Volume 8, Issue 1 (1-2020)
J. Pediatr. Rev 2020, 8(1): 29-34 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Abolhassan Choobdar F, Milani H, Behrouzi K, Khalesi N, Haghighi B, Manafi A, et al . Anti-Rh17 Alloimmunization: A Rare Case of Severe Hemolytic Disease of the Newborn and Review of the Literature. J. Pediatr. Rev 2020; 8 (1) :29-34
URL: http://jpr.mazums.ac.ir/article-1-216-en.html
URL: http://jpr.mazums.ac.ir/article-1-216-en.html
Farhad Abolhassan Choobdar1 

 , Hani Milani *2
, Hani Milani *2 

 , Kamran Behrouzi3
, Kamran Behrouzi3 

 , Nasrin Khalesi1
, Nasrin Khalesi1 

 , Behzad Haghighi4
, Behzad Haghighi4 

 , Ali Manafi4
, Ali Manafi4 

 , Mohammad Naderisorki5
, Mohammad Naderisorki5 

 , Sorraya Shojaee1
, Sorraya Shojaee1 




 , Hani Milani *2
, Hani Milani *2 

 , Kamran Behrouzi3
, Kamran Behrouzi3 

 , Nasrin Khalesi1
, Nasrin Khalesi1 

 , Behzad Haghighi4
, Behzad Haghighi4 

 , Ali Manafi4
, Ali Manafi4 

 , Mohammad Naderisorki5
, Mohammad Naderisorki5 

 , Sorraya Shojaee1
, Sorraya Shojaee1 


1- Department of Neonatology, Aliasghar Children Hospital, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Neonatology, Kamali Hospital, Alborz University of Medical Sciences, Karaj, Iran. ,h.milani@abzums.ac.ir
3- Department of Neonatology, Kamali Hospital, Alborz University of Medical Sciences, Karaj, Iran.
4- Department of Pediatric Intensive Care, Aliasghar Children Hospital, Iran University of Medical Sciences, Tehran, Iran.
5- Thalassemia Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Neonatology, Kamali Hospital, Alborz University of Medical Sciences, Karaj, Iran. ,
3- Department of Neonatology, Kamali Hospital, Alborz University of Medical Sciences, Karaj, Iran.
4- Department of Pediatric Intensive Care, Aliasghar Children Hospital, Iran University of Medical Sciences, Tehran, Iran.
5- Thalassemia Research Center, Faculty of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 729 kb]
(3043 Downloads)
| Abstract (HTML) (6654 Views)
After the exchange transfusion, the bilirubin level reached 8.6mg/dL, with a platelet count of 50000/μL and a hemoglobin level of 10.6g/dL. The infant received a second dose of 1g/kg IVIG, checked for rebounds while treatment continued with intensive phototherapy. Thrombocytopenia resolved spontaneously. The results of antibody screening tests performed by Gel method determined the existence of anti-Rh17 in maternal serum. Maternal RBC extended phenotype was blood group B RH D--.
Kell: K Negative, Cellano k positive; Duffy: Fy a positive, Fy b Negative; Kidd: Jk a positive, JK b positive; MNS: S positive, s Positive On day 9 of life, the baby was discharged from NICU in good health, and scheduled for close follow-up visits. Her auditory brainstem response performed at 3 and 10 months of age showed normal hearing patterns. Currently, at the age of 11 months, the infant has normal neurological development and meet all her age-specific developmental milestones.
Since the D-- phenotype is derived from a homologous deletion of the RHCE genes3, Rh phenotyping and genotyping tests were requested for family members which were not finally performed because parents were unwilling to accept further extended studies. The mother referred to IBTO to be registered in the rare blood groups database (Table 1).
3. Discussion and Review of the Literature
Since the first recognition of the Rh antigen system by Landsteiner and Wiener in 1940, more than 50 subtypes of this system have been identified (1).
Rh system is the most complicated blood group in human (2). Of these 50 antigens, D, C, c, E, e antigens, have the most important functions (3). Encoding genes of Rh D and Rh CE are located near each other on chromosome one (5). Rh D-- is a rare phenotype in which D antigen is strongly expressed but none of the C, c, E, e, antigens are. While in D—phenotype, expression of C, c, E, e antigens are completely ablated, the expression of D antigen is intensified due to a large insertion of RHD gene into RHCE genes. In other words, D-- phenotype may be suspected in any case with stronger agglutination reactions in Rh D typing (6).
Hemolytic Disease of Neonate (HDN) is caused by the transmission of maternal IgG antibody from placenta and destruction of neonatal RBCs. D antigen is responsible for more than 50% of human HDN, and others are caused by K, c, C/G, E, and Fya antigens (7). Encountering fetal antigens is the most reason of maternal sensitization to Rh antigen. Keilhauer in 1957 described the diagnostic test for finding fetal RBC in mother bloodstream which is based on Hb F resistance to acid (8).
The main cause of fetomaternal hemorrhage (FMH) is the childbirth (9). And the amount of transfusion blood is the most determinants factor for sensitization. Transmission of 0.1 mL RBC may create less than 3% sensitization, and by transfer of 0.4 mL of neonatal RBC, this value reaches 22% (10). However, Rh sensitization occurs in less than 1.5% of pregnant women (11).
Historically, the hemolytic disease was the most common cause of severe anemia, hyperbilirubinemia, and immune hydrops fetalis. However, prenatal prophylactic measures such as close fetal surveillance with ultrasonography and middle cerebral artery flow velocimetry, and administration of high titer of anti-D immunoglobulin G to sensitized pregnant women, along with postnatal early diagnosis and treatment, greatly diminishes both the incidence and the severity of the disease. With the reduction of Rh isoimmunization (alloimmunization), DAT-positive ABO incompatibility is now the single most prominent cause of immune hemolytic disease in the neonate (12).
The individuals with D-- phenotype produce multiple Rh antibodies against C, c, E, or e antigens (AntiRh17 antibody also known as Anti-Hr0) if they are sensitized to Rh antigens because of previous blood or even platelet transfusion of incompatible product, or fetomaternal bleeding in the previous pregnancy (13). While this will significantly raise the risk for severe hemolytic transfusion reactions in healthy subjects, prior sensitization in a pregnant woman put her fetus at risk for severe alloimmune hemolytic disease of newborn, severe anemia, and hyperbilirubinemia (5, 14).
Anti-Rh 17 antibody is an antibody against Hro (Rh17) antigen and is produced in persons lacking any Rh group antigens, but D antigen (15). HDN caused by this antibody is very rare over the world, and this report is the first report from Iran. In the event of an occurrence, the intrauterine blood transfusion is one of the treatments. In a case report, Dietenbeck et al. from England used intrauterine blood transfusion for treatment HDN due to anti-Rh17 antibody (16).
After birth, like our patient, the treatment is based on the control of complications and transfusion of full match blood, as needed. Shah et al. used IVIG and blood transfusion for treatment of HDN in a 36 4/7-weeks’ gestation female infant. Mother’s anti-Rh17 tube titer was 1:256. IVIG binds and occupies sites on the surface of the RBCs and decreases hemolysis (17).
In Japan, Hirose et al. reported eight cases of HDN due to anti-Rh17 antibody from 1979 to 2002. In this report, anti-Rh17 titer increased during pregnancy, and maximal titer ranged between 1:128 and 1:4000. Based on this report, the prevalence of HDN due to anti-Rh17 was higher than in other places like England. Bramit et al. reported successful treatment of anti-Rh17 HDN with compatible blood transfusion (18).
Denomme et al. presented a woman with a history of hemolytic disease of the newborn due to anti-Rh17 in her third pregnancy. Intrauterine transfusion with washed maternal packed cells was done seven times. Pregnancy was terminated at 38 weeks, and a 2560-g infant was delivered successfully (19). Rh17 antigen-negative blood or washed maternal blood is routinely used to treat the hemolytic disease of the newborn due to anti-Rh17, but Li et al. successfully treated a 10-hour neonate with least incompatible blood for exchange transfusion (20).
4. Conclusion
As for our patient, the postnatal management of non-RhD alloimmunization should be based on the principles applied in the management of the RhD-immunized newborn. Such modalities as the administration of IVIG and double volume exchange transfusion can be employed safely and successfully in these patients (12, 21).
This case would improve the insight and knowledge about managing severe hemolytic anemia due to minor group alloantibodies, and would also help to overcome practical challenges in preparing rare packed RBC units such as group B Rh D-- for exchange transfusion. Because of the rarity of this kind of HDN, other new reports may lead to better diagnosis and treatment.
Ethical Considerations
Compliance with ethical guidelines
The parents of the patient provided informed written consent for the release of results and data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contribution's
Patient management: Farhad Abolhasan Choobdar, Nasrin Khalesi, and Hani Milani; fellow on the case and gathered the case data and drafted the manuscript: Hani Milani and Kamran Behrouzi; Manuscript writing: Farhad Abolhasan Choobdar, Behzad Haghighi and Ali Manafi; Revised and modified the manuscript: Farhad Abolhasan Choobdar, Nasrin Khalesi, Behzad Haghighi, Mohammad Naderisorki, and Ali Manafi; read and approved the final manuscript: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Saeed Hosseini for technical support in determining the specificity of the antibody and for her assistance in the preparation of the specific packed RBC unit for exchange transfusion.
References
Full-Text: (22776 Views)
1. Introduction
To date, more than 50 Red Blood Cell (RBC) antigens have been identified that cause hemolytic disease of the newborn. The Rh blood group proteins are a highly antigenic group of proteins, capable of causing severe alloimmunization with diverse clinical manifestations from mild anemia and hyperbilirubinemia to severe hydrops fetalis and death (1). The most important antigens in this regard are D/d, C/c, E/e, and Kell antigens. The Rh antigens are inherited as linked group of RHD and RHCE genes located on chromosome 1. Based on the expression of the major D antigen on their RBCs, people are typed as Rh-negative or positive. RBCs will also express C or c, E or e antigens. The most frequently occurring forms of RHCE and RHD encoding are 8 haplotypes; Dce, dce, DCe, dCe, DcE, dcE, DCE, and dCE (1-3).
Rarely Rh deletion of C/c and E/e loci can occur, leading to the D-- blood group phenotype. However, the haplotype is very rare; frequencies of D-- blood group have been estimated at 0.0005 in Sweden, 0·0047 in Iceland, and 0.0032 in Japan (4). The phenotype of D-- is a very rare Rh phenotype in which D antigen is expressed, but neither C/c nor E/e antigens are. The anti-Rh17 antibody is commonly found in the sera of D-- phenotype individuals, if there is a history of sensitization such as pregnancy (fetomaternal hemorrhage) or transfusion with red blood cells that express C/c or E/e antigens. As in Rh incompatibility, alloimmunization may occur when the fetus is expressing either C/c, E/e antigens, that is absent on maternal red cells. The maternal immune system is stimulated by positive fetal RBCs, to produce antibodies (IgG), which passes readily through the placenta and destroy the antigen-positive fetal red blood cells.
2. Case Presentation
A term (39 weeks), small for gestational age, 2530g, female infant, was admitted because of pallor and hyperbilirubinemia in the first hour of life. The baby was born by cesarean section due to preeclampsia and meconium-stained amniotic fluid. The parents were related (first cousins). Her prenatal lab tests, as well as maternal and family history, were unremarkable. The mother was 28 years old, gravid 2, labor 2, without abortion. The baby and the mother were both B blood group Rh-positive. There was no history of transfusion or previous abortion.
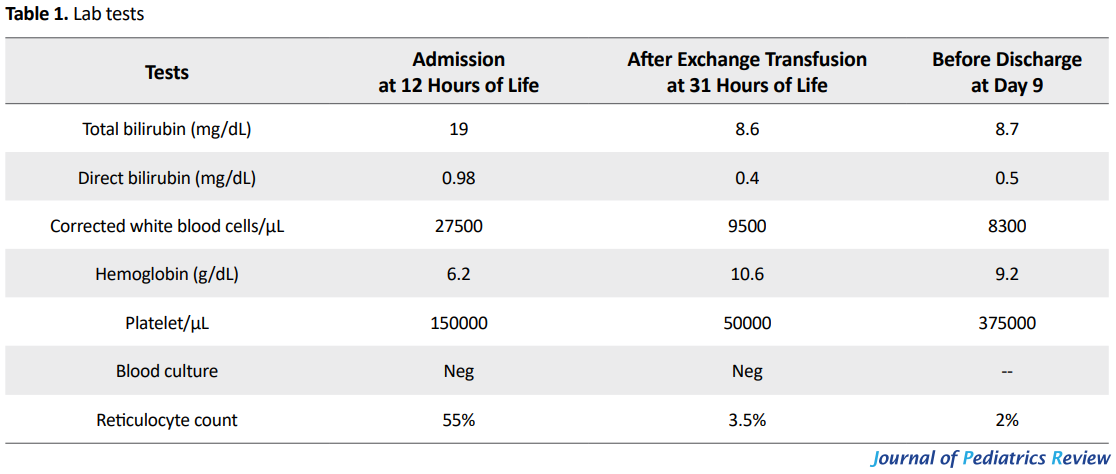
On the second hour of baby’s life, the lab tests were as follows: total serum bilirubin of 14.2mg/dL (direct bilirubin of 0.7mg/dL), hemoglobin of 6.9g/dL, and a reticulocyte count of 14.2%. The baby was transferred from a level I nursery where she was born, to our unit (NICU level III) at 12 hours of her life. At the time of admission, in her physical exam and laboratory tests, she had nondysmorphic appearance, pallor, jaundice, hepatomegaly of 4cm under costal margin and a palpable spleen (2cm under costal margin).
No apparent sign of kernicterus was detected. Her complete blood count report comprised with hemoglobin of 6.2g/dL, Nucleated Red Blood Cells (NRBC) of 95%, corrected white blood cells of 27500/μL, platelet count of 150000/μL, positive Direct Antiglobulin Test (DAT), total serum bilirubin of 19mg/dL with a serum conjugated bilirubin of 0.98mg/dL. Her qualitatively measured (Glucose 6-Phosphate Dehydrogenase) G6PD was reported to be normal. Also, her peripheral blood smear revealed signs of hemolysis, including polychromasia, anisocytosis, reticulocytosis, and nucleated RBCs. Maternal indirect coombs test known as Indirect Antiglobulin Test (IAT) was strongly positive.
Blood samples were sent for cross-match, antibody screening, RBC phenotyping, minor groups, and their antibodies. Treatment started with intensive phototherapy and Intravenous Immunoglobulin (IVIG) and planned for exchange transfusion, by the presumed diagnosis of alloimmune hemolytic disease of the newborn due to minor blood group incompatibility. Seeking the blood for exchange transfusion, the blood bank technician reported that on cross-matching, the sample reacted with all blood units tested and no cross-match compatible unit of blood was available for exchange transfusion.
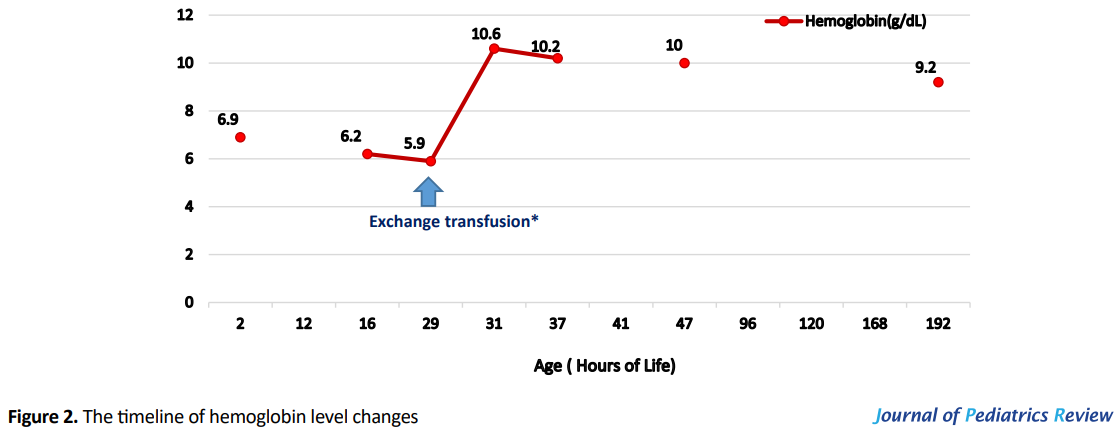
Samples were then sent to Iran Blood Transfusion Organization (IBTO), and the maternal blood type revealed to be B Rh D--. The IBTO reported that there were only 2 bags of about 100 mL frozen glycerolized packed RBCs available in IBTO blood bank, reserved for probable future autotransfusion of an individual with B Rh D-- blood type. Double volume exchange transfusion performed at 29 hours of life with reconstituted blood (Packed RBC group B Rh D-- and fresh frozen plasma AB Rh positive), after official consent taken by phone from the owner of the reserved blood. Peak total bilirubin was 21.5mg/dL before exchange transfusion. Figures 1 and 2 display serial bilirubin and hemoglobin level changes through the admission period.
To date, more than 50 Red Blood Cell (RBC) antigens have been identified that cause hemolytic disease of the newborn. The Rh blood group proteins are a highly antigenic group of proteins, capable of causing severe alloimmunization with diverse clinical manifestations from mild anemia and hyperbilirubinemia to severe hydrops fetalis and death (1). The most important antigens in this regard are D/d, C/c, E/e, and Kell antigens. The Rh antigens are inherited as linked group of RHD and RHCE genes located on chromosome 1. Based on the expression of the major D antigen on their RBCs, people are typed as Rh-negative or positive. RBCs will also express C or c, E or e antigens. The most frequently occurring forms of RHCE and RHD encoding are 8 haplotypes; Dce, dce, DCe, dCe, DcE, dcE, DCE, and dCE (1-3).
Rarely Rh deletion of C/c and E/e loci can occur, leading to the D-- blood group phenotype. However, the haplotype is very rare; frequencies of D-- blood group have been estimated at 0.0005 in Sweden, 0·0047 in Iceland, and 0.0032 in Japan (4). The phenotype of D-- is a very rare Rh phenotype in which D antigen is expressed, but neither C/c nor E/e antigens are. The anti-Rh17 antibody is commonly found in the sera of D-- phenotype individuals, if there is a history of sensitization such as pregnancy (fetomaternal hemorrhage) or transfusion with red blood cells that express C/c or E/e antigens. As in Rh incompatibility, alloimmunization may occur when the fetus is expressing either C/c, E/e antigens, that is absent on maternal red cells. The maternal immune system is stimulated by positive fetal RBCs, to produce antibodies (IgG), which passes readily through the placenta and destroy the antigen-positive fetal red blood cells.
2. Case Presentation
A term (39 weeks), small for gestational age, 2530g, female infant, was admitted because of pallor and hyperbilirubinemia in the first hour of life. The baby was born by cesarean section due to preeclampsia and meconium-stained amniotic fluid. The parents were related (first cousins). Her prenatal lab tests, as well as maternal and family history, were unremarkable. The mother was 28 years old, gravid 2, labor 2, without abortion. The baby and the mother were both B blood group Rh-positive. There was no history of transfusion or previous abortion.
On the second hour of baby’s life, the lab tests were as follows: total serum bilirubin of 14.2mg/dL (direct bilirubin of 0.7mg/dL), hemoglobin of 6.9g/dL, and a reticulocyte count of 14.2%. The baby was transferred from a level I nursery where she was born, to our unit (NICU level III) at 12 hours of her life. At the time of admission, in her physical exam and laboratory tests, she had nondysmorphic appearance, pallor, jaundice, hepatomegaly of 4cm under costal margin and a palpable spleen (2cm under costal margin).
No apparent sign of kernicterus was detected. Her complete blood count report comprised with hemoglobin of 6.2g/dL, Nucleated Red Blood Cells (NRBC) of 95%, corrected white blood cells of 27500/μL, platelet count of 150000/μL, positive Direct Antiglobulin Test (DAT), total serum bilirubin of 19mg/dL with a serum conjugated bilirubin of 0.98mg/dL. Her qualitatively measured (Glucose 6-Phosphate Dehydrogenase) G6PD was reported to be normal. Also, her peripheral blood smear revealed signs of hemolysis, including polychromasia, anisocytosis, reticulocytosis, and nucleated RBCs. Maternal indirect coombs test known as Indirect Antiglobulin Test (IAT) was strongly positive.
Blood samples were sent for cross-match, antibody screening, RBC phenotyping, minor groups, and their antibodies. Treatment started with intensive phototherapy and Intravenous Immunoglobulin (IVIG) and planned for exchange transfusion, by the presumed diagnosis of alloimmune hemolytic disease of the newborn due to minor blood group incompatibility. Seeking the blood for exchange transfusion, the blood bank technician reported that on cross-matching, the sample reacted with all blood units tested and no cross-match compatible unit of blood was available for exchange transfusion.
Samples were then sent to Iran Blood Transfusion Organization (IBTO), and the maternal blood type revealed to be B Rh D--. The IBTO reported that there were only 2 bags of about 100 mL frozen glycerolized packed RBCs available in IBTO blood bank, reserved for probable future autotransfusion of an individual with B Rh D-- blood type. Double volume exchange transfusion performed at 29 hours of life with reconstituted blood (Packed RBC group B Rh D-- and fresh frozen plasma AB Rh positive), after official consent taken by phone from the owner of the reserved blood. Peak total bilirubin was 21.5mg/dL before exchange transfusion. Figures 1 and 2 display serial bilirubin and hemoglobin level changes through the admission period.
After the exchange transfusion, the bilirubin level reached 8.6mg/dL, with a platelet count of 50000/μL and a hemoglobin level of 10.6g/dL. The infant received a second dose of 1g/kg IVIG, checked for rebounds while treatment continued with intensive phototherapy. Thrombocytopenia resolved spontaneously. The results of antibody screening tests performed by Gel method determined the existence of anti-Rh17 in maternal serum. Maternal RBC extended phenotype was blood group B RH D--.
Kell: K Negative, Cellano k positive; Duffy: Fy a positive, Fy b Negative; Kidd: Jk a positive, JK b positive; MNS: S positive, s Positive On day 9 of life, the baby was discharged from NICU in good health, and scheduled for close follow-up visits. Her auditory brainstem response performed at 3 and 10 months of age showed normal hearing patterns. Currently, at the age of 11 months, the infant has normal neurological development and meet all her age-specific developmental milestones.
Since the D-- phenotype is derived from a homologous deletion of the RHCE genes3, Rh phenotyping and genotyping tests were requested for family members which were not finally performed because parents were unwilling to accept further extended studies. The mother referred to IBTO to be registered in the rare blood groups database (Table 1).
3. Discussion and Review of the Literature
Since the first recognition of the Rh antigen system by Landsteiner and Wiener in 1940, more than 50 subtypes of this system have been identified (1).
Rh system is the most complicated blood group in human (2). Of these 50 antigens, D, C, c, E, e antigens, have the most important functions (3). Encoding genes of Rh D and Rh CE are located near each other on chromosome one (5). Rh D-- is a rare phenotype in which D antigen is strongly expressed but none of the C, c, E, e, antigens are. While in D—phenotype, expression of C, c, E, e antigens are completely ablated, the expression of D antigen is intensified due to a large insertion of RHD gene into RHCE genes. In other words, D-- phenotype may be suspected in any case with stronger agglutination reactions in Rh D typing (6).
Hemolytic Disease of Neonate (HDN) is caused by the transmission of maternal IgG antibody from placenta and destruction of neonatal RBCs. D antigen is responsible for more than 50% of human HDN, and others are caused by K, c, C/G, E, and Fya antigens (7). Encountering fetal antigens is the most reason of maternal sensitization to Rh antigen. Keilhauer in 1957 described the diagnostic test for finding fetal RBC in mother bloodstream which is based on Hb F resistance to acid (8).
The main cause of fetomaternal hemorrhage (FMH) is the childbirth (9). And the amount of transfusion blood is the most determinants factor for sensitization. Transmission of 0.1 mL RBC may create less than 3% sensitization, and by transfer of 0.4 mL of neonatal RBC, this value reaches 22% (10). However, Rh sensitization occurs in less than 1.5% of pregnant women (11).
Historically, the hemolytic disease was the most common cause of severe anemia, hyperbilirubinemia, and immune hydrops fetalis. However, prenatal prophylactic measures such as close fetal surveillance with ultrasonography and middle cerebral artery flow velocimetry, and administration of high titer of anti-D immunoglobulin G to sensitized pregnant women, along with postnatal early diagnosis and treatment, greatly diminishes both the incidence and the severity of the disease. With the reduction of Rh isoimmunization (alloimmunization), DAT-positive ABO incompatibility is now the single most prominent cause of immune hemolytic disease in the neonate (12).
The individuals with D-- phenotype produce multiple Rh antibodies against C, c, E, or e antigens (AntiRh17 antibody also known as Anti-Hr0) if they are sensitized to Rh antigens because of previous blood or even platelet transfusion of incompatible product, or fetomaternal bleeding in the previous pregnancy (13). While this will significantly raise the risk for severe hemolytic transfusion reactions in healthy subjects, prior sensitization in a pregnant woman put her fetus at risk for severe alloimmune hemolytic disease of newborn, severe anemia, and hyperbilirubinemia (5, 14).
Anti-Rh 17 antibody is an antibody against Hro (Rh17) antigen and is produced in persons lacking any Rh group antigens, but D antigen (15). HDN caused by this antibody is very rare over the world, and this report is the first report from Iran. In the event of an occurrence, the intrauterine blood transfusion is one of the treatments. In a case report, Dietenbeck et al. from England used intrauterine blood transfusion for treatment HDN due to anti-Rh17 antibody (16).
After birth, like our patient, the treatment is based on the control of complications and transfusion of full match blood, as needed. Shah et al. used IVIG and blood transfusion for treatment of HDN in a 36 4/7-weeks’ gestation female infant. Mother’s anti-Rh17 tube titer was 1:256. IVIG binds and occupies sites on the surface of the RBCs and decreases hemolysis (17).
In Japan, Hirose et al. reported eight cases of HDN due to anti-Rh17 antibody from 1979 to 2002. In this report, anti-Rh17 titer increased during pregnancy, and maximal titer ranged between 1:128 and 1:4000. Based on this report, the prevalence of HDN due to anti-Rh17 was higher than in other places like England. Bramit et al. reported successful treatment of anti-Rh17 HDN with compatible blood transfusion (18).
Denomme et al. presented a woman with a history of hemolytic disease of the newborn due to anti-Rh17 in her third pregnancy. Intrauterine transfusion with washed maternal packed cells was done seven times. Pregnancy was terminated at 38 weeks, and a 2560-g infant was delivered successfully (19). Rh17 antigen-negative blood or washed maternal blood is routinely used to treat the hemolytic disease of the newborn due to anti-Rh17, but Li et al. successfully treated a 10-hour neonate with least incompatible blood for exchange transfusion (20).
4. Conclusion
As for our patient, the postnatal management of non-RhD alloimmunization should be based on the principles applied in the management of the RhD-immunized newborn. Such modalities as the administration of IVIG and double volume exchange transfusion can be employed safely and successfully in these patients (12, 21).
This case would improve the insight and knowledge about managing severe hemolytic anemia due to minor group alloantibodies, and would also help to overcome practical challenges in preparing rare packed RBC units such as group B Rh D-- for exchange transfusion. Because of the rarity of this kind of HDN, other new reports may lead to better diagnosis and treatment.
Ethical Considerations
Compliance with ethical guidelines
The parents of the patient provided informed written consent for the release of results and data.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contribution's
Patient management: Farhad Abolhasan Choobdar, Nasrin Khalesi, and Hani Milani; fellow on the case and gathered the case data and drafted the manuscript: Hani Milani and Kamran Behrouzi; Manuscript writing: Farhad Abolhasan Choobdar, Behzad Haghighi and Ali Manafi; Revised and modified the manuscript: Farhad Abolhasan Choobdar, Nasrin Khalesi, Behzad Haghighi, Mohammad Naderisorki, and Ali Manafi; read and approved the final manuscript: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Dr. Saeed Hosseini for technical support in determining the specificity of the antibody and for her assistance in the preparation of the specific packed RBC unit for exchange transfusion.
References
- Landsteiner K, Wiener A. An agglutinable factor in human blood recognized by immune sera for rhesus blood. Proceedings of the Society for Experimental Biology and Medicine. 1940; 43(1):223. [DOI:10.3181/00379727-43-11151]
- Cartron JP. RH blood group system and molecular basis of Rh-deficiency. Best Practice & Research Clinical Haematology. 1999; 12(4):655-89. [DOI:10.1053/beha.1999.0047]
- Westhoff CM. The structure and function of the Rh antigen complex. Seminars in Hematology. 2007; 44(1):42-50. [DOI:10.1053/j.seminhematol.2006.09.010] [PMID] [PMCID]
- Daniels G. Rh Blood group system, human blood groups, 3rd edition. Hoboken: John Wiley & Sons, Ltd, Publication; 2013.
- Cherif-Zahar B, Bloy C, Le Van Kim CA, Blanchard D, Bailly P, Hermand P, et al. Molecular cloning and protein structure of a human blood group Rh polypeptide. Proceedings of the National Academy of Sciences. 1990; 87(16):6243-7. [DOI:10.1073/pnas.87.16.6243] [PMID] [PMCID]
- Avent ND, Reid ME. The Rh blood group system: A review. Blood. 2000; 95(2):375-87. [PMID]
- Heddle NM, Klama L, Frassetto R, O’Hoski P, Leaman B. A retrospective study to determine the risk of red cell alloimmunization and transfusion during pregnancy. Transfusion. 1993; 33(3):217-20. [DOI:10.1046/j.1537-2995.1993.33393174447.x] [PMID]
- Kleihauer E, Braun H, Betke K. [Demonstration von fetalem hämoglobin in den erythrocyten eines Blutausstrichs (German)]. Journal of Molecular Medicine. 1957; 35(12):637-8. [DOI:10.1007/BF01481043] [PMID]
- Harvey G, Klein, Anstee DJ, Haemolytic disease of the fetus and the newborn, 12th edition. Hoboken: John Wiley & Sons, Ltd, Publication; 2013. [DOI:10.1002/9781118689943.ch12]
- Woodrow J. Rh immunization and its prevention. the immune response in the mother. In: jensen K, Killmann S, editors. Series haematologica, vol 3. Copenhagen: Munksgaard; 1970.
- Crowther CA, Keirse MJ. Anti-D administration in pregnancy for preventing rhesus alloimmunisation. Cochrane. 2000; (2):CD000020. [DOI:10.1002/14651858.CD000020]
- Fanaroff AAM, Fanaroff RJA, Martin RJ, Klaus MHF, Avroy A. Neonatal-perinatal medicine: Diseases of the fetus and infant. Maryland Heights: Mosby; 2002.
- Yun JW, Kang ES, Ki CS, Koh KC, Kim DW. Sensitization to multiple Rh antigens by transfusion of random donor platelet concentrates in a-D-phenotype patient. Annals of Laboratory Medicine. 2012; 32(6):429-32. [DOI:10.3343/alm.2012.32.6.429] [PMID] [PMCID]
- Dean L. Blood groups and red cell antigens. Bethesda: National Center for Biotechnology Information; 2005.
- Mario. E, Oyen RR, Morsh WL. Summary of the clinical significance of blood group alloantibodies. Seminars in Hematology. 2000; 37(2):197-216. [DOI:10.1016/S0037-1963(00)90044-1]
- Hirose M, Nakanishi K, Kaku S, Moro H, Hodohara K, Aotani H, et al. Fetal hemolytic disease due to anti-Rh17 alloimmunization. Fetal Diagnosis and Therapy. 2004; 19(2):182-6. [DOI:10.1159/000075147] [PMID]
- Shah SI, Caprio M, Strauss R, Moskowitz N. Management of a full-term infant with hemolytic disease of the newborn due to an anti-Rh17 antibody in a mother with D-phenotype. American Journal of Hematology. 2005; 80(1):88-9. [DOI:10.1002/ajh.20370] [PMID]
- Brumit MC, Carnahan GE, Stubbs JR, Storry JR, Reid ME. moderate Hemolytic Disease of the Newborn (HDN) due to anti-Rh17 produced by a black female with an e variant phenotype. Immunohematology. 2002; 18(2):40-2. [PMID]
- Denomme GA, Ryan G, Seaward PG, Kelly EN, Fernandes BJ. Maternal ABO-mismatched blood for intrauterine transfusion of severe hemolytic disease of the newborn due to anti-Rh17. Transfusion. 2004; 44(9):1357-60. [DOI:10.1111/j.1537-2995.2004.04082.x] [PMID]
- Li BJ, Jiang YJ, Yuan F, Ye HX. Exchange transfusion of least incompatible blood for severe hemolytic disease of newborn due to antiRh17. Transfusion Medicine. 2010; 20(1):66-9. [DOI:10.1111/j.1365-3148.2009.00946.x] [PMID]
- Rath ME, Smits‐Wintjens VE, Lindenburg I, Folman CC, Brand A, Kamp IL, et al. Postnatal outcome in neonates with severe Rhesus c compared to Rhesus D hemolytic disease. Transfusion. 2013; 53(7):1580-5. [DOI:10.1111/j.1537-2995.2012.03937.x] [PMID]
Type of Study: Case Report and Review of Literature |
Subject:
Pediatric Hematology and Oncology
Received: 2018/12/3 | Accepted: 2019/07/21 | Published: 2020/01/1
Received: 2018/12/3 | Accepted: 2019/07/21 | Published: 2020/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |