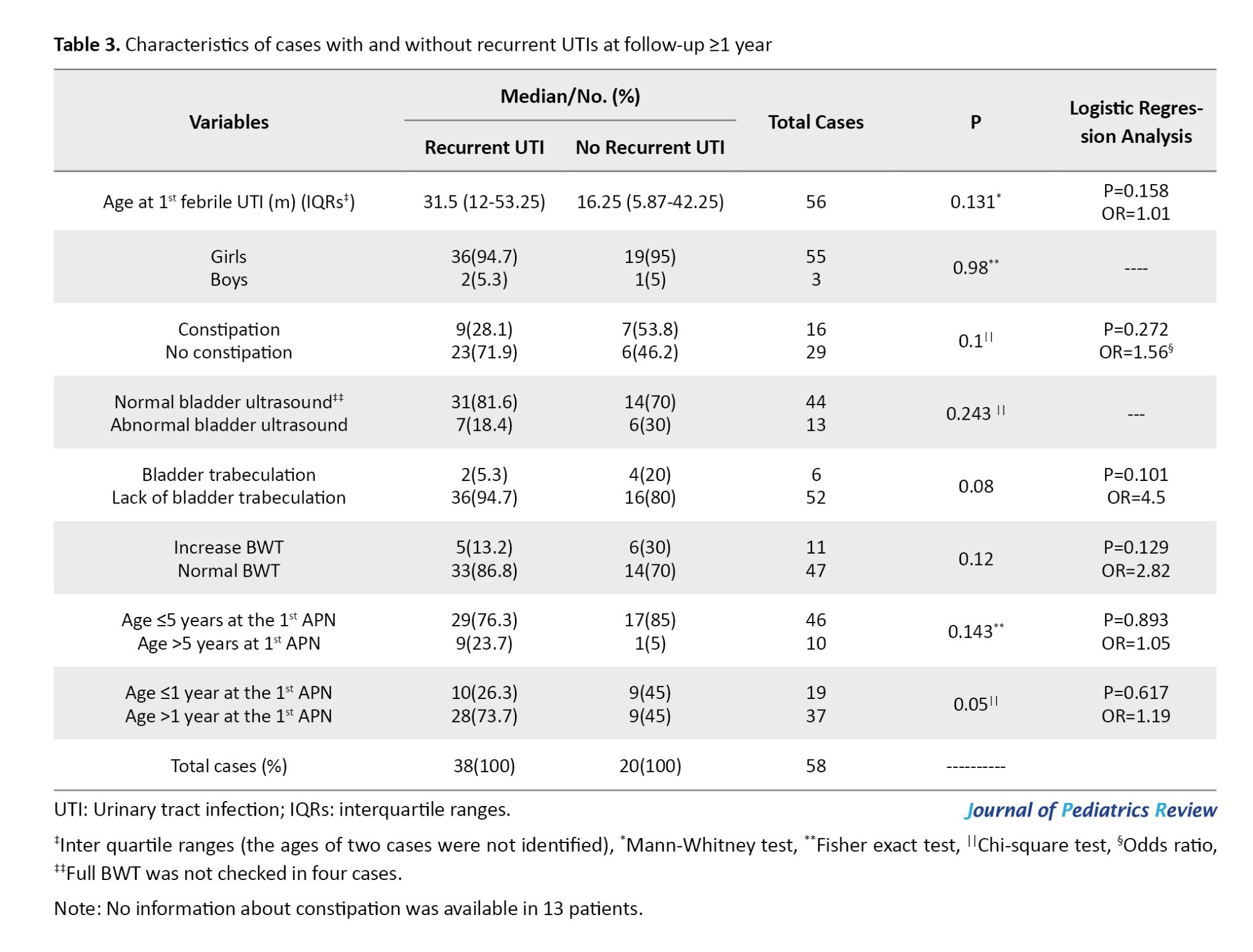
Of 75 cases with recurrent UTIs, the age of 17 patients (22.7%) at the first episode of APN was ≥5 years. The LUTS were reported in 14 cases (82.3%), including daytime incontinence (n=5), enuresis (n=3), decreased voiding frequency (n=3), holding maneuver (n=2), and urgency (n=1). Urodynamic studies were performed in three cases (17.6%), which were abnormal in one. Bladder US was abnormal in six cases (35.3%). In total, 58 out of 139 patients (41.7%) followed up for ≥12 months. They consisted of 55 girls (94.8%). Recurrent UTI was noted in 65.5% (n=38), and 52.6% was as APN. Patients who followed up for ≥12 months were divided into groups with and without recurrent UTIs.
Demographic characteristics, frequency of chronic constipation, and bladder US findings were compared between groups. Recurrent UTI was more prevalent in those older than one year at the first APN compared to cases ≤1 year (75.6% vs 52.6%, respectively; P=0.05). None of the other variables correlated with recurrent UTIs (P≥0.05). There was no risk factor for recurrent UTIs (Table 3).
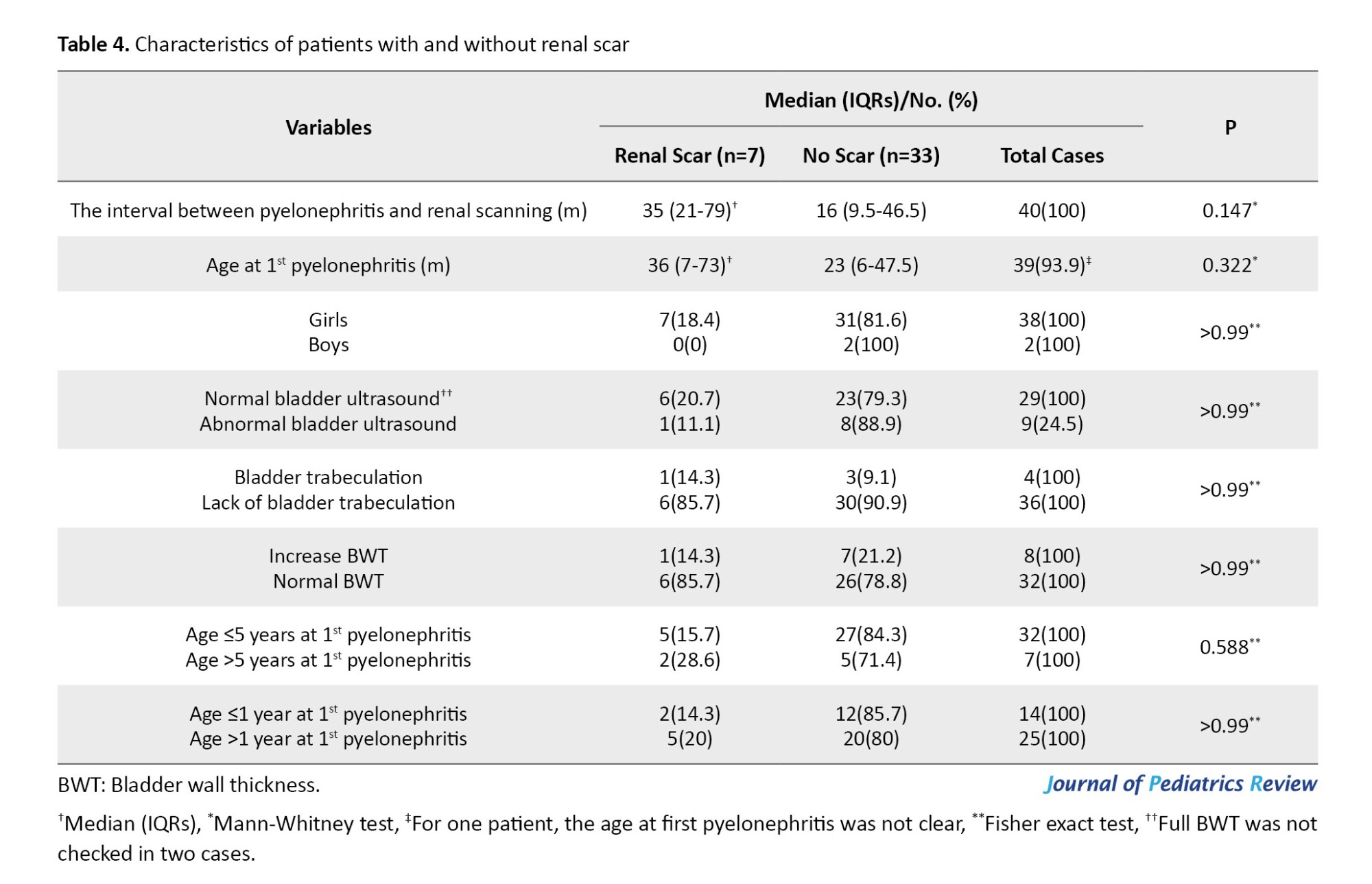
In total, 40 cases (28.8%) underwent a renal scan during follow-up. Renal scars developed in 7 out of the 40 cases, which represents 17.5%. The interval between the first APN and renal scanning was longer in patients with a renal scar than those without (median of 35 and 16 months, respectively; P=0.147). The interval between the first APN and renal scan was not a risk factor for renal scar development (P=0.2, odds ratio=1.01, 95% CI, 0.99%, 1.04%).
b
The present study evaluated the rate of recurrent UTIs and frequency of renal scarring in children presented by APNs. Enrolled cases had no abnormal findings in kidney US [hydronephrosis, hydro ureter, decreased kidney size, renal scarring, or renal cortical thinning. In addition, VCUG demonstrated the absence of VUR and PUV. Recurrence of UTIs was found in 54%, and renal scarring was detected in 17.5% of patients who underwent a Tc99m-DMSA scan. Gender, age at the first APN, and abnormal findings in bladder US were not risk factors for either recurrent UTI or renal scarring. In addition, chronic constipation was not a risk factor for the recurrence of infection.
There is no consensus and evidence-based guidelines for imaging children after their first UTI [13, 14]. After one episode of APN, 10%-15% of children develop renal scarring [15, 16]. Recurrent UTI and VUR significantly increase the risk of renal scarring. The majority of pyelonephritis in children occurred before 1-2 years of age, with a male predominance. In contrast to Lee et al. [17], we demonstrated a female predominance in 94.2% of the total, 93.8% of ≤2 years cases (61 out of 65 cases).
Age <6 months at the first UTI is suggested as an independent risk factor for recurrent UTIs (odds ratio=2.9) [6]. In our series, age <6 months was not an independent risk factor for the recurrence of UTI (P=0.455, odds ratio=1.42). Conway et al. [18] reported that ages between 3-4 years and 4-5 years were associated with recurrent UTIs. In the current study, ages between 3-4 years and 4-5 years were not independent risk factors for the recurrence of UTI (P=0.564, odds ratio=1.33, P=0.791, and odds ratio =1.7, respectively). Of the 177 eligible cases, 15(8.5%) and 18 patients (10.2%) were between 3-4 years and 4-5 years, respectively.
In our participants who followed up for ≥12 months, recurrent UTIs were more common in patients >1 vs ≤1 year (75.7% compared to 52.6%; P=0.05). Meanwhile, age >1 year was not an independent risk factor for the recurrence of UTIs (P=0.617 and odds ratio=1.19).
VUR and BBD are common risk factors for the recurrence of UTI, and circumcision reduces the risk in males [19, 20]. The role of constipation in the pathogenesis of pyelonephritis is well documented [21]. Shaikh et al. [7] reported that recurrent UTIs occurred in 51% of children with both bladder and bowel dysfunction (BBD) and VUR, 20% of those with VUR alone, 35% of those with BBD alone, and 32% of children with neither BBD nor VUR. Garout et al. [22] reported recurrent UTIs in 45.1% of children <5 years, 78% of girls, and 71% of boys presenting the first infections within the first year of life. Children who develop renal scars are older at the time of APN than those without scars [22]. In our series, the median ages of patients with and without renal scarring were 36 and 23 months, respectively (P=0.322).
APN caused by non-E coli organisms was more likely to be associated with renal scarring than E-coli (83% vs 57%) [23]. In our series, the etiologies of UTIs at the first APN were recorded in 23 cases (57.5%) who underwent a Tc99m-DMSA scan. Renal scarring was found in one of three (33.3%) with non-E coli and four of 20 cases (20%) with E-coli organisms (P=0.539). The recurrence rates of UTIs for non-E coli and E coli organisms were 50% (7 of 14) and 57.9% (44 out of 76), respectively (P=0.584).
Beiraghdar et al. reported a female predominance for UTI after age 2 years [24]. They reported a significantly higher frequency of renal scarring in children aged <1 year at the first presentation. In our series, 775 patients were referred by APNs, including 670(86.5%) girls and 105(13.5%) boys (female/male=6.38) (Figure 1), which indicates a female predominance in children with APNs. Of 758 cases with identified age at first APN, 317(41.8%) and 455 patients (60%) presented before age 1 and 2 years, respectively. Gender distribution in the first and second groups was 255(80.45%) girls and 62(19.55%) boys (female/male=4.1), and 385 girls (84.6%) and 70 boys (15.4%; female/male=5.5), respectively. In our series, there was no significant difference in the frequency of renal scarring between patients ≤1 year vs >1 year (14.3% vs 20%, respectively; P>0.99) [Table 4]). All boys, girls <3 years and girls 3-7 years with febrile UTIS were recommended to undergo cystography and ultrasonography following the first UTI [25].
Male gender, younger age at the first APN, VUR, recurrent UTIs, genetic predisposition, and delayed antibiotic treatment have been suggested risk factors for renal scarring after APN [26, 27]. Renal scarring develops in 15% to 60% of children following APN [28]. Suggested risk factors for renal scarring after APN include age at diagnosis, gender, history of recurrent UTIs, total white blood cell count, erythrocyte sedimentation rate, and creactive protein level at diagnosis of APN, bacterial virulence, and genetic factors [29-31].
Previous studies have mentioned VUR and BBD as factors with the highest 2-year recurrence rates for UTIs [32]. A group of our participants had evidence of bladder dysfunction (n=24 [17.2%]). They included seven patients with neurogenic bladder and 17 out of 30 (56.7%) children ≥5 years with LUTS suggestive of bladder dysfunction. Recurrent UTIs were found in 66.6% and 53.8% of patients with and without bladder dysfunction, respectively (P=0.225).
New renal scars develop in 52.1% of patients with VUR receiving continuous antibiotic prophylaxis [33]. In our cases, renal scans were not repeated to determine the new renal scar development. However, baseline DMSA scans reported renal scarring in 17.5% of cases. The frequency rate of renal scarring in the study by Su et al. [33] was about three times what we found. The reason for such a difference is that their patients included VUR cases, but our cases consisted of cases without VUR.
Neurogenic bladder, older age, the presence of VUR, increased BWT, and low bladder compliance are factors associated with recurrent UTIs [34]. In our series, patients with recurrent UTIs were older than those without (median age of 31.5 compared to 16.25 months, respectively; P=0.131). The increased BWT did not correlate with recurrent UTIs. It was reported in 20.3% and 18% of patients with and without recurrent UTIs, respectively (P=0.743).
A study by Bandari et al. [35] found that female gender, children <5 years, and infection with E-coli microorganism were the common risk factors associated with recurrent UTI and renal scarring. We found renal scarring in 33.3% of patients with non-E coli and 20% with E-coli UTIs (P=0.539). In addition, renal scarring was not correlated with age ≤5 vs >5 years (15.6% compared to 28.6%, respectively; P=0.588), and female gender (P>0.99; Table 4).
A recently published study [36] evaluated the frequency of renal scarring in children with febrile UTIs. The median age of patients was 7 years. The study included cases with and without VUR. Girls included 86.3% of the cases. In total, 66.9% had only one episode of APN. VUR was found in 46.8% of patients who underwent VCUG. Nine cases with VUR (60%) had renal scarring. Renal scarring was reported in 16.9% (n=27) and was significantly more prevalent in those with recurrent UTIs compared to than without recurrent UTIs (26.4 % and 12.5 %, respectively; P=0.04).
In our study, the median age was 26 months, 94.2% were girls, and 54% had recurrent UTIs. Although the participants in the study by Gökceoğlu et al. [36] consisted of patients diagnosed with VUR and cases without VUR, the frequency of renal scarring was similar to our finding (16.9% in their study and 17.5% in our study). A positive point of the mentioned study was assessing the role of recurrent UTIs in renal scarring. We missed this since most cases underwent the DMSA scan once, following the first APNs. The positive point in the current study compared to the mentioned study is excluding cases with a diagnosis of VUR. The role of VUR as a confounding variable for recurrent UTIs and renal scarring is well established.
Gökceoğlu et al. [36] found higher rates of renal scarring in children with recurrent UTI and bladder trabeculation and thickening of the bladder wall on US examination. In our series, rates of renal scarring in patients with bladder trabeculation were 25% (one of four) and 12.5% (one of eight) for cases with increased BWT. In total, 25% of cases with and 9.1% without scar, and 12.5% of patients with and 21.2% without scar had bladder trabeculation and increased BWT on US examination, respectively (P>0.99 for both).
Different studies focused on evaluating the risk factors of renal scarring following APN in children. The question is that other studies have found some risk factors [24, 28, 29, 31, 36]. Different methodologies may be the most important reason. Their participants were children with and without VUR. They did not exclude cases with VUR or abnormal kidney US from their studies. In addition, some of these studies include a special age group of children [24, 29].
Conclusion
Recurrent UTI is common in children with a history of APN and normal kidney ultrasound, as well as a normal VCUG (no VUR or PUV). In this special group of patients, recurrence of UTIs and renal scarring did not correlate with gender, age at the first presentation, abnormal findings in bladder US, and chronic constipation.
Study limitations
The limitations of the current study were losing follow-up in a group of patients, a lack of performing Tc99m-DMSA scan after recurrent APNs, and no assessment for renal scarring in about 70% of cases due to losing follow-up or unwillingness of parents.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.MEDICAL.REC.1397.317).
Funding
This study was funded by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No.: 961766).
Authors contributions
Conceptualization, supervision, and writing: All authors; Methodology and data validation: Mitra Naseri.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors highly appreciate the Research Development Section of Mashhad University of Medical Sciences, Mashhad, Iran, for providing scientific and financial support.
References
By the age of 16 years, at least one episode of urinary tract infection (UTI) will happen in up to 11% of girls and 7% of boys. Recurrence of infection is common, 1/2 to 1/3 of cases will have at least one episode of recurrent UTI within 1 to 2 years. A renal scar develops in 3% to 15% of patients following febrile UTI [1-3]. Acute pyelonephritis (APN) potentially can result in renal scarring [4, 5]. Uncircumcised conditions in boys, bladder bowel dysfunction (BBD), constipation, and urological abnormalities are among the factors that increase the incidence of recurrent UTIs [6, 7].
The incidence of UTI in male children after circumcision was reduced by 90% [8, 9]. Non-neurogenic neurogenic bladder results in ineffective bladder emptying and UTI [10]. Establishing a suitable approach and identifying children at risk of renal parenchymal damage is not a simple task [11]. The First outcome of the current study is to define the rate of recurrent urinary tract infections and renal scarring in specific groups of children presented by APN. Secondly, this study determines the risk factors for these two conditions.
Methods
Study design and population
A cross-sectional study was performed from October 2003 to 2016 to evaluate the frequency of recurrent UTIs following APN in children ≤18 years old. The census method determined the sample size. The eligible cases were children with a confirmed diagnosis of APN. Ultrasound (US) examination and voiding cystourethrogram (VCUG) were recommended for all cases with a diagnosis of APN. The inclusion criteria were a normal kidney US and normal VCUG. Meanwhile, the exclusion criteria were abnormal VCUG, including presence of vesicoureteral reflux (VUR) or posterior urethral valve (PUV); abnormal kidney US, including presence of dilated renal pelvis (hydronephrosis), dilated ureter (hydroureter), decreased kidney size, decreased renal cortical thickness, or presence of renal scar.
Patients who underwent a technetium-99m dimercaptosuccinic acid scan (Tc99m-DMSA) scan were evaluated for renal scarring. The roles of confounders in recurrent UTI and renal scarring were assessed. They included age at the presentation of APN (median, ≤ one year vs > one year, ≤5 compared to >5 years), gender, abnormal bladder US, increased bladder wall thickness (BWT), bladder trabeculation, and chronic constipation.
The study was done in the nephrology clinic of a tertiary academic children’s hospital. Sampling of urine was by urinary bags and midstream methods in non- toilet-trained and toilet-trained, respectively. The growth of a single organism with a colony-forming unit/mL ≥105 was considered a positive urine culture (UTI). Leukocyturia was defined as a white blood cell count ≥5 in the high-power field of urinary sediment in a centrifuged urine sample. APN was defined as a UTI accompanied by body temperature ≥38.5 °C.
In patients whose urine samples were obtained by urine bags (non-toilet-trained patients), APN was confirmed in the presence of the following criteria: The presence of fever (body temperature ≥38.5 °C) + leukocyturia and positive urine culture. Figure 1 illustrates the methods of selecting the enrolled cases. Meanwhile, patients with a history of APN were included.

We recommended VCUG for any case of APN without considering age at presentation. Kidney-bladder US was done in the first days of diagnosis of UTI. Six months or later after APN, patients performed a Tc-99m DMSA scan to detect renal scarring. In addition, the following definitions were used in the study: Normal bladder US (Full BWT ≤3 mm and post-void urinary residual volume ≤5 mL); normal kidney US (absence of hydronephrosis [anterior-posterior diameter of renal pelvis ≥5 mm], hydro-ureter [ureteral diameter ≥4 mm], renal scarring, decreased renal cortical thickness, and decreased renal size); normal VCUG (absence of VUR and PUV); recurrence of UTI (at least one episode of UTI at follow-up, either cystitis or APN); renal scar (renal cortical uptake defect on Tc-99m-DMSA scan); grading of renal scar (no more than two scarred areas [grade I], more than two scars with some areas of normal parenchyma between them [grade II], generalized damage to the whole kidney [grade III]); shrunken kidney with little or no uptake of Tc-99M-DMSA (i.e. <10% of the overall function [grade IV]). Meanwhile, renal scars grades III and IV were considered severe scars [12].
Follow-up of the patients
Hospital admission was recommended for all children ≤2 years following APN. Outpatient management was considered in children >2 years. The second group was admitted to the hospital in the presence of high fever (body temperature ≥39 °C), vomiting, lack of response to oral antibiotic treatment (persistent fever for ≥48 h after starting the treatment), or unreliable parents. Antibiotic treatment was recommended for two weeks.
Patients were recommended to perform urine analysis (U/A) and culture one week after completing the course of treatment, then monthly for 3 months, and afterward in intervals of 2-3 months. Follow-up was stopped if there was no episode of recurrent UTIs for one year. In the case of recurrent APNs, prophylactic antibiotics were recommended for at least six months after the last UTI. Urodynamic studies were recommended in patients suspected of voiding dysfunction or neurogenic bladder. Presence of lower UTI (LUTs) in the absence of UTI was emphasized, and was considered as voiding dysfunction. These symptoms included enuresis, daytime wetting, urgency, urge incontinence, giggle incontinence, decreased and increased voiding frequency, and holding maneuver.
Statistical analysis
Descriptive statistics were expressed as Mean±SD or median and interquartile ranges (IQRs) and proportions. One-sample Kolmogorov-Smirnov test was used to define the normality of variables. Comparison of groups based on chi-square, Fisher exact, Mann-Whitney, and Post hoc Bonferroni tests. Meanwhile, P<0.05 was considered significant. The logistic regression analysis test was used to define risk factors. Also, a P<0.05 associated with an odds ratio >1 was considered a risk factor.
Results
Of 775 cases presented by APNs, 177 patients (22.8%) had normal kidney US and normal VCUG (Figure 1). The first episodes of UTIs were as APN in 154 patients (87%). In 23 cases (13%), the first presentation was as cystitis, and APN occurred during follow-up. A total of 38 cases (21.5%) lost follow-up. The recurrent rates of UTIs were evaluated in 139 patients (Figure 1).
Of 139 cases, 48.9% (n=68) were referred to the nephrology clinic following the second APNs. Their median age was 26 months (IQRs=10.6-55.5 months), and 94.2% (n=131) were girls. The median follow-up was 8 months (3-24 months). The etiologies of UTIs were Escherichia coli and non- E-coli organisms in 76(54.6%) and 14(10.1%) cases, respectively. The etiologies were not recorded in the medical files of 49 cases (35.3%). The recurrent UTIs occurred in 75 patients (54%).
The median follow-up in patients with recurrent UTIs was 12 months (IQR of 6-31 months). Those with no recurrent UTI followed up for a median of 4.75 months (IQR of 2-19 months; P=0.002). In total, 29.7% (n=19) of patients without and 52% (n=39) of the cases with recurrent UTIs had a follow-up of ≥1 year.
According to the duration of the follow-up, patients were divided into three groups (Table 1).

As Table 1 shows, recurrent UTIs were reported in 36%, 69.5%, and 67.3% of patients with follow-ups of 1-6, 7-12, and >12 months, respectively. Of 75 cases with recurrent UTIs, 49.3% (n=37) had a recurrence of infections in a follow-up of >1 year. The number of episodes of UTIs was significantly higher in patients with a 7-12-month vs 1-6-month follow-up (P=0.01) and in follow-up >12 months vs 1-6 months (P<0.0001; Table 2).

Patients with recurrent UTIs
Of 75 cases with recurrent UTIs, five cases (6.7%) were patients with a diagnosis of neurogenic bladder due to myelomeningocele (n=4) or cerebral palsy (n=1). Nine patients (12%) had LUTS, and urodynamic evaluations suggested voiding dysfunction as the etiology of APN and recurrent UTIs. Chronic constipation affected 21 cases (28%), including seven cases with either voiding dysfunction or neurogenic bladder. Two cases were uncircumcised boys (2.7%), and one patient had a horseshoe kidney. We found a possible factor for recurrent UTIs in 31 cases (41.3%).
Of 75 cases with recurrent UTIs, the age of 17 patients (22.7%) at the first episode of APN was ≥5 years. The LUTS were reported in 14 cases (82.3%), including daytime incontinence (n=5), enuresis (n=3), decreased voiding frequency (n=3), holding maneuver (n=2), and urgency (n=1). Urodynamic studies were performed in three cases (17.6%), which were abnormal in one. Bladder US was abnormal in six cases (35.3%). In total, 58 out of 139 patients (41.7%) followed up for ≥12 months. They consisted of 55 girls (94.8%). Recurrent UTI was noted in 65.5% (n=38), and 52.6% was as APN. Patients who followed up for ≥12 months were divided into groups with and without recurrent UTIs.
Demographic characteristics, frequency of chronic constipation, and bladder US findings were compared between groups. Recurrent UTI was more prevalent in those older than one year at the first APN compared to cases ≤1 year (75.6% vs 52.6%, respectively; P=0.05). None of the other variables correlated with recurrent UTIs (P≥0.05). There was no risk factor for recurrent UTIs (Table 3).

In total, 40 cases (28.8%) underwent a renal scan during follow-up. Renal scars developed in 7 out of the 40 cases, which represents 17.5%. The interval between the first APN and renal scanning was longer in patients with a renal scar than those without (median of 35 and 16 months, respectively; P=0.147). The interval between the first APN and renal scan was not a risk factor for renal scar development (P=0.2, odds ratio=1.01, 95% CI, 0.99%, 1.04%).
b
The present study evaluated the rate of recurrent UTIs and frequency of renal scarring in children presented by APNs. Enrolled cases had no abnormal findings in kidney US [hydronephrosis, hydro ureter, decreased kidney size, renal scarring, or renal cortical thinning. In addition, VCUG demonstrated the absence of VUR and PUV. Recurrence of UTIs was found in 54%, and renal scarring was detected in 17.5% of patients who underwent a Tc99m-DMSA scan. Gender, age at the first APN, and abnormal findings in bladder US were not risk factors for either recurrent UTI or renal scarring. In addition, chronic constipation was not a risk factor for the recurrence of infection.
There is no consensus and evidence-based guidelines for imaging children after their first UTI [13, 14]. After one episode of APN, 10%-15% of children develop renal scarring [15, 16]. Recurrent UTI and VUR significantly increase the risk of renal scarring. The majority of pyelonephritis in children occurred before 1-2 years of age, with a male predominance. In contrast to Lee et al. [17], we demonstrated a female predominance in 94.2% of the total, 93.8% of ≤2 years cases (61 out of 65 cases).
Age <6 months at the first UTI is suggested as an independent risk factor for recurrent UTIs (odds ratio=2.9) [6]. In our series, age <6 months was not an independent risk factor for the recurrence of UTI (P=0.455, odds ratio=1.42). Conway et al. [18] reported that ages between 3-4 years and 4-5 years were associated with recurrent UTIs. In the current study, ages between 3-4 years and 4-5 years were not independent risk factors for the recurrence of UTI (P=0.564, odds ratio=1.33, P=0.791, and odds ratio =1.7, respectively). Of the 177 eligible cases, 15(8.5%) and 18 patients (10.2%) were between 3-4 years and 4-5 years, respectively.
In our participants who followed up for ≥12 months, recurrent UTIs were more common in patients >1 vs ≤1 year (75.7% compared to 52.6%; P=0.05). Meanwhile, age >1 year was not an independent risk factor for the recurrence of UTIs (P=0.617 and odds ratio=1.19).
VUR and BBD are common risk factors for the recurrence of UTI, and circumcision reduces the risk in males [19, 20]. The role of constipation in the pathogenesis of pyelonephritis is well documented [21]. Shaikh et al. [7] reported that recurrent UTIs occurred in 51% of children with both bladder and bowel dysfunction (BBD) and VUR, 20% of those with VUR alone, 35% of those with BBD alone, and 32% of children with neither BBD nor VUR. Garout et al. [22] reported recurrent UTIs in 45.1% of children <5 years, 78% of girls, and 71% of boys presenting the first infections within the first year of life. Children who develop renal scars are older at the time of APN than those without scars [22]. In our series, the median ages of patients with and without renal scarring were 36 and 23 months, respectively (P=0.322).
APN caused by non-E coli organisms was more likely to be associated with renal scarring than E-coli (83% vs 57%) [23]. In our series, the etiologies of UTIs at the first APN were recorded in 23 cases (57.5%) who underwent a Tc99m-DMSA scan. Renal scarring was found in one of three (33.3%) with non-E coli and four of 20 cases (20%) with E-coli organisms (P=0.539). The recurrence rates of UTIs for non-E coli and E coli organisms were 50% (7 of 14) and 57.9% (44 out of 76), respectively (P=0.584).
Beiraghdar et al. reported a female predominance for UTI after age 2 years [24]. They reported a significantly higher frequency of renal scarring in children aged <1 year at the first presentation. In our series, 775 patients were referred by APNs, including 670(86.5%) girls and 105(13.5%) boys (female/male=6.38) (Figure 1), which indicates a female predominance in children with APNs. Of 758 cases with identified age at first APN, 317(41.8%) and 455 patients (60%) presented before age 1 and 2 years, respectively. Gender distribution in the first and second groups was 255(80.45%) girls and 62(19.55%) boys (female/male=4.1), and 385 girls (84.6%) and 70 boys (15.4%; female/male=5.5), respectively. In our series, there was no significant difference in the frequency of renal scarring between patients ≤1 year vs >1 year (14.3% vs 20%, respectively; P>0.99) [Table 4]). All boys, girls <3 years and girls 3-7 years with febrile UTIS were recommended to undergo cystography and ultrasonography following the first UTI [25].

Male gender, younger age at the first APN, VUR, recurrent UTIs, genetic predisposition, and delayed antibiotic treatment have been suggested risk factors for renal scarring after APN [26, 27]. Renal scarring develops in 15% to 60% of children following APN [28]. Suggested risk factors for renal scarring after APN include age at diagnosis, gender, history of recurrent UTIs, total white blood cell count, erythrocyte sedimentation rate, and creactive protein level at diagnosis of APN, bacterial virulence, and genetic factors [29-31].
Previous studies have mentioned VUR and BBD as factors with the highest 2-year recurrence rates for UTIs [32]. A group of our participants had evidence of bladder dysfunction (n=24 [17.2%]). They included seven patients with neurogenic bladder and 17 out of 30 (56.7%) children ≥5 years with LUTS suggestive of bladder dysfunction. Recurrent UTIs were found in 66.6% and 53.8% of patients with and without bladder dysfunction, respectively (P=0.225).
New renal scars develop in 52.1% of patients with VUR receiving continuous antibiotic prophylaxis [33]. In our cases, renal scans were not repeated to determine the new renal scar development. However, baseline DMSA scans reported renal scarring in 17.5% of cases. The frequency rate of renal scarring in the study by Su et al. [33] was about three times what we found. The reason for such a difference is that their patients included VUR cases, but our cases consisted of cases without VUR.
Neurogenic bladder, older age, the presence of VUR, increased BWT, and low bladder compliance are factors associated with recurrent UTIs [34]. In our series, patients with recurrent UTIs were older than those without (median age of 31.5 compared to 16.25 months, respectively; P=0.131). The increased BWT did not correlate with recurrent UTIs. It was reported in 20.3% and 18% of patients with and without recurrent UTIs, respectively (P=0.743).
A study by Bandari et al. [35] found that female gender, children <5 years, and infection with E-coli microorganism were the common risk factors associated with recurrent UTI and renal scarring. We found renal scarring in 33.3% of patients with non-E coli and 20% with E-coli UTIs (P=0.539). In addition, renal scarring was not correlated with age ≤5 vs >5 years (15.6% compared to 28.6%, respectively; P=0.588), and female gender (P>0.99; Table 4).
A recently published study [36] evaluated the frequency of renal scarring in children with febrile UTIs. The median age of patients was 7 years. The study included cases with and without VUR. Girls included 86.3% of the cases. In total, 66.9% had only one episode of APN. VUR was found in 46.8% of patients who underwent VCUG. Nine cases with VUR (60%) had renal scarring. Renal scarring was reported in 16.9% (n=27) and was significantly more prevalent in those with recurrent UTIs compared to than without recurrent UTIs (26.4 % and 12.5 %, respectively; P=0.04).
In our study, the median age was 26 months, 94.2% were girls, and 54% had recurrent UTIs. Although the participants in the study by Gökceoğlu et al. [36] consisted of patients diagnosed with VUR and cases without VUR, the frequency of renal scarring was similar to our finding (16.9% in their study and 17.5% in our study). A positive point of the mentioned study was assessing the role of recurrent UTIs in renal scarring. We missed this since most cases underwent the DMSA scan once, following the first APNs. The positive point in the current study compared to the mentioned study is excluding cases with a diagnosis of VUR. The role of VUR as a confounding variable for recurrent UTIs and renal scarring is well established.
Gökceoğlu et al. [36] found higher rates of renal scarring in children with recurrent UTI and bladder trabeculation and thickening of the bladder wall on US examination. In our series, rates of renal scarring in patients with bladder trabeculation were 25% (one of four) and 12.5% (one of eight) for cases with increased BWT. In total, 25% of cases with and 9.1% without scar, and 12.5% of patients with and 21.2% without scar had bladder trabeculation and increased BWT on US examination, respectively (P>0.99 for both).
Different studies focused on evaluating the risk factors of renal scarring following APN in children. The question is that other studies have found some risk factors [24, 28, 29, 31, 36]. Different methodologies may be the most important reason. Their participants were children with and without VUR. They did not exclude cases with VUR or abnormal kidney US from their studies. In addition, some of these studies include a special age group of children [24, 29].
Conclusion
Recurrent UTI is common in children with a history of APN and normal kidney ultrasound, as well as a normal VCUG (no VUR or PUV). In this special group of patients, recurrence of UTIs and renal scarring did not correlate with gender, age at the first presentation, abnormal findings in bladder US, and chronic constipation.
Study limitations
The limitations of the current study were losing follow-up in a group of patients, a lack of performing Tc99m-DMSA scan after recurrent APNs, and no assessment for renal scarring in about 70% of cases due to losing follow-up or unwillingness of parents.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Mashhad University of Medical Sciences, Mashhad, Iran (Code: IR.MUMS.MEDICAL.REC.1397.317).
Funding
This study was funded by Mashhad University of Medical Sciences, Mashhad, Iran (Grant No.: 961766).
Authors contributions
Conceptualization, supervision, and writing: All authors; Methodology and data validation: Mitra Naseri.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors highly appreciate the Research Development Section of Mashhad University of Medical Sciences, Mashhad, Iran, for providing scientific and financial support.
References
- Paintsil E. Update on recent guidelines for the management of urinary tract infections in children: The shifting paradigm. Curr Opin Pediatr. 2013; ;25(1):88-94. [DOI:10.1097/MOP.0b013e32835c14cc] [PMID]
- Larcombe J. Urinary tract infection in children: Recurrent infections. BMJ Clin Evid. 2015; 2015:0306. [PMID]
- White B. Diagnosis and treatment of urinary tract infections in children. Am Fam Physician. 2011; 83(4):409-15. [PMID]
- Elder JS. Urinary tract infection. In: Kliegman RM, Stanton, BF, Geme JS, Schor NF, editors. Nelson Textbook of Pediatrics. Philadelphia: Elsevier Saunders; 2015. [Link]
- Panaretto K, Craig J, Knight J, Howman-Giles R, Sureshkumar P, Roy L. Risk factors for recurrent urinary tract infection in preschool children. J Paediatr Child Health. 1999; 35(5):454-9. [DOI:10.1046/j.1440-1754.1999.355417.x] [PMID]
- Tewary K, Narchi H. Recurrent urinary tract infections in children: Preventive interventions other than prophylactic antibiotics. World J Methodol. 2015; 5(2):13-9. [DOI:10.5662/wjm.v5.i2.13] [PMID]
- Shaikh N, Hoberman A, Keren R, Gotman N, Docimo SG, Mathews R, et al. Recurrent urinary tract infections in children with bladder and bowel dysfunction. Pediatrics. 2016; 137(1):e20152982. [DOI:10.1542/peds.2015-2982] [PMID]
- Bader M, McCarthy L. What is the efficacy of circumcision in boys with complex urinary tract abnormalities? Pediatr Nephrol. 2013; 28(12):2267-72. [DOI:10.1007/s00467-013-2410-2] [PMID]
- Shaikh N, Morone NE, Bost JE, Farrell MH. Prevalence of urinary tract infection in childhood: A meta-analysis. Pediatr Infect Dis J. 2008; 27(4):302-8. [DOI:10.1097/INF.0b013e31815e4122] [PMID]
- Mingin GC, Hinds A, Nguyen HT, Baskin LS. Children with a febrile urinary tract infection and a negative radiologic workup: Factors predictive of recurrence. Urology. 2004; 63(3):562-5; discussion 565. [DOI:10.1016/j.urology.2003.10.055] [PMID]
- Simões e Silva AC, Oliveira EA, Mak RH. Urinary tract infection in pediatrics: An overview. J Pediatr. 2020; 96(S1):65-79. [DOI:10.1016/j.jped.2019.10.006] [PMID]
- Goldraich NP, Rames OL, Goldraich IH. Urography versus Tc-99m DMSA scan in children with vesicoureteral reflux. Pediatr Nephrol. 1989; 3(1):1-5. [DOI:10.1007/BF00859614] [PMID]
- Schlager TA. Urinary tract infections in infants and children. Infect Dis Clin North Am. 2003; 17(2):353-65. [DOI:10.1016/S0891-5520(03)00009-6] [PMID]
- La Scola C, De Mutiis C, Hewitt IK, Puccio G, Toffolo A, Zucchetta P, et al. Different guidelines for imaging after first UTI in febrile infants: Yield, cost, and radiation. Pediatrics. 2013; 131(3):e665-71. [DOI:10.1542/peds.2012-0164] [PMID]
- Chisht ASi, Maul EC, Nazario RJ, Bennett JS, Kiesslinga SG. A guideline for the inpatient care of children with pyelonephritis. Ann Saudi Med. 2010; 30(5):341-9. [DOI:10.4103/0256-4947.68549] [PMID]
- Shaikh N, Ewing AL, Bhatnagar S, Hoberman A. Risk of renal scarring in children with a first urinary tract infection: A systematic review. Pediatrics. 2010; 126(6):1084-91.[DOI:10.1542/peds.2010-0685] [PMID]
- Lee KY. New Insights for Febrile Urinary Tract Infection (Acute Pyelonephritis) in Children. Child Kidney Dis. 2016; 20(2):37-44. [DOI:10.3339/jkspn.2016.20.2.37]
- Conway PH, Cnaan A, Zaoutis T, Henry BV, Grundmeier RW, Keren R. Recurrent urinary tract infections in children: Risk factors and association with prophylactic antimicrobials. JAMA. 2007; 298(2):179-86. [DOI:10.1001/jama.298.2.179] [PMID]
- Keren R, Shaikh N, Pohl H, Gravens-Mueller L, Ivanova A, Zaoutis L, et al. Risk factors for recurrent urinary tract infection and renal scarring. Pediatrics. 2015; 136(1):e13-21. [DOI:10.1542/peds.2015-0409] [PMID]
- Loening-Baucke V. Urinary incontinence and urinary tract infection and their resolution with treatment of chronic constipation of childhood. Pediatrics. 1997; 100(2 Pt 1):228-32. [DOI:10.1542/peds.100.2.228] [PMID]
- Jakobsson B, Berg U, Svensson L. Renal scarring after acute pyelonephritis. Arch Dis Child. 1994; 70(2):111-5. [DOI:10.1136/adc.70.2.111] [PMID]
- Garout WA, Kurdi HS, Shilli AH, Kari JA. Urinary tract infection in children younger than 5 years: Etiology and associated urological anomalies. Saudi Med J 2015; 36(4):497-501. [DOI:10.15537/smj.2015.4.10770] [PMID]
- Orellana P, Baquedano P, Rangarajan V, Zhao JH, Eng ND, Fettich J, et al. Relationship between acute pyelonephritis, renal scarring, and vesicoureteral reflux. Results of a coordinated research project. Pediatr Nephrol. 2004; 19(10):1122-6. [DOI:10.1007/s00467-004-1501-5] [PMID]
- Beiraghdar F, Panahi Y, Einollahi B, Moharamzad Y, Nemati E, Amirsalari S. Predisposing factors for renal scarring in children with urinary tract infection. Saudi J Kidney Dis Transpl. 2012; 23(3):532-7. [PMID]
- Team UG. Cincinnati Children’s Hospital Medical Center: Evidence-based care guideline for medical management of first urinary tract infection in children 12 years of age or less. Guideline. 2006; 7:1-23. [Link]
- Pohl HG, Belman AB. The "top-down" approach to the evaluation of children with febrile urinary tract infection. Adv Urol. 2009; 2009:783409. [DOI:10.1155/2009/783409] [PMID]
- Yılmaz S, Özçakar ZB, Kurt Şükür ED, Bulum B, Kavaz A, Elhan AH, et al. Vesicoureteral reflux and renal scarring risk in children after the First Febrile Urinary Tract Infection. Nephron. 2016; 132(3):175-80. [DOI:10.1159/000443536] [PMID]
- Faust WC, Diaz M, Pohl HG. Incidence of post pyelonephritic renal scarring: A met analysis of the dimercaptosuccinic acid literature. J Urol. 2009; 181(1):290-7; discussion 297-8. [DOI:10.1016/j.juro.2008.09.039] [PMID]
- Pecile P, Miorin E, Romanello C, Vidal E, Contardo M, Valent F, et al. Age-related renal parenchymal lesions in children with first febrile urinary tract infections. Pediatrics. 2009; 124(1):23-9. [DOI:10.1542/peds.2008-1192] [PMID]
- Wullt B, Bergsten G, Fischer H, Godaly G, Karpman D, Leijonhufvud I, et al The host response to urinary tract infection. Infect Dis Clin North Am. 2003; 17(2):279-301. [DOI:10.1016/S0891-5520(03)00028-X] [PMID]
- Lee JH, Son CH, Lee MS, Park YS. Vesicoureteral reflux increases the risk of renal scars: A study of unilateral reflux. Pediatr Nephrol. 2006; 21(9):1281-4. [DOI:10.1007/s00467-006-0147-x] [PMID]
- Mattoo TK, Shaikh N, Nelson CP. Contemporary management of urinary tract infection in children. Pediatrics. 2021; 147(2):e2020012138. [DOI:10.1542/peds.2020-012138] [PMID]
- Su D, Zhuo Z, Zhang J, Zhan Z, Huang H. Risk factors for new renal scarring in children with vesicoureteral reflux receiving continuous antibiotic prophylaxis. Sci Rep. 2024; 14(1):1784. [DOI:10.1038/s41598-024-52161-w] [PMID]
- Jiang M, Deng J, Zhou G, Li S, Liu G. Risk factors for recurrent urinary tract infection in children with neurogenic bladder following clean intermittent catheterization. Urology. 2022; 164:224-229. [DOI:10.1016/j.urology.2021.12.027] [PMID]
- Bandari B, Sindgikar SP, Kumar SS, Vijaya MS, Shankar R. Renal scarring following urinary tract infections in children. Sudan J Paediatr. 2019; 19(1):25-30. [DOI:10.24911/SJP.106-1554791193] [PMID]
- Gökceoğlu AU, Taş N. Renal scarring in children with febrile urinary tract infection. J Pediatr (Rio J). 2025; 101(3):370-4. [PMID]
Type of Study: Original Article |
Subject:
Pediatric Nephrology
Received: 2024/11/10 | Accepted: 2025/01/23 | Published: 2025/04/1
Received: 2024/11/10 | Accepted: 2025/01/23 | Published: 2025/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









