Volume 13, Issue 4 (10-2025)
J. Pediatr. Rev 2025, 13(4): 303-318 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Mirmohammadkhani M, Paknazar F, Ghods E. Epidemiologic Features of Kawasaki Disease in Middle East Countries: A Systematic Review and Meta-analysis. J. Pediatr. Rev 2025; 13 (4) :303-318
URL: http://jpr.mazums.ac.ir/article-1-747-en.html
URL: http://jpr.mazums.ac.ir/article-1-747-en.html
1- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran. & Department of Epidemiology and Biostatistics, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran.
2- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran & Department of Community Medicine, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran. ,ghodsemla@yahoo.com
2- Social Determinants of Health Research Center, Semnan University of Medical Sciences, Semnan, Iran & Department of Community Medicine, School of Medicine, Semnan University of Medical Sciences, Semnan, Iran. ,
Keywords: Kawasaki disease (KD), Systematic review, Meta-analysis, Epidemiology, Middle East, Prevalence
Full-Text [PDF 2428 kb]
(354 Downloads)
| Abstract (HTML) (808 Views)
Full-Text: (332 Views)
Introduction
Kawasaki disease (KD) is one of the most important causes of vasculitis in children, and manifests as a febrile systemic inflammatory disorder [1-3]. Approximately 20–25% of untreated children develop coronary artery lesions (CALs), including aneurysms, making it the most common cause of acquired heart disease in children from developed countries [1, 4-6]. Several studies have been conducted to clarify KD’s etiology worldwide; however, it still has an unknown etiology and remains a clinical diagnosis without a pathognomonic diagnostic test, making it difficult to identify all children at risk for developing CALs, even in countries with extensive nationwide studies [3, 6-12]. Typical KD is characterized by the presence of specific clinical criteria, while atypical KD (or incomplete KD) presents with fewer symptoms that do not meet the full diagnostic criteria. The atypical form is important since it may be diagnosed later or with more difficulty, which can lead to late initiation of treatment and the development of complications [1, 13]. The prevalence of atypical KD may vary from 15% to 36% and is more prevalent in younger patients [14].
Affecting all racial and ethnic groups, KD has been reported in all countries [2-4]. The disease is usually diagnosed in early childhood, with an age range from 3 months to 10 years, and boys are slightly more likely to be affected than girls. The mainstay of treatment for KD is intravenous immunoglobulin, and the patients generally require hospitalization for diagnosis and treatment. Among ethnic groups, epidemiological studies have shown that children with Asian and Pacific Islander descent have the highest incidence of KD [1]. The incidence of KD in the United States is approximately 25 per 100,000 children <5 years of age. In Japan, the incidence is estimated at approximately 250 per 100,000 [15]. Most countries have recently observed a gradual increase in KD incidence. The reasons for this increase are unclear and may reflect a true rise in cases or improved ascertainment due to increased awareness among healthcare providers and greater access to specialist care resulting from economic growth and industrialization [7, 16]. Recent studies in Japan suggest that the proportion of Japanese patients with CAL has decreased over time [1].
Many studies have been conducted to investigate the epidemiological features of KD in developed countries worldwide, with many based on data from accurate disease registration systems in those countries [17]. These studies provide useful and practical information about the incidence of the KD and its temporal and spatial distribution, which can help control the disease and make effective management decisions for its surveillance at the national level. The studies in Asian countries are limited to some countries with registration systems, such as Japan. But in most Asian countries, there is not enough scientific evidence about the epidemiology of the disease that can be relied upon by physicians and health officials, including those in the Middle East. Due to the relatively low prevalence of KD in some Middle Eastern countries, there is basically no national research on the epidemiology of KD. In other countries, most studies are case reports or case series.
The Middle East is a geopolitical region encompassing the Arabian Peninsula (including Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, the United Arab Emirates (UAE), Jordan, and Yemen), the Levant countries (including Cyprus, Israel, Jordan, Lebanon, Syria, and the Palestinian territories), Turkey, Egypt, Iran, and Iraq. In the 1980s, the term “Greater Middle East” came to encompass Pakistan and Afghanistan from Asia, as well as the Maghreb, Sudan, and Mauritania from Africa. In terms of the number of speakers, the two top languages in Middle East countries are Arabic and Persian [18-20]. Considering the geography of the Middle East and the cultural affinity of the countries in this region, pooled data on epidemiological features of KD in this region remain limited. A search of scientific sources shows that accurate and reliable information on the KD incidence or prevalence is lacking in many Middle East countries, while a few countries have detailed information from some national data or their disease registration systems. This is also true for some other basic epidemiological indicators such as age, gender, time, and spatial distribution of disease. To address this knowledge gap, joint research projects, such as the Kawarabi project, are being developed and implemented in some Middle East countries; however, the results have not yet been published [21].
The findings of studies in the Middle East on important epidemiological characteristics of KD (including age, gender, and seasonal incidence) and CAL (as the most important complication of KD) conducted so far can be examined and retrieved comprehensively, which is crucial for identifying the KD’s burden and distribution, ultimately enabling better prevention strategies, tailored treatment protocols, and improved outcomes for affected individuals [3, 5, 6]. Therefore, given the lack of national studies in some countries in Middle East, this study aims to review and retrieve the valid scientific evidence regarding the most important epidemiological characteristics of KD in Middle East countries and summarize them in the form of pooled indicators so that it can be generalized to other countries in the region as the most valid reportable scientific evidence obtained so far.
Methods
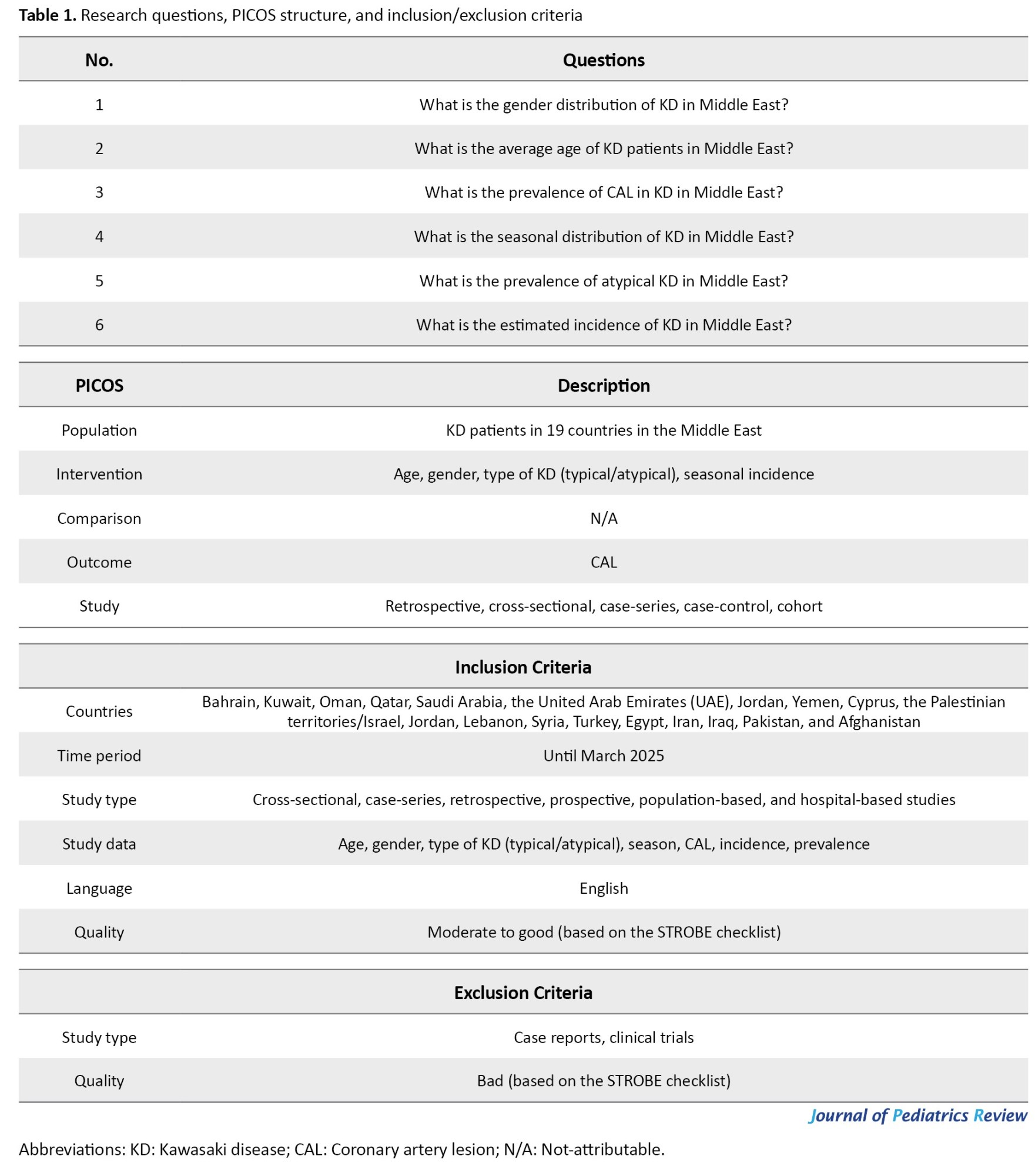
This is a systematic review and meta-analysis study. To prepare a profile of the KD epidemiology in the Middle East countries, we focused on 17 main countries belonging to the main Middle East region, plus two eastern neighboring countries of Iran, Afghanistan, and Pakistan. Information on the epidemiological characteristics at the national level can only be extracted from disease registration systems or through field survey studies. Given the lack of such systems or studies in the vast majority of countries in the Middle East, and the fact that most KD patients are hospitalized, we included all hospital-based studies in our review study, including retrospective, case-series, cohort, and cross-sectional studies. The goal was to reach acceptable estimates of the most important epidemiological indicators, including age and gender, prevalence of atypical KD and CAL, as well as the seasonal pattern of KD occurrence by summarizing the characteristics of the patients in the Middle East. The clinical trials and case reports were excluded because of the high risk of selection bias in these studies. To estimate the incidence of KD, it was not possible to obtain a pooled summary index for the Middle East due to a lack of eligible population-based studies or reports. Thus, we just conducted a concise narrative review of the available scientific evidence about the incidence of KD. For this systematic review and meta-analysis, the main questions were formulated based on the population, intervention, comparison, outcomes and study (PICOS) framework (Table 1).
By using relevant keywords with Boolean operators, truncation, and wildcards, the search was conducted in databases including Google Scholar, PubMed, and Web of Science for relevant studies published in English up to March 2025. In addition, the reference lists of the selected studies were screened to identify additional studies. The search strategy was as follows:
{(Kawasaki [Title/Abstract] OR KD [Title/Abstract] OR Kawasaki diseases [MeSH terms]) OR (Kawasaki AND incidence) [Title] OR (Kawasaki AND prevalence) [Title] OR (Kawasaki AND epidemiology) [Title]} AND {(Middle East OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR the United Arab Emirates OR UAE OR Jordan OR Yemen OR Cyprus OR Israel OR Lebanon OR Syria OR Palestinian OR Turkey OR Egypt OR Iran OR Iraq OR Pakistan OR Afghanistan) [Title/Abstract]}
In this study, we used the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart as a guideline to depict the flow of information through the different phases of the systematic review. It maps out the number of records identified, included, or excluded, and the reasons for exclusions [22]. Evaluation and confirmation of the quality of studies were performed using the STROBE checklist, and articles that did not meet the required quality criteria were excluded. The quality evaluation was initially conducted by two authors (Majid Mirmohammadkhani and Elahe Ghods). In case of disagreement, it was resolved based on the opinion of the third author (Fatemeh Paknazar). Then, the data from each eligible article were extracted and recorded in Excel software.
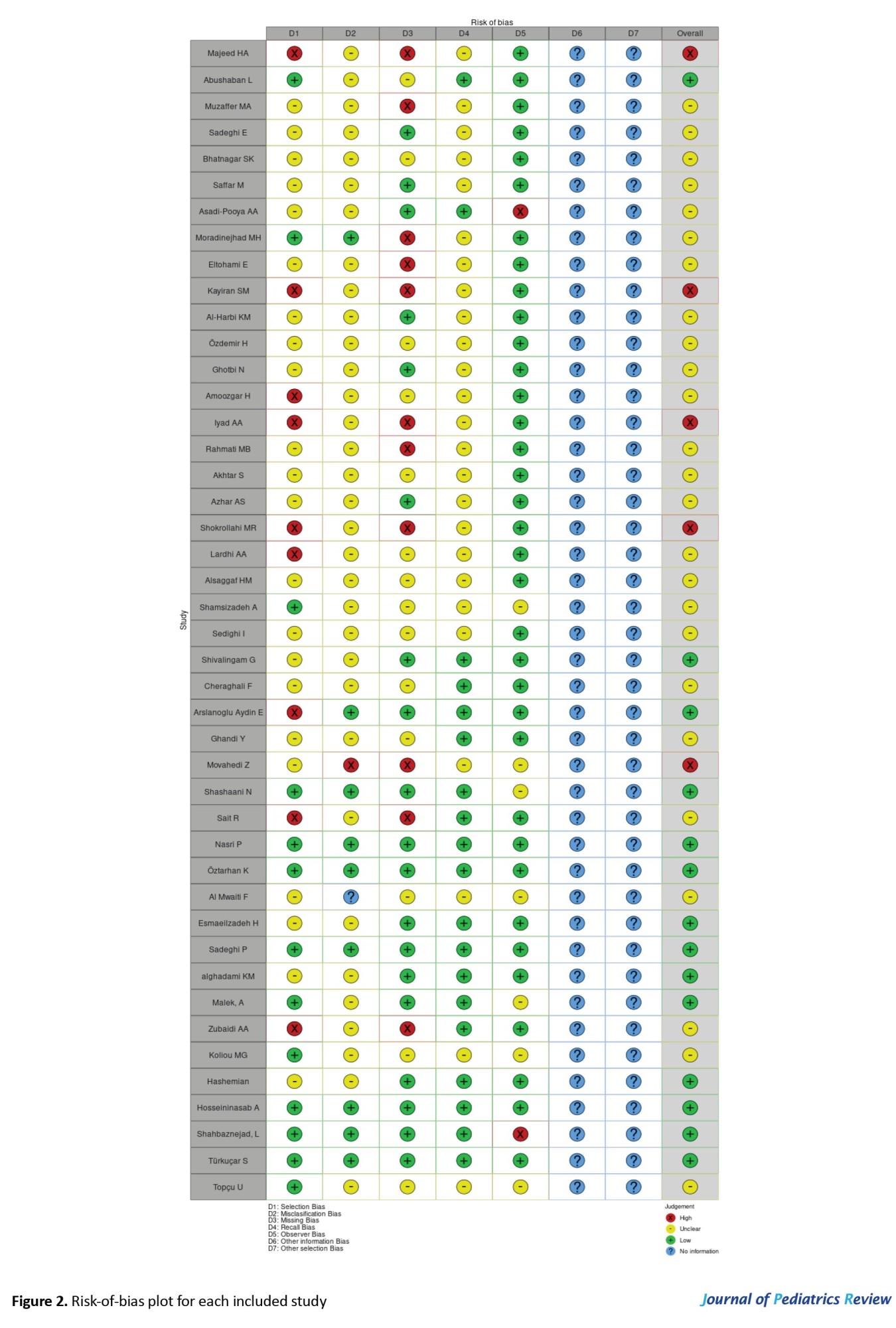
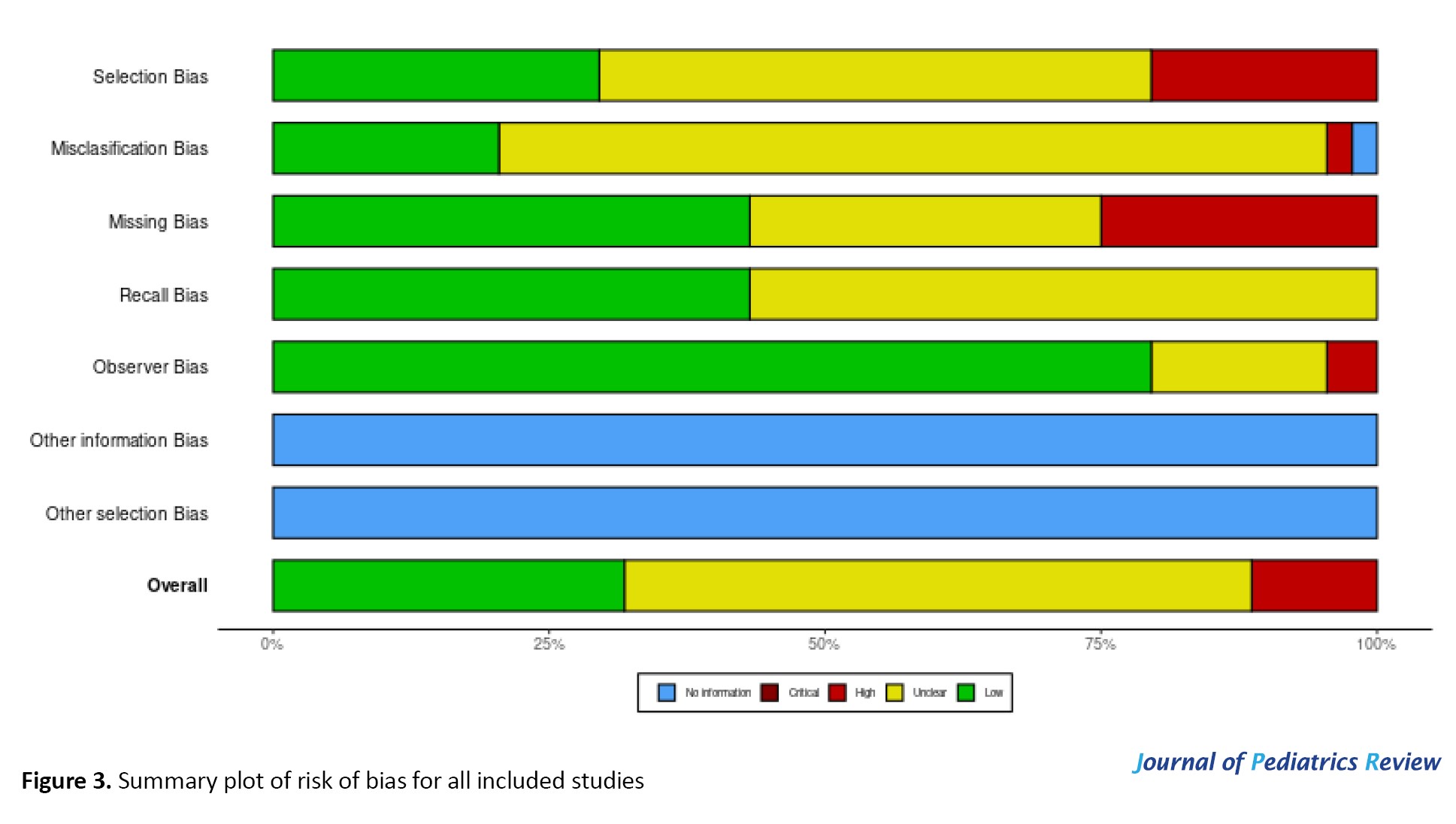
In prevalence studies, two main biases are selection bias (related to the study population) and information bias. Prevalence studies can be population-based or non-population-based (generally hospital-based). Most of the studies included in this meta-analysis were hospital-based. Berkson’s bias is a type of selection bias that can arise when the sample is taken not from the general population but from a subpopulation. The important types of information bias include misclassification, observer, and recall bias [23-25]. We evaluated the mentioned types of bias for each included study by using the Robvis tool and demonstrated by creating the risk-of-bias plots [26]. STATA software, version 17 was used for meta-analysis, applying “meta” and “metaprop” commands. To examine publication bias, a funnel plot was created using the data on age and gender. Forest plots were used to show the estimated values. Heterogeneity was measured using the I-squared (I2) statistic.
Results
By searching for the studies using the terms related to disease, epidemiology, and region in Google Scholar (n=23777) and Web of Science (n=3033), out of 26,810 articles, 57 articles belonged to the target countries. Also, based on our search in PubMed using MeSH (medical subject headings) terms, of 7,740 articles related to KD, only 36 articles were related to the target countries. From the total number of 93 articles, after removing duplicates, 49 articles remained, of which 44 [4, 27-69] were selected after removing low-quality articles, articles with overlapping data (n=2), and articles with high selection bias (n=3) (Figure 1).
Kawasaki disease (KD) is one of the most important causes of vasculitis in children, and manifests as a febrile systemic inflammatory disorder [1-3]. Approximately 20–25% of untreated children develop coronary artery lesions (CALs), including aneurysms, making it the most common cause of acquired heart disease in children from developed countries [1, 4-6]. Several studies have been conducted to clarify KD’s etiology worldwide; however, it still has an unknown etiology and remains a clinical diagnosis without a pathognomonic diagnostic test, making it difficult to identify all children at risk for developing CALs, even in countries with extensive nationwide studies [3, 6-12]. Typical KD is characterized by the presence of specific clinical criteria, while atypical KD (or incomplete KD) presents with fewer symptoms that do not meet the full diagnostic criteria. The atypical form is important since it may be diagnosed later or with more difficulty, which can lead to late initiation of treatment and the development of complications [1, 13]. The prevalence of atypical KD may vary from 15% to 36% and is more prevalent in younger patients [14].
Affecting all racial and ethnic groups, KD has been reported in all countries [2-4]. The disease is usually diagnosed in early childhood, with an age range from 3 months to 10 years, and boys are slightly more likely to be affected than girls. The mainstay of treatment for KD is intravenous immunoglobulin, and the patients generally require hospitalization for diagnosis and treatment. Among ethnic groups, epidemiological studies have shown that children with Asian and Pacific Islander descent have the highest incidence of KD [1]. The incidence of KD in the United States is approximately 25 per 100,000 children <5 years of age. In Japan, the incidence is estimated at approximately 250 per 100,000 [15]. Most countries have recently observed a gradual increase in KD incidence. The reasons for this increase are unclear and may reflect a true rise in cases or improved ascertainment due to increased awareness among healthcare providers and greater access to specialist care resulting from economic growth and industrialization [7, 16]. Recent studies in Japan suggest that the proportion of Japanese patients with CAL has decreased over time [1].
Many studies have been conducted to investigate the epidemiological features of KD in developed countries worldwide, with many based on data from accurate disease registration systems in those countries [17]. These studies provide useful and practical information about the incidence of the KD and its temporal and spatial distribution, which can help control the disease and make effective management decisions for its surveillance at the national level. The studies in Asian countries are limited to some countries with registration systems, such as Japan. But in most Asian countries, there is not enough scientific evidence about the epidemiology of the disease that can be relied upon by physicians and health officials, including those in the Middle East. Due to the relatively low prevalence of KD in some Middle Eastern countries, there is basically no national research on the epidemiology of KD. In other countries, most studies are case reports or case series.
The Middle East is a geopolitical region encompassing the Arabian Peninsula (including Bahrain, Kuwait, Oman, Qatar, Saudi Arabia, the United Arab Emirates (UAE), Jordan, and Yemen), the Levant countries (including Cyprus, Israel, Jordan, Lebanon, Syria, and the Palestinian territories), Turkey, Egypt, Iran, and Iraq. In the 1980s, the term “Greater Middle East” came to encompass Pakistan and Afghanistan from Asia, as well as the Maghreb, Sudan, and Mauritania from Africa. In terms of the number of speakers, the two top languages in Middle East countries are Arabic and Persian [18-20]. Considering the geography of the Middle East and the cultural affinity of the countries in this region, pooled data on epidemiological features of KD in this region remain limited. A search of scientific sources shows that accurate and reliable information on the KD incidence or prevalence is lacking in many Middle East countries, while a few countries have detailed information from some national data or their disease registration systems. This is also true for some other basic epidemiological indicators such as age, gender, time, and spatial distribution of disease. To address this knowledge gap, joint research projects, such as the Kawarabi project, are being developed and implemented in some Middle East countries; however, the results have not yet been published [21].
The findings of studies in the Middle East on important epidemiological characteristics of KD (including age, gender, and seasonal incidence) and CAL (as the most important complication of KD) conducted so far can be examined and retrieved comprehensively, which is crucial for identifying the KD’s burden and distribution, ultimately enabling better prevention strategies, tailored treatment protocols, and improved outcomes for affected individuals [3, 5, 6]. Therefore, given the lack of national studies in some countries in Middle East, this study aims to review and retrieve the valid scientific evidence regarding the most important epidemiological characteristics of KD in Middle East countries and summarize them in the form of pooled indicators so that it can be generalized to other countries in the region as the most valid reportable scientific evidence obtained so far.
Methods
This is a systematic review and meta-analysis study. To prepare a profile of the KD epidemiology in the Middle East countries, we focused on 17 main countries belonging to the main Middle East region, plus two eastern neighboring countries of Iran, Afghanistan, and Pakistan. Information on the epidemiological characteristics at the national level can only be extracted from disease registration systems or through field survey studies. Given the lack of such systems or studies in the vast majority of countries in the Middle East, and the fact that most KD patients are hospitalized, we included all hospital-based studies in our review study, including retrospective, case-series, cohort, and cross-sectional studies. The goal was to reach acceptable estimates of the most important epidemiological indicators, including age and gender, prevalence of atypical KD and CAL, as well as the seasonal pattern of KD occurrence by summarizing the characteristics of the patients in the Middle East. The clinical trials and case reports were excluded because of the high risk of selection bias in these studies. To estimate the incidence of KD, it was not possible to obtain a pooled summary index for the Middle East due to a lack of eligible population-based studies or reports. Thus, we just conducted a concise narrative review of the available scientific evidence about the incidence of KD. For this systematic review and meta-analysis, the main questions were formulated based on the population, intervention, comparison, outcomes and study (PICOS) framework (Table 1).

By using relevant keywords with Boolean operators, truncation, and wildcards, the search was conducted in databases including Google Scholar, PubMed, and Web of Science for relevant studies published in English up to March 2025. In addition, the reference lists of the selected studies were screened to identify additional studies. The search strategy was as follows:
{(Kawasaki [Title/Abstract] OR KD [Title/Abstract] OR Kawasaki diseases [MeSH terms]) OR (Kawasaki AND incidence) [Title] OR (Kawasaki AND prevalence) [Title] OR (Kawasaki AND epidemiology) [Title]} AND {(Middle East OR Bahrain OR Kuwait OR Oman OR Qatar OR Saudi Arabia OR the United Arab Emirates OR UAE OR Jordan OR Yemen OR Cyprus OR Israel OR Lebanon OR Syria OR Palestinian OR Turkey OR Egypt OR Iran OR Iraq OR Pakistan OR Afghanistan) [Title/Abstract]}
In this study, we used the preferred reporting items for systematic reviews and meta-analyses (PRISMA) flowchart as a guideline to depict the flow of information through the different phases of the systematic review. It maps out the number of records identified, included, or excluded, and the reasons for exclusions [22]. Evaluation and confirmation of the quality of studies were performed using the STROBE checklist, and articles that did not meet the required quality criteria were excluded. The quality evaluation was initially conducted by two authors (Majid Mirmohammadkhani and Elahe Ghods). In case of disagreement, it was resolved based on the opinion of the third author (Fatemeh Paknazar). Then, the data from each eligible article were extracted and recorded in Excel software.
In prevalence studies, two main biases are selection bias (related to the study population) and information bias. Prevalence studies can be population-based or non-population-based (generally hospital-based). Most of the studies included in this meta-analysis were hospital-based. Berkson’s bias is a type of selection bias that can arise when the sample is taken not from the general population but from a subpopulation. The important types of information bias include misclassification, observer, and recall bias [23-25]. We evaluated the mentioned types of bias for each included study by using the Robvis tool and demonstrated by creating the risk-of-bias plots [26]. STATA software, version 17 was used for meta-analysis, applying “meta” and “metaprop” commands. To examine publication bias, a funnel plot was created using the data on age and gender. Forest plots were used to show the estimated values. Heterogeneity was measured using the I-squared (I2) statistic.
Results
By searching for the studies using the terms related to disease, epidemiology, and region in Google Scholar (n=23777) and Web of Science (n=3033), out of 26,810 articles, 57 articles belonged to the target countries. Also, based on our search in PubMed using MeSH (medical subject headings) terms, of 7,740 articles related to KD, only 36 articles were related to the target countries. From the total number of 93 articles, after removing duplicates, 49 articles remained, of which 44 [4, 27-69] were selected after removing low-quality articles, articles with overlapping data (n=2), and articles with high selection bias (n=3) (Figure 1).
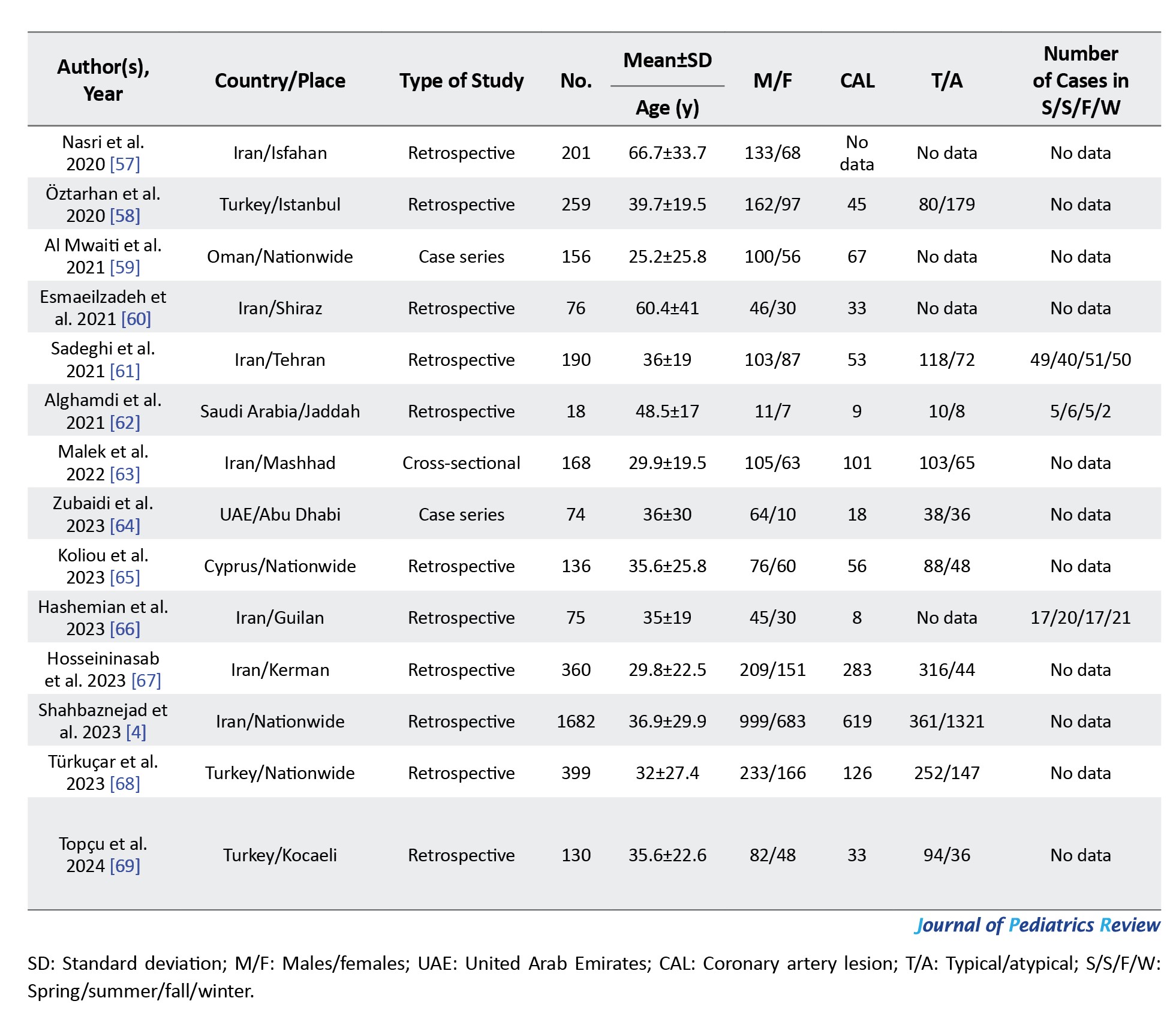
The list of final included articles can be seen in Table 2.
Risk-of-bias plots are demonstrated in Figures 2 and 3.


Risk-of-bias plots are demonstrated in Figures 2 and 3.
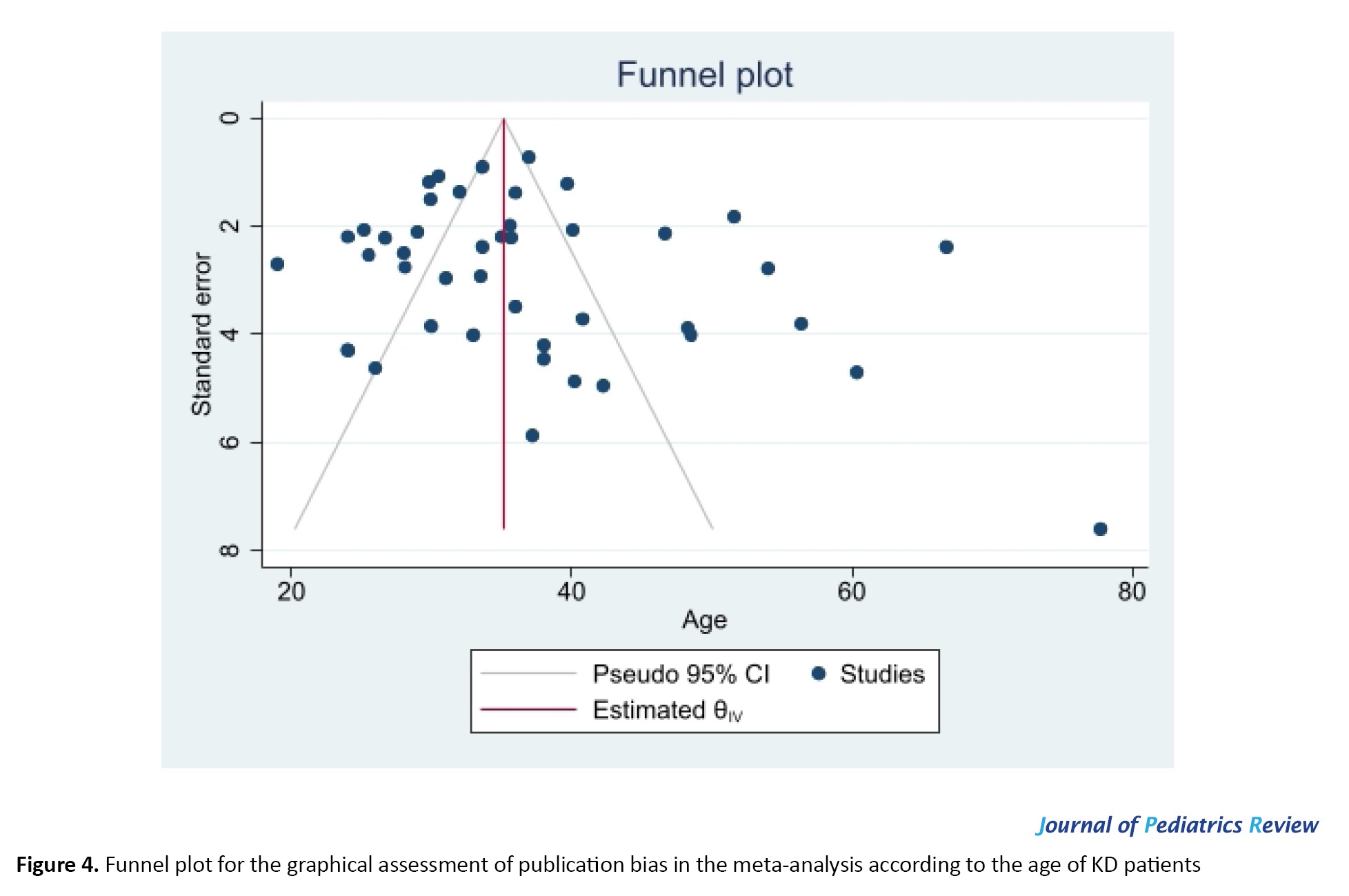
Figure 4 presents the funnel plot for the graphical assessment of publication bias in the meta-analysis according to the age of KD patients.
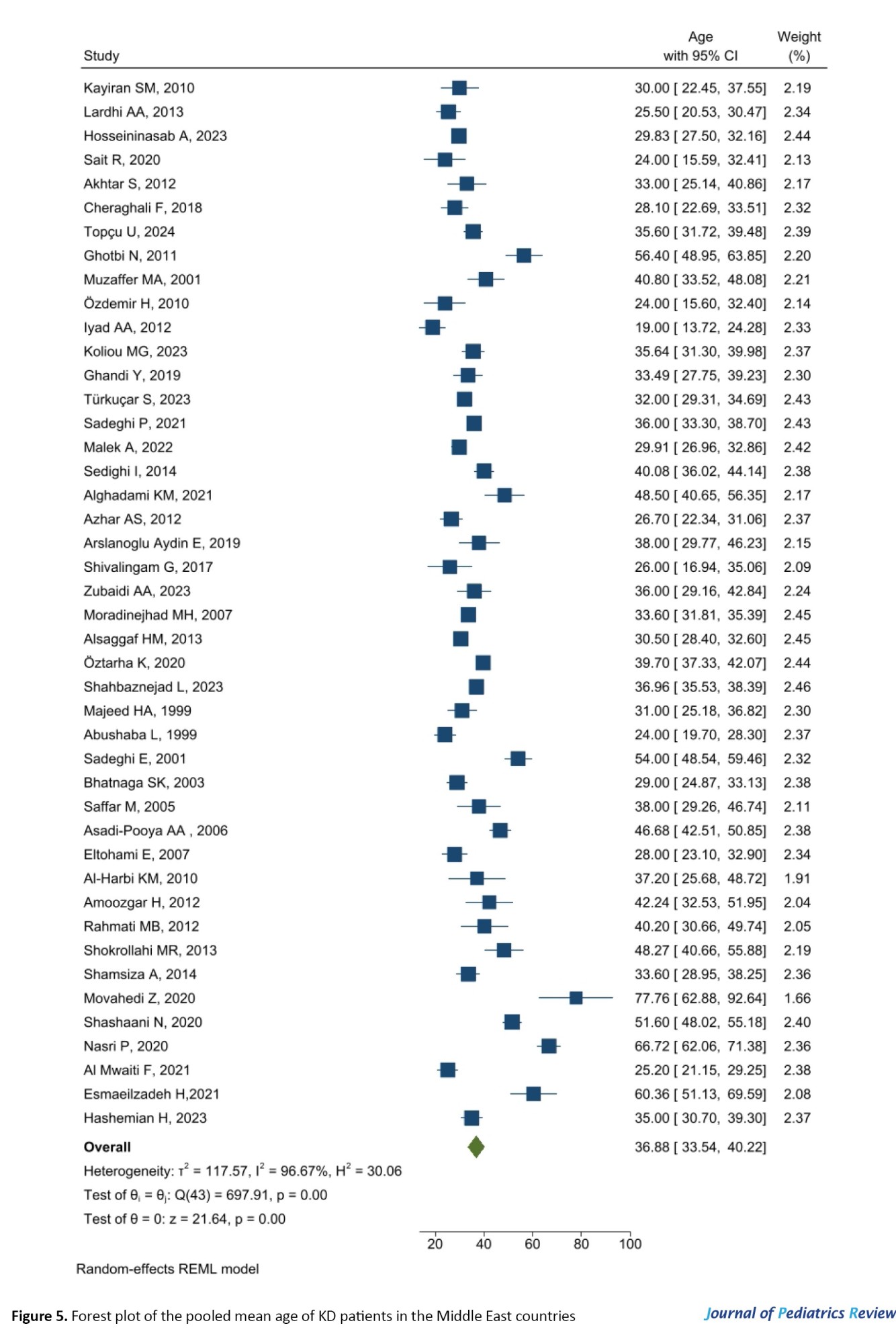
Figure 5 presents the forest plot of the pooled mean age of KD patients in the Middle East countries estimated at 36.88 months with a 95% confidence interval (CI) of 33.54% to 40.22%.
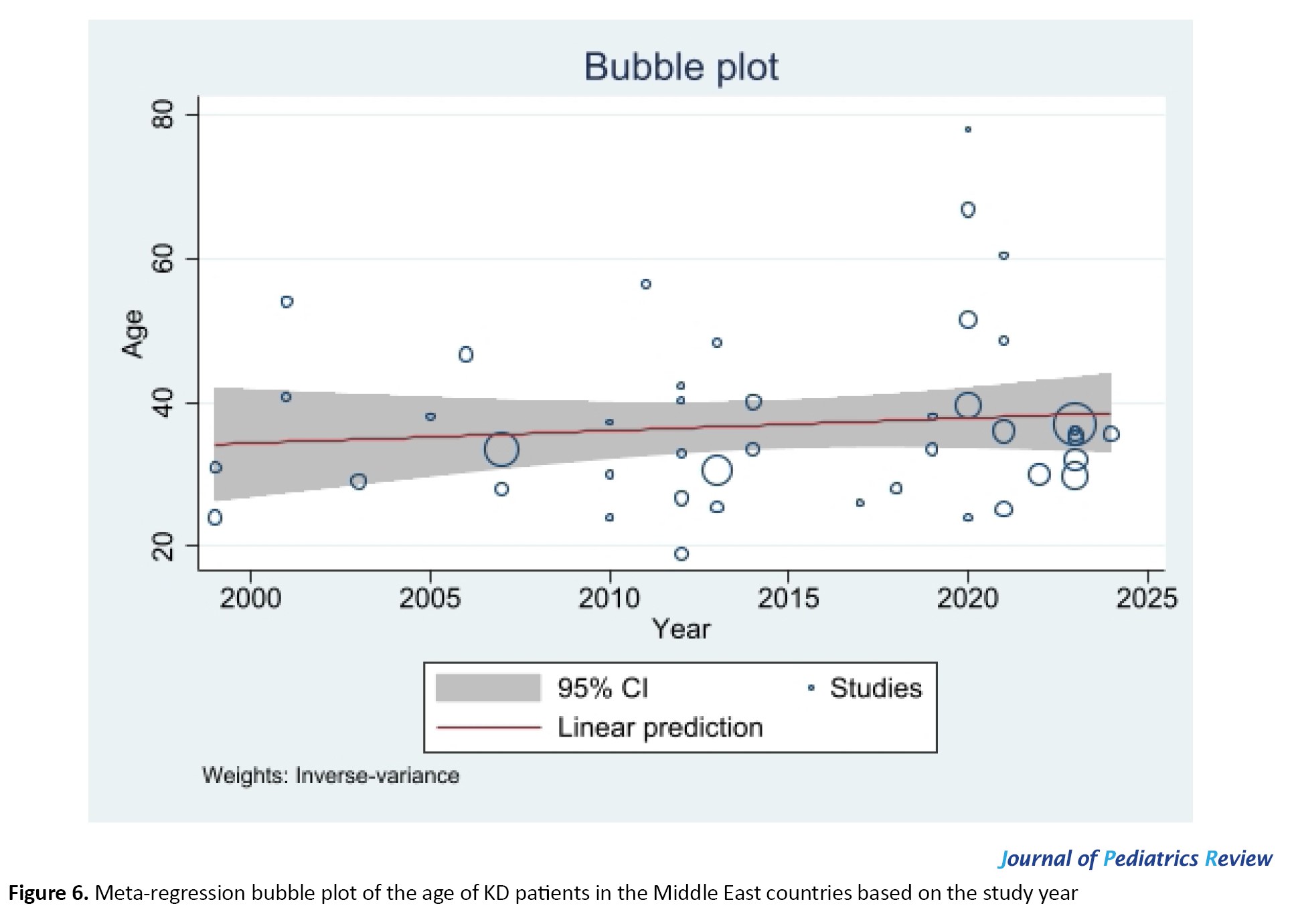
Significant heterogeneity was reported for the articles included in the meta-analysis (I2=96.67%, P<0.001). Meta-regression did not show a significant effect of the year of study on age (P=0.453). Figure 6 shows the Bubble plot for the meta-regression.
As can be seen, the results of some studies were outliers compared to others. A possible reason can be due to differences in patient selection strategies. By eliminating case-control and cohort studies (only one study from each one) that may differ in nature from the rest of the studies, the degree of heterogeneity was slightly reduced but was still significant and noticeable for both case series (I2=93.18%, P<0.001) and retrospective studies (I2=96.44%, P<0.001). Country can be considered as another source of heterogeneity regarding the test of group differences (chi-squared=48.81, P<0.001).
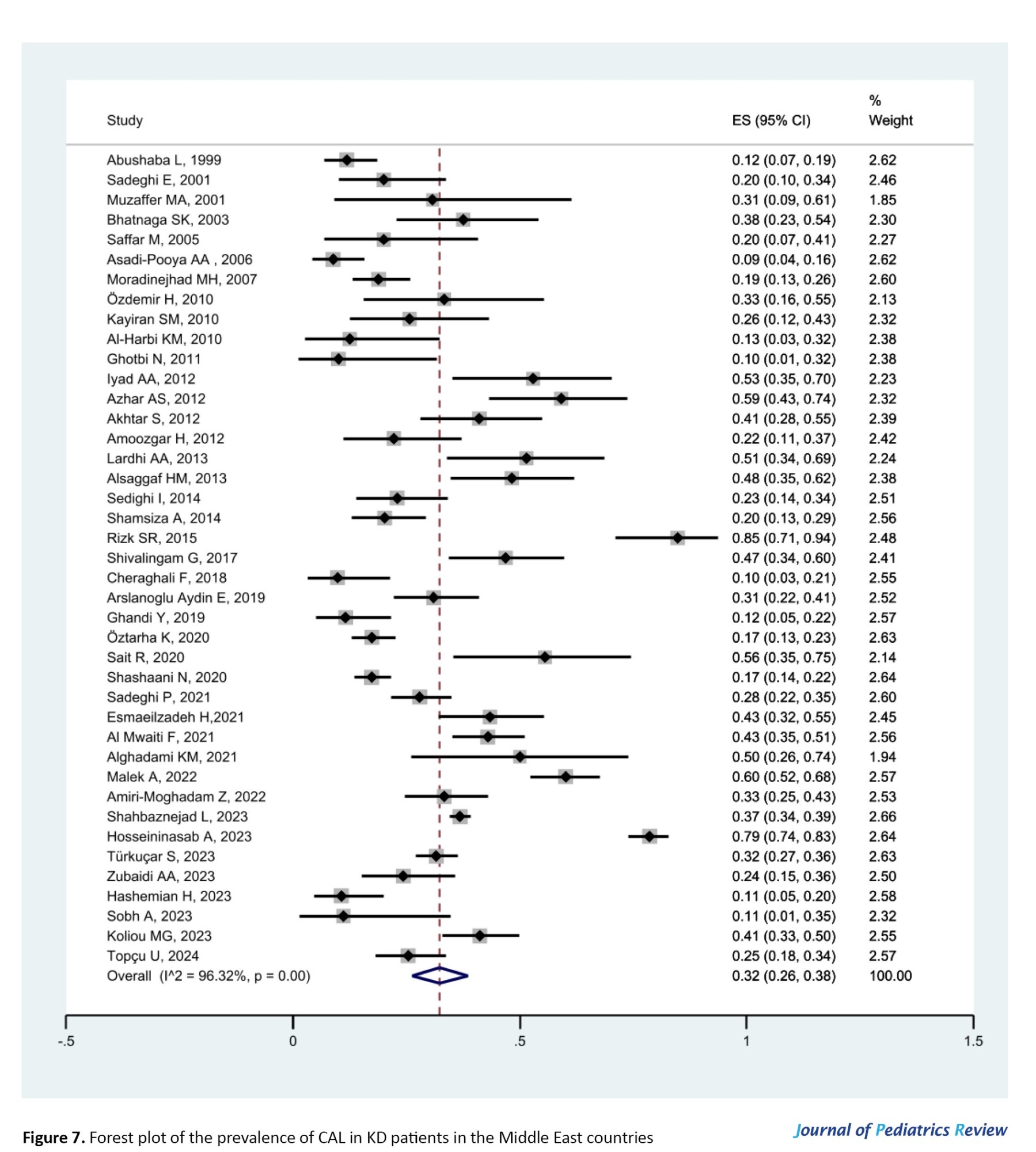
According to meta-analysis results, the percentage of boys in total patients was reported to be 63% with a 95% CI of 61% to 65% (I2=44.57%, P<0.001), which indicates a male to female sex ratio of 1.7:1. Among all articles, 41 and 26 articles had data on CAL and type of KD, respectively, and were included for meta-analysis. Figure 7 presents the forest plot of the prevalence of CAL in KD patients in the Middle East countries (estimated prevalence: 32%; 95% CI, 26%, 38%; I2=96.32%, P<0.001).
According to meta-analysis results, the percentage of boys in total patients was reported to be 63% with a 95% CI of 61% to 65% (I2=44.57%, P<0.001), which indicates a male to female sex ratio of 1.7:1. Among all articles, 41 and 26 articles had data on CAL and type of KD, respectively, and were included for meta-analysis. Figure 7 presents the forest plot of the prevalence of CAL in KD patients in the Middle East countries (estimated prevalence: 32%; 95% CI, 26%, 38%; I2=96.32%, P<0.001).
Figure 8 presents the forest plot of the prevalence of atypical KD (estimated prevalence: 38%; 95% CI, 26%, 50%; I2=98.5%, P<0.001).
Due to the heterogeneity of study estimates for the prevalence of both CAL and KD type, meta-regression was performed, which did not show significant effect for time, country, and type of study.
Among studies, 20 had information on the season of KD occurrence that was included in the meta-analysis. The results for the prevalence of KD in four seasons of spring, summer, autumn and winter were 30%, 20%, 19% and 31%, respectively. No significant heterogeneity was reported for the articles included in the meta-analysis for the seasonal pattern of KD. The number of articles with reliable information on the KD incidence was not sufficient for meta-analysis, so meta-analysis was not conducted.
Discussion
The age and sex distribution patterns of KD have implications for early diagnosis and treatment strategies, emphasizing the importance of high awareness among pediatricians, especially those working with younger male children with prolonged fever and associated symptoms. Age and male gender are critical risk factors for the development of coronary artery aneurysms (CAAs) associated with KD [70]. According to the results of the present study, the pooled mean age of KD patients in the Middle East countries was estimated to be 36.88 months (95% CI, 33.54%, 40.22%). The mean age of onset for KD typically ranges from 1 to 5 years. However, this age may vary depending on the study population [31, 34, 58, 63, 71]. The findings from a nationwide study in Japan indicated specific distributions related to age, showing a single-peak incidence rate predominantly among infants aged 9-11 months (with 81.7% of cases occurring in children under 4 years old) [72].
The male-to-female ratio of KD has been reported as approximately 1.5 to 2:1, indicating a higher incidence in males worldwide. According to meta-analysis results in our study, a male-to-female ratio of 1.7:1 was reported for KD in Middle East countries, indicating a male predominance similar to Eastern Asia, the USA, and European countries [33, 37]. The male-to-female ratio of a disease can provide insights into hormonal and genetic factors, health behaviors, and the trends in the disease pathogenesis [73]. This sex ratio can be attributed to biological factors, including differences in immune response and susceptibility to KD.
Cardiac involvement is one of the most serious complications of KD, with an increased risk of CAAs [44, 58]. In patients with typical KD, cardiac complications occur in approximately 15-25% of cases [31, 74], depending on the duration and effectiveness of treatment. However, in cases with atypical KD, where fewer clinical criteria are met, the risk of cardiac involvement may be underestimated. Recent findings indicate that even patients with atypical presentations can experience significant cardiac complications [63]. This necessitates careful monitoring and management of all children diagnosed with KD to mitigate long-term cardiovascular risks. Clinicians should maintain a high index of suspicion for cardiac involvement, regardless of the clinical presentation.
Age can influence the risk of cardiac involvement. Older children with KD may be at a higher risk of developing significant cardiovascular complications, including CAL, compared to younger children. This emphasizes the importance of recognizing KD in older children and being vigilant for atypical symptoms and potential cardiac issues. Increased vigilance for atypical presentations, particularly in older children or those with less classical manifestations, is essential for improving outcomes. In our study, the prevalence of CAL and atypical KD in the Middle East countries was estimated to be 32% and 38%, respectively. According to statistics, approximately 20–25% of untreated children develop CAL [1]. The results of our study confirm the higher prevalence of cardiac diseases in the Middle East. In a study conducted in Japan, the incidence of KD was found to be 1.4-1.7 times higher in males than in females; however, this proportion has declined over time for both genders [72]. The identification of atypical KD is crucial, as these cases may present with milder or non-specific symptoms and can lead to significant cardiovascular complications if not appropriately recognized and treated.
The CALs were observed in more than 25% of KD patients in a 20-year cohort study, with three cases showing persistent lesions in long-term follow-up [69]. Compared to global statistics that reported the prevalence of atypical KD between 15% and 36%, its prevalence in our study for Middle Eastern countries was estimated at 38%, which is slightly higher than the global rate [14]. The primarily winter-spring KD prevalence, as well as the well-documented Japanese epidemics with wave-like spread, also support an infectious trigger. In Iran, one study showed that 37% of cases occurred in the spring [16]. Based on the results of the present review study, the prevalence of KD in the Middle East countries in the four seasons of spring, summer, autumn, and winter was 30%, 20%, 19% and 31%, respectively. The incidence of KD also varies significantly across different populations and geographic regions [7, 75]. This variation underscores the need for more comprehensive epidemiological studies to understand the factors contributing to these differences, such as genetic predisposition, environmental influences, and healthcare access. Understanding the incidence is crucial for developing targeted public health interventions and awareness programs, particularly in areas where KD is less recognized. In a study, a total of 17 nationwide surveys were carried out biannually since 1970. The results showed that the annual incidence of KD for children younger than 5 years of age in 2001 and 2002 was 138.8 and 151.2 per 100000 children [72]. In Northeast Asian countries, including Japan, South Korea, China, and Taiwan, the incidence of KD is 10-30 times higher than in the United States [72, 76], where the rate of KD among children under 5 years old was reported to be 18-25 per 100,000 [77]. Data in Iran for 186,069 patients reported a mean annual incidence of 2.85 per 100,000 children under five years old. The annual incidence rates varied from 7.28 for children under 1 year to 0.78 for those over 5 years [4].
Due to a lack of population-based research in most countries in the Middle East, which are the best relevant studies for our purpose, we have to use hospital-based studies instead, including case series, which are subject to various types of selection bias including Berkson’s bias, which was limitation for our meta-analysis. The most important source of heterogeneity in our meta-analysis seems to be related to the type of studies included, which cannot be addressed by subgroup analysis or meta-regression. The interpretation of the results should be considered in light of the above limitations. Conducting population-based research on the epidemiological characteristics of the KD in the Middle East countries is recommended.
Conclusion
A comprehensive understanding of KD characteristics, including its prevalence rate, age at onset, sex and seasonal distribution, cardiac involvement, and the distinction between typical and atypical cases, is essential for improving diagnosis, treatment, and outcomes in affected children. Although our results align with global statistics regarding age, gender, and seasonal distribution of the KD, the prevalence of CAL in KD patients and atypical form of the KD were higher in the Middle East. To accurately estimate the incidence of KD in the region, further population-based research is needed. This can be facilitated through consortia-based scientific activities aimed at establishing disease registration facilities in these countries.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contribution
Conceptualization and supervision: Majid Mirmohammadkhani and Elahe Ghods; Methodology: Majid Mirmohammadkhani and Fatemeh Paknazar; Data collection: Elahe Ghods and Fatemeh Paknazar; Data analysis: Majid Mirmohammadkhani and Fatemeh Paknazar; Funding acquisition and resources, investigation, and writing: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to express their sincere gratitude to Mahdiyeh Mousavi, Pediatric Rheumatologist for her invaluable scientific guidance.
Among studies, 20 had information on the season of KD occurrence that was included in the meta-analysis. The results for the prevalence of KD in four seasons of spring, summer, autumn and winter were 30%, 20%, 19% and 31%, respectively. No significant heterogeneity was reported for the articles included in the meta-analysis for the seasonal pattern of KD. The number of articles with reliable information on the KD incidence was not sufficient for meta-analysis, so meta-analysis was not conducted.
Discussion
The age and sex distribution patterns of KD have implications for early diagnosis and treatment strategies, emphasizing the importance of high awareness among pediatricians, especially those working with younger male children with prolonged fever and associated symptoms. Age and male gender are critical risk factors for the development of coronary artery aneurysms (CAAs) associated with KD [70]. According to the results of the present study, the pooled mean age of KD patients in the Middle East countries was estimated to be 36.88 months (95% CI, 33.54%, 40.22%). The mean age of onset for KD typically ranges from 1 to 5 years. However, this age may vary depending on the study population [31, 34, 58, 63, 71]. The findings from a nationwide study in Japan indicated specific distributions related to age, showing a single-peak incidence rate predominantly among infants aged 9-11 months (with 81.7% of cases occurring in children under 4 years old) [72].
The male-to-female ratio of KD has been reported as approximately 1.5 to 2:1, indicating a higher incidence in males worldwide. According to meta-analysis results in our study, a male-to-female ratio of 1.7:1 was reported for KD in Middle East countries, indicating a male predominance similar to Eastern Asia, the USA, and European countries [33, 37]. The male-to-female ratio of a disease can provide insights into hormonal and genetic factors, health behaviors, and the trends in the disease pathogenesis [73]. This sex ratio can be attributed to biological factors, including differences in immune response and susceptibility to KD.
Cardiac involvement is one of the most serious complications of KD, with an increased risk of CAAs [44, 58]. In patients with typical KD, cardiac complications occur in approximately 15-25% of cases [31, 74], depending on the duration and effectiveness of treatment. However, in cases with atypical KD, where fewer clinical criteria are met, the risk of cardiac involvement may be underestimated. Recent findings indicate that even patients with atypical presentations can experience significant cardiac complications [63]. This necessitates careful monitoring and management of all children diagnosed with KD to mitigate long-term cardiovascular risks. Clinicians should maintain a high index of suspicion for cardiac involvement, regardless of the clinical presentation.
Age can influence the risk of cardiac involvement. Older children with KD may be at a higher risk of developing significant cardiovascular complications, including CAL, compared to younger children. This emphasizes the importance of recognizing KD in older children and being vigilant for atypical symptoms and potential cardiac issues. Increased vigilance for atypical presentations, particularly in older children or those with less classical manifestations, is essential for improving outcomes. In our study, the prevalence of CAL and atypical KD in the Middle East countries was estimated to be 32% and 38%, respectively. According to statistics, approximately 20–25% of untreated children develop CAL [1]. The results of our study confirm the higher prevalence of cardiac diseases in the Middle East. In a study conducted in Japan, the incidence of KD was found to be 1.4-1.7 times higher in males than in females; however, this proportion has declined over time for both genders [72]. The identification of atypical KD is crucial, as these cases may present with milder or non-specific symptoms and can lead to significant cardiovascular complications if not appropriately recognized and treated.
The CALs were observed in more than 25% of KD patients in a 20-year cohort study, with three cases showing persistent lesions in long-term follow-up [69]. Compared to global statistics that reported the prevalence of atypical KD between 15% and 36%, its prevalence in our study for Middle Eastern countries was estimated at 38%, which is slightly higher than the global rate [14]. The primarily winter-spring KD prevalence, as well as the well-documented Japanese epidemics with wave-like spread, also support an infectious trigger. In Iran, one study showed that 37% of cases occurred in the spring [16]. Based on the results of the present review study, the prevalence of KD in the Middle East countries in the four seasons of spring, summer, autumn, and winter was 30%, 20%, 19% and 31%, respectively. The incidence of KD also varies significantly across different populations and geographic regions [7, 75]. This variation underscores the need for more comprehensive epidemiological studies to understand the factors contributing to these differences, such as genetic predisposition, environmental influences, and healthcare access. Understanding the incidence is crucial for developing targeted public health interventions and awareness programs, particularly in areas where KD is less recognized. In a study, a total of 17 nationwide surveys were carried out biannually since 1970. The results showed that the annual incidence of KD for children younger than 5 years of age in 2001 and 2002 was 138.8 and 151.2 per 100000 children [72]. In Northeast Asian countries, including Japan, South Korea, China, and Taiwan, the incidence of KD is 10-30 times higher than in the United States [72, 76], where the rate of KD among children under 5 years old was reported to be 18-25 per 100,000 [77]. Data in Iran for 186,069 patients reported a mean annual incidence of 2.85 per 100,000 children under five years old. The annual incidence rates varied from 7.28 for children under 1 year to 0.78 for those over 5 years [4].
Due to a lack of population-based research in most countries in the Middle East, which are the best relevant studies for our purpose, we have to use hospital-based studies instead, including case series, which are subject to various types of selection bias including Berkson’s bias, which was limitation for our meta-analysis. The most important source of heterogeneity in our meta-analysis seems to be related to the type of studies included, which cannot be addressed by subgroup analysis or meta-regression. The interpretation of the results should be considered in light of the above limitations. Conducting population-based research on the epidemiological characteristics of the KD in the Middle East countries is recommended.
Conclusion
A comprehensive understanding of KD characteristics, including its prevalence rate, age at onset, sex and seasonal distribution, cardiac involvement, and the distinction between typical and atypical cases, is essential for improving diagnosis, treatment, and outcomes in affected children. Although our results align with global statistics regarding age, gender, and seasonal distribution of the KD, the prevalence of CAL in KD patients and atypical form of the KD were higher in the Middle East. To accurately estimate the incidence of KD in the region, further population-based research is needed. This can be facilitated through consortia-based scientific activities aimed at establishing disease registration facilities in these countries.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contribution
Conceptualization and supervision: Majid Mirmohammadkhani and Elahe Ghods; Methodology: Majid Mirmohammadkhani and Fatemeh Paknazar; Data collection: Elahe Ghods and Fatemeh Paknazar; Data analysis: Majid Mirmohammadkhani and Fatemeh Paknazar; Funding acquisition and resources, investigation, and writing: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to express their sincere gratitude to Mahdiyeh Mousavi, Pediatric Rheumatologist for her invaluable scientific guidance.
References
- Lo MS SM, Newburger JW. Kawasaki Disease. In: Kliegman RM SGJ, Blum NJ, Shah SS, Tasker RC, Wilson KM, editors. Nelson Textbook Of Pediatrics. Philadelphia: Elsevier; 2024. [Link]
- Kawasaki T. Acute febrile muco-cutaneous lymph node syndrome in young children with unique digital desquamation. Jpn J Allergol. 1967; 16:178-222. [Link]
- Rowley AH, Shulman ST. The epidemiology and pathogenesis of Kawasaki disease. Front Pediatr. 2018; 6:374. [DOI:10.3389/fped.2018.00374] [PMID]
- Shahbaznejad L, Hosseininasab A, Mahboobi L, Mohammadi H, Esmaeili H, Farrokhi Far SM, et al. Epidemiological data of national Kawasaki disease registry in Iran, 2007-2019. Front Pediatr. 2023; 10:988371. [DOI:10.3389/fped.2022.988371] [PMID]
- Manlhiot C, Mueller B, O’Shea S, Majeed H, Bernknopf B, Labelle M, et al. Environmental epidemiology of Kawasaki disease: Linking disease etiology, pathogenesis and global distribution. Plos One. 2018; 13(2):e0191087. [DOI:10.1371/journal.pone.0191087] [PMID]
- Jone PN, Tremoulet A, Choueiter N, Dominguez SR, Harahsheh AS, Mitani Y, et al. Update on diagnosis and management of Kawasaki Disease: A scientific statement from the American Heart Association. Circulation. 2024; 150(23):e481-500. [DOI:10.1161/CIR.0000000000001295] [PMID]
- Lin MT, Wu MH. The global epidemiology of Kawasaki disease: Review and future perspectives. Glob Cardiol Sci Pract. 2017; 2017(3):e201720. [DOI:10.21542/gcsp.2017.20] [PMID]
- Osiejewska A, Wojtachnio D, Bartoszewicz J, Grądzik A, Nowakowska I, Kudan M, et al. Kawasaki disease: A comprehensive review. J Educ Health Sport. 2022; 12(8):317-27. [DOI:10.12775/JEHS.2022.12.08.032]
- Owens AM, Plewa MC. Kawasaki Disease. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. [PMID]
- Wu MH, Lin MT, Chen HC, Kao FY, Huang SK. Postnatal risk of acquiring kawasaki disease: A nationwide birth cohort database study. J Pediatr. 2017; 180:80-6.e2. [DOI:10.1016/j.jpeds.2016.09.052] [PMID]
- Burns JC. The etiologies of Kawasaki disease. J Clin Invest. 2024; 134(5):e176938. [DOI:10.1172/JCI176938] [PMID]
- Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110(17):2747-71. [DOI:10.1161/01.CIR.0000145143.19711.78] [PMID]
- Arjmand R, Soltani H, Manesh NY, Shahbabaie MA. A systematic review of atypical presentations of Kawasaki Disease. Int J Enteric Pathog. 2023; 11(4):151-61. [DOI:10.34172/ijep.5613]
- Singh S, Inban P, Mishra A, Yadav AS, Singh T, Singh R, et al. Atypical Kawasaki disease in a 16-month-old baby: A case report and literature review. Cureus. 2023; 15(5):e39336. [DOI:10.7759/cureus.39336]
- Son MBF, Newburger JW. Kawasaki disease. Pediatr Rev. 2018; 39(2):78-90. [DOI:10.1542/pir.2016-0182] [PMID]
- Mosaiebi Z, Movahedian AH, Heidarzadeh M, Hojati M, Mousavi SGA. [Evaluation of clinical and paraclinical findings of Kawazaki patients among children admitted in Kashan Shahid Beheshti hospital during 1998-2008 (Persian)]. Feyz Med Sci J. 2010; 14(3):249-55. [Link]
- Koyama Y, Miura M, Kobayashi T, Hokosaki T, Suganuma E, Numano F, et al. A registry study of Kawasaki disease patients with coronary artery aneurysms (KIDCAR): A report on a multicenter prospective registry study three years after commencement. Eur J Pediatr. 2023; 182(2):633-40. [DOI:10.1007/s00431-022-04719-x] [PMID]
- Pappé I. The modern Middle East: A social and cultural history. London: Routledge; 2014. [DOI:10.4324/9781315858340]
- Kabasakal H, Dastmalchian A. Introduction to the special issue on leadership and culture in the Middle East. Appl Psychol Int Rev. 2001; 50(4):479-88. [Link]
- Güney A, Gökcan F. The ‘greater Middle East’ as a ‘modern’ geopolitical imagination in American foreign policy. Geopolitics. 2010; 15(1):22-38. [DOI:10.1080/14650040903420370]
- Arab Y, Choueiter N, Dahdah N, El-Kholy N, Abu Al-Saoud SY, Abu-Shukair ME, et al. Kawasaki disease Arab initiative [Kawarabi]: Establishment and results of a multicenter survey. Pediatr Cardiol. 2022; 43(6):1239-46. [DOI:10.1007/s00246-022-02844-w]
- PRISMA. PRISMA 2020 flow diagram [Internet]. 2025 [Updated 15 April 2025]. Available from: [Link]
- Buitrago-Garcia D, Salanti G, Low N. Studies of prevalence: How a basic epidemiology concept has gained recognition in the COVID-19 pandemic. BMJ Open. 2022; 12(10):e061497. [DOI:10.1136/bmjopen-2022-061497] [PMID]
- Westreich D. Berkson’s bias, selection bias, and missing data. Epidemiology. 2012; 23(1):159-64. [DOI:10.1097/EDE.0b013e31823b6296] [PMID]
- Atkinson D, Peijnenburg J. Beyond Berkson: Further light on the selection bias. Notre Dame J Formal Logic. 2025; 66(1):143-52. [DOI:10.1215/00294527-2024-0039]
- McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods. 2021; 12(1):55-61. [DOI:10.1002/jrsm.1411] [PMID]
- Majeed HA, Shaltout AA, Khuffash FA, Othaman B. Kawasaki disease in Kuwait: A prospective study of 41 cases. Ann Saudi Med. 1990; 10(6):642-5. [DOI:10.5144/0256-4947.1990.642]
- Abushaban L, Salama A, Uthaman B, Kumar A, Selvan J. Do we have a less severe form of Kawasaki disease or is it the gammaglobulin effect? Int J Cardiol. 1999; 69(1):71-6. [DOI:10.1016/S0167-5273(99)00014-5] [PMID]
- Muzaffer MA, Al-Mayouf SM. Pattern of clinical features of Kawasaki. Saudi Med J. 2002; 23(4):409-12. [PMID]
- Sadeghi E, Amin R, Ajamee G. Kawasaki syndrome: The Iranian experience. East Mediterr Health J. 2001; 7(1-2):16-25. [PMID]
- Bhatnagar SK, Paul G, Subramanian R, Al Hosni MS, Al Khusaiby SM. Kawasaki disease in Oman-a clinical study. J Trop Pediatr. 2003; 49(6):361-6. [DOI:10.1093/tropej/49.6.361] [PMID]
- Saffar MJ, Reshidighader F. Kawasaki disease in East Mazandaran, Islamic Republic of Iran, 1997-2002. East Mediterr Health J. 2005; 11(1-2):28-35. [PMID]
- Asadi-Pooya AA, Borzoee M, Amoozgar H. The experience with 113 patients with Kawasaki disease in Fars Province, Iran. Turk J Pediatr. 2006; 48(2):109-14. [Link]
- Moradinezhad MH, Kiani A. Kawasaki disease in 159 Iranian children. Iran J Ped. 2007; 17(3):241-6. [Link]
- Eltohami E, Ahmed H, Numan M, Gendi S, Salam M, Al Hroob A, et al. Epidemiology of Kawasaki disease in Qatar (An Arabian Gulf Country). Qatar Med J. 2007; 2007(2):17. [DOI:10.5339/qmj.2007.2.17]
- Kayiran SM, Dindar A, Gurakan B. An evaluation of children with Kawasaki disease in Istanbul: A retrospective follow-up study. Clinics. 2010; 65(12):1261-5. [DOI:10.1590/S1807-59322010001200007] [PMID]
- Al-Harbi KM. Kawasaki disease in Western Saudi Arabia. Saudi Med J. 2010; 31(11):1217-20. [PMID]
- Özdemir H, Çiftçi E, Tapısız A, İnce E, Tutar E, Atalay S, et al. Clinical and epidemiological characteristics of children with Kawasaki disease in Turkey. J Trop Pediatr. 2010; 56(4):260-2. [DOI:10.1093/tropej/fmp110] [PMID]
- Ghotbi N, Molanaei S. P049 Epidemiological Survey of Kawasaki Disease in Kurdish Patients in Sanandaj of Iran. Int J Cardiol. 2011; 147(Supplement 1):S23. [DOI:10.1016/S0167-5273(11)70075-4]
- Amoozgar H, Mohammadi H, Shakibazad N. Risk factors of coronary involvement in kawasaki disease in recent outbreak in South Iran, Shiraz. J Pediatr Sci. 2012; 4(4):1-7. [Link]
- Iyad A-A, Al-Wahsh S, Khuri-Bulos N. Kawasaki disease in Jordan: Demographics, presentation, and outcome. Cardiol Young. 2012; 22(4):390-5. [DOI:10.1017/S1047951111001818] [PMID]
- Rahmati MB, Jahanshahi KA, Jahangiri Z, Mahboobi H, Khorgoei T. Clinical aspects and laboratory tests of Kawasaki Disease in Iran. Electron Physician. 2012; 4(1):461-4. [Link]
- Akhtar S, Alam MM, Ahmed MA. Cardiac involvement in Kawasaki disease in Pakistani children. Ann Pediatr Cardiol. 2012; 5(2):129-32. [DOI:10.4103/0974-2069.99612] [PMID]
- Azhar AS, Al-Attas A. Risk factors for coronary artery lesions in Kawasaki disease. Med Glas (Zenica). 2013; 10(2):254-7. [PMID]
- Shokrollahi MR, Heydari H, Nasehi L, Movahedi Z. A survey of clinical and laboratory manifestations of Kawasaki disease in children admitted to Children Hospital in Qom, Iran. Life Sci J. 2013; 10(10s):1-6. [Link]
- Lardhi AA. Kawasaki disease: A university hospital experience. Saudi J Med Med Sci. 2013; 1(1):35-9. [DOI:10.4103/1658-631X.112923]
- Alsaggaf HM. Clinical experience of Kawasaki disease in two tertiary care centers in Jeddah, Saudi Arabia. Med Sci. 2013; 20(4):3-12. [DOI:10.4197/Med.20-4.1]
- Shamsizadeh A, Kajbaf TZ, Razavi M, Cheraghian B. Clinical and epidemiological characteristics of Kawasaki disease. Jundishapur J Microbiol. 2014; 7(8):e11014. [DOI:10.5812/jjm.11014] [PMID]
- Sedighi I, Biglari M, Olfat M, Yadolahi H, Tanasan A, Torabian S. Clinical characteristics and outcomes of Iranian Patients With Kawasaki. J Compr Pediatr. 2014; 5(1):e13971. [DOI:10.17795/compreped-13971]
- Shivalingam G, Prashanth G, Hebbal K, Aguiar R. Clinical presentation and cardiovascular outcome in complete versus incomplete Kawasaki disease. Indian Pediatr. 2017; 54(10):844-7. [DOI:10.1007/s13312-017-1147-6] [PMID]
- Cheraghali F, Hajimoradloo N, Roshandel G, Meftah M, Azadfar S. Prevalence of Kawasaki Disease in Children Admitted to Taleghani Medical Center in Gorgan, Iran.J Clin Basic Resh. 2018; 2(4):54-9. [DOI:10.29252/jcbr.2.4.54]
- Arslanoglu Aydin E, Ertugrul I, Bilginer Y, Batu ED, Sonmez HE, Demir S, et al. The factors affecting the disease course in Kawasaki disease. Rheumatol Int. 2019; 39(8):1343-9. [DOI:10.1007/s00296-019-04336-2] [PMID]
- Ghandi Y, Habibi D, Kahbazi M, Dorreh F, Lotfi M. Clinical Characteristics of Kawasaki Disease in Markazi Province, Iran. J Compr Ped. 2019; 11(1):e85695. [DOI:10.5812/compreped.85695]
- Movahedi Z, Ashouri S. What’s the relation between paranasal sinusitis (based on PNS-CT scan) and Kawasaki disease: A comparative study in children in Tehran, Iran [Preprint]. 2020. [Link]
- Shashaani N, Shiari R, Karimi A, Salehi S, Ghanaei R, Hassas Yeganeh M, et al. Determination of the relationship between Kobayashi, Sano, and Egami Criteria and Prevalence of Intravenous Immunoglobulin Resistance and Coronary Artery Aneurysm in Iranian Children with Kawasaki Disease. Open Access Rheumatol. 2020; 12:187-92. [DOI:10.2147/OARRR.S255138] [PMID]
- Sait R, Azar AJ, Loney T, Hassan S. Kawasaki disease in children from Dubai, United Arab Emirates (2012-2020): A single-centre retrospective clinical case series. BMJ Paediatr Open. 2022; 6(1):e001649. [DOI:10.1136/bmjpo-2022-001649] [PMID]
- Nasri P, Adibmajlesi Z, Rahimi H, Saneian H, Famouri F, Khademian M, et al. Gastrointestinal manifestations in children with Kawasaki disease in Isfahan, Iran. Arch Pediatr Infect Dis. 2020; 8(2):e103072. [DOI:10.5812/pedinfect.103072]
- Öztarhan K, Varlı YZ, Ayaz N. Usefulness of Kawasaki disease risk scoring systems to the Turkish population. Anatol J Cardiol. 2020; 24(2):97-106. [DOI:10.14744/AnatolJCardiol.2020.37560] [PMID]
- Al Mwaiti F, Alhinai Z, AlAbrawi S, Abdwani R, Al Senidi K. 1025. Prediction of Intravenous Immunoglobulin Resistance and Coronary Artery Dilatation in Kawasaki Disease: A Multicenter Study from Oman. Open Forum Infect Dis. 2021; 8(Supplement_1):S604. [DOI:10.1093/ofid/ofab466.1219]
- Esmaeilzadeh H, Mortazavi N, Salehi A, Fatemian H, Dehghani SM, Vali M, et al. Effect of COVID-19 on Kawasaki disease: Decrease age of onset and increase skin manifestation. BMC Pediatr. 2021; 21(1):571. [DOI:10.1186/s12887-021-03060-w] [PMID]
- Sadeghi P, Izadi A, Mojtahedi SY, Khedmat L, Jafari M, Afshin A, et al. A 10-year cross-sectional retrospective study on Kawasaki disease in Iranian children: Incidence, clinical manifestations, complications, and treatment patterns. BMC Infect Dis. 2021; 21(1):368. [DOI:10.1186/s12879-021-06046-2] [PMID]
- Alghamdi KT, Waggass RA, Aga SS, Alrohaili AA, Alaidroos AH, Alghamdi MD, et al. The most common clinical features of kawasaki disease patients in King Abdulaziz Medical City. Cureus. 2021; 13(5):e15127. [DOI:10.7759/cureus.15127]
- Malek A, Ghodsi A, Hamedi A. The negative predictive value of harada scoring for coronary artery dilatation or aneurysm in children with Kawasaki Disease: A cross-sectional study. Iran J Med Sci. 2022; 47(4):379-84. [PMID]
- Zubaidi AA, Ghatasheh G, Karuppaswamy V, Narchi H. Epidemiology of Kawasaki Disease, Its Incomplete Form and Outcomes: A Single-Institution Experience in the United Arab Emirates. Cureus. 2023; 15(12):e51320. [DOI:10.7759/cureus.51320]
- Koliou MG, Aristidou A, Mazeri S, Georgiou E, Agathocleous M, Kousparou M, et al. Epidemiology and risk factors for resistance to treatment of Kawasaki disease in Cyprus. Sci Rep. 2023; 13(1):352. [DOI:10.1038/s41598-023-27694-1] [PMID]
- Hashemian H, Ebrahimzadeh F. Clinical, laboratory, and demographic profile of children with kawasaki disease admitted to a Tertiary Referral Hospital in Iran. Caspian J Pediatr. 2023; 9(1):14. [DOI:10.22088/CJP.BUMS.9.1.14]
- Hosseininasab A, Pashang F, Rukerd MRZ, Mirkamali H, Nakhaie M, Sayyadi A. Kawasaki disease in children: A retrospective cross-sectional study. Reumatologia. 2023; 61(3):152-60. [DOI:10.5114/reum/163170] [PMID]
- Türkuçar S, Kaya Ü, Çakmak F, Haşlak F, Demir F, Karabulut E, et al. Risk factors for coronary arterial involvement in Turkish children with Kawasaki disease: A multicenter retrospective study. Turk J Pediatr. 2023; 65(1):64-72.[DOI:10.24953/turkjped.2021.1132] [PMID]
- Topçu U, Sahin N, Kayabey Ö, Babaoğlu K. Retrospective evaluation of 130 cases with kawasaki disease follow-up in a tertiary care center in Turkey between 1999 and 2019: A 20-year experience. Postgrad Med. 2024; 136(2):189-97. [DOI:10.1080/00325481.2024.2325334] [PMID]
- Mărginean CO, Meliţ LE, Mărginean MO. The peculiarities of Kawasaki disease at the extremes of age: Two case reports. Medicine. 2019; 98(42):e17595. [DOI:10.1097/MD.0000000000017595] [PMID]
- Yavuz L, AlHamdani S, Alasrawi S, Wafadari D, Al-Fraihat A, Bebars MA, et al. Kawasaki disease (KD) and multisystem inflammatory syndrome in children (MIS-C) in a Middle Eastern patient cohort. Pediatr Rheumatol Online J. 2023; 21(1):64. [DOI:10.1186/s12969-023-00834-7] [PMID]
- Nakamura Y, Yanagawa H. The worldwide epidemiology of Kawasaki disease. Prog Pediatr Cardiol. 2004; 19(2):99-108. [DOI:10.1016/j.ppedcard.2004.08.002]
- Muenchhoff M, Goulder PJ. Sex differences in pediatric infectious diseases. J Infect Dis. 2014; 209(Suppl 3):S120-6. [DOI:10.1093/infdis/jiu232] [PMID]
- Amiri-Moghadam Z. Frequency of heart involvement in Kawasaki disease during a decade in Iran. Novelty Clin Med. 2022; 1(1):50-4. [Link]
- Pilania RK, Jindal AK, Bhattarai D, Naganur SH, Singh S. Cardiovascular involvement in Kawasaki disease is much more than mere coronary arteritis. Front Pediatr. 2020; 8:526969. [DOI:10.3389/fped.2020.526969] [PMID]
- McCrindle BW, Rowley AH, Newburger JW, Burns JC, Bolger AF, Gewitz M, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: A scientific statement for health professionals from the American Heart Association. circulation. 2017; 135(17):e927-e99. [DOI:10.1161/CIR.0000000000000484] [PMID]
- Elakabawi K, Lin J, Jiao F, Guo N, Yuan Z. Kawasaki disease: global burden and genetic background. Cardiol Res. 2020; 11(1):9-14. [DOI:10.14740/cr993] [PMID]
Type of Study: Meta-analysis Review |
Received: 2025/04/16 | Accepted: 2025/09/2 | Published: 2025/10/18
Received: 2025/04/16 | Accepted: 2025/09/2 | Published: 2025/10/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |