Volume 8, Issue 3 (7-2020)
J. Pediatr. Rev 2020, 8(3): 175-180 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sobouti B, Otukesh H, Seirafianpour F, Nakhaie S, Rahimzadeh N, Sayyahfar S et al . Post-Kidney Transplantation Epstein-Barr Virus Infection in Children: Case Series Study. J. Pediatr. Rev 2020; 8 (3) :175-180
URL: http://jpr.mazums.ac.ir/article-1-265-en.html
URL: http://jpr.mazums.ac.ir/article-1-265-en.html
Behnam Sobouti1 

 , Hasan Otukesh2
, Hasan Otukesh2 

 , Farnoosh Seirafianpour3
, Farnoosh Seirafianpour3 

 , Shahrbanoo Nakhaie4
, Shahrbanoo Nakhaie4 
 , Nahid Rahimzadeh2
, Nahid Rahimzadeh2 
 , Shirin Sayyahfar1
, Shirin Sayyahfar1 
 , Rozita Hoseini *5
, Rozita Hoseini *5 




 , Hasan Otukesh2
, Hasan Otukesh2 

 , Farnoosh Seirafianpour3
, Farnoosh Seirafianpour3 

 , Shahrbanoo Nakhaie4
, Shahrbanoo Nakhaie4 
 , Nahid Rahimzadeh2
, Nahid Rahimzadeh2 
 , Shirin Sayyahfar1
, Shirin Sayyahfar1 
 , Rozita Hoseini *5
, Rozita Hoseini *5 


1- Department of Pediatric Infectious Diseases, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Pediatric Nephrology, Iran University of Medical Sciences, Tehran, Iran.
3- Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Pediatric Gastroenterology, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Pediatric Nephrology, Iran University of Medical Sciences, Tehran, Iran. ,rozitahoseini@yahoo.com
2- Department of Pediatric Nephrology, Iran University of Medical Sciences, Tehran, Iran.
3- Student Research Committee, School of Medicine, Iran University of Medical Sciences, Tehran, Iran.
4- Department of Pediatric Gastroenterology, Iran University of Medical Sciences, Tehran, Iran.
5- Department of Pediatric Nephrology, Iran University of Medical Sciences, Tehran, Iran. ,
Full-Text [PDF 395 kb]
(2820 Downloads)
| Abstract (HTML) (6260 Views)
Full-Text: (1870 Views)
1. Introduction
One of the main problems following organ transplantation is the spread of various microbial infections, especially opportunistic ones. One of these infections is caused by the Epstein-Barr Virus (EBV) (1-3). The virus exacerbates the immune system and treatment with immunosuppressive medications could aggravate this infection (4, 5). Some researchers believe that the virus could disrupt and stimulate the immune system and is prone to reject kidney transplantation (6). Individuals who are transplanted will be infected through various manners, including a virus-infected donor or blood transfusion. Only in the United States, approximately 50% of children aged <5 years are infected with EBV (7). The infection is transmitted through contact with oral secretions and remains in the body for life after infection (8). The prevalence of this disease is directly related to a younger age at the time of transplantation, as well as living status in high-income, underdeveloped, and populous countries (9, 10). The active infection with this virus could be manifested as mononucleosis, fever, leukopenia, and lymphocytosis. Post-transplant Lymphoproliferative Disease (PTLD) (11) could also occur after being infected with this virus (12). After the spread of the virus in the entire body, some serious complications appear, such as spleen rupture, aplastic anemia, hemolytic anemia, thrombocytopenia, neutropenia, as well as neurological, respiratory, cardiac, renal, and hepatic conditions (13, 14). This virus is a significant cause of lymphoma, especially Burkitt type (15). Overall, EBV virus plays a putative pathophysiological role in the development of Post-Transplant Lymphoproliferative Disease (PTLD). In children undergoing kidney transplantation, EBV-related conditions, such as PTLD are among the main problems associated with several immunosuppressive medications, like tacrolimus (16). Studies have revealed that 80%-90% of patients, in the first year after transplantation are infected with the secondary infection of EBV (17). According to the guidelines, in children undergoing kidney transplantation, the virus load should be monitored and controlled, especially in those with a negative serum level who received tissue from a positive donor (18).
All the explored presentations and studies have supported the effect of EBV infection on the rejection of the transplant or PTLD, requiring a more precise assessment of the patients. Studies on the rate of EBV infection in Iranian children after transplantation are scarce; thus, we aimed to determine the prevalence of EBV infection in children undergoing kidney transplantation by exploring the virus titers before and after the transplantation. We also conducted a brief review concerning post-kidney transplantation EBV infection in children.
2. Methods
In this study, all children who underwent kidney transplantation at Ali-e-Asghar Hospital from 23 September 2015 to 22 September 2016 were included. They were 16 children with advanced renal disease who were candidates for renal transplantation and were assessed, retrospectively. A checklist was used to collect the required data by reviewing the hospital records. The pointed information included age, gender, the cause of renal insufficiency, pre-transplantation viral serology in patients, transplantation time, childhood underlying illnesses, patient’s medications, and post-transplant serology results in donors and recipients. The EBV serology was assessed by virus Deoxyribonucleic Acid (DNA) quantitative assessment using the Polymerase Chain Reaction (PCR) technique. For this purpose, the Artus EBV RG PCR Kit and the real-time PCR kit were used with the sensitivity of 200 copies/UL and the specificity of 95%.
The obtained data were analyzed by SPSS. Moreover, quantitative results were expressed by Mean±SD, and the normality of variables was established by the Kolmogorov-Smirnov test. For comparing the categorical variables, Chi-squared test or Fisher’s Exact test were employed. For comparing the quantitative variables, Independent Samples t-test or Mann-Whitney U test were implemented. P<0.05 was considered as statistically significant. The relevant ethics code was also obtained for this study (Code: IR.IUMS.FMD.REC.1396).
3. Results
In total, 16 children (Mean±SD age: 11.26±3.4 years at the time of transplantation, 75% male) were assessed in the present study. The main causes of kidney insufficiency included cystinosis in 4 (25.0%) children, Focal Necrotizing Glomerulonephritis (FNGN) in 3 (18.8%) children, polycystic kidney in one (6.3%) child, hypoplastic kidney in one (6.3%) child, neurogenic bladder in one (6.3%) child, bilateral reflux in one (6.3%) child, Posterior Urethral Valve (PUV) in one (6.3%) child, and unknown causes for 4 (25%) cases (Table1).
One of the main problems following organ transplantation is the spread of various microbial infections, especially opportunistic ones. One of these infections is caused by the Epstein-Barr Virus (EBV) (1-3). The virus exacerbates the immune system and treatment with immunosuppressive medications could aggravate this infection (4, 5). Some researchers believe that the virus could disrupt and stimulate the immune system and is prone to reject kidney transplantation (6). Individuals who are transplanted will be infected through various manners, including a virus-infected donor or blood transfusion. Only in the United States, approximately 50% of children aged <5 years are infected with EBV (7). The infection is transmitted through contact with oral secretions and remains in the body for life after infection (8). The prevalence of this disease is directly related to a younger age at the time of transplantation, as well as living status in high-income, underdeveloped, and populous countries (9, 10). The active infection with this virus could be manifested as mononucleosis, fever, leukopenia, and lymphocytosis. Post-transplant Lymphoproliferative Disease (PTLD) (11) could also occur after being infected with this virus (12). After the spread of the virus in the entire body, some serious complications appear, such as spleen rupture, aplastic anemia, hemolytic anemia, thrombocytopenia, neutropenia, as well as neurological, respiratory, cardiac, renal, and hepatic conditions (13, 14). This virus is a significant cause of lymphoma, especially Burkitt type (15). Overall, EBV virus plays a putative pathophysiological role in the development of Post-Transplant Lymphoproliferative Disease (PTLD). In children undergoing kidney transplantation, EBV-related conditions, such as PTLD are among the main problems associated with several immunosuppressive medications, like tacrolimus (16). Studies have revealed that 80%-90% of patients, in the first year after transplantation are infected with the secondary infection of EBV (17). According to the guidelines, in children undergoing kidney transplantation, the virus load should be monitored and controlled, especially in those with a negative serum level who received tissue from a positive donor (18).
All the explored presentations and studies have supported the effect of EBV infection on the rejection of the transplant or PTLD, requiring a more precise assessment of the patients. Studies on the rate of EBV infection in Iranian children after transplantation are scarce; thus, we aimed to determine the prevalence of EBV infection in children undergoing kidney transplantation by exploring the virus titers before and after the transplantation. We also conducted a brief review concerning post-kidney transplantation EBV infection in children.
2. Methods
In this study, all children who underwent kidney transplantation at Ali-e-Asghar Hospital from 23 September 2015 to 22 September 2016 were included. They were 16 children with advanced renal disease who were candidates for renal transplantation and were assessed, retrospectively. A checklist was used to collect the required data by reviewing the hospital records. The pointed information included age, gender, the cause of renal insufficiency, pre-transplantation viral serology in patients, transplantation time, childhood underlying illnesses, patient’s medications, and post-transplant serology results in donors and recipients. The EBV serology was assessed by virus Deoxyribonucleic Acid (DNA) quantitative assessment using the Polymerase Chain Reaction (PCR) technique. For this purpose, the Artus EBV RG PCR Kit and the real-time PCR kit were used with the sensitivity of 200 copies/UL and the specificity of 95%.
The obtained data were analyzed by SPSS. Moreover, quantitative results were expressed by Mean±SD, and the normality of variables was established by the Kolmogorov-Smirnov test. For comparing the categorical variables, Chi-squared test or Fisher’s Exact test were employed. For comparing the quantitative variables, Independent Samples t-test or Mann-Whitney U test were implemented. P<0.05 was considered as statistically significant. The relevant ethics code was also obtained for this study (Code: IR.IUMS.FMD.REC.1396).
3. Results
In total, 16 children (Mean±SD age: 11.26±3.4 years at the time of transplantation, 75% male) were assessed in the present study. The main causes of kidney insufficiency included cystinosis in 4 (25.0%) children, Focal Necrotizing Glomerulonephritis (FNGN) in 3 (18.8%) children, polycystic kidney in one (6.3%) child, hypoplastic kidney in one (6.3%) child, neurogenic bladder in one (6.3%) child, bilateral reflux in one (6.3%) child, Posterior Urethral Valve (PUV) in one (6.3%) child, and unknown causes for 4 (25%) cases (Table1).
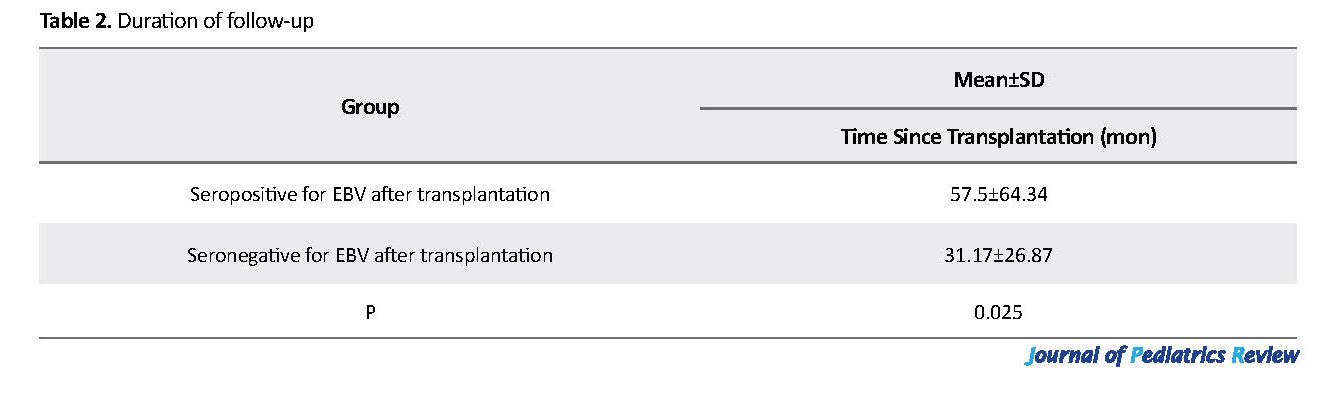
Regarding underlying disorders, 10 (62.4%) children had no history of diagnosed disorders; one (6.3%) child had Attention-Deficit/Hyperactivity Disorder (ADHD), one (6.3%) child had hypothyroidism, and 4 (25.0%) children had hypertension. Overall, 7 (43.7%) children received the kidney from survived ones, and 4 (25.0%) from deceased donors, while the donors remained unknown in other children (31.3%). The Mean±SD of time since transplantation was 34.92±31.93 months ranging from 5 to 103 months (Table 2).
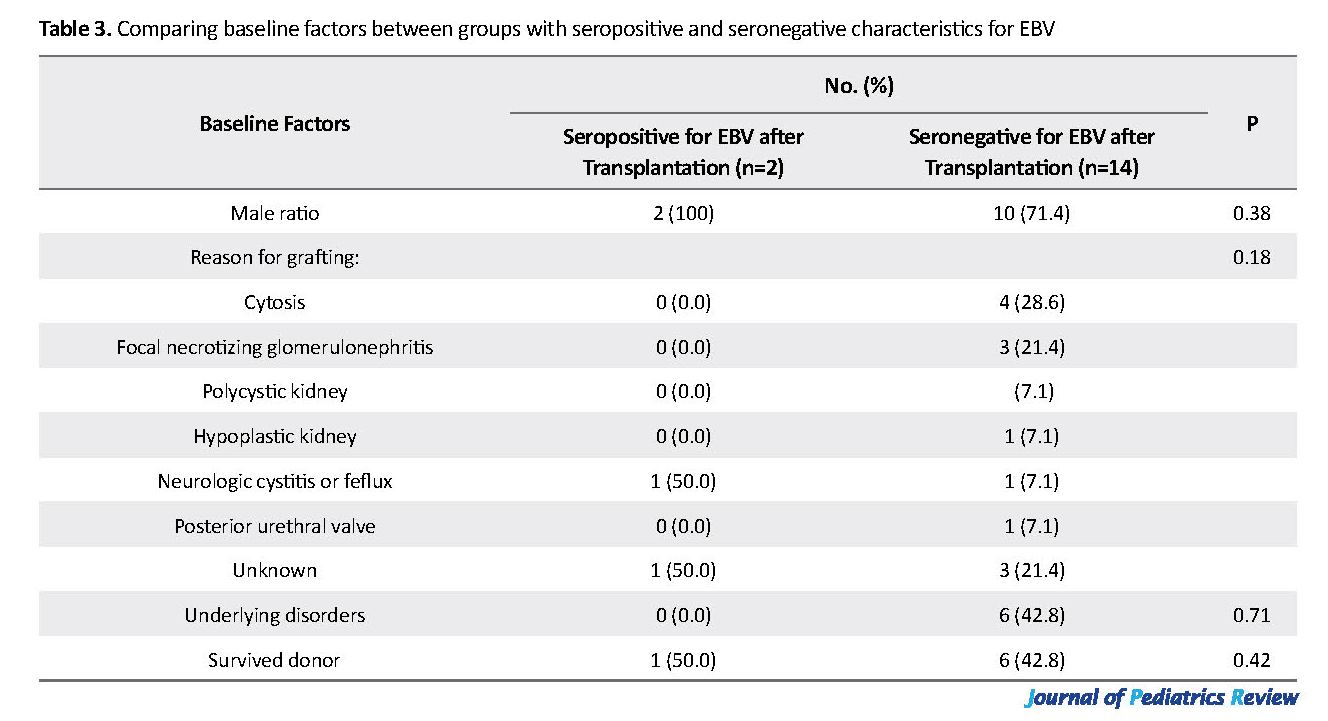
All explored patients were seronegative for EBV infection before transplantation. The immunosuppression regime after transplantation was the same in all children, including Cellcept, tacrolimus, and prednisolone. Of the studied children, only two (12.5%) males had positive serology after transplantation (with the loads of 278 copies/mL & 14655 copies/mL); however, none resulted in the rejection of kidney transplantation. We detected no significant association between the odds of serological positivity for EBV and patients’ gender (P=0.38), causes for kidney insufficiency before transplantation (P=0.18), baseline underlying disorders (P=0.71), initial medications (P=0.91), the type of donor (P=0.42), and the mean age (P=0.19). However, the duration after transplantation was significantly longer in the children with positive serology after transplantation (P=0.025) (Table 3).
4. Discussion
The present study determined the prevalence of EBV infection and transplant rejection among 16 children in terms of age, gender, cause of failure, the pre-transplantation serology of virus, childhood underlying diseases, patient’s medications, donor, and post-transplant viral serology. We observed that all of the investigated children had negative serology for EBV before transplantation. Eventually, only two children had positive post-transplantation serology; none of which presented complications and symptoms of the virus infection. Besides, none of these children experienced organ rejection. In other words, low seropositivity for EBV after transplantation could explain the lack of evidence for serious complications of post-transplantation, like PTLD in our study subjects. In a similar study by Comak et al., prior to transplantation, EBV was positive in 86.7% of the cases. The follow-up period was 36 months; half of the explored children with negative EBV developed primary EBV infection, and two patients with primary infection developed PTLD (18). Suzuki et al. studied 32 children, of whom, 6 (18.7%) were EBV-seronegative before the transplantation that the conversion to seropositivity occurred in 5 of them after grafting (19). In an study by Armstrong et al. rises to EBV antibody titers occurred in 32% of children after transplantation (20).
Every year, some children develop advanced renal disease. Advances in surgical procedures, such as kidney transplantation and immunosuppression therapy have dramatically reduced the mortality rate induced by kidney failure. A problem developed after organ transplantation is introducing various microbial infections, especially opportunistic infections, like EBV. The virus exacerbates the immune system and treatment with immunosuppressive drugs aggravates this infection. Some researchers believe that the virus could disrupt and stimulate the immune system and is prone to reject kidney transplantation. EBV significantly affects the progression of patients to PTLD after transplantation and is the strongest predictor of this condition. The higher the EBV virus load is in the body, the greater the risk of PTLD generation.
The literature indicates a wide range of seropositivity for EBV within post-transplantation period from 3% to 96% regardless of the seopositivity for this virus before transplantation. Moreover, the prevalence rate of PTLD development after transplantation also varied; however, the strong association between this complication and seropositivity to EBV was clearly presented. As per some studies, several factors could influence the rate of EBV-seropositivity as well as the occurrence of its related PTLD after transplantation. Such conditions include the employed immunosuppressive regimen, patients’ age, the industrialization level of country, and the time past since renal transplantation (1, 21-25). Different studies reported an association between age and the rate of EBV seropositivity. In line with our study, a relationship was suggested between the time after transplantation and the risk for viral seropositivity; however, unlike our study, a high rate of EBV infection, approaching 100% within three months after transplantation was reported (21).
5. Conclusion
Of the investigated children undergoing renal transplantation, none were seropositive to EBV before transplantation; while 12.5% were convert to EBV seropositivity after transplantation. None of the studied children experienced severe post-transplantation complications, such as PTLD and rejection. The odds of EBV seropositivity was only linked to the time interval since the transplantation. These results represent an appropriate use of medication and suitable patient training in this center. This study can be extended to the whole population of Iran because the research patients were selected from all cities of Iran; they referred to Ali-e-Asghar Hospital in Tehran to receive treatment. The limitation of this study was the small sample size; thus, conducting further studies with a high sample size is recommended.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information. Moreover, they were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed in designing, running, and writing all parts of the research.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors would like to express their gratitude to Ali Asghar Clinical Research Development Center.
References
Le J, Durand CM, Agha I, Brennan DC. Epstein-Barr virus and renal transplantation. Transplantation Reviews. 2017; 31(1):55-60. [DOI:10.1016/j.trre.2016.12.001] [PMID]
Paulsen G, Cumagun P, Mixon E, Fowler K, Feig D, Shimamura M. Cytomegalovirus and Epstein‐Barr Virus infections among pediatric kidney transplant recipients at a center using universal Valganciclovir Prophylaxis. Pediatric Transplantation. 2019; 23(3):e13382. [DOI:10.1111/petr.13382] [PMID] [PMCID]
Laurent A, Klich A, Roy P, Lina B, Kassai B, Bacchetta J, Cochat P. Pediatric renal transplantation: A retrospective single‐center study on epidemiology and morbidity due to EBV. Pediatric Transplantation. 2018; 22(3):e13151. [DOI:10.1111/petr.13151] [PMID]
Allen U, Alfieri C, Preiksaitis J, Humar A, Moore D, Tapiero B, et al. Epstein-Barr Virus infection in transplant recipients: Sumary of a workshop on surveillance, prevention and treatment. Canadian Journal of Infectious Diseases and Medical Microbiology. 2002; 13(2):89-99. [DOI:10.1155/2002/634318] [PMID] [PMCID
Huang FA, Danziger-Isakov L. Infectious disease risks in pediatric renal transplantation. Pediatric Nephrology. 2019; 34(7):1155-66. [DOI:10.1007/s00467-018-3951-1] [PMID]
Martinez OM, Krams SM. The immune response to Epstein Barr Virus and implications for posttransplant lymphoproliferative disorder. Transplantation. 2017; 101(9):2009. [DOI:10.1097/TP.0000000000001767] [PMID] [PMCID]
Condon LM, Cederberg LE, Rabinovitch MD, Liebo RV, Go JC, Delaney AS, et al. Age-specific prevalence of Epstein-Barr Virus infection among Minnesota children: Effects of race/ethnicity and family environment. Clinical Infectious Diseases. 2014; 59(4):501-8. [DOI:10.1093/cid/ciu342] [PMID]
Chesnokova LS, Hutt-Fletcher LM. Epstein-Barr virus infection mechanisms. Chinese Journal of Cancer. 2014; 33(11):545. [DOI:10.5732/cjc.014.10168] [PMID] [PMCID]
Gequelin LC, Riediger IN, Nakatani SM, Biondo AW, Bonfim CM. Epstein-Barr Virus: General factors, virus-related diseases and measurement of viral load after transplant. Revista Brasileira de Hematologia e Hemoterapia. 2011; 33(5):383-8. [DOI:10.5581/1516-8484.20110103] [PMID] [PMCID]
Higgins CD, Swerdlow AJ, Macsween KF, Harrison N, Williams H, McAulay K, Thomas R, Reid S, Conacher M, Britton K, Crawford DH. A study of risk factors for acquisition of Epstein-Barr Virus and its subtypes. Journal of Infectious Diseases. 2007; 195(4):474-82. [DOI:10.1086/510854] [PMID]
Ladfors SW, Lindahl JK, Hansson S, Brandström P, Andersson R, Jertborn M, et al. Long-lasting chronic high load carriage of Epstein-Barr Virus is more common in young pediatric renal transplant recipients. Pediatric Nephrology. 2019; 4:1-3. [DOI:10.1007/s00467-019-04401-9] [PMID] [PMCID]
Green M, Michaels MG. Epstein-Barr Virus infection and posttransplant lymphoproliferative disorder. American Journal of Transplantation. 2013; 13(s3):41-54. [DOI:10.1111/ajt.12004] [PMID]
Alpert G, Fleisher GR. Complications of infection with Epstein-Barr Virus during childhood: A study of children admitted to the hospital. Pediatric Infectious Disease. 1984; 3(4):304-7. [DOI:10.1097/00006454-198407000-00005] [PMID]
Jenson HB. Acute complications of Epstein-Barr Virus infectious mononucleosis. Current Opinion in Pediatrics. 2000; 12(3):263-8. [DOI:10.1097/00008480-200006000-00016] [PMID]
Rowe M, Fitzsimmons L, Bell AI. Epstein-Barr Virus and Burkitt lymphoma. Chinese Journal of Cancer. 2014; 33(12):609. [DOI:10.5732/cjc.014.10190] [PMID] [PMCID]
Lim WH, Russ GR, Coates PT. Review of Epstein-Barr Virus and post‐transplant lymphoproliferative disorder post‐solid organ transplantation. Nephrology. 2006; 11(4):355-66. [DOI:10.1111/j.1440-1797.2006.00596.x] [PMID]
Höcker B, Fickenscher H, Delecluse HJ, Böhm S, Küsters U, Schnitzler P, et al. Epidemiology and morbidity of Epstein-Barr Virus infection in pediatric renal transplant recipients: A multicenter, prospective study. Clinical Infectious Diseases. 2012; 56(1):84-92. [DOI:10.1093/cid/cis823] [PMID]
Comak E, Akman S, Ongut G, Colak D, Koyun M, Dogan CS, et al. Epstein-Barr Virus infection in children with renal transplantation: 17 years experience at a single center. Renal Failure. 2014; 36(5):760-6. [DOI:10.3109/0886022X.2014.890861] [PMID]
Suzuki T, Ikezumi Y, Okubo S, Uchiyama M, Takahashi K, Shiraga H, et al. Epstein-Barr Virus DNA load and seroconversion in pediatric renal transplantation with tacrolimus immunosuppression. Pediatric Transplantation. 2007; 11(7):749-54. [DOI:10.1111/j.1399-3046.2007.00738.x] [PMID]
Armstrong JA, Evans AS, Rao N, Ho M. Viral infections in renal transplant recipients. Infection and Immunity. 1976; 14(4):970-5. [DOI:10.1128/IAI.14.4.970-975.1976] [PMID] [PMCID]
Savoie A, Perpête C, Carpentier L, Joncas J, Alfieri C. Direct correlation between the load of Epstein-Barr Virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood, 1994; 83(9):2715-22. [DOI:10.1182/blood.V83.9.2715.bloodjournal8392715] [PMID]
Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, ten Berge IJ. Epstein-Barr Virus-positive posttransplant lymphoproliferative disease after solid organ transplantation: Pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015; 2(1):e48. [DOI:10.1097/TXD.0000000000000557] [PMID] [PMCID]
Sampaio MS, Cho YW, Shah T, Bunnapradist S, Hutchinson IV. Impact of Epstein-Barr Virus donor and recipient serostatus on the incidence of post-transplant lymphoproliferative disorder in kidney transplant recipients. Nephrol Dial Transplant. 2012; 27:2971-9. [DOI:10.1093/ndt/gfr769] [PMID]
Allen UD, Preiksaitis JK, AST Infectious Diseases Community of Practice. Epstein-Barr Virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013; 13(Suppl 4):107-20. [DOI:10.1111/ajt.12104] [PMID]
Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. British Journal of Haematology. 2010; 149:675-92. [DOI:10.1111/j.1365-2141.2010.08160.x] [PMID]
The present study determined the prevalence of EBV infection and transplant rejection among 16 children in terms of age, gender, cause of failure, the pre-transplantation serology of virus, childhood underlying diseases, patient’s medications, donor, and post-transplant viral serology. We observed that all of the investigated children had negative serology for EBV before transplantation. Eventually, only two children had positive post-transplantation serology; none of which presented complications and symptoms of the virus infection. Besides, none of these children experienced organ rejection. In other words, low seropositivity for EBV after transplantation could explain the lack of evidence for serious complications of post-transplantation, like PTLD in our study subjects. In a similar study by Comak et al., prior to transplantation, EBV was positive in 86.7% of the cases. The follow-up period was 36 months; half of the explored children with negative EBV developed primary EBV infection, and two patients with primary infection developed PTLD (18). Suzuki et al. studied 32 children, of whom, 6 (18.7%) were EBV-seronegative before the transplantation that the conversion to seropositivity occurred in 5 of them after grafting (19). In an study by Armstrong et al. rises to EBV antibody titers occurred in 32% of children after transplantation (20).
Every year, some children develop advanced renal disease. Advances in surgical procedures, such as kidney transplantation and immunosuppression therapy have dramatically reduced the mortality rate induced by kidney failure. A problem developed after organ transplantation is introducing various microbial infections, especially opportunistic infections, like EBV. The virus exacerbates the immune system and treatment with immunosuppressive drugs aggravates this infection. Some researchers believe that the virus could disrupt and stimulate the immune system and is prone to reject kidney transplantation. EBV significantly affects the progression of patients to PTLD after transplantation and is the strongest predictor of this condition. The higher the EBV virus load is in the body, the greater the risk of PTLD generation.
The literature indicates a wide range of seropositivity for EBV within post-transplantation period from 3% to 96% regardless of the seopositivity for this virus before transplantation. Moreover, the prevalence rate of PTLD development after transplantation also varied; however, the strong association between this complication and seropositivity to EBV was clearly presented. As per some studies, several factors could influence the rate of EBV-seropositivity as well as the occurrence of its related PTLD after transplantation. Such conditions include the employed immunosuppressive regimen, patients’ age, the industrialization level of country, and the time past since renal transplantation (1, 21-25). Different studies reported an association between age and the rate of EBV seropositivity. In line with our study, a relationship was suggested between the time after transplantation and the risk for viral seropositivity; however, unlike our study, a high rate of EBV infection, approaching 100% within three months after transplantation was reported (21).
5. Conclusion
Of the investigated children undergoing renal transplantation, none were seropositive to EBV before transplantation; while 12.5% were convert to EBV seropositivity after transplantation. None of the studied children experienced severe post-transplantation complications, such as PTLD and rejection. The odds of EBV seropositivity was only linked to the time interval since the transplantation. These results represent an appropriate use of medication and suitable patient training in this center. This study can be extended to the whole population of Iran because the research patients were selected from all cities of Iran; they referred to Ali-e-Asghar Hospital in Tehran to receive treatment. The limitation of this study was the small sample size; thus, conducting further studies with a high sample size is recommended.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information. Moreover, they were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors contributed in designing, running, and writing all parts of the research.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors would like to express their gratitude to Ali Asghar Clinical Research Development Center.
References
Le J, Durand CM, Agha I, Brennan DC. Epstein-Barr virus and renal transplantation. Transplantation Reviews. 2017; 31(1):55-60. [DOI:10.1016/j.trre.2016.12.001] [PMID]
Paulsen G, Cumagun P, Mixon E, Fowler K, Feig D, Shimamura M. Cytomegalovirus and Epstein‐Barr Virus infections among pediatric kidney transplant recipients at a center using universal Valganciclovir Prophylaxis. Pediatric Transplantation. 2019; 23(3):e13382. [DOI:10.1111/petr.13382] [PMID] [PMCID]
Laurent A, Klich A, Roy P, Lina B, Kassai B, Bacchetta J, Cochat P. Pediatric renal transplantation: A retrospective single‐center study on epidemiology and morbidity due to EBV. Pediatric Transplantation. 2018; 22(3):e13151. [DOI:10.1111/petr.13151] [PMID]
Allen U, Alfieri C, Preiksaitis J, Humar A, Moore D, Tapiero B, et al. Epstein-Barr Virus infection in transplant recipients: Sumary of a workshop on surveillance, prevention and treatment. Canadian Journal of Infectious Diseases and Medical Microbiology. 2002; 13(2):89-99. [DOI:10.1155/2002/634318] [PMID] [PMCID
Huang FA, Danziger-Isakov L. Infectious disease risks in pediatric renal transplantation. Pediatric Nephrology. 2019; 34(7):1155-66. [DOI:10.1007/s00467-018-3951-1] [PMID]
Martinez OM, Krams SM. The immune response to Epstein Barr Virus and implications for posttransplant lymphoproliferative disorder. Transplantation. 2017; 101(9):2009. [DOI:10.1097/TP.0000000000001767] [PMID] [PMCID]
Condon LM, Cederberg LE, Rabinovitch MD, Liebo RV, Go JC, Delaney AS, et al. Age-specific prevalence of Epstein-Barr Virus infection among Minnesota children: Effects of race/ethnicity and family environment. Clinical Infectious Diseases. 2014; 59(4):501-8. [DOI:10.1093/cid/ciu342] [PMID]
Chesnokova LS, Hutt-Fletcher LM. Epstein-Barr virus infection mechanisms. Chinese Journal of Cancer. 2014; 33(11):545. [DOI:10.5732/cjc.014.10168] [PMID] [PMCID]
Gequelin LC, Riediger IN, Nakatani SM, Biondo AW, Bonfim CM. Epstein-Barr Virus: General factors, virus-related diseases and measurement of viral load after transplant. Revista Brasileira de Hematologia e Hemoterapia. 2011; 33(5):383-8. [DOI:10.5581/1516-8484.20110103] [PMID] [PMCID]
Higgins CD, Swerdlow AJ, Macsween KF, Harrison N, Williams H, McAulay K, Thomas R, Reid S, Conacher M, Britton K, Crawford DH. A study of risk factors for acquisition of Epstein-Barr Virus and its subtypes. Journal of Infectious Diseases. 2007; 195(4):474-82. [DOI:10.1086/510854] [PMID]
Ladfors SW, Lindahl JK, Hansson S, Brandström P, Andersson R, Jertborn M, et al. Long-lasting chronic high load carriage of Epstein-Barr Virus is more common in young pediatric renal transplant recipients. Pediatric Nephrology. 2019; 4:1-3. [DOI:10.1007/s00467-019-04401-9] [PMID] [PMCID]
Green M, Michaels MG. Epstein-Barr Virus infection and posttransplant lymphoproliferative disorder. American Journal of Transplantation. 2013; 13(s3):41-54. [DOI:10.1111/ajt.12004] [PMID]
Alpert G, Fleisher GR. Complications of infection with Epstein-Barr Virus during childhood: A study of children admitted to the hospital. Pediatric Infectious Disease. 1984; 3(4):304-7. [DOI:10.1097/00006454-198407000-00005] [PMID]
Jenson HB. Acute complications of Epstein-Barr Virus infectious mononucleosis. Current Opinion in Pediatrics. 2000; 12(3):263-8. [DOI:10.1097/00008480-200006000-00016] [PMID]
Rowe M, Fitzsimmons L, Bell AI. Epstein-Barr Virus and Burkitt lymphoma. Chinese Journal of Cancer. 2014; 33(12):609. [DOI:10.5732/cjc.014.10190] [PMID] [PMCID]
Lim WH, Russ GR, Coates PT. Review of Epstein-Barr Virus and post‐transplant lymphoproliferative disorder post‐solid organ transplantation. Nephrology. 2006; 11(4):355-66. [DOI:10.1111/j.1440-1797.2006.00596.x] [PMID]
Höcker B, Fickenscher H, Delecluse HJ, Böhm S, Küsters U, Schnitzler P, et al. Epidemiology and morbidity of Epstein-Barr Virus infection in pediatric renal transplant recipients: A multicenter, prospective study. Clinical Infectious Diseases. 2012; 56(1):84-92. [DOI:10.1093/cid/cis823] [PMID]
Comak E, Akman S, Ongut G, Colak D, Koyun M, Dogan CS, et al. Epstein-Barr Virus infection in children with renal transplantation: 17 years experience at a single center. Renal Failure. 2014; 36(5):760-6. [DOI:10.3109/0886022X.2014.890861] [PMID]
Suzuki T, Ikezumi Y, Okubo S, Uchiyama M, Takahashi K, Shiraga H, et al. Epstein-Barr Virus DNA load and seroconversion in pediatric renal transplantation with tacrolimus immunosuppression. Pediatric Transplantation. 2007; 11(7):749-54. [DOI:10.1111/j.1399-3046.2007.00738.x] [PMID]
Armstrong JA, Evans AS, Rao N, Ho M. Viral infections in renal transplant recipients. Infection and Immunity. 1976; 14(4):970-5. [DOI:10.1128/IAI.14.4.970-975.1976] [PMID] [PMCID]
Savoie A, Perpête C, Carpentier L, Joncas J, Alfieri C. Direct correlation between the load of Epstein-Barr Virus-infected lymphocytes in the peripheral blood of pediatric transplant patients and risk of lymphoproliferative disease. Blood, 1994; 83(9):2715-22. [DOI:10.1182/blood.V83.9.2715.bloodjournal8392715] [PMID]
Nijland ML, Kersten MJ, Pals ST, Bemelman FJ, ten Berge IJ. Epstein-Barr Virus-positive posttransplant lymphoproliferative disease after solid organ transplantation: Pathogenesis, clinical manifestations, diagnosis, and management. Transplantation Direct. 2015; 2(1):e48. [DOI:10.1097/TXD.0000000000000557] [PMID] [PMCID]
Sampaio MS, Cho YW, Shah T, Bunnapradist S, Hutchinson IV. Impact of Epstein-Barr Virus donor and recipient serostatus on the incidence of post-transplant lymphoproliferative disorder in kidney transplant recipients. Nephrol Dial Transplant. 2012; 27:2971-9. [DOI:10.1093/ndt/gfr769] [PMID]
Allen UD, Preiksaitis JK, AST Infectious Diseases Community of Practice. Epstein-Barr Virus and posttransplant lymphoproliferative disorder in solid organ transplantation. Am J Transplant. 2013; 13(Suppl 4):107-20. [DOI:10.1111/ajt.12104] [PMID]
Parker A, Bowles K, Bradley JA, Emery V, Featherstone C, Gupte G, et al. Diagnosis of post-transplant lymphoproliferative disorder in solid organ transplant recipients - BCSH and BTS Guidelines. British Journal of Haematology. 2010; 149:675-92. [DOI:10.1111/j.1365-2141.2010.08160.x] [PMID]
Type of Study: Original Article |
Subject:
Pediatric Infectious Diseases
Received: 2019/10/8 | Accepted: 2019/12/16 | Published: 2020/07/1
Received: 2019/10/8 | Accepted: 2019/12/16 | Published: 2020/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |