Volume 10, Issue 1 (1-2022)
J. Pediatr. Rev 2022, 10(1): 1-16 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Amiri B, Namakin K, Soltani M, Shetty S, Riahi S M. Effect of Vitamin D Supplementation on Serum Lipid Profiles in Children and Adolescence: A Meta-analysis. J. Pediatr. Rev 2022; 10 (1) :1-16
URL: http://jpr.mazums.ac.ir/article-1-417-en.html
URL: http://jpr.mazums.ac.ir/article-1-417-en.html
1- Department of Pediatrics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
2- Cardivascular Disease Research Center, Department of Pediatrics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
3- Razi Clinical Research Development Unit (RCRDU), Birjand University of Medical Sciences, Birjand, Iran.
4- Department of Oral and Maxillofacial Surgery, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India.
5- Cardiovascular Diseases Research Center, Department of Epidemiology and Biostatistics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran. , riahim61@gmail.com
2- Cardivascular Disease Research Center, Department of Pediatrics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
3- Razi Clinical Research Development Unit (RCRDU), Birjand University of Medical Sciences, Birjand, Iran.
4- Department of Oral and Maxillofacial Surgery, Manipal College of Dental Sciences, Mangalore, Manipal Academy of Higher Education (MAHE), Manipal, India.
5- Cardiovascular Diseases Research Center, Department of Epidemiology and Biostatistics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran. , riahim61@gmail.com
Full-Text [PDF 1195 kb]
(864 Downloads)
| Abstract (HTML) (1755 Views)
Full-Text: (432 Views)
1. Introduction
Chronic diseases and their nascent risk factors begin in the early years of life; thus, early prevention of disease from childhood could halt the nascent activity (1). Amongst non-communicable diseases, cardiovascular diseases display significant morbidity and mortality (2). The early onset of atherosclerosis in addition to the other risk factors of cardiovascular diseases, such as dyslipidemia will continue to persist in adulthood, and cause a host of other diseases (3). Due to continued lipid deposition and proliferation of smooth muscle and connective tissue, fatty streaks and fibrous plaques increase in size and extent and some undergo qualitative changes (4). The most serious change is rupture, which exposes the blood to lipid-rich thrombogenic material and precipitates an occlusive thrombus, which in turn leads to myocardial infarction or sudden cardiac death (5).
Vitamin D deficiency is a major global health problem and is an important risk factor for diabetes, cancer, dyslipidemia, and Cardiovascular Disease (CVD) (6, 7, 8). Hypovitaminosis D is a risk factor for glucose intolerance and the impairment in the functional regulation of pancreatic β-cells. Vit D supplementation in early infancy can reduce the subsequent risk of type 1 diabetes by about 30% (9, 10). Vitamin D has a significant role in children and adolescents, including the prevention of infectious diseases, immune-related diseases (type 1 diabetes and asthma), cardiovascular disease, and the onset of osteomalacia and rickets (11). Vitamin D receptors exist in more than 36 tissues of the body; hence, vitamin D actions will expand over almost all cell systems and organs (e.g., immune cells, brain, colon, prostate, and breast) (12). Vitamin D regulates phosphorus and calcium homeostasis, regulates blood pressure, and modification of immune competence, and minimizes the propensity for autoimmune diseases, infectious diseases, and risk of cancer (13). Some reports suggested that there is an interrelationship between lipids and cholecalciferol. A pathway that produces a common substrate 7-dehydrocholesterol (7-DHC) in the skin, synthesizes Cholecalciferol and cholesterol. After exposure to sunlight, 7-DHC is converted to previtamin D3 and then by a heat-dependent process to cholecalciferol. On the other hand, 7-DHC by the enzyme, 7-dehydrocholesterol reductase converts into cholesterol (14, 15). It is important to identify dyslipidemia early to stop the progression of cardiovascular disease and protect the future health of children (16). It is still difficult to find a strong theory for such a relationship, except if we know vitamin D supplementation’s effect on serum lipids in placebo-controlled randomized cases. Unfortunately, the associated studies had different consequences, which some indicate a positive effect (17), and others show a negative effect (14). To the best of our knowledge, there is no systematic review and meta-analysis of randomized controlled trials (RCTs) on the effect of vitamin D supplementation on serum lipid profiles in children and adolescents. This meta-analysis was conducted to summarize the existing evidence of RCTs to evaluate the impact of vitamin D supplementation on lipid profiles in children and adolescents.
2. Material and Methods
Databases and search strategy
This study was conducted according to PRISMA guidelines (18), and the study protocol was submitted to the Birjand University of Medical Sciences, Iran (Code: Ir.bums.REC.1398.68).
PICO question: children or adolescent (P), vitamin D (I), control group (C), lipids (Chol, LDL, VLDL, HDL, TG) (O), clinical trial (S)
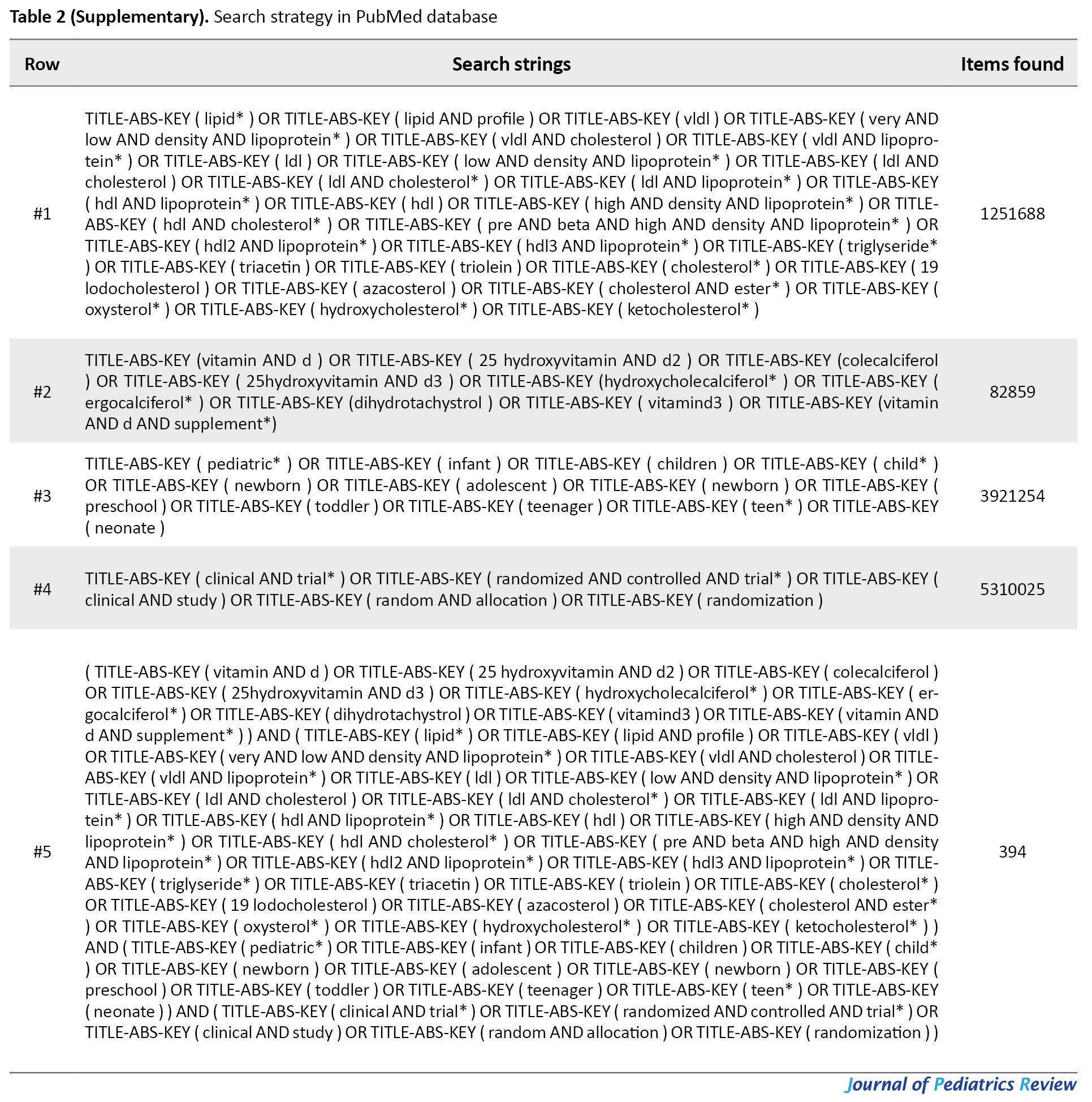
For this systematic review and meta-analysis, an electronic search of the literature published up to December 21, 2019, was conducted in the English databases (i.e., Web of Science, PubMed, Scopus, Google Scholar, EMBASE, ScienceDirect, and Persian databases (i.e., Magiran, Scientific Information Database (SID)) by two authors (A.B & R.SM) independently, to identify all articles related to RCTs examining the effect of vitamin D supplementation on the blood lipid profile using the following search terms: Vitamin D or 25-hydroxyvitamin D or Vitamin D3 or Cholecalciferol or Ergocalciferol or Calcitriol or Calcidiol and Lipids or Cholesterol or Triglycerides or HDL or LDL or VLDL. Medical Subject Headings (MESH) and Embase Subject Headings (EMTREE) were used to developing a search strategy and for each database, the strategy was revised. Search strategies are detailed in Tables 1-5 (Supplementary).

.jpg)
.jpg)
.jpg)
Eligibility Criteria
Clinical trials were considered eligible if they were conducted among children and adolescents (<18 years), and had evaluated the association between vitamin D and lipid profile.
Study selection
Based on the eligibility criteria, two authors (A.B & R.SM) independently reviewed the titles and abstracts of search results. Any disagreements were resolved by discussion. After the removal of duplicates, the full-text articles of potentially relevant publications were assessed to determine the final list of included studies. The excluded full-text articles were archived with the reasons for exclusion. The flow of the records was depicted in a PRISMA diagram.
Data extraction
Characteristics of the included studies were assessed by two authors (A.B & R.SM) independently using a standardized data collection form. The following data were extracted from the previous studies: first authors, year of publication, country, Follow-up (per week), age, gender, study design, the dosage of vitamin D supplementation, the sample size in control and intervention groups, comparison groups, and lipid profiles (i.e., total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG).
Risk of bias assessment
We used the JBI (Joanna Briggs Institute) guidelines to evaluate the risk of bias in the included study by two authors (A.B & R.SM) independently. Any incongruities were resolved by discussion. The JBI guidelines have a total of 13 questions and the response options for each field were yes, no, unclear, and not available (NA) (19). Quality assessment of clinical trial studies concerning method and risk of bias included sequence generation, blinding, incomplete outcome data, allocation concealment, selective outcome reporting, and other sources of bias. Articles with quality scores <60% were considered with a high risk of bias and excluded.
Data synthesis and analysis
Measurement of the treatment effect
Random-effects meta-analysis models were used to estimate pooled standardized mean differences (SMDs) and 95% confidence intervals (CIs) for all outcomes, such as HDL, LDL, TG, and total cholesterol. The SMD was calculated by (μ1-μ2)/SD, where µ1 is the mean of the intervention group, µ2 is the Mean of the control group, and SD is the pooled standard deviation. The standardized mean differences serve as an easy method to judge the magnitude of the effect, considering the general rules of thumb described by Cohen that suggest that SMD values of 0.2–0.5 are considered small, values of 0.5–0.8 are considered medium, and values >0.8 are considered large (20).
Assessment of heterogeneity
Statistical heterogeneity among studies was evaluated by statistical tests (i.e., Cochran Q and I2 statistics). I2 statistics is between 0 and 100 percent, and values of 60% or more were considered heterogeneous (21, 22).
Subgroups analysis, meta-regression, and sensitivity analysis
Subgroup analysis was performed on healthy and obese groups. Meta-regression analysis was conducted to explore covariates that might explain the heterogeneity between clinical trials (23). Meta-regression was conducted for qualitative covariates, such as daily intake of Vit D, publication year, and sample size. Sensitivity analyses were carried out for assessing the robustness of the findings based on primary analyses of data. We evaluated the influence of key assumptions, such as different methods of analysis, population study, protocol deviations, and outliers on the overall conclusions in all lipid profiles. The analyses were conducted by STATA 12.0 (Stata Corporation, College Station, TX, USA). All Statistical tests were two-sided, and a P<0.05 was considered statistically significant.
Assessment of publication biases
Publication bias was investigated by Egger’s test to judge publication bias for each outcome.
3. Results
Characteristics of included studies
We identified 3931 publications after a thorough search of Web of Science, PubMed, Scopus, Google Scholar, EMBASE, ScienceDirect, Magiran, and SID databases. After screening titles and abstracts and removing duplicates, we reviewed the remaining 46 full-text articles for eligibility. Subsequently, 33 articles were excluded for the following reasons: only abstract available (n=5), data not usable (n=13), non-original studies e.g. reviews, letters, case reports, etc. (n=9), inappropriate design (n=2), and inappropriate outcomes (n=4). Lastly, 13 articles were retained for the analysis in our meta-analysis. Publication years of articles ranged from 2014 to 2019. Two articles had been conducted in Iran, one article in Saudi Arabia, two articles in America, three articles in Argentina, four articles in Denmark, and one article in Egypt. All trials had been conducted using parallel design. The sample size varied from 11 to 189 participants with an overall number of 1173 subjects. Characteristics of the included studies are summarized in Table 1.
.jpg)
Also, the study selection process by to the Preferred Reporting Items for Systematic reviews and Meta-Analyses for Protocols (PRISMA) is shown in Figure 1.
.jpg)
High-density lipoprotein cholesterol (HDL-C)
Twelve papers reported the HDL-C level of participants. Three studies showed a statistically significant increase in HDL-C levels with vitamin D supplementation. Also, four studies showed a not statistically significant reduction in HDL-C levels, and four studies did not show a statistically significant increase in LDL-C levels with vitamin D supplementation (Figure 2B).
.jpg)
Using a random-effects model, the pooled results for lipid profiles showed that Vit D supplementation significantly increased HDL-C levels (SMD 0.23; 95% CI: 0.02, 0.45) (Table 2).
.jpg)
As the heterogeneity of studies was moderate, we conducted a subgroup analysis between HDL-C levels among the healthy group and the obese group. The results demonstrated a constant trend in HDL-C levels by use of vitamin D supplementation. Besides, the results of subgroup analysis showed a significant increase in HDL-cholesterol levels among the healthy group (SMD 0.26; 95% CI: 0.01, 0.50) than the obese group (SMD 0.23; 95% CI: 0.02, 0.45) (Figure 2C). Moreover, the meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and HDL-C level (b=-0.00005, P=0.38) (Figure 3).
.jpg)
Low-density lipoprotein cholesterol (LDL-C)
Seven papers reported the LDL-C level of participants. Six studies disclosed a no significant reduction in LDL-C levels and one study no statistically significant increase in LDL-C levels with vitamin D supplementation (Figure 2A). We found no significant association between Vit D supplementation and LDL-C levels (SMD -0.10; 95% CI: −0.29, 0.09) (Table 2) Using the random-effect model. Furthermore, the meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and LDL-C levels (b=-0.00006, P=0.264).
Total cholesterol (TC)
Eight papers reported the TC level of participants. Three studies showed no statistically significant reduction in TC levels and five studies showed no statistically significant increase in TC levels with vitamin D supplementation (Figure 2D).

The result showed no significant association between Vit D supplementation and TC levels (SMD -0.01; 95% CI, −0.20, 0.18) using the random-effects model. Also, the meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and TC levels (b=0.00007, P=0.196).
Triglycerides (TG)
Eleven papers reported on the TG level of participants. Eight studies showed a not statistically significant reduction in TG levels and three studies showed a statistically insignificant increase in TG levels with vitamin D supplementation (Figure 2E). The results show that, no significant association between Vit D supplementation and TG level (SMD -0.10; 95% CI, −0.22, 0.02) (Table 2) using a random-effects model. Also, The meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and TG level (b=0.00001, P=0.791).
4. Discussion
Supplementation of Vit D can be effective on serum lipid profiles through direct and indirect mechanisms (14, 24). It has been suggested that vitamin D has a link to the onset of cardiovascular diseases, such as hypertension (HTN), and CVD through its diverse role in blood pressure control, endothelial function, calcification of the coronary vessels, and increased vascular resistance (25, 26, 27). This study was conducted to evaluate the effect of Vit D supplementation on lipid profiles in children and adolescents. The findings of the current systematic review and meta-analysis showed that Vit D supplementation had an increasing effect on HDL-C levels in children and adolescents, which was in line with the study by Sethuraman et al. (28). A systematic review and meta-analysis that examined the effect of calcium and Vit D co-supplementation on lipids concentrations in overweight/obese subjects revealed that Vit D and calcium co-supplementation increased the blood concentrations of HDL-C (29). Another systematic review and meta-analysis conducted on adults indicated that lower doses of Vit D supplementation alone were effective in increasing HDL-C levels in a short period (30). A meta-analysis in 2017 among women with gestational diabetes revealed that Vit D supplementation had a beneficial effect on the levels of serum LDL; however, no effect was seen in the total cholesterol, HDL-C, or TG (31).
A meta-analysis of 17 cross-sectional studies noted that higher circulating levels of 25(OH) D are associated with a more favorable lipid profile in the pediatric group (32). Jorde et al. cross-sectional study on 8018 nonsmoking participants revealed significant positive associations between serum 25(OH)D and HDL-C, LDL-C, TC levels, and a significant negative association between serum 25(OH)D and TG levels after adjustment for age, gender, BMI, and month of blood sampling (33). Saedisomeolia et al. assessed 108 participants with T2D and demonstrated that serum levels of 25OHD had an inverse, but no significant association with TC and TG levels and a positive correlation with LDL-C and HDL-C levels after adjusting for confounding variables (25).
These studies are heterogeneous concerning the characteristics of subjects, Vit D dose, and study duration. Also, some common causes may be attributed to both the high serum Vit D levels and favorable lipid profile. Individuals who exercise routinely and eat nutritious food would have increased Vit D levels, and also, may have other healthy habits that could positively affect lipid profiles. The widespread difference in the quantity and formulation of supplemental Vit D may be the most important contributor to the heterogeneity found in our results (34, 35).
In our study, there was no significant association between Vit D supplementation and LDL-C, TC, and TG levels, while our results are not similar to other findings. A meta-analysis of RCTs that evaluated the effects of vitamin D supplementation on blood lipids, showed that Vit D supplementation provided a statistically significant increase in LDL-C. There was also a tendency to an increase in TC with supplementation of Vit D and the reductions in HDL-C and TG both were non-significant (35). Another meta-analysis in type 2 diabetics showed that Vit D supplementation lowered LDL-c and total cholesterol levels but had no positive effect on HDL-C and TG levels (36). Al-Daghri et al. demonstrated that Vit D supplementation decreased TG, LDL-C, and total cholesterol levels (37), whereas Manoy et al. reported that HDL-C and LDL-C improved after supplementation but total cholesterol and TG were not different (38). According to Zitterman et al. and Martins et al., Vit D might decrease serum TG and it is well-identified that the clearance of VLDL may lead to increased levels of LDL-C and HDL-C. Moreover, it is possible that the binding of 25-OH-D to LDL-C could result in a reduction in LDL-C clearance (39, 40). The inconsistencies of these studies might be partially explained by differences in age groups and eating nutritious food (41).
Several mechanisms can explain the effect of vitamin D on lipids. In vitro, Vit D metabolites can affect lipoprotein lipase (42), lower TG, and increase HDL levels. Vit D has anti-inflammatory effects and may reduce insulin resistance by reducing low-grade chronic inflammation (43, 44), thereby increasing HDLC and lowering TG levels.
Strengths and limitations
This systematic review and meta-analysis have several strengths. First, this is the first meta-analysis to assess the effect of Vit D supplementation on serum lipid profiles in children and adolescents. The inclusion of data from 13 RCTs provided enough power and robustness to detect the effect of Vit D on serum lipid profiles. Lastly, the source of heterogeneity and publication bias was not observed and sensitivity analysis was performed for the robustness of the finding. Additionally, this study has several limitations that must be acknowledged: First, the season of data collection was not available in trials; thus, examination of the role of Vit D on serum lipid profile by season was not possible. Second, no included studies had assessed potential differences regarding the impact of Vit D supplementation based on race and gender. Finally, the dietary pattern had not been mentioned in some included studies; however, nutrition training was similar in the intervention and control groups.
5. Conclusion
The result of this study indicated that Vit D supplementation could increase HDL levels with low effect size, but does not appear to significantly affect LDL-C, TG, and TC levels. Therefore, it is important to report that higher serum Vit D is correlated with a more positive effect on HDL levels in children and adolescents. In future research, higher doses of Vit D should be considered.
Summary of evidence
Our study did not find a significant relationship between Vit D supplementation and LDL levels. Vit D supplementation significantly increased HDL levels. There was no significant relationship between Vit D supplementation and the levels of TC and TG. Finally, due to the very low impact of Vit D consumption on the lipid profile of children and adolescents, health policymakers should look for the effect and study other compounds.
Ethical Considerations
Compliance with ethical guidelines
The research was approved on October 14, 2019 by Birjand University of Medical Sciences (IR.BUMS.REC.1398.215).
Funding
This study did not receive any specific funding from the public, commercial or non-profit financial sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no competing of interests.
Acknowledgments
This article is taken from the dissertation of a residency course in the pediatrics field at Birjand University of Medical Sciences. We thank the participation of the Razi Clinical Research Development Unit (RCRDU).
References
Chronic diseases and their nascent risk factors begin in the early years of life; thus, early prevention of disease from childhood could halt the nascent activity (1). Amongst non-communicable diseases, cardiovascular diseases display significant morbidity and mortality (2). The early onset of atherosclerosis in addition to the other risk factors of cardiovascular diseases, such as dyslipidemia will continue to persist in adulthood, and cause a host of other diseases (3). Due to continued lipid deposition and proliferation of smooth muscle and connective tissue, fatty streaks and fibrous plaques increase in size and extent and some undergo qualitative changes (4). The most serious change is rupture, which exposes the blood to lipid-rich thrombogenic material and precipitates an occlusive thrombus, which in turn leads to myocardial infarction or sudden cardiac death (5).
Vitamin D deficiency is a major global health problem and is an important risk factor for diabetes, cancer, dyslipidemia, and Cardiovascular Disease (CVD) (6, 7, 8). Hypovitaminosis D is a risk factor for glucose intolerance and the impairment in the functional regulation of pancreatic β-cells. Vit D supplementation in early infancy can reduce the subsequent risk of type 1 diabetes by about 30% (9, 10). Vitamin D has a significant role in children and adolescents, including the prevention of infectious diseases, immune-related diseases (type 1 diabetes and asthma), cardiovascular disease, and the onset of osteomalacia and rickets (11). Vitamin D receptors exist in more than 36 tissues of the body; hence, vitamin D actions will expand over almost all cell systems and organs (e.g., immune cells, brain, colon, prostate, and breast) (12). Vitamin D regulates phosphorus and calcium homeostasis, regulates blood pressure, and modification of immune competence, and minimizes the propensity for autoimmune diseases, infectious diseases, and risk of cancer (13). Some reports suggested that there is an interrelationship between lipids and cholecalciferol. A pathway that produces a common substrate 7-dehydrocholesterol (7-DHC) in the skin, synthesizes Cholecalciferol and cholesterol. After exposure to sunlight, 7-DHC is converted to previtamin D3 and then by a heat-dependent process to cholecalciferol. On the other hand, 7-DHC by the enzyme, 7-dehydrocholesterol reductase converts into cholesterol (14, 15). It is important to identify dyslipidemia early to stop the progression of cardiovascular disease and protect the future health of children (16). It is still difficult to find a strong theory for such a relationship, except if we know vitamin D supplementation’s effect on serum lipids in placebo-controlled randomized cases. Unfortunately, the associated studies had different consequences, which some indicate a positive effect (17), and others show a negative effect (14). To the best of our knowledge, there is no systematic review and meta-analysis of randomized controlled trials (RCTs) on the effect of vitamin D supplementation on serum lipid profiles in children and adolescents. This meta-analysis was conducted to summarize the existing evidence of RCTs to evaluate the impact of vitamin D supplementation on lipid profiles in children and adolescents.
2. Material and Methods
Databases and search strategy
This study was conducted according to PRISMA guidelines (18), and the study protocol was submitted to the Birjand University of Medical Sciences, Iran (Code: Ir.bums.REC.1398.68).
PICO question: children or adolescent (P), vitamin D (I), control group (C), lipids (Chol, LDL, VLDL, HDL, TG) (O), clinical trial (S)
For this systematic review and meta-analysis, an electronic search of the literature published up to December 21, 2019, was conducted in the English databases (i.e., Web of Science, PubMed, Scopus, Google Scholar, EMBASE, ScienceDirect, and Persian databases (i.e., Magiran, Scientific Information Database (SID)) by two authors (A.B & R.SM) independently, to identify all articles related to RCTs examining the effect of vitamin D supplementation on the blood lipid profile using the following search terms: Vitamin D or 25-hydroxyvitamin D or Vitamin D3 or Cholecalciferol or Ergocalciferol or Calcitriol or Calcidiol and Lipids or Cholesterol or Triglycerides or HDL or LDL or VLDL. Medical Subject Headings (MESH) and Embase Subject Headings (EMTREE) were used to developing a search strategy and for each database, the strategy was revised. Search strategies are detailed in Tables 1-5 (Supplementary).


.jpg)
.jpg)
.jpg)
Eligibility Criteria
Clinical trials were considered eligible if they were conducted among children and adolescents (<18 years), and had evaluated the association between vitamin D and lipid profile.
Study selection
Based on the eligibility criteria, two authors (A.B & R.SM) independently reviewed the titles and abstracts of search results. Any disagreements were resolved by discussion. After the removal of duplicates, the full-text articles of potentially relevant publications were assessed to determine the final list of included studies. The excluded full-text articles were archived with the reasons for exclusion. The flow of the records was depicted in a PRISMA diagram.
Data extraction
Characteristics of the included studies were assessed by two authors (A.B & R.SM) independently using a standardized data collection form. The following data were extracted from the previous studies: first authors, year of publication, country, Follow-up (per week), age, gender, study design, the dosage of vitamin D supplementation, the sample size in control and intervention groups, comparison groups, and lipid profiles (i.e., total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), and triglyceride (TG).
Risk of bias assessment
We used the JBI (Joanna Briggs Institute) guidelines to evaluate the risk of bias in the included study by two authors (A.B & R.SM) independently. Any incongruities were resolved by discussion. The JBI guidelines have a total of 13 questions and the response options for each field were yes, no, unclear, and not available (NA) (19). Quality assessment of clinical trial studies concerning method and risk of bias included sequence generation, blinding, incomplete outcome data, allocation concealment, selective outcome reporting, and other sources of bias. Articles with quality scores <60% were considered with a high risk of bias and excluded.
Data synthesis and analysis
Measurement of the treatment effect
Random-effects meta-analysis models were used to estimate pooled standardized mean differences (SMDs) and 95% confidence intervals (CIs) for all outcomes, such as HDL, LDL, TG, and total cholesterol. The SMD was calculated by (μ1-μ2)/SD, where µ1 is the mean of the intervention group, µ2 is the Mean of the control group, and SD is the pooled standard deviation. The standardized mean differences serve as an easy method to judge the magnitude of the effect, considering the general rules of thumb described by Cohen that suggest that SMD values of 0.2–0.5 are considered small, values of 0.5–0.8 are considered medium, and values >0.8 are considered large (20).
Assessment of heterogeneity
Statistical heterogeneity among studies was evaluated by statistical tests (i.e., Cochran Q and I2 statistics). I2 statistics is between 0 and 100 percent, and values of 60% or more were considered heterogeneous (21, 22).
Subgroups analysis, meta-regression, and sensitivity analysis
Subgroup analysis was performed on healthy and obese groups. Meta-regression analysis was conducted to explore covariates that might explain the heterogeneity between clinical trials (23). Meta-regression was conducted for qualitative covariates, such as daily intake of Vit D, publication year, and sample size. Sensitivity analyses were carried out for assessing the robustness of the findings based on primary analyses of data. We evaluated the influence of key assumptions, such as different methods of analysis, population study, protocol deviations, and outliers on the overall conclusions in all lipid profiles. The analyses were conducted by STATA 12.0 (Stata Corporation, College Station, TX, USA). All Statistical tests were two-sided, and a P<0.05 was considered statistically significant.
Assessment of publication biases
Publication bias was investigated by Egger’s test to judge publication bias for each outcome.
3. Results
Characteristics of included studies
We identified 3931 publications after a thorough search of Web of Science, PubMed, Scopus, Google Scholar, EMBASE, ScienceDirect, Magiran, and SID databases. After screening titles and abstracts and removing duplicates, we reviewed the remaining 46 full-text articles for eligibility. Subsequently, 33 articles were excluded for the following reasons: only abstract available (n=5), data not usable (n=13), non-original studies e.g. reviews, letters, case reports, etc. (n=9), inappropriate design (n=2), and inappropriate outcomes (n=4). Lastly, 13 articles were retained for the analysis in our meta-analysis. Publication years of articles ranged from 2014 to 2019. Two articles had been conducted in Iran, one article in Saudi Arabia, two articles in America, three articles in Argentina, four articles in Denmark, and one article in Egypt. All trials had been conducted using parallel design. The sample size varied from 11 to 189 participants with an overall number of 1173 subjects. Characteristics of the included studies are summarized in Table 1.
.jpg)
Also, the study selection process by to the Preferred Reporting Items for Systematic reviews and Meta-Analyses for Protocols (PRISMA) is shown in Figure 1.
.jpg)
High-density lipoprotein cholesterol (HDL-C)
Twelve papers reported the HDL-C level of participants. Three studies showed a statistically significant increase in HDL-C levels with vitamin D supplementation. Also, four studies showed a not statistically significant reduction in HDL-C levels, and four studies did not show a statistically significant increase in LDL-C levels with vitamin D supplementation (Figure 2B).
.jpg)
Using a random-effects model, the pooled results for lipid profiles showed that Vit D supplementation significantly increased HDL-C levels (SMD 0.23; 95% CI: 0.02, 0.45) (Table 2).
.jpg)
As the heterogeneity of studies was moderate, we conducted a subgroup analysis between HDL-C levels among the healthy group and the obese group. The results demonstrated a constant trend in HDL-C levels by use of vitamin D supplementation. Besides, the results of subgroup analysis showed a significant increase in HDL-cholesterol levels among the healthy group (SMD 0.26; 95% CI: 0.01, 0.50) than the obese group (SMD 0.23; 95% CI: 0.02, 0.45) (Figure 2C). Moreover, the meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and HDL-C level (b=-0.00005, P=0.38) (Figure 3).
.jpg)
Low-density lipoprotein cholesterol (LDL-C)
Seven papers reported the LDL-C level of participants. Six studies disclosed a no significant reduction in LDL-C levels and one study no statistically significant increase in LDL-C levels with vitamin D supplementation (Figure 2A). We found no significant association between Vit D supplementation and LDL-C levels (SMD -0.10; 95% CI: −0.29, 0.09) (Table 2) Using the random-effect model. Furthermore, the meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and LDL-C levels (b=-0.00006, P=0.264).
Total cholesterol (TC)
Eight papers reported the TC level of participants. Three studies showed no statistically significant reduction in TC levels and five studies showed no statistically significant increase in TC levels with vitamin D supplementation (Figure 2D).

The result showed no significant association between Vit D supplementation and TC levels (SMD -0.01; 95% CI, −0.20, 0.18) using the random-effects model. Also, the meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and TC levels (b=0.00007, P=0.196).
Triglycerides (TG)
Eleven papers reported on the TG level of participants. Eight studies showed a not statistically significant reduction in TG levels and three studies showed a statistically insignificant increase in TG levels with vitamin D supplementation (Figure 2E). The results show that, no significant association between Vit D supplementation and TG level (SMD -0.10; 95% CI, −0.22, 0.02) (Table 2) using a random-effects model. Also, The meta-regression analysis demonstrated no significant association between the dosage of Vit D supplementation and TG level (b=0.00001, P=0.791).
4. Discussion
Supplementation of Vit D can be effective on serum lipid profiles through direct and indirect mechanisms (14, 24). It has been suggested that vitamin D has a link to the onset of cardiovascular diseases, such as hypertension (HTN), and CVD through its diverse role in blood pressure control, endothelial function, calcification of the coronary vessels, and increased vascular resistance (25, 26, 27). This study was conducted to evaluate the effect of Vit D supplementation on lipid profiles in children and adolescents. The findings of the current systematic review and meta-analysis showed that Vit D supplementation had an increasing effect on HDL-C levels in children and adolescents, which was in line with the study by Sethuraman et al. (28). A systematic review and meta-analysis that examined the effect of calcium and Vit D co-supplementation on lipids concentrations in overweight/obese subjects revealed that Vit D and calcium co-supplementation increased the blood concentrations of HDL-C (29). Another systematic review and meta-analysis conducted on adults indicated that lower doses of Vit D supplementation alone were effective in increasing HDL-C levels in a short period (30). A meta-analysis in 2017 among women with gestational diabetes revealed that Vit D supplementation had a beneficial effect on the levels of serum LDL; however, no effect was seen in the total cholesterol, HDL-C, or TG (31).
A meta-analysis of 17 cross-sectional studies noted that higher circulating levels of 25(OH) D are associated with a more favorable lipid profile in the pediatric group (32). Jorde et al. cross-sectional study on 8018 nonsmoking participants revealed significant positive associations between serum 25(OH)D and HDL-C, LDL-C, TC levels, and a significant negative association between serum 25(OH)D and TG levels after adjustment for age, gender, BMI, and month of blood sampling (33). Saedisomeolia et al. assessed 108 participants with T2D and demonstrated that serum levels of 25OHD had an inverse, but no significant association with TC and TG levels and a positive correlation with LDL-C and HDL-C levels after adjusting for confounding variables (25).
These studies are heterogeneous concerning the characteristics of subjects, Vit D dose, and study duration. Also, some common causes may be attributed to both the high serum Vit D levels and favorable lipid profile. Individuals who exercise routinely and eat nutritious food would have increased Vit D levels, and also, may have other healthy habits that could positively affect lipid profiles. The widespread difference in the quantity and formulation of supplemental Vit D may be the most important contributor to the heterogeneity found in our results (34, 35).
In our study, there was no significant association between Vit D supplementation and LDL-C, TC, and TG levels, while our results are not similar to other findings. A meta-analysis of RCTs that evaluated the effects of vitamin D supplementation on blood lipids, showed that Vit D supplementation provided a statistically significant increase in LDL-C. There was also a tendency to an increase in TC with supplementation of Vit D and the reductions in HDL-C and TG both were non-significant (35). Another meta-analysis in type 2 diabetics showed that Vit D supplementation lowered LDL-c and total cholesterol levels but had no positive effect on HDL-C and TG levels (36). Al-Daghri et al. demonstrated that Vit D supplementation decreased TG, LDL-C, and total cholesterol levels (37), whereas Manoy et al. reported that HDL-C and LDL-C improved after supplementation but total cholesterol and TG were not different (38). According to Zitterman et al. and Martins et al., Vit D might decrease serum TG and it is well-identified that the clearance of VLDL may lead to increased levels of LDL-C and HDL-C. Moreover, it is possible that the binding of 25-OH-D to LDL-C could result in a reduction in LDL-C clearance (39, 40). The inconsistencies of these studies might be partially explained by differences in age groups and eating nutritious food (41).
Several mechanisms can explain the effect of vitamin D on lipids. In vitro, Vit D metabolites can affect lipoprotein lipase (42), lower TG, and increase HDL levels. Vit D has anti-inflammatory effects and may reduce insulin resistance by reducing low-grade chronic inflammation (43, 44), thereby increasing HDLC and lowering TG levels.
Strengths and limitations
This systematic review and meta-analysis have several strengths. First, this is the first meta-analysis to assess the effect of Vit D supplementation on serum lipid profiles in children and adolescents. The inclusion of data from 13 RCTs provided enough power and robustness to detect the effect of Vit D on serum lipid profiles. Lastly, the source of heterogeneity and publication bias was not observed and sensitivity analysis was performed for the robustness of the finding. Additionally, this study has several limitations that must be acknowledged: First, the season of data collection was not available in trials; thus, examination of the role of Vit D on serum lipid profile by season was not possible. Second, no included studies had assessed potential differences regarding the impact of Vit D supplementation based on race and gender. Finally, the dietary pattern had not been mentioned in some included studies; however, nutrition training was similar in the intervention and control groups.
5. Conclusion
The result of this study indicated that Vit D supplementation could increase HDL levels with low effect size, but does not appear to significantly affect LDL-C, TG, and TC levels. Therefore, it is important to report that higher serum Vit D is correlated with a more positive effect on HDL levels in children and adolescents. In future research, higher doses of Vit D should be considered.
Summary of evidence
Our study did not find a significant relationship between Vit D supplementation and LDL levels. Vit D supplementation significantly increased HDL levels. There was no significant relationship between Vit D supplementation and the levels of TC and TG. Finally, due to the very low impact of Vit D consumption on the lipid profile of children and adolescents, health policymakers should look for the effect and study other compounds.
Ethical Considerations
Compliance with ethical guidelines
The research was approved on October 14, 2019 by Birjand University of Medical Sciences (IR.BUMS.REC.1398.215).
Funding
This study did not receive any specific funding from the public, commercial or non-profit financial sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no competing of interests.
Acknowledgments
This article is taken from the dissertation of a residency course in the pediatrics field at Birjand University of Medical Sciences. We thank the participation of the Razi Clinical Research Development Unit (RCRDU).
References
- Conceição-Machado MEPd, Silva LR, Santana MLP, Pinto EJ, Silva RdCR, Moraes LTL, et al. Hypertriglyceridemic waist phenotype: association with metabolic abnormalities in adolescents. Jornal de Pediatria. 2013; 89(1):56-63. [DOI:10.1016/j.jped.2013.02.009] [PMID]
- Lennon SL, Quindry J, Hamilton KL, French J, Staib J, Mehta JL, et al. Loss of exercise-induced cardioprotection after cessation of exercise. Journal of Applied Physiology. 2004; 96(4):1299-305. [DOI:10.1152/japplphysiol.00920.2003] [PMID]
- Lauer RM, Clarke WR. Use of cholesterol measurements in childhood for the prediction of adult hypercholesterolemia: The Muscatine Study. JAMA. 1990; 264(23):3034-8. [DOI:10.1001/jama.264.23.3034]
- Strong JP, Malcom GT, McMahan CA, Tracy RE, Newman III WP, Herderick EE, et al. Prevalence and extent of atherosclerosis in adolescents and young adults: Implications for prevention from the Pathobiological Determinants of Atherosclerosis in Youth Study. JAMA. 1999; 281(8):727-35. [DOI:10.1001/jama.281.8.727] [PMID]
- Virmani R, Burke A, Farb A. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. European Heart Journal. 1998; 19(5):678-80.
- Parker J, Hashmi O, Dutton D, Mavrodaris A, Stranges S, Kandala N-B, et al. Levels of vitamin D and cardiometabolic disorders: Systematic review and meta-analysis. Maturitas. 2010; 65(3):225-36. [DOI:10.1016/j.maturitas.2009.12.013] [PMID]
- Braegger C, Campoy C, Colomb V, Decsi T, Domellof M, Fewtrell M, et al. Vitamin D in the healthy European paediatric population. Journal of Pediatric Gastroenterology and Nutrition. 2013; 56(6):692-701. [DOI:10.1097/MPG.0b013e31828f3c05] [PMID]
- Karhapää P, Pihlajamäki J, Pörsti I, Kastarinen M, Mustonen J, Niemelä O, et al. Diverse associations of 25-hydroxyvitamin D and 1, 25-dihydroxy-vitamin D with dyslipidaemias. Journal of Internal Medicine. 2010; 268(6):604-10. [DOI:10.1111/j.1365-2796.2010.02279.x] [PMID]
- Hyppönen E. Vitamin D and increasing incidence of type 1 diabetes-evidence for an association? Diabetes, Obesity and Metabolism. 2010; 12(9):737-43. [DOI:10.1111/j.1463-1326.2010.01211.x] [PMID]
- Zipitis CS, Akobeng AK. Vitamin D supplementation in early childhood and risk of type 1 diabetes: A systematic review and meta-analysis. Archives of disease in childhood. 2008; 93(6):512-7. [DOI:10.1136/adc.2007.128579] [PMID]
- Christakos S, DeLuca HF. Minireview: vitamin D: Is there a role in extraskeletal health? Endocrinology. 2011; 152(8):2930-6. [DOI:10.1210/en.2011-0243] [PMID] [PMCID]
- Bringhurst F, Demay MB, Krane SM, Kronenberg HM. Bone and mineral metabolism in health and disease. In: Kasper D, Fauci A, Hauser S, Longo D, Jameson J, Loscalzo J. eds. Harrison's Principles of Internal Medicine, 19e. McGraw Hill; 2014. https://accessmedicine.mhmedical.com/content.aspx?sectionid=79753494&bookid=1130&jumpsec-2
- Kao KT, Abidi N, Ranasinha S, Brown J, Rodda C, McCallum Z, et al. Low vitamin D is associated with hypertension in paediatric obesity. Journal of Paediatrics and Child Health. 2015; 51(12):1207-13. [DOI:10.1111/jpc.12935] [PMID]
- Jorde R, Grimnes G. Vitamin D and metabolic health with special reference to the effect of vitamin D on serum lipids. Progress in Lipid Research. 2011; 50(4):303-12. [DOI:10.1016/j.plipres.2011.05.001] [PMID]
- Kuan V, Martineau AR, Griffiths CJ, Hyppönen E, Walton R. DHCR7 mutations linked to higher vitamin D status allowed early human migration to northern latitudes. BMC Evolutionary Biology. 2013; 13(1):1-10. [DOI:10.1186/1471-2148-13-144] [PMID] [PMCID]
- Berenson GS, Srinivasan SR, Bao W, Newman WP, Tracy RE, Wattigney WA. Association between multiple cardiovascular risk factors and atherosclerosis in children and young adults. New England Journal of Medicine. 1998; 338(23):1650-6. [DOI:10.1056/NEJM199806043382302] [PMID]
- Hirschler V, Maccallini G, Sanchez MS, Castaño L, Molinari C, Group SAdlCS. Improvement in high-density lipoprotein cholesterol levels in argentine Indian school children after vitamin D supplementation. Hormone Research in Paediatrics. 2013; 80(5):335-42. [DOI:10.1159/000355511] [PMID]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Journal of Clinical Epidemiology. 2009; 62(10):e1-e34. [DOI:10.1016/j.jclinepi.2009.06.006] [PMID]
- Porritt K, Gomersall J, Lockwood C. JBI’s systematic reviews: Study selection and critical appraisal. American Journal of Nursing (AJN). 2014; 114(6):47-52. [DOI:10.1097/01.NAJ.0000450430.97383.64] [PMID]
- Cohen J. Statistical power analysis for the behavioral sciences: Academic press; 2013. [DOI:10.4324/9780203771587]
- Higgins J. Assessing risk of bias in included studies. Altman DG, Higgins JPT, SG, eds. Cochrane handbook for systematic reviews of interventions Version 501. The Cochrane Collaboration. Chichester, UK: Wiley-Blackwell; 2008. https://handbook-5-1.cochrane.org/chapter_8/8_assessing_risk_of_bias_in_included_studies.htm
- Riahi SM, Mokhayeri Y. Methodological issues in a meta-analysis. Current Medical Research and Opinion. 2017; 33(10):1813. [DOI:10.1080/03007995.2017.1359152] [PMID]
- Yousefi M, Parvaie P, Riahi SM. Salivary factors related to caries in pregnancy: A systematic review and meta-analysis. Journal of the American Dental Association. 2020; 151(8):576-88. e4. [DOI:10.1016/j.aime.2020.04.021] [PMID]
- Wang J-H, Keisala T, Solakivi T, Minasyan A, Kalueff AV, Tuohimaa P. Serum cholesterol and expression of ApoAI, LXRβ and SREBP2 in vitamin D receptor knock-out mice. Journal of Steroid Biochemistry and Molecular Biology. 2009; 113(3-5):222-6. [DOI:10.1016/j.jsbmb.2009.01.003] [PMID]
- Saedisomeolia A, Taheri E, Djalali M, Moghadam AM, Qorbani M. Association between serum level of vitamin D and lipid profiles in type 2 diabetic patients in Iran. Journal of Diabetes & Metabolic Disorders. 2014; 13(1):1-5. [DOI:10.1186/2251-6581-13-7] [PMID] [PMCID]
- Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. Journal of the American College of Cardiology. 2011; 58(2):186-92. [DOI:10.1016/j.jacc.2011.02.051] [PMID] [PMCID]
- Alam U, Najam O, Al-Himdani S, Benoliel S, Jinadev P, Berry JL, et al. Marked vitamin D deficiency in patients with diabetes in the UK: Ethnic and seasonal differences and an association with dyslipidaemia. Diabetic medicine : A Journal of the British Diabetic Association. 2012; 29(10):1343-5. [DOI:10.1111/j.1464-5491.2012.03692.x] [PMID]
- Sethuraman U, Zidan MA, Hanks L, Bagheri M, Ashraf A. Impact of vitamin D treatment on 25 hydroxy vitamin D levels and insulin homeostasis in obese African American adolescents in a randomized trial. Journal of Clinical & Translational Endocrinology. 2018; 12:13-9. [DOI:10.1016/j.jcte.2018.03.002] [PMID] [PMCID]
- Kashkooli S, Choghakhori R, Hasanvand A, Abbasnezhad A. Effect of calcium and vitamin D co-supplementation on lipid profile of overweight/obese subjects: A systematic review and meta-analysis of the randomized clinical trials. Obesity Medicine. 2019; 15:100124. [DOI:10.1016/j.obmed.2019.100124]
- Mirhosseini N, Rainsbury J, Kimball SM. Vitamin D supplementation, serum 25 (OH) D concentrations and cardiovascular disease risk factors: A systematic review and meta-analysis. Frontiers in cardiovascular medicine. 2018; 5:87. [DOI:10.3389/fcvm.2018.00087] [PMID] [PMCID]
- Akbari M, Mosazadeh M, Lankarani KB, Tabrizi R, Samimi M, Karamali M, et al. The effects of vitamin D supplementation on glucose metabolism and lipid profiles in patients with gestational diabetes: A systematic review and meta-analysis of randomized controlled trials. Hormone and Metabolic Research. 2017; 49(09):647-53. [DOI:10.1055/s-0043-115225] [PMID]
- Kelishadi R, Farajzadegan Z, Bahreynian M. Association between vitamin D status and lipid profile in children and adolescents: A systematic review and meta-analysis. International Journal of Food Sciences and Nutrition. 2014; 65(4):404-10. [DOI:10.3109/09637486.2014.886186] [PMID]
- Jorde R, Figenschau Y, Hutchinson M, Emaus N, Grimnes G. High serum 25-hydroxyvitamin D concentrations are associated with a favorable serum lipid profile. European Journal of Clinical Nutrition. 2010; 64(12):1457-64. [DOI:10.1038/ejcn.2010.176] [PMID]
- Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. American Journal of Clinical Nutrition. 2006; 84(1):18-28. [DOI:10.1093/ajcn/84.1.18] [PMID]
- Wang H, Xia N, Yang Y, Peng D-Q. Influence of vitamin D supplementation on plasma lipid profiles: A meta-analysis of randomized controlled trials. Lipids in Health and Disease. 2012; 11(1):1-9. [DOI:10.1186/1476-511X-11-42] [PMID] [PMCID]
- Jafari T, Fallah AA, Barani A. Effects of vitamin D on serum lipid profile in patients with type 2 diabetes: A meta-analysis of randomized controlled trials. Clinical Nutrition. 2016; 35(6):1259-68. [DOI:10.1016/j.clnu.2016.03.001] [PMID]
- Al-Daghri NM, Mohammed AK, Al-Attas OS, Ansari MGA, Wani K, Hussain SD, et al. Vitamin D receptor gene polymorphisms modify cardiometabolic response to vitamin D supplementation in T2DM patients. Scientific Reports. 2017; 7(1):1-10. [DOI:10.1038/s41598-017-08621-7] [PMID] [PMCID]
- Manoy P, Yuktanandana P, Tanavalee A, Anomasiri W, Ngarmukos S, Tanpowpong T, et al. Vitamin D supplementation improves quality of life and physical performance in osteoarthritis patients. Nutrients. 2017; 9(8):799. [DOI:10.3390/nu9080799] [PMID] [PMCID]
- Martins D, Wolf M, Pan D, Zadshir A, Tareen N, Thadhani R, et al. Prevalence of cardiovascular risk factors and the serum levels of 25-hydroxyvitamin D in the United States: Data from the Third National Health and Nutrition Examination Survey. Archives of Internal Medicine. 2007; 167(11):1159-65. [DOI:10.1001/archinte.167.11.1159] [PMID]
- Zittermann A, Frisch S, Berthold HK, Götting C, Kuhn J, Kleesiek K, et al. Vitamin D supplementation enhances the beneficial effects of weight loss on cardiovascular disease risk markers. American Journal of Clinical Nutrition. 2009; 89(5):1321-7. [DOI:10.3945/ajcn.2008.27004] [PMID]
- Ashraf AP, Alvarez JA, Gower BA, Saenz KH, McCormick KL. Associations of serum 25-hydroxyvitamin D and components of the metabolic syndrome in obese adolescent females. Obesity. 2011; 19(11):2214-21. [DOI:10.1038/oby.2011.110] [PMID] [PMCID]
- Querfeld U, Hoffmann MM, KLAUS G, Eifinger F, Ackerschott M, Michalk D, et al. Antagonistic effects of vitamin D and parathyroid hormone on lipoprotein lipase in cultured adipocytes. Journal of the American Society of Nephrology. 1999; 10(10):2158-64. [DOI:10.1681/ASN.V10102158] [PMID]
- Chagas CEA, Borges MC, Martini LA, Rogero MM. Focus on vitamin D, inflammation and type 2 diabetes. Nutrients. 2012; 4(1):52-67. [DOI:10.3390/nu4010052] [PMID] [PMCID]
- Hewison M. An update on vitamin D and human immunity. Clinical Endocrinology. 2012; 76(3):315-25. [DOI:10.1111/j.1365-2265.2011.04261.x] [PMID]
- Magge SN, Prasad D, Zemel BS, Kelly A. Vitamin D3 supplementation in obese, African-American, vitamin D deficient adolescents. Journal of Clinical & Translational Endocrinology. 2018; 12:1-7. [DOI:10.1016/j.jcte.2018.03.001] [PMID] [PMCID]
- Tavakoli F, Namakin K, Zardast M. Vitamin D supplementation and high-density lipoprotein cholesterol: A study in healthy school children. Iranian Journal of Pediatrics. 2016; 26(4):e3311. [DOI:10.5812/ijp.3311] [PMID] [PMCID]
- Al-Daghri NM, Amer OE, Khattak MN, Sabico S, Ansari MGA, Al-Saleh Y, et al. Effects of different vitamin D supplementation strategies in reversing metabolic syndrome and its component risk factors in adolescents. Journal of Steroid Biochemistry and Molecular Biology. 2019; 191:105378. [DOI:10.1016/j.jsbmb.2019.105378] [PMID]
- Hafez M, Musa N, Atty SA, Ibrahem M, Wahab NA. Effect of vitamin D supplementation on lipid profile in vitamin D-deficient children with type 1 diabetes and dyslipidemia. Hormone Research in Paediatrics. 2019; 91(5):311-8. [DOI:10.1159/000500829] [PMID]
- Hirschler V, Molinari C, Maccallini G, Hidalgo M, Gonzalez C. Healthier lipid profiles with vitamin D supplementation in a pilot study in argentinean children of two ethnicities. International Journal for Vitamin and Nutrition Research. 2016; 86(1-2):48-55. [DOI:10.1024/0300-9831/a000289] [PMID]
- Namakin K, Tavakoli F, Zardast M. Effect of vitamin D supplementation on lipid profile in children aged 10-14 years old. International Journal of Pediatrics. 2015; 3(5.2):987-94. https://ijp.mums.ac.ir/article_5141_484f0d283e3de92a8452548ef81b257a.pdf
- Smith TJ, Tripkovic L, Hauger H, Damsgaard CT, Mølgaard C, Lanham-New SA, et al. Winter cholecalciferol supplementation at 51° N has no effect on markers of cardiometabolic risk in healthy adolescents aged 14-18 years. Journal of Nutrition. 2018; 148(8):1269-75. [DOI:10.1093/jn/nxy079] [PMID]
- Hauger H, Mølgaard C, Mortensen C, Ritz C, Frøkiær H, Smith TJ, et al. Winter cholecalciferol supplementation at 55° N has no effect on markers of cardiometabolic risk in healthy children aged 4-8 years. Journal of Nutrition. 2018; 148(8):1261-8. [DOI:10.1093/jn/nxy080] [PMID]
- Hirschler V, Maccallini G, I Tamborenea M, Gonzalez C, Sanchez M, Molinari C, et al. Improvement in lipid profile after vitamin D supplementation in indigenous argentine school children. Cardiovascular & Hematological Agents in Medicinal Chemistry. 2014; 12(1):42-9. https://europepmc.org/article/med/24845422
Type of Study: Meta-analysis Review |
Subject:
Pediatrics
Received: 2021/06/23 | Accepted: 2021/11/22 | Published: 2022/01/1
Received: 2021/06/23 | Accepted: 2021/11/22 | Published: 2022/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








