Volume 10, Issue 3 (7-2022)
J. Pediatr. Rev 2022, 10(3): 257-266 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Saleh V, Afroundeh R. Effects of 8-Week Anaerobic Gymnastics Training on Weight Loss and Related Growth Factors in Obese Children: A Clinical Trial. J. Pediatr. Rev 2022; 10 (3) :257-266
URL: http://jpr.mazums.ac.ir/article-1-438-en.html
URL: http://jpr.mazums.ac.ir/article-1-438-en.html
1- Department of Physical Education and Sport Sciences, Faculty of Education and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran. , v_saleh1365@yahoo.com
2- Physical Education and Sport Sciences, Faculty of Education and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran.
2- Physical Education and Sport Sciences, Faculty of Education and Psychology, University of Mohaghegh Ardabili, Ardabil, Iran.
Full-Text [PDF 798 kb]
(882 Downloads)
| Abstract (HTML) (1908 Views)
Full-Text: (352 Views)
1. Background
Overweight and obesity are prominent threats to pediatric health and the worldwide prevalence of childhood obesity has dramatically been increasing. Childhood obesity is induced by multiple factors, such as environmental factors, genetics, medical status, and drugs. It is administered by surgical treatment, diet regimen, and exercise (1, 2). Higher physical activity and consequently higher energy expenditure is a beneficial method to prevent childhood obesity (3).
Many studies have demonstrated the beneficial effects of physical activity on resting metabolic rate (RMR), maximal oxygen consumption (VO2 max), body composition, lipid profile, and plasma leptin (4). Additionally, exercise training improves cardiovascular health (5). Also, exercise has positive effects on brain health by increasing the density of capillaries because of angiogenesis, that is, the sprouting of new capillaries from pre-existing vessels, and via activating specific processes that promote synaptic plasticity which is one mechanism through which exercise improves brain function (6). Growth factors with angiogenic and neurotrophic properties, such as vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) are implicated in vascular and neurological repair in both human and animal studies (7, 8).
Vascular permeability and vasodilatation are increased by VEGF and pathogenesis of cardiovascular risk factors, arteriosclerosis, obesity, and metabolism morbidity; meanwhile, mortality is related to VEGF (9, 10). In addition, adipose tissue as an endocrine organ produces considerable amounts of VEGF (11). Previous studies have indicated that overweight and obese individuals show elevated serum VEGF levels (12, 13). Also, studies have found that subjects who lost at least 1.85% of their baseline fat mass in a 12-month exercise intervention experienced significant reductions in some biomarkers of angiogenesis, compared to sedentary controls (14).
The neurotrophin family, including BDNF, is considered a key player in controlling the growth, survival, and preservation of neurons (15). BDNF and its receptors are expressed in the hypothalamus and peripheral tissues, such as smooth and skeletal muscle, liver, pancreas, and adipose tissue (16). BDNF is considered the main constituent of the hypothalamic axis that governs body weight and plays a relevant role in regulating energy homeostasis, glucose metabolism, and eating behavior through the central nervous system (17). Many studies have observed a low-level serum concentration of BDNF in obese people and subjects with metabolic syndrome (18, 19). It has been demonstrated that serum BDNF increases during exercise and muscle-derived BDNF enhances fatty acid oxidation in skeletal muscle while inducing weight loss in children (17, 20). However, Roth et al. (21) maintained that serum BDNF concentration is higher in obese compared to normal-weight children.
It has been suggested that exercise intensity has an effect on blood BDNF and VEGF levels so high-intensity exercises that produces higher blood lactate levels induce a higher increase in plasma BDNF concentrations (22, 23, 24). To understand the mechanisms that are responsible for increasing the BDNF level, Schiffer et al. (25) used a method called lactate clamp; after the infusion of sodium-lactate, BDNF and lactate increased significantly and reached baseline values at the end of the experiment. They reported that blood lactate increases during high-intensity exercise after the infusion of sodium-lactate; however, no metabolic acidosis is observed, suggesting that the mechanism underlying blood BDNF augmentation is lactate per se (25).
Based on the contradictory results of previous studies, this study aims to investigate the effects of 8-week anaerobic gymnastics training (AGT) on salivary growth factors (BDNF and VEGF), RMR, and weight loss in obese children.
2. Materials and Methods
Participants
A total of 30 obese boys in the age ranged 8-12 years who enrolled in the elementary level of gymnastics participated in this study. The number of samples was selected based on the G*Power software and they were randomly divided into experimental and control groups. Obese subjects were diagnosed according to the American Council on Exercise lists (Jackson and Pollock equation for 3-point subcutaneous fat measurement considering the fat percentage of 26 and above as obesity) (26) without concomitant diseases and the body mass index (BMI) expressed in percentage. Children above the 95th percentile were considered obese. The exclusion criteria included evidence of any disease, drug therapy, structural abnormality, and prohibition of exercise testing. The study protocol was approved by the Ethics Committee of Ardabil University of Medical Science (IR.ARUMS.REC.1397.290) and the Iranian Registry of Clinical Trials (IRCT20190917044807N1). This study was performed under the Declaration of Helsinki 1975 (Revised 2013). The study procedures and any possible risks during the study were explained to the participants’ parents and they signed a written consent form. The baseline characteristics of both experimental and control groups are provided in Table 1.
.jpg)
Experimental protocol
The experimental group performed a set of given gymnastic training 3 sessions per week for a total of 8 weeks. Each session was 45 minutes, including a 10-minute warm-up, 30 minutes of the main exercise, and a 5-minute cooldown. The main part of the training included 30 s continuous jump (30 s CJ), 30 s vertical continuous jump on a box (30 s VCJB), running jumping rolling (RJR) (Figure 1), and specific aerobic gymnast anaerobic test (SAGAT) (Figure 2) (27, 28).
.jpg)
.jpg)
Note 1: Each repetition was as follows: after the start command, the subject runs 4 meters toward Point B to perform jumping over a box with a height of 50 cm, then continues to run toward Point C to perform front-rolling. Following the rolling, the subject must change the direction and run fast to reach Point D to jump over the box, then run to Point E to perform front-rolling, and then at the end, run to the starting point (Point A). After completing 5 repetitions (first set), the subject recovers for 3 min and then starts the second set.
Note 2: After the start command, the subject taps the floor and runs 7 meters toward Point B. At this point, the subject taps the floor again and returns 2 meters toward Point A (Line 1). At this point, the subject performs tuck jumps, push-ups, and sit-ups exercises, each task for one time, and then returns to Point B and taps the floor. This is the end of the first repetition and the start of the second repetition. The subject runs 7 meters to Point A, taps the floor, returns 2 meters toward Point B (Line 2), performs the exercises described above, and then returns to Point A and taps the floor to end the second repetition and start the third repetition. This pattern continues until a total of 6 repetitions are completed.
Anthropometrical measurements
The participants’ heights were measured using a stadiometer with an error coefficient of 1% cm (SECA213; SECA, Hamburg, Germany). Their weight was measured using a portable scale with an error coefficient of 1% kg (H20B; Biospace, Seoul, Korea). To measure waist and hip circumference, the subjects were asked to stand up straight and breathe out. The smallest circumference between the umbilicus and the xiphoid process was considered waist and the largest circumference around the buttocks was considered hip. These circumferences were measured by measuring tape.
Measurements of body fat percentage, body fat weight, lean body weight, and body mass index
Harpenden caliper was applied to tight (quadriceps), chest (pectoral), and belly (abdomen), and Jackson/Pollock 3-Site equation was used to predict body fat percentage (BF%). An online body composition calculator was used to obtain the BF%. Body fat weight (BFW) and lean body weight (LBW) were calculated as Equation 1 (26):

BMI in children and adults is calculated in one way. This calculation is done according to the Equation 2:
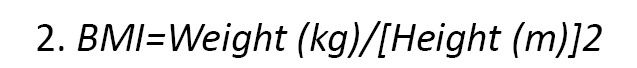
However, the BMI for children is expressed in percentages. Recommended BMI for boys aged 2-19 years are as follows:
>95th percentile: overweight;
85th to <95th percentile: risk of overweight;
<5th percentile: Underweight.
Maximal oxygen consumption measurement
As subjects were children in the age range of 8 to 12 years, a modified Balke protocol was used to evaluate their VO2 max (29). This continuous protocol is well-suited for the unfit and obese children (29). After warming up, each subject performed the modified Balke protocol, which progressively increases the grade from 2% to more than 10% at 2% increments each for 1 min until the subject cannot maintain a constant speed of 3.5 mph and reaches exhaustion (29). The VO2 max was also measured before and after 8 weeks in both groups.
Resting metabolic rate measurement
RMR test was administered for all subjects before noon between 9:00 and 11:00. The RMR was determined by a gas analyzer system via the open-circuit technique while the subject was sitting (30). After entering the laboratory, subjects rested in a chair for 15 min in an isolated temperature-controlled room (21°C to 24°C). After the first 15 min rest, the second 15 min started and subjects were fitted with a Hans Rudolf face mask which was connected to the gas analyzer system. The RMR was obtained by Equation 3 using the average of the last 10 min of the measurement period (30).

3. RMR=3.941 [VO2()]+1.106 [VCO2 ()]=Kcal/min
Saliva brain-driven neurotrophic factor and vascular endothelial growth factor measurement
Saliva samples were collected between 09:00 and 11:00. The subjects should not consume anything and brush their teeth before the sample collection. They also should rinse their mouths with water and then swallow to increase hydration (31). Saliva samples were collected via unstimulated passive drool over 5 min, where the children dripped saliva through a 5 cm plastic straw into a pre-weighed polypropylene cryovial tube (5 mL capacity). Saliva was carefully dripped into the collecting tubes with minimal orofacial movement. After collection, the samples were analyzed in the laboratory (31).
Human BDNF PicoKine™ ELISA Kit (Catalog No. EK0307; R&D Systems, Austria) and Human VEGF PicoKine™ ELISA Kit (Catalog No.: EK0539; R&D Systems, Austria) were used for measuring BDNF and VEGF, respectively. Collected saliva samples were centrifuged for 15 min at 4000 rpm. The evaluation was performed according to the manufacturer’s instructions for the use of buffers, diluents, and materials. The analysis of BDNF and VEGF was performed using a sandwich enzyme-linked immunosorbent assay. Fluorescence was measured at 450 nm with a microplate reader.
2.8. Statistical Analysis
The data are expressed as Mean±SD. All analyses were performed using the SPSS software, version 23. The Kolmogorov-Smirnov test was used to test the normality of the distribution. Independent and paired t tests were used to examine significant differences between and within groups, respectively. A value of P<0.05 was considered statistically significant.
3. Results
Subjects’ characteristics and any of the dependent variables between the two groups were not significantly different at the baseline (P≥0.05) (Table 2).
.jpg)
There were significant reductions in weight, waist-hip ratio, BF%, BFW, and LBW after 8 weeks of training in the experimental group (P<0.05) but not in the control group (P>0.05).
According to Table 2, VO2 max level significantly increased (P=0.03), while maximal heart rate and BMI decreased, respectively (P=0.01, P=0.03) in the experimental group from pretest to posttest. In addition, there was a significant difference in posttest maximal heart rate between the experimental and the control group (P=0.04) (Table 2).
According to Figure 3, the level of BDNF increased significantly after 8 weeks of training (P=0.003) and there was a significant difference in the posttest BDNF level between experimental and control groups (P=0.03).
.jpg)
The level of VEGF increased after the 8-week intervention, however, it was not significant (P=0.179). No significant difference was observed in the posttest VEGF level between the experimental and the control group (P=0.55) (Figure 4).
.jpg)
4. Discussion
Our study results suggest that regular anaerobic gymnastics exercises increase BDNF production and improve body composition, VO2 max, and maximal heart rate in obese children. A previous study observed that after the infusion of sodium-lactate, BDNF increases and provides an increase in blood lactate without metabolic acidosis, which is accompanied by high-intensity and lactate exercise (25). Also, physical activity increases the expression of growth factors, such as BDNF and VEGF. These factors promote the production of neurons and have an effect on cardiorespiratory indices and body composition (21, 32, 33); however, there are contradictions in the results (21).
Gymnastics training is becoming a very popular and basic exercise among children and is a highly energy-demanding physical activity. Therefore, the body requires high energy expenditure and oxygen during gymnastic training, leading to serial hypoxia and hypoglycemia. The resulted hypoxia and hypoglycemia stimulate the synthesis of hypoxia-inducible factor 1-alpha (HIF1A) and sirtuin proteins while factors, such as BDNF, nerve growth factor (NGF), and VEGF are produced under the stimulation of these proteins (34). Recent studies have demonstrated that increased levels of BDNF after exercise lead to increased oxidation of glucose and triglycerides, resulting in increased body temperature, energy, and oxygen consumption (35). In the present study, weight, BF%, and BFW decreased significantly after 8 weeks of AGT; this result is consistent with previous studies (35, 36). Furthermore, the waist-hip ratio showed a significant reduction in the experimental group, which might suggest that the subcutaneous, as well as the visceral abdominal adipose tissues are also decreased in this group as a result of AGT. Furthermore, after 8 weeks of AGT, the level of VEGF in the experimental group increased, however, not significantly. This result is contradictory to previous studies as they maintain that overweight and obese individuals display elevated serum VEGF levels (12, 13, 37). They explained that adipose tissue as an endocrine organ produces VEGF in a considerable amount and adipose tissue is highly plastic and requires vascularization to expand (11). This extra adipose tissue causes an increase in the level of VEGF in obese individuals and weight loss and reduction of adipose tissue causes a decrease in the level of VEGF in obese individuals (37). In the present study, a slight increase and no decline in the level of VEGF was observed that might be related to the effects of the exercise on this factor. This is because exercise increases both skeletal muscle mass and blood circulation and both processes require the up-regulation of angiogenesis. The value of MHR in the progressive modified Balke test was also less than the pretest, and its changes were significant. The Heart rate is controlled by the autonomic nervous system (38) and increasing parasympathetic activity decreases heart rate. Even though we did not assess parasympathetic activity, there is a possibility that vagal activity increases after 8 weeks of AGT used in the present study. VO2 max increased significantly after training in the experimental group. These results indicate amelioration in heart function that is in line with the results of previous studies (39).
There are controversial findings regarding BDNF and its effects and relationship with obesity. Some researchers have reported lower levels of serum or plasma BDNF in obese people compared to normal weight subjects (19), while Roth et al. (21) found higher BDNF serum concentrations in obese compared to lean children and suggested a relationship between BDNF and fat mass. In the present research, we observed notable increases in BDNF levels in the experimental group after the intervention. Also, weight, BF%, and BFW were reduced. Inconsistent findings in this area across different studies might be due to the differences in obesity stages and grades of studied people. For instance, the studies that demonstrated lower levels of BDNF were on children and extremely obese subjects (4, 19). Whereas a study by Roth et al. (21), showed higher levels of BDNF in obese children in the early stage of obesity or overweight have been compared with partially lean children (21) Recently, a neurotrophic hypothesis has suggested that neurotrophins have a different role in the early or late stages of metabolic diseases. Accodringly, neurotrophin levels are high in the early stage of metabolic diseases to compensate and attenuate emerging inflammatory events, but when metabolic disease criteria are developed, the concentration of neurotrophins begins to gradually reduce because of pro-inflammatory cytokines’ effects on the neurotrophins. Therefore, hyponeurotrophinemia appears during the developed stage of the disease (40, 41, 42). It has been observed that increased levels of BDNF after exercise lead to increased oxidation of glucose and triglycerides, resulting in increased body temperature, energy, and oxygen consumption (35).
Our study had some limitations, such as the lack of female participants and the small sample size. To explore practical usage and the mechanisms that appear to increase salivary BDNF and VEGF in children, we suggest using large sample sizes and inclusion of female participants for further investigations.
5. Conclusion
According to our findings, we concluded that weight loss because of lactate-producing gymnastics exercise may cause a high serum concentration of BDNF. High BDNF may help in maintaining a reduced weight after intervention for obesity and may increase the oxidation of fat. The inhibitory effect of weight loss on VEGF may have abolished the stimulatory effect of exercise and prevented a significant increase in VEGF.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information. Moreover, they were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
We would like to thank all the children and their parents who participated in this study.
References
Overweight and obesity are prominent threats to pediatric health and the worldwide prevalence of childhood obesity has dramatically been increasing. Childhood obesity is induced by multiple factors, such as environmental factors, genetics, medical status, and drugs. It is administered by surgical treatment, diet regimen, and exercise (1, 2). Higher physical activity and consequently higher energy expenditure is a beneficial method to prevent childhood obesity (3).
Many studies have demonstrated the beneficial effects of physical activity on resting metabolic rate (RMR), maximal oxygen consumption (VO2 max), body composition, lipid profile, and plasma leptin (4). Additionally, exercise training improves cardiovascular health (5). Also, exercise has positive effects on brain health by increasing the density of capillaries because of angiogenesis, that is, the sprouting of new capillaries from pre-existing vessels, and via activating specific processes that promote synaptic plasticity which is one mechanism through which exercise improves brain function (6). Growth factors with angiogenic and neurotrophic properties, such as vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) are implicated in vascular and neurological repair in both human and animal studies (7, 8).
Vascular permeability and vasodilatation are increased by VEGF and pathogenesis of cardiovascular risk factors, arteriosclerosis, obesity, and metabolism morbidity; meanwhile, mortality is related to VEGF (9, 10). In addition, adipose tissue as an endocrine organ produces considerable amounts of VEGF (11). Previous studies have indicated that overweight and obese individuals show elevated serum VEGF levels (12, 13). Also, studies have found that subjects who lost at least 1.85% of their baseline fat mass in a 12-month exercise intervention experienced significant reductions in some biomarkers of angiogenesis, compared to sedentary controls (14).
The neurotrophin family, including BDNF, is considered a key player in controlling the growth, survival, and preservation of neurons (15). BDNF and its receptors are expressed in the hypothalamus and peripheral tissues, such as smooth and skeletal muscle, liver, pancreas, and adipose tissue (16). BDNF is considered the main constituent of the hypothalamic axis that governs body weight and plays a relevant role in regulating energy homeostasis, glucose metabolism, and eating behavior through the central nervous system (17). Many studies have observed a low-level serum concentration of BDNF in obese people and subjects with metabolic syndrome (18, 19). It has been demonstrated that serum BDNF increases during exercise and muscle-derived BDNF enhances fatty acid oxidation in skeletal muscle while inducing weight loss in children (17, 20). However, Roth et al. (21) maintained that serum BDNF concentration is higher in obese compared to normal-weight children.
It has been suggested that exercise intensity has an effect on blood BDNF and VEGF levels so high-intensity exercises that produces higher blood lactate levels induce a higher increase in plasma BDNF concentrations (22, 23, 24). To understand the mechanisms that are responsible for increasing the BDNF level, Schiffer et al. (25) used a method called lactate clamp; after the infusion of sodium-lactate, BDNF and lactate increased significantly and reached baseline values at the end of the experiment. They reported that blood lactate increases during high-intensity exercise after the infusion of sodium-lactate; however, no metabolic acidosis is observed, suggesting that the mechanism underlying blood BDNF augmentation is lactate per se (25).
Based on the contradictory results of previous studies, this study aims to investigate the effects of 8-week anaerobic gymnastics training (AGT) on salivary growth factors (BDNF and VEGF), RMR, and weight loss in obese children.
2. Materials and Methods
Participants
A total of 30 obese boys in the age ranged 8-12 years who enrolled in the elementary level of gymnastics participated in this study. The number of samples was selected based on the G*Power software and they were randomly divided into experimental and control groups. Obese subjects were diagnosed according to the American Council on Exercise lists (Jackson and Pollock equation for 3-point subcutaneous fat measurement considering the fat percentage of 26 and above as obesity) (26) without concomitant diseases and the body mass index (BMI) expressed in percentage. Children above the 95th percentile were considered obese. The exclusion criteria included evidence of any disease, drug therapy, structural abnormality, and prohibition of exercise testing. The study protocol was approved by the Ethics Committee of Ardabil University of Medical Science (IR.ARUMS.REC.1397.290) and the Iranian Registry of Clinical Trials (IRCT20190917044807N1). This study was performed under the Declaration of Helsinki 1975 (Revised 2013). The study procedures and any possible risks during the study were explained to the participants’ parents and they signed a written consent form. The baseline characteristics of both experimental and control groups are provided in Table 1.
.jpg)
Experimental protocol
The experimental group performed a set of given gymnastic training 3 sessions per week for a total of 8 weeks. Each session was 45 minutes, including a 10-minute warm-up, 30 minutes of the main exercise, and a 5-minute cooldown. The main part of the training included 30 s continuous jump (30 s CJ), 30 s vertical continuous jump on a box (30 s VCJB), running jumping rolling (RJR) (Figure 1), and specific aerobic gymnast anaerobic test (SAGAT) (Figure 2) (27, 28).
.jpg)
.jpg)
Note 1: Each repetition was as follows: after the start command, the subject runs 4 meters toward Point B to perform jumping over a box with a height of 50 cm, then continues to run toward Point C to perform front-rolling. Following the rolling, the subject must change the direction and run fast to reach Point D to jump over the box, then run to Point E to perform front-rolling, and then at the end, run to the starting point (Point A). After completing 5 repetitions (first set), the subject recovers for 3 min and then starts the second set.
Note 2: After the start command, the subject taps the floor and runs 7 meters toward Point B. At this point, the subject taps the floor again and returns 2 meters toward Point A (Line 1). At this point, the subject performs tuck jumps, push-ups, and sit-ups exercises, each task for one time, and then returns to Point B and taps the floor. This is the end of the first repetition and the start of the second repetition. The subject runs 7 meters to Point A, taps the floor, returns 2 meters toward Point B (Line 2), performs the exercises described above, and then returns to Point A and taps the floor to end the second repetition and start the third repetition. This pattern continues until a total of 6 repetitions are completed.
Anthropometrical measurements
The participants’ heights were measured using a stadiometer with an error coefficient of 1% cm (SECA213; SECA, Hamburg, Germany). Their weight was measured using a portable scale with an error coefficient of 1% kg (H20B; Biospace, Seoul, Korea). To measure waist and hip circumference, the subjects were asked to stand up straight and breathe out. The smallest circumference between the umbilicus and the xiphoid process was considered waist and the largest circumference around the buttocks was considered hip. These circumferences were measured by measuring tape.
Measurements of body fat percentage, body fat weight, lean body weight, and body mass index
Harpenden caliper was applied to tight (quadriceps), chest (pectoral), and belly (abdomen), and Jackson/Pollock 3-Site equation was used to predict body fat percentage (BF%). An online body composition calculator was used to obtain the BF%. Body fat weight (BFW) and lean body weight (LBW) were calculated as Equation 1 (26):

BMI in children and adults is calculated in one way. This calculation is done according to the Equation 2:
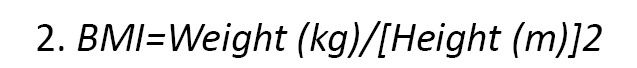
However, the BMI for children is expressed in percentages. Recommended BMI for boys aged 2-19 years are as follows:
>95th percentile: overweight;
85th to <95th percentile: risk of overweight;
<5th percentile: Underweight.
Maximal oxygen consumption measurement
As subjects were children in the age range of 8 to 12 years, a modified Balke protocol was used to evaluate their VO2 max (29). This continuous protocol is well-suited for the unfit and obese children (29). After warming up, each subject performed the modified Balke protocol, which progressively increases the grade from 2% to more than 10% at 2% increments each for 1 min until the subject cannot maintain a constant speed of 3.5 mph and reaches exhaustion (29). The VO2 max was also measured before and after 8 weeks in both groups.
Resting metabolic rate measurement
RMR test was administered for all subjects before noon between 9:00 and 11:00. The RMR was determined by a gas analyzer system via the open-circuit technique while the subject was sitting (30). After entering the laboratory, subjects rested in a chair for 15 min in an isolated temperature-controlled room (21°C to 24°C). After the first 15 min rest, the second 15 min started and subjects were fitted with a Hans Rudolf face mask which was connected to the gas analyzer system. The RMR was obtained by Equation 3 using the average of the last 10 min of the measurement period (30).

3. RMR=3.941 [VO2()]+1.106 [VCO2 ()]=Kcal/min
Saliva brain-driven neurotrophic factor and vascular endothelial growth factor measurement
Saliva samples were collected between 09:00 and 11:00. The subjects should not consume anything and brush their teeth before the sample collection. They also should rinse their mouths with water and then swallow to increase hydration (31). Saliva samples were collected via unstimulated passive drool over 5 min, where the children dripped saliva through a 5 cm plastic straw into a pre-weighed polypropylene cryovial tube (5 mL capacity). Saliva was carefully dripped into the collecting tubes with minimal orofacial movement. After collection, the samples were analyzed in the laboratory (31).
Human BDNF PicoKine™ ELISA Kit (Catalog No. EK0307; R&D Systems, Austria) and Human VEGF PicoKine™ ELISA Kit (Catalog No.: EK0539; R&D Systems, Austria) were used for measuring BDNF and VEGF, respectively. Collected saliva samples were centrifuged for 15 min at 4000 rpm. The evaluation was performed according to the manufacturer’s instructions for the use of buffers, diluents, and materials. The analysis of BDNF and VEGF was performed using a sandwich enzyme-linked immunosorbent assay. Fluorescence was measured at 450 nm with a microplate reader.
2.8. Statistical Analysis
The data are expressed as Mean±SD. All analyses were performed using the SPSS software, version 23. The Kolmogorov-Smirnov test was used to test the normality of the distribution. Independent and paired t tests were used to examine significant differences between and within groups, respectively. A value of P<0.05 was considered statistically significant.
3. Results
Subjects’ characteristics and any of the dependent variables between the two groups were not significantly different at the baseline (P≥0.05) (Table 2).
.jpg)
There were significant reductions in weight, waist-hip ratio, BF%, BFW, and LBW after 8 weeks of training in the experimental group (P<0.05) but not in the control group (P>0.05).
According to Table 2, VO2 max level significantly increased (P=0.03), while maximal heart rate and BMI decreased, respectively (P=0.01, P=0.03) in the experimental group from pretest to posttest. In addition, there was a significant difference in posttest maximal heart rate between the experimental and the control group (P=0.04) (Table 2).
According to Figure 3, the level of BDNF increased significantly after 8 weeks of training (P=0.003) and there was a significant difference in the posttest BDNF level between experimental and control groups (P=0.03).
.jpg)
The level of VEGF increased after the 8-week intervention, however, it was not significant (P=0.179). No significant difference was observed in the posttest VEGF level between the experimental and the control group (P=0.55) (Figure 4).
.jpg)
4. Discussion
Our study results suggest that regular anaerobic gymnastics exercises increase BDNF production and improve body composition, VO2 max, and maximal heart rate in obese children. A previous study observed that after the infusion of sodium-lactate, BDNF increases and provides an increase in blood lactate without metabolic acidosis, which is accompanied by high-intensity and lactate exercise (25). Also, physical activity increases the expression of growth factors, such as BDNF and VEGF. These factors promote the production of neurons and have an effect on cardiorespiratory indices and body composition (21, 32, 33); however, there are contradictions in the results (21).
Gymnastics training is becoming a very popular and basic exercise among children and is a highly energy-demanding physical activity. Therefore, the body requires high energy expenditure and oxygen during gymnastic training, leading to serial hypoxia and hypoglycemia. The resulted hypoxia and hypoglycemia stimulate the synthesis of hypoxia-inducible factor 1-alpha (HIF1A) and sirtuin proteins while factors, such as BDNF, nerve growth factor (NGF), and VEGF are produced under the stimulation of these proteins (34). Recent studies have demonstrated that increased levels of BDNF after exercise lead to increased oxidation of glucose and triglycerides, resulting in increased body temperature, energy, and oxygen consumption (35). In the present study, weight, BF%, and BFW decreased significantly after 8 weeks of AGT; this result is consistent with previous studies (35, 36). Furthermore, the waist-hip ratio showed a significant reduction in the experimental group, which might suggest that the subcutaneous, as well as the visceral abdominal adipose tissues are also decreased in this group as a result of AGT. Furthermore, after 8 weeks of AGT, the level of VEGF in the experimental group increased, however, not significantly. This result is contradictory to previous studies as they maintain that overweight and obese individuals display elevated serum VEGF levels (12, 13, 37). They explained that adipose tissue as an endocrine organ produces VEGF in a considerable amount and adipose tissue is highly plastic and requires vascularization to expand (11). This extra adipose tissue causes an increase in the level of VEGF in obese individuals and weight loss and reduction of adipose tissue causes a decrease in the level of VEGF in obese individuals (37). In the present study, a slight increase and no decline in the level of VEGF was observed that might be related to the effects of the exercise on this factor. This is because exercise increases both skeletal muscle mass and blood circulation and both processes require the up-regulation of angiogenesis. The value of MHR in the progressive modified Balke test was also less than the pretest, and its changes were significant. The Heart rate is controlled by the autonomic nervous system (38) and increasing parasympathetic activity decreases heart rate. Even though we did not assess parasympathetic activity, there is a possibility that vagal activity increases after 8 weeks of AGT used in the present study. VO2 max increased significantly after training in the experimental group. These results indicate amelioration in heart function that is in line with the results of previous studies (39).
There are controversial findings regarding BDNF and its effects and relationship with obesity. Some researchers have reported lower levels of serum or plasma BDNF in obese people compared to normal weight subjects (19), while Roth et al. (21) found higher BDNF serum concentrations in obese compared to lean children and suggested a relationship between BDNF and fat mass. In the present research, we observed notable increases in BDNF levels in the experimental group after the intervention. Also, weight, BF%, and BFW were reduced. Inconsistent findings in this area across different studies might be due to the differences in obesity stages and grades of studied people. For instance, the studies that demonstrated lower levels of BDNF were on children and extremely obese subjects (4, 19). Whereas a study by Roth et al. (21), showed higher levels of BDNF in obese children in the early stage of obesity or overweight have been compared with partially lean children (21) Recently, a neurotrophic hypothesis has suggested that neurotrophins have a different role in the early or late stages of metabolic diseases. Accodringly, neurotrophin levels are high in the early stage of metabolic diseases to compensate and attenuate emerging inflammatory events, but when metabolic disease criteria are developed, the concentration of neurotrophins begins to gradually reduce because of pro-inflammatory cytokines’ effects on the neurotrophins. Therefore, hyponeurotrophinemia appears during the developed stage of the disease (40, 41, 42). It has been observed that increased levels of BDNF after exercise lead to increased oxidation of glucose and triglycerides, resulting in increased body temperature, energy, and oxygen consumption (35).
Our study had some limitations, such as the lack of female participants and the small sample size. To explore practical usage and the mechanisms that appear to increase salivary BDNF and VEGF in children, we suggest using large sample sizes and inclusion of female participants for further investigations.
5. Conclusion
According to our findings, we concluded that weight loss because of lactate-producing gymnastics exercise may cause a high serum concentration of BDNF. High BDNF may help in maintaining a reduced weight after intervention for obesity and may increase the oxidation of fat. The inhibitory effect of weight loss on VEGF may have abolished the stimulatory effect of exercise and prevented a significant increase in VEGF.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles are considered in this article. The participants were informed about the purpose of the research and its implementation stages. They were also assured about the confidentiality of their information. Moreover, they were free to leave the study whenever they wished, and if desired, the research results would be available to them.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
We would like to thank all the children and their parents who participated in this study.
References
- Bennett B, Sothern MS. Diet, exercise, behavior: The promise and limits of lifestyle change. Seminars in Pediatric Surgery. 2009; 18(3):152–8. [DOI:10.1053/j.sempedsurg.2009.04.005] [PMID] [PMCID]
- Güngör NK. Overweight and obesity in children and adolescents. Journal of Clinical Research in Pediatric Endocrinology. 2014; 6(3):129-43. [DOI:10.4274/jcrpe.1471] [PMID] [PMCID]
- Han JC, Lawlor DA, Kimm SY. Childhood obesity. The Lancet. 2010; 375(9727):1737-48. [DOI:10.1016/S0140-6736(10)60171-7]
- Gutin B, Ramsey L, Barbeau P, Cannady W, Ferguson M, Litaker M, et al. Plasma leptin concentrations in obese children: Changes during 4-mo periods with and without physical training. The American Journal of Clinical Nutrition. 1999; 69(3):388-94. [PMID]
- Lee DC, Sui X, Ortega FB, Kim YS, Church TS, Winett RA, et al. Comparisons of leisure-time physical activity and cardiorespiratory fitness as predictors of all-cause mortality in men and women. British Journal of Sports Medicine. 2011; 45(6):504-10. [DOI:10.1136/bjsm.2009.066209] [PMID]
- Ding YH, Li J, Zhou Y, Rafols JA, Clark JC, Ding Y. Cerebral angiogenesis and expression of angiogenic factors in aging rats after exercise. Current Neurovascular Research. 2006; 3(1):15-23. [PMID]
- Kermani P, Rafii D, Jin DK, Whitlock P, Schaffer W, Chiang A, et al. Neurotrophins promote revascularization by local recruitment of TrkB+ endothelial cells and systemic mobilization of hematopoietic progenitors. The Journal of Clinical Investigation. 2005; 115(3):653-63. [PMID] [PMCID]
- Spartano NL, Davis-Plourde KL, Himali JJ, Murabito JM, Vasan RS, Beiser AS, et al. Self-reported physical activity and relations to growth and neurotrophic factors in diabetes mellitus: The framingham offspring study. Journal of Diabetes Research. 2019; 2019:2718465. [DOI:10.1155/2019/2718465] [PMID] [PMCID]
- Paillard T, Rolland Y, de Souto Barreto P. Protective effects of physical exercise in Alzheimer’s disease and Parkinson’s disease: A narrative review. Journal of Clinical Neurology (Seoul, Korea). 2015; 11(3):212-9. [PMID]
- Ferrara N. Vascular endothelial growth factor and the regulation of angiogenesis. Recent Progress in Hormone Research. 2000; 55:15-36. [PMID]
- Cao Y. Angiogenesis modulates adipogenesis and obesity. The Journal of Clinical Investigation. 2007; 117(9):2362-8. [PMID] [PMCID]
- García de la Torre N, Rubio MA, Bordiú E, Cabrerizo L, Aparicio E, Hernández C, et al. Effects of weight loss after bariatric surgery for morbid obesity on vascular endothelial growth factor-A, adipocytokines, and insulin. The Journal of Clinical Endocrinology and Metabolism. 2008; 93(11):4276-81. [PMID]
- Silha JV, Krsek M, Sucharda P, Murphy LJ. Angiogenic factors are elevated in overweight and obese individuals. International Journal of Obesity (2005). 29(11):1308–14. [PMID]
- Duggan C, Xiao L, Wang CY, McTiernan A. Effect of a 12-month exercise intervention on serum biomarkers of angiogenesis in postmenopausal women: A randomized controlled trial. Cancer epidemiology, Biomarkers & Prevention : A Publication of the American Association for Cancer Research, Cosponsored by The American Society of Preventive Oncology. 2014; 23(4):648-57. [PMID] [PMCID]
- Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: A key factor with multipotent impact on brain signaling and synaptic plasticity. Cellular and Molecular Neurobiology. 2018; 38(3):579-93. [PMID] [PMCID]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species.The International Journal of Neuropsychopharmacology. 2011; 14(3):347-53. [PMID]
- Cho HC, Kim J, Kim S, Son YH, Lee N, Jung SH. The concentrations of serum, plasma and platelet BDNF are all increased by treadmill VO2max performance in healthy college men. Neuroscience Letters. 2012; 519(1):78-83. [DOI:10.1016/j.neulet.2012.05.025] [PMID]
- Araki S, Yamamoto Y, Dobashi K, Asayama K, Kusuhara K. Decreased plasma levels of brain-derived neurotrophic factor and its relationship with obesity and birth weight in obese Japanese children. Obesity Research & Clinical Practice. 2014; 8(1):e63-9. [DOI:10.1016/j.orcp.2012.07.003] [PMID]
- El-Gharbawy AH, Adler-Wailes DC, Mirch MC, Theim KR, Ranzenhofer L, Tanofsky-Kraff M, et al. Serum brain-derived neurotrophic factor concentrations in lean and overweight children and adolescents. The Journal of Clinical Endocrinology and Metabolism. 2006; 91(9):3548-52. [DOI:10.1210/jc.2006-0658] [PMID]
- Habibian M, Khosravi H, Farzanegi P. [The effects of 8 weeks of vitamin C intake and regular aerobic exercise on serum brain-derived neurotrophic factor and insulin-like growth factor-1 levels in obese girls (Prsian)]. Iranian Journal of Nutrition Sciences & Food Technology. 2016; 11(3):21-30. [Link]
- Roth CL, Elfers C, Gebhardt U, Müller HL, Reinehr T. Brain-derived neurotrophic factor and its relation to leptin in obese children before and after weight loss. Metabolism. 2013; 62(2):226-34. [PMID]
- Ferris LT, Williams JS, Shen CL. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Medicine and Science in Sports and Exercise. 2007; 39(4):728-34. [DOI:10.1249/mss.0b013e31802f04c7] [PMID]
- Gold SM, Schulz KH, Hartmann S, Mladek M, Lang UE, Hellweg R, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. Journal of Neuroimmunology. 2003; 138(1-2):99-105. [DOI:10.1016/S0165-5728(03)00121-8]
- Rojas Vega S, Strüder HK, Vera Wahrmann B, Schmidt A, Bloch W, Hollmann W. Acute BDNF and cortisol response to low intensity exercise and following ramp incremental exercise to exhaustion in humans. Brain Research. 2006; 1121(1):59-65. [DOI:10.1016/j.brainres.2006.08.105] [PMID]
- Schiffer T, Schulte S, Sperlich B, Achtzehn S, Fricke H, Strüder HK. Lactate infusion at rest increases BDNF blood concentration in humans. Neuroscience Letters. 2011; 488(3):234-7. [DOI:10.1016/j.neulet.2010.11.035] [PMID]
- Jackson AS, Pollock ML. Generalized equations for predicting body density of men. The British Journal of Nutrition. 1978; 40(3):497-504. [PMID]
- Alves CR, Borelli MT, Paineli Vde S, Azevedo Rde A, Borelli CC, Lancha Junior AH, et al. Development of a specific anaerobic field test for aerobic gymnastics. PloS One. 2015; 10(4):e0123115. [PMID] [PMCID]
- Čular D, Ivančev V, Zagatto AM, Milić M, Beslija T, Sellami M, et al. Validity and reliability of the 30-s continuous jump for anaerobic power and capacity assessment in combat sport. Frontiers in Physiology. 2018; 9:543. [PMID] [PMCID]
- Washington RL, Bricker JT, Alpert BS, Daniels SR, Deckelbaum RJ, Fisher EA, et al. Guidelines for exercise testing in the pediatric age group. From the Committee on Atherosclerosis and Hypertension in Children, Council on Cardiovascular Disease in the Young, the American Heart Association. Circulation. 1994; 90(4):2166-79. [PMID]
- Consolazio CF, Johnson RE, Pecora LJ. Physiological Measurements of Metabolic Function in Man. New York: McGraw-Hill; 1963. [Link]
- Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: A new laboratory tool for diagnostic and basic investigation. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2007; 383(1-2):30-40. [DOI:10.1016/j.cca.2007.04.011] [PMID]
- El-Alameey IR, Ahmed HH, Abushady MM. Role of lifestyle intervention program in regulating brain derived neurotrophic factor in obese children with metabolic syndrome components. Biomedical and Pharmacology Journal. 2019; 12(3):1317-28. [DOI:10.13005/bpj/1760]
- Fediani Y, Dewi MR, Irfannuddin M, Saleh MI, Dhaini S. The effect of regular aerobic exercise on urinary brain-derived neurotrophic factor in children. Paediatrica Indonesiana. 2014; 54(6):351-7. [DOI:10.14238/pi54.6.2014.351-7]
- Jones NM, Lee EM, Brown TG, Jarrott B, Beart PM. Hypoxic preconditioning produces differential expression of hypoxia-inducible factor-1α (HIF-1α) and its regulatory enzyme HIF prolyl hydroxylase 2 in neonatal rat brain. Neuroscience Letters. 2006; 404(1-2):72-7. [PMID]
- Huang T, Larsen KT, Ried-Larsen M, Møller NC, Andersen LB. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scandinavian Journal of Medicine & Science in Sports. 2014; 24(1):1-10. [DOI:10.1111/sms.12069] [PMID]
- Meyer AA, Kundt G, Lenschow U, Schuff-Werner P, Kienast W. Improvement of early vascular changes and cardiovascular risk factors in obese children after a six-month exercise program. Journal of The American College of Cardiology. 2006; 48(9):1865-70. [DOI:10.1016/j.jacc.2006.07.035] [PMID]
- Duggan C, Tapsoba Jde D, Wang CY, McTiernan A. Dietary weight loss and exercise effects on serum biomarkers of angiogenesis in overweight postmenopausal women: A randomized controlled trial. Cancer Research. 2016; 76(14):4226-35. [DOI:10.1158/0008-5472.CAN-16-0399] [PMID] [PMCID]
- Almeida MB, Araújo CGS. Effects of aerobic training on heart rate. Revista Brasileira de Medicina do Esporte. 2003; 9(2):113-20. [DOI:10.1590/S1517-86922003000200006]
- Gutin B, Barbeau P, Owens S, Lemmon CR, Bauman M, Allison J, et al. Effects of exercise intensity on cardiovascular fitness, total body composition, and visceral adiposity of obese adolescents. The American Journal of Clinical Nutrition. 2002; 75(5):818-26. [PMID]
- Lee IT, Lee WJ, Tsai IC, Liang KW, Lin SY, Wan CJ, et al. Brain-derived neurotrophic factor not associated with metabolic syndrome but inversely correlated with vascular cell adhesion molecule-1 in men without diabetes. Clinica Chimica Acta; International Journal of Clinical Chemistry. 2012; 413(9-10):944-8. [PMID]
- Hristova MG. Metabolic syndrome-from the neurotrophic hypothesis to a theory. Medical Hypotheses. 2013; 81(4):627-34. [DOI:10.1016/j.mehy.2013.07.018] [PMID]
- Marti A, Santos J, Gratacos M, Moreno-Aliaga M, Maiz A, Martinez J, et al. Association between leptin receptor (LEPR) and brain-derived neurotrophic factor (BDNF) gene variants and obesity: A case-control study. Nutritional Neuroscience. 2009; 12(4):183-8. [PMID]
Type of Study: Research Article |
Subject:
Physiology
Received: 2021/10/29 | Accepted: 2021/11/27 | Published: 2022/07/1
Received: 2021/10/29 | Accepted: 2021/11/27 | Published: 2022/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








