Volume 8, Issue 2 (4-2020)
J. Pediatr. Rev 2020, 8(2): 65-78 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Orimadegun A E, Adepoju A A, Myer L. A Systematic Review and Meta-analysis of Sex Differences in Morbidity and Mortality of Acute Lower Respiratory Tract Infections Among African Children. J. Pediatr. Rev 2020; 8 (2) :65-78
URL: http://jpr.mazums.ac.ir/article-1-268-en.html
URL: http://jpr.mazums.ac.ir/article-1-268-en.html
1- Institute of Child Health, College of Medicine, University of Ibadan, Ibadan, Nigeria. , aorimadegun@hotmail.co.uk
2- Department of Paediatrics, College of Medicine, University of Ibadan, Ibadan, Nigeria.
3- Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
2- Department of Paediatrics, College of Medicine, University of Ibadan, Ibadan, Nigeria.
3- Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
Keywords: Respiratory tract infections, Pneumonia, Respiratory syncytial viruses, Sex characteristics
Full-Text [PDF 707 kb]
(2314 Downloads)
| Abstract (HTML) (7610 Views)
References
Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016; 388(10053):1459-544. [DOI:10.1016/S0140-6736(16)31012-1]
Full-Text: (8075 Views)
1. Context
Lower respiratory tract infections (LRTIs) are common diseases in children worldwide, accounting for high morbidity, hospital admissions, and healthcare costs, especially in developing countries (1). In children aged 1-59 months, pneumonia accounts for about 1.071 million deaths (range: 0.977–1.176), which comprise 14.1% of all-cause mortality. Pneumonia along with diarrhea (9.9%; 0.751 million death, range: 0.538–1.031), and malaria (7.4%; 0.564 million death, range: 0.432–0.709) are among the three most common diseases claiming most lives of children <5 years of age (2). Although the global mortality rate among children aged <5 years is declining, there are marked variations in the magnitude and trends across regions and countries of the world, and the mortality rates range from 8% to 15% among African children (3-5). Generally, the highest overall mortality rates were reported from studies on children with either HIV, severe malnutrition, unvaccinated, and very severe pneumonia (3, 6, 7).
Recently, sustainable solutions to the high number of child deaths associated with LRTI have been advocated through management (8) and prevention of pneumonia (9, 10). It is, therefore, essential to know the incidence, likely etiological agents, and burden of LRTI mortality in Africa as it relates to demographic risk factors (11, 12). Conventional knowledge maintains that male children develop LRTIs more frequently than females, and are at higher risk of mortality and morbidity (13). Despite these assumptions, epidemiological data on the evidence of sex differences for LRTI in children in sub-Saharan Africa are strikingly limited. In a review of the 52 studies on LRTI, published by Falagas (13) in 2007, only 7 were carried out on children, and none was done in Africa. Even for non-African countries, the association between sex and LRTI has been inconsistently reported. More recently, Jackson’s systematic review found that the odds of having severe ALRI was 1.5 (95% CI: 1.0 to 2.3) times higher in males than females, but only one included report was from Africa (14).
2. Objective
To date, no systematic review of published literature has assessed the relationship between sex and LRTI in African children. This review aimed to determine the quality of available evidence systematically and to present summary estimates of the strength of the association of sex with the incidence, etiology, and outcomes of LRTI in children using narrative and meta-analysis methods.
Lower respiratory tract infections (LRTIs) are common diseases in children worldwide, accounting for high morbidity, hospital admissions, and healthcare costs, especially in developing countries (1). In children aged 1-59 months, pneumonia accounts for about 1.071 million deaths (range: 0.977–1.176), which comprise 14.1% of all-cause mortality. Pneumonia along with diarrhea (9.9%; 0.751 million death, range: 0.538–1.031), and malaria (7.4%; 0.564 million death, range: 0.432–0.709) are among the three most common diseases claiming most lives of children <5 years of age (2). Although the global mortality rate among children aged <5 years is declining, there are marked variations in the magnitude and trends across regions and countries of the world, and the mortality rates range from 8% to 15% among African children (3-5). Generally, the highest overall mortality rates were reported from studies on children with either HIV, severe malnutrition, unvaccinated, and very severe pneumonia (3, 6, 7).
Recently, sustainable solutions to the high number of child deaths associated with LRTI have been advocated through management (8) and prevention of pneumonia (9, 10). It is, therefore, essential to know the incidence, likely etiological agents, and burden of LRTI mortality in Africa as it relates to demographic risk factors (11, 12). Conventional knowledge maintains that male children develop LRTIs more frequently than females, and are at higher risk of mortality and morbidity (13). Despite these assumptions, epidemiological data on the evidence of sex differences for LRTI in children in sub-Saharan Africa are strikingly limited. In a review of the 52 studies on LRTI, published by Falagas (13) in 2007, only 7 were carried out on children, and none was done in Africa. Even for non-African countries, the association between sex and LRTI has been inconsistently reported. More recently, Jackson’s systematic review found that the odds of having severe ALRI was 1.5 (95% CI: 1.0 to 2.3) times higher in males than females, but only one included report was from Africa (14).
2. Objective
To date, no systematic review of published literature has assessed the relationship between sex and LRTI in African children. This review aimed to determine the quality of available evidence systematically and to present summary estimates of the strength of the association of sex with the incidence, etiology, and outcomes of LRTI in children using narrative and meta-analysis methods.
Protocol registration and data sources
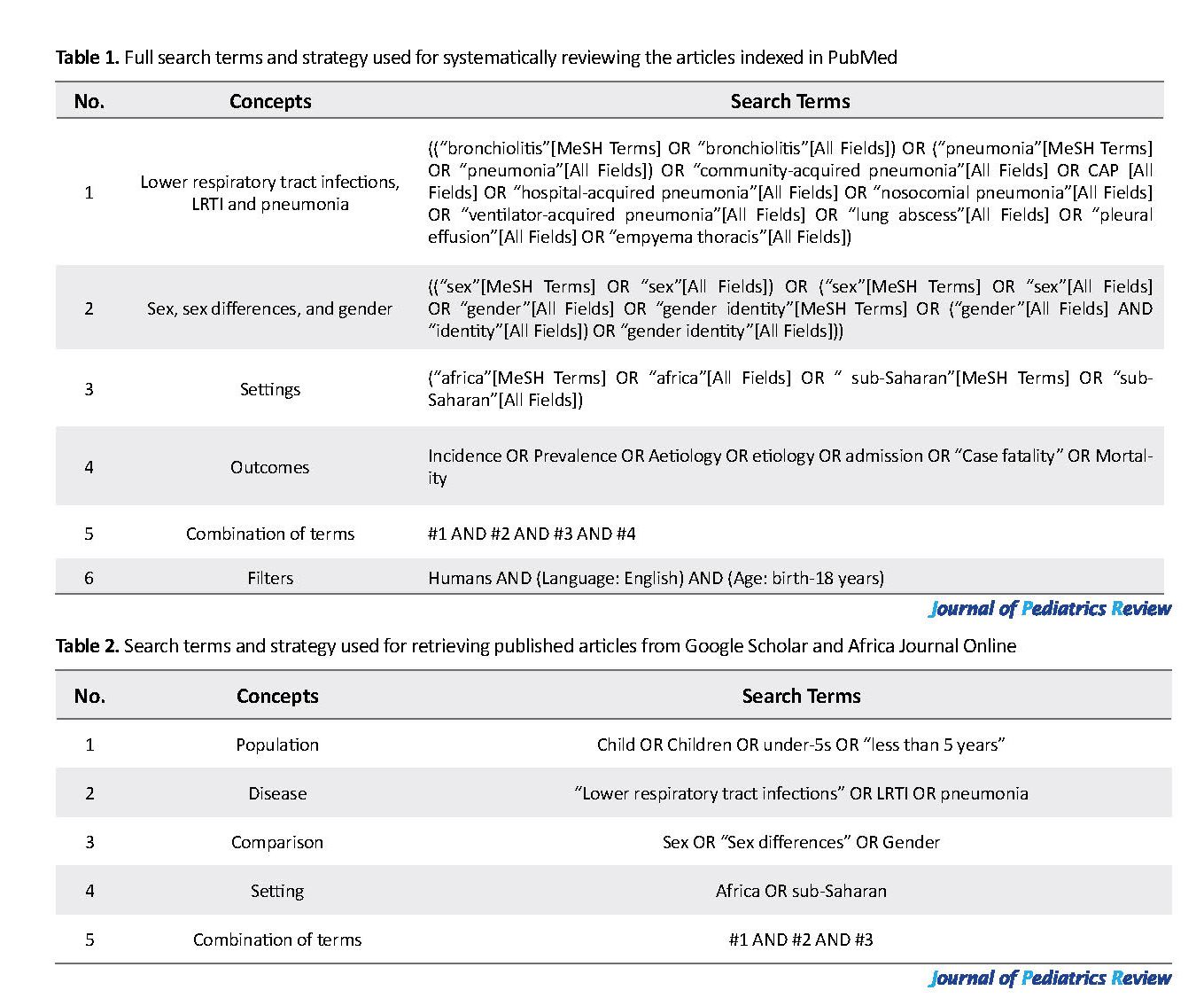
The protocol for this systematic review has been approved and registered with PROSPERO (CRD42019122494). We searched for literature on acute lower respiratory infections in African children using (https://www.ncbi.nlm.nih.gov/pubmed/), African Journals Online (www.ajol.info), and Google scholar (https://scholar.google.co.za/) from 1971-2016. These databases were searched for studies that report data on the incidence, etiology, and outcomes of LRTI for both male and female children. The terms used and details of the search steps were presented in Table 1 and Table 2.
The protocol for this systematic review has been approved and registered with PROSPERO (CRD42019122494). We searched for literature on acute lower respiratory infections in African children using (https://www.ncbi.nlm.nih.gov/pubmed/), African Journals Online (www.ajol.info), and Google scholar (https://scholar.google.co.za/) from 1971-2016. These databases were searched for studies that report data on the incidence, etiology, and outcomes of LRTI for both male and female children. The terms used and details of the search steps were presented in Table 1 and Table 2.
Study selection and eligibility criteria
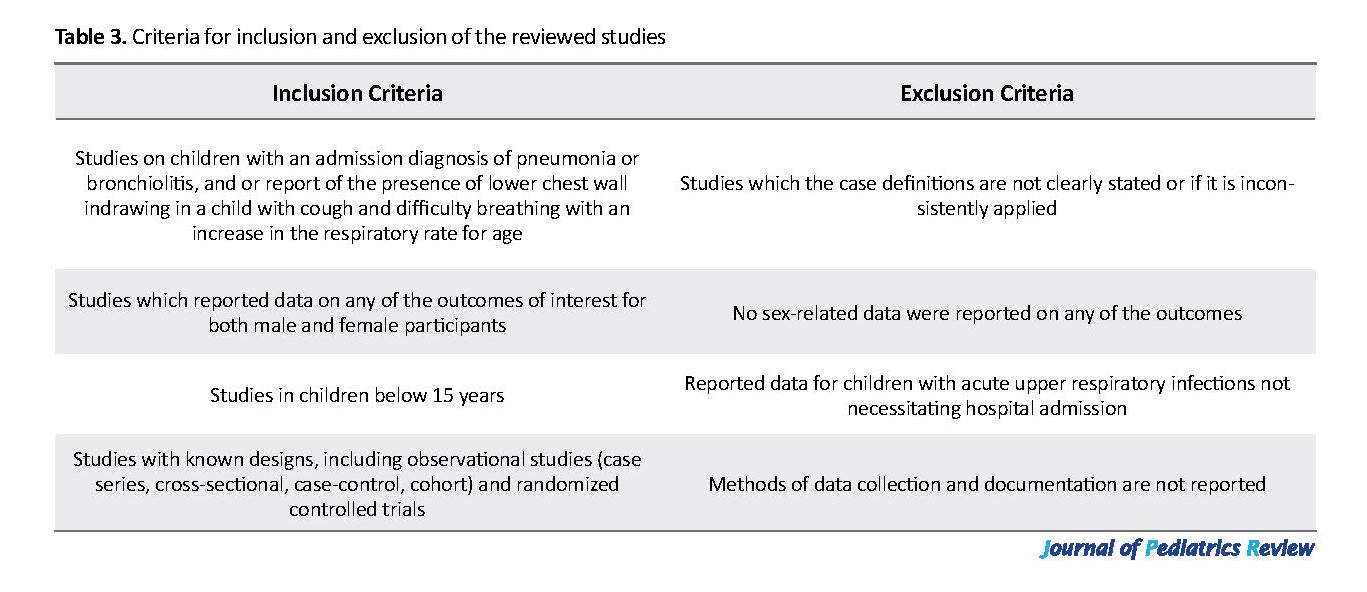
We included the published peer-reviewed journal articles reporting data on any of our outcomes of interest, namely incidence, etiology, and case fatality. The titles and abstracts of the articles that were initially identified have been reviewed, to select studies with objectives or focus on our desired results, for a more detailed examination. The decision to include a study was based on whether the data on the incidence, etiology, and case fatality of acute LRTI were included in the abstract or body of the article. Subsequently, each eligible article was read to identify the relevant individual patient data in full text. Only those studies that met the inclusion criteria (Table 3) were thoroughly reviewed and analyzed. We limited the articles reviewed to only those studies involving human subjects, written in English, and research conducted in Africa.
We acknowledged the fact that different researchers used different case definitions for the LRTI and the outcomes. Thus, we defined acute LRTI episode in the health facility setting as “any child with an admission diagnosis of pneumonia or bronchiolitis” as the primary manifestations of LRTI in children. In studies conducted outside health facilities, the presence of lower chest wall indrawing in children with cough and difficulty breathing at an increased rate of breathing for age was used to define the case, as in the WHO case definition for pneumonia (11, 15).
3. Data Extraction
The conduct of this review was carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (16). After iterative database searches and screenings of all titles and abstracts to identify full-text articles for detailed review, a data abstraction form was developed. One author (Adebola E. Orimadegun) extracted data while the second author (Landon Myer) cross-checked all extracted data compiled using Microsoft Excel 2010. To ensure the accuracy of the extracted data, the second author (Landon Myer) compared the extracted information with the original data published in the selected complete texts (or in the supporting documents submitted by the authors). Any identified errors were discussed and corrected, if necessary.
Assessment of quality of studies
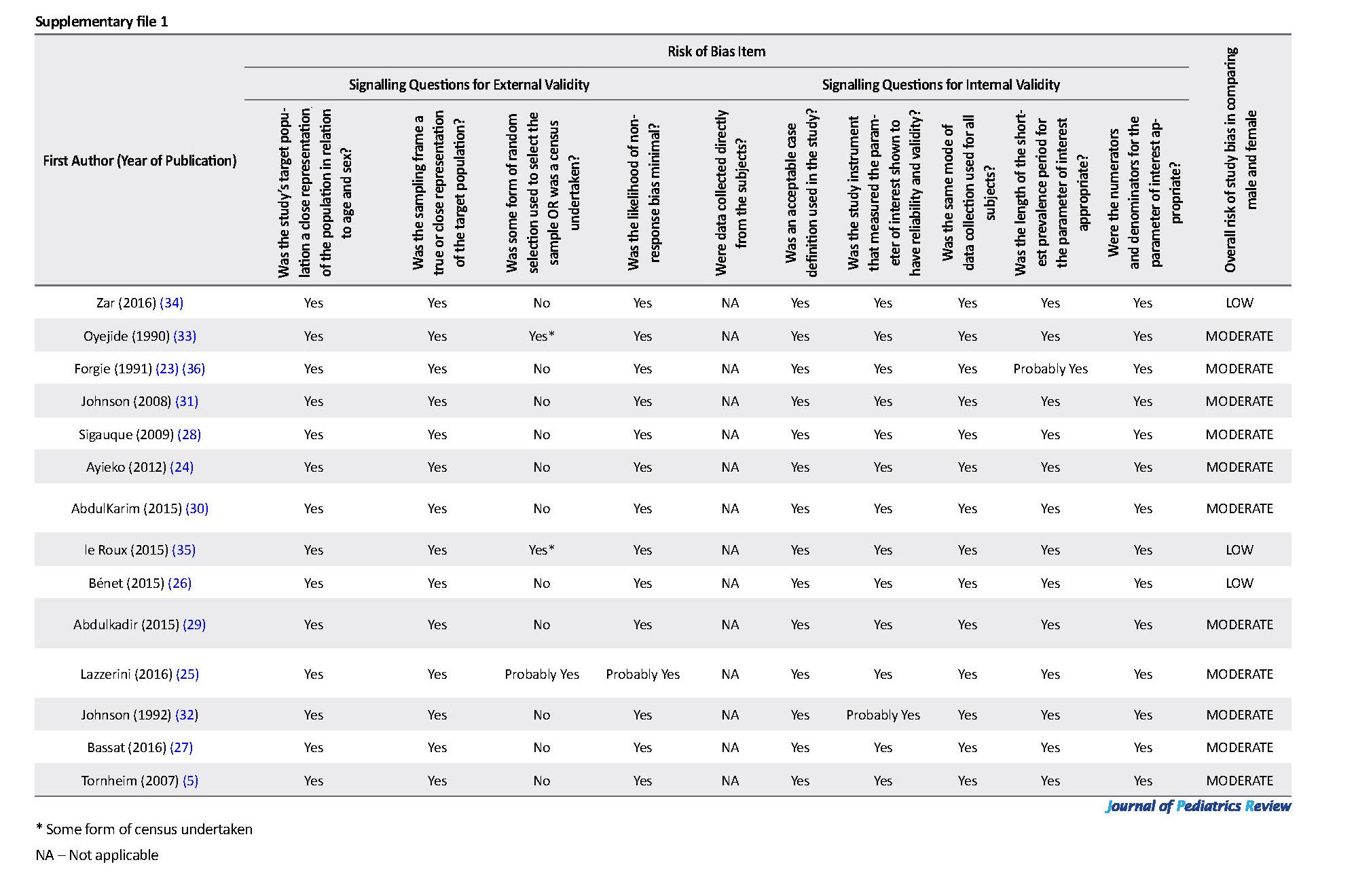
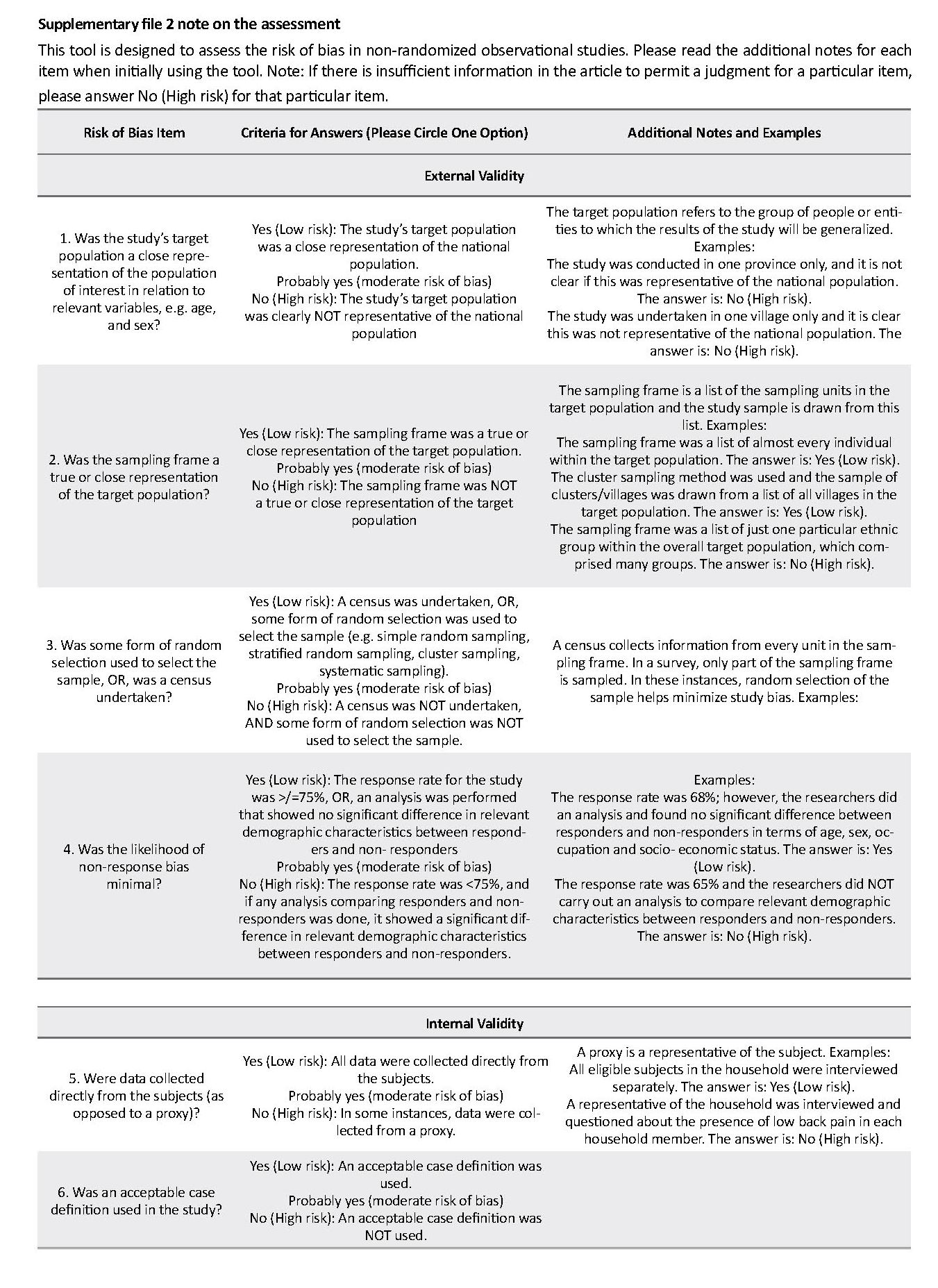
We assessed the quality of selected studies and potential risk of bias with the Newcastle-Ottawa Scale (17, 18), following the Cochrane Handbook (19). This tool includes 10 items that assess measurement bias, selection bias, and analysis bias-related (all rated as either high, moderate, or low risk) and an overall assessment of the risk of bias rated as either low, moderate, or high (Supplementary file 1). We followed the format of the Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACRO-BAT-NRSI) (20) by using 11 “signaling” questions (Supplementary file 1). Each question has a single answer, either yes (low risk of bias), probably yes (moderate risk of bias), no (serious to the critical risk of bias), or insufficient information to assess (unable to allocate the risk of bias). Based on the answers to the signaling questions on external and internal validity, the overall risk of bias was assigned to each study as either “low” (suggesting that the study is comparable to a well-performed randomized trial); “moderate” (suggesting that the study is sound for a non-randomized study; or “high” (indicating the study is too problematic to provide useful evidence concerning sex). If there is not enough information to make a reasonable overall assessment, the study should be assigned “no information” and not used for data synthesis. Only those studies that have less than moderate overall bias risks were used for data synthesis.
Data synthesis and analysis
The data extracted from the included studies regarding sex differences were summarised (Supplementary file 2). Since we anticipated significant clinical and methodological heterogeneity, we narratively summarized the potential effect of sex on the incidence, etiology, and case fatality of LRTI in individual studies. Gaps in the research have also been highlighted. We conducted a meta-analysis to pool data for case-fatality because it is the only outcome with reasonably well-recorded data from studies with a low or moderate risk of bias. We evaluated heterogeneity using the Chi square-based Q statistic (significant for P<0.1) (21). The funnel plot and Egger’s test were used to check for small-study effects, a potential cause of publication bias (22). For studies that have reported on Respiratory Syncytia Virus (RSV) and case fatality, the findings of the study were further summarized using an unadjusted odds ratio with a 95% confidence interval (CI). Statistical analysis was performed in Stata v. 12.1 (StataCorp, Texas USA) and using metan commands to produce forest plots.
4. Results
Characteristics of studies, design, and participants
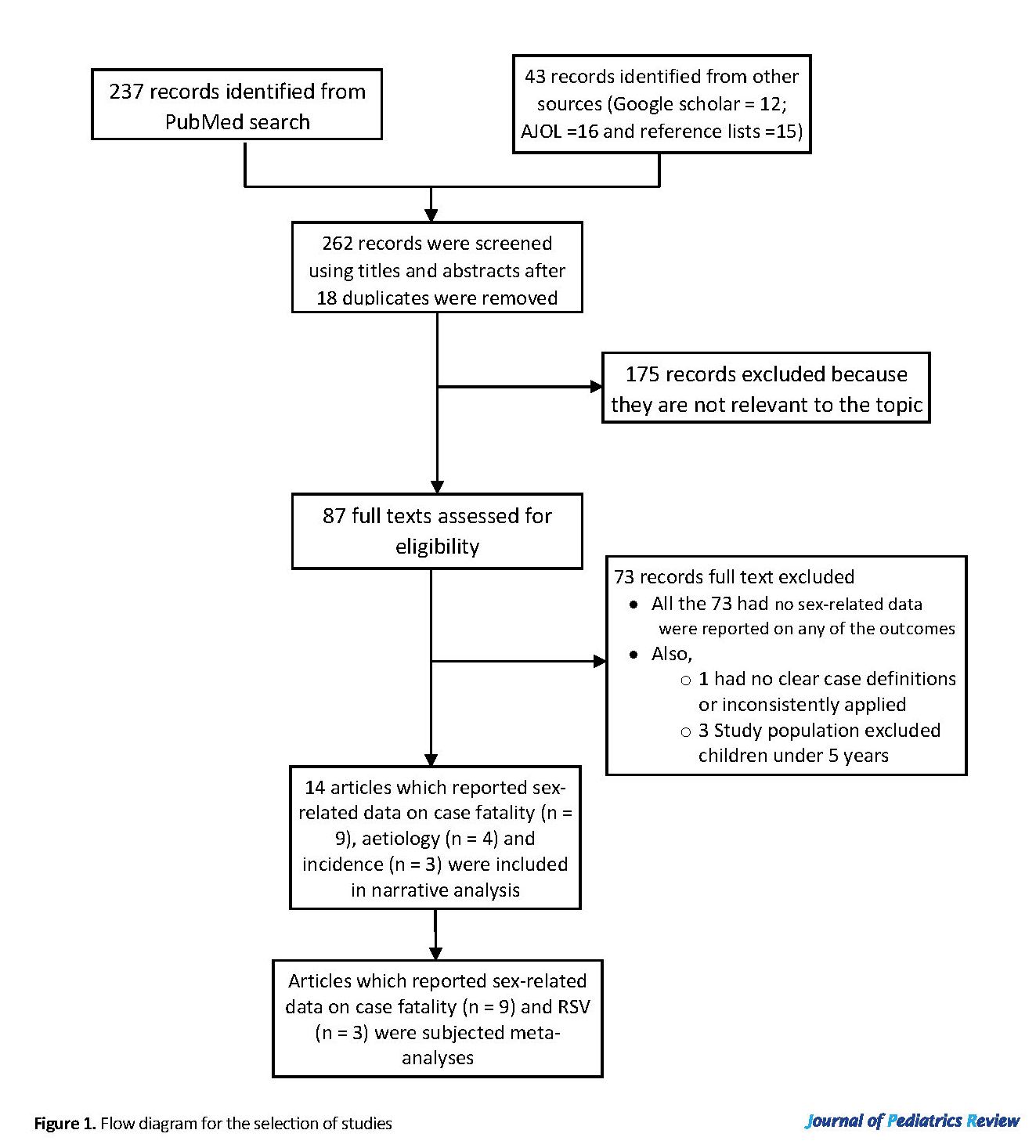
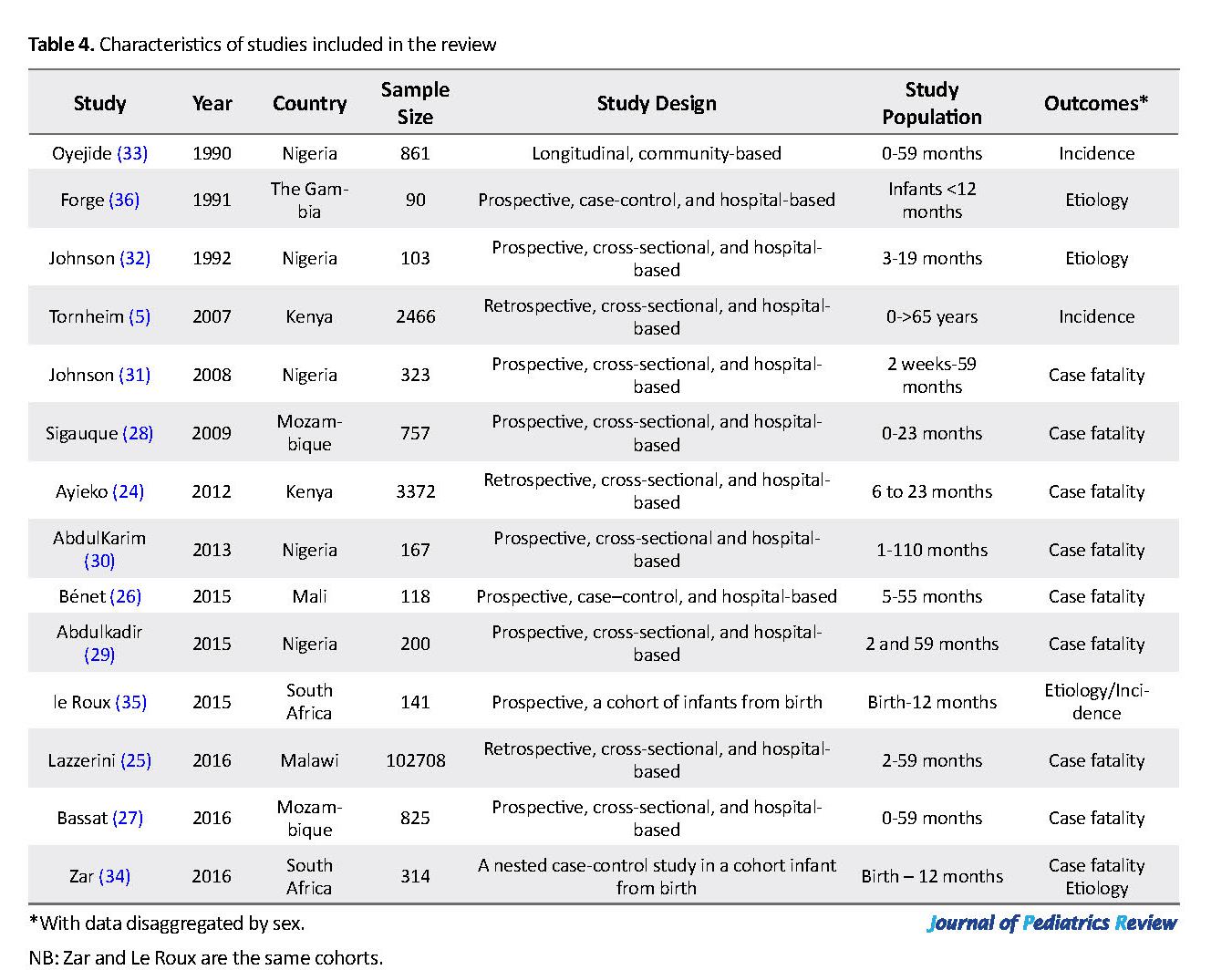
A total of 262 studies with sex-related data were retrieved (Figure 1); 175 reports were screened out due to missing eligibility criteria. There were no sex-related data on any of the outcomes of interest in the full-texts of 73 out of 87 full-text articles assessed for eligibility; these were also excluded from further review. We identified only 14 studies that reported on the incidence, etiology, and or case fatality of LRTI among African children disaggregated by sex. These studies were conducted in the Gambia (23), Kenya (5, 24), Malawi (25), Mali (26), Mozambique (27, 28), Nigeria (29-33), and South Africa (34, 35) (Table 4). However, one Nigeria (33) study was excluded from narrative analysis for the high incidence of LRTI, which was considered an outlier (ranged from 6.1 to 8.1 episodes per child-year an incidence ratio of 1.08 [male=7.2; female=6.7]).
We included the published peer-reviewed journal articles reporting data on any of our outcomes of interest, namely incidence, etiology, and case fatality. The titles and abstracts of the articles that were initially identified have been reviewed, to select studies with objectives or focus on our desired results, for a more detailed examination. The decision to include a study was based on whether the data on the incidence, etiology, and case fatality of acute LRTI were included in the abstract or body of the article. Subsequently, each eligible article was read to identify the relevant individual patient data in full text. Only those studies that met the inclusion criteria (Table 3) were thoroughly reviewed and analyzed. We limited the articles reviewed to only those studies involving human subjects, written in English, and research conducted in Africa.
We acknowledged the fact that different researchers used different case definitions for the LRTI and the outcomes. Thus, we defined acute LRTI episode in the health facility setting as “any child with an admission diagnosis of pneumonia or bronchiolitis” as the primary manifestations of LRTI in children. In studies conducted outside health facilities, the presence of lower chest wall indrawing in children with cough and difficulty breathing at an increased rate of breathing for age was used to define the case, as in the WHO case definition for pneumonia (11, 15).
3. Data Extraction
The conduct of this review was carried out following the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) checklist (16). After iterative database searches and screenings of all titles and abstracts to identify full-text articles for detailed review, a data abstraction form was developed. One author (Adebola E. Orimadegun) extracted data while the second author (Landon Myer) cross-checked all extracted data compiled using Microsoft Excel 2010. To ensure the accuracy of the extracted data, the second author (Landon Myer) compared the extracted information with the original data published in the selected complete texts (or in the supporting documents submitted by the authors). Any identified errors were discussed and corrected, if necessary.
Assessment of quality of studies
We assessed the quality of selected studies and potential risk of bias with the Newcastle-Ottawa Scale (17, 18), following the Cochrane Handbook (19). This tool includes 10 items that assess measurement bias, selection bias, and analysis bias-related (all rated as either high, moderate, or low risk) and an overall assessment of the risk of bias rated as either low, moderate, or high (Supplementary file 1). We followed the format of the Cochrane Risk of Bias Assessment Tool for Non-Randomized Studies of Interventions (ACRO-BAT-NRSI) (20) by using 11 “signaling” questions (Supplementary file 1). Each question has a single answer, either yes (low risk of bias), probably yes (moderate risk of bias), no (serious to the critical risk of bias), or insufficient information to assess (unable to allocate the risk of bias). Based on the answers to the signaling questions on external and internal validity, the overall risk of bias was assigned to each study as either “low” (suggesting that the study is comparable to a well-performed randomized trial); “moderate” (suggesting that the study is sound for a non-randomized study; or “high” (indicating the study is too problematic to provide useful evidence concerning sex). If there is not enough information to make a reasonable overall assessment, the study should be assigned “no information” and not used for data synthesis. Only those studies that have less than moderate overall bias risks were used for data synthesis.
Data synthesis and analysis
The data extracted from the included studies regarding sex differences were summarised (Supplementary file 2). Since we anticipated significant clinical and methodological heterogeneity, we narratively summarized the potential effect of sex on the incidence, etiology, and case fatality of LRTI in individual studies. Gaps in the research have also been highlighted. We conducted a meta-analysis to pool data for case-fatality because it is the only outcome with reasonably well-recorded data from studies with a low or moderate risk of bias. We evaluated heterogeneity using the Chi square-based Q statistic (significant for P<0.1) (21). The funnel plot and Egger’s test were used to check for small-study effects, a potential cause of publication bias (22). For studies that have reported on Respiratory Syncytia Virus (RSV) and case fatality, the findings of the study were further summarized using an unadjusted odds ratio with a 95% confidence interval (CI). Statistical analysis was performed in Stata v. 12.1 (StataCorp, Texas USA) and using metan commands to produce forest plots.
4. Results
Characteristics of studies, design, and participants
A total of 262 studies with sex-related data were retrieved (Figure 1); 175 reports were screened out due to missing eligibility criteria. There were no sex-related data on any of the outcomes of interest in the full-texts of 73 out of 87 full-text articles assessed for eligibility; these were also excluded from further review. We identified only 14 studies that reported on the incidence, etiology, and or case fatality of LRTI among African children disaggregated by sex. These studies were conducted in the Gambia (23), Kenya (5, 24), Malawi (25), Mali (26), Mozambique (27, 28), Nigeria (29-33), and South Africa (34, 35) (Table 4). However, one Nigeria (33) study was excluded from narrative analysis for the high incidence of LRTI, which was considered an outlier (ranged from 6.1 to 8.1 episodes per child-year an incidence ratio of 1.08 [male=7.2; female=6.7]).
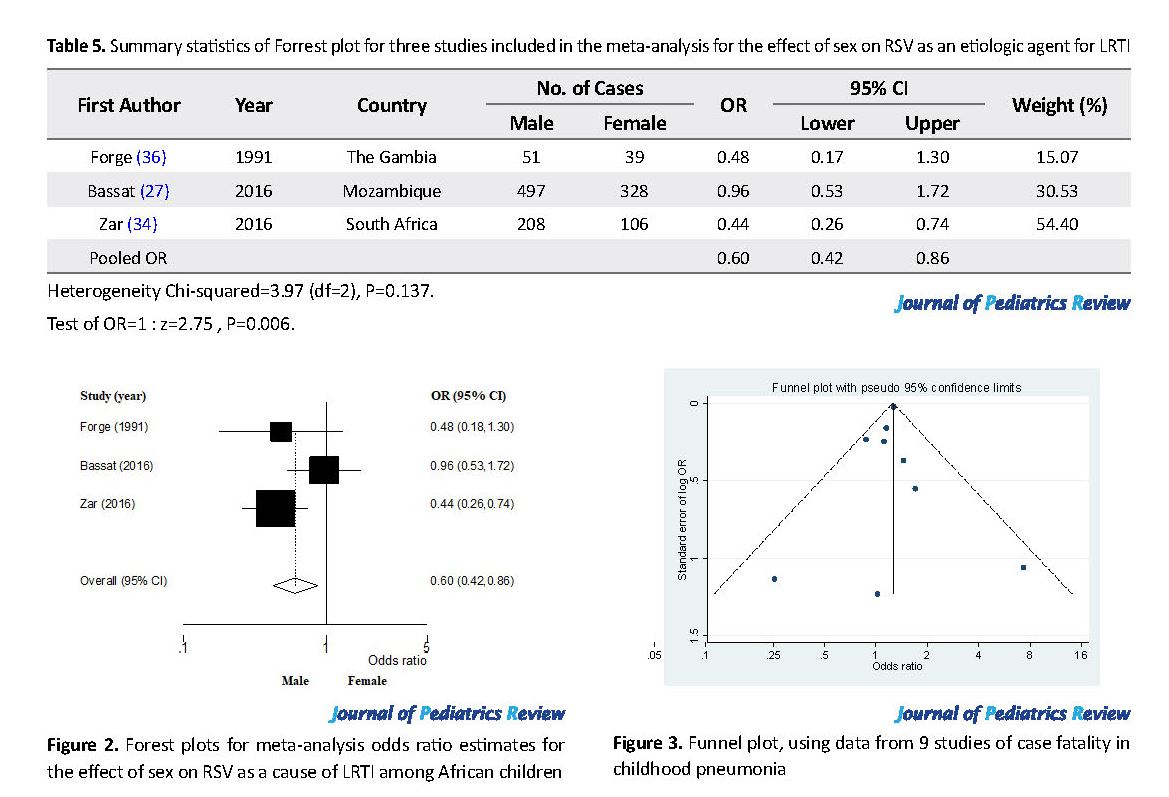
The publication dates for all the 14 studies ranged from 1990 to 2016. We found data on sex differences for case fatality in 9 (26-31, 34), etiology in 3 (23, 34, 35), and incidence of pneumonia in 3 (5, 28, 34) articles. There was no article on bronchiolitis with data on sex differences. All studies focused on LRTI as defined by clinical presentations and or radiological findings.
The study population was children aged less than 5 years in all articles but two studies, including a Nigerian research (30), which extended the participants’ age to 110 months and a study from Kenya (5) which included adults, too. Also, most of the studies were health facility-based (n=12/13), and data were prospectively collected in 11 studies (26-31). Nine studies were cross-sectional in design (5, 24, 25, 27-32), 3 were case-control studies (26, 34, 36) and only 2 studies (33, 35) involved follow up of their participants for at least 1 year. Our assessment showed that none of the studies in the review had a very high risk of bias. Four studies (5, 25, 33, 36) were classified as having a moderate risk of bias, while the remaining 10 studies have a low risk of bias.
The study population was children aged less than 5 years in all articles but two studies, including a Nigerian research (30), which extended the participants’ age to 110 months and a study from Kenya (5) which included adults, too. Also, most of the studies were health facility-based (n=12/13), and data were prospectively collected in 11 studies (26-31). Nine studies were cross-sectional in design (5, 24, 25, 27-32), 3 were case-control studies (26, 34, 36) and only 2 studies (33, 35) involved follow up of their participants for at least 1 year. Our assessment showed that none of the studies in the review had a very high risk of bias. Four studies (5, 25, 33, 36) were classified as having a moderate risk of bias, while the remaining 10 studies have a low risk of bias.
Sex difference in the incidence of pneumonia
Generally, pneumonia was reported more frequently in males than females in 13 out of 14 studies. Two studies reported the sex-specific incidence of pneumonia. The overall incidence of LRTI was lower in female than male children in South Africa, with an incidence ratio of 0.49 (35). The Kenya study reported the incidence (per 100000 person-years) of pneumonia for children aged 0-4 years 5-9 years and 10-14 years as female/male rate ratio of 0.84 (95% CI; 0.75-0.95), 0.98 (95% CI; 0.64-1.52), and 1.51 (95% CI; 0.81-2.88), respectively. The authors reported a lower risk of pneumonia in female than male children younger than 5 years (RR=0.84, 95% CI; 0.75-0.95). These two studies used either clinical or WHO case definitions of LRTI. It was, however, difficult to pool the incidence data for the two studies because the participants’ ages varied widely with apparent high heterogeneity. Also, the numbers of male and female children were not presented in the report from Kenya.
Generally, pneumonia was reported more frequently in males than females in 13 out of 14 studies. Two studies reported the sex-specific incidence of pneumonia. The overall incidence of LRTI was lower in female than male children in South Africa, with an incidence ratio of 0.49 (35). The Kenya study reported the incidence (per 100000 person-years) of pneumonia for children aged 0-4 years 5-9 years and 10-14 years as female/male rate ratio of 0.84 (95% CI; 0.75-0.95), 0.98 (95% CI; 0.64-1.52), and 1.51 (95% CI; 0.81-2.88), respectively. The authors reported a lower risk of pneumonia in female than male children younger than 5 years (RR=0.84, 95% CI; 0.75-0.95). These two studies used either clinical or WHO case definitions of LRTI. It was, however, difficult to pool the incidence data for the two studies because the participants’ ages varied widely with apparent high heterogeneity. Also, the numbers of male and female children were not presented in the report from Kenya.
Sex differences in etiology of LRTI in children
Out of the 14 studies, 4 studies (28.6%) were reviewed, investigated, and presented sex-related data on the etiology of LRTI (23, 27, 32, 34). In microbiology-based studies, the leading reported bacterial cause was Streptococcus pneumonia, identified in two studies (27, 32). Streptococcus pneumonia accounted for 4.5% of 557 and 6.1% of 380 pneumonia cases in males and females, respectively. Other reported bacterial pathogens were Haemophilus influenzae type B in two studies (32, 34) (male=37/247 [10.9%]; female=1/276 [0.4%]), and Staphylococcus aureus in one study (32) (male=7/55 [12.7%]; female=7/48 [14.6%]). Sex disaggregated results on rhinovirus and Pneumocystis jirovecii were presented by two (27, 34) studies out of the four studies, while only one study (34) reported sex distribution for the influenza virus, parainfluenza, and bocavirus as pathogens. Influenza virus, parainfluenza, and bocavirus were identified more frequently in male than female infants, male constitutes over 60% children from whom isolates were obtained. Bassat and Lanaspa (27) identified rhinovirus as the dominant pathogen in both male (n=125/497; 25.2%) and female (n=69/328; 21.0%) children followed by adenovirus (male 68/497; female 34/328), Pneumocystis jirovecii (male 28/497; female 29/328) and Streptococcus pneumonia (male 24/497; female 22/328).
Out of the 14 studies, 4 studies (28.6%) were reviewed, investigated, and presented sex-related data on the etiology of LRTI (23, 27, 32, 34). In microbiology-based studies, the leading reported bacterial cause was Streptococcus pneumonia, identified in two studies (27, 32). Streptococcus pneumonia accounted for 4.5% of 557 and 6.1% of 380 pneumonia cases in males and females, respectively. Other reported bacterial pathogens were Haemophilus influenzae type B in two studies (32, 34) (male=37/247 [10.9%]; female=1/276 [0.4%]), and Staphylococcus aureus in one study (32) (male=7/55 [12.7%]; female=7/48 [14.6%]). Sex disaggregated results on rhinovirus and Pneumocystis jirovecii were presented by two (27, 34) studies out of the four studies, while only one study (34) reported sex distribution for the influenza virus, parainfluenza, and bocavirus as pathogens. Influenza virus, parainfluenza, and bocavirus were identified more frequently in male than female infants, male constitutes over 60% children from whom isolates were obtained. Bassat and Lanaspa (27) identified rhinovirus as the dominant pathogen in both male (n=125/497; 25.2%) and female (n=69/328; 21.0%) children followed by adenovirus (male 68/497; female 34/328), Pneumocystis jirovecii (male 28/497; female 29/328) and Streptococcus pneumonia (male 24/497; female 22/328).
RSV was the most frequently reported viral cause of pneumonia for both males (n=79/745, 10.6%) and females (n=70/599, 11.7%) children. It was identified in three studies from The Gambia (23), Nigeria (32) and South Africa (34). These parts of the sex-related data on etiologic agents were subjected to meta-analysis, and the pooled sex effect (with 95% CI and the P-value) and heterogeneity test were as presented in Table 5 and Figure 2. Only the study from Mozambique (27) has the 95% confidence interval for the odds ratio crossing the “line of no difference”, while others showed a significant odds ratio in favor of male children. Overall, the effect of sex averaged for all the three studies was in favor of the male sex, i.e., odds of identifying RSV was significantly lower in male than female children (OR=0.60; 95% CI: 0.42, 0.86).
Sex differences in mortality among children with LRTI
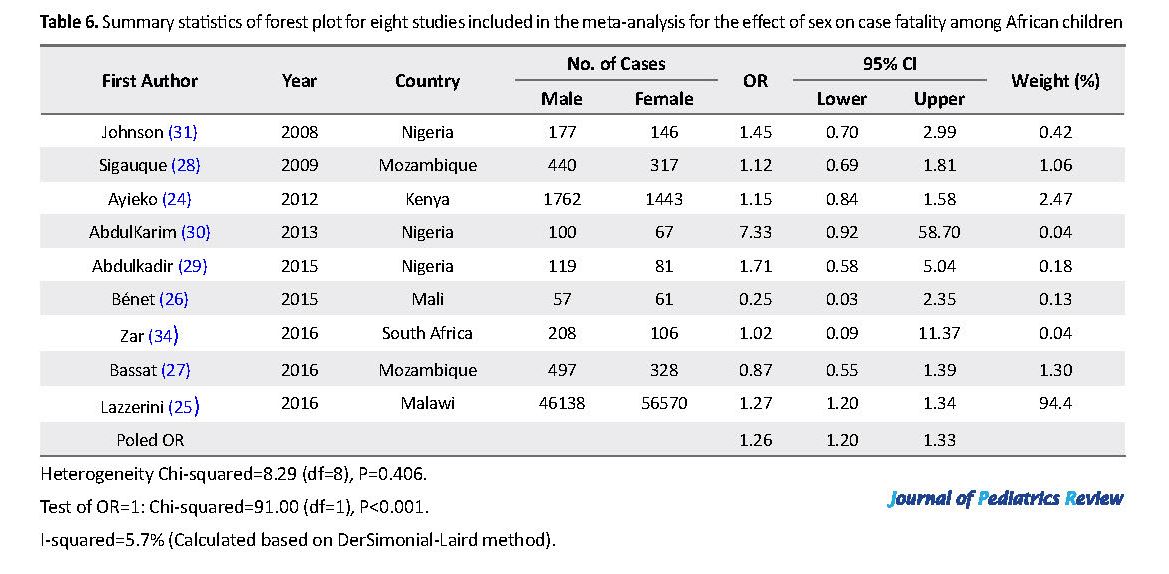
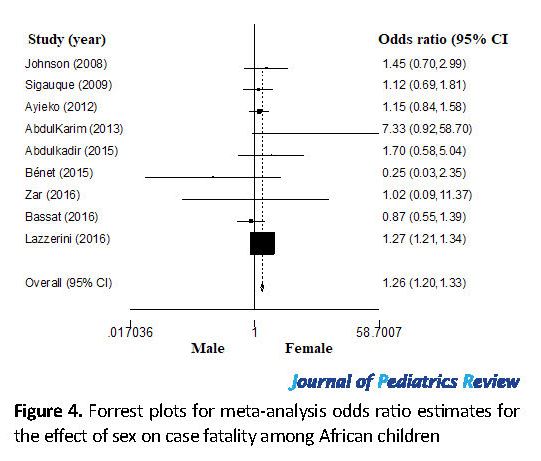
We identified 9 studies from Nigeria (n=3), Mozambique (n=2), Kenya (n=1), Mali (n=1), Malawi (n=1), and South Africa (1) with sex-related data on case fatality (Table 6). The distribution of the included publications in the funnel plots (Figure 3) shows symmetrical scattered points on either side of the overall effect line. This observation was supported by the results of the Egger’s test (P>0.05), suggesting no remarkable publication biases. The meta-analysis odds ratio estimates for the effect of sex on case fatality with statistical tests for heterogeneity are shown in Figure 4. The pooled estimate indicates that the odds of fatality were significantly higher for male than female children (OR=1.26; 95% CI: 1.20-1.33).
5. Discussion
This novel review synthesizes published studies from African countries on sex differences in incidence, etiology, and case fatality of LRTI in children. We observed that despite the increasing evidence that gender is an essential factor which influences the diseases and response to treatments (13, 37, 38), data on sex-related differences in incidence, etiology, and outcomes of LRTI are scarce. We found only two studies (5, 35) that reported the incidence of pneumonia between males and females, and they both reported a higher rate for male than female children. There were inconsistencies in the reports of pathogens identified in children with LRTI so that RSV was the leading cause in the three studies that disaggregated etiologic agents by sex. Our summary statistics indicated that the odds of RSV infection was significantly higher in female than male children. We further observed that only 9 studies reported an association between sex and case fatality and the overall estimate revealed that the odds of death was higher in male children (OR=1.26; 95% CI 1.20-1.33).
To our knowledge, our study is the first comprehensive attempt to systematically assess the effect of sex on the incidence, etiology, and mortality of ALRI in children of the African population. Excess pneumonia found among males compared with females has been previously described (39, 40). However, it remains unclear whether sex differences in pneumonia incidence is due to variability in susceptibility between boys and girls or to selection bias from preferential care-seeking. The incidence reported for male children (0.36 episodes per child-year) in the South Africa study is considerably higher than the estimated rate (0.33 episodes per child-year) for African children aged less than 5 years (41), and more recent estimate is 0.22 episodes per child-year (interquartile range: 0.11-0.51) for all low and middle–income countries (LMIC) derived from 35 community-based studies published between 1990 and 2012 (42). The considerable variation in the incidence between the studies selected for our review was most probably due to the distinct study designs and or real differences in the prevalence of pneumonia in the various study settings. For instance, the Nigeria study (33) had an error in respect of the application of case definition and that affected the computation of incidence that the study estimate of 6.1-8.1 episodes per child-year was remarkably higher than any rate ever reported.
The difficulties associated with pooling data for estimation of the incidence of pneumonia have been highlighted (42). These problems included the scarcity of longitudinal studies in LMIC, the necessity of conducting such studies over a full calendar year, active and frequent screening of a large number of children, as well as the correctness of application of case definition by the assessor (42). All of these issues limited our ability to pool data in this review. Similarly, we were unable to combine sex-related data on the etiology of pneumonia in children, given the heterogeneity of the data (41).
In our review, bacterial and viral etiologies of pneumonia were inconsistently reported for male and female children. The leading bacterial and viral causes for pneumonia found in our review agreed with previous reports (43-46). However, literature does not explain why the predisposition to some pathogen has sex preference. Generally, in Africa, there is the need to conduct more studies on the etiology of pneumonia with sex-specific data in children. Such reviews will help define the new distribution of pneumonia-causing etiologies in both sexes that may have important implications for empirical diagnosis and treatments. It is necessary to note that determining the cause of pneumonia in children is often challenging due to difficulties in obtaining direct lung samples. The expectorant easily gets contaminated by oropharyngeal organisms, but the patient’s age and probably the child’s sex can help narrow the list of probable etiology (47). Therefore, interpreting the results of studies on the etiologies of pneumonia requires an understanding of the limitations imposed by methods of identification and socio-demographic peculiarities of the affected population (48).
In this review, we adopted known methods to select studies and synthesize evidence and ensure transparency in our report. These techniques allow readers to focus on the merits of decisions made in compiling the information presented. Although this systematic review draws primarily from evidence published in journals written in English, synthesizing and pooling of evidence cover studies from a large number of countries across sub-Saharan Africa, including Francophone settings in West Africa. Therefore, our inferences could still be considered generalizable to the broader sub-Saharan context.
There are three limitations to interpreting the findings of this review. First, there was considerable variability in the sample size of the studies, ranging from 90 (36) to 102708 (25). This is reflected in the wide confidence intervals for some of the reported odds ratio estimates included in the meta-analysis, and one study from Malawi contributed 94.4% of the variation. It appears that one research substantially influenced the overall estimate of the odds of deaths, but a repeated analysis without that study had no significant impact on the assessment for publication bias. Second, there are flaws in some of the data presented in a few studies (35). For example, a direct comparison of incidence rates in two studies that reported sex-related data was difficult because of variation in definitions of LRTI incidence. Finally, we were unable to verify response rates and whether non-participants were different from participants in terms of socio-demographic characteristics in all the observational studies. Despite these concerns, our review shows that sex-related differences should be considered seriously by clinical researchers and physicians in working with children with pneumonia.
We identified 9 studies from Nigeria (n=3), Mozambique (n=2), Kenya (n=1), Mali (n=1), Malawi (n=1), and South Africa (1) with sex-related data on case fatality (Table 6). The distribution of the included publications in the funnel plots (Figure 3) shows symmetrical scattered points on either side of the overall effect line. This observation was supported by the results of the Egger’s test (P>0.05), suggesting no remarkable publication biases. The meta-analysis odds ratio estimates for the effect of sex on case fatality with statistical tests for heterogeneity are shown in Figure 4. The pooled estimate indicates that the odds of fatality were significantly higher for male than female children (OR=1.26; 95% CI: 1.20-1.33).
5. Discussion
This novel review synthesizes published studies from African countries on sex differences in incidence, etiology, and case fatality of LRTI in children. We observed that despite the increasing evidence that gender is an essential factor which influences the diseases and response to treatments (13, 37, 38), data on sex-related differences in incidence, etiology, and outcomes of LRTI are scarce. We found only two studies (5, 35) that reported the incidence of pneumonia between males and females, and they both reported a higher rate for male than female children. There were inconsistencies in the reports of pathogens identified in children with LRTI so that RSV was the leading cause in the three studies that disaggregated etiologic agents by sex. Our summary statistics indicated that the odds of RSV infection was significantly higher in female than male children. We further observed that only 9 studies reported an association between sex and case fatality and the overall estimate revealed that the odds of death was higher in male children (OR=1.26; 95% CI 1.20-1.33).
To our knowledge, our study is the first comprehensive attempt to systematically assess the effect of sex on the incidence, etiology, and mortality of ALRI in children of the African population. Excess pneumonia found among males compared with females has been previously described (39, 40). However, it remains unclear whether sex differences in pneumonia incidence is due to variability in susceptibility between boys and girls or to selection bias from preferential care-seeking. The incidence reported for male children (0.36 episodes per child-year) in the South Africa study is considerably higher than the estimated rate (0.33 episodes per child-year) for African children aged less than 5 years (41), and more recent estimate is 0.22 episodes per child-year (interquartile range: 0.11-0.51) for all low and middle–income countries (LMIC) derived from 35 community-based studies published between 1990 and 2012 (42). The considerable variation in the incidence between the studies selected for our review was most probably due to the distinct study designs and or real differences in the prevalence of pneumonia in the various study settings. For instance, the Nigeria study (33) had an error in respect of the application of case definition and that affected the computation of incidence that the study estimate of 6.1-8.1 episodes per child-year was remarkably higher than any rate ever reported.
The difficulties associated with pooling data for estimation of the incidence of pneumonia have been highlighted (42). These problems included the scarcity of longitudinal studies in LMIC, the necessity of conducting such studies over a full calendar year, active and frequent screening of a large number of children, as well as the correctness of application of case definition by the assessor (42). All of these issues limited our ability to pool data in this review. Similarly, we were unable to combine sex-related data on the etiology of pneumonia in children, given the heterogeneity of the data (41).
In our review, bacterial and viral etiologies of pneumonia were inconsistently reported for male and female children. The leading bacterial and viral causes for pneumonia found in our review agreed with previous reports (43-46). However, literature does not explain why the predisposition to some pathogen has sex preference. Generally, in Africa, there is the need to conduct more studies on the etiology of pneumonia with sex-specific data in children. Such reviews will help define the new distribution of pneumonia-causing etiologies in both sexes that may have important implications for empirical diagnosis and treatments. It is necessary to note that determining the cause of pneumonia in children is often challenging due to difficulties in obtaining direct lung samples. The expectorant easily gets contaminated by oropharyngeal organisms, but the patient’s age and probably the child’s sex can help narrow the list of probable etiology (47). Therefore, interpreting the results of studies on the etiologies of pneumonia requires an understanding of the limitations imposed by methods of identification and socio-demographic peculiarities of the affected population (48).
In this review, we adopted known methods to select studies and synthesize evidence and ensure transparency in our report. These techniques allow readers to focus on the merits of decisions made in compiling the information presented. Although this systematic review draws primarily from evidence published in journals written in English, synthesizing and pooling of evidence cover studies from a large number of countries across sub-Saharan Africa, including Francophone settings in West Africa. Therefore, our inferences could still be considered generalizable to the broader sub-Saharan context.
There are three limitations to interpreting the findings of this review. First, there was considerable variability in the sample size of the studies, ranging from 90 (36) to 102708 (25). This is reflected in the wide confidence intervals for some of the reported odds ratio estimates included in the meta-analysis, and one study from Malawi contributed 94.4% of the variation. It appears that one research substantially influenced the overall estimate of the odds of deaths, but a repeated analysis without that study had no significant impact on the assessment for publication bias. Second, there are flaws in some of the data presented in a few studies (35). For example, a direct comparison of incidence rates in two studies that reported sex-related data was difficult because of variation in definitions of LRTI incidence. Finally, we were unable to verify response rates and whether non-participants were different from participants in terms of socio-demographic characteristics in all the observational studies. Despite these concerns, our review shows that sex-related differences should be considered seriously by clinical researchers and physicians in working with children with pneumonia.
6. Conclusions
There is little sex-specific data on the incidence, etiology, and case fatality of pneumonia in studies published from Africa. It seems that male patients die more frequently than females. However, female patients seem to suffer more commonly from RSV infection. Clinicians should be aware of these differences and take them under consideration when managing children with LRTIs. Also, researchers should be encouraged to include and report on sex differences as separately defined variables in LRTI studies. This review shows clearly that male children carry a considerable burden of pneumonia morbidity and mortality in Africa, making them a group that would benefit significantly from existing and newer preventive interventions.
Ethical Considerations
Compliance with ethical guidelines
The protocol for this systematic review has been approved and registered with PROSPERO (CRD42019122494).
Funding
This work was a product of a short-term visiting scholarship at the University of Cape Town supported by the Postgraduate Academic Mobility for African Physician Scientists of the EACEA Mobility Program (Project No: 2013-4692).
Authors contributions
Adebola E. Orimadegun and Landon Myer conceptualized and developed the original idea, prepared the study protocol, abstracted and analyzed data, and wrote the manuscript. Adedayo A. Adepoju contributed significantly to the assessment of study quality, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors are grateful to PAMAPS Project, Professor O. O. Akinyinka, University of Ibadan and the Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
There is little sex-specific data on the incidence, etiology, and case fatality of pneumonia in studies published from Africa. It seems that male patients die more frequently than females. However, female patients seem to suffer more commonly from RSV infection. Clinicians should be aware of these differences and take them under consideration when managing children with LRTIs. Also, researchers should be encouraged to include and report on sex differences as separately defined variables in LRTI studies. This review shows clearly that male children carry a considerable burden of pneumonia morbidity and mortality in Africa, making them a group that would benefit significantly from existing and newer preventive interventions.
Ethical Considerations
Compliance with ethical guidelines
The protocol for this systematic review has been approved and registered with PROSPERO (CRD42019122494).
Funding
This work was a product of a short-term visiting scholarship at the University of Cape Town supported by the Postgraduate Academic Mobility for African Physician Scientists of the EACEA Mobility Program (Project No: 2013-4692).
Authors contributions
Adebola E. Orimadegun and Landon Myer conceptualized and developed the original idea, prepared the study protocol, abstracted and analyzed data, and wrote the manuscript. Adedayo A. Adepoju contributed significantly to the assessment of study quality, drafting of the manuscript, and critical revision of the manuscript for important intellectual content.
Conflict of interest
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Acknowledgements
The authors are grateful to PAMAPS Project, Professor O. O. Akinyinka, University of Ibadan and the Division of Epidemiology and Biostatistics, School of Public Health and Family Medicine, University of Cape Town, Cape Town, South Africa.
References
Wang H, Naghavi M, Allen C, Barber RM, Bhutta ZA, Carter A, et al. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: A systematic analysis for the Global Burden of Disease Study 2015. The Lancet. 2016; 388(10053):1459-544. [DOI:10.1016/S0140-6736(16)31012-1]
Liu L, Johnson HL, Cousens S, Perin J, Scott S, Lawn JE, et al. Global, regional, and national causes of child mortality: An updated systematic analysis for 2010 with time trends since 2000. The Lancet. 2012; 379(9832):2151-61. [DOI:10.1016/S0140-6736(12)60560-1]
O’Callaghan-Gordo C, Bassat Q, Morais L, Diez-Padrisa N, Machevo S, Nhampossa T, et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: A malaria endemic area with high prevalence of human immunodeficiency virus. The Pediatric Infectious Disease Journal. 2011; 30(1):39-44. [DOI:10.1097/INF.0b013e3181f232fe] [PMID]
Hussey G, Hitchcock J, Schaaf H, Coetzee G, Hanslo D, van Schalkwyk E, et al. Epidemiology of invasive Haemophilus influenzae infections in Cape Town, South Africa. Annals of Tropical Paediatrics. 1994; 14(2):97-103 [DOI:10.1080/02724936.1994.11747700] [PMID]
Tornheim JA, Manya AS, Oyando N, Kabaka S, Breiman RF, Feikin DR. The epidemiology of hospitalized pneumonia in rural Kenya: The potential of surveillance data in setting public health priorities. International Journal of Infectious Diseases. 2007; 11(6):536-43. [DOI:10.1016/j.ijid.2007.03.006] [PMID]
Campbell JD, Kotloff KL, Sow SO, Tapia M, Keita MM, Keita T, et al. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. The Pediatric Infectious Disease Journal. 2004; 23(7):642-9. [DOI:10.1097/01.inf.0000130951.85974.79] [PMID]
Nantanda R, Hildenwall H, Peterson S, Kaddu-Mulindwa D, Kalyesubula I, Tumwine JK. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Annals of Tropical Paediatrics. 2008; 28(4):253-60. [DOI:10.1179/146532808X375404] [PMID]
Saffar MJ, Rezai MS. Management of lower respiratory tract illnesses in developing countries: A narrative review. Journal of Pediatrics Review. 2014; 2(2):47-56.
Kirkwood BR, Gove S, Rogers S, Lob-Levyt J, Arthur P, Campbell H. Potential interventions for the prevention of childhood pneumonia in developing countries: A systematic review. Bulletin of the World Health Organization. 1995; 73(6):793-8. [PMCID] [PMID]
Victora CG, Kirkwood BR, Ashworth A, Black RE, Rogers S, Sazawal S, et al. Potential interventions for the prevention of childhood pneumonia in developing countries: Improving nutrition. The American Journal of Clinical Nutrition. 1999; 70(3):309-20 [DOI:10.1093/ajcn/70.3.309] [PMID]
World Health Organization. Recommendations for management of common childhood conditions: Evidence for technical update of pocket book recommendations. Geneva: World Health Organization; 2012.
Falade AG, Ayede AI. Epidemiology, aetiology and management of childhood acute community-acquired pneumonia in developing countries: A review. African Journal of Medicine and Medical Sciences. 2011; 40(4):293-308. [PMID]
Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respiratory Medicine. 2007; 101(9):1845-63. [DOI:10.1016/j.rmed.2007.04.011] [PMID]
Jackson S, Mathews KH, Pulanic D, Falconer R, Rudan I, Campbell H, et al. Risk factors for severe acute lower respiratory infections in children: A systematic review and meta-analysis. Croatian Medical Journal. 2013; 54(2):110-21 [DOI:10.3325/cmj.2013.54.110] [PMID] [PMCID]
World Health Organization. Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. Geneva: World Health Organization; 1991.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015; 4:1. [DOI:10.1186/2046-4053-4-1] [PMID] [PMCID]
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010; 25(9):603-5. [DOI:10.1007/s10654-010-9491-z] [PMID]
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. 2014 [Updated 2014 June 10]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Manchester: The Cochrane Collaboration; 2011.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. British Medical Journal. 2016; 355:i4919. [DOI:10.1136/bmj.i4919] [PMID] [PMCID]
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002; 21(11):1539-58. [DOI:10.1002/sim.1186] [PMID]
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L, Moreno SG. Assessing publication bias in meta-analyses in the presence of between-study heterogeneity. Journal of the Royal Statistical Society: Series A (Statistics in Society). 2010; 173(3):575-91. [DOI:10.1111/j.1467-985X.2009.00629.x]
Forgie IM, O’Neill KP, Lloyd-Evans N, Leinonen M, Campbell H, Whittle HC, et al. Etiology of acute lower respiratory tract infections in Gambian children: I. Acute lower respiratory tract infections in infants presenting at the hospital. The Pediatric Infectious Disease Journal. 1991; 10(1):33-41. [DOI:10.1097/00006454-199101000-00008] [PMID]
Ayieko P, Okiro EA, Edwards T, Nyamai R, English M. Variations in mortality in children admitted with pneumonia to Kenyan hospitals. PLoS One. 2012; 7(11):e47622. [DOI:10.1371/journal.pone.0047622] [PMID] [PMCID]
Lazzerini M, Seward N, Lufesi N, Banda R, Sinyeka S, Masache G, et al. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001-12: A retrospective observational study. Lancet Glob Health. 2016; 4(1):e57-68. [DOI:10.1016/S2214-109X(15)00215-6]
Bénet T, Sylla M, Messaoudi M, Sanchez Picot V, Telles JN, Diakite AA, et al. Etiology and Factors Associated with Pneumonia in Children under 5 Years of Age in Mali: A Prospective Case-Control Study. PLoS One. 2015; 10(12):e0145447. [DOI:10.1371/journal.pone.0145447] [PMID] [PMCID]
Bassat Q, Lanaspa M, Machevo S, O’Callaghan-Gordo C, Madrid L, Nhampossa T, et al. Hypoxaemia in Mozambican children <5 years of age admitted to hospital with clinical severe pneumonia: Clinical features and performance of predictor models. Tropical Medicine & International Health. 2016; 21(9):1147-56. [DOI:10.1111/tmi.12738] [PMID]
Sigauque B, Roca A, Bassat Q, Morais L, Quinto L, Berenguera A, et al. Severe pneumonia in Mozambican young children: Clinical and radiological characteristics and risk factors. Journal of Tropical Pediatrics. 2009; 55(6):379-87. [DOI:10.1093/tropej/fmp030] [PMID]
Abdulkadir MB, Ibraheem RM, Gobir AA, Johnson WBR. Hypoxaemia as a measure of disease severity in young hospitalised Nigerian children with pneumonia: A cross-sectional study. South African Journal of Child Health. 2015; 9(2):53-6.
Abdulkarim A, Ibraheem R, Adegboye A, Johnson W, Adeboye M. Childhood pneumonia at the University of Ilorin Teaching Hospital, Ilorin Nigeria. Nigerian Journal of Paediatrics. 2013; 40(3):284-9. [DOI:10.4314/njp.v40i3,16]
Johnson AW, Osinusi K, Aderele WI, Gbadero DA, Olaleye OD, Adeyemi-Doro FA. Etiologic agents and outcome determinants of community-acquired pneumonia in urban children: a hospital-based study. Journal of the National Medical Association. 2008; 100(4):370-85 [DOI:10.1016/S0027-9684(15)31269-4]
Johnson WB, Aderele WI, Gbadero DA. Host factors and acute lower respiratory infections in pre-school children. Journal of Tropical Pediatrics. 1992; 38(3):132-6 [DOI:10.1093/tropej/38.3.132] [PMID]
Oyejide C, Osinusi K. Acute respiratory tract infection in children in Idikan community, Ibadan, Nigeria: Severity, risk factors, and frequency of occurrence. Review of Infectious Diseases. 1990; 12(Supplement 8):S1042-SI046 [DOI:10.1093/clinids/12.Supplement_8.S1042] [PMID]
Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. The Lancet Respiratory Medicine. 2016; 4(6):463-72. [DOI:10.1016/S2213-2600(16)00096-5]
le Roux DM, Myer L, Nicol MP, Zar HJ. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: The Drakenstein Child Health Study. Lancet Glob Health. 2015; 3(2):e95-e103. [DOI:10.1016/S2214-109X(14)70360-2]
Forgie IM, O’Neill KP, Lloyd-Evans N, Leinonen M, Campbell H, Whittle HC, et al. Etiology of acute lower respiratory tract infections in Gambian children: II. Acute lower respiratory tract infection in children ages one to nine years presenting at the hospital. The Pediatric Infectious Disease Journal. 1991; 10(1):42-7. [DOI:10.1097/00006454-199101000-00009] [PMID]
Legato MJ, Johnson PA, Manson JE. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016; 316(18):1865-6. [DOI:10.1001/jama.2016.13995] [PMID]
Legato MJ. Gender-specific medicine in the genomic era. Clinical Science (London). 2016; 130(1):1-7. [DOI:10.1042/CS20150551] [PMID]
Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries: Coordinated Data Group of BOSTID Researchers. Review of Infectious Diseases. 1990; 12(Suppl 8):S870-88 [DOI:10.1093/clinids/12.Supplement_S870] [PMID]
Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. The Pediatric Infectious Disease Journal. 2004; 23(Suppl 1):S11-8. [DOI:10.1097/01.inf.0000108188.37237.48] [PMID]
Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008; 86(5):408-16 [DOI:10.2471/BLT.07.048769] [PMID] [PMCID]
Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. Journal of Global Health. 2013; 3(1):010401. [PMCID] [PMID]
Shann F. Etiology of severe pneumonia in children in developing countries. The Pediatric Infectious Disease Journal. 1986; 5(2):247-52 [DOI:10.1097/00006454-198603000-00017] [PMID]
Sinaniotis CA. Viral pneumoniae in children: Incidence and aetiology. Paediatric Respiratory Reviews. 2004; 5(Suppl A):S197-200. [DOI:10.1016/S1526-0542(04)90037-1]
Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine & International Health. 1998; 3(4):268-80 [DOI:10.1046/j.1365-3156.1998.00213.x] [PMID]
Cardinale F, Cappiello AR, Mastrototaro MF, Pignatelli M, Esposito S. Community-acquired pneumonia in children. Early Human Development. 2013; 89(Suppl 3):S49-52. [DOI:10.1016/j.earlhumdev.2013.07.023] [PMID]
Chen K, Jia R, Li L, Yang C, Shi Y. The aetiology of community associated pneumonia in children in Nanjing, China and aetiological patterns associated with age and season. BMC Public Health. 2015; 15:113. [DOI:10.1186/s12889-015-1422-1] [PMID] [PMCID]
Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clinical Infectious Diseases. 2011; 52(Suppl 4):S296-304. [DOI:10.1093/cid/cir045] [PMID]
O’Callaghan-Gordo C, Bassat Q, Morais L, Diez-Padrisa N, Machevo S, Nhampossa T, et al. Etiology and epidemiology of viral pneumonia among hospitalized children in rural Mozambique: A malaria endemic area with high prevalence of human immunodeficiency virus. The Pediatric Infectious Disease Journal. 2011; 30(1):39-44. [DOI:10.1097/INF.0b013e3181f232fe] [PMID]
Hussey G, Hitchcock J, Schaaf H, Coetzee G, Hanslo D, van Schalkwyk E, et al. Epidemiology of invasive Haemophilus influenzae infections in Cape Town, South Africa. Annals of Tropical Paediatrics. 1994; 14(2):97-103 [DOI:10.1080/02724936.1994.11747700] [PMID]
Tornheim JA, Manya AS, Oyando N, Kabaka S, Breiman RF, Feikin DR. The epidemiology of hospitalized pneumonia in rural Kenya: The potential of surveillance data in setting public health priorities. International Journal of Infectious Diseases. 2007; 11(6):536-43. [DOI:10.1016/j.ijid.2007.03.006] [PMID]
Campbell JD, Kotloff KL, Sow SO, Tapia M, Keita MM, Keita T, et al. Invasive pneumococcal infections among hospitalized children in Bamako, Mali. The Pediatric Infectious Disease Journal. 2004; 23(7):642-9. [DOI:10.1097/01.inf.0000130951.85974.79] [PMID]
Nantanda R, Hildenwall H, Peterson S, Kaddu-Mulindwa D, Kalyesubula I, Tumwine JK. Bacterial aetiology and outcome in children with severe pneumonia in Uganda. Annals of Tropical Paediatrics. 2008; 28(4):253-60. [DOI:10.1179/146532808X375404] [PMID]
Saffar MJ, Rezai MS. Management of lower respiratory tract illnesses in developing countries: A narrative review. Journal of Pediatrics Review. 2014; 2(2):47-56.
Kirkwood BR, Gove S, Rogers S, Lob-Levyt J, Arthur P, Campbell H. Potential interventions for the prevention of childhood pneumonia in developing countries: A systematic review. Bulletin of the World Health Organization. 1995; 73(6):793-8. [PMCID] [PMID]
Victora CG, Kirkwood BR, Ashworth A, Black RE, Rogers S, Sazawal S, et al. Potential interventions for the prevention of childhood pneumonia in developing countries: Improving nutrition. The American Journal of Clinical Nutrition. 1999; 70(3):309-20 [DOI:10.1093/ajcn/70.3.309] [PMID]
World Health Organization. Recommendations for management of common childhood conditions: Evidence for technical update of pocket book recommendations. Geneva: World Health Organization; 2012.
Falade AG, Ayede AI. Epidemiology, aetiology and management of childhood acute community-acquired pneumonia in developing countries: A review. African Journal of Medicine and Medical Sciences. 2011; 40(4):293-308. [PMID]
Falagas ME, Mourtzoukou EG, Vardakas KZ. Sex differences in the incidence and severity of respiratory tract infections. Respiratory Medicine. 2007; 101(9):1845-63. [DOI:10.1016/j.rmed.2007.04.011] [PMID]
Jackson S, Mathews KH, Pulanic D, Falconer R, Rudan I, Campbell H, et al. Risk factors for severe acute lower respiratory infections in children: A systematic review and meta-analysis. Croatian Medical Journal. 2013; 54(2):110-21 [DOI:10.3325/cmj.2013.54.110] [PMID] [PMCID]
World Health Organization. Technical bases for the WHO recommendations on the management of pneumonia in children at first-level health facilities. Geneva: World Health Organization; 1991.
Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015; 4:1. [DOI:10.1186/2046-4053-4-1] [PMID] [PMCID]
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. European Journal of Epidemiology. 2010; 25(9):603-5. [DOI:10.1007/s10654-010-9491-z] [PMID]
Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses [Internet]. 2014 [Updated 2014 June 10]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
Higgins JP, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011]. Manchester: The Cochrane Collaboration; 2011.
Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. British Medical Journal. 2016; 355:i4919. [DOI:10.1136/bmj.i4919] [PMID] [PMCID]
Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statistics in Medicine. 2002; 21(11):1539-58. [DOI:10.1002/sim.1186] [PMID]
Peters JL, Sutton AJ, Jones DR, Abrams KR, Rushton L, Moreno SG. Assessing publication bias in meta-analyses in the presence of between-study heterogeneity. Journal of the Royal Statistical Society: Series A (Statistics in Society). 2010; 173(3):575-91. [DOI:10.1111/j.1467-985X.2009.00629.x]
Forgie IM, O’Neill KP, Lloyd-Evans N, Leinonen M, Campbell H, Whittle HC, et al. Etiology of acute lower respiratory tract infections in Gambian children: I. Acute lower respiratory tract infections in infants presenting at the hospital. The Pediatric Infectious Disease Journal. 1991; 10(1):33-41. [DOI:10.1097/00006454-199101000-00008] [PMID]
Ayieko P, Okiro EA, Edwards T, Nyamai R, English M. Variations in mortality in children admitted with pneumonia to Kenyan hospitals. PLoS One. 2012; 7(11):e47622. [DOI:10.1371/journal.pone.0047622] [PMID] [PMCID]
Lazzerini M, Seward N, Lufesi N, Banda R, Sinyeka S, Masache G, et al. Mortality and its risk factors in Malawian children admitted to hospital with clinical pneumonia, 2001-12: A retrospective observational study. Lancet Glob Health. 2016; 4(1):e57-68. [DOI:10.1016/S2214-109X(15)00215-6]
Bénet T, Sylla M, Messaoudi M, Sanchez Picot V, Telles JN, Diakite AA, et al. Etiology and Factors Associated with Pneumonia in Children under 5 Years of Age in Mali: A Prospective Case-Control Study. PLoS One. 2015; 10(12):e0145447. [DOI:10.1371/journal.pone.0145447] [PMID] [PMCID]
Bassat Q, Lanaspa M, Machevo S, O’Callaghan-Gordo C, Madrid L, Nhampossa T, et al. Hypoxaemia in Mozambican children <5 years of age admitted to hospital with clinical severe pneumonia: Clinical features and performance of predictor models. Tropical Medicine & International Health. 2016; 21(9):1147-56. [DOI:10.1111/tmi.12738] [PMID]
Sigauque B, Roca A, Bassat Q, Morais L, Quinto L, Berenguera A, et al. Severe pneumonia in Mozambican young children: Clinical and radiological characteristics and risk factors. Journal of Tropical Pediatrics. 2009; 55(6):379-87. [DOI:10.1093/tropej/fmp030] [PMID]
Abdulkadir MB, Ibraheem RM, Gobir AA, Johnson WBR. Hypoxaemia as a measure of disease severity in young hospitalised Nigerian children with pneumonia: A cross-sectional study. South African Journal of Child Health. 2015; 9(2):53-6.
Abdulkarim A, Ibraheem R, Adegboye A, Johnson W, Adeboye M. Childhood pneumonia at the University of Ilorin Teaching Hospital, Ilorin Nigeria. Nigerian Journal of Paediatrics. 2013; 40(3):284-9. [DOI:10.4314/njp.v40i3,16]
Johnson AW, Osinusi K, Aderele WI, Gbadero DA, Olaleye OD, Adeyemi-Doro FA. Etiologic agents and outcome determinants of community-acquired pneumonia in urban children: a hospital-based study. Journal of the National Medical Association. 2008; 100(4):370-85 [DOI:10.1016/S0027-9684(15)31269-4]
Johnson WB, Aderele WI, Gbadero DA. Host factors and acute lower respiratory infections in pre-school children. Journal of Tropical Pediatrics. 1992; 38(3):132-6 [DOI:10.1093/tropej/38.3.132] [PMID]
Oyejide C, Osinusi K. Acute respiratory tract infection in children in Idikan community, Ibadan, Nigeria: Severity, risk factors, and frequency of occurrence. Review of Infectious Diseases. 1990; 12(Supplement 8):S1042-SI046 [DOI:10.1093/clinids/12.Supplement_8.S1042] [PMID]
Zar HJ, Barnett W, Stadler A, Gardner-Lubbe S, Myer L, Nicol MP. Aetiology of childhood pneumonia in a well vaccinated South African birth cohort: a nested case-control study of the Drakenstein Child Health Study. The Lancet Respiratory Medicine. 2016; 4(6):463-72. [DOI:10.1016/S2213-2600(16)00096-5]
le Roux DM, Myer L, Nicol MP, Zar HJ. Incidence and severity of childhood pneumonia in the first year of life in a South African birth cohort: The Drakenstein Child Health Study. Lancet Glob Health. 2015; 3(2):e95-e103. [DOI:10.1016/S2214-109X(14)70360-2]
Forgie IM, O’Neill KP, Lloyd-Evans N, Leinonen M, Campbell H, Whittle HC, et al. Etiology of acute lower respiratory tract infections in Gambian children: II. Acute lower respiratory tract infection in children ages one to nine years presenting at the hospital. The Pediatric Infectious Disease Journal. 1991; 10(1):42-7. [DOI:10.1097/00006454-199101000-00009] [PMID]
Legato MJ, Johnson PA, Manson JE. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016; 316(18):1865-6. [DOI:10.1001/jama.2016.13995] [PMID]
Legato MJ. Gender-specific medicine in the genomic era. Clinical Science (London). 2016; 130(1):1-7. [DOI:10.1042/CS20150551] [PMID]
Selwyn BJ. The epidemiology of acute respiratory tract infection in young children: comparison of findings from several developing countries: Coordinated Data Group of BOSTID Researchers. Review of Infectious Diseases. 1990; 12(Suppl 8):S870-88 [DOI:10.1093/clinids/12.Supplement_S870] [PMID]
Henrickson KJ, Hoover S, Kehl KS, Hua W. National disease burden of respiratory viruses detected in children by polymerase chain reaction. The Pediatric Infectious Disease Journal. 2004; 23(Suppl 1):S11-8. [DOI:10.1097/01.inf.0000108188.37237.48] [PMID]
Rudan I, Boschi-Pinto C, Biloglav Z, Mulholland K, Campbell H. Epidemiology and etiology of childhood pneumonia. Bulletin of the World Health Organization. 2008; 86(5):408-16 [DOI:10.2471/BLT.07.048769] [PMID] [PMCID]
Rudan I, O’Brien KL, Nair H, Liu L, Theodoratou E, Qazi S, et al. Epidemiology and etiology of childhood pneumonia in 2010: Estimates of incidence, severe morbidity, mortality, underlying risk factors and causative pathogens for 192 countries. Journal of Global Health. 2013; 3(1):010401. [PMCID] [PMID]
Shann F. Etiology of severe pneumonia in children in developing countries. The Pediatric Infectious Disease Journal. 1986; 5(2):247-52 [DOI:10.1097/00006454-198603000-00017] [PMID]
Sinaniotis CA. Viral pneumoniae in children: Incidence and aetiology. Paediatric Respiratory Reviews. 2004; 5(Suppl A):S197-200. [DOI:10.1016/S1526-0542(04)90037-1]
Weber MW, Mulholland EK, Greenwood BM. Respiratory syncytial virus infection in tropical and developing countries. Tropical Medicine & International Health. 1998; 3(4):268-80 [DOI:10.1046/j.1365-3156.1998.00213.x] [PMID]
Cardinale F, Cappiello AR, Mastrototaro MF, Pignatelli M, Esposito S. Community-acquired pneumonia in children. Early Human Development. 2013; 89(Suppl 3):S49-52. [DOI:10.1016/j.earlhumdev.2013.07.023] [PMID]
Chen K, Jia R, Li L, Yang C, Shi Y. The aetiology of community associated pneumonia in children in Nanjing, China and aetiological patterns associated with age and season. BMC Public Health. 2015; 15:113. [DOI:10.1186/s12889-015-1422-1] [PMID] [PMCID]
Bartlett JG. Diagnostic tests for agents of community-acquired pneumonia. Clinical Infectious Diseases. 2011; 52(Suppl 4):S296-304. [DOI:10.1093/cid/cir045] [PMID]
Type of Study: Meta-analysis Review |
Subject:
Pediatric Pulmonology
Received: 2019/10/28 | Accepted: 2019/11/24 | Published: 2020/04/1
Received: 2019/10/28 | Accepted: 2019/11/24 | Published: 2020/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |