Volume 13, Issue 4 (10-2025)
J. Pediatr. Rev 2025, 13(4): 331-338 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Gooran M, Farhadi R, Ghaffari J, Taji M, Dabbaghzadeh A. Nebulized Salbutamol for Treatment of Transient Tachypnea of the Newborn: A Randomized Placebo-controlled Clinical Trial in Iran. J. Pediatr. Rev 2025; 13 (4) :331-338
URL: http://jpr.mazums.ac.ir/article-1-718-en.html
URL: http://jpr.mazums.ac.ir/article-1-718-en.html
1- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
2- Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,siamakdabbaghzadeh@gmail.com
2- Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,
Keywords: Transient tachypnea of the newborn (TTN), Salbutamol nebulizer, Silverman-Andersen respiratory severity (RSS) score
Full-Text [PDF 477 kb]
(307 Downloads)
| Abstract (HTML) (402 Views)
Assessments
Data, including gender, mode of delivery, birth weight, Apgar score, history of prelabor rupture of membranes (PROM), history of diabetes and asthma in mothers, type of respiratory support (CPAP, oxygen therapy alone), partial pressure of oxygen (PO2) and carbon dioxide (PCO2), and arterial blood gas (ABG), were first recorded. Empirical antibiotic therapy was initiated after culture sampling in cases that were unresponsive to oxygen supplementation and supportive management. The severity of TTN was assigned based on the RSS (a score of 4-6 and >6) [22]. The RSS assesses five parameters of respiratory effort, yielding a total score ranging from 0 (representing comfortable breathing) to 10 (representing severe respiratory distress). If the RSS was ≥6, the nasal CPAP or supplemental oxygen was initiated. When the respiratory rate reached <60 and abdominal distention was not present, feeding was initiated.
The primary outcomes included the duration of initial nasal CPAP and supplemental oxygen therapy, post-treatment PO2 and PCO2 levels, length of hospitalization, and age at initiation of feeding, determined by achieving an RSS score <4 along with stabilization of heart rate, respiratory rate, and oxygen saturation (SpO2). ABG was analyzed 4 hours after the administration of 4 doses of salbutamol. Supplementary oxygen or CPAP was used to reach an oxygen saturation of 90-95%. The RDS score was checked after hospital admission and before administration of drugs.
Intervention
In the salbutamol group, 0.1 mL of salbutamol (Ventolin, salbutamol sulfate 5 mg/mL) was nebulized in 3 mL of 0.9% normal saline. Salbutamol was then administered at a standard dose of 0.15 mg/kg via daily nebulization using a jet nebulizer with a continuous oxygen flow of 5 liters per minute for 10 minutes and every 6 hours for one day. The placebo group was given 2 mL of 0.9% saline as a placebo, administered via nebulizer at the same intervals and duration as those for the salbutamol group. The complications of salbutamol treatment (agitation, irritability, tachycardia, arrhythmia, blood pressure variability, vomiting, hypokalemia, and muscle cramps) were assessed at all times of drug administration and for 24 hours thereafter. If any complications happened, therapeutic or supportive care was done, or salbutamol was discontinued.
Data analysis
After recording demographic characteristics and clinical findings, analyses were performed in SPSS software, version 23. Qualitative variables were presented as frequencies (percentages), while quantitative variables were expressed as Mean±SD for normally distributed data and as median with 25th and 75th percentiles for abnormally distributed data. Comparisons between groups were conducted using the independent t-test for normally distributed quantitative variables and the Mann-Whitney U test for abnormally distributed quantitative variables. The chi-square test was applied for qualitative variables. P<0.05 was considered statistically significant.
Results
The baseline demographic and clinical characteristics of newborns are presented in Table 1.
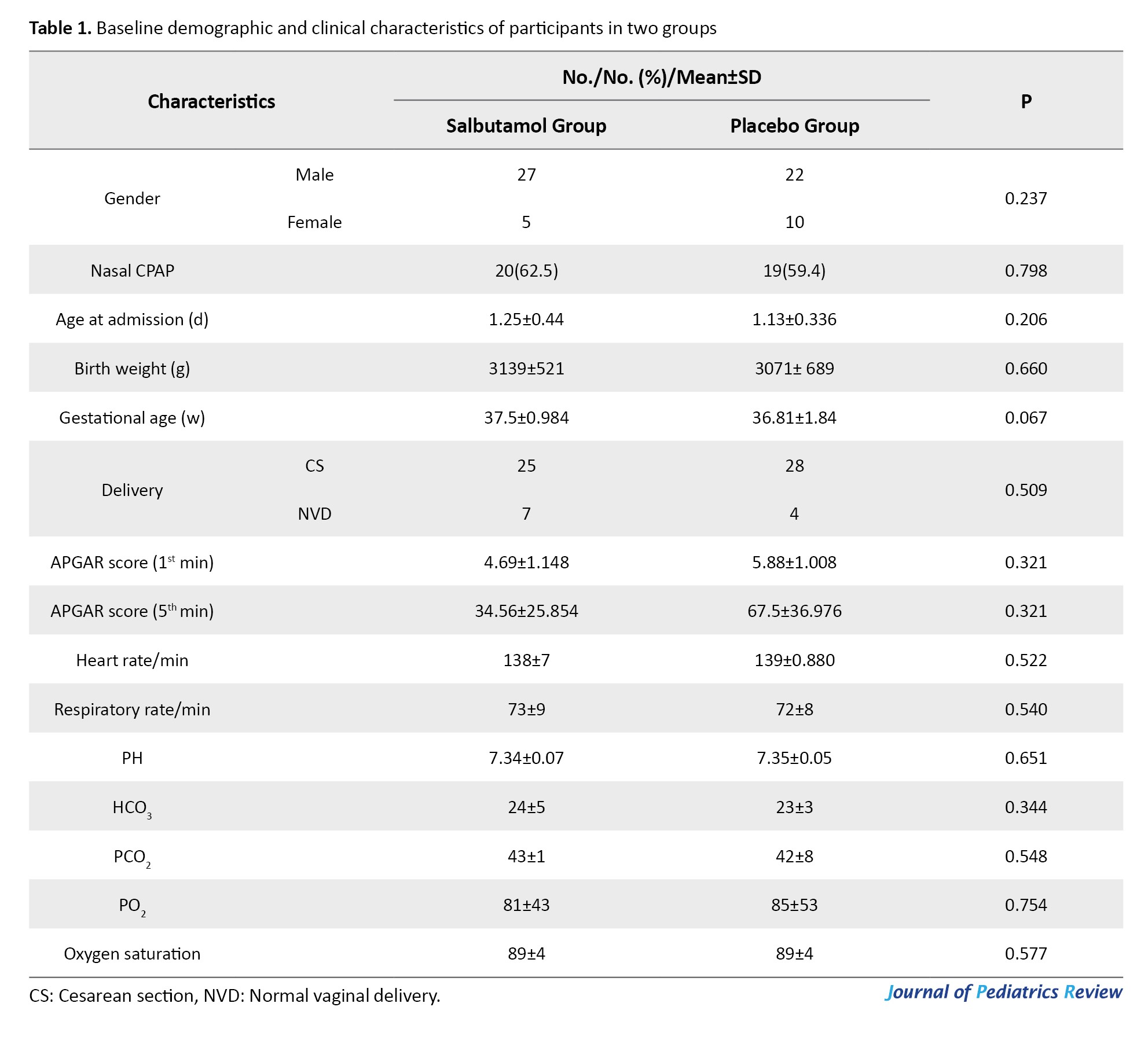
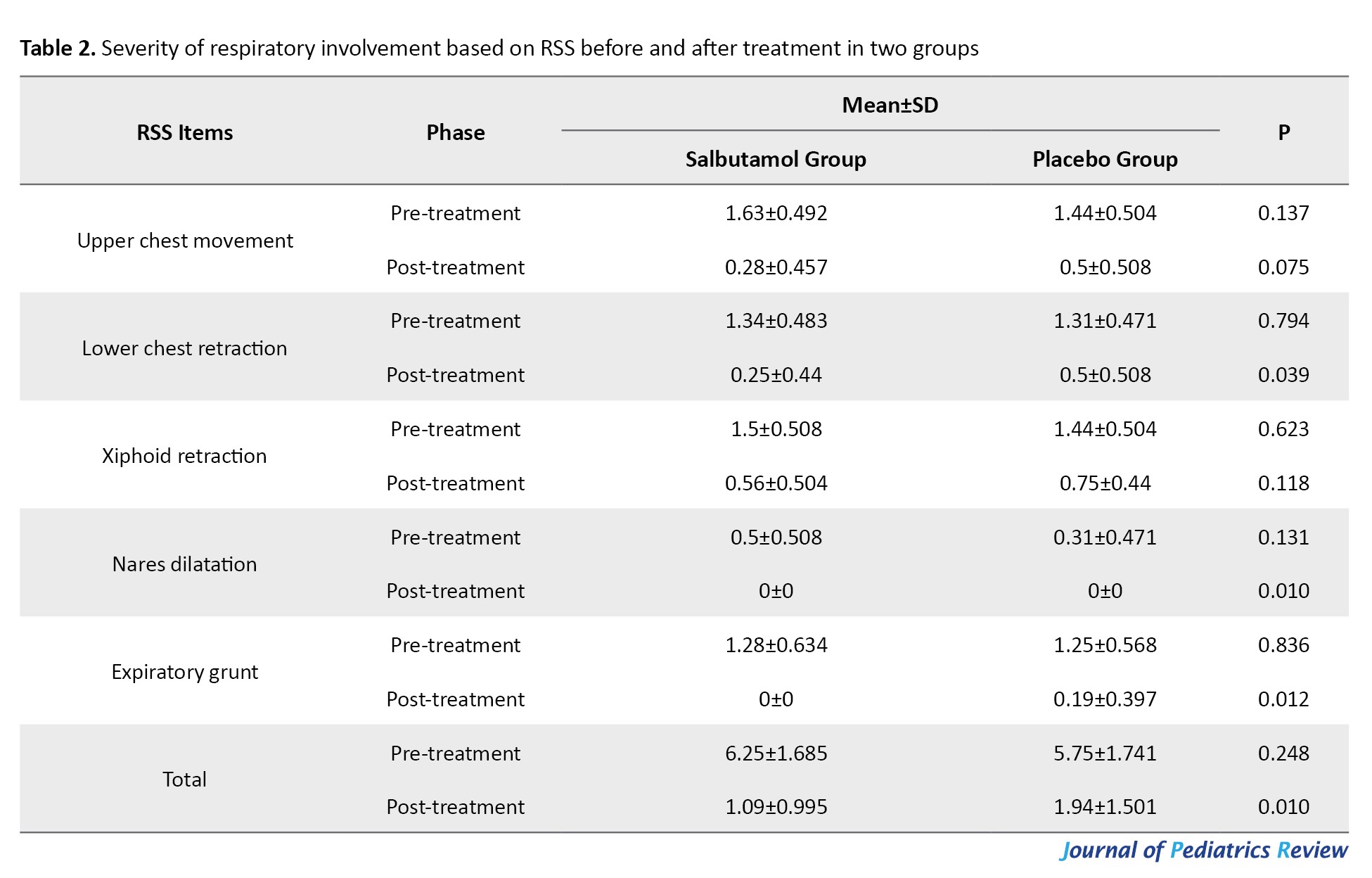
No significant differences were observed between the groups regarding gender, mode of delivery, birth weight, or type of respiratory support (P>0.05). The mean birth weight was 3071±689 g in the placebo group and 3139±539 g in the salbutamol group (P=0.207). No significant difference was observed in TTN severity (P=0.248) at baseline based on the total RSS; however, a significant difference was found after treatment (P=0.01) (Table 2).
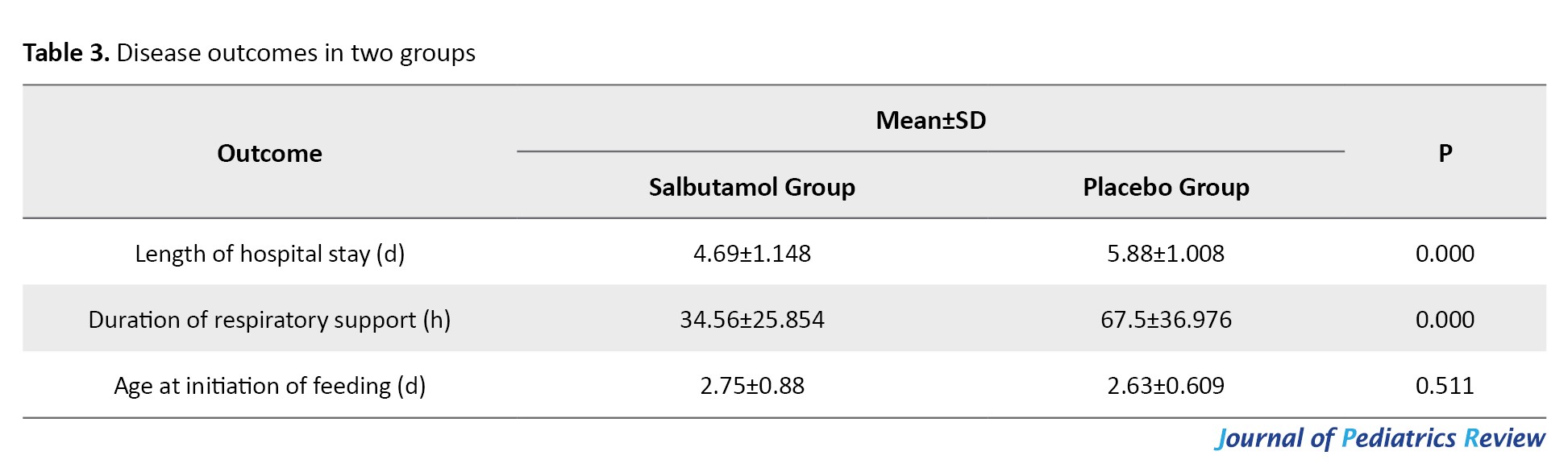
Regarding the disease outcomes, the intervention group had significantly shorter length of hospital stay and duration of respiratory support (P<0.05), whereas the age at initiation of feeding did not differ significantly between groups (Table 3).
While upper chest movement, lower chest retraction, and xiphoid retraction did not differ significantly between groups, nares dilatation and expiratory grunt showed significant improvement in the salbutamol group (P<0.05).
Patients were categorized based on their pre-treatment RSS into two subgroups: A group with a score of 4-5 and a group with a score of 6 or higher. The disease outcomes in these two subgroups are presented in Table 4.
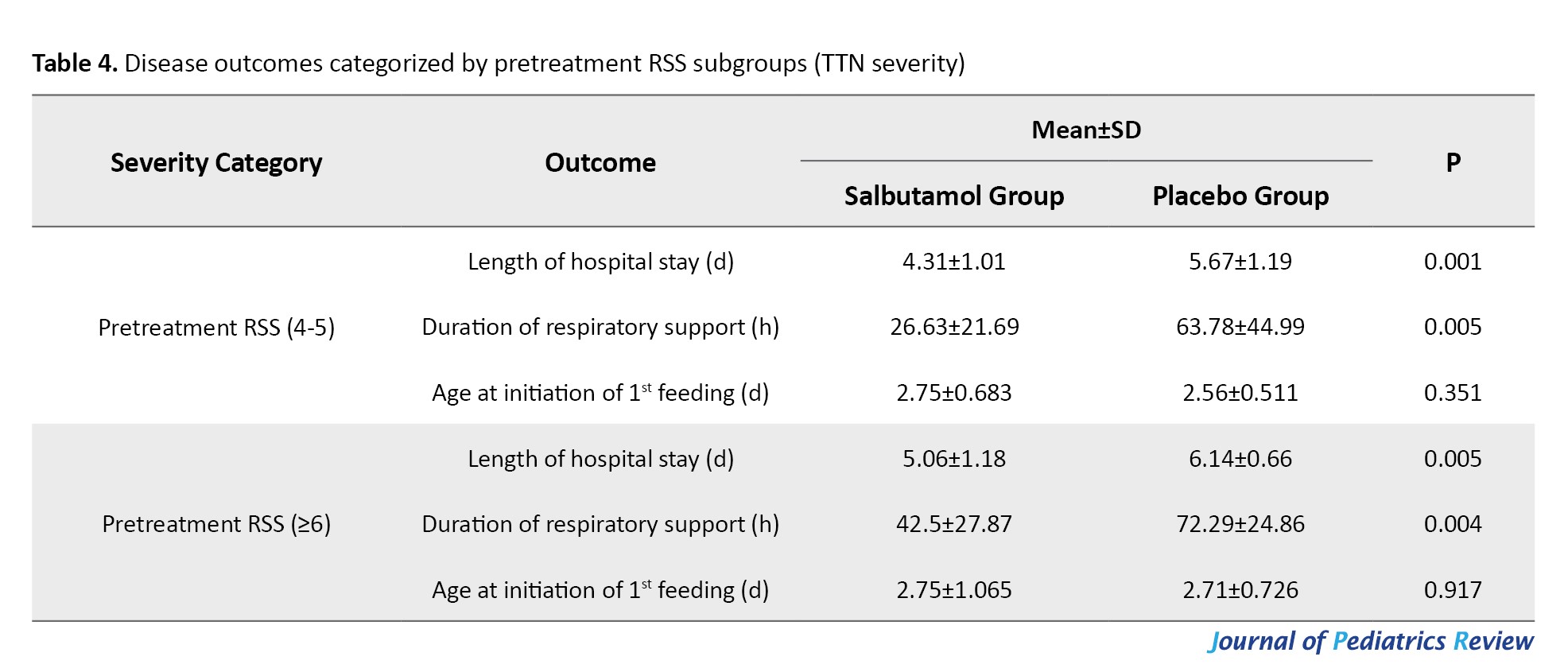
The length of hospital stay and duration of respiratory support were significantly lower in both RSS subgroups of the salbutamol group compared to those in the placebo group (P<0.05). The age at initiation of feeding was similar between the salbutamol and placebo groups in two RSS subgroups (P>0.05). No adverse effect of salbutamol was reported in this study.
Discussion
This study aimed to investigate the efficacy of nebulized salbutamol in newborns with transient tachypnea. Our findings indicate that after treatment, the total RSS improved significantly in the salbutamol group compared to the placebo group. Also, nares dilatation and expiratory grunt domains of RSS showed significant improvement in the salbutamol group. Additionally, the salbutamol group experienced significantly shorter length of hospital stay and respiratory support duration compared to the placebo group.
Salama et al. conducted a study on 150 neonates with gestational age of 35-39 weeks in three groups (50 received a single dose of salbutamol, 50 received a double dose of salbutamol, and 50 served as controls). They concluded that inhaled salbutamol reduced the duration of supplemental oxygen therapy, hospitalization, and time of initiation of feeding, without any reported adverse effects [20]. In a study by Kim et al. on 40 hospitalized neonates with TTN (28 receiving inhaled salbutamol and 12 receiving placebo), the duration of supplemental oxygen therapy and empirical antibiotic treatment was significantly shorter in the salbutamol group. However, the duration of tachypnea, time of initiation of enteral feeding, and length of hospital stay were similar between groups. No adverse effects were reported in any groups [10]. In the triple-blind clinical trial by Malakian et al., TTN score and severity were compared between the groups that received inhaled salbutamol or normal saline. The salbutamol group demonstrated shorter durations of hospitalization and oxygen therapy, as well as earlier initiation of oral feeding [9]. These findings are consistent with our results. In our study, the total TTN score reduced significantly in the salbutamol group similar to the mentioned studies. It seems that the acute respiratory benefit of salbutamol is acceptable. However, long-term results and the safety of the drug should also be considered.
Basiri et al. in a study on 52 newborns in two groups of nebulized sodium chloride (control) and salbutamol reported significantly lower TTN scores and shorter duration of respiratory support in the salbutamol group but there was no significant difference in length of hospital stay, duration of antibiotic therapy, time to start oral feeding and maximum oral feeding time between two groups [11]. El-Badawy et al. prospectively compared 100 full-term neonates with TTN in four equal groups receiving nebulized budesonide, epinephrine, salbutamol, and normal saline. After 48 hours, there was a significant decrease in the respiratory rate in the salbutamol group. The 48-hour TTN clinical score was significantly lower in the salbutamol group compared to the normal saline control group [15]. Khushdil et al. evaluated 100 newborns with TTN in four groups of nebulized salbutamol, furosemide, furosemide + nebulized salbutamol, and only supportive care (control). The mean duration of oxygen dependency and the need for mechanical ventilation were significantly lower in the salbutamol + furosemide group compared to the control group. The mean duration of oxygen dependency was not significantly different in the salbutamol group compared to the control group [17]. Mussavi et al. studied neonates with TTN who received either nebulized albuterol or a placebo. In treatment group, duration of CPAP and respiratory distress score decreased significantly and the PO2 increased significantly. No adverse effects were observed in any groups [13]. In the randomized controlled clinical trial by Al Lahony et al., after 4 hours of inhaled therapy, the placebo group exhibited higher respiratory rates, oxygen requirements, TTN scores, need for respiratory support, and longer hospitalization, whereas the salbutamol group showed significantly higher arterial pH and PO2. No significant differences were observed between groups in heart rate or serum potassium levels [12]. Babaei et al. included 80 neonates with TTN who received either nebulized salbutamol or a placebo. The salbutamol group experienced significantly shorter durations of tachypnea, hospitalization, oxygen therapy, and time to initiate enteral feeding. However, no significant differences were observed between groups in the duration of mechanical ventilation, need for or duration of CPAP, or incidence of pneumothorax [16]. Talaat et al. studied 100 neonates with TTN in two groups of inhaled salbutamol and normal saline. The salbutamol group demonstrated significantly shorter durations of respiratory support and hospitalization, as well as lower respiratory rates, fraction of inspired oxygen, and TTN score [18]. Mohammadzadeh et al. evaluated the effects of inhaled salbutamol versus placebo in 70 neonates with TTN. They concluded that inhaled salbutamol facilitated earlier initiation of enteral feeding, reduced the duration of respiratory support, and shortened hospitalization in neonates with moderate to severe TTN [7]. In our study, the salbutamol group demonstrated significantly shorter durations of hospitalization and respiratory support compared to the placebo group, whereas the time to initiate feeding was similar between groups. Discrepancies among different studies may be attributed to differences in study settings and sample sizes. It remains unclear whether salbutamol had a long-term effect on the duration of hospitalization, respiratory support, or initiation of feeding.
Conclusion
In conclusion, nebulized salbutamol seems to improve acute respiratory distress in neonates with TTN. This improvement is evident in neonates with both lower (RSS ≤6) and higher (RSS >6) disease severity. Further studies with larger sample sizes are recommended to evaluate the long-term effects of nebulized salbutamol.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Written informed consent was obtained from the parents or legal guardians of all neonates prior to enrollment. The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.REC.1402.166) and was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20091201002801N6).
Funding
This study was extracted from the thesis in pediatrics of Maedeh Gooran Oorimi, approved by the Department of Pediatrics, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. This study was supported by the University of Medical Sciences, Sari, Iran (Grant No.: 13885).
Authors contributions
Conceptualization: Abbas Dabbaghzadeh; Methodology: Roya Farhadi, Javad Ghaffari and Abbas Dabbaghzadeh; Data curation and writing: Maedeh Gooran and Marziyeh Taji; Supervision: Javad Ghaffari and Abbas Dabbaghzadeh; Investigation and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the medical staff of Bouali and Imam Khomeini hospitals in Sari and the parents of all neonates who participated in this study for their assistance and cooperation.
Full-Text: (57 Views)
Introduction
Transient tachypnea of the newborn (TTN) is a common, self-limited respiratory disorder resulting from delayed clearance of fetal lung fluid after birth [1, 2]. This condition is frequently observed in full-term or late-preterm infants, with an incidence rate of 5.7 per 1000 newborns [3, 4]. Symptoms of TTN usually appear within the first few minutes to hours after birth, and include tachypnea, nasal flaring, grunting, intercostal, subcostal, and suprasternal retraction [5, 6]. Respiratory distress is often resolved within 3-5 days, provided there are no complications [7, 8]. TTN is generally benign and self-limited, but in some newborns, it can cause pneumothorax, pulmonary hypertension, and some secondary problems such as increased monitoring, maternal-infant separation, unnecessary antibiotic therapy, prolonged hospital stay, and respiratory failure. There is no definitive treatment for TTN. The standard management of TTN involves supportive respiratory measures, which may include supplemental oxygen, nasal continuous positive airway pressure (CPAP), or, in severe refractory cases, mechanical ventilation [9, 10]. The use of prophylactic antibiotics is rational until a blood culture is reported [11, 4]. Drugs such as diuretics, inhaled racemic epinephrine, and inhaled beta-2 agonists may be effective in treating TTN, but none are definitive cures [12-14]. Salbutamol, a short-acting selective β2-adrenergic receptor agonist, enhances the clearance of lung fluid by stimulating epithelial sodium channel activity and improving tachypnea in newborns with TTN [15, 16].
Recent studies have reported that salbutamol can significantly improve the TTN score, reduce the duration of hospitalization and the need for supportive oxygen therapy, and facilitate earlier feeding in newborns [3, 15, 17-20]. Several studies have assessed the efficacy of salbutamol in patients with varying severities of TTN; however, no study has been performed in northern Iran. Therefore, this study aimed to evaluate the efficacy of inhaled salbutamol in treating TTN in newborns and assess the outcome based on the different severities of TTN. The novelty of this study lay in determining the TTN severity based on the Silverman-Andersen respiratory severity score (RSS), a measure that had not been applied previously.
Materials and Methods
Study design and participants
In this double-blind randomized clinical trial, newborns with TTN admitted to the neonatal intensive care unit (NICU) of Bouali and Imam Khomeini tertiary hospitals in Sari, Mazandaran Province, northern Iran, were evaluated from March 2022 to April 2023. Newborns with a gestational age of >34 weeks who met the TTN diagnostic criteria, including tachypnea (a respiratory rate >60 breaths per minute) with or without cyanosis, respiratory distress, and chest x-ray findings in favor of TTN [21], were enrolled in this study. TTN diagnosis based on chest x-ray findings was done by a neonatologist. In case of meconium aspiration, pneumonia, complex congenital heart disease, tachycardia (heart rate >180 beats per minute), an RSS <4, a need for respiratory support with a ventilator after birth, sepsis, respiratory distress syndrome (RDS) diagnosis during hospitalization and administration of surfactants, the samples were excluded. Empirical antibiotic therapy, supplemental oxygen therapy, and supportive care were applied for all patients. Based on previous studies, the sample size was calculated as 60, with 30 allocated to each group [12] at a 95% confidence interval (CI) level and considering a test power of 85%.
Patients were randomly selected using the permuted block randomization method with blocks of 4 and randomly divided into two parallel groups of salbutamol (n=32) and placebo (n=32) on the sealed envelope website. Patients were stratified into two groups based on the severity of TTN (RSS score >6 and RSS score 4-6). The patients were blinded to the drug administered and group allocation. Placebo and drug had the same color and shape in appearance and were coded by the researcher, and each patient had a drug code. The drug administration was done by an experienced nurse who was blind to the group allocation. Figure 1 presents the flowchart of sampling and allocation processes.
Transient tachypnea of the newborn (TTN) is a common, self-limited respiratory disorder resulting from delayed clearance of fetal lung fluid after birth [1, 2]. This condition is frequently observed in full-term or late-preterm infants, with an incidence rate of 5.7 per 1000 newborns [3, 4]. Symptoms of TTN usually appear within the first few minutes to hours after birth, and include tachypnea, nasal flaring, grunting, intercostal, subcostal, and suprasternal retraction [5, 6]. Respiratory distress is often resolved within 3-5 days, provided there are no complications [7, 8]. TTN is generally benign and self-limited, but in some newborns, it can cause pneumothorax, pulmonary hypertension, and some secondary problems such as increased monitoring, maternal-infant separation, unnecessary antibiotic therapy, prolonged hospital stay, and respiratory failure. There is no definitive treatment for TTN. The standard management of TTN involves supportive respiratory measures, which may include supplemental oxygen, nasal continuous positive airway pressure (CPAP), or, in severe refractory cases, mechanical ventilation [9, 10]. The use of prophylactic antibiotics is rational until a blood culture is reported [11, 4]. Drugs such as diuretics, inhaled racemic epinephrine, and inhaled beta-2 agonists may be effective in treating TTN, but none are definitive cures [12-14]. Salbutamol, a short-acting selective β2-adrenergic receptor agonist, enhances the clearance of lung fluid by stimulating epithelial sodium channel activity and improving tachypnea in newborns with TTN [15, 16].
Recent studies have reported that salbutamol can significantly improve the TTN score, reduce the duration of hospitalization and the need for supportive oxygen therapy, and facilitate earlier feeding in newborns [3, 15, 17-20]. Several studies have assessed the efficacy of salbutamol in patients with varying severities of TTN; however, no study has been performed in northern Iran. Therefore, this study aimed to evaluate the efficacy of inhaled salbutamol in treating TTN in newborns and assess the outcome based on the different severities of TTN. The novelty of this study lay in determining the TTN severity based on the Silverman-Andersen respiratory severity score (RSS), a measure that had not been applied previously.
Materials and Methods
Study design and participants
In this double-blind randomized clinical trial, newborns with TTN admitted to the neonatal intensive care unit (NICU) of Bouali and Imam Khomeini tertiary hospitals in Sari, Mazandaran Province, northern Iran, were evaluated from March 2022 to April 2023. Newborns with a gestational age of >34 weeks who met the TTN diagnostic criteria, including tachypnea (a respiratory rate >60 breaths per minute) with or without cyanosis, respiratory distress, and chest x-ray findings in favor of TTN [21], were enrolled in this study. TTN diagnosis based on chest x-ray findings was done by a neonatologist. In case of meconium aspiration, pneumonia, complex congenital heart disease, tachycardia (heart rate >180 beats per minute), an RSS <4, a need for respiratory support with a ventilator after birth, sepsis, respiratory distress syndrome (RDS) diagnosis during hospitalization and administration of surfactants, the samples were excluded. Empirical antibiotic therapy, supplemental oxygen therapy, and supportive care were applied for all patients. Based on previous studies, the sample size was calculated as 60, with 30 allocated to each group [12] at a 95% confidence interval (CI) level and considering a test power of 85%.
Patients were randomly selected using the permuted block randomization method with blocks of 4 and randomly divided into two parallel groups of salbutamol (n=32) and placebo (n=32) on the sealed envelope website. Patients were stratified into two groups based on the severity of TTN (RSS score >6 and RSS score 4-6). The patients were blinded to the drug administered and group allocation. Placebo and drug had the same color and shape in appearance and were coded by the researcher, and each patient had a drug code. The drug administration was done by an experienced nurse who was blind to the group allocation. Figure 1 presents the flowchart of sampling and allocation processes.
Assessments
Data, including gender, mode of delivery, birth weight, Apgar score, history of prelabor rupture of membranes (PROM), history of diabetes and asthma in mothers, type of respiratory support (CPAP, oxygen therapy alone), partial pressure of oxygen (PO2) and carbon dioxide (PCO2), and arterial blood gas (ABG), were first recorded. Empirical antibiotic therapy was initiated after culture sampling in cases that were unresponsive to oxygen supplementation and supportive management. The severity of TTN was assigned based on the RSS (a score of 4-6 and >6) [22]. The RSS assesses five parameters of respiratory effort, yielding a total score ranging from 0 (representing comfortable breathing) to 10 (representing severe respiratory distress). If the RSS was ≥6, the nasal CPAP or supplemental oxygen was initiated. When the respiratory rate reached <60 and abdominal distention was not present, feeding was initiated.
The primary outcomes included the duration of initial nasal CPAP and supplemental oxygen therapy, post-treatment PO2 and PCO2 levels, length of hospitalization, and age at initiation of feeding, determined by achieving an RSS score <4 along with stabilization of heart rate, respiratory rate, and oxygen saturation (SpO2). ABG was analyzed 4 hours after the administration of 4 doses of salbutamol. Supplementary oxygen or CPAP was used to reach an oxygen saturation of 90-95%. The RDS score was checked after hospital admission and before administration of drugs.
Intervention
In the salbutamol group, 0.1 mL of salbutamol (Ventolin, salbutamol sulfate 5 mg/mL) was nebulized in 3 mL of 0.9% normal saline. Salbutamol was then administered at a standard dose of 0.15 mg/kg via daily nebulization using a jet nebulizer with a continuous oxygen flow of 5 liters per minute for 10 minutes and every 6 hours for one day. The placebo group was given 2 mL of 0.9% saline as a placebo, administered via nebulizer at the same intervals and duration as those for the salbutamol group. The complications of salbutamol treatment (agitation, irritability, tachycardia, arrhythmia, blood pressure variability, vomiting, hypokalemia, and muscle cramps) were assessed at all times of drug administration and for 24 hours thereafter. If any complications happened, therapeutic or supportive care was done, or salbutamol was discontinued.
Data analysis
After recording demographic characteristics and clinical findings, analyses were performed in SPSS software, version 23. Qualitative variables were presented as frequencies (percentages), while quantitative variables were expressed as Mean±SD for normally distributed data and as median with 25th and 75th percentiles for abnormally distributed data. Comparisons between groups were conducted using the independent t-test for normally distributed quantitative variables and the Mann-Whitney U test for abnormally distributed quantitative variables. The chi-square test was applied for qualitative variables. P<0.05 was considered statistically significant.
Results
The baseline demographic and clinical characteristics of newborns are presented in Table 1.

No significant differences were observed between the groups regarding gender, mode of delivery, birth weight, or type of respiratory support (P>0.05). The mean birth weight was 3071±689 g in the placebo group and 3139±539 g in the salbutamol group (P=0.207). No significant difference was observed in TTN severity (P=0.248) at baseline based on the total RSS; however, a significant difference was found after treatment (P=0.01) (Table 2).

Regarding the disease outcomes, the intervention group had significantly shorter length of hospital stay and duration of respiratory support (P<0.05), whereas the age at initiation of feeding did not differ significantly between groups (Table 3).

While upper chest movement, lower chest retraction, and xiphoid retraction did not differ significantly between groups, nares dilatation and expiratory grunt showed significant improvement in the salbutamol group (P<0.05).
Patients were categorized based on their pre-treatment RSS into two subgroups: A group with a score of 4-5 and a group with a score of 6 or higher. The disease outcomes in these two subgroups are presented in Table 4.

The length of hospital stay and duration of respiratory support were significantly lower in both RSS subgroups of the salbutamol group compared to those in the placebo group (P<0.05). The age at initiation of feeding was similar between the salbutamol and placebo groups in two RSS subgroups (P>0.05). No adverse effect of salbutamol was reported in this study.
Discussion
This study aimed to investigate the efficacy of nebulized salbutamol in newborns with transient tachypnea. Our findings indicate that after treatment, the total RSS improved significantly in the salbutamol group compared to the placebo group. Also, nares dilatation and expiratory grunt domains of RSS showed significant improvement in the salbutamol group. Additionally, the salbutamol group experienced significantly shorter length of hospital stay and respiratory support duration compared to the placebo group.
Salama et al. conducted a study on 150 neonates with gestational age of 35-39 weeks in three groups (50 received a single dose of salbutamol, 50 received a double dose of salbutamol, and 50 served as controls). They concluded that inhaled salbutamol reduced the duration of supplemental oxygen therapy, hospitalization, and time of initiation of feeding, without any reported adverse effects [20]. In a study by Kim et al. on 40 hospitalized neonates with TTN (28 receiving inhaled salbutamol and 12 receiving placebo), the duration of supplemental oxygen therapy and empirical antibiotic treatment was significantly shorter in the salbutamol group. However, the duration of tachypnea, time of initiation of enteral feeding, and length of hospital stay were similar between groups. No adverse effects were reported in any groups [10]. In the triple-blind clinical trial by Malakian et al., TTN score and severity were compared between the groups that received inhaled salbutamol or normal saline. The salbutamol group demonstrated shorter durations of hospitalization and oxygen therapy, as well as earlier initiation of oral feeding [9]. These findings are consistent with our results. In our study, the total TTN score reduced significantly in the salbutamol group similar to the mentioned studies. It seems that the acute respiratory benefit of salbutamol is acceptable. However, long-term results and the safety of the drug should also be considered.
Basiri et al. in a study on 52 newborns in two groups of nebulized sodium chloride (control) and salbutamol reported significantly lower TTN scores and shorter duration of respiratory support in the salbutamol group but there was no significant difference in length of hospital stay, duration of antibiotic therapy, time to start oral feeding and maximum oral feeding time between two groups [11]. El-Badawy et al. prospectively compared 100 full-term neonates with TTN in four equal groups receiving nebulized budesonide, epinephrine, salbutamol, and normal saline. After 48 hours, there was a significant decrease in the respiratory rate in the salbutamol group. The 48-hour TTN clinical score was significantly lower in the salbutamol group compared to the normal saline control group [15]. Khushdil et al. evaluated 100 newborns with TTN in four groups of nebulized salbutamol, furosemide, furosemide + nebulized salbutamol, and only supportive care (control). The mean duration of oxygen dependency and the need for mechanical ventilation were significantly lower in the salbutamol + furosemide group compared to the control group. The mean duration of oxygen dependency was not significantly different in the salbutamol group compared to the control group [17]. Mussavi et al. studied neonates with TTN who received either nebulized albuterol or a placebo. In treatment group, duration of CPAP and respiratory distress score decreased significantly and the PO2 increased significantly. No adverse effects were observed in any groups [13]. In the randomized controlled clinical trial by Al Lahony et al., after 4 hours of inhaled therapy, the placebo group exhibited higher respiratory rates, oxygen requirements, TTN scores, need for respiratory support, and longer hospitalization, whereas the salbutamol group showed significantly higher arterial pH and PO2. No significant differences were observed between groups in heart rate or serum potassium levels [12]. Babaei et al. included 80 neonates with TTN who received either nebulized salbutamol or a placebo. The salbutamol group experienced significantly shorter durations of tachypnea, hospitalization, oxygen therapy, and time to initiate enteral feeding. However, no significant differences were observed between groups in the duration of mechanical ventilation, need for or duration of CPAP, or incidence of pneumothorax [16]. Talaat et al. studied 100 neonates with TTN in two groups of inhaled salbutamol and normal saline. The salbutamol group demonstrated significantly shorter durations of respiratory support and hospitalization, as well as lower respiratory rates, fraction of inspired oxygen, and TTN score [18]. Mohammadzadeh et al. evaluated the effects of inhaled salbutamol versus placebo in 70 neonates with TTN. They concluded that inhaled salbutamol facilitated earlier initiation of enteral feeding, reduced the duration of respiratory support, and shortened hospitalization in neonates with moderate to severe TTN [7]. In our study, the salbutamol group demonstrated significantly shorter durations of hospitalization and respiratory support compared to the placebo group, whereas the time to initiate feeding was similar between groups. Discrepancies among different studies may be attributed to differences in study settings and sample sizes. It remains unclear whether salbutamol had a long-term effect on the duration of hospitalization, respiratory support, or initiation of feeding.
Conclusion
In conclusion, nebulized salbutamol seems to improve acute respiratory distress in neonates with TTN. This improvement is evident in neonates with both lower (RSS ≤6) and higher (RSS >6) disease severity. Further studies with larger sample sizes are recommended to evaluate the long-term effects of nebulized salbutamol.
Ethical Considerations
Compliance with ethical guidelines
This study was conducted in accordance with the ethical principles outlined in the Declaration of Helsinki. Written informed consent was obtained from the parents or legal guardians of all neonates prior to enrollment. The study was approved by the Ethics Committee of Mazandaran University of Medical Sciences, Sari, Iran (Code: IR.MAZUMS.REC.1402.166) and was registered by the Iranian Registry of Clinical Trials (IRCT) (Code: IRCT20091201002801N6).
Funding
This study was extracted from the thesis in pediatrics of Maedeh Gooran Oorimi, approved by the Department of Pediatrics, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. This study was supported by the University of Medical Sciences, Sari, Iran (Grant No.: 13885).
Authors contributions
Conceptualization: Abbas Dabbaghzadeh; Methodology: Roya Farhadi, Javad Ghaffari and Abbas Dabbaghzadeh; Data curation and writing: Maedeh Gooran and Marziyeh Taji; Supervision: Javad Ghaffari and Abbas Dabbaghzadeh; Investigation and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the medical staff of Bouali and Imam Khomeini hospitals in Sari and the parents of all neonates who participated in this study for their assistance and cooperation.
References
- Kiliçbay F, Gaffari T, Ünsal G. Assessment of risk factors to predict the duration of tachypnea in the management of infants hospitalized with transient tachypnea of newborns. Turk J Sci Health. 2022; 3(2):114-22. [DOI:10.51972/tfsd.1091894]
- Bhering CA, Moraes Ramos JR. Transient Tachypnea of the Newborn. In: Moreira de Sá RA, Fonseca EBd, editors. Perinatology. Cham: Springer; 2022. [DOI:10.1007/978-3-030-83434-0_61]
- Rocha G. Inhaled pharmacotherapy for neonates: A narrative review. Turk Arch Pediatr. 2022; 57(1):5-17. [DOI:10.5152/TurkArchPediatr.2021.21125] [PMID]
- Alhassen Z, Vali P, Guglani L, Lakshminrusimha S, Ryan RM. Recent advances in pathophysiology and management of transient tachypnea of newborn. J Perinatol. 2021; 41(1):6-16. [DOI:10.1038/s41372-020-0757-3] [PMID]
- Chiruvolu A, Claunch KM, Garcia AJ, Petrey B, Hammonds K, Mallett LH. Effect of continuous positive airway pressure versus nasal cannula on late preterm and term infants with transient tachypnea of the newborn. J Perinatol. 2021; 41(7):1675-80. [DOI:10.1038/s41372-021-01068-9] [PMID]
- Bruschettini M, Hassan KO, Romantsik O, Banzi R, Calevo MG, Moresco L. Interventions for the management of transient tachypnoea of the newborn - an overview of systematic reviews. Cochrane Database Syst Rev. 2022; 2(2):CD013563. [DOI:10.1002/14651858.CD013563.pub2] [PMID]
- Mohammadzadeh I, Akbarian-Rad Z, Heidari F, Zahedpasha Y, Haghshenas-Mojaveri M. The effect of inhaled salbutamol in transient of tachypnea of the newborn: A randomized clinical trial. Inn J Pediatr. 2017; 27(5):e9633. [DOI:10.5812/ijp.9633]
- Ekmen S, Doğan E. Prediction of the course of transient tachypnea of the newborn by blood laboratory parameters at the time of admission. Inn J Pediatr. 2021; 31(3):e112224. [DOI:10.5812/ijp.112224]
- Malakian A, Dehdashtian M, Aramesh MR, Aletayeb MH, Heidari S. The effect of inhaled salbutamol on the outcomes of transient tachypnea of the newborn. J Chin Med Assoc. 2018; 81(11):990-7. [DOI:10.1016/j.jcma.2018.01.015] [PMID]
- Kim MJ, Yoo JH, Jung JA, Byun SY. The effects of inhaled albuterol in transient tachypnea of the newborn. Allergy Asthma Immunol Res. 2014; 6(2):126-30. [DOI:10.4168/aair.2014.6.2.126] [PMID]
- Basiri B, Sadeghi N, Sabzehei MK, Ashari FE. Effects of inhaled salbutamol on transient tachypnea of the newborn. Respir Care. 2022; 67(4):433-9. [DOI:10.4187/respcare.09284] [PMID]
- Al Lahony DM, Elsayed HM, Mohammed IS. The effects of inhaled β-adrenergic agonists in transient tachypnea of the newborn. Menoufia Med J. 2021; 33(3):847-51.[Link]
- Mussavi M, Asadollahi K, Kayvan M, Sadeghvand S. Effects of nebulized albuterol in transient tachypnea of the newborn a clinical trial. Inn J Pediatr. 2017; 27(3):e8211 [DOI:10.5812/ijp.8211]
- Buchiboyina A, Jasani B, Deshmukh M, Patole S. Strategies for managing transient tachypnoea of the newborn - a systematic review. J Matern Fetal Neonatal Med. 2017; 30(13):1524-32. [DOI:10.1080/14767058.2016.1193143] [PMID]
- El-Badawy AM, Ibrahim AM, El Rahman M, Awny MM. Efficacy of budesonide, epinephrine and salbutamol inhalation for treatment of transient tachypnea of newborn: Prospective Controlled Study. Asian J Pediatr Res. 2021; 7(3):10-19. [DOI:10.9734/ajpr/2021/v7i330217]
- Babaei H, Dabiri S, Mohammadi Pirkashani L, Mohsenpour H. Effects of salbutamol on the treatment of transient tachypnea of the newborn. Iran J Neonatol. 2019; 10(1):42-9. [DOI:10.22038/ijn.2018.31294.1430]
- Khushdil A, Ahmed Z, Ahmed M, Nazir S, Waqar T. Effects of salbutamol and furosemide in the treatment of transient tachypnea of newborn-a randomized controlled trial. Pak Armed Forces Med J. 2022; 72(1):101-04. [DOI:10.51253/pafmj.v72i1.6076]
- Talaat AA, Abohashish M, Farid TM, Salah MM. Evaluation of inhaled beta-2 agonist in management of transient tachypnea of the newborn. Bull Natl Res Cent. 2020; 44(1):1-5. [DOI:10.1186/s42269-020-0271-y]
- Armangil D, Yurdakök M, Korkmaz A, Yiğit S, Tekinalp G. Inhaled beta-2 agonist salbutamol for the treatment of transient tachypnea of the newborn. J Pediatr. 2011; 159(3):398-403.e1. [DOI:10.1016/j.jpeds.2011.02.028] [PMID]
- Salama AA, El-Seheimy LAF, Elsamanoudy MI. Inhaled salbutamol for the treatment of transient tachypnea of the newborn. Int J Med Arts. 2020; 2(2):457-61. [DOI:10.21608/ijma.2020.21436.1064]
- Kasap B, Duman N, Özer E, Tatli M, Kumral A, Özkan H. Transient tachypnea of the newborn: Predictive factors. Pediatr Int. 2008; 50(1):81-4. [DOI:10.1111/j.1442-200X.2007.02535.x]
- Nussbaum C, Lengauer M, Puchwein-Schwepcke AF, Weiss VB, Spielberger B, Genzel-Boroviczény O. Noninvasive Ventilation in Preterm Infants: Factors influencing weaning decisions and the role of the silverman-andersen score. Children. 2022; 9(9):1292. [DOI:10.3390/children9091292] [PMID]
Type of Study: Original Article |
Subject:
Allergy and Clinical Immunology
Received: 2025/01/25 | Accepted: 2025/09/2 | Published: 2025/10/18
Received: 2025/01/25 | Accepted: 2025/09/2 | Published: 2025/10/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |










