Volume 7, Issue 4 (10-2019)
J. Pediatr. Rev 2019, 7(4): 199-210 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemipour M, Samei P, Kelishadi R, Hovsepian S, Hani Tabaei Zavareh N. A Systematic Review on the Risk Factors of Congenital Hypothyroidism. J. Pediatr. Rev 2019; 7 (4) :199-210
URL: http://jpr.mazums.ac.ir/article-1-196-en.html
URL: http://jpr.mazums.ac.ir/article-1-196-en.html
Mahin Hashemipour1 

 , Payam Samei *2
, Payam Samei *2 

 , Roya Kelishadi3
, Roya Kelishadi3 

 , Silva Hovsepian4
, Silva Hovsepian4 

 , Neda Hani Tabaei Zavareh5
, Neda Hani Tabaei Zavareh5 




 , Payam Samei *2
, Payam Samei *2 

 , Roya Kelishadi3
, Roya Kelishadi3 

 , Silva Hovsepian4
, Silva Hovsepian4 

 , Neda Hani Tabaei Zavareh5
, Neda Hani Tabaei Zavareh5 


1- Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.; Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.; Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran. ,payam.sameii@gmail.com
3- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.; Imam Hossein Children's Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
5- Department of Public Health, Massachusetts College of Pharmacy and Health Sciences, Boston, Massachusetts, United States.
2- Isfahan Endocrine and Metabolism Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.; Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran. ,
3- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
4- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.; Imam Hossein Children's Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
5- Department of Public Health, Massachusetts College of Pharmacy and Health Sciences, Boston, Massachusetts, United States.
Full-Text [PDF 616 kb]
(4002 Downloads)
| Abstract (HTML) (9048 Views)
6. Results
Most of the studies have cross-sectional, case-control, and prospective design. Most of them evaluated the possible risk factors for primary CH. Reported risk factors for transient CH were as follows: iodine deficiency or excess, prematurity, old maternal age, male gender, retinopathy of prematurity, twin pregnancy, maternal autoimmune thyroid disease, intrauterine growth retardation, and cesarean delivery (19, 24, 26, 33, 51, 56, 63). Reported risk factors for permanent CH with dysgenesis of the thyroid gland were as follows: female gender, familial history of CH, birth in geographical areas with a high rate of the disease, old maternal age, ethnicity (Caucasians) but not seasonality (5, 15, 42, 50, 56, 61).
Reported risk factors for permanent CH with dyshormonogenesis were the familial history of CH and origin of both parents from the high-risk geographical region (15). In five papers, the effects of risk factors on the TSH level during screening were evaluated (14, 25, 28, 6, 33).
7. Discussion
In this review study, we studied all reported studies in the field of CH risk factors. Most of the reviewed studies were cross-sectional and evaluated the risk factors of primary CH. Few studies were investigating the risk factors of permanent vs. transient or different etiologies of CH. Though some of the reported risk factors for permanent and temporary CH and various etiologies of permanent CH were similar, some of them were specific for the mentioned groups. By considering the reported group differences, we could design more studies for better understanding of different subgroups of CH. As mentioned previously, though there were studies regarding CH-related risk factors (6, 34, 36, 44, 47, 49, 56, 59, 63) there was no comprehensive review in this field. Moreover, for some important risk factors such as seasonality or gender differences, the results of studies were not in agreement. We classified the risk factors in the following categories; known risk factors with enough and appropriate evidence, known risk factors with controversial results, and risk factors with limited evidence which need more evaluations.
The role of some risk factors such as ethnicity, thyroid disorders in families, other birth defects, pre-term and post-term delivery, low- and high-birth weight, parental consanguinity and twin or multiple pregnancies for CH have been clearly determined in many studies (12, 13, 20, 23-25, 32, 33, 45, 48, 52, 54, 55, 62, 65, 66). Though there were also few studies which did not report such an association, almost all of them support the role of above-mentioned risk factors for CH. However, the additive effect of the risk factors for the occurrence of CH should be investigated in future research.
Iodine deficiency or excess (18, 26, 34), gender (31, 17), seasonality (5, 13, 20-22, 30, 67) maternal age (12, 37, 44, 61, 63), type of delivery (28, 62) and maternal anti-thyroid drug use (6, 27) were the risk factors with controversial reports. Though their role as CH-related risk factors has been demonstrated in previous studies, the findings are not conclusive.
Iodine deficiency is one of the most critical risk factors for CH, but by the elimination of iodine deficiency in different countries, it seems that iodine excess is considered as a risk factor for CH (18, 26, 34). Iodine excess could be a result of different factors such as using iodinated salt and different pharmacological agents using for therapeutic or diagnostic procedures in specific disorders.
Satoh et al. in Japan evaluated the rate of thyroid dysfunction in neonates born to mothers who have undergone Hysterosalpingography (HSG) involving an oil-soluble iodinated contrast medium. According to their findings in the thyroid dysfunction group, the median dosage of ethiodized oil was significantly higher than in the normal thyroid function group. They recommended that when infertile women undergo HSG, the administrated dosage of oil-soluble iodinated contrast medium should be reduced to minimize the risk of thyroid dysfunction in fetus or neonates (57).
Previous studies showed an association between gender and CH. Many reports have indicated that CH is frequently found in girls (12, 15, 17, 6, 31, 36, 44, 46, 53, 56). According to previous studies, the female to male ratio was approximately 1.0 among hereditary cases of CH (31). Moreover, this ratio was about 2.0 for the CH cases with both athyreosis and ectopic groups (17). Castanet et al. reported that the female preponderance over males for isolated CH was similar to those with the ectopic thyroid gland or athyreosis (68). Accordingly, the preponderance of female gender for CH is mainly related to thyroid dysgenesis. These results were also reported in another study (15). According to our findings, girls were at higher risk of CH than boys. But there are also studies which did not show such an association (49).
Recently, Rezaeian et al. in Hamedan, Iran studied the potential interactions that could change the effect of gender on congenital hypothyroidism (53). They indicated that odds ratio estimates of CH for investigated factors (except for birth season) did not differ substantially between girls and boys. Similarly, Ng et al. found no significant difference between girls and boys regarding gestation and birth weight in all etiological subgroups such as athyreosis and ectopic groups (48). Rezaeian and colleagues have finally indicated that birth season might act as an interaction to increase the risk of CH in girls (53).
However, it is unclear why girls have a higher incidence rate of CH than boys, while there is no difference in the proportion of other risk factors between them. So, the reasons for gender differences deserve further investigations. The results of the reviewed literature regarding the seasonal relationship were inconsistent, too. Gu et al. in Japan reported that temperature and season had a significant effect on CH. According to them, from January to December, males and females had one and two peaks, respectively (30). In the British Midland, higher incidence of CH was reported in fall between October and December (20).
Some studies did not report any seasonal pattern for CH. Rosenthal et al. observed no seasonal difference in the incidence of CH in the Northwest of England, in Asian families compared with non-Asians (69). No evidence of seasonal variations was reported during the CH screening program in Saudi Arabia and Italy (21, 22). Kaiserman et al. in Israel conducted a 10-year temporal analysis of primary CH; the average monthly incidence showed a small peak in August, but, monthly incidence of CH had no significant periodicity (16).
There were different studies from Iran on this topic, too. Ordookhani et al. reported a significant correlation between winter and CH. Hashemipour et al. reported higher and lower incidence rates of CH in summer and the last month of autumn, respectively (16). Their findings were not similar to others. They suggested that other factors such as exposure to different chemical compounds, seasonal environmental factors, and differences in climate might play a role in the etiology of CH.
In previous studies in Iran, Aminzadeh et al. investigated the association between seasonal changes in temperature and the prevalence of congenital Hypothyroidism (CH) in Southwest Iran and reported that the prevalence of CH had a significant negative correlation with temperature. The odds of being affected increased by 4% for each 1°C drop in temperature (39). Findings of other studies from Iran showed a higher incidence of CH in autumn and winter.
The impact of environmental factors such as climatic conditions and seasonal changes in the incidence of CH is still unclear. In a recent study in Iran, Khanjani et al. for the first time evaluated the effects of several climatic factors such as temperature, humidity, and rainfall on the incidence of CH. They did not find any significant association between CH and climate factors, in Kerman Province, whereas they reported the highest rate of CH in October (autumn) and lowest in June (summer) (67).
It seems that the reported discrepancy may be due to differences in climate, living conditions, and various levels of iodine in different geographical areas. It is also suggested that different environmental and genetic factors could interact with seasonality and consequently could affect the incidence of CH in each region.
Some studies reported advanced maternal age as a risk factor for CH (12, 37, 44). But some of them have reported such an association only for thyroid dysgenesis (64). According to the documents, the maternal age of more than 35 years could be a risk factor for CH (47).
Type of delivery was another conflicting risk factor. McElduff et al. in their investigation among 2031 infants have indicated that TSH levels were greater among babies delivered by cesarean section (28). Rezaeian and colleagues have also reported a higher incidence rate of CH in both emergency and elective cesarean sections (47). Whereas Ordookhani et al. reported that umbilical cord blood TSH and rates of hyperthyrotropinemia are lower in cesarean section than in vaginal deliveries. They showed that povidone-iodine disinfection at delivery has an effect neither on TSH concentrations nor on the rate of hyperthyrotropinemia in the iodine-replete area of Iran (6).
Similarly, Dalili et al. have reported that the frequency of Normal Vaginal Delivery (NVD) was significantly higher in neonates with CH compared to the normal population (49). It seems that different conditions related to the type of delivery, including the iodine condition of the population, method of delivery and using different disinfectant have an impact on the association of type of delivery and CH occurrence.
Some studies reported that maternal anti-thyroid drug use and its pattern could affect thyroid function of neonates (27, 55). Lian et al. in China reported that the risk of abnormal thyroid function of infants whose hyperthyroid mothers did not take anti-thyroid drugs until the third trimester of pregnancy might be increased (26). In one study, using thyroid hormones by mother was not considered as a risk factor for CH.
Some of the reported risk factors, including environmental pollutants (25, 58), dietary component of mothers during prenatal period (41, 64), neonatal jaundice (47), maternal anemia (48), intrauterine growth retardation (6), lower weight gain during pregnancy (60), urbanization (62), parental occupation and education (35, 47), gestational diabetes (6, 46, 59), and smoking (14, 47, 60) have limited evidence. It seems that more studies for investigating the association of the mentioned risk factors with CH are necessary. Of the above-mentioned risk factors, some have high priority, including environmental pollutants, smoking, gestational diabetes, and maternal anemia due to their effectiveness in preventative medicine.
So far, few studies have investigated the effect of environmental factors on CH incidence. Ouhoummane et al. in Canada compared the thyroid function of newborns from 11 municipalities where drinking water was disinfected by Chlorine Dioxide (ClO2) with that of newborns from 15 municipalities using chlorine disinfection. There was no significant increase in the TSH level and rate of CH when all newborns exposed to ClO2 were considered. However, for newborns with low-birth weight, mean TSH level was significantly higher among those exposed to ClO2 than for those in the reference group. They concluded that ClO2 was a risk factor for CH in preterm and low-birth-weight neonates (26). In another study in Iran, Mehrnejat et al. found no significant relationship between nitrate concentration in drinking water and the incidence of CH through linear regression analysis (58).
In two studies, the dietary component of mothers has been reported as risk factors for CH, including Cu deficiency and some other nutritional components (41, 64). It seems that evaluating the association of prenatal dietary components is helpful for identification of CH-related modifiable risk factors. The limitation of the current review was the heterogeneity of papers so that we could not do meta-analysis in this field. The strength of this review was its novelty. There was not any systematic review regarding the risk factors of CH.
8. Conclusions
The findings of the current review provide us basic information about reported CH-related risk factors from different countries. Using this information, we could plan more etiologic studies to investigate the pathogenesis of the disorder, design interventional studies for the known modifiable risk factors, and reduce the rate of CH in our region. Besides, for risk factors with limited evidence, more studies should be performed.
Moreover, the discrepancies between different studies regarding CH-related risk factors may also be due to the interaction of different risk factors in different populations with different genetic background and different environmental factors. Also, neonatal, maternal, and pregnancy-related determinants are responsible for the occurrence of CH, which should be investigated through more complex statistical analysis.
Ethical Considerations
Compliance with ethical guidelines
There are no ethical considerations to be noted in this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed in designing, running, and writing all parts of the research.
Conflicts of interest
The authors declare no conflict of interest.
References
Full-Text: (2878 Views)
1. Context
Congenital Hypothyroidism (CH) is the most common endocrine disorder and causes of preventable mental retardation in children. It is defined as thyroid hormone deficiency at birth (1, 2). It is classified as primary and secondary. Primary causes include defects in thyroid gland development (thyroid dysgenesis) or deficiencies in thyroid hormone synthesis (thyroid dyshormonogenesis) (1, 2).
CH screening program is considered the most practical and effective method of CH diagnosis as the disorder has not any specific signs and symptoms at birth or during neonatal period. It is a routine practice in developed countries and many developing countries. Findings of CH screening from different regions and countries indicate great variability in the incidence and etiology of CH. In accordance with CH screening, etiological factors, and various risk factors of the disorder have been identified and reported in previous studies (3, 4).
Findings of the studies demonstrate the importance of etiological studies for better understanding of the pathogenesis of CH, as well as its related risk factors to conduct further preventative strategies. The investigation of modifiable risk factors for CH is important because of the potential to prevent CH, especially in regions with a high rate of CH.
Based on the current evidence, several individual and environmental factors affect CH such as gender, birth weight, race, age, consanguinity, parental education, type of labor, birth order, twin and drug usage during pregnancy (5-8). It is believed that many other risk factors might influence the occurrence of CH (5-12). Confirming the causality between these risk factors and CH and identifying them might be helpful even in decreasing the incidence of CH. More practically, it can help have a higher index of suspicion for CH in neonates with the identified risk factors.
2. Objective
Though there are different studies in this field, the results are not conclusive enough, and it is suggested that systematically reviewing of CH-related risk factors would provide us more appropriate information for designing our future etiological and preventative research. So we aimed to review the reported CH-related risk factors systematically.
3. Data Sources
In this study, we systematically reviewed all studies which investigated CH risk factors. The protocol of this study was approved by the Ethics Committee of Isfahan University of Medical Sciences. An electronic search was conducted in international electronic databases, including PubMed, Cochrane, Scopus, ISI, Web of Science, Ovid, Science direct, as well as Persian databases such as IranMedex, IranDoc, and Scientific Information Database (SID). The keywords of “congenital hypothyroidism” (Mesh) AND “risk factor” (Mesh) were used in the Title and the abstract. The latest search was conducted on the 29th September 2017.
4. Study Selection
In this review, all types of human studies on the risk factors related to the occurrence or high rate of CH were included without any time limitation. The included articles were in English and Persian. The search was performed without any time limitation until September 2017. Inappropriate or repeated papers were excluded. The titles of all searched articles were reviewed and studied, and repeated items were excluded. Two researchers carefully studied the full text of selected articles and excluded irrelevant papers. A secondary search was conducted from the references of the selected papers.
5. Data Extraction
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used for reporting systematic reviews. The quality of the documents was evaluated independently by two research experts regarding the objective of each study, methods, sample size, sampling method, data collection tool, variable evaluation status, and evaluated target group. Disagreements were resolved by consensus, mutual discussion, and consulting with an expert in the field of CH. From each finally included article, the following information was extracted; authors, place of the study, ethnicity, year of publication, sample size, study design, and reported risk factors.
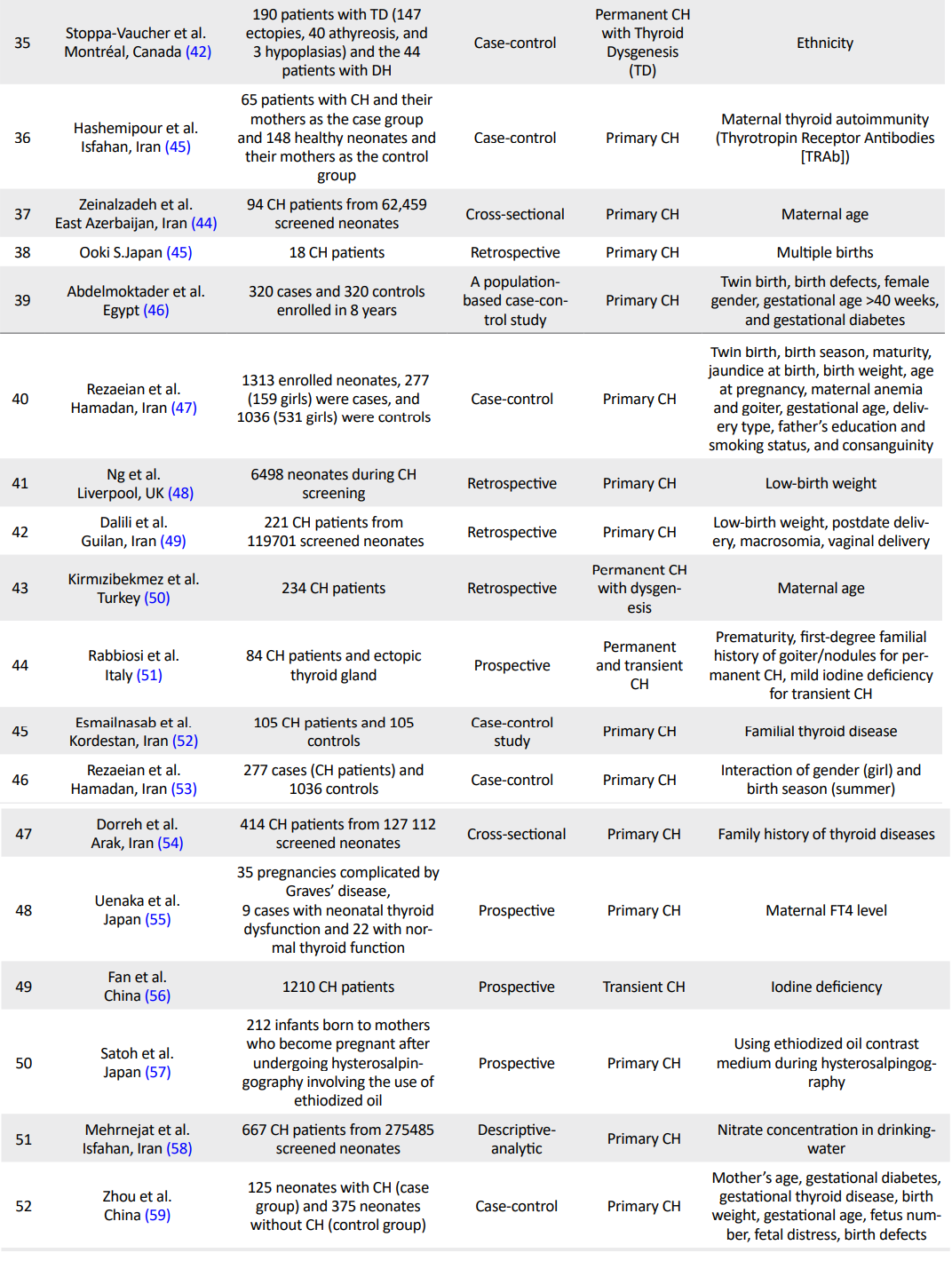
In this review, 373 papers (PubMed: 199; Scopus: 36; ISI: 53, SID: 55, Ovid: 11; Science Direct: 19) were identified through electronic database search. A total of 98 articles were assessed for eligibility, of which 60 qualified articles were selected for final evaluation (Figure 1). Details of all selected studies were presented in Table 1 (5, 8, 9, 12-67).
Congenital Hypothyroidism (CH) is the most common endocrine disorder and causes of preventable mental retardation in children. It is defined as thyroid hormone deficiency at birth (1, 2). It is classified as primary and secondary. Primary causes include defects in thyroid gland development (thyroid dysgenesis) or deficiencies in thyroid hormone synthesis (thyroid dyshormonogenesis) (1, 2).
CH screening program is considered the most practical and effective method of CH diagnosis as the disorder has not any specific signs and symptoms at birth or during neonatal period. It is a routine practice in developed countries and many developing countries. Findings of CH screening from different regions and countries indicate great variability in the incidence and etiology of CH. In accordance with CH screening, etiological factors, and various risk factors of the disorder have been identified and reported in previous studies (3, 4).
Findings of the studies demonstrate the importance of etiological studies for better understanding of the pathogenesis of CH, as well as its related risk factors to conduct further preventative strategies. The investigation of modifiable risk factors for CH is important because of the potential to prevent CH, especially in regions with a high rate of CH.
Based on the current evidence, several individual and environmental factors affect CH such as gender, birth weight, race, age, consanguinity, parental education, type of labor, birth order, twin and drug usage during pregnancy (5-8). It is believed that many other risk factors might influence the occurrence of CH (5-12). Confirming the causality between these risk factors and CH and identifying them might be helpful even in decreasing the incidence of CH. More practically, it can help have a higher index of suspicion for CH in neonates with the identified risk factors.
2. Objective
Though there are different studies in this field, the results are not conclusive enough, and it is suggested that systematically reviewing of CH-related risk factors would provide us more appropriate information for designing our future etiological and preventative research. So we aimed to review the reported CH-related risk factors systematically.
3. Data Sources
In this study, we systematically reviewed all studies which investigated CH risk factors. The protocol of this study was approved by the Ethics Committee of Isfahan University of Medical Sciences. An electronic search was conducted in international electronic databases, including PubMed, Cochrane, Scopus, ISI, Web of Science, Ovid, Science direct, as well as Persian databases such as IranMedex, IranDoc, and Scientific Information Database (SID). The keywords of “congenital hypothyroidism” (Mesh) AND “risk factor” (Mesh) were used in the Title and the abstract. The latest search was conducted on the 29th September 2017.
4. Study Selection
In this review, all types of human studies on the risk factors related to the occurrence or high rate of CH were included without any time limitation. The included articles were in English and Persian. The search was performed without any time limitation until September 2017. Inappropriate or repeated papers were excluded. The titles of all searched articles were reviewed and studied, and repeated items were excluded. Two researchers carefully studied the full text of selected articles and excluded irrelevant papers. A secondary search was conducted from the references of the selected papers.
5. Data Extraction
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) checklist was used for reporting systematic reviews. The quality of the documents was evaluated independently by two research experts regarding the objective of each study, methods, sample size, sampling method, data collection tool, variable evaluation status, and evaluated target group. Disagreements were resolved by consensus, mutual discussion, and consulting with an expert in the field of CH. From each finally included article, the following information was extracted; authors, place of the study, ethnicity, year of publication, sample size, study design, and reported risk factors.
In this review, 373 papers (PubMed: 199; Scopus: 36; ISI: 53, SID: 55, Ovid: 11; Science Direct: 19) were identified through electronic database search. A total of 98 articles were assessed for eligibility, of which 60 qualified articles were selected for final evaluation (Figure 1). Details of all selected studies were presented in Table 1 (5, 8, 9, 12-67).
6. Results
Most of the studies have cross-sectional, case-control, and prospective design. Most of them evaluated the possible risk factors for primary CH. Reported risk factors for transient CH were as follows: iodine deficiency or excess, prematurity, old maternal age, male gender, retinopathy of prematurity, twin pregnancy, maternal autoimmune thyroid disease, intrauterine growth retardation, and cesarean delivery (19, 24, 26, 33, 51, 56, 63). Reported risk factors for permanent CH with dysgenesis of the thyroid gland were as follows: female gender, familial history of CH, birth in geographical areas with a high rate of the disease, old maternal age, ethnicity (Caucasians) but not seasonality (5, 15, 42, 50, 56, 61).
Reported risk factors for permanent CH with dyshormonogenesis were the familial history of CH and origin of both parents from the high-risk geographical region (15). In five papers, the effects of risk factors on the TSH level during screening were evaluated (14, 25, 28, 6, 33).
7. Discussion
In this review study, we studied all reported studies in the field of CH risk factors. Most of the reviewed studies were cross-sectional and evaluated the risk factors of primary CH. Few studies were investigating the risk factors of permanent vs. transient or different etiologies of CH. Though some of the reported risk factors for permanent and temporary CH and various etiologies of permanent CH were similar, some of them were specific for the mentioned groups. By considering the reported group differences, we could design more studies for better understanding of different subgroups of CH. As mentioned previously, though there were studies regarding CH-related risk factors (6, 34, 36, 44, 47, 49, 56, 59, 63) there was no comprehensive review in this field. Moreover, for some important risk factors such as seasonality or gender differences, the results of studies were not in agreement. We classified the risk factors in the following categories; known risk factors with enough and appropriate evidence, known risk factors with controversial results, and risk factors with limited evidence which need more evaluations.
The role of some risk factors such as ethnicity, thyroid disorders in families, other birth defects, pre-term and post-term delivery, low- and high-birth weight, parental consanguinity and twin or multiple pregnancies for CH have been clearly determined in many studies (12, 13, 20, 23-25, 32, 33, 45, 48, 52, 54, 55, 62, 65, 66). Though there were also few studies which did not report such an association, almost all of them support the role of above-mentioned risk factors for CH. However, the additive effect of the risk factors for the occurrence of CH should be investigated in future research.
Iodine deficiency or excess (18, 26, 34), gender (31, 17), seasonality (5, 13, 20-22, 30, 67) maternal age (12, 37, 44, 61, 63), type of delivery (28, 62) and maternal anti-thyroid drug use (6, 27) were the risk factors with controversial reports. Though their role as CH-related risk factors has been demonstrated in previous studies, the findings are not conclusive.
Iodine deficiency is one of the most critical risk factors for CH, but by the elimination of iodine deficiency in different countries, it seems that iodine excess is considered as a risk factor for CH (18, 26, 34). Iodine excess could be a result of different factors such as using iodinated salt and different pharmacological agents using for therapeutic or diagnostic procedures in specific disorders.
Satoh et al. in Japan evaluated the rate of thyroid dysfunction in neonates born to mothers who have undergone Hysterosalpingography (HSG) involving an oil-soluble iodinated contrast medium. According to their findings in the thyroid dysfunction group, the median dosage of ethiodized oil was significantly higher than in the normal thyroid function group. They recommended that when infertile women undergo HSG, the administrated dosage of oil-soluble iodinated contrast medium should be reduced to minimize the risk of thyroid dysfunction in fetus or neonates (57).
Previous studies showed an association between gender and CH. Many reports have indicated that CH is frequently found in girls (12, 15, 17, 6, 31, 36, 44, 46, 53, 56). According to previous studies, the female to male ratio was approximately 1.0 among hereditary cases of CH (31). Moreover, this ratio was about 2.0 for the CH cases with both athyreosis and ectopic groups (17). Castanet et al. reported that the female preponderance over males for isolated CH was similar to those with the ectopic thyroid gland or athyreosis (68). Accordingly, the preponderance of female gender for CH is mainly related to thyroid dysgenesis. These results were also reported in another study (15). According to our findings, girls were at higher risk of CH than boys. But there are also studies which did not show such an association (49).
Recently, Rezaeian et al. in Hamedan, Iran studied the potential interactions that could change the effect of gender on congenital hypothyroidism (53). They indicated that odds ratio estimates of CH for investigated factors (except for birth season) did not differ substantially between girls and boys. Similarly, Ng et al. found no significant difference between girls and boys regarding gestation and birth weight in all etiological subgroups such as athyreosis and ectopic groups (48). Rezaeian and colleagues have finally indicated that birth season might act as an interaction to increase the risk of CH in girls (53).
However, it is unclear why girls have a higher incidence rate of CH than boys, while there is no difference in the proportion of other risk factors between them. So, the reasons for gender differences deserve further investigations. The results of the reviewed literature regarding the seasonal relationship were inconsistent, too. Gu et al. in Japan reported that temperature and season had a significant effect on CH. According to them, from January to December, males and females had one and two peaks, respectively (30). In the British Midland, higher incidence of CH was reported in fall between October and December (20).
Some studies did not report any seasonal pattern for CH. Rosenthal et al. observed no seasonal difference in the incidence of CH in the Northwest of England, in Asian families compared with non-Asians (69). No evidence of seasonal variations was reported during the CH screening program in Saudi Arabia and Italy (21, 22). Kaiserman et al. in Israel conducted a 10-year temporal analysis of primary CH; the average monthly incidence showed a small peak in August, but, monthly incidence of CH had no significant periodicity (16).
There were different studies from Iran on this topic, too. Ordookhani et al. reported a significant correlation between winter and CH. Hashemipour et al. reported higher and lower incidence rates of CH in summer and the last month of autumn, respectively (16). Their findings were not similar to others. They suggested that other factors such as exposure to different chemical compounds, seasonal environmental factors, and differences in climate might play a role in the etiology of CH.
In previous studies in Iran, Aminzadeh et al. investigated the association between seasonal changes in temperature and the prevalence of congenital Hypothyroidism (CH) in Southwest Iran and reported that the prevalence of CH had a significant negative correlation with temperature. The odds of being affected increased by 4% for each 1°C drop in temperature (39). Findings of other studies from Iran showed a higher incidence of CH in autumn and winter.
The impact of environmental factors such as climatic conditions and seasonal changes in the incidence of CH is still unclear. In a recent study in Iran, Khanjani et al. for the first time evaluated the effects of several climatic factors such as temperature, humidity, and rainfall on the incidence of CH. They did not find any significant association between CH and climate factors, in Kerman Province, whereas they reported the highest rate of CH in October (autumn) and lowest in June (summer) (67).
It seems that the reported discrepancy may be due to differences in climate, living conditions, and various levels of iodine in different geographical areas. It is also suggested that different environmental and genetic factors could interact with seasonality and consequently could affect the incidence of CH in each region.
Some studies reported advanced maternal age as a risk factor for CH (12, 37, 44). But some of them have reported such an association only for thyroid dysgenesis (64). According to the documents, the maternal age of more than 35 years could be a risk factor for CH (47).
Type of delivery was another conflicting risk factor. McElduff et al. in their investigation among 2031 infants have indicated that TSH levels were greater among babies delivered by cesarean section (28). Rezaeian and colleagues have also reported a higher incidence rate of CH in both emergency and elective cesarean sections (47). Whereas Ordookhani et al. reported that umbilical cord blood TSH and rates of hyperthyrotropinemia are lower in cesarean section than in vaginal deliveries. They showed that povidone-iodine disinfection at delivery has an effect neither on TSH concentrations nor on the rate of hyperthyrotropinemia in the iodine-replete area of Iran (6).
Similarly, Dalili et al. have reported that the frequency of Normal Vaginal Delivery (NVD) was significantly higher in neonates with CH compared to the normal population (49). It seems that different conditions related to the type of delivery, including the iodine condition of the population, method of delivery and using different disinfectant have an impact on the association of type of delivery and CH occurrence.
Some studies reported that maternal anti-thyroid drug use and its pattern could affect thyroid function of neonates (27, 55). Lian et al. in China reported that the risk of abnormal thyroid function of infants whose hyperthyroid mothers did not take anti-thyroid drugs until the third trimester of pregnancy might be increased (26). In one study, using thyroid hormones by mother was not considered as a risk factor for CH.
Some of the reported risk factors, including environmental pollutants (25, 58), dietary component of mothers during prenatal period (41, 64), neonatal jaundice (47), maternal anemia (48), intrauterine growth retardation (6), lower weight gain during pregnancy (60), urbanization (62), parental occupation and education (35, 47), gestational diabetes (6, 46, 59), and smoking (14, 47, 60) have limited evidence. It seems that more studies for investigating the association of the mentioned risk factors with CH are necessary. Of the above-mentioned risk factors, some have high priority, including environmental pollutants, smoking, gestational diabetes, and maternal anemia due to their effectiveness in preventative medicine.
So far, few studies have investigated the effect of environmental factors on CH incidence. Ouhoummane et al. in Canada compared the thyroid function of newborns from 11 municipalities where drinking water was disinfected by Chlorine Dioxide (ClO2) with that of newborns from 15 municipalities using chlorine disinfection. There was no significant increase in the TSH level and rate of CH when all newborns exposed to ClO2 were considered. However, for newborns with low-birth weight, mean TSH level was significantly higher among those exposed to ClO2 than for those in the reference group. They concluded that ClO2 was a risk factor for CH in preterm and low-birth-weight neonates (26). In another study in Iran, Mehrnejat et al. found no significant relationship between nitrate concentration in drinking water and the incidence of CH through linear regression analysis (58).
In two studies, the dietary component of mothers has been reported as risk factors for CH, including Cu deficiency and some other nutritional components (41, 64). It seems that evaluating the association of prenatal dietary components is helpful for identification of CH-related modifiable risk factors. The limitation of the current review was the heterogeneity of papers so that we could not do meta-analysis in this field. The strength of this review was its novelty. There was not any systematic review regarding the risk factors of CH.
8. Conclusions
The findings of the current review provide us basic information about reported CH-related risk factors from different countries. Using this information, we could plan more etiologic studies to investigate the pathogenesis of the disorder, design interventional studies for the known modifiable risk factors, and reduce the rate of CH in our region. Besides, for risk factors with limited evidence, more studies should be performed.
Moreover, the discrepancies between different studies regarding CH-related risk factors may also be due to the interaction of different risk factors in different populations with different genetic background and different environmental factors. Also, neonatal, maternal, and pregnancy-related determinants are responsible for the occurrence of CH, which should be investigated through more complex statistical analysis.
Ethical Considerations
Compliance with ethical guidelines
There are no ethical considerations to be noted in this article.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors' contributions
All authors contributed in designing, running, and writing all parts of the research.
Conflicts of interest
The authors declare no conflict of interest.
References
- Rastogi MV, LaFranchi SH. Congenital hypothyroidism. Orphanet Journal of Rare Diseases. 2010; 5:17. [DOI:10.1186/1750-1172-5-17] [PMID] [PMCID]
- Wassner AJ, Brown RS. Congenital hypothyroidism: Recent advances. Current Opinion in Endocrinology, Diabetes and Obesity. 2015; 22(5):407-12. [DOI:10.1097/MED.0000000000000181] [PMID]
- Heindel JJ, Zoeller RT. Thyroid hormone and brain development: translating molecular mechanisms to population risk. Thyroid. 2003; 13(11):1001-10. [DOI:10.1089/105072503770867165] [PMID]
- Delange F. Neonatal screening for congenital hypothyroidism: Results and perspectives. Hormones. 1997; 48(2):51-61. [DOI:10.1159/000185485]
- Deladoëy J, Bélanger N, Van Vliet G. Random variability in congenital hypothyroidism from thyroid dysgenesis over 16 years in Quebec. The Journal of Clinical Endocrinology & Metabolism. 2007; 92(8):3158-61. [DOI:10.1210/jc.2007-0527] [PMID]
- Medda E, Olivieri A, Stazi MA, Grandolfo ME, Fazzini C, Baserga M, et al. Risk factors for congenital hypothyroidism: Results of a population case-control study (1997–2003). European Journal of Endocrinology. 2005; 153(6):765-73. [DOI:10.1530/eje.1.02048] [PMID]
- Pearce MS, Korada M, Day J, Turner S, Allison D, Kibirige M, et al. Increasing incidence, but lack of seasonality, of elevated TSH levels, on newborn screening, in the North of England. Journal of Thyroid Research. 2010; 2010(101948):1-5. [DOI:10.4061/2010/101948] [PMID] [PMCID]
- Hinton CF, Harris KB, Borgfeld L, Drummond-Borg M, Eaton R, Lorey F, et al. Trends in incidence rates of congenital hypothyroidism related to select demographic factors: Data from the United States, California, Massachusetts, New York, and Texas. Pediatrics. 2010; 125(Suppl. 2):S37-47. [DOI:10.1542/peds.2009-1975D] [PMID]
- Mao HQ, Yang RL, Liu ZH. [Correlation of congenital hypothyroidism with birth weight and gestational age in newborn infants (Chinese). Journal of Zhejiang University. Medical Sciences. 2007; 36(4):378-81. [PMID]
- Silva SA, Chagas AJ, Goulart EM, Silva GA, Marçal LV, Gomes MN, et al. Screening for congenital hypothyroidism in extreme premature and/or very low birth weight newborns: The importance of a specific protocol. Journal of Pediatric Endocrinology and Metabolism. 2010; 23(1-2):45-52. [DOI:10.1515/JPEM.2010.23.1-2.45]
- Bijarnia S, Wilcken B, Wiley VC. Newborn screening for congenital hypothyroidism in very-low-birth-weight babies: The need for a second test. Journal of Inherited Metabolic Disease 2011; 34(3):827-33. [DOI:10.1007/s10545-011-9286-8] [PMID]
- Waller DK, Anderson JL, Lorey F, Cunningham GC. Risk factors for congenital hypothyroidism: An investigation of infant’s birth weight, ethnicity, and gender in California, 1990-1998. Teratology. 2000; 62(1):36-41. [DOI:10.1002/1096-9926(200007)62:13.0.CO;2-W]
- Thalhammer O. Screening for congenital hypothyroidism in Austria (author’s transl). Klinische Padiatrie. 1981; 193(5):375-7. [DOI:10.1055/s-2008-1034501] [PMID]
- Meberg A, Marstein S. Smoking during pregnancy-effects on the fetal thyroid function. Acta Paediatrica. 1986; 75(5):762-6. [DOI:10.1111/j.1651-2227.1986.tb10287.x]
- Virtanen M, Mäenpää J, Pikkarainen J, Pitkänen L, Perheentupa J. Aetiology of congenital hypothyroidism in Finland. Acta Paediatrica. 1989; 78(1):67-73. [DOI:10.1111/j.1651-2227.1989.tb10889.x]
- Kaiserman I, Siebner R, Kletter G, Sack J. A ten-year temporal analysis of primary congenital hypothyroidism in Israel. Early Human Development 1991; 26(3):193-201. [DOI:10.1016/0378-3782(91)90159-Z]
- Lorey FW, Cunningham GC. Birth prevalence of primary congenital hypothyroidism by sex and ethnicity. Human Biology. 1992; 64(4):531-8. [PMID]
- Sorcini M, Fazzini C, Olivieri A, Grandolfo M, Medda E, Stazi M, et al. [Neonatal screening in congenital hypothyroidism in Italy. The national registry (Italian)]. Annali Dell’Istituto Superiore Di Sanita. 1994; 30(3):275-87. [PMID]
- Dussault JH. Screening for congenital hypothyroidism. Clinical Obstetrics and Gynecology. 1997; 40(1):117-23. [DOI:10.1097/00003081-199703000-00012] [PMID]
- Hall S, Hutchesson A, Kirk J. Congenital hypothyroidism, seasonality and consanguinity in the West Midlands, England. Acta Paediatrica. 1999; 88(2):212-5. [DOI:10.1111/j.1651-2227.1999.tb01084.x] [PMID]
- Rocchi MB, Perlini C, Ciatti R, Burroni M. Is the birthdate a risk factor for congenital hypothyroidism? A statistical answer based on personal experience. Minerva Pediatrica. 2001; 53(6):531-6. [PMID]
- Henry G, Sobki SH, Othman JM. Screening for congenital hypothyroidism. Saudi Medical Journal. 2002; 23(5):529-35. [PMID]
- Ordookhani A, Mirmiran PA, Hedayati M, Hajipour R, Azizi F. [Screening for congenital hypothyroidism in Tehran and Damavand: An interim report on descriptive and etiologic findings, 1998-2001 (Persian)]. Iranian Journal of Endocrinology and Metabolism. 2002; 4(3):153-60.
- Büyükgebiz A. Congenital hypothyroidism clinical aspects and late consequences. Pediatric Endocrinology Reviews. 2003; 1:185-90. [PMID]
- Ouhoummane N, Levallois P, Gingras S. Thyroid function of newborns and exposure to chlorine dioxide by-products. Archives of Environmental Health. 2004; 59(11):582-7. [DOI:10.1080/00039890409603437] [PMID]
- Ordookhani A, Hedayati M, Mirmiran P, Ainy E, Sabet-Saeedy H, Azizi F. [Etiologies of transient congenital hypothyroidism in Tehran and Damavand (Persian)]. Iranian Journal of Endocrinology and Metabolism. 2004; 6(2):107-13.
- Lian XL, Bai Y, Xun YH, Dai WX, Guo ZS. [Effects of maternal hyperthyroidism and antithyroid drug therapy on thyroid function of newborn infants (Chinese)]. Zhongguo yi xue ke xue yuan xue bao. 2005; 27(6):756-60. [PMID]
- McElduff A, McElduff P, Wiley V, Wilcken B. Neonatal thyrotropin as measured in a congenital hypothyroidism screening program: Influence of the mode of delivery. The Journal of Clinical Endocrinology and Metabolism. 2005; 90(12):6361-3. [DOI:10.1210/jc.2005-0786] [PMID]
- Ordookhani A, Pearce EN, Mirmiran P, Azizi F, Braverman LE. The effect of type of delivery and povidone-iodine application at delivery on cord dried-blood-specimen thyrotropin level and the rate of hyperthyrotropinemia in mature and normal-birth-weight neonates residing in an iodine-replete area: Report of Tehran Province, 1998-2005. Thyroid. 2007; 17(11):1097-102. [DOI:10.1089/thy.2007.0058] [PMID]
- Hashemipour M, Amini M, Kelishadi R, Hovsepian S, Haghighi S, Hosseini M, et al. Seasonal variation in the incidence of congenital hypothyroidism in Isfahan, Iran. Saudi Medical Journal. 2007; 28(10):1582-6. [PMID]
- Gu YH, Kato T, Harada S, Inomata H, Saito T, Aoki K. Seasonality in the incidence of congenital hypothyroidism in Japan: Gender-specific patterns and correlation with temperature. Thyroid. 2007; 17(9):869-74. [DOI:10.1089/thy.2006.0317] [PMID]
- Hashemipour M, Amini M, Talaie M, Kelishadi R, Hovespian S, Iranpour R, et al. Parental consanguinity among parents of neonates with congenital hypothyroidism in Isfahan. Eastern Mediterranean Health Journal. 2007; 13(3):567-74.
- Olivieri A, Medda E, De Angelis S, Valensise H, De Felice M, Fazzini C, et al. High risk of congenital hypothyroidism in multiple pregnancies. Journal of Clinical Endocrinology & Metabolism. 2007; 92(8):3141-7. [DOI:10.1210/jc.2007-0238] [PMID]
- Rowland K, Crotteau C, Kovach F, Hoekzema G. Clinical inquiries. What are the causes of elevated TSH in a newborn? The Journal of Family Practice. 2008; 57(3):185-7. [PMID]
- Eftekhari N, Gh A, Khaksari M, Salari Z. [The prevalence rate of congenital hypothyroidism in Kerman/Iran in 2005-2007 (Persian)]. Journal of Kerman University of Medical Sciences. 2015; 15(3):243-50.
- Sepandi M, Holakoei Naeini K, Yarahmadi S, Haghtdust A, Nedjat S, Taghdir M. [Risk factors for congenital hypothyroidism in Fars Province, Iran, 2003-2006 (Persian)]. Journal of School of Public Health and Institute of Public Health Research. 2009; 7(1):35-45.
- Cranston MM, Ryan MA, Smith TC, Sevick CJ, Brodine SK. Hypothyroidism among military infants born in countries of varied iodine nutrition status. BMC Endocrine Disorders. 2010; 10:2. [DOI:10.1186/1472-6823-10-2] [PMID] [PMCID]
- Hashemipour M, Nasri P, Hovsepian S, Hadian R, Heidari K, Attar HM, et al. Urine and milk iodine concentrations in healthy and congenitally hypothyroid neonates and their mothers. Endokrynologia Polska. 2010; 61(4):371-6. [PMID]
- Aminzadeh M, Chomeili B, Riahi K, Dehdashtian M, Cheraghian B, Valavi E. Effect of temperature changes on the occurrence of congenital hypothyroidism. Journal of Medical Screening. 2010; 17(3):121-4. [DOI:10.1258/jms.2010.010026] [PMID]
- Hashemipour M, Hasani N, Amini M, Heidari K, Sajadi A, Dastanpour M, et al. Thyroid function abnormalities among first-degree relatives of Iranian congenital hypothyroidism neonates. Pediatrics international. 2010; 52(3):467-71. [DOI:10.1111/j.1442-200X.2009.03016.x] [PMID]
- Safar alizade F, Sadify R, Parto Azam H. [Prevalence of congenital hypothyroidism and its relation with some risk factors in Khoy health service centers (Persian)]. Journal of Urmia Nursing and Midwifery Faculty. 2010; 8(1):35-9.
- Stoppa-Vaucher S, Van Vliet G, Deladoëy J. Variation by ethnicity in the prevalence of congenital hypothyroidism due to thyroid dysgenesis. Thyroid. 2011; 21(1):13-8. [DOI:10.1089/thy.2010.0205] [PMID] [PMCID]
- Hashemipour M, Abari SS, Mostofizadeh N, Haghjooy-Javanmard S, Esmail N, Hovsepian S, et al. The role of maternal thyroid stimulating hormone receptor blocking antibodies in the etiology of congenital hypothyroidism in Isfahan, Iran. International Journal of Preventive Medicine. 2012; 3(2):128-33. [PMID] [PMCID]
- Zeinalzadeh AH, Talebi M. Neonatal screening for congenital hypothyroidism in East Azerbaijan, Iran: The first report. Journal of Medical Screening. 2012; 19(3):123-6. [DOI:10.1258/jms.2012.012024] [PMID]
- Ooki S. Congenital hypothyroidism after assisted reproductive technology in Japan: Comparison between multiples and singletons, 2005-2009. International Journal of Pediatric Endocrinology. 2013; 2013:5. [DOI:10.1186/1687-9856-2013-5] [PMID] [PMCID]
- Abdelmoktader AM. Risk factors for congenital hypothyroidism in Egypt: Results of a population case-control study (2003–2010). Annals of Saudi Medicine. 2013; 33(3):273-6. [DOI:10.5144/0256-4947.2013.273] [PMID] [PMCID]
- Rezaeian S, Poorolajal J, Moghimbegi A, Esmailnasab N. Risk factors of congenital hypothyroidism using propensity score: A matched case-control study. Journal of Research in Health Sciences. 2013; 13(2):151-6.
- Ng SM, Wong SC, Paize F, Chakkarapani E, Newland P, Isherwood D, et al. Multivariate analyses of factors that affect neonatal screening thyroid stimulating hormone. Journal of Pediatric Endocrinology & Metabolism. 2011; 24(9-10):727-32. [DOI:10.1515/JPEM.2011.234] [PMID]
- Dalili S, Rezvany SM, Dadashi A, Medghalchi A, Mohammadi H, Dalili H, et al. Congenital hypothyroidism: A review of the risk factors. Acta Medica Iranica. 2012; 50(11):735-9.
- Kırmızıbekmez H, Güven A, Yıldız M, Cebeci AN, Dursun F. Developmental defects of the thyroid gland: Relationship with advanced maternal age. Journal of Clinical Research in Pediatric Endocrinology. 2012; 4(2):72-5. [DOI:10.4274/Jcrpe.560] [PMID] [PMCID]
- Rabbiosi S, Vigone MC, Cortinovis F, Zamproni I, Fugazzola L, Persani L, et al. Congenital hypothyroidism with eutopic thyroid gland: Analysis of clinical and biochemical features at diagnosis and after re-evaluation. The Journal of Clinical Endocrinology and Metabolism. 2013; 98(4):1395-402. [DOI:10.1210/jc.2012-3174] [PMID]
- Esmailnasab N, Moasses G, Afkhamzadeh A. [Investigation of the risk factors for congenital hypothyroidism in the newborns in Kurdistan Province (Persian)]. Scientific Journal of Kurdistan University of Medical Sciences. 2012; 17(4):103-8.
- Rezaeian S, Moghimbeigi A, Esmailnasab N. Gender differences in risk factors of congenital hypothyroidism: An interaction hypothesis examination. International Journal of Endocrinology and Metabolism. 2014; 12(2):e13946. [DOI:10.5812/ijem.13946] [PMID] [PMCID]
- Dorreh F, Chaijan PY, Javaheri J, Zeinalzadeh AH. Epidemiology of congenital hypothyroidism in Markazi Province, Iran. Journal of Clinical Research İn Pediatric Endocrinology. 2014; 6(2):105-10. [DOI:10.4274/jcrpe.1287] [PMID] [PMCID]
- Uenaka M, Tanimura K, Tairaku S, Morioka I, Ebina Y, Yamada H. Risk factors for neonatal thyroid dysfunction in pregnancies complicated by Graves’ disease. European Journal of Obstetrics, Gynecology, and Reproductive Biology. 2014; 177:89-93. [DOI:10.1016/j.ejogrb.2014.03.007] [PMID]
- Fan X, Chen S, Qian J, Sooranna S, Luo J, Li C, et al. Incidence and interrelated factors in patients with congenital hypothyroidism as detected by newborn screening in Guangxi, China. Global Pediatric Health. 2015; 2:2333794X14567193. [DOI:10.1177/2333794X14567193] [PMID] [PMCID]
- Satoh M, Aso K, Katagiri Y. Thyroid dysfunction in neonates born to mothers who have undergone hysterosalpingography involving an oil-soluble iodinated contrast medium. Hormone Research in Paediatrics. 2015; 84(6):370-5. [DOI:10.1159/000439381] [PMID]
- Mehrnejat N, Yazdanpanah H, Nobari RF, Hashemipour M, Maracy M, Moafi M, et al. Spatial analysis of neonatal congenital hypothyroidism and nitrate as an environmental pollutant in Isfahan Province during 2010-2013. International Journal of Preventive Medicine. 2015; 6. [DOI:10.4103/2008-7802.162952] [PMID] [PMCID]
- Zhou J, Luo J, Zhao H, Wang J, Lin F, Zhang H, et al. [Risk factors of 125 cases of neonatal congenital hypothyroidism during perinatal period (Chinese)]. Zhonghua liu xing bing xue za zhi Zhonghua liuxingbingxue zazhi. 2015; 36(7):747-51. [PMID]
- Trumpff C, Vandevijvere S, Moreno-Reyes R, Vanderpas J, Tafforeau J, Van Oyen H, et al. Neonatal thyroid-stimulating hormone level is influenced by neonatal, maternal, and pregnancy factors. Nutrition Research. 2015; 35(11):975-81. [DOI:10.1016/j.nutres.2015.09.002] [PMID]
- Dayal D, Prasad R. Congenital hypothyroidism: Current perspectives. Research and Reports in Endocrine Disorders. 2015; 5(5):91-102. [DOI:10.2147/RRED.S56402]
- Keshavarzian E, Valipoor AA, Maracy MR. The incidence of congenital hypothyroidism and its determinants from 2012 to 2014 in Shadegan, Iran: A case-control study. Epidemiology and Health 2016; 38:e2016021. [DOI:10.4178/epih.e2016021] [PMID] [PMCID]
- Aguiar L, Garb J, Reiter E, Visintainer P, Singh R, Allen H, et al. Can one predict resolution of neonatal hyperthyrotropinemia? The Journal of Pediatrics. 2016; 174:71-7.e1. [DOI:10.1016/j.jpeds.2016.04.011] [PMID]
- Blasig S, Kühnen P, Schuette A, Blankenstein O, Mittag J, Schomburg L. Positive correlation of thyroid hormones and serum copper in children with congenital hypothyroidism. Journal of Trace Elements in Medicine and Biology. 2016; 37:90-5. [DOI:10.1016/j.jtemb.2016.05.007] [PMID]
- Yang HH, Qiu L, Zhao JQ, Yang N, Gong LF, Kong YY. [Epidemiologic characteristics and risk factors for congenital hypothyroidism from 1989 to 2014 in Beijing (Chinese)]. Zhonghua yu fang yi xue za zhi. 2016; 50(8):728-32. [DOI:10.3760/cma.j.issn.0253-9624.2016.08.011]
- Anastasovska V, Kocova M. Ethnicity and incidence of congenital hypothyroidism in the capital of Macedonia. Journal of Pediatric Endocrinology and Metabolism. 2017; 30(4):405-9. [DOI:10.1515/jpem-2016-0178]
- Khanjani N, Ahmadzadeh A, Bakhtiari B, Madadizadeh F. The role of season and climate in the incidence of congenital hypothyroidism in Kerman province, Southeastern Iran. Journal of Pediatric Endocrinology and Metabolism. 2017; 30(2):149-57. [DOI:10.1515/jpem-2016-0178] [PMID]
- Castanet M, Polak M, Bonaiti-Pellie C, Lyonnet S, Czernichow P, Leger J. Nineteen years of national screening for congenital hypothyroidism: Familial cases with thyroid dysgenesis sug-gest the involvement of genetic factors. The Journal of Clinical Endocrinology & Metabolism. 2001; 86(5):2009-14. [DOI:10.1210/jcem.86.5.7501] [PMID]
- Rosenthal M, Addison GM, Price DA. Congenital hypothyroidism: Increased incidence in Asian families. Archives of Disease in Childhood. 1988; 63(7):790-3. [DOI:10.1136/adc.63.7.790] [PMID] [PMCID]
Type of Study: Systematic Review |
Subject:
Endocrinology
Received: 2018/07/21 | Accepted: 2019/02/3 | Published: 2019/10/1
Received: 2018/07/21 | Accepted: 2019/02/3 | Published: 2019/10/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |








.PNG)