Volume 8, Issue 2 (4-2020)
J. Pediatr. Rev 2020, 8(2): 93-100 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Ahmadzadeh Amiri A, Alaee A, Ahmadzadeh Amiri A. Neuroimaging in Pediatric Optic Neuritis: A Narrative Review. J. Pediatr. Rev 2020; 8 (2) :93-100
URL: http://jpr.mazums.ac.ir/article-1-245-en.html
URL: http://jpr.mazums.ac.ir/article-1-245-en.html
1- Department of Pediatric, Bahrami Children’s Hospital, Tehran University of Medical Sciences, Tehran, Iran.
2- Department of Radiology, School of Allied Medical Sciences, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Ophthalmology, Clinical Research Development Unit, Bu-Ali Sina Hospital, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. ,Ahmadzdh@yahoo.com
2- Department of Radiology, School of Allied Medical Sciences, Mazandaran University of Medical Sciences, Sari, Iran.
3- Department of Ophthalmology, Clinical Research Development Unit, Bu-Ali Sina Hospital, School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran. ,
Full-Text [PDF 377 kb]
(3739 Downloads)
| Abstract (HTML) (6173 Views)
Full-Text: (32055 Views)
1. Context
Pediatric Optic Neuritis (PON) is commonly presented as a spectrum of neuroinflammatory disorders such as Monophasic Optic Neuritis (MON), Isolated Recurrent Optic Neuritis (IRON), Neuromyelitis Optica (NMO), and Acute Disseminated Encephalomyelitis (ADEM) as well as in chronic diseases such as Multiple Sclerosis (MS).
Optic neuritis is an inflammation of the optic nerve clinically characterized by decreased visual acuity, field defects, diminished color vision, and positive Marcus Gunn in unilateral cases (1). Neuroimaging enhancement and latency of conduction on visual evoked potentials are essential findings to confirm the diagnosis. Despite the identical name, children are not little adults. Children suffer differently from adults regarding this disease. Children might present with bilateral painless optic nerve head swelling and more severe visual impairment after a prodromal viral illness. Visual recovery in children is more expectable.
Most pediatric patients with PON are older than 12 years. However, the correlation between puberty and risk associations is unknown. Children with PON are less likely to develop MS compared to adults, but they are prone to experience a preliminary representation of ADEM (2). Opticneuritisin children with its peculiar clinical features has a better prognosis than in adulthood. The main concern for therapeutics is the relationship ofopticneuritiswith multiple sclerosis (3). Diagnosis of PON is more difficult as there are unilateral, subclinical, spontaneous resolving, and poor history presentation. Also, children’s desire to pretend vision loss in order to obtain glasses is being misdiagnosed as spurious, and subsequently delays diagnosis and treatment. In a child with PON, a guided medical workup helps to differentiate the various metabolic, infectious, autoimmune, and treatable space-occupying lesions. Generally, the patients are treated with intravenous methylprednisolone, although the decision to treat varies between practitioners (4).
Among the newly developed neuroimaging techniques, Magnetic Resonance Imaging (MRI) is considered a reliable, noninvasive, and reproducible approach for the diagnosis and management of PON. Brain MRI is more sensitive to white matter lesions than the grey matter ones. MRI with high sensitivity serves as an essential component of subclinical disease activity and diagnostic criteria, especially for MS and Neuromyelitis Optica (NMO) spectrum disorders (5, 6). However, the disadvantages of MRI make it hard to detect the nature of demyelinating lesions and is an expensive modality. MRI helps to rule out the other differential diagnoses such as brain tumors that may appear similar to demyelinating lesions, without the necessity to use invasive procedures. Furthermore, it is particularly useful to detect the evolution of clinically silent lesions (7).
In this study, we just present and discuss the findings of recent investigations in which the impact on PON was assessed. However, few studies exist about this association in children growing up.
2. Evidence Acquisition
A PubMed literature search limited to the English language from 1995 to 2019 was accomplished using the following search terms: “Neuroimaging", “Pediatric Optic Neuritis", “Multiple Sclerosis", and “Magnetic Resonance Imaging". In this step, qualitative results from research studies were obtained. The articles were then reviewed to exclude those related to brain diseases (such as raised intracranial pressure), adult cases, and studies in healthy subjects as these were not the aim of this review.
For the significance of this review, case control, randomized controlled trials, cohort studies, evidence from meta-analyses, and systematic reviews were included. Case reports or case series were only included if there was specified evidence by two or more articles to merge unusual findings as an index of future investigation. We excluded articles considering the expert viewpoints and letters to the editor. A total of 325 potentially relevant records were identified. Following the exclusion of 239 citations, 86 full-text papers were retrieved for detailed examination. Finally, a total of 27 articles matched the eligibility criteria.
3. Results
Neuroimaging can disclose the cranial MRI sequences with axial T2, axial FLAIR, sagittal T2, and contrast-enhanced axial T1.
Generally, the brain MRI of demyelination in children has one of the following four patterns: poorly demarcated changes in white and or gray matter, well-demarcated white matter changes, confluent lesions in white matter, nonspecific small (<0.3 cm) lesions or nothing. These lesions more commonly involve certain regions of juxtacortical and cortical gray matter, deep and periventricular white matter, thalamus, corpus callosum, basal ganglia, cerebellum, and brainstem (8).
In orbital MRI, optic nerve lesions can be found by thin (2-3 mm) fat suppression imaging. This imaging can be achieved with fat-saturated fast-spin echo technique or fat-saturated T1-weighted imaging following contrast enhancement (9-12).
Optic nerve imaging by Short Tau Inversion Recovery (STIR) sequences may display particular high-signal intensity foci in the optic nerve. Contrast administration can enhance these lesions, but it does not occur in a healthy optic nerve. Specific MR findings of optic nerve lesions with greater length involvement or lesion within the optic canal may predict a poor visual outcome, although this is still controversial among some investigators.
The Signal Intensity Ratio (SIR) of the optic nerve to the white matter on STIR is a distinctive measure for acute optic neuritis. Patients with acute optic neuritis have higher SIRave and SIRmax than in control patients (13).NMO lesions have different types of MRI findings. They are longitudinally extensive and involve several optic nerve segments. On the contrary, MS lesions are often localized focally in one optic nerve segment (14).Diffusion-weighted and diffusion-tensor imaging may yield more pathologic information about optic nerve than conventional anatomic imaging, such as T2 signal intensity and enhancement. However, the application of these advanced technologies is too time-consuming and laborious for everyday clinical use.
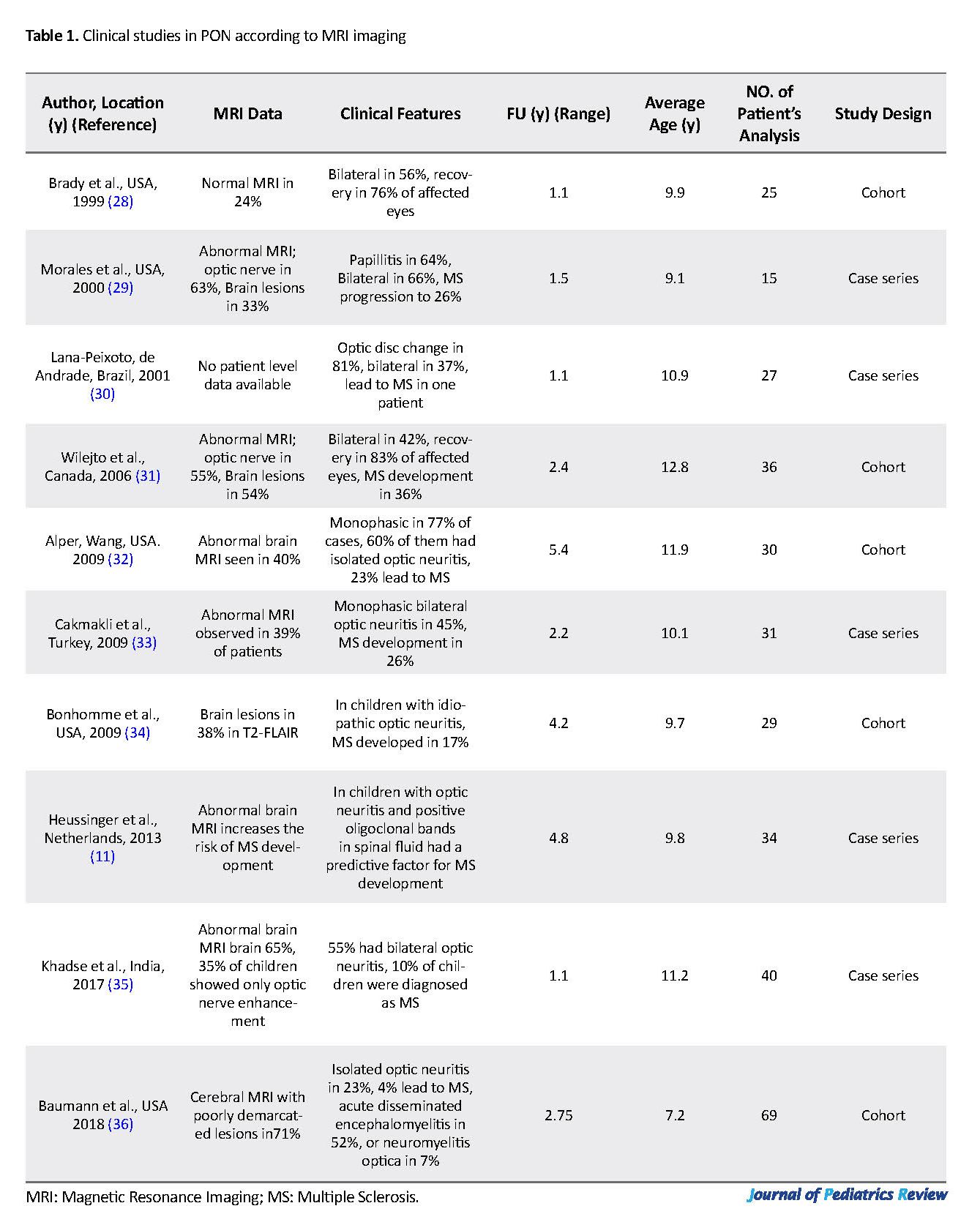
Three-dimensional Double Inversion Recovery (3D DIR) is preferred over 2-dimensional STIR for the detection of optic nerve signal abnormalities. Multiplanar DIR sequences have the foremost efficiency for the diagnosis of PON (9). In the pediatric population, MS may initially be expressed as PON. However, limited data are available about the rate of progression of isolated optic neuritis to MS. Table 1 lists the results of some relevant studies concentrated on the clinical and radio-imaging features of PON. Most of these investigations were case series or longitudinal observations. The highest rate of MS development was 36%, as reported by Wilejto et al. in Canadian children with PON (30).
Diagnosis of MS was proposed by McDonald criteria, which could also assist the early diagnosis of MS in teenagers (5, 15). Brain MRI is considered a sensitive modality for the detection of white matter lesions. The MRI in MS agreement suggests essential sequences for brain MRI include 2D or 3D contrast-enhanced T1-weighted, axial proton density, and or T2-Fluid-Attenuated Inversion Recovery (FLAIR)/T2-weighted, sagittal 2D, or 3D T2-FLAIR (16).
Diffusion tensor imaging and magnetization transfer ratio are two modern non-conventional MRI imaging techniques that were sensitive to optic nerve damage, especially in patients with prior episodes of PON. Axial diffusion-weighted imaging can differentiate an acute gadolinium-enhanced MS lesion from an acute restricted ischemic lesion (17, 18).
Although spinal MRI is highly sensitive in detecting silent lesions, it is not routinely recommended in patients without spinal cord symptoms (19). In the absence of spinal cord signs, spine imaging can probably be postponed unless an antibody against aquaporin 4 testing is positive (20).
The diagnosis of PON and MS is supported by the contrast agent administration to detect active lesions specified by the blood-brain barrier breakdown. Repeated use of contrast agents in serial MRI in a young population may bring some concerns regarding the deposition of considerable gadolinium amount in the brain (21).The MRI can predict MS in pediatric patients with demyelinating brain lesions. A recent prospective study in Canadian pediatric patients suggests that the presence of at least one black hole (a persistent hypointensity for more than 3 months on T1-weighted imaging) and at least one periventricular lesion (Dawson’s finger) were predictive parameters of MS in pediatric patients (22).
Generally, MS brain lesions become visible as well-defined, high signal ovoid-shaped areas on T2-weighted and T2-FLAIR images extend throughout the white matter, usually in the juxtacortical and periventricular regions, corpus callosum, cerebellum, and brainstem. T2-weighted imaging is a suitable technique for the detection of supratentorial, infratentorial, and spinal cord lesions; however, T2-FLAIR imaging offers a better sequence for cortical, juxtacortical, and periventricular lesions (23, 24).
Proton density-weighted imaging approaches better detect periventricular lesions with lesion-tissue contrast. This sequence is especially helpful in patients with an incomplete myelinated brain as in young children.The STIR imaging technique can identify delicate spinal cord lesions. STIR scans also give fat suppression, so properly gain sensitivity for the optic nerve and spinal cord imaging (24). Contrast-enhanced T1-weighted sequences allow differentiation of newly formed active lesions from inactive lesions with displaying different schemas of contrast enhancement. This lesion enhancement lasts for about 3 weeks and may change depending on the treatment modalities (25).
Pediatric Optic Neuritis (PON) is commonly presented as a spectrum of neuroinflammatory disorders such as Monophasic Optic Neuritis (MON), Isolated Recurrent Optic Neuritis (IRON), Neuromyelitis Optica (NMO), and Acute Disseminated Encephalomyelitis (ADEM) as well as in chronic diseases such as Multiple Sclerosis (MS).
Optic neuritis is an inflammation of the optic nerve clinically characterized by decreased visual acuity, field defects, diminished color vision, and positive Marcus Gunn in unilateral cases (1). Neuroimaging enhancement and latency of conduction on visual evoked potentials are essential findings to confirm the diagnosis. Despite the identical name, children are not little adults. Children suffer differently from adults regarding this disease. Children might present with bilateral painless optic nerve head swelling and more severe visual impairment after a prodromal viral illness. Visual recovery in children is more expectable.
Most pediatric patients with PON are older than 12 years. However, the correlation between puberty and risk associations is unknown. Children with PON are less likely to develop MS compared to adults, but they are prone to experience a preliminary representation of ADEM (2). Opticneuritisin children with its peculiar clinical features has a better prognosis than in adulthood. The main concern for therapeutics is the relationship ofopticneuritiswith multiple sclerosis (3). Diagnosis of PON is more difficult as there are unilateral, subclinical, spontaneous resolving, and poor history presentation. Also, children’s desire to pretend vision loss in order to obtain glasses is being misdiagnosed as spurious, and subsequently delays diagnosis and treatment. In a child with PON, a guided medical workup helps to differentiate the various metabolic, infectious, autoimmune, and treatable space-occupying lesions. Generally, the patients are treated with intravenous methylprednisolone, although the decision to treat varies between practitioners (4).
Among the newly developed neuroimaging techniques, Magnetic Resonance Imaging (MRI) is considered a reliable, noninvasive, and reproducible approach for the diagnosis and management of PON. Brain MRI is more sensitive to white matter lesions than the grey matter ones. MRI with high sensitivity serves as an essential component of subclinical disease activity and diagnostic criteria, especially for MS and Neuromyelitis Optica (NMO) spectrum disorders (5, 6). However, the disadvantages of MRI make it hard to detect the nature of demyelinating lesions and is an expensive modality. MRI helps to rule out the other differential diagnoses such as brain tumors that may appear similar to demyelinating lesions, without the necessity to use invasive procedures. Furthermore, it is particularly useful to detect the evolution of clinically silent lesions (7).
In this study, we just present and discuss the findings of recent investigations in which the impact on PON was assessed. However, few studies exist about this association in children growing up.
2. Evidence Acquisition
A PubMed literature search limited to the English language from 1995 to 2019 was accomplished using the following search terms: “Neuroimaging", “Pediatric Optic Neuritis", “Multiple Sclerosis", and “Magnetic Resonance Imaging". In this step, qualitative results from research studies were obtained. The articles were then reviewed to exclude those related to brain diseases (such as raised intracranial pressure), adult cases, and studies in healthy subjects as these were not the aim of this review.
For the significance of this review, case control, randomized controlled trials, cohort studies, evidence from meta-analyses, and systematic reviews were included. Case reports or case series were only included if there was specified evidence by two or more articles to merge unusual findings as an index of future investigation. We excluded articles considering the expert viewpoints and letters to the editor. A total of 325 potentially relevant records were identified. Following the exclusion of 239 citations, 86 full-text papers were retrieved for detailed examination. Finally, a total of 27 articles matched the eligibility criteria.
3. Results
Neuroimaging can disclose the cranial MRI sequences with axial T2, axial FLAIR, sagittal T2, and contrast-enhanced axial T1.
Generally, the brain MRI of demyelination in children has one of the following four patterns: poorly demarcated changes in white and or gray matter, well-demarcated white matter changes, confluent lesions in white matter, nonspecific small (<0.3 cm) lesions or nothing. These lesions more commonly involve certain regions of juxtacortical and cortical gray matter, deep and periventricular white matter, thalamus, corpus callosum, basal ganglia, cerebellum, and brainstem (8).
In orbital MRI, optic nerve lesions can be found by thin (2-3 mm) fat suppression imaging. This imaging can be achieved with fat-saturated fast-spin echo technique or fat-saturated T1-weighted imaging following contrast enhancement (9-12).
Optic nerve imaging by Short Tau Inversion Recovery (STIR) sequences may display particular high-signal intensity foci in the optic nerve. Contrast administration can enhance these lesions, but it does not occur in a healthy optic nerve. Specific MR findings of optic nerve lesions with greater length involvement or lesion within the optic canal may predict a poor visual outcome, although this is still controversial among some investigators.
The Signal Intensity Ratio (SIR) of the optic nerve to the white matter on STIR is a distinctive measure for acute optic neuritis. Patients with acute optic neuritis have higher SIRave and SIRmax than in control patients (13).NMO lesions have different types of MRI findings. They are longitudinally extensive and involve several optic nerve segments. On the contrary, MS lesions are often localized focally in one optic nerve segment (14).Diffusion-weighted and diffusion-tensor imaging may yield more pathologic information about optic nerve than conventional anatomic imaging, such as T2 signal intensity and enhancement. However, the application of these advanced technologies is too time-consuming and laborious for everyday clinical use.
Three-dimensional Double Inversion Recovery (3D DIR) is preferred over 2-dimensional STIR for the detection of optic nerve signal abnormalities. Multiplanar DIR sequences have the foremost efficiency for the diagnosis of PON (9). In the pediatric population, MS may initially be expressed as PON. However, limited data are available about the rate of progression of isolated optic neuritis to MS. Table 1 lists the results of some relevant studies concentrated on the clinical and radio-imaging features of PON. Most of these investigations were case series or longitudinal observations. The highest rate of MS development was 36%, as reported by Wilejto et al. in Canadian children with PON (30).
Diagnosis of MS was proposed by McDonald criteria, which could also assist the early diagnosis of MS in teenagers (5, 15). Brain MRI is considered a sensitive modality for the detection of white matter lesions. The MRI in MS agreement suggests essential sequences for brain MRI include 2D or 3D contrast-enhanced T1-weighted, axial proton density, and or T2-Fluid-Attenuated Inversion Recovery (FLAIR)/T2-weighted, sagittal 2D, or 3D T2-FLAIR (16).
Diffusion tensor imaging and magnetization transfer ratio are two modern non-conventional MRI imaging techniques that were sensitive to optic nerve damage, especially in patients with prior episodes of PON. Axial diffusion-weighted imaging can differentiate an acute gadolinium-enhanced MS lesion from an acute restricted ischemic lesion (17, 18).
Although spinal MRI is highly sensitive in detecting silent lesions, it is not routinely recommended in patients without spinal cord symptoms (19). In the absence of spinal cord signs, spine imaging can probably be postponed unless an antibody against aquaporin 4 testing is positive (20).
The diagnosis of PON and MS is supported by the contrast agent administration to detect active lesions specified by the blood-brain barrier breakdown. Repeated use of contrast agents in serial MRI in a young population may bring some concerns regarding the deposition of considerable gadolinium amount in the brain (21).The MRI can predict MS in pediatric patients with demyelinating brain lesions. A recent prospective study in Canadian pediatric patients suggests that the presence of at least one black hole (a persistent hypointensity for more than 3 months on T1-weighted imaging) and at least one periventricular lesion (Dawson’s finger) were predictive parameters of MS in pediatric patients (22).
Generally, MS brain lesions become visible as well-defined, high signal ovoid-shaped areas on T2-weighted and T2-FLAIR images extend throughout the white matter, usually in the juxtacortical and periventricular regions, corpus callosum, cerebellum, and brainstem. T2-weighted imaging is a suitable technique for the detection of supratentorial, infratentorial, and spinal cord lesions; however, T2-FLAIR imaging offers a better sequence for cortical, juxtacortical, and periventricular lesions (23, 24).
Proton density-weighted imaging approaches better detect periventricular lesions with lesion-tissue contrast. This sequence is especially helpful in patients with an incomplete myelinated brain as in young children.The STIR imaging technique can identify delicate spinal cord lesions. STIR scans also give fat suppression, so properly gain sensitivity for the optic nerve and spinal cord imaging (24). Contrast-enhanced T1-weighted sequences allow differentiation of newly formed active lesions from inactive lesions with displaying different schemas of contrast enhancement. This lesion enhancement lasts for about 3 weeks and may change depending on the treatment modalities (25).
Brain MR imaging of MS children is different from adults. They have a higher lesion loading on their initial scans. Lesions are larger, confluent, with indistinct borders, and deeper in grey matter, more easily decrease in T2-bright lesions. And the tumefactive lesions (lesions of >2 cm, with surrounding edema) are more common in younger children with MS (26, 27).
4. Conclusions
Brain MRI should be performed in all patients, if possible, during the two weeks after the initial diagnosis of PON.
Fat suppression MRI imaging can detect optic nerve lesions, attained either with fast spin-echo technique or contrast-enhanced T1-weighted imaging. Although the abnormal gadolinium-enhanced MRI is not diagnostic for demyelinating optic neuritis-these findings can be seen in other conditions such as neoplastic infiltrative, cytomegalovirus, rheumatics optic neuropathy—contrast enhancement and STIR may help to distinguish an acute inflammatory lesion from an acute restricted ischemic lesion. The implication of modern orbital MRI sequences in standard clinical practice assesses the patient for compressive lesions, meningeal enhancement, and inflammatory or demyelinating lesions elsewhere in the brain to obtain relevant information for assessment, treatment, and prognosis of PON. The presence of lesions, commonly oval-shaped, and located in the region of periventricular white matter is an ominous prognostic factor for the possibility of future development of MS.
Ethical Considerations
Compliance with ethical guidelines
This research had no ethical consideration.
Funding
This research received no specific grant.
Authors contributions
All three authors equally contributed to the literature search, compiling, and approving the final manuscript.
Conflict of interest
The authors have no financial or personal relations that could create a conflict of interest.
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Bu-Ali Sina Hospital for cooperation in search strategies.
References
Hierons R, Lyle TK. Bilateral retrobulbar optic neuritis. Brain. 1959; 82(1):56-67. [DOI:10.1093/brain/82.1.56] [PMID]
Rizzo JF III, Lessell S. Risk of developing multiple sclerosis after uncomplicated optic neuritis: A long-term prospective study. Neurology. 1988; 38(2):185-90. [DOI:10.1212/WNL.38.2.185] [PMID]
Pérez-Cambrodí RJ, Gómez-Hurtado Cubillana A, Merino-Suárez ML, Piñero-Llorens DP, Laria-Ochaita C. Optic neuritis in pediatric population: A review in current tendencies of diagnosis and management. Journal of Optometry. 2014; 7(3):125-30. [DOI:10.1016/j.optom.2013.12.008] [PMID] [PMCID]
Volpe NJ. The optic neuritis treatment trial: A definitive answer and profound impact with unexpected results. Archives of Ophthalmology. 2008; 126(7):996-9. [DOI:10.1001/archopht.126.7.996] [PMID]
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for Neuromyelitis Optica spectrum disorders. Neurology. 2015; 85(2):177-89. [DOI:10.1212/WNL.0000000000001729] [PMID] [PMCID]
Tornatore C, Phillips JT, Khan O, Miller AE, Barnes CJ. Practice patterns of US neurologists in patients with CIS, RRMS, or RIS: A consensus study. Neurology Clinical Practice. 2012; 2(1):48-57. [DOI:10.1212/CPJ.0b013e31824cb09b] [PMID] [PMCID]
Verhey LH, Branson HM, Laughlin S, Shrof MM, Benseler SM, Feldman BM, et al. Development of a standardized MRI scoring tool for CNS demyelination in children. American Journal of Neuroradiology. 2013; 34(6):1271-7. [DOI:10.3174/ajnr.A3382] [PMID]
Hodel J, Outteryck O, Bocher AL, Zéphir H, Lambert O, Benadjaoud MA, et al. Comparison of 3D double inversion recovery and 2D STIR FLAIR MR sequences for the imaging of optic neuritis: Pilot study. European Radiology. 2014; 24(12):3069-75. [DOI:10.1007/s00330-014-3342-3] [PMID]
Pino-Lopez L, Wenz H, Böhme J, Maros M, Schlichtenbrede F, Groden C, et al. Contrast-enhanced fat-suppressed FLAIR for the characterization of leptomeningeal inflammation in optic neuritis. Multiple Sclerosis Journal. 2019; 25(6):792-800. [DOI:10.1177/1352458518770268] [PMID]
Heussinger N, Kontopantelis E, Rompel O, Paulides M, Trollmann R. Predicting multiple sclerosis following isolated optic neuritis in children. European Journal of Neurology. 2013; 20(9):1292-6. [DOI:10.1111/ene.12184] [PMID]
Soelberg K, Skejoe HPB, Grauslund J, Smith TJ, Lillevang ST, Jarius S, et al. Magnetic resonance imaging findings at the first episode of acute optic neuritis. Multiple Sclerosis and Related Disorders Journal. 2018; 20:30-6. [DOI:10.1016/j.msard.2017.12.018] [PMID]
Onodera M, Yama N, Hashimoto M, Shonai T, Aratani K, Takashima H, et al. The signal intensity ratio of the optic nerve to ipsilateral frontal white matter is of value in the diagnosis of acute optic neuritis. European Radiology. 2016; 26(8):2640-5. [DOI:10.1007/s00330-015-4114-4] [PMID]
Mealy MA, Whetstone A, Orman G, Izbudak I, Calabresi PA, Levy M. Longitudinally extensive optic neuritis as an MRI biomarker distinguishes neuromyelitis optica from multiple sclerosis. Journal of the Neurological Sciences. 2015; 355(1-2):59-63. [DOI:10.1016/j.jns.2015.05.013] [PMID] [PMCID]
Sadaka Y, Verhey LH, Shroff MM, Branson HM, Arnold DL, Narayanan S, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Annals of Neurology. 2012; 72(2):211-23. [DOI:10.1002/ana.23575] [PMID]
Rovira A, Wattjes MP, Tintore M, Tur C, Yousry TA, Sormani MP, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nature Reviews Neurology. 2015; 11(8):471-82. [DOI:10.1038/nrneurol.2015.106] [PMID]
Naismith RT, Xu J, Tutlam NT, Lancia S, Trinkaus K, Song SK, et al. Diffusion tensor imaging in acute optic neuropathies: Predictor of clinical outcomes. Archives of Neurology. 2012; 69(1):65-71. [DOI:10.1001/archneurol.2011.243] [PMID] [PMCID]
Wang Y, van der Walt A, Paine M, Klistorner A, Butzkueven H, Egan GF, et al. Optic nerve magnetization transfer ratio after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2012; 7(12):e52291. [DOI:10.1371/journal.pone.0052291] [PMID] [PMCID]
Dalton CM, Brex PA, Miszkiel KA, Fernando K, MacManus DG, Plant GT, et al. Spinal cord MRI in clinically isolated optic neuritis. Journal of Neurology, Neurosurgery, and Psychiatry. 2003; 74(11):1577-80. [DOI:10.1136/jnnp.74.11.1577] [PMID] [PMCID]
Borchert M, Liu GT, Pineles S, Waldman AT. Pediatric Optic Neuritis: What is new. Journal of Neuro-Ophthalmology. 2017; 37(Suppl 1):S14-22. [DOI:10.1097/WNO.0000000000000551] [PMID] [PMCID]
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015; 276(1):228-32. [DOI:10.1148/radiol.2015142690] [PMID]
Verhey LH, Branson HM, Shroff MM, Callen DJ, Sled JG, Narayanan S, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: A prospective national cohort study. The Lancet Neurology. 2011; 10(12):1065-73. [DOI:10.1016/S1474-4422(11)70250-2]
Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial; UBC MS/MRI Study Group, IFNB Multiple Sclerosis Study Group. Neurology. 1993; 43(4):662-7. [DOI:10.1212/WNL.43.4.662] [PMID]
Pirko I. Neuroimaging of demyelinating diseases. Continuum: Lifelong Learning in Neurology. 2008; 14(4, Neuroimaging):118-43. [DOI:10.1212/01.CON.0000333203.65171.52]
Verhey LH, Narayanan S, Banwell B. Standardized magnetic resonance imaging acquisition and reporting in pediatric multiple sclerosis. Neuroimaging Clinics of North America. 2013; 23(2):217-26. [DOI:10.1016/j.nic.2012.12.003] [PMID]
Waubant E, Chabas D. Pediatric multiple sclerosis. Current Treatment Options in Neurology. 2009; 11(3):203-10. [DOI:10.1007/s11940-009-0024-6] [PMID]
Chabas D, Castillo-Trivino T, Mowry EM, Strober JB, Glenn OA, Waubant E. Vanishing MS T2-bright lesions before puberty: A distinct MRI phenotype. Neurology. 2008; 71(14):1090-3. [DOI:10.1212/01.wnl.0000326896.66714.ae] [PMID]
Brady KM, Brar AS, Lee AG, Coats DK, Paysse EA, Steinkuller PG. Optic neuritis in children: Clinical features and visual outcome. Journal of AAPOS. 1999; 3(2):98-103. [DOI:10.1016/S1091-8531(99)70078-9]
Morales DS, Siatkowski RM, Howard CW, Warman R. Optic neuritis in children. Journal of Pediatric Ophthalmology and Strabismus. 2000; 37(5):254-9. [PMID]
Lana-Peixoto MA, Andrade GC. The clinical profile of children optic neutitis. Arquivos de Neuro-Psiquiatria. 2001; 59(2-B):311-7. [DOI:10.1590/S0004-282X2001000300001] [PMID]
Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006; 67(2):258-62. [DOI:10.1212/01.wnl.0000224757.69746.fb] [PMID]
Alper G, Wang L. Demyelinating optic neuritis in children. Journal of Child Neurology. 2009; 24(1):45-8. [DOI:10.1177/0883073808321052] [PMID] [PMCID]
Cakmakli G, Kurne A, Güven A, Serdaroğlu A, Topaloğlu H, Teber S, et al. Childhood optic neuritis: The pediatric neurologist’s perspective. European Journal of Paediatric Neurology. 2009; 13(5):452-7. [DOI:10.1016/j.ejpn.2008.09.003] [PMID]
Bonhomme GR, Waldman AT, Balcer LJ, Daniels AB, Tennekoon GI, Forman S, et al. Pediatric optic neuritis: Brain MRI abnormalities and risk of multiple sclerosis. Neurology. 2009; 72(10):881-5. [DOI:10.1212/01.wnl.0000344163.65326.48] [PMID]
Khadse R, Ravindran M, Pawar N, Maharajan P, Rengappa R. Clinical profile and neuroimaging in pediatric optic neuritis in Indian population: A case series. Indian Journal of Ophthalmology. 2017; 65(3):242-5. [DOI:10.4103/ijo.IJO_939_16] [PMID] [PMCID]
Baumann M, Grams A, Djurdjevic T, Wendel TM, Lechner C, Behring B, et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. Journal of Neurology. 2018; 265(4):845-55. [DOI:10.1007/s00415-018-8781-3] [PMID]
4. Conclusions
Brain MRI should be performed in all patients, if possible, during the two weeks after the initial diagnosis of PON.
Fat suppression MRI imaging can detect optic nerve lesions, attained either with fast spin-echo technique or contrast-enhanced T1-weighted imaging. Although the abnormal gadolinium-enhanced MRI is not diagnostic for demyelinating optic neuritis-these findings can be seen in other conditions such as neoplastic infiltrative, cytomegalovirus, rheumatics optic neuropathy—contrast enhancement and STIR may help to distinguish an acute inflammatory lesion from an acute restricted ischemic lesion. The implication of modern orbital MRI sequences in standard clinical practice assesses the patient for compressive lesions, meningeal enhancement, and inflammatory or demyelinating lesions elsewhere in the brain to obtain relevant information for assessment, treatment, and prognosis of PON. The presence of lesions, commonly oval-shaped, and located in the region of periventricular white matter is an ominous prognostic factor for the possibility of future development of MS.
Ethical Considerations
Compliance with ethical guidelines
This research had no ethical consideration.
Funding
This research received no specific grant.
Authors contributions
All three authors equally contributed to the literature search, compiling, and approving the final manuscript.
Conflict of interest
The authors have no financial or personal relations that could create a conflict of interest.
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Bu-Ali Sina Hospital for cooperation in search strategies.
References
Hierons R, Lyle TK. Bilateral retrobulbar optic neuritis. Brain. 1959; 82(1):56-67. [DOI:10.1093/brain/82.1.56] [PMID]
Rizzo JF III, Lessell S. Risk of developing multiple sclerosis after uncomplicated optic neuritis: A long-term prospective study. Neurology. 1988; 38(2):185-90. [DOI:10.1212/WNL.38.2.185] [PMID]
Pérez-Cambrodí RJ, Gómez-Hurtado Cubillana A, Merino-Suárez ML, Piñero-Llorens DP, Laria-Ochaita C. Optic neuritis in pediatric population: A review in current tendencies of diagnosis and management. Journal of Optometry. 2014; 7(3):125-30. [DOI:10.1016/j.optom.2013.12.008] [PMID] [PMCID]
Volpe NJ. The optic neuritis treatment trial: A definitive answer and profound impact with unexpected results. Archives of Ophthalmology. 2008; 126(7):996-9. [DOI:10.1001/archopht.126.7.996] [PMID]
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Annals of Neurology. 2011; 69(2):292-302. [DOI:10.1002/ana.22366] [PMID] [PMCID]
Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for Neuromyelitis Optica spectrum disorders. Neurology. 2015; 85(2):177-89. [DOI:10.1212/WNL.0000000000001729] [PMID] [PMCID]
Tornatore C, Phillips JT, Khan O, Miller AE, Barnes CJ. Practice patterns of US neurologists in patients with CIS, RRMS, or RIS: A consensus study. Neurology Clinical Practice. 2012; 2(1):48-57. [DOI:10.1212/CPJ.0b013e31824cb09b] [PMID] [PMCID]
Verhey LH, Branson HM, Laughlin S, Shrof MM, Benseler SM, Feldman BM, et al. Development of a standardized MRI scoring tool for CNS demyelination in children. American Journal of Neuroradiology. 2013; 34(6):1271-7. [DOI:10.3174/ajnr.A3382] [PMID]
Hodel J, Outteryck O, Bocher AL, Zéphir H, Lambert O, Benadjaoud MA, et al. Comparison of 3D double inversion recovery and 2D STIR FLAIR MR sequences for the imaging of optic neuritis: Pilot study. European Radiology. 2014; 24(12):3069-75. [DOI:10.1007/s00330-014-3342-3] [PMID]
Pino-Lopez L, Wenz H, Böhme J, Maros M, Schlichtenbrede F, Groden C, et al. Contrast-enhanced fat-suppressed FLAIR for the characterization of leptomeningeal inflammation in optic neuritis. Multiple Sclerosis Journal. 2019; 25(6):792-800. [DOI:10.1177/1352458518770268] [PMID]
Heussinger N, Kontopantelis E, Rompel O, Paulides M, Trollmann R. Predicting multiple sclerosis following isolated optic neuritis in children. European Journal of Neurology. 2013; 20(9):1292-6. [DOI:10.1111/ene.12184] [PMID]
Soelberg K, Skejoe HPB, Grauslund J, Smith TJ, Lillevang ST, Jarius S, et al. Magnetic resonance imaging findings at the first episode of acute optic neuritis. Multiple Sclerosis and Related Disorders Journal. 2018; 20:30-6. [DOI:10.1016/j.msard.2017.12.018] [PMID]
Onodera M, Yama N, Hashimoto M, Shonai T, Aratani K, Takashima H, et al. The signal intensity ratio of the optic nerve to ipsilateral frontal white matter is of value in the diagnosis of acute optic neuritis. European Radiology. 2016; 26(8):2640-5. [DOI:10.1007/s00330-015-4114-4] [PMID]
Mealy MA, Whetstone A, Orman G, Izbudak I, Calabresi PA, Levy M. Longitudinally extensive optic neuritis as an MRI biomarker distinguishes neuromyelitis optica from multiple sclerosis. Journal of the Neurological Sciences. 2015; 355(1-2):59-63. [DOI:10.1016/j.jns.2015.05.013] [PMID] [PMCID]
Sadaka Y, Verhey LH, Shroff MM, Branson HM, Arnold DL, Narayanan S, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Annals of Neurology. 2012; 72(2):211-23. [DOI:10.1002/ana.23575] [PMID]
Rovira A, Wattjes MP, Tintore M, Tur C, Yousry TA, Sormani MP, et al. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis-clinical implementation in the diagnostic process. Nature Reviews Neurology. 2015; 11(8):471-82. [DOI:10.1038/nrneurol.2015.106] [PMID]
Naismith RT, Xu J, Tutlam NT, Lancia S, Trinkaus K, Song SK, et al. Diffusion tensor imaging in acute optic neuropathies: Predictor of clinical outcomes. Archives of Neurology. 2012; 69(1):65-71. [DOI:10.1001/archneurol.2011.243] [PMID] [PMCID]
Wang Y, van der Walt A, Paine M, Klistorner A, Butzkueven H, Egan GF, et al. Optic nerve magnetization transfer ratio after acute optic neuritis predicts axonal and visual outcomes. PLoS One. 2012; 7(12):e52291. [DOI:10.1371/journal.pone.0052291] [PMID] [PMCID]
Dalton CM, Brex PA, Miszkiel KA, Fernando K, MacManus DG, Plant GT, et al. Spinal cord MRI in clinically isolated optic neuritis. Journal of Neurology, Neurosurgery, and Psychiatry. 2003; 74(11):1577-80. [DOI:10.1136/jnnp.74.11.1577] [PMID] [PMCID]
Borchert M, Liu GT, Pineles S, Waldman AT. Pediatric Optic Neuritis: What is new. Journal of Neuro-Ophthalmology. 2017; 37(Suppl 1):S14-22. [DOI:10.1097/WNO.0000000000000551] [PMID] [PMCID]
Kanda T, Fukusato T, Matsuda M, Toyoda K, Oba H, Kotoku J, et al. Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: Evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology. 2015; 276(1):228-32. [DOI:10.1148/radiol.2015142690] [PMID]
Verhey LH, Branson HM, Shroff MM, Callen DJ, Sled JG, Narayanan S, et al. MRI parameters for prediction of multiple sclerosis diagnosis in children with acute CNS demyelination: A prospective national cohort study. The Lancet Neurology. 2011; 10(12):1065-73. [DOI:10.1016/S1474-4422(11)70250-2]
Paty DW, Li DK. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. II. MRI analysis results of a multicenter, randomized, double-blind, placebo-controlled trial; UBC MS/MRI Study Group, IFNB Multiple Sclerosis Study Group. Neurology. 1993; 43(4):662-7. [DOI:10.1212/WNL.43.4.662] [PMID]
Pirko I. Neuroimaging of demyelinating diseases. Continuum: Lifelong Learning in Neurology. 2008; 14(4, Neuroimaging):118-43. [DOI:10.1212/01.CON.0000333203.65171.52]
Verhey LH, Narayanan S, Banwell B. Standardized magnetic resonance imaging acquisition and reporting in pediatric multiple sclerosis. Neuroimaging Clinics of North America. 2013; 23(2):217-26. [DOI:10.1016/j.nic.2012.12.003] [PMID]
Waubant E, Chabas D. Pediatric multiple sclerosis. Current Treatment Options in Neurology. 2009; 11(3):203-10. [DOI:10.1007/s11940-009-0024-6] [PMID]
Chabas D, Castillo-Trivino T, Mowry EM, Strober JB, Glenn OA, Waubant E. Vanishing MS T2-bright lesions before puberty: A distinct MRI phenotype. Neurology. 2008; 71(14):1090-3. [DOI:10.1212/01.wnl.0000326896.66714.ae] [PMID]
Brady KM, Brar AS, Lee AG, Coats DK, Paysse EA, Steinkuller PG. Optic neuritis in children: Clinical features and visual outcome. Journal of AAPOS. 1999; 3(2):98-103. [DOI:10.1016/S1091-8531(99)70078-9]
Morales DS, Siatkowski RM, Howard CW, Warman R. Optic neuritis in children. Journal of Pediatric Ophthalmology and Strabismus. 2000; 37(5):254-9. [PMID]
Lana-Peixoto MA, Andrade GC. The clinical profile of children optic neutitis. Arquivos de Neuro-Psiquiatria. 2001; 59(2-B):311-7. [DOI:10.1590/S0004-282X2001000300001] [PMID]
Wilejto M, Shroff M, Buncic JR, Kennedy J, Goia C, Banwell B. The clinical features, MRI findings, and outcome of optic neuritis in children. Neurology. 2006; 67(2):258-62. [DOI:10.1212/01.wnl.0000224757.69746.fb] [PMID]
Alper G, Wang L. Demyelinating optic neuritis in children. Journal of Child Neurology. 2009; 24(1):45-8. [DOI:10.1177/0883073808321052] [PMID] [PMCID]
Cakmakli G, Kurne A, Güven A, Serdaroğlu A, Topaloğlu H, Teber S, et al. Childhood optic neuritis: The pediatric neurologist’s perspective. European Journal of Paediatric Neurology. 2009; 13(5):452-7. [DOI:10.1016/j.ejpn.2008.09.003] [PMID]
Bonhomme GR, Waldman AT, Balcer LJ, Daniels AB, Tennekoon GI, Forman S, et al. Pediatric optic neuritis: Brain MRI abnormalities and risk of multiple sclerosis. Neurology. 2009; 72(10):881-5. [DOI:10.1212/01.wnl.0000344163.65326.48] [PMID]
Khadse R, Ravindran M, Pawar N, Maharajan P, Rengappa R. Clinical profile and neuroimaging in pediatric optic neuritis in Indian population: A case series. Indian Journal of Ophthalmology. 2017; 65(3):242-5. [DOI:10.4103/ijo.IJO_939_16] [PMID] [PMCID]
Baumann M, Grams A, Djurdjevic T, Wendel TM, Lechner C, Behring B, et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. Journal of Neurology. 2018; 265(4):845-55. [DOI:10.1007/s00415-018-8781-3] [PMID]
Type of Study: Narrative Review |
Subject:
Radiology
Received: 2019/06/12 | Accepted: 2019/08/28 | Published: 2020/04/1
Received: 2019/06/12 | Accepted: 2019/08/28 | Published: 2020/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |