Volume 13, Issue 3 (7-2025)
J. Pediatr. Rev 2025, 13(3): 183-192 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Kalejahi P, Noorazar S G. Magnesium Status and Supplementation in Children With Attention-deficit/Hyperactivity Disorder: A Systematic Review. J. Pediatr. Rev 2025; 13 (3) :183-192
URL: http://jpr.mazums.ac.ir/article-1-620-en.html
URL: http://jpr.mazums.ac.ir/article-1-620-en.html
1- Research Center of Psychiatry and Behavioral Sciences, Tabriz University of Medical Sciences, Tabriz, Iran. , parinaz.kalejahi@gmail.com
2- Research Center of Psychiatry and Behavioral Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
2- Research Center of Psychiatry and Behavioral Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Full-Text [PDF 593 kb]
(11135 Downloads)
| Abstract (HTML) (1273 Views)
Full-Text: (4646 Views)
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental condition marked by challenges in attention, impulsiveness, hyperactivity, and emotional regulation [1]. These deficits can lead to impairments in various areas of life, and many children continue to exhibit significant symptoms even as they grow into adolescence and adulthood [2]. The global incidence of ADHD is estimated to be around 5-7% in children and adolescents, and around 2.5% among adults [3, 4].
The origin of this disorder is still unknown, but it seems that genetic and environmental factors are involved in its development [5]. Environmental factors, such as nutritional factors and diet patterns, have been demonstrated to significantly contribute to the development of ADHD symptoms. As a result, there is a growing interest in using nutritional approaches to help manage ADHD. Scientific research in this area is increasing, and it is hoped that this will lead to new insights and treatment options for individuals with ADHD [6, 7].
Research in this area suggests that children with this disorder may have lower levels of important minerals, like magnesium, compared to healthy children [8, 9]. However, supplementing these nutrients has been shown to have a positive impact on managing the symptoms of the disorder [9, 10]. Magnesium is a critical cation in the central nervous system that plays a crucial role in various functions, including signal transmission and intracellular signaling [9].
Magnesium, in turn, plays a crucial role as a voltage-gated antagonist to the glutamate, N-methyl-D-aspartate (NMDA) receptor and also functions as an antagonist to calcium entry through voltage-gated channels of all types. It is worth noting that while magnesium levels in the brain tend to be more stable and decrease more gradually than in other tissues, even small reductions can have a profound effect on neuronal functioning [11].
Magnesium has been found to have a direct impact on dopamine release and the stimulatory effect of glutamate on dopamine release at the presynaptic level [12-14]. Low levels of magnesium may increase glutamatergic neurotransmission, potentially fostering an environment that promotes excitotoxicity. This may cause oxidative stress and neuronal loss [15]. Despite magnesium’s critical role in the central nervous system, there is limited research on its effects on ADHD [16]. Also, magnesium deficiency is linked to behavioral symptoms, such as anxiety and irritability, which can lead to a worsening of ADHD symptoms. Some studies have shown that magnesium supplements may be effective in improving some mental and neurological conditions, which could be explored as a treatment option for ADHD. Therefore, this systematic review aimed to evaluate existing research on the serum levels of magnesium in children with ADHD, as well as clinical trials on magnesium supplementation in these children.
Methods
Review design
This study was conducted adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) protocol.
Articles published until December 2023 were retrieved through a search in PUBMED/Medline, Google Scholar, and Scopus databases. Unpublished studies and documents were also manually searched and reviewed. Correspondence was also conducted with the corresponding author if the findings of an article were not available.
Search strategy
Keywords used to search for articles were in accordance with the MeSH glossary. Free keywords were also used in addition to MeSH keywords to search for articles. The following keywords were used alone or cross-linked, depending on which database was searched:
(Attention deficit disorder with hyperactivity [MeSH terms]) OR (ADHD [Title/Abstract]) OR (deficit-hyperactivity disorders, attention [Title/Abstract]) OR (hyperkinetic syndrome [Title/Abstract]) OR (attention deficit disorder [Title/Abstract]) AND (magnesium [Title/Abstract]) OR (mg [Title/Abstract])
Inclusion and exclusion criteria
Inclusion criteria based on the PICOS framework (population/intervention/comparators/outcomes/time/setting) were as follows:
Population: Studies on children with ADHD published in English;
Intervention: Supplementation with magnesium and, for descriptive studies, assessment of magnesium in serum, urine, or hair;
Outcome: Improvement in ADHD symptoms in the clinical trial;
Time/date: Publication date up to December 2023 without any location restrictions.
The exclusion criteria were as follows:
1) Reviews, systematic reviews, or meta-analyses, 2) Not enough information, 3) Studies in languages other than English or Persian, 4) Incomplete articles or obscure statistical results.
Data selection and extraction
We imported publication titles and abstracts into ENDNOTE, version 7 and removed duplicates. Two independent reviewers screened and evaluated all publications. We first screened article titles and abstracts and then reviewed full publications based on pre-specified criteria.
The selected studies were entered into Excel software. The data form was designed as follows: Published information included the author’s name, year of publication, country, type of intervention, type of magnesium measurement, final sample size, participants, possible side effects, and final results.
Quality assessment
The risk of bias in the included studies was assessed using the National Institutes of Health (NIH) quality assessment tool and Cochrane collaboration tool (Tables 1 and 2).
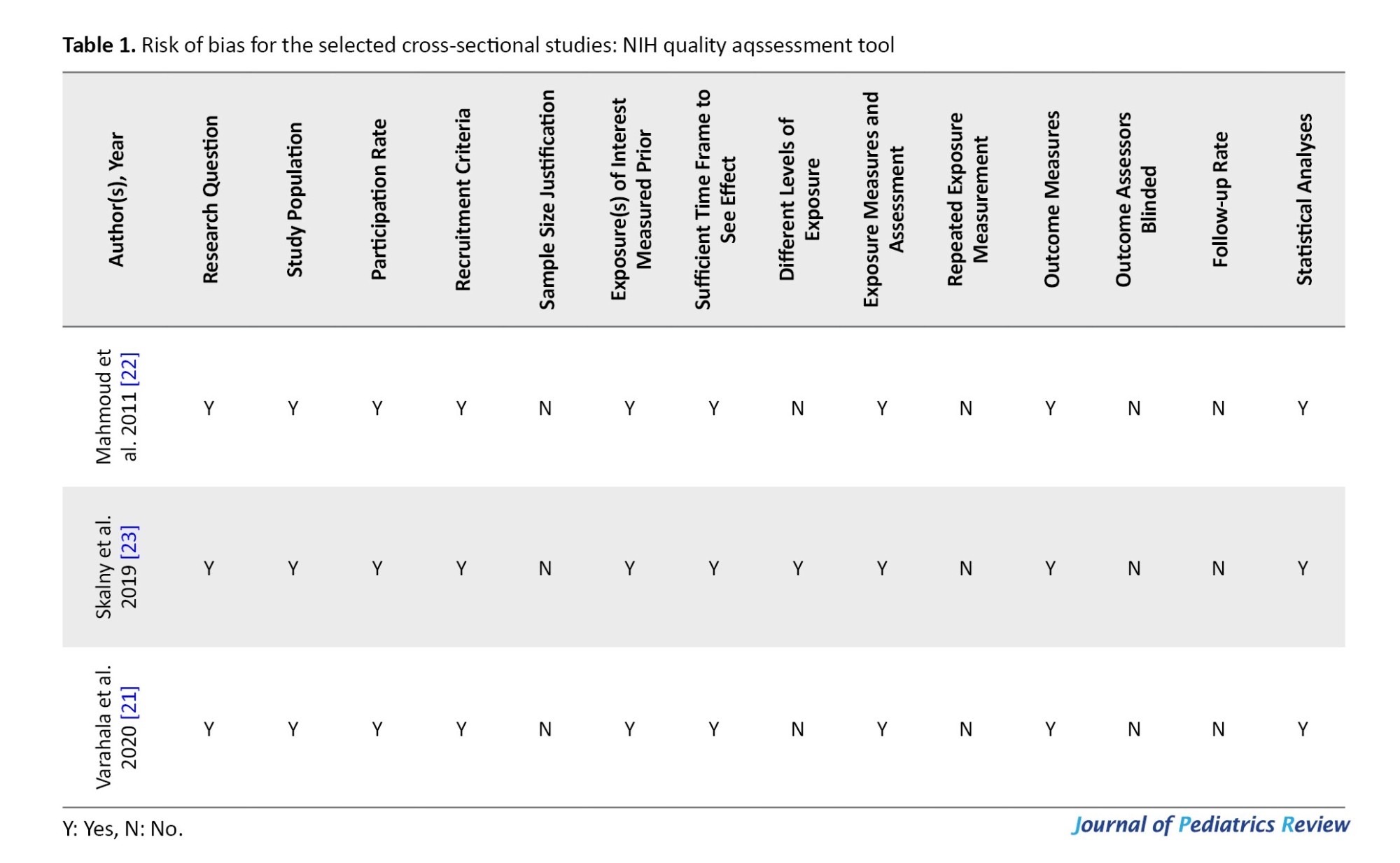
The NIH quality assessment tool for assessing bias in cross-sectional studies was used, which includes the following subscales: Study population, participation rate, recruitment criteria, sample size justification, exposure(s) of interest measured prior, sufficient time frame to observe the effect, different levels of exposure, exposure measures and assessment, repeated exposure measurement, outcome measures, outcome assessors blinded, and follow-up rate.
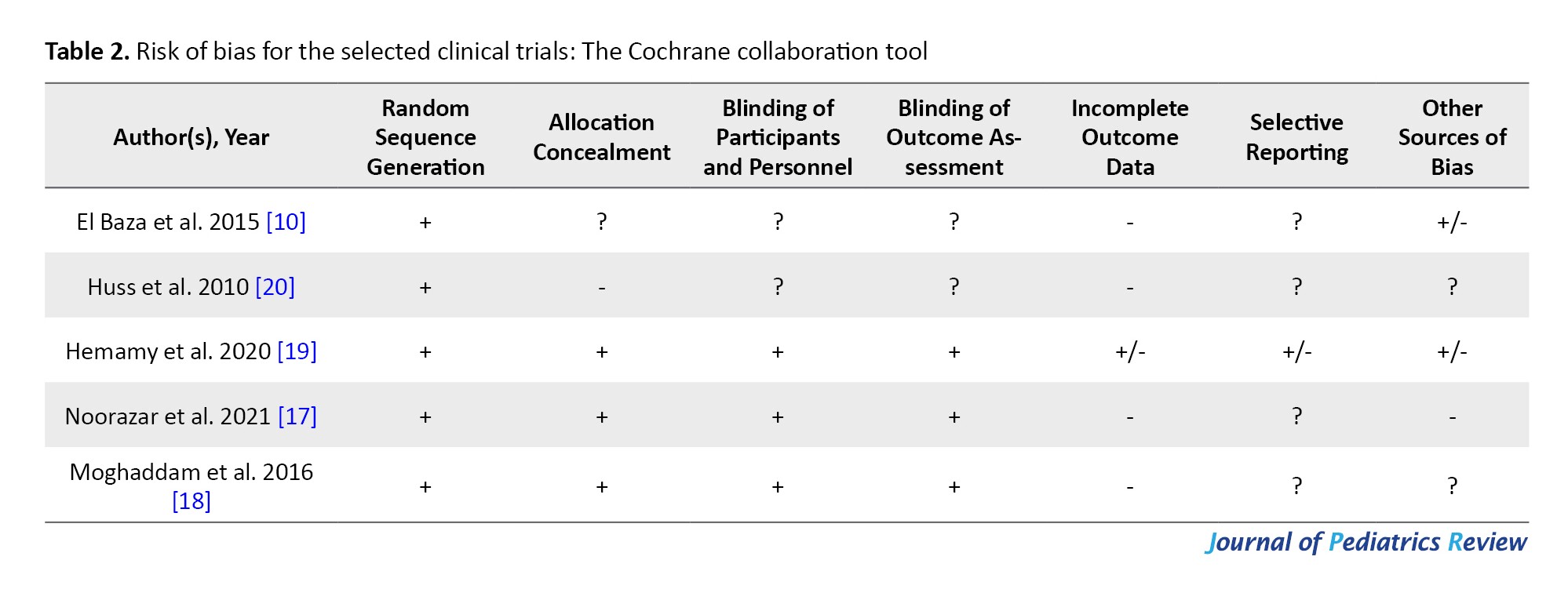
To evaluate the quality of intervention articles, the Cochrane tool was employed, which has seven subscales: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective bias, and other sources of bias.
Results
Study selection
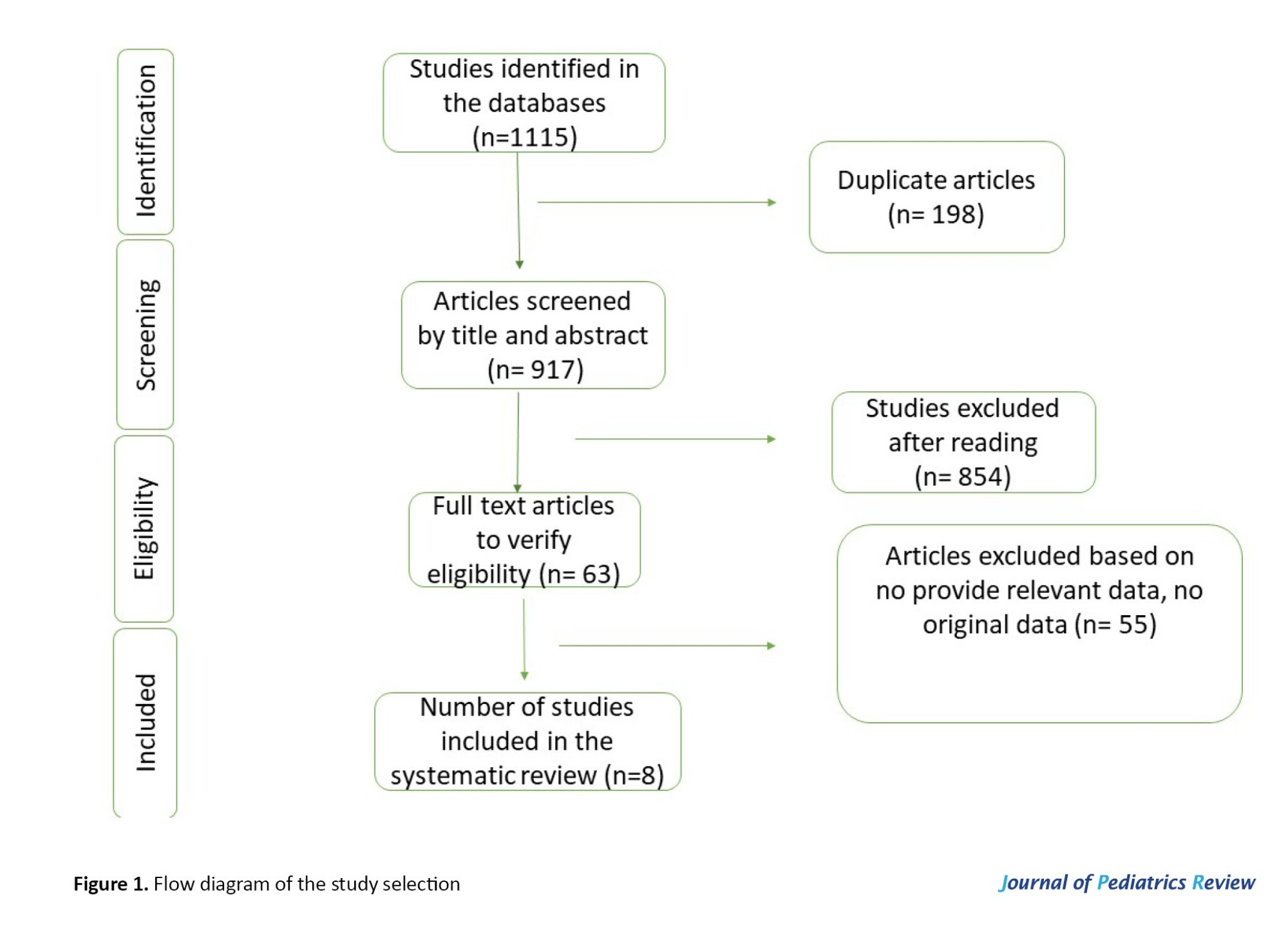
We found 1115 articles and, after removing duplicates, 917 articles were selected for the next step. After reviewing the studies by two separate researchers, 63 studies were selected, and finally, 8 studies were selected to be included in the review study (Figure 1).
Study characteristics
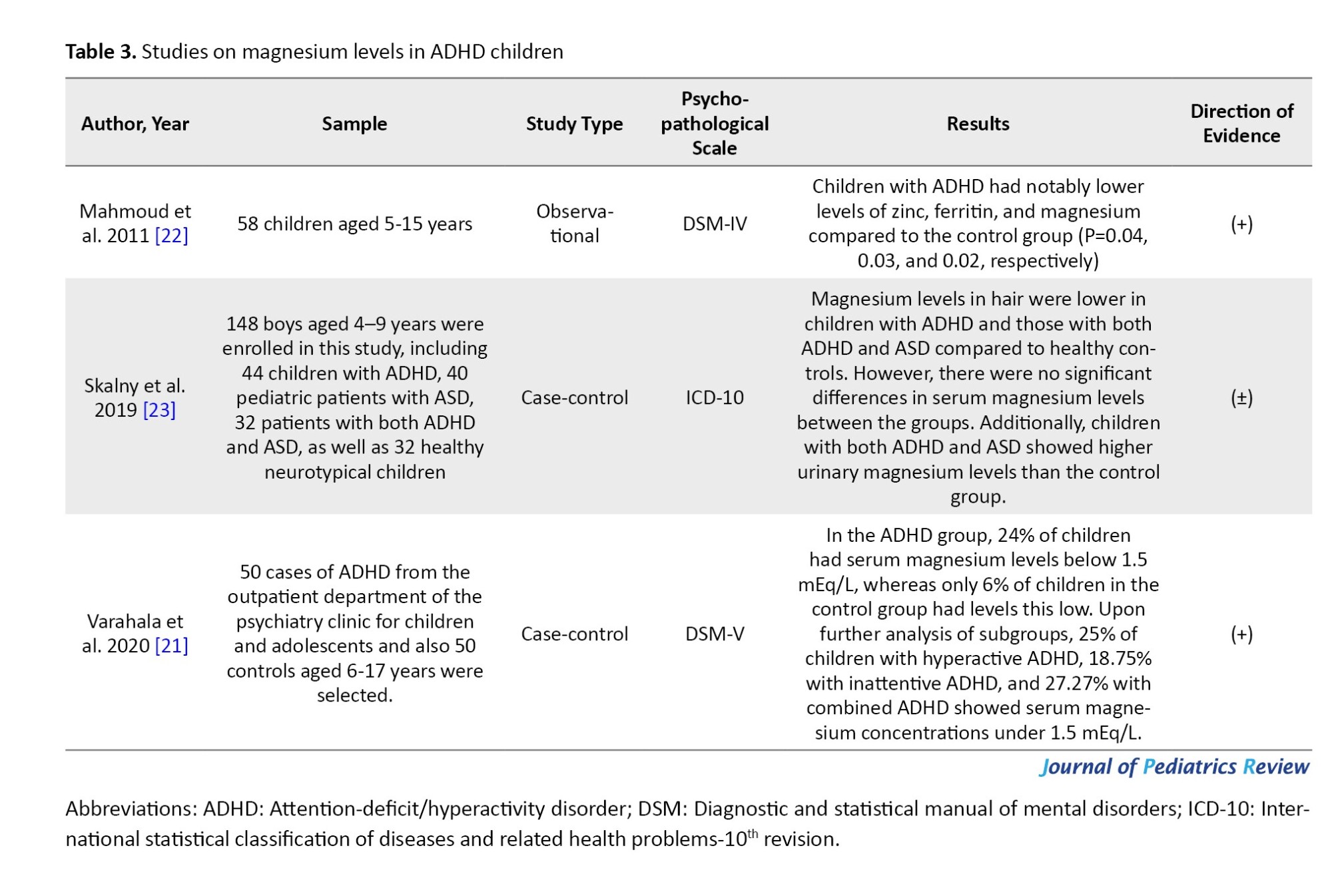
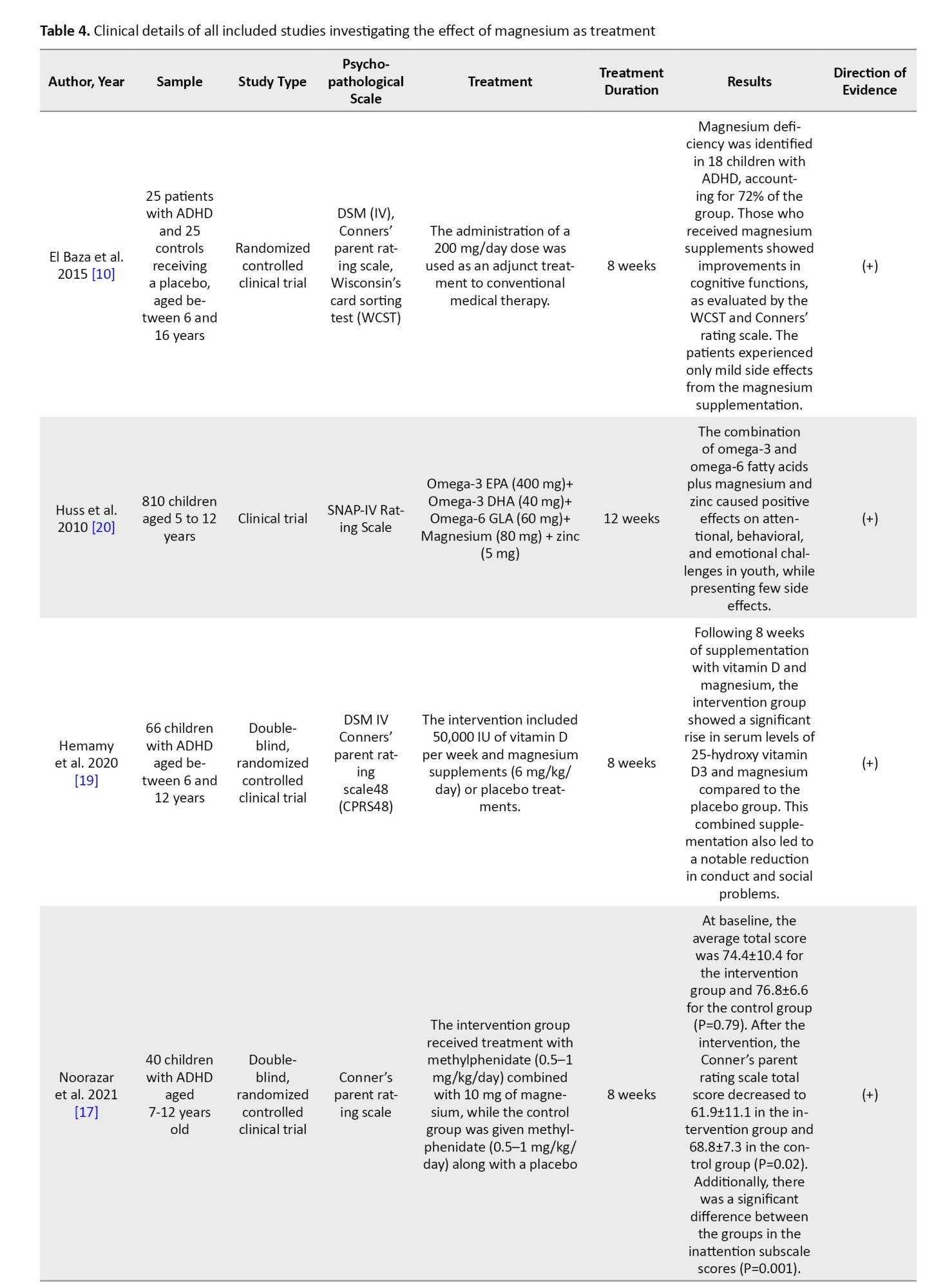
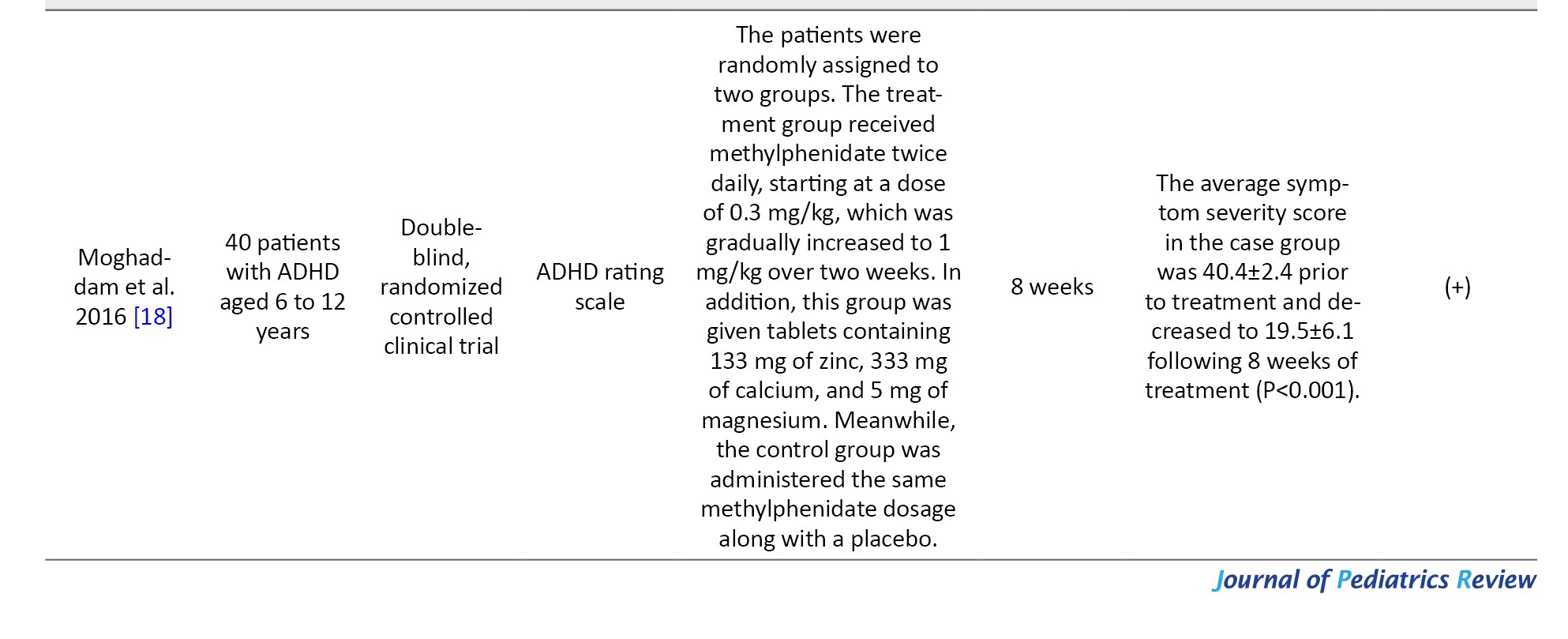
Out of the studies that were selected, 5 were clinical trials [10, 17-20] and 3 were descriptive studies that compared the serum magnesium as well as magnesium concentrations in the hair of children diagnosed with ADHD with those of healthy children [8, 21, 22]. In three clinical trial studies, magnesium supplementation was given as a single therapy [10, 17], while in the remaining 3 studies, magnesium was supplemented along with other nutrients, such as vitamin D, zinc, calcium, and omega-3 [18-20]. The duration of the intervention, as reported in the studies, was either 8 or 12 weeks. The total number of people was 1285, and the studies were conducted from 2010 to 2021 in the following countries: Iran (3 studies) [17-19], Egypt (2 studies) [10, 22], Germany (1 study) [20], Russia (1 study) [23], and India (1 study) [21]. Tables 3 and 4 show the characteristics and results of the selected studies.
Assessment of magnesium concentrations in the serum and hair of children diagnosed with ADHD
In Mahmoud et al.’s study, 58 hyperactive children and 25 healthy children in the age range of 5-15 years were selected. Serum levels of magnesium were lower in hyperactive children compared to healthy children, and ferritin and zinc levels were also reported to be lower in these children [22].
In the next study conducted by Moghaddam et al., 148 children in the age group of 4-9 years were studied, and ADHD and autism spectrum disorder (ASD) children were compared with the healthy group. The levels of magnesium in the hair in ADHD and ASD children were lower than in healthy children, but the serum levels of magnesium in the groups [23]. In Varahala et al.'s study, 100 ADHD and healthy children aged 6-17 were studied, and ADHD children had lower serum magnesium levels compared to healthy children [21].
Effect of magnesium supplementation on ADHD children
Five interventional studies were included in this review:
In El Baza et al.’s study, a total of 50 hyperactive children aged 6-16 years were supplemented with magnesium (200 mg/day as an adjunctive treatment to the standard medical treatment) or a placebo for 8 weeks, and after 8 weeks, significant improvement was seen in the children’s cognitive function [10].
In Huss et al.’s study, 810 children aged 5-12 years were supplemented with omega-3 and omega-6, zinc, and magnesium (80 mg/day) for 12 weeks, and the result showed an improvement in attentional, behavioral, and emotional problems of children [20].
In a 2020 study by Hemamy et al., 66 children with ADHD were supplemented with vitamin D (50,000 IU/week) and magnesium (6 mg/kg/day) for 8 weeks. After 8 weeks of taking both vitamin D and magnesium, the intervention group experienced a significant increase in serum levels of 25-hydroxy vitamin D3 and magnesium compared to the placebo group. This supplementation also resulted in a marked reduction in conduct and social difficulties [19].
In a 2021 study by Noorazar et al., 40 children received magnesium supplementation (10 mg/day) for 8 weeks. After 8 weeks, a significant decrease was observed in the total score of the Conner’s test and the inattention score in the intervention group [17].
Moghaddam et al. in 2016, 40 ADHD children were supplemented with zinc, magnesium (5 mg/day), and calcium for 8 weeks, and after the intervention, a significant reduction was observed in the severity of hyperactivity symptoms [18].
Discussion
In the selected descriptive studies, it was observed that children diagnosed with ADHD displayed lower serum and hair levels of magnesium, while urinary magnesium levels were comparatively elevated in comparison to their healthy counterparts [8, 22, 23]. These findings suggest an altered magnesium status in ADHD-afflicted individuals. Clinical trial studies indicate that magnesium supplementation for 8 to 12 weeks can mitigate clinical symptoms of ADHD while also reducing other associated symptoms, such as anxiety and social problems in affected children [10, 17-19].
The pivotal role of magnesium in the pathway of neurotransmitter function is of great significance in ADHD. The intricate interaction of magnesium with neurotransmitters is fundamental in regulating signal transmission, which, in turn, plays a vital role in the cognitive and behavioral functioning of individuals [11, 24, 25].
Magnesium’s interaction with the NMDA receptor (NMDAR) is one of its key neurological functions. The malfunctioning of the NMDAR can lead to complex alterations in the levels of neurotransmitters, such as dopamine and epinephrine in the brain, which can significantly contribute to the manifestation of ADHD symptoms [26, 27]. It is noteworthy that the primary pharmacotherapeutic agents employed in the treatment of ADHD, methylphenidate and atomoxetine, have been demonstrated to regulate the function of NMDARs, which are essential for learning and memory processes. Furthermore, clinical trials have shown that pharmacological agents that directly target NMDARs, such as memantine and amantadine, exhibit efficacy in ameliorating the symptoms of ADHD. These findings suggest that NMDAR function may be a critical target for the development of novel pharmacotherapeutic agents for ADHD [28-30].
Magnesium has been identified as a regulator of presynaptic glutamate release, and its effects on glutamate neurotransmission have been linked to the pathophysiology of ADHD. Research has shown that an increase in glutamatergic activity in the frontal and striatal brain regions, as well as elevated levels of glutamate in the anterior cingulate cortex, may be associated with ADHD. Therefore, the regulatory effects of magnesium on glutamate neurotransmission can be regarded as another therapeutic intervention for this disorder [15].
Another effect of magnesium can be related to the brain-derived neurotrophic factor (BDNF) mechanism [31]. Research suggests that decreased levels of BDNF may play a role in the neurodevelopmental deficits observed in individuals with ADHD. Moreover, it is believed that this deficiency may also contribute to the persistence of the disorder into adulthood. In this field, studies have shown that magnesium can increase the levels of this neurotrophin. The role of magnesium as a calcium antagonist and voltage-dependent blocker of the NMDA channel may be connected to its effect on BDNF [32, 33].
Magnesium can also play a role in the dopamine neurotransmitter pathways [34]. Studies conducted on animals have demonstrated that magnesium is capable of activating tyrosine hydroxylase (TH) [35]. TH is a key enzyme that plays a vital role in the biosynthesis of several important neurotransmitters, such as dopamine, norepinephrine, and epinephrine. It converts the amino acid tyrosine into L-DOPA, which is a precursor for the formation of these neurotransmitters. This conversion is considered to be the rate-limiting step in the biosynthesis of catecholamines. Without the activity of TH, the production of these neurotransmitters would be severely impaired [13]. In addition, magnesium has a fascinating impact on the generation of cyclic adenosine monophosphate response element binding protein (CREB) within the brain. This protein plays a crucial role in regulating the activity of genes that influence the functioning of the human brain, particularly those involved in dopamine production. By binding to specific DNA sequences known as cAMP response elements (CREs), CREB can either enhance or diminish gene transcription. It has been found that children with ADHD who suffer from magnesium deficiency may experience adverse effects on their health, and magnesium supplementation can have a positive impact on these children. The available findings strongly support the hypothesis that magnesium supplementation can help alleviate the symptoms of ADHD in affected children [26].
Conclusion
Magnesium may play a significant role in managing ADHD symptoms. Individuals with ADHD often have lower serum magnesium levels, and magnesium supplementation can lead to improvements in hyperactivity, impulsivity, and attention deficits. Magnesium is crucial for neurotransmitter function, which may explain its potential benefits in ADHD management.
However, the evidence is mixed, and more extensive, high-quality research is needed to confirm these findings and establish optimal supplementation guidelines.
Limitations
This study also had limitations. Some studies had small sample sizes, limiting the generalizability of their findings. Also, studies often do not account for factors, like concurrent medications or comorbidities, that could influence results. In addition, different methods for measuring magnesium levels (serum, hair, and urine) can lead to inconsistent results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (Code: TBZMED.REC 139).
Funding
This study was supported by the Research Center of Psychiatry and Behavioral Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Authors contributions
Conceptualization: Parinaz Kalejahi, Supervision, data collection, funding acquisition and resources: Gholamreza Noorazar; Methodology, investigation and writing: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Razi Medical Center, Tabriz University of Medical Sciences, for their cooperation in conducting this research.
References
Attention-deficit/hyperactivity disorder (ADHD) is a prevalent neurodevelopmental condition marked by challenges in attention, impulsiveness, hyperactivity, and emotional regulation [1]. These deficits can lead to impairments in various areas of life, and many children continue to exhibit significant symptoms even as they grow into adolescence and adulthood [2]. The global incidence of ADHD is estimated to be around 5-7% in children and adolescents, and around 2.5% among adults [3, 4].
The origin of this disorder is still unknown, but it seems that genetic and environmental factors are involved in its development [5]. Environmental factors, such as nutritional factors and diet patterns, have been demonstrated to significantly contribute to the development of ADHD symptoms. As a result, there is a growing interest in using nutritional approaches to help manage ADHD. Scientific research in this area is increasing, and it is hoped that this will lead to new insights and treatment options for individuals with ADHD [6, 7].
Research in this area suggests that children with this disorder may have lower levels of important minerals, like magnesium, compared to healthy children [8, 9]. However, supplementing these nutrients has been shown to have a positive impact on managing the symptoms of the disorder [9, 10]. Magnesium is a critical cation in the central nervous system that plays a crucial role in various functions, including signal transmission and intracellular signaling [9].
Magnesium, in turn, plays a crucial role as a voltage-gated antagonist to the glutamate, N-methyl-D-aspartate (NMDA) receptor and also functions as an antagonist to calcium entry through voltage-gated channels of all types. It is worth noting that while magnesium levels in the brain tend to be more stable and decrease more gradually than in other tissues, even small reductions can have a profound effect on neuronal functioning [11].
Magnesium has been found to have a direct impact on dopamine release and the stimulatory effect of glutamate on dopamine release at the presynaptic level [12-14]. Low levels of magnesium may increase glutamatergic neurotransmission, potentially fostering an environment that promotes excitotoxicity. This may cause oxidative stress and neuronal loss [15]. Despite magnesium’s critical role in the central nervous system, there is limited research on its effects on ADHD [16]. Also, magnesium deficiency is linked to behavioral symptoms, such as anxiety and irritability, which can lead to a worsening of ADHD symptoms. Some studies have shown that magnesium supplements may be effective in improving some mental and neurological conditions, which could be explored as a treatment option for ADHD. Therefore, this systematic review aimed to evaluate existing research on the serum levels of magnesium in children with ADHD, as well as clinical trials on magnesium supplementation in these children.
Methods
Review design
This study was conducted adhering to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) protocol.
Articles published until December 2023 were retrieved through a search in PUBMED/Medline, Google Scholar, and Scopus databases. Unpublished studies and documents were also manually searched and reviewed. Correspondence was also conducted with the corresponding author if the findings of an article were not available.
Search strategy
Keywords used to search for articles were in accordance with the MeSH glossary. Free keywords were also used in addition to MeSH keywords to search for articles. The following keywords were used alone or cross-linked, depending on which database was searched:
(Attention deficit disorder with hyperactivity [MeSH terms]) OR (ADHD [Title/Abstract]) OR (deficit-hyperactivity disorders, attention [Title/Abstract]) OR (hyperkinetic syndrome [Title/Abstract]) OR (attention deficit disorder [Title/Abstract]) AND (magnesium [Title/Abstract]) OR (mg [Title/Abstract])
Inclusion and exclusion criteria
Inclusion criteria based on the PICOS framework (population/intervention/comparators/outcomes/time/setting) were as follows:
Population: Studies on children with ADHD published in English;
Intervention: Supplementation with magnesium and, for descriptive studies, assessment of magnesium in serum, urine, or hair;
Outcome: Improvement in ADHD symptoms in the clinical trial;
Time/date: Publication date up to December 2023 without any location restrictions.
The exclusion criteria were as follows:
1) Reviews, systematic reviews, or meta-analyses, 2) Not enough information, 3) Studies in languages other than English or Persian, 4) Incomplete articles or obscure statistical results.
Data selection and extraction
We imported publication titles and abstracts into ENDNOTE, version 7 and removed duplicates. Two independent reviewers screened and evaluated all publications. We first screened article titles and abstracts and then reviewed full publications based on pre-specified criteria.
The selected studies were entered into Excel software. The data form was designed as follows: Published information included the author’s name, year of publication, country, type of intervention, type of magnesium measurement, final sample size, participants, possible side effects, and final results.
Quality assessment
The risk of bias in the included studies was assessed using the National Institutes of Health (NIH) quality assessment tool and Cochrane collaboration tool (Tables 1 and 2).

The NIH quality assessment tool for assessing bias in cross-sectional studies was used, which includes the following subscales: Study population, participation rate, recruitment criteria, sample size justification, exposure(s) of interest measured prior, sufficient time frame to observe the effect, different levels of exposure, exposure measures and assessment, repeated exposure measurement, outcome measures, outcome assessors blinded, and follow-up rate.

To evaluate the quality of intervention articles, the Cochrane tool was employed, which has seven subscales: Random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective bias, and other sources of bias.
Results
Study selection
We found 1115 articles and, after removing duplicates, 917 articles were selected for the next step. After reviewing the studies by two separate researchers, 63 studies were selected, and finally, 8 studies were selected to be included in the review study (Figure 1).

Study characteristics
Out of the studies that were selected, 5 were clinical trials [10, 17-20] and 3 were descriptive studies that compared the serum magnesium as well as magnesium concentrations in the hair of children diagnosed with ADHD with those of healthy children [8, 21, 22]. In three clinical trial studies, magnesium supplementation was given as a single therapy [10, 17], while in the remaining 3 studies, magnesium was supplemented along with other nutrients, such as vitamin D, zinc, calcium, and omega-3 [18-20]. The duration of the intervention, as reported in the studies, was either 8 or 12 weeks. The total number of people was 1285, and the studies were conducted from 2010 to 2021 in the following countries: Iran (3 studies) [17-19], Egypt (2 studies) [10, 22], Germany (1 study) [20], Russia (1 study) [23], and India (1 study) [21]. Tables 3 and 4 show the characteristics and results of the selected studies.

Assessment of magnesium concentrations in the serum and hair of children diagnosed with ADHD
In Mahmoud et al.’s study, 58 hyperactive children and 25 healthy children in the age range of 5-15 years were selected. Serum levels of magnesium were lower in hyperactive children compared to healthy children, and ferritin and zinc levels were also reported to be lower in these children [22].


In the next study conducted by Moghaddam et al., 148 children in the age group of 4-9 years were studied, and ADHD and autism spectrum disorder (ASD) children were compared with the healthy group. The levels of magnesium in the hair in ADHD and ASD children were lower than in healthy children, but the serum levels of magnesium in the groups [23]. In Varahala et al.'s study, 100 ADHD and healthy children aged 6-17 were studied, and ADHD children had lower serum magnesium levels compared to healthy children [21].
Effect of magnesium supplementation on ADHD children
Five interventional studies were included in this review:
In El Baza et al.’s study, a total of 50 hyperactive children aged 6-16 years were supplemented with magnesium (200 mg/day as an adjunctive treatment to the standard medical treatment) or a placebo for 8 weeks, and after 8 weeks, significant improvement was seen in the children’s cognitive function [10].
In Huss et al.’s study, 810 children aged 5-12 years were supplemented with omega-3 and omega-6, zinc, and magnesium (80 mg/day) for 12 weeks, and the result showed an improvement in attentional, behavioral, and emotional problems of children [20].
In a 2020 study by Hemamy et al., 66 children with ADHD were supplemented with vitamin D (50,000 IU/week) and magnesium (6 mg/kg/day) for 8 weeks. After 8 weeks of taking both vitamin D and magnesium, the intervention group experienced a significant increase in serum levels of 25-hydroxy vitamin D3 and magnesium compared to the placebo group. This supplementation also resulted in a marked reduction in conduct and social difficulties [19].
In a 2021 study by Noorazar et al., 40 children received magnesium supplementation (10 mg/day) for 8 weeks. After 8 weeks, a significant decrease was observed in the total score of the Conner’s test and the inattention score in the intervention group [17].
Moghaddam et al. in 2016, 40 ADHD children were supplemented with zinc, magnesium (5 mg/day), and calcium for 8 weeks, and after the intervention, a significant reduction was observed in the severity of hyperactivity symptoms [18].
Discussion
In the selected descriptive studies, it was observed that children diagnosed with ADHD displayed lower serum and hair levels of magnesium, while urinary magnesium levels were comparatively elevated in comparison to their healthy counterparts [8, 22, 23]. These findings suggest an altered magnesium status in ADHD-afflicted individuals. Clinical trial studies indicate that magnesium supplementation for 8 to 12 weeks can mitigate clinical symptoms of ADHD while also reducing other associated symptoms, such as anxiety and social problems in affected children [10, 17-19].
The pivotal role of magnesium in the pathway of neurotransmitter function is of great significance in ADHD. The intricate interaction of magnesium with neurotransmitters is fundamental in regulating signal transmission, which, in turn, plays a vital role in the cognitive and behavioral functioning of individuals [11, 24, 25].
Magnesium’s interaction with the NMDA receptor (NMDAR) is one of its key neurological functions. The malfunctioning of the NMDAR can lead to complex alterations in the levels of neurotransmitters, such as dopamine and epinephrine in the brain, which can significantly contribute to the manifestation of ADHD symptoms [26, 27]. It is noteworthy that the primary pharmacotherapeutic agents employed in the treatment of ADHD, methylphenidate and atomoxetine, have been demonstrated to regulate the function of NMDARs, which are essential for learning and memory processes. Furthermore, clinical trials have shown that pharmacological agents that directly target NMDARs, such as memantine and amantadine, exhibit efficacy in ameliorating the symptoms of ADHD. These findings suggest that NMDAR function may be a critical target for the development of novel pharmacotherapeutic agents for ADHD [28-30].
Magnesium has been identified as a regulator of presynaptic glutamate release, and its effects on glutamate neurotransmission have been linked to the pathophysiology of ADHD. Research has shown that an increase in glutamatergic activity in the frontal and striatal brain regions, as well as elevated levels of glutamate in the anterior cingulate cortex, may be associated with ADHD. Therefore, the regulatory effects of magnesium on glutamate neurotransmission can be regarded as another therapeutic intervention for this disorder [15].
Another effect of magnesium can be related to the brain-derived neurotrophic factor (BDNF) mechanism [31]. Research suggests that decreased levels of BDNF may play a role in the neurodevelopmental deficits observed in individuals with ADHD. Moreover, it is believed that this deficiency may also contribute to the persistence of the disorder into adulthood. In this field, studies have shown that magnesium can increase the levels of this neurotrophin. The role of magnesium as a calcium antagonist and voltage-dependent blocker of the NMDA channel may be connected to its effect on BDNF [32, 33].
Magnesium can also play a role in the dopamine neurotransmitter pathways [34]. Studies conducted on animals have demonstrated that magnesium is capable of activating tyrosine hydroxylase (TH) [35]. TH is a key enzyme that plays a vital role in the biosynthesis of several important neurotransmitters, such as dopamine, norepinephrine, and epinephrine. It converts the amino acid tyrosine into L-DOPA, which is a precursor for the formation of these neurotransmitters. This conversion is considered to be the rate-limiting step in the biosynthesis of catecholamines. Without the activity of TH, the production of these neurotransmitters would be severely impaired [13]. In addition, magnesium has a fascinating impact on the generation of cyclic adenosine monophosphate response element binding protein (CREB) within the brain. This protein plays a crucial role in regulating the activity of genes that influence the functioning of the human brain, particularly those involved in dopamine production. By binding to specific DNA sequences known as cAMP response elements (CREs), CREB can either enhance or diminish gene transcription. It has been found that children with ADHD who suffer from magnesium deficiency may experience adverse effects on their health, and magnesium supplementation can have a positive impact on these children. The available findings strongly support the hypothesis that magnesium supplementation can help alleviate the symptoms of ADHD in affected children [26].
Conclusion
Magnesium may play a significant role in managing ADHD symptoms. Individuals with ADHD often have lower serum magnesium levels, and magnesium supplementation can lead to improvements in hyperactivity, impulsivity, and attention deficits. Magnesium is crucial for neurotransmitter function, which may explain its potential benefits in ADHD management.
However, the evidence is mixed, and more extensive, high-quality research is needed to confirm these findings and establish optimal supplementation guidelines.
Limitations
This study also had limitations. Some studies had small sample sizes, limiting the generalizability of their findings. Also, studies often do not account for factors, like concurrent medications or comorbidities, that could influence results. In addition, different methods for measuring magnesium levels (serum, hair, and urine) can lead to inconsistent results.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Tabriz University of Medical Sciences, Tabriz, Iran (Code: TBZMED.REC 139).
Funding
This study was supported by the Research Center of Psychiatry and Behavioral Sciences, Tabriz University of Medical Sciences, Tabriz, Iran.
Authors contributions
Conceptualization: Parinaz Kalejahi, Supervision, data collection, funding acquisition and resources: Gholamreza Noorazar; Methodology, investigation and writing: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank the Clinical Research Development Unit of Razi Medical Center, Tabriz University of Medical Sciences, for their cooperation in conducting this research.
References
- Gaub M, Carlson CL. Gender differences in ADHD: A meta-analysis and critical review. J Am Acad Child Adolesc Psychiatry. 1997; 36(8):1036-45. [DOI:10.1097/00004583-199708000-00011]
- Newcorn JH, Halperin JM, Jensen PS, Abikoff HB, Arnold LE, Cantwell DP, et al. Symptom profiles in children with ADHD: Effects of comorbidity and gender. J Am Acad Child Adolesc Psychiatry. 2001; 40(2):137-46. [DOI:10.1097/00004583-200102000-00008]
- Polanczyk GV, Willcutt EG, Salum GA, Kieling C, Rohde LA. ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. Int J Epidemiol. 2014; 43(2):434-42. [DOI:10.1093/ije/dyt261]
- Sayal K, Prasad V, Daley D, Ford T, Coghill D. ADHD in children and young people: Prevalence, care pathways, and service provision. Lancet Psychiatry. 2018; 5(2):175-86. [Link]
- Nigg JT, Sibley MH, Thapar A, Karalunas SL. Development of ADHD: Etiology, heterogeneity, and early life course. Annu Rev Dev Psychol. 2020; 2(1):559-83. [DOI:10.1146/annurev-devpsych-060320-093413]
- Lange KW, Lange KM, Nakamura Y, Reissmann A. Nutrition in the management of ADHD: A review of recent research. Curr Nutr Rep. 2023; 12(3):383-94. [DOI:10.1007/s13668-023-00487-8]
- Bloch MH, Mulqueen J. Nutritional supplements for the treatment of ADHD. Child Adolesc Psychiatr Clin. 2014; 23(4):883-97. [DOI:10.1016/j.chc.2014.05.002]
- Effatpanah M, Rezaei M, Effatpanah H, Effatpanah Z, Varkaneh HK, Mousavi SM, et al. Magnesium status and attention deficit hyperactivity disorder (ADHD): A meta-analysis. Psychiatry research. 2019; 274:228-34. [DOI:10.1016/j.psychres.2019.02.043]
- Botturi A, Ciappolino V, Delvecchio G, Boscutti A, Viscardi B, Brambilla P. The role and the effect of magnesium in mental disorders: A systematic review. Nutrients. 2020; 12(6):1661. [DOI:10.3390/nu12061661]
- El Baza F, AlShahawi HA, Zahra S, AbdelHakim RA. Magnesium supplementation in children with attention deficit hyperactivity disorder. Egypt J Med Hum Genet. 2016; 17(1):63-70. [Link]
- Cuciureanu MD, Vink R. Magnesium and stress. In: Vink R, Nechifor M, editors. Magnesium in the central nervous system. Adelaide: University of Adelaide Press; 2011. [PMID]
- Ghanbari A, Warchomicka F, Sommitsch C, Zamanian A. Investigation of the oxidation mechanism of dopamine functionalization in an AZ31 magnesium alloy for biomedical applications. Coatings. 2019; 9(9):584. [DOI:10.3390/coatings9090584]
- Watanabe M, George SR, Seeman P. Regulation of anterior pituitary D2 dopamine receptors by magnesium and sodium ions. J Neurochem. 1985; 45(6):1842-9. [DOI:10.1111/j.1471-4159.1985.tb10542.x]
- Cardoso CC, Lobato KR, Binfaré RW, Ferreira PK, Rosa AO, Santos AR, et al. Evidence for the involvement of the monoaminergic system in the antidepressant-like effect of magnesium. Prog Neuro Psychopharmacol Biol Psychiatry. 2009; 33(2):235-42. [DOI:10.1016/j.pnpbp.2008.11.007]
- Nowak LP, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984; 307(5950):462-5. [DOI:10.1038/307462a0]
- Papadopol V, Nechifor M. Magnesium in neuroses and neuroticism. In: Vink R, Nechifor M, editors. Magnesium in the Central Nervous System. Adelaide: University of Adelaide Press; 2011. [PMID]
- Noorazar SG, Kalejahi P, Setayesh S, Amiri S, Yasamineh N. The efficacy of magnesium supplementation in children with attention deficit hyperactivity disorder under treatment with methylphenidate: A randomized controlled trial. Crescent J Med Biol Sci. 2021. 8(1):73-76. [Link]
- Moghaddam MF, Rakhshani T, Khosravi M. Effectiveness of methylphenidate supplemented by zinc, calcium, and magnesium for treatment of ADHD patients in the city of Zahedan. Shiraz E Med J. 2016. 17(9):e40019. [DOI:10.17795/semj40019]
- Hemamy M, Heidari-Beni M, Askari G, Karahmadi M, Maracy M. Effect of vitamin D and magnesium supplementation on behavior problems in children with attention-deficit hyperactivity disorder. Int J Prevent Med. 2020; 11(1):4. [DOI:10.4103/ijpvm.IJPVM_546_17]
- Huss M, Völp A, Stauss-Grabo M. Supplementation of polyunsaturated fatty acids, magnesium and zinc in children seeking medical advice for attention-deficit/hyperactivity problems-an observational cohort study. Lipids Health Dis. 2010; 9(1):105. [DOI:10.1186/1476-511X-9-105]
- Varahala AM, Gajula R. Serum magnesium levels in attention deficit hyperactive disorder in 6-17 years age group: A study in tertiary care center. Int J Contemp Pediatr. 2020; 7(3):504-10. [DOI:10.18203/2349-3291.ijcp20200210]
- Mahmoud MM, El-Mazary AA, Maher RM, Saber MM. Zinc, ferritin, magnesium and copper in a group of Egyptian children with attention deficit hyperactivity disorder. Ital J Pediatr. 2011; 37(1):60. [DOI:10.1186/1824-7288-37-60]
- Skalny AV, Mazaletskaya AL, Ajsuvakova OP, Bjørklund G, Skalnaya MG, Chernova LN, et al. Magnesium status in children with attention-deficit/hyperactivity disorder and/or autism spectrum disorder. Soa Chongsonyon Chongsin Uihak. 2020; 31(1):41-5. [DOI:10.5765/jkacap.190036] [PMID]
- Kirkland AE, Sarlo GL, Holton KF. The role of magnesium in neurological disorders. Nutrients. 2018; 10(6):730. [DOI:10.3390/nu10060730]
- Spasov AA, Iezhitsa IN, Kravchenko MS, Kharitonova MV. Features of central neurotransmission in animals in conditions of dietary magnesium deficiency and after its correction. Neurosci Behav Physiol. 2009; 39(7):645-53. [DOI:10.1007/s11055-009-9182-y]
- Hou H, Wang L, Fu T, Papasergi M, Yule DI, Xia H. Magnesium acts as a second messenger in the regulation of NMDA receptor-mediated CREB signaling in neurons. Mol Neurobiol. 2020; 57(6):2539-50. [DOI:10.1007/s12035-020-01871-z]
- Wang JK. Presynaptic glutamate receptors modulate dopamine release from striatal synaptosomes. J Neurochem. 1991; 57(3):819-22. [DOI:10.1111/j.1471-4159.1991.tb08224.x]
- Guilarte TR, Chen MK. Manganese inhibits NMDA receptor channel function: Implications to psychiatric and cognitive effects. Neurotoxicology. 2007; 28(6):1147-52. [DOI:10.1016/j.neuro.2007.06.005]
- Pei-Chen Chang J, Lane HY, E Tsai G. Attention deficit hyperactivity disorder and N-methyl-D-aspartate (NMDA) dysregulation. Curr Pharm Design. 2014; 20(32):5180-5. [Link]
- R Durrant A, Heresco-Levy U. The role of N-methyl-d-aspartate receptor-mediated neurotransmission in attention deficit (hyperactivity) disorder (ADHD/ADD). Curr Psychopharmacol. 2014; 3(3):184-94. [Link]
- Razmjou S, Litteljohn D, Hayley S. The interactive effects of Ketamine and Magnesium on Brain-Derived Neurotrophic Factor (BDNF) and depressive-like behavior (P4. 080). Neurology. 2015; 84(14_supplement):P4-080. [DOI:10.1212/WNL.84.14_supplement.P4.080]
- Liu DY, Shen XM, Yuan FF, Guo OY, Zhong Y, Chen JG, et al. The physiology of BDNF and its relationship with ADHD. Mol Neurobiol. 2015; 52(3):1467-76. [DOI:10.1007/s12035-014-8956-6]
- Lee N, Park S, Kim J. Effects of hippotherapy on brain function, BDNF level, and physical fitness in children with ADHD. J Exerc Nutr Biochem. 2015; 19(2):115. [DOI:10.5717/jenb.2015.15061209] [PMID]
- Shen Y, Dai L, Tian H, Xu R, Li F, Li Z, et al. Treatment of magnesium-L-threonate elevates the magnesium level in the cerebrospinal fluid and attenuates motor deficits and dopamine neuron loss in a mouse model of Parkinson’s disease. Neuropsychiatr Dis Treat. 2019; 15:3143-53. [DOI:10.2147/NDT.S230688]
- Iuvone PM. Calcium, ATP, and magnesium activate soluble tyrosine hydroxylase from rat striatum. J Neurochem. 1984; 43(5):1359-68. [DOI:10.1111/j.1471-4159.1984.tb05395.x]
Type of Study: Systematic Review |
Subject:
Pediatric Neurology
Received: 2024/04/16 | Accepted: 2025/07/19 | Published: 2025/07/19
Received: 2024/04/16 | Accepted: 2025/07/19 | Published: 2025/07/19
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |









