Volume 13, Issue 3 (7-2025)
J. Pediatr. Rev 2025, 13(3): 235-248 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mehrem E S, Kamel R M, Sallam M A, Shabana M, Salem S, Gad Allah M A et al . Analysis of Pulmonary Functions in Pediatrics With Spastic Cerebral Palsy: A Pediatric Innovative Study. J. Pediatr. Rev 2025; 13 (3) :235-248
URL: http://jpr.mazums.ac.ir/article-1-724-en.html
URL: http://jpr.mazums.ac.ir/article-1-724-en.html
Elsayed S. Mehrem *1 

 , Roshdy M. Kamel2
, Roshdy M. Kamel2 

 , Mohamed A. Sallam3
, Mohamed A. Sallam3 

 , Magdy Shabana4
, Magdy Shabana4 

 , Shymaa Salem5
, Shymaa Salem5 

 , Mohamed A. Gad Allah3
, Mohamed A. Gad Allah3 

 , Osama Yassin6
, Osama Yassin6 




 , Roshdy M. Kamel2
, Roshdy M. Kamel2 

 , Mohamed A. Sallam3
, Mohamed A. Sallam3 

 , Magdy Shabana4
, Magdy Shabana4 

 , Shymaa Salem5
, Shymaa Salem5 

 , Mohamed A. Gad Allah3
, Mohamed A. Gad Allah3 

 , Osama Yassin6
, Osama Yassin6 


1- Department of Physical Therapy, College of Applied Medical Sciences, Qassim University, Buraydah, Saudi Arabia. , sayed7555@yahoo.com
2- Department of Basic Sciences, Faculty of Physical Therapy, Benha University, Qalyubia, Egypt. & Department of Physical Therapy, Faculty of Allied Medical Sciences, Al-Ahliyya Amman University, Amman, Jordan.
3- Department of Physical Therapy for Internal Medicine, Faculty of Physical Therapy, Deraya University, Minia, Egypt.
4- Department of Musculoskeletal, Faculty of Physical Therapy, German International University, Cairo, Egypt. & Department of Musculoskeletal, Cairo University Hospitals, Cairo University, Cairo, Egypt.
5- Department of Physical Therapy for Neurology and its Surgery, Faculty of Physical Therapy, Sphinx University, Assuit, Egypt.
6- Lecturer of physical therapy for Pediatrics, Faculty of Physical Therapy, Sphinx University, Asyut- Egypt.
2- Department of Basic Sciences, Faculty of Physical Therapy, Benha University, Qalyubia, Egypt. & Department of Physical Therapy, Faculty of Allied Medical Sciences, Al-Ahliyya Amman University, Amman, Jordan.
3- Department of Physical Therapy for Internal Medicine, Faculty of Physical Therapy, Deraya University, Minia, Egypt.
4- Department of Musculoskeletal, Faculty of Physical Therapy, German International University, Cairo, Egypt. & Department of Musculoskeletal, Cairo University Hospitals, Cairo University, Cairo, Egypt.
5- Department of Physical Therapy for Neurology and its Surgery, Faculty of Physical Therapy, Sphinx University, Assuit, Egypt.
6- Lecturer of physical therapy for Pediatrics, Faculty of Physical Therapy, Sphinx University, Asyut- Egypt.
Full-Text [PDF 987 kb]
(326 Downloads)
| Abstract (HTML) (736 Views)
Full-Text: (289 Views)
Introduction
Cerebral palsy (CP) is a physical disability that commonly affects children. It is characterized by persistent movement and postural disorders caused by non-progressive damage in the developing brain of a fetus or infant. The motor deficits associated with CP can lead to deficits in sensation, perception, communication, and behavior [1]. Despite advances in medical treatment, respiratory health remains a major challenge for individuals with CP [2].
These challenges include oropharyngeal dysfunction, which can result in recurrent aspiration, airway obstruction, hypoventilation, spine and chest wall abnormalities, and frequent respiratory infections. Efficient respiration relies on the coordination of various muscle actions, including those of the diaphragm, abdomen, chest, neck, and throat, as well as the stability of the head and trunk. This coordination allows for forceful inhalation and exhalation, as well as an efficient cough [3].
Worldwide, CP affects between one and nearly four out of every 1,000 live births (LB) [4]. In Arabic-speaking nations, its prevalence ranges from 0.62 to 3.6 per 1,000 LB [5]. Roughly one in eight children with CP admitted to hospitals need care for respiratory conditions. Moreover, their hospitalizations for respiratory issues last 2.5 times longer than those of children without CP [6].
Children with CP often experience respiratory problems due to restricted chest wall movement and weakened respiratory muscles. As a result, they may have poor alveolar ventilation, impaired airway clearance, and difficulty breathing [7]. These complications are among the leading causes of hospitalization and early mortality in individuals with CP. Wang et al. highlight a strong connection between respiratory muscle strength and an individual’s ability to perform daily self-care and adapt socially. Consequently, enhancing respiratory function could greatly improve both lifespan and overall quality of life [8].
Individuals with CP are at a higher risk of developing pulmonary infections. However, identifying and diagnosing respiratory issues in these individuals can be challenging. Limited communication with the patient, difficulties in conducting medical tests, and mild initial symptoms can all contribute to delays in diagnosis and treatment. This delay can put this vulnerable population at higher risk of developing complications. Nevertheless, experienced caregivers or parents are usually able to detect early, subtle signs of respiratory problems [8].
Pulmonary function tests (PFTs) help evaluate lung performance in individuals with potential risk factors for lung disease, exposure to harmful substances at work, or signs of pulmonary toxicity [9].
Spirometry, a key physiological assessment, measures a person’s ability to inhale and exhale air over a specific period. It is widely used to diagnose respiratory conditions, like asthma and chronic obstructive pulmonary disease (COPD), as well as to track the progression of various lung disorders [10].
Geratherm Respiratory Blue Cherry software is a digital spirometer designed to precisely assess dynamic lung volumes and capacities during both forced exhalation and inhalation. Its configuration offers key data regarding the test phase and timing, in alignment with the guidelines set by the American Thoracic Society (ATS), the European Respiratory Society (ERS) [11], and the global lung function initiative (GLI). The GLI has developed internationally recognized spirometry reference equations, which have been validated across multiple countries [12, 13] and supported by various professional societies [14, 15].
This is crucial because the data in GLI 2012 equations include Arab ethnicity from North Africa [16]. Emerging evidence suggests that children with CP have significantly lower respiratory status values than typically developing children. This respiratory decline is not directly caused by brain damage but is observed in children with neurological impairments [17]. However, most studies describing respiratory problems in patients with neurological impairment do not specifically focus on CP, which makes it difficult to interpret the results. Additionally, CP is a complex disorder with various types, which may require studying subgroups to better understand the condition [18].
The literature indicates that children with typical development (TD) have higher spirometry readings compared to those with CP. Moreover, the TD group exhibited superior values in forced vital capacity (FVC), forced expired volume in one second (FEV1), and peak expiratory flow (PEF) than the CP group [19, 20]. Additionally, research by Kwon and Lee found that children with spastic diplegic CP had significantly reduced respiratory function—specifically in maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), FVC, and FEV1—compared to their typically developing peers. However, there were no notable differences between the groups in FEV1/FVC ratios and PEF values [21].
The lack of standardized reference values for specific populations hinders accurate data identification, tracking intervention outcomes, and making prognoses. Consequently, some researchers opt to assess individuals with TD to create a comparative baseline between the control and experimental groups [19].
The present study differs from earlier research in terms of patient characteristics (such as age, functional abilities, and spasticity level) and the assessment method, as it utilized the norm-referenced Geratherm Respiratory Blue Cherry software.
This study aimed to evaluate the pulmonary function of children with CP and compare their results to normative values based on age and gender. Additionally, it sought to investigate differences in lung function based on the distribution of paralysis, such as diplegia and hemiplegia. Finally, the study intended to examine the relationship between the body mass index (BMI) Z-score and measures of pulmonary function.
Methods
This observational cross-sectional study was conducted over six months from January 2024 to Jun 2024. Sixteen children of both genders, aged between 3 and 16 years, diagnosed with spastic CP (10 with diaplegia and 6 with hemiplegia) participated in the study. They were selected from a pediatric rehabilitation center in Egypt.
Prior to collecting data, the study received approval from the Research Ethical Committee at the Faculty of Physical Therapy, and informed consent was obtained from the participants’ parents. Participants were screened based on the following inclusion criteria: A diagnosis of spastic CP (either diplegia or hemiplegia), a spasticity grade of 1 or 1+ on the modified Ashworth scale, adequate orientation to follow instructions for using the Geratherm Respiratory Blue Cherry software, and a normal mental status with an average intelligence quotient.
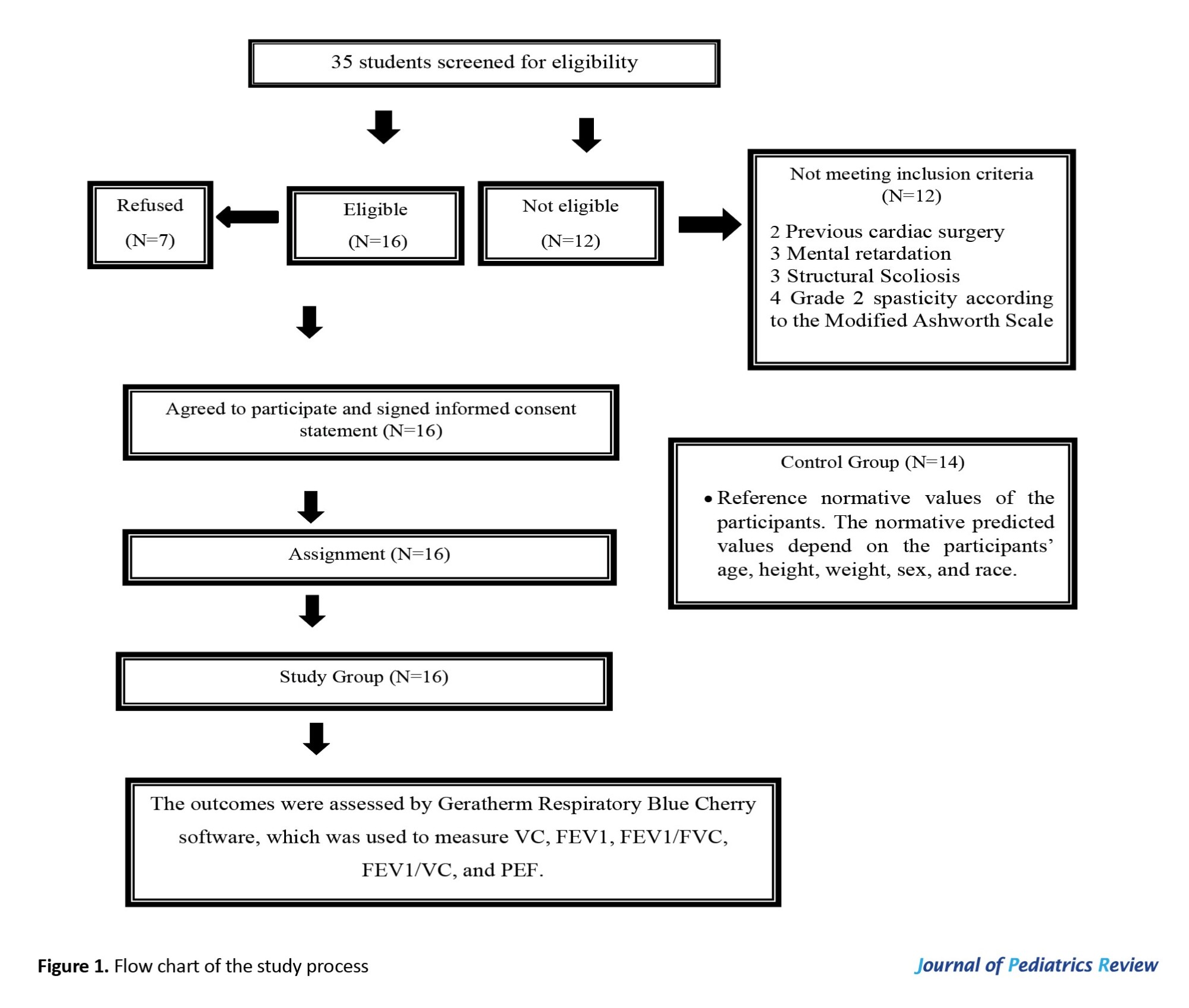
The exclusion criteria included any cognitive or neurological condition (other than CP), respiratory infections, and a history of previous surgical operations that can affect pulmonary functions, as well as mental retardation, scoliosis, and evidence of lung disease. All of these cases were confirmed by medical records and examined by a neurologist, pediatrician, and physical therapist. As shown in Figure 1, the subjects included in this study were divided into two groups of equal numbers: The study group (A), which consists of sixteen children with CP, and the control group (B), which represents the reference normative values of the participants.
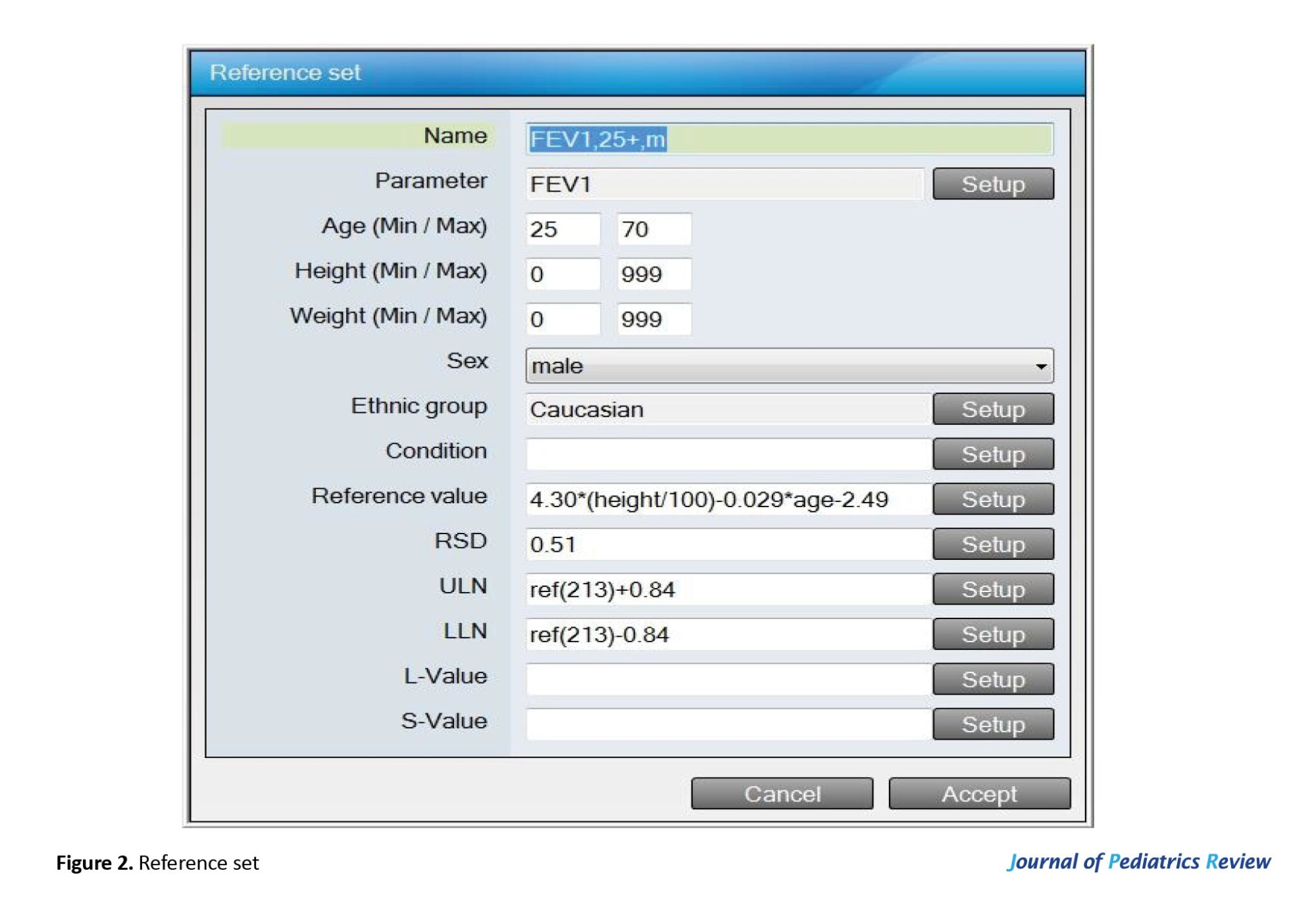
The reference values of the measured parameters (VC, FEV1, FEV1/FVC, FEV1/VC, and PEF) depend on the participants’ age, height, weight, sex, and race. For example, the FEV1 value is derived from the equation set for males older than 25, as presented in Figure 2.
Instruments
Geratherm respiratory blue cherry software
The outcomes were evaluated using a medical device called Blue Cherry, developed by Geratherm Respiratory. Blue Cherry is an electronic spirometer with a built-in mini-computer that measures the ventilator function parameters in subjects through timed measurements of dynamic lung volumes and capacities during forced expiration and inspiration. The software utilizes formulas and equations to calculate reference values based on the age, height, weight, sex, and race of the participants. Blue Cherry is a registered trademark of Geratherm Respiration and consists of both hardware and software components. The device uses a standard driver provided by Microsoft.
In 2005, the ATS and the ERS issued standardized guidelines for lung function testing [11]. Building on this, the GLI was formed in Berlin in September 2008. The goal of the GLI is to improve international spirometry reference equations by incorporating the following elements:
1. The equations must be derived from individual lung function data gathered under standardized testing conditions using validated equipment and software.
2. Advanced statistical methods should be used to develop equations that apply continuously across all age groups, from young children to the elderly.
3. The equations should support flexible interpretation using normal range limits, accounting for differences in sex, ethnicity, age, and lung function variables.
4. They should have practical clinical applications and be compatible with commercially available devices.
5. Results should clearly indicate where an individual falls within the “normal range”.
Geratherm Respiratory Blue Cherry software settings provide essential information about the stage and test timer according to recommendations by ATS/ERS, which is illustrated in Figure 3. These tools use a set of equations that have been validated in different countries [12, 13] and endorsed by different societies [14, 15]. This is particularly important because the data on Arab ethnicity included in these equations were sourced only from North Africa [16].
Nasal clip
It was utilized to measure ventilatory parameters.
Weight-height scale
It was used to determine each patient’s weight and height to calculate predicted normal values for spirometry tests.
Procedure of PFTs
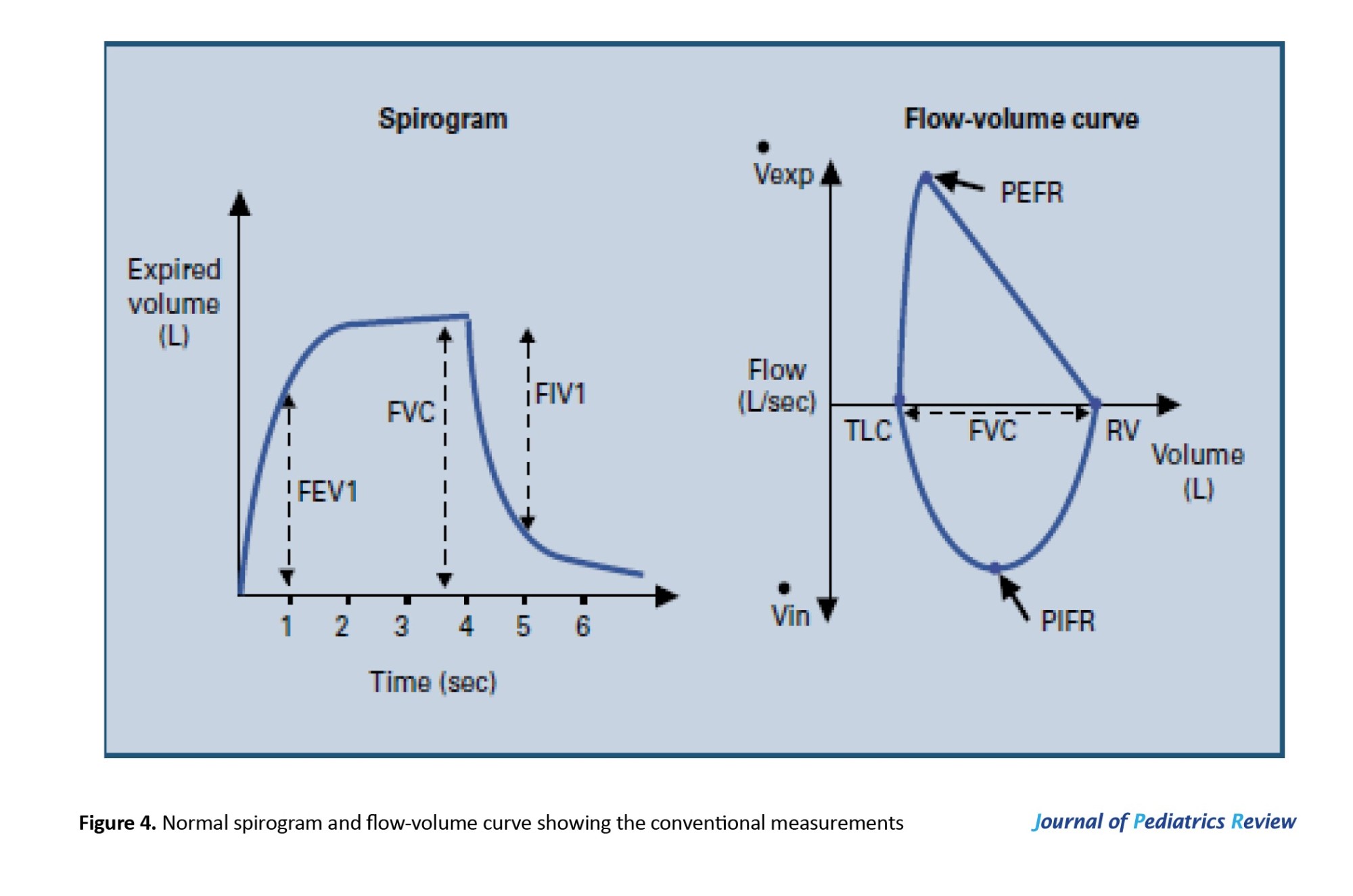
The PFTs were carried out on each patient after measuring their weight and height to determine the predicted normal values of the tested parameters. The tests were conducted while the patients were seated with their backs well supported. Each test was repeated three times, and the highest score was recorded by the computerized spirometer (Figure 4).
The ventilatory function tests were carried out to measure the following parameters:
1. VC: This is the maximum volume of air that can be exhaled or inhaled during either a maximally forced (FVC) or a slow (VC) maneuver.
2. FEV1: This refers to the volume of air that can be forcefully exhaled during the first second after a full inhalation. It serves as a valuable indicator of how efficiently the lungs can expel air and is frequently used to assess the severity of respiratory conditions.
FEV1/VC (or FEV1/FVC)
This ratio represents FEV1 as a percentage of either the vital capacity (VC) or the FVC, depending on which is greater. It provides an important measure of airflow limitation. A low FVC paired with a normal or elevated FEV1/VC ratio suggests a restrictive lung disorder. Conversely, a reduced FEV1/VC ratio points to an obstructive airflow issue. This ratio is regarded as the most accurate and reliable indicator for detecting airflow obstruction.
PEF
This is the highest rate of airflow achieved during the initial phase of a forced exhalation.
Sample size
In the pilot study, a power analysis was used to estimate the sample size. Ten patients were divided into two equal groups: The study group (FEV1/VC) (Mean±SD 91.2±2.049) and the control group (Mean±SD 90.2±4.817). The control group represents the reference normative values of the measured parameter (FEV1/VC), depending on the participant’s age, height, weight, sex, and race, using a two-tailed hypothesis with α=0.05 and power=95%. The G*Power software version 3.1.9.4 determined a sample size of 32 patients, with the sample evenly split between the two groups.
Statistical analysis
Statistical analysis was carried out using SPSS software, version 26 (SPSS Inc. in Chicago, Illinois, USA). The assumption of normality was evaluated using the Shapiro-Wilk test. The Mean±SD, minimum, maximum, and mean rank were calculated for the patients’ characteristics and dependent variables. A one-way analysis of variance (ANOVA) and the Kruskal-Wallis H test were conducted to assess differences between the three independent groups. An independent t-test was utilized to compare the means of the study groups (diaplegia and hemiplegia). A post hoc analysis was conducted using Tukey’s test and the Mann-Whitney U test to compare means and mean ranks between two independent groups. The significance level was set at <0.05.
Results
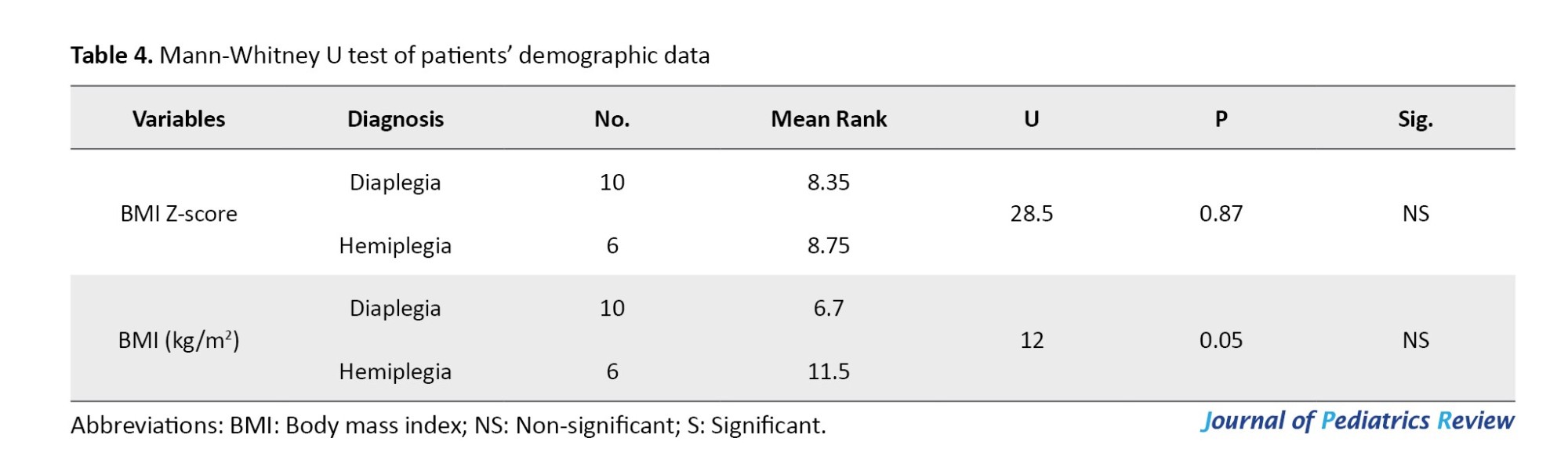
The current study involved 16 children of both genders who were diagnosed with spastic CP (10 diaplegia and 6 hemiplegia). The patients’ characteristics are presented in Table 1.
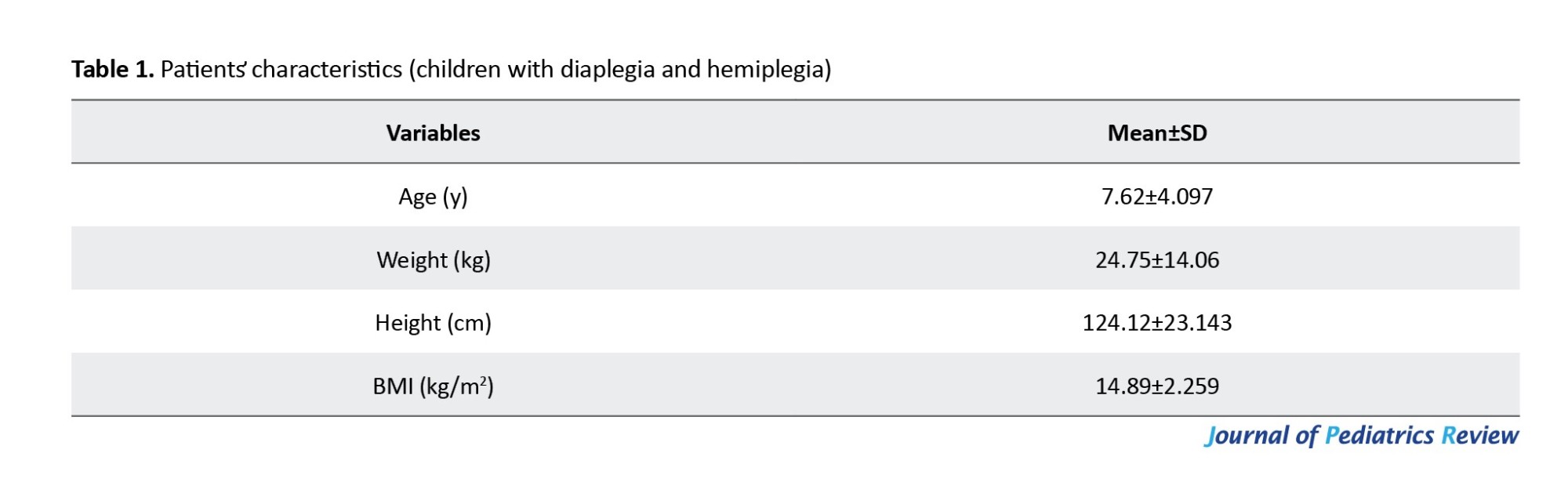
The Shapiro-Wilk test revealed no significant difference between groups in demographic characteristics (age, weight, and height) (P>0.05). While BMI and BMI Z-score data are not normally distributed, as shown in Table 2, the pulmonary functions of the study groups—including VC, FEV1, FEV1/FVC, FEV1/VC, and PEF—were compared to the normative reference values of the participants based on GLI reference equations.
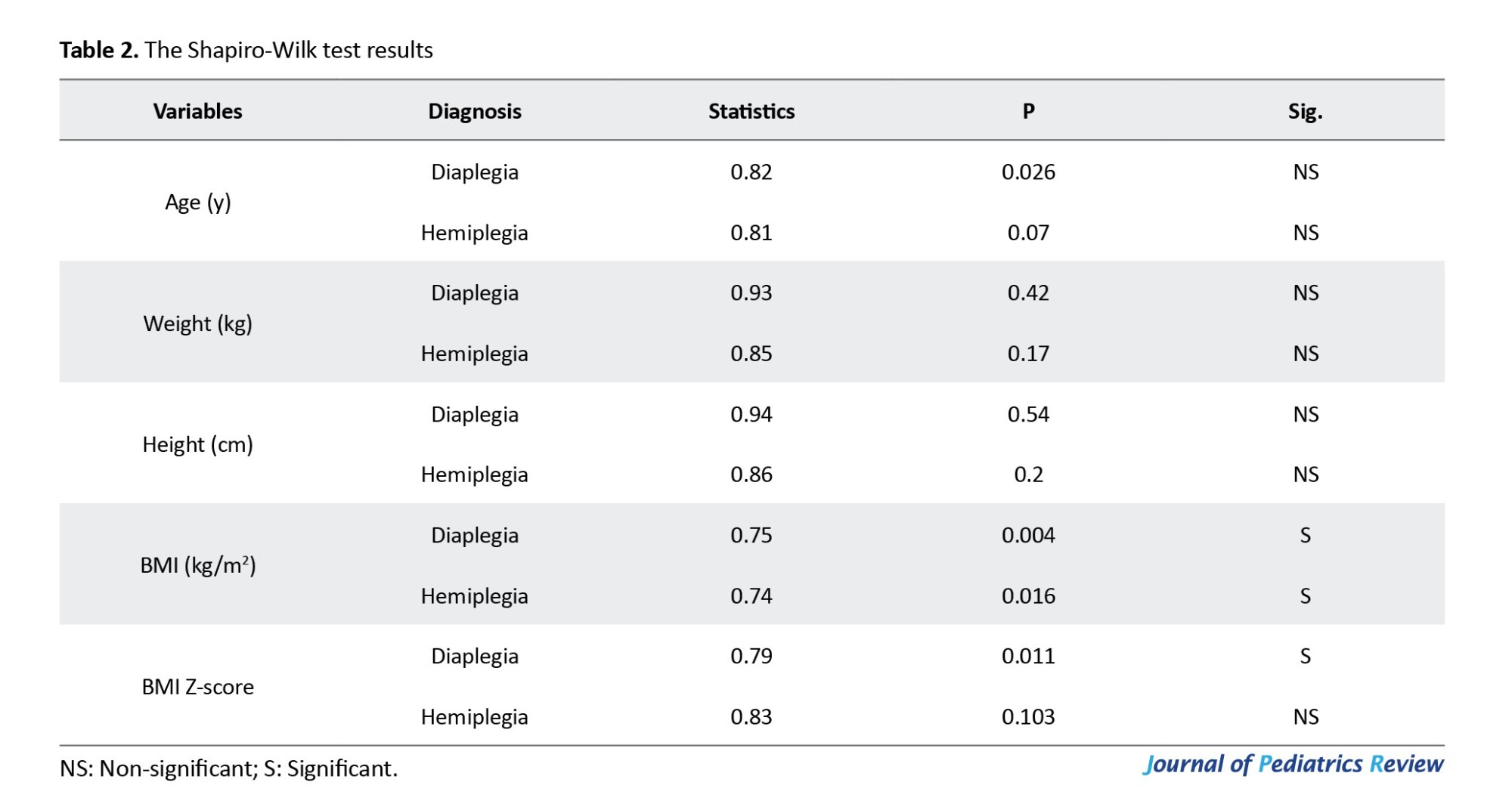
The independent t-test revealed no statistically significant differences between the groups in terms of age and height (P=0.07 and P=0.067, respectively). However, there was a statistically significant difference regarding weight (P=0.29), as presented in Table 3.
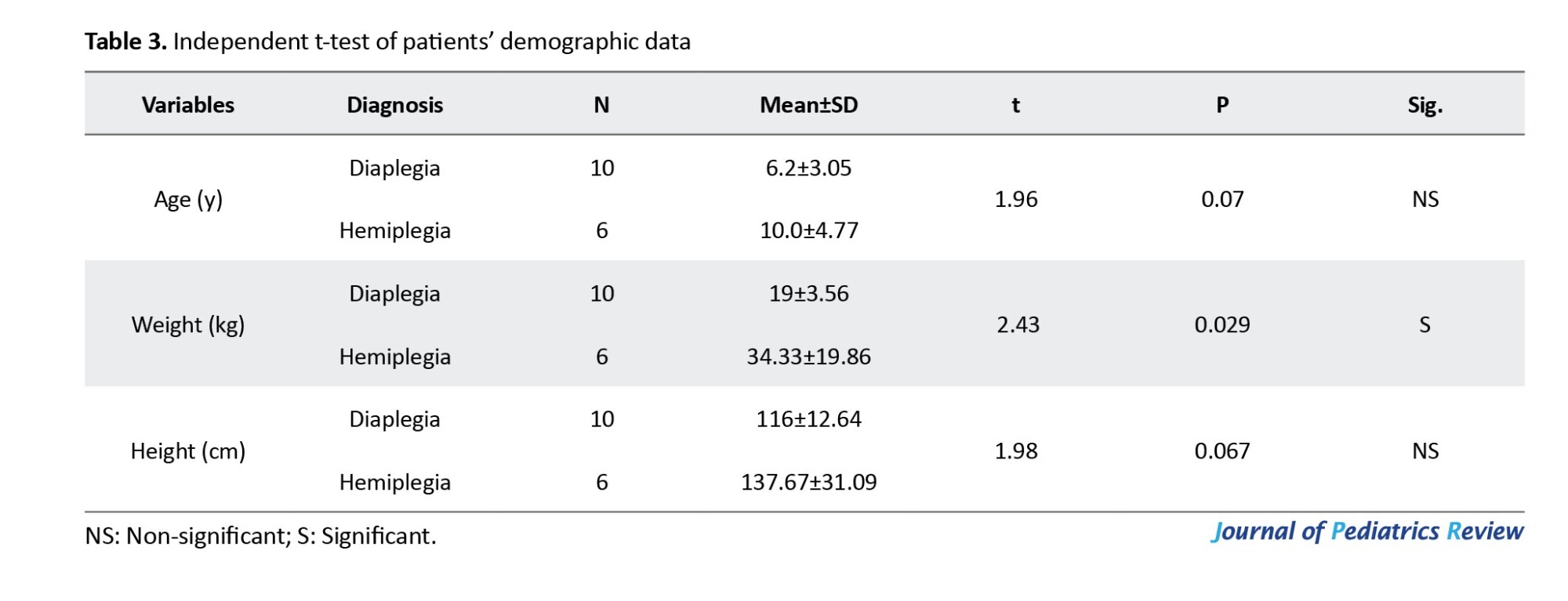
Additionally, there was no statistically significant difference in terms of BMI Z-score and BMI, as shown in Table 4.
Spirometry results
As shown in Table 5, the unpaired t-test indicated that there was a significant difference between the groups in terms of VC, FEV1, and PEF, with P=0.038, P=0.044, and P=0.00125, respectively. However, there was no significant difference between the groups regarding FEV1/FVC and FEV1/VC, with P=0.066 and P=0.076, respectively.
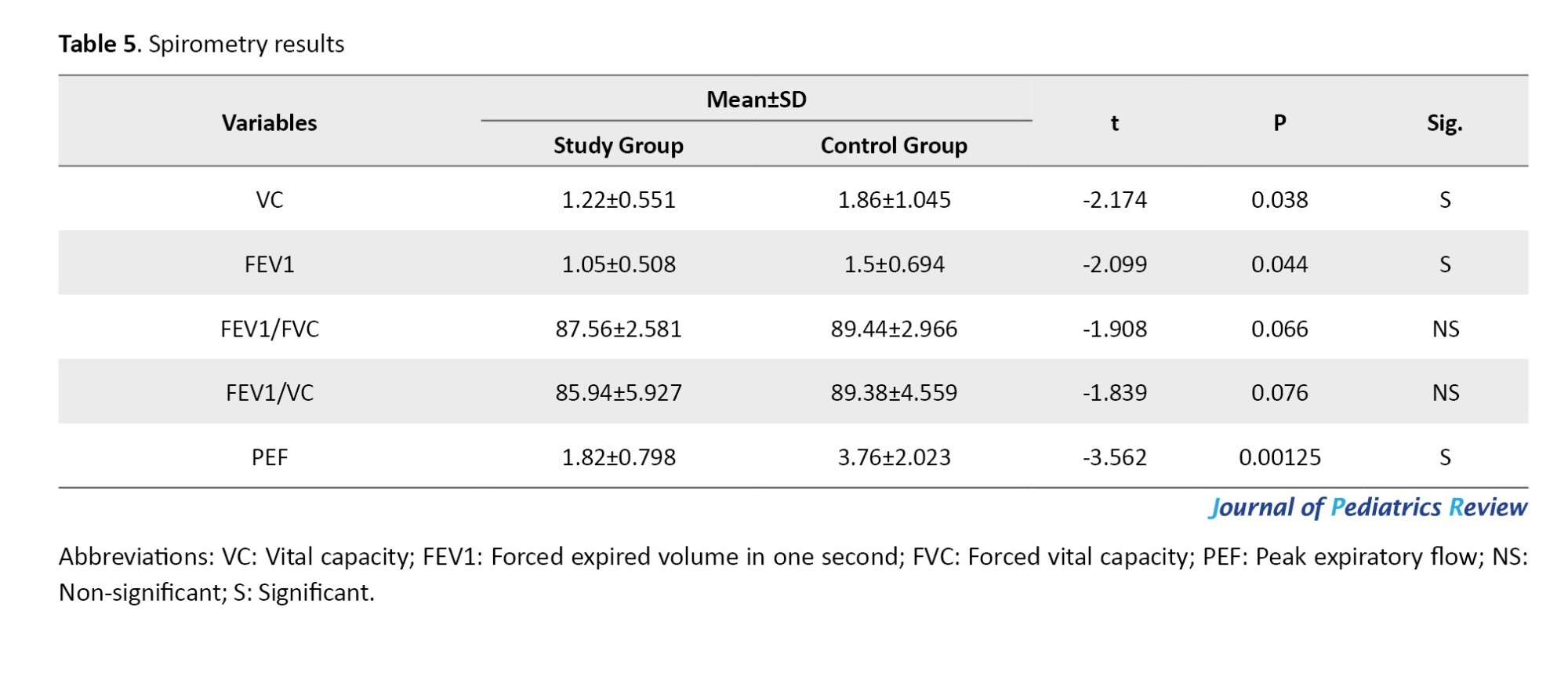
As shown in Table 6, the Shapiro-Wilk test revealed no significant difference in the data of FEV1/FVC and PEF for each group, with P<0.05. However, the data for VC, FEV1, and FEV1/VC were not normally distributed.
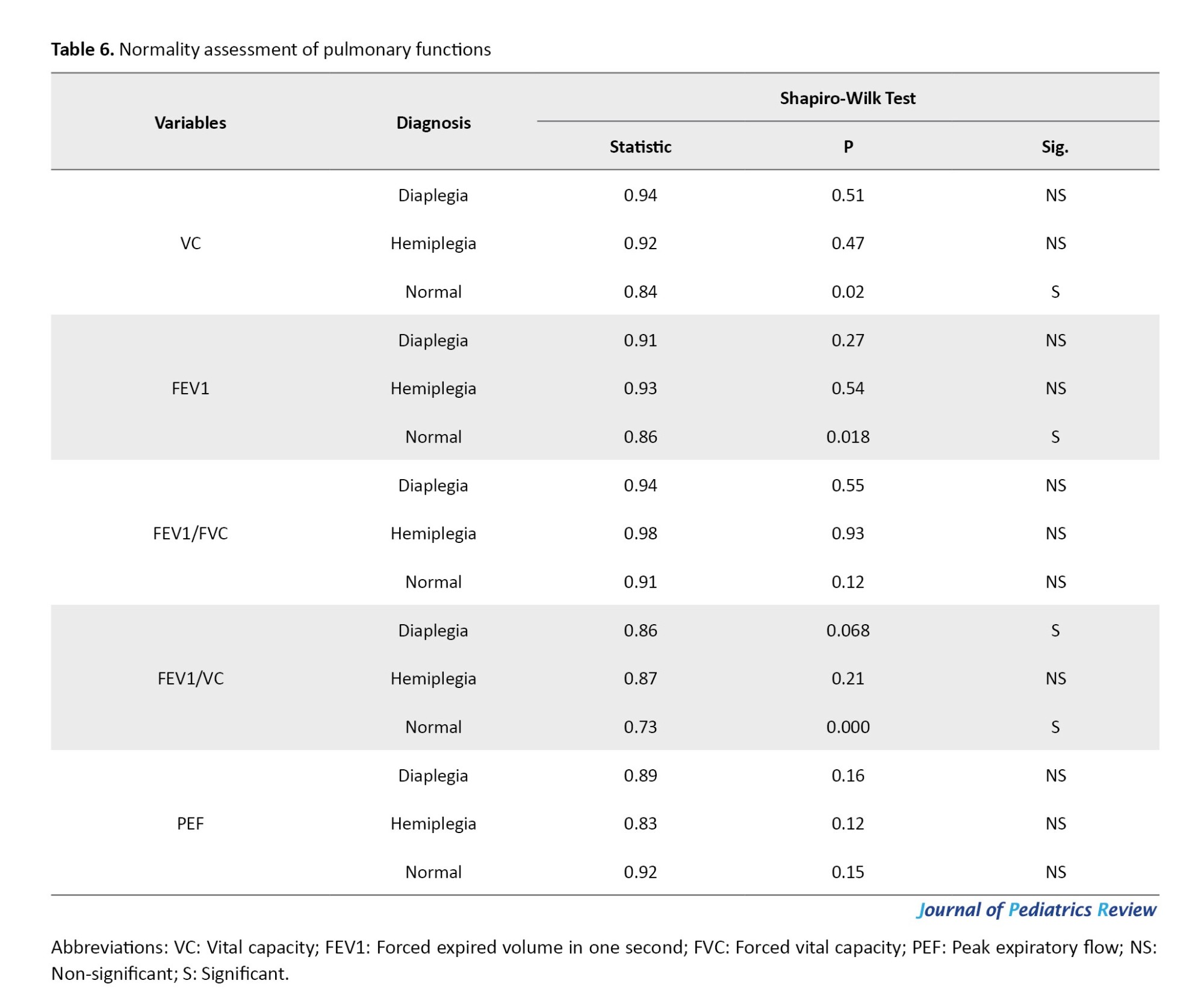
FEV1/FVC ratios did not differ significantly among the groups, as indicated by the ANOVA (P=0.138). However, there was a statistically significant difference in PEF among the groups (P=0.005), as presented in Table 7.
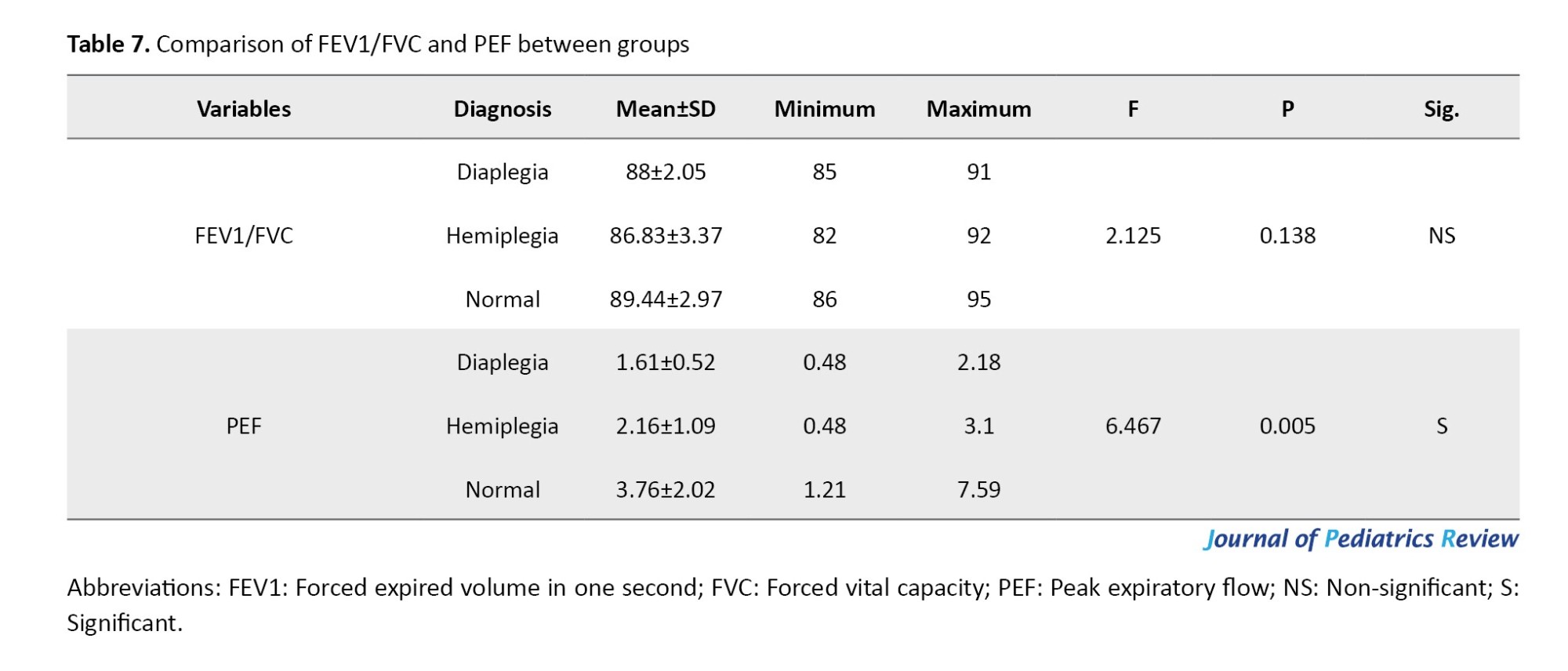
Following the significant ANOVA result for PEF, post-hoc comparisons revealed no statistically significant difference between the hemiplegic CP group and either the diaplegic CP group or the normative values of the same age and sex (P=0.77 and P=0.098, respectively). However, there was a statistically significant difference between patients with diaplegia and the normative values of the same age and sex (P=0.005), as presented in Table 8.
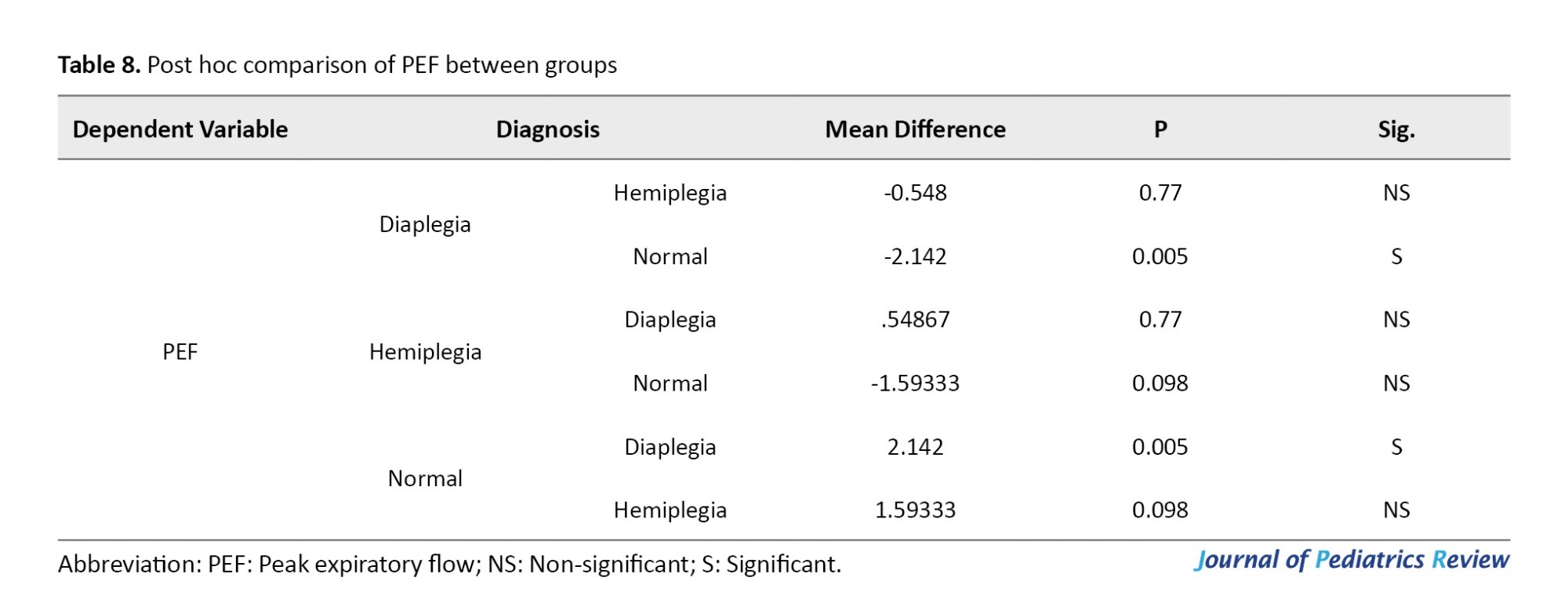
The Kruskal-Wallis test indicated a statistically significant difference among the groups in terms of VC and FEV1, where P=0.037 and P=0.016, respectively. Additionally, it indicated no statistically significant difference among the groups regarding the FEV1/VC ratio (P=0.131), as shown in Table 9.
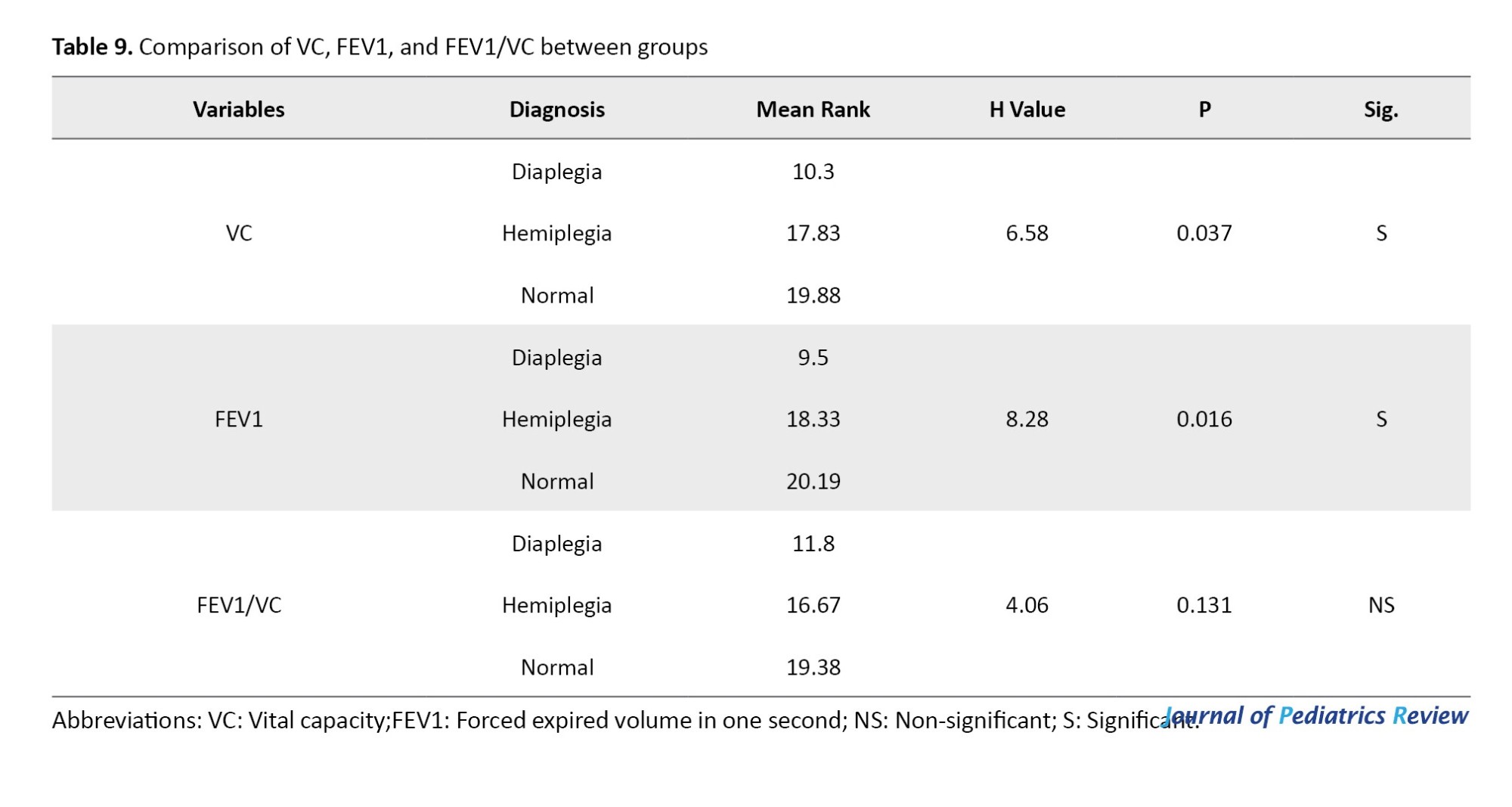
As presented in Table 10, following the significant Kruskal-Wallis test results for VC and FEV1/VC, post-hoc comparisons using the Mann–Whitney U test revealed no statistically significant difference between the hemiplegic CP group and either the diaplegic CP group (P=0.159 and P=0.193, respectively) or the normative values of the same age and sex (P=0.712 and P=0.941, respectively). However, there was a statistically significant difference between patients with diaplegia and the normative values of the same age and sex (P=0.01 and P=0.002, respectively).
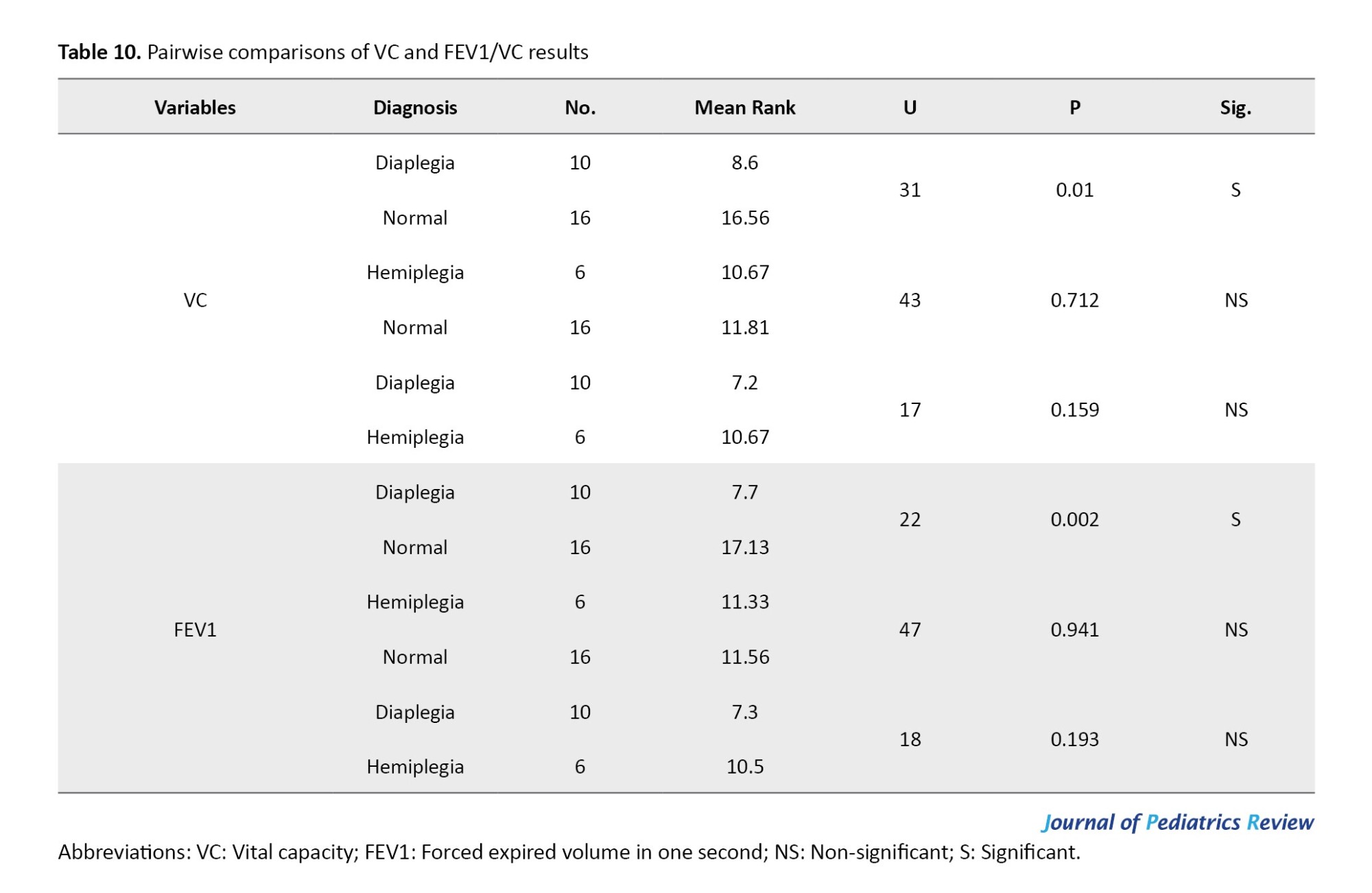
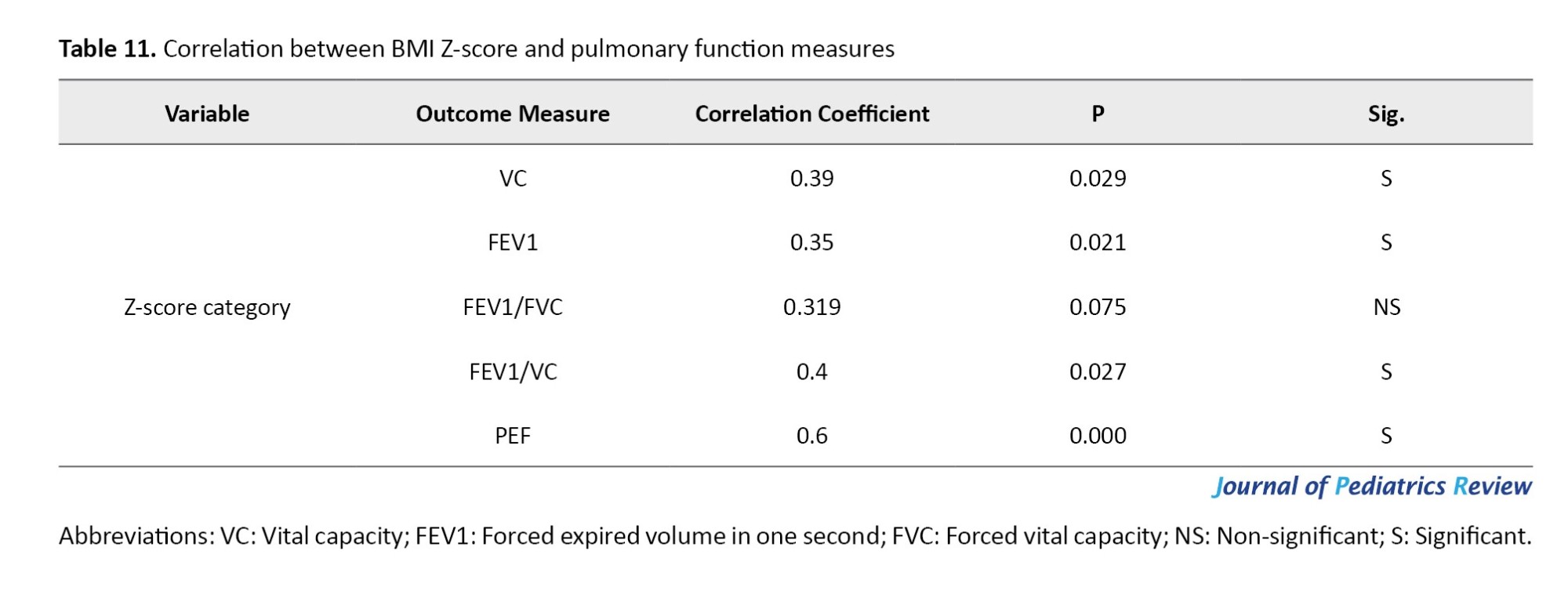
The BMI Z-Score category has a positive correlation with most pulmonary function measures, as indicated by Spearman’s rank correlation coefficient. Specifically, the correlations with PEF, VC, FEV1, and the FEV1/VC ratio are 0.6, 0.4, 0.35, and 0.39, respectively. This suggests that a lower abnormal Z-score category is associated with poorer lung function. However, the FEV1/FVC ratio did not demonstrate a significant correlation with the Z-scores, as indicated by P=0.075, as shown in Table 11.
Discussion
The findings of this study indicate that children with CP have restrictive respiratory deficits, as measured by Geratherm Respiratory Blue Cherry software. The results for VC, FEV1, and PEF revealed statistically significant differences between groups, with P=0.038, P=0.044, and P=0.00125, respectively. However, there was no statistically significant difference between groups regarding FEV1/FVC and FEV1/VC, with P=0.066 and P=0.076, respectively.
Regarding PEF, there was a statistically significant difference between patients with diaplegia and normative values of the same age and sex (P=0.005). In terms of VC and FEV1/VC, the pairwise comparisons revealed a statistically significant difference between patients with diaplegia and normative values of the same age and sex (P=0.01 and P=0.002, respectively).
Individuals with CP primarily experience impairments related to their neuromuscular and musculoskeletal systems. However, the leading cause of mortality among them is respiratory dysfunction, which poses a 14 times higher risk of death due to respiratory diseases compared to the general population [22-24]. Respiratory impairments also represent the primary cause of hospital admissions among young people with CP [6, 25].
One possible reason for the reduced values of VC, FEV1, and PEF in people with CP is the weakness of the respiratory muscles. Recent studies have shown that CP can cause a decrease in the movement of the diaphragm, the expansion of the chest wall, and the function of the lungs. Therefore, a treatment that targets the mobility of the diaphragm may help increase its movement and thus improve respiratory function in people with CP [26, 27].
The diaphragm works in combination with the abdominal muscles and the trunk to control breathing and posture [28]. The primary reason for a weak or paralyzed diaphragm in individuals with CP is damage to the phrenic motor neurons, motor cortex, respiratory center, and postural control center of the brain. This damage results in a decrease in the automatic stimulating impulses to the diaphragmatic muscle fibers [29, 30].
The difference observed between the two groups can likely be explained by reduced physical activity and chest mobility in children with motor disabilities. This aligns with the findings of Ersoz et al., who found that restricted chest mobility in children with CP stemmed primarily from muscle weakness, spasticity, and impaired neuro-motor control, rather than issues with costo-vertebral joint movement [31].
This muscle weakness can be exacerbated by reduced physical activity. It is plausible that limited physical activity and trunk control contribute to diminished respiratory function, hindering lung expansion due to restricted chest mobility. This restriction is characteristic of restrictive lung diseases, which are primarily caused by impaired elasticity in the lungs and chest wall, leading to reduced lung volumes and capacities [32].
The results of the current study showed that the BMI Z-score has a positive correlation with most pulmonary function measures, as indicated by Spearman’s rank correlation coefficient. Specifically, the correlations with PEF, VC, FEV1, and the FEV1/VC ratio are 0.6, 0.4, 0.35, and 0.39, respectively. This suggests that a lower abnormal Z-score is associated with poorer lung function.
Impaired respiratory function in children with CP may be due to malnutrition, as the patients’ average BMI was only 14.89 kg/m2. In high-income countries, about 20% of children and adolescents with CP are malnourished due to dietary issues, gastroesophageal reflux, and excessive energy expenditure. When in a catabolic state, respiratory muscles can atrophy, leading to a decrease in lung function, an increase in airway colonization, and a reduced ability to fight infections [33, 34].
The pathophysiological mechanisms that explain variations in respiratory function related to body composition can be understood through several factors. One major factor is low muscle mass, which significantly affects the strength and endurance of respiratory muscles. A decrease in muscle mass not only hampers the mechanics of breathing but also reduces pulmonary function in underweight individuals [35]. Additionally, nutritional deficiencies commonly observed in those with low body weight further compromise lung function, as inadequate intake of essential nutrients can negatively impact overall health, including respiratory health. Addressing these issues is vital for comprehensively understanding respiratory function in both obesity and leanness [36].
Finally, there was no significant difference between groups regarding FEV1/FVC and FEV1/VC, with P=0.066 and P=0.076, respectively. This result is similar to a study conducted on patients with severe Parkinson’s disease, which found a restrictive dysfunction of respiratory muscles [37].
In line with other researchers, the Tiffenau index (FEV1/VC) was also unaffected by disability level in patients with multiple sclerosis, indicating a restrictive pattern of pulmonary dysfunction [38]. Moreover, a study conducted by Yoon et al. compared the respiratory function of individuals with spinal cord injury (SCI) or stroke and normal elderly individuals. The results showed that there was no significant difference between the groups in terms of FEV1/FVC [39]. Similarly, FEV1/FVC was found to be normal in all subgroups of patients with amyotrophic lateral sclerosis [40].
Conclusion
Respiratory assessments of children with CP suggest a tendency toward a restrictive breathing pattern, primarily indicated by decreased VC, FEV1, and PEF. Further research with larger sample sizes is recommended, incorporating classifications based on various factors, such as functional gait level, topographic classification, and prematurity.
It is important to mention some limitations of this study. The sample size used was small, and the sample was convenient rather than random. In addition, we did not consider a variety of clinical factors related to pulmonary function, such as oxygen saturation and chest wall excursion.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Faculty of Physical Therapy, Deraya University, Minia, Egypt (Code: P.T./REC/230004/4/12/2023).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interests
The authors declared no conflict of interest.
Acknowledgements
The authors acknowledge all children and their parents for their cooperation.
References
Cerebral palsy (CP) is a physical disability that commonly affects children. It is characterized by persistent movement and postural disorders caused by non-progressive damage in the developing brain of a fetus or infant. The motor deficits associated with CP can lead to deficits in sensation, perception, communication, and behavior [1]. Despite advances in medical treatment, respiratory health remains a major challenge for individuals with CP [2].
These challenges include oropharyngeal dysfunction, which can result in recurrent aspiration, airway obstruction, hypoventilation, spine and chest wall abnormalities, and frequent respiratory infections. Efficient respiration relies on the coordination of various muscle actions, including those of the diaphragm, abdomen, chest, neck, and throat, as well as the stability of the head and trunk. This coordination allows for forceful inhalation and exhalation, as well as an efficient cough [3].
Worldwide, CP affects between one and nearly four out of every 1,000 live births (LB) [4]. In Arabic-speaking nations, its prevalence ranges from 0.62 to 3.6 per 1,000 LB [5]. Roughly one in eight children with CP admitted to hospitals need care for respiratory conditions. Moreover, their hospitalizations for respiratory issues last 2.5 times longer than those of children without CP [6].
Children with CP often experience respiratory problems due to restricted chest wall movement and weakened respiratory muscles. As a result, they may have poor alveolar ventilation, impaired airway clearance, and difficulty breathing [7]. These complications are among the leading causes of hospitalization and early mortality in individuals with CP. Wang et al. highlight a strong connection between respiratory muscle strength and an individual’s ability to perform daily self-care and adapt socially. Consequently, enhancing respiratory function could greatly improve both lifespan and overall quality of life [8].
Individuals with CP are at a higher risk of developing pulmonary infections. However, identifying and diagnosing respiratory issues in these individuals can be challenging. Limited communication with the patient, difficulties in conducting medical tests, and mild initial symptoms can all contribute to delays in diagnosis and treatment. This delay can put this vulnerable population at higher risk of developing complications. Nevertheless, experienced caregivers or parents are usually able to detect early, subtle signs of respiratory problems [8].
Pulmonary function tests (PFTs) help evaluate lung performance in individuals with potential risk factors for lung disease, exposure to harmful substances at work, or signs of pulmonary toxicity [9].
Spirometry, a key physiological assessment, measures a person’s ability to inhale and exhale air over a specific period. It is widely used to diagnose respiratory conditions, like asthma and chronic obstructive pulmonary disease (COPD), as well as to track the progression of various lung disorders [10].
Geratherm Respiratory Blue Cherry software is a digital spirometer designed to precisely assess dynamic lung volumes and capacities during both forced exhalation and inhalation. Its configuration offers key data regarding the test phase and timing, in alignment with the guidelines set by the American Thoracic Society (ATS), the European Respiratory Society (ERS) [11], and the global lung function initiative (GLI). The GLI has developed internationally recognized spirometry reference equations, which have been validated across multiple countries [12, 13] and supported by various professional societies [14, 15].
This is crucial because the data in GLI 2012 equations include Arab ethnicity from North Africa [16]. Emerging evidence suggests that children with CP have significantly lower respiratory status values than typically developing children. This respiratory decline is not directly caused by brain damage but is observed in children with neurological impairments [17]. However, most studies describing respiratory problems in patients with neurological impairment do not specifically focus on CP, which makes it difficult to interpret the results. Additionally, CP is a complex disorder with various types, which may require studying subgroups to better understand the condition [18].
The literature indicates that children with typical development (TD) have higher spirometry readings compared to those with CP. Moreover, the TD group exhibited superior values in forced vital capacity (FVC), forced expired volume in one second (FEV1), and peak expiratory flow (PEF) than the CP group [19, 20]. Additionally, research by Kwon and Lee found that children with spastic diplegic CP had significantly reduced respiratory function—specifically in maximal inspiratory pressure (MIP), maximal expiratory pressure (MEP), FVC, and FEV1—compared to their typically developing peers. However, there were no notable differences between the groups in FEV1/FVC ratios and PEF values [21].
The lack of standardized reference values for specific populations hinders accurate data identification, tracking intervention outcomes, and making prognoses. Consequently, some researchers opt to assess individuals with TD to create a comparative baseline between the control and experimental groups [19].
The present study differs from earlier research in terms of patient characteristics (such as age, functional abilities, and spasticity level) and the assessment method, as it utilized the norm-referenced Geratherm Respiratory Blue Cherry software.
This study aimed to evaluate the pulmonary function of children with CP and compare their results to normative values based on age and gender. Additionally, it sought to investigate differences in lung function based on the distribution of paralysis, such as diplegia and hemiplegia. Finally, the study intended to examine the relationship between the body mass index (BMI) Z-score and measures of pulmonary function.
Methods
This observational cross-sectional study was conducted over six months from January 2024 to Jun 2024. Sixteen children of both genders, aged between 3 and 16 years, diagnosed with spastic CP (10 with diaplegia and 6 with hemiplegia) participated in the study. They were selected from a pediatric rehabilitation center in Egypt.
Prior to collecting data, the study received approval from the Research Ethical Committee at the Faculty of Physical Therapy, and informed consent was obtained from the participants’ parents. Participants were screened based on the following inclusion criteria: A diagnosis of spastic CP (either diplegia or hemiplegia), a spasticity grade of 1 or 1+ on the modified Ashworth scale, adequate orientation to follow instructions for using the Geratherm Respiratory Blue Cherry software, and a normal mental status with an average intelligence quotient.
The exclusion criteria included any cognitive or neurological condition (other than CP), respiratory infections, and a history of previous surgical operations that can affect pulmonary functions, as well as mental retardation, scoliosis, and evidence of lung disease. All of these cases were confirmed by medical records and examined by a neurologist, pediatrician, and physical therapist. As shown in Figure 1, the subjects included in this study were divided into two groups of equal numbers: The study group (A), which consists of sixteen children with CP, and the control group (B), which represents the reference normative values of the participants.

The reference values of the measured parameters (VC, FEV1, FEV1/FVC, FEV1/VC, and PEF) depend on the participants’ age, height, weight, sex, and race. For example, the FEV1 value is derived from the equation set for males older than 25, as presented in Figure 2.

Instruments
Geratherm respiratory blue cherry software
The outcomes were evaluated using a medical device called Blue Cherry, developed by Geratherm Respiratory. Blue Cherry is an electronic spirometer with a built-in mini-computer that measures the ventilator function parameters in subjects through timed measurements of dynamic lung volumes and capacities during forced expiration and inspiration. The software utilizes formulas and equations to calculate reference values based on the age, height, weight, sex, and race of the participants. Blue Cherry is a registered trademark of Geratherm Respiration and consists of both hardware and software components. The device uses a standard driver provided by Microsoft.
In 2005, the ATS and the ERS issued standardized guidelines for lung function testing [11]. Building on this, the GLI was formed in Berlin in September 2008. The goal of the GLI is to improve international spirometry reference equations by incorporating the following elements:
1. The equations must be derived from individual lung function data gathered under standardized testing conditions using validated equipment and software.
2. Advanced statistical methods should be used to develop equations that apply continuously across all age groups, from young children to the elderly.
3. The equations should support flexible interpretation using normal range limits, accounting for differences in sex, ethnicity, age, and lung function variables.
4. They should have practical clinical applications and be compatible with commercially available devices.
5. Results should clearly indicate where an individual falls within the “normal range”.
Geratherm Respiratory Blue Cherry software settings provide essential information about the stage and test timer according to recommendations by ATS/ERS, which is illustrated in Figure 3. These tools use a set of equations that have been validated in different countries [12, 13] and endorsed by different societies [14, 15]. This is particularly important because the data on Arab ethnicity included in these equations were sourced only from North Africa [16].

Nasal clip
It was utilized to measure ventilatory parameters.
Weight-height scale
It was used to determine each patient’s weight and height to calculate predicted normal values for spirometry tests.
Procedure of PFTs
The PFTs were carried out on each patient after measuring their weight and height to determine the predicted normal values of the tested parameters. The tests were conducted while the patients were seated with their backs well supported. Each test was repeated three times, and the highest score was recorded by the computerized spirometer (Figure 4).

The ventilatory function tests were carried out to measure the following parameters:
1. VC: This is the maximum volume of air that can be exhaled or inhaled during either a maximally forced (FVC) or a slow (VC) maneuver.
2. FEV1: This refers to the volume of air that can be forcefully exhaled during the first second after a full inhalation. It serves as a valuable indicator of how efficiently the lungs can expel air and is frequently used to assess the severity of respiratory conditions.
FEV1/VC (or FEV1/FVC)
This ratio represents FEV1 as a percentage of either the vital capacity (VC) or the FVC, depending on which is greater. It provides an important measure of airflow limitation. A low FVC paired with a normal or elevated FEV1/VC ratio suggests a restrictive lung disorder. Conversely, a reduced FEV1/VC ratio points to an obstructive airflow issue. This ratio is regarded as the most accurate and reliable indicator for detecting airflow obstruction.
PEF
This is the highest rate of airflow achieved during the initial phase of a forced exhalation.
Sample size
In the pilot study, a power analysis was used to estimate the sample size. Ten patients were divided into two equal groups: The study group (FEV1/VC) (Mean±SD 91.2±2.049) and the control group (Mean±SD 90.2±4.817). The control group represents the reference normative values of the measured parameter (FEV1/VC), depending on the participant’s age, height, weight, sex, and race, using a two-tailed hypothesis with α=0.05 and power=95%. The G*Power software version 3.1.9.4 determined a sample size of 32 patients, with the sample evenly split between the two groups.
Statistical analysis
Statistical analysis was carried out using SPSS software, version 26 (SPSS Inc. in Chicago, Illinois, USA). The assumption of normality was evaluated using the Shapiro-Wilk test. The Mean±SD, minimum, maximum, and mean rank were calculated for the patients’ characteristics and dependent variables. A one-way analysis of variance (ANOVA) and the Kruskal-Wallis H test were conducted to assess differences between the three independent groups. An independent t-test was utilized to compare the means of the study groups (diaplegia and hemiplegia). A post hoc analysis was conducted using Tukey’s test and the Mann-Whitney U test to compare means and mean ranks between two independent groups. The significance level was set at <0.05.
Results
The current study involved 16 children of both genders who were diagnosed with spastic CP (10 diaplegia and 6 hemiplegia). The patients’ characteristics are presented in Table 1.

The Shapiro-Wilk test revealed no significant difference between groups in demographic characteristics (age, weight, and height) (P>0.05). While BMI and BMI Z-score data are not normally distributed, as shown in Table 2, the pulmonary functions of the study groups—including VC, FEV1, FEV1/FVC, FEV1/VC, and PEF—were compared to the normative reference values of the participants based on GLI reference equations.

The independent t-test revealed no statistically significant differences between the groups in terms of age and height (P=0.07 and P=0.067, respectively). However, there was a statistically significant difference regarding weight (P=0.29), as presented in Table 3.

Additionally, there was no statistically significant difference in terms of BMI Z-score and BMI, as shown in Table 4.

Spirometry results
As shown in Table 5, the unpaired t-test indicated that there was a significant difference between the groups in terms of VC, FEV1, and PEF, with P=0.038, P=0.044, and P=0.00125, respectively. However, there was no significant difference between the groups regarding FEV1/FVC and FEV1/VC, with P=0.066 and P=0.076, respectively.

As shown in Table 6, the Shapiro-Wilk test revealed no significant difference in the data of FEV1/FVC and PEF for each group, with P<0.05. However, the data for VC, FEV1, and FEV1/VC were not normally distributed.

FEV1/FVC ratios did not differ significantly among the groups, as indicated by the ANOVA (P=0.138). However, there was a statistically significant difference in PEF among the groups (P=0.005), as presented in Table 7.

Following the significant ANOVA result for PEF, post-hoc comparisons revealed no statistically significant difference between the hemiplegic CP group and either the diaplegic CP group or the normative values of the same age and sex (P=0.77 and P=0.098, respectively). However, there was a statistically significant difference between patients with diaplegia and the normative values of the same age and sex (P=0.005), as presented in Table 8.

The Kruskal-Wallis test indicated a statistically significant difference among the groups in terms of VC and FEV1, where P=0.037 and P=0.016, respectively. Additionally, it indicated no statistically significant difference among the groups regarding the FEV1/VC ratio (P=0.131), as shown in Table 9.

As presented in Table 10, following the significant Kruskal-Wallis test results for VC and FEV1/VC, post-hoc comparisons using the Mann–Whitney U test revealed no statistically significant difference between the hemiplegic CP group and either the diaplegic CP group (P=0.159 and P=0.193, respectively) or the normative values of the same age and sex (P=0.712 and P=0.941, respectively). However, there was a statistically significant difference between patients with diaplegia and the normative values of the same age and sex (P=0.01 and P=0.002, respectively).

The BMI Z-Score category has a positive correlation with most pulmonary function measures, as indicated by Spearman’s rank correlation coefficient. Specifically, the correlations with PEF, VC, FEV1, and the FEV1/VC ratio are 0.6, 0.4, 0.35, and 0.39, respectively. This suggests that a lower abnormal Z-score category is associated with poorer lung function. However, the FEV1/FVC ratio did not demonstrate a significant correlation with the Z-scores, as indicated by P=0.075, as shown in Table 11.

Discussion
The findings of this study indicate that children with CP have restrictive respiratory deficits, as measured by Geratherm Respiratory Blue Cherry software. The results for VC, FEV1, and PEF revealed statistically significant differences between groups, with P=0.038, P=0.044, and P=0.00125, respectively. However, there was no statistically significant difference between groups regarding FEV1/FVC and FEV1/VC, with P=0.066 and P=0.076, respectively.
Regarding PEF, there was a statistically significant difference between patients with diaplegia and normative values of the same age and sex (P=0.005). In terms of VC and FEV1/VC, the pairwise comparisons revealed a statistically significant difference between patients with diaplegia and normative values of the same age and sex (P=0.01 and P=0.002, respectively).
Individuals with CP primarily experience impairments related to their neuromuscular and musculoskeletal systems. However, the leading cause of mortality among them is respiratory dysfunction, which poses a 14 times higher risk of death due to respiratory diseases compared to the general population [22-24]. Respiratory impairments also represent the primary cause of hospital admissions among young people with CP [6, 25].
One possible reason for the reduced values of VC, FEV1, and PEF in people with CP is the weakness of the respiratory muscles. Recent studies have shown that CP can cause a decrease in the movement of the diaphragm, the expansion of the chest wall, and the function of the lungs. Therefore, a treatment that targets the mobility of the diaphragm may help increase its movement and thus improve respiratory function in people with CP [26, 27].
The diaphragm works in combination with the abdominal muscles and the trunk to control breathing and posture [28]. The primary reason for a weak or paralyzed diaphragm in individuals with CP is damage to the phrenic motor neurons, motor cortex, respiratory center, and postural control center of the brain. This damage results in a decrease in the automatic stimulating impulses to the diaphragmatic muscle fibers [29, 30].
The difference observed between the two groups can likely be explained by reduced physical activity and chest mobility in children with motor disabilities. This aligns with the findings of Ersoz et al., who found that restricted chest mobility in children with CP stemmed primarily from muscle weakness, spasticity, and impaired neuro-motor control, rather than issues with costo-vertebral joint movement [31].
This muscle weakness can be exacerbated by reduced physical activity. It is plausible that limited physical activity and trunk control contribute to diminished respiratory function, hindering lung expansion due to restricted chest mobility. This restriction is characteristic of restrictive lung diseases, which are primarily caused by impaired elasticity in the lungs and chest wall, leading to reduced lung volumes and capacities [32].
The results of the current study showed that the BMI Z-score has a positive correlation with most pulmonary function measures, as indicated by Spearman’s rank correlation coefficient. Specifically, the correlations with PEF, VC, FEV1, and the FEV1/VC ratio are 0.6, 0.4, 0.35, and 0.39, respectively. This suggests that a lower abnormal Z-score is associated with poorer lung function.
Impaired respiratory function in children with CP may be due to malnutrition, as the patients’ average BMI was only 14.89 kg/m2. In high-income countries, about 20% of children and adolescents with CP are malnourished due to dietary issues, gastroesophageal reflux, and excessive energy expenditure. When in a catabolic state, respiratory muscles can atrophy, leading to a decrease in lung function, an increase in airway colonization, and a reduced ability to fight infections [33, 34].
The pathophysiological mechanisms that explain variations in respiratory function related to body composition can be understood through several factors. One major factor is low muscle mass, which significantly affects the strength and endurance of respiratory muscles. A decrease in muscle mass not only hampers the mechanics of breathing but also reduces pulmonary function in underweight individuals [35]. Additionally, nutritional deficiencies commonly observed in those with low body weight further compromise lung function, as inadequate intake of essential nutrients can negatively impact overall health, including respiratory health. Addressing these issues is vital for comprehensively understanding respiratory function in both obesity and leanness [36].
Finally, there was no significant difference between groups regarding FEV1/FVC and FEV1/VC, with P=0.066 and P=0.076, respectively. This result is similar to a study conducted on patients with severe Parkinson’s disease, which found a restrictive dysfunction of respiratory muscles [37].
In line with other researchers, the Tiffenau index (FEV1/VC) was also unaffected by disability level in patients with multiple sclerosis, indicating a restrictive pattern of pulmonary dysfunction [38]. Moreover, a study conducted by Yoon et al. compared the respiratory function of individuals with spinal cord injury (SCI) or stroke and normal elderly individuals. The results showed that there was no significant difference between the groups in terms of FEV1/FVC [39]. Similarly, FEV1/FVC was found to be normal in all subgroups of patients with amyotrophic lateral sclerosis [40].
Conclusion
Respiratory assessments of children with CP suggest a tendency toward a restrictive breathing pattern, primarily indicated by decreased VC, FEV1, and PEF. Further research with larger sample sizes is recommended, incorporating classifications based on various factors, such as functional gait level, topographic classification, and prematurity.
It is important to mention some limitations of this study. The sample size used was small, and the sample was convenient rather than random. In addition, we did not consider a variety of clinical factors related to pulmonary function, such as oxygen saturation and chest wall excursion.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Faculty of Physical Therapy, Deraya University, Minia, Egypt (Code: P.T./REC/230004/4/12/2023).
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to the conception and design of the study, data collection and analysis, interception of the results and drafting of the manuscript. Each author approved the final version of the manuscript for submission.
Conflict of interests
The authors declared no conflict of interest.
Acknowledgements
The authors acknowledge all children and their parents for their cooperation.
References
- Boel L, Pernet K, Toussaint M, Ides K, Leemans G, Haan J, et al. Respiratory morbidity in children with cerebral palsy: An overview. Dev Med Child Neurol. 2019; 61(6):646-53. [DOI:10.1111/dmcn.14060] [PMID]
- Himmelmann K, Sundh V. Survival with cerebral palsy over five decades in western Sweden. Dev Med Child Neurol. 2015; 57(8):762-7. [DOI:10.1111/dmcn.12718] [PMID]
- Proesmans M, Vreys M, Huenaerts E, Haest E, Coremans S, Vermeulen F, et al. Respiratory morbidity in children with profound intellectual and multiple disability. Pediatr Pulmonol. 2015; 50(10):1033-8. [DOI:10.1002/ppul.23114] [PMID]
- Stavsky M, Mor O, Mastrolia SA, Greenbaum S, Than NG, Erez O. Cerebral palsy-trends in epidemiology and recent development in prenatal mechanisms of disease, treatment, and prevention. Front Pediatr. 2017; 5:21. [DOI:10.3389/fped.2017.00021] [PMID] [PMCID]
- Mushta SM, King C, Goldsmith S, Smithers-Sheedy H, Badahdah AM, Rashid H, et al. Epidemiology of cerebral palsy among children and adolescents in Arabic-speaking countries: A systematic review and meta-analysis. Brain Sci. 2022; 12(7):859. [DOI:10.3390/brainsci12070859] [PMID] [PMCID]
- Meehan E, Reid SM, Williams K, Freed GL, Sewell JR, Vidmar S, et al. Hospital admissions in children with cerebral palsy: A data linkage study. Dev Med Child Neurol. 2017; 59(5):512-9. [DOI:10.1111/dmcn.13350] [PMID]
- Young NL, McCormick AM, Gilbert T, Ayling-Campos A, Burke T, Fehlings D, et al. Reasons for hospital admissions among youth and young adults with cerebral palsy. Arch Phys Med Rehabil. 2011; 92(1):46-50. [DOI:10.1016/j.apmr.2010.10.002] [PMID]
- Wang HY, Chen CC, Hsiao SF. Relationships between respiratory muscle strength and daily living function in children with cerebral palsy. Res Dev Disabil. 2012; 33(4):1176-82. [DOI:10.1016/j.ridd.2012.02.004] [PMID]
- Stanojevic S, Kaminsky DA, Miller MR, Thompson B, Aliverti A, Barjaktarevic I, et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J. 2022; 60(1):2101499. [DOI:10.1183/13993003.01499-2021] [PMID]
- Sankari A, Bascom A, Oomman S, Badr MS. Sleep disordered breathing in chronic spinal cord injury. J Clin Sleep Med. 2014; 10(1):65-72. [DOI:10.5664/jcsm.3362] [PMID] [PMCID]
- Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J. 2005; 26(5):948-68. [DOI:10.1183/09031936.05.00035205] [PMID]
- Busi LE, Sly PD. Validation of the GLI‐2012 spirometry reference equations in Argentinian children. Pediatr Pulmonol. 2018; 53(2):204-8. [DOI:10.1002/ppul.23923]
- Mandadzhieva S, Marinov B, Kostianev S. Global lung function initiative (GLI) prediction equations fit the normal lung function in Bulgarian and Romany children. Eur Respir J. 2018; 52(suppl 62):PA4577. [DOI:10.1183/13993003]
- Culver BH, Graham BL, Coates AL, Wanger J, Berry CE, Clarke PK, et al. Recommendations for a standardized pulmonary function report. An official American thoracic society technical statement. Am J Respir Crit Care Med. 2017; 196(11):1463-72. [DOI:10.1164/rccm.201710-1981ST] [PMID]
- Quanjer PH, Brazzale DJ, Boros PW, Pretto JJ. Implications of adopting the global lungs initiative 2012 all-age reference equations for spirometry. Eur Respir J. 2013; 42(4):1046-54. [DOI:10.1183/09031936.00195512] [PMID]
- Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. Multi-ethnic reference values for spirometry for the 3-95-yr age range: The global lung function 2012 equations. Eur Respir J. 2012; 40(6):1324-43. [DOI:10.1183/ 09031936.00080312] [PMID] [PMCID]
- Marpole R, Blackmore AM, Gibson N, Cooper MS, Langdon K, Wilson AC. Evaluation and management of respiratory illness in children with cerebral palsy. Front Pediatr. 2020; 8:333. [DOI:10.3389/fped.2020.00333] [PMID] [PMCID]
- Holmes Jr L. Pediatric cerebral palsy life expectancy: Has survival improved over time?. Pediatr Ther. 2013; 3(01):146. [DOI:10.4172/2161 0665.1000146]
- Almeida KC, Rasera CC, Ripka WL, Mélo TR, Neves EB. Respiratory function in children with cerebral palsy. Arch Med. 2023; 23(1). [DOI:10.30554/archmed.23.1.4545.2023]
- Gupt SK, Borah D, Athani BD. Comparison of pulmonary function in children with cerebral palsy and normally developed children. J Clin Diagn Res. 2019; 13(8):OC25-7. [DOI:10.7860/JCDR/2019/41971.13086]
- Kwon YH, Lee HY. Differences in respiratory pressure and pulmonary function among children with spastic diplegic and hemiplegic cerebral palsy in comparison with normal controls. J Phys Ther Sci. 2015; 27(2):401-3. [DOI:10.1589/jpts.27.401] [PMID] [PMCID]
- Blair E, Langdon K, McIntyre S, Lawrence D, Watson L. Survival and mortality in cerebral palsy: Observations to the sixth decade from a data linkage study of a total population register and National Death Index. BMC Neurol. 2019; 19(1):111. [DOI:10.1186/s12883-019- 1343-1] [PMID] [PMCID]
- Ryan JM, Peterson MD, Ryan N, Smith KJ, O'connell NE, Liverani S, et al. Mortality due to cardiovascular disease, respiratory disease, and cancer in adults with cerebral palsy. Dev Med Child Neurol. 2019; 61(8):924-8. [DOI:10.1111/dmcn.14176] [PMID] [PMCID]
- Pilla M, Langlois NE, Byard RW. Causes of death in a series of decedents with cerebral palsy in a medicolegal context. Aust J Forensic Sci. 2018; 50(4):428-34. [DOI:10.1080/00450618.2016.1259432]
- Carter B, Bennett CV, Jones H, Bethel J, Perra O, Wang T, et al. Healthcare use by children and young adults with cerebral palsy. Dev Med Child Neurol. 2021; 63(1):75-80. [DOI:10.1111/dmcn.14536] [PMID]
- Bennett S, Siritaratiwat W, Tanrangka N, Bennett MJ, Kanpittaya J. Diaphragmatic mobility in children with spastic cerebral palsy and differing motor performance levels. Respir Physiol Neurobiol. 2019; 266:163-70. [DOI:10.1016/j.resp.2019.05.010] [PMID]
- Kwon HY, Kim BJ. Correlation between the dimensions of diaphragm movement, respiratory functions and pressures in accordance with the gross motor function classification system levels in children with cerebral palsy. J Exerc Rehabil. 2018; 14(6):998-1004. [DOI:10.12965/jer.1836422.211] [PMID] [PMCID]
- Massery M. Multisystem clinical implications of impaired breathing mechanics and postural control. In: Frownfalter D, Dean E. Cardiovascular and pulmonary physical therapy: Evidence to practice. Amsterdam: Elsevier Health Sciences; 2022. [Link]
- Nogués MA, Roncoroni AJ, Benarroch E. Breathing control in neurological diseases. Clin Auton Res. 2002; 12(6):440-9. [DOI:10.1007/s10286-002-0067-1] [PMID]
- Horn EM, Waldrop TG. Suprapontine control of respiration. Respir Physiol. 1998; 114(3):201-11. [DOI:10.1016/S0034-5687(98)00087-5] [PMID]
- Ersöz M, Selcuk B, Gündüz R, Kurtaran A, Akyüz M. Decreased chest mobility in children with spastic cerebral palsy. Turk J Pediatr. 2006; 48(4):344-50. [Link]
- Kwon YH, Lee HY. Differences of respiratory function according to level of the gross motor function classification system in children with cerebral palsy. J Phys Ther Sci. 2014; 26(3):389-91. [DOI:10.1589/jpts.26.389] [PMID] [PMCID]
- Reddihough DS, Collins KJ. The epidemiology and causes of cerebral palsy. Aust J Physiother. 2003; 49(1):7-12.[DOI:10.1016/S0004-9514(14)60183-5] [PMID]
- Reddihough DS, Baikie G, Walstab JE. Cerebral palsy in Victoria, Australia: mortality and causes of death. J Paediatr Child Health. 2001; 37(2):183-6. [DOI:10.1046/j.1440-1754.2001.00644.x] [PMID]
- Martínez-Luna N, Orea-Tejeda A, González-Islas D, Flores-Cisneros L, Keirns-Davis C, Sánchez-Santillán R, et al. Association between body composition, sarcopenia and pulmonary function in chronic obstructive pulmonary disease. BMC Pulm Med. 2022; 22(1):106. [DOI:10.1186/s12890-022-01907-1] [PMID] [PMCID]
- Gozzi-Silva SC, Teixeira FME, Duarte AJDS, Sato MN, Oliveira LM. Immunomodulatory role of nutrients: How can pulmonary dysfunctions improve? Front Nutr. 2021; 8:674258. [DOI:10.3389/fnut.2021.674258] [PMID] [PMCID]
- De Pandis MF, Starace A, Stefanelli F, Marruzzo P, Meoli I, De Simone G, et al. Modification of respiratory function parameters in patients with severe Parkinson's disease. Neurol Sci. 2002; 23(Suppl 2):S69-70. [DOI:10.1007/s100720200074] [PMID]
- Buyse B, Demedts M, Meekers J, Vandegaer L, Rochette F, Kerkhofs L. Respiratory dysfunction in multiple sclerosis: A prospective analysis of 60 patients. Eur Respir J. 1997; 10(1):139-45. [DOI:10.1183/09031936.97.10010139] [PMID]
- Yoon J, Park J, Lee D, Roh H. Comparisons of respiratory function and activities of daily living between spinal cord injury and stroke patients and normal elderly people. J Phys Ther Sci. 2012; 24(6):465-9. [DOI:10.1589/jpts.24.465]
- Chandrasoma B, Balfe D, Naik T, Elsayegh A, Lewis M, Mosenifar Z. Pulmonary function in patients with amyotrophic lateral sclerosis at disease onset. Monaldi Arch Chest Dis. 2012; 77(3-4):129-33. [DOI:10.4081/monaldi.2012.146] [PMID]
Type of Study: Research Article |
Subject:
Pediatric Rehabilitation
Received: 2025/02/10 | Accepted: 2025/07/20 | Published: 2025/07/19
Received: 2025/02/10 | Accepted: 2025/07/20 | Published: 2025/07/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






