Volume 12, Issue 2 (4-2024)
J. Pediatr. Rev 2024, 12(2): 153-170 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Yousefifard M, Ahmadzadeh K, Madani Neishaboori A, Rafiei Alavi S N, Rafiei Alavi S R, Ahmadzadeh H, et al . A Systematic Review on the Risk Factors of Steroid-Sensitive Nephrotic Syndrome Relapse in the Pediatric Population. J. Pediatr. Rev 2024; 12 (2) :153-170
URL: http://jpr.mazums.ac.ir/article-1-622-en.html
URL: http://jpr.mazums.ac.ir/article-1-622-en.html
Mahmoud Yousefifard1 

 , Koohyar Ahmadzadeh1
, Koohyar Ahmadzadeh1 

 , Arian Madani Neishaboori1
, Arian Madani Neishaboori1 

 , Seyedeh Niloufar Rafiei Alavi1
, Seyedeh Niloufar Rafiei Alavi1 

 , Seyedeh Romina Rafiei Alavi2
, Seyedeh Romina Rafiei Alavi2 

 , Hooman Ahmadzadeh3
, Hooman Ahmadzadeh3 

 , Amirmohammad Toloui1
, Amirmohammad Toloui1 

 , Mohammed I M Gubari4
, Mohammed I M Gubari4 

 , Michael E. Jones5
, Michael E. Jones5 

 , Nematollah Ataei6
, Nematollah Ataei6 

 , Mojtaba Fazel6
, Mojtaba Fazel6 

 , Mostafa Hosseini *7
, Mostafa Hosseini *7 




 , Koohyar Ahmadzadeh1
, Koohyar Ahmadzadeh1 

 , Arian Madani Neishaboori1
, Arian Madani Neishaboori1 

 , Seyedeh Niloufar Rafiei Alavi1
, Seyedeh Niloufar Rafiei Alavi1 

 , Seyedeh Romina Rafiei Alavi2
, Seyedeh Romina Rafiei Alavi2 

 , Hooman Ahmadzadeh3
, Hooman Ahmadzadeh3 

 , Amirmohammad Toloui1
, Amirmohammad Toloui1 

 , Mohammed I M Gubari4
, Mohammed I M Gubari4 

 , Michael E. Jones5
, Michael E. Jones5 

 , Nematollah Ataei6
, Nematollah Ataei6 

 , Mojtaba Fazel6
, Mojtaba Fazel6 

 , Mostafa Hosseini *7
, Mostafa Hosseini *7 


1- Physiology Research Center, Iran University of Medical Sciences, Tehran, Iran.
2- Department of Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Medicine, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Community Medicine, College of Medicine, University of Sulaimani, Sulaimani, Iraq.
5- Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK.
6- Pediatrics Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran.
7- Pediatrics Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran. ,mhossein110@yahoo.com
2- Department of Medicine, School of Medicine, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
3- Department of Medicine, School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
4- Department of Community Medicine, College of Medicine, University of Sulaimani, Sulaimani, Iraq.
5- Division of Genetics and Epidemiology, The Institute of Cancer Research, London, UK.
6- Pediatrics Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran.
7- Pediatrics Chronic Kidney Disease Research Center, Tehran University of Medical Sciences, Tehran, Iran. ,
Full-Text [PDF 594 kb]
(1147 Downloads)
| Abstract (HTML) (2916 Views)
Full-Text: (1100 Views)
Introduction
Nephrotic syndrome is a collection of symptoms caused by kidney injury. These symptoms include proteinuria, decreased serum albumin level, elevated serum lipids, and edema [1, 2]. The most efficient treatment for nephrotic syndrome is corticosteroid therapy, and the patients are divided into four groups steroid sensitive, steroid-resistant, steroid-tolerant, and steroid-dependent based on their response to treatment [3, 4].
Steroid-sensitive nephrotic syndrome (SSNS), which affects a large proportion of patients, is defined as the response to corticosteroid therapy within the first eight weeks of the treatment. Although patients with SSNS have a good prognosis, 40% to 50% of them experience multiple relapses or become steroid-dependent [1, 2, 3, 4]. These patients suffer from several complications, such as the Cushing symptoms, hypertension, elevated serum lipid level, hyperglycemia, severe infections, growth retardation, and osteopenia [5].
Identifying affecting and predictive factors of SSNS outcomes may greatly aid physicians in proper patient management. Several available studies have reported possible risk factors and predictive factors of adverse outcomes in patients with nephrotic syndrome. For instance, Mishra et al. [6] have shown that patients aged between 1 and 3 years old have a higher risk of relapse; however, Bakr et al. [7] reported no relation between patients’ age at debut and relapses. Ali et al. [8] have demonstrated that hematuria at debut may be a risk factor for relapse. However, Noer [9] have not reported a predictive value for hematuria. Discrepancies can also be seen in reports on the predictive value of hypertension [9, 10] and time from treatment to response [8, 9].
The currently existing evidence on the risk factors of SSNS relapse is inconsistent, and no conclusion has been reached in this regard. This study adopts a systematic approach and gathers available evidence on the risk factors of SSNS relapse in children and adolescents.
Methods
Study design
The current study was designed to investigate the risk factors of SSNS relapse in the pediatric and adolescent populations. The patient, exposure, comparison, and outcome framework in the present study was defined as follows: Patients (P) described children and adolescent patients with SSNS; exposure (E) showed risk factors of frequent relapse or relapse; comparison (C) demonstrated infrequently relapsing nephrotic syndrome (IFRNS) patients or non-relapsing patients; and outcome (O) showed SSNS frequent relapse or relapses. Two reviewers independently performed all study steps, and a third reviewer was consulted in case of disagreement.
Search strategy
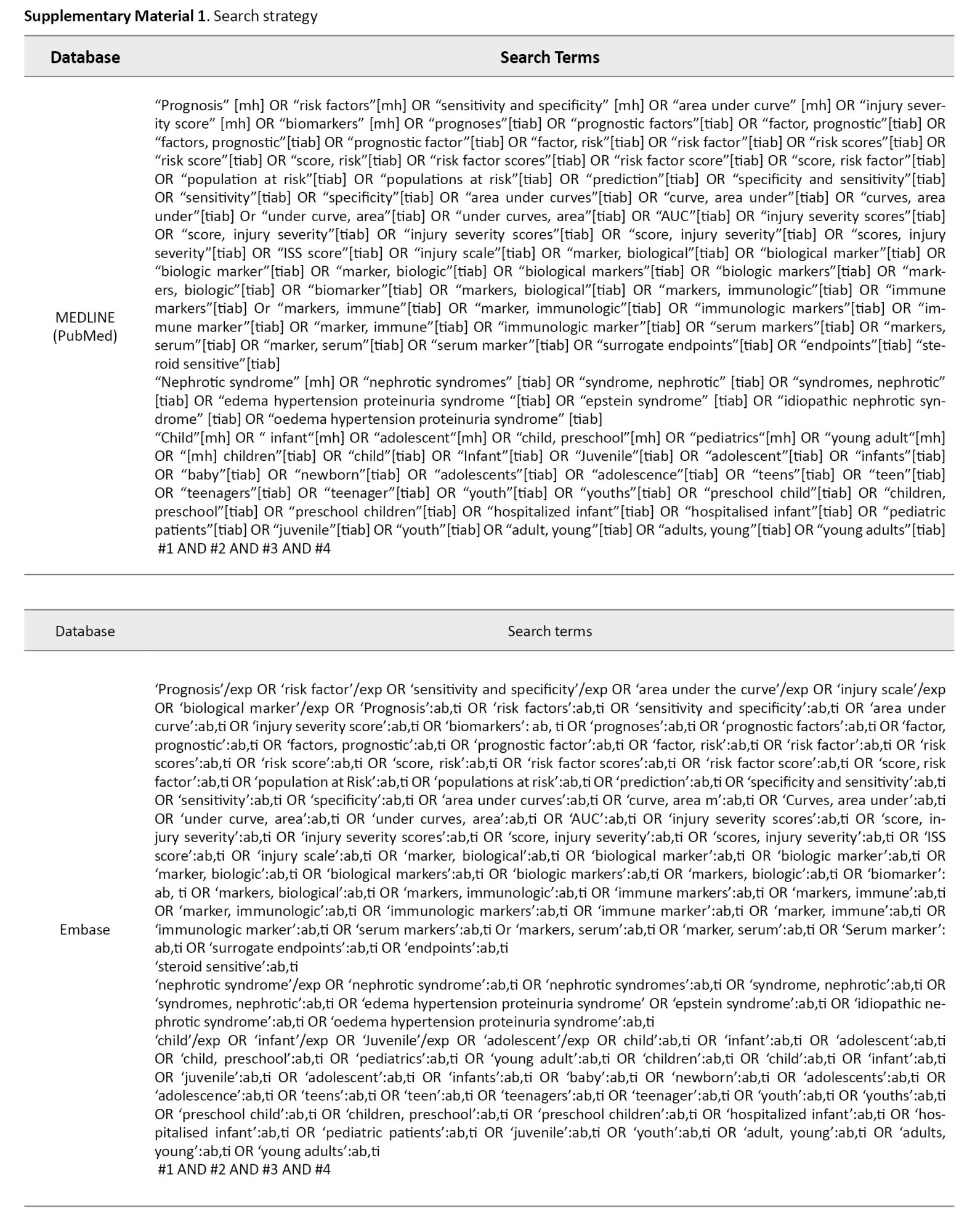
A search was conducted on the electronic databases of Medline, Embase, Web of Science, and Scopus until February 18th, 2024. Relevant keywords were selected through Emtree terms of Embase database and MeSH terms of PubMed gateway, consultation with the experts, and review of related studies. Moreover, grey literature was addressed by a manual search in Google and Google Scholar search engines and forward (citation tracking) and backward (reference tracking) screening. Supplementary Material 1 shows the utilized search strategies.

Selection criteria
The observational studies investigating the value of patient baseline and clinical characteristics in predicting SSNS relapse were included. The target population was children and adolescents under 18 years old without language, location, racial or sexual limitations.
Articles not having a no-relapse group, not assessing relapse as an outcome, not reporting data collection protocol, articles without accurate definitions of the relapse, studies with mixed adult and pediatric populations with no stratification of the results by age group, case reports, and review articles were excluded from this study.
Data extraction
Two independent reviewers screened and selected articles based on the inclusion and exclusion criteria. Data from the included articles were summarized using a checklist based on preferred reporting items for systematic reviews and meta-analyses statement protocols [11]. The extracted data included study design, sample size, number of patients in relapse and non-relapse groups, follow-up, studied risk factors, reported data for each risk factor, and definitions of nephrotic syndrome, SSNS, relapse, and frequent relapse. Studied risk factors included patient characteristics (age at debut, gender), hematuria at debut, serum creatinine, serum albumin, serum cholesterol, serum protein, urine 24 h protein) blood pressure/pre-existing hypertension, and disease characteristics (number of relapses, time to response). Any disagreements in the screening or data extraction processes were resolved by consulting a third reviewer.
Quality assessment and certainty of evidence
The quality of the included studies was evaluated using National Heart, Lung, and Blood Institute (NHLBI) protocols. Two independent researchers performed the quality assessment, and disagreements were resolved using the third reviewer’s opinion. The NHLBI quality control checklists are available at NHLBI. Certainty of evidence was assessed using the grading of recommendations, assessment, development, and evaluation framework [12].
Terms definitions
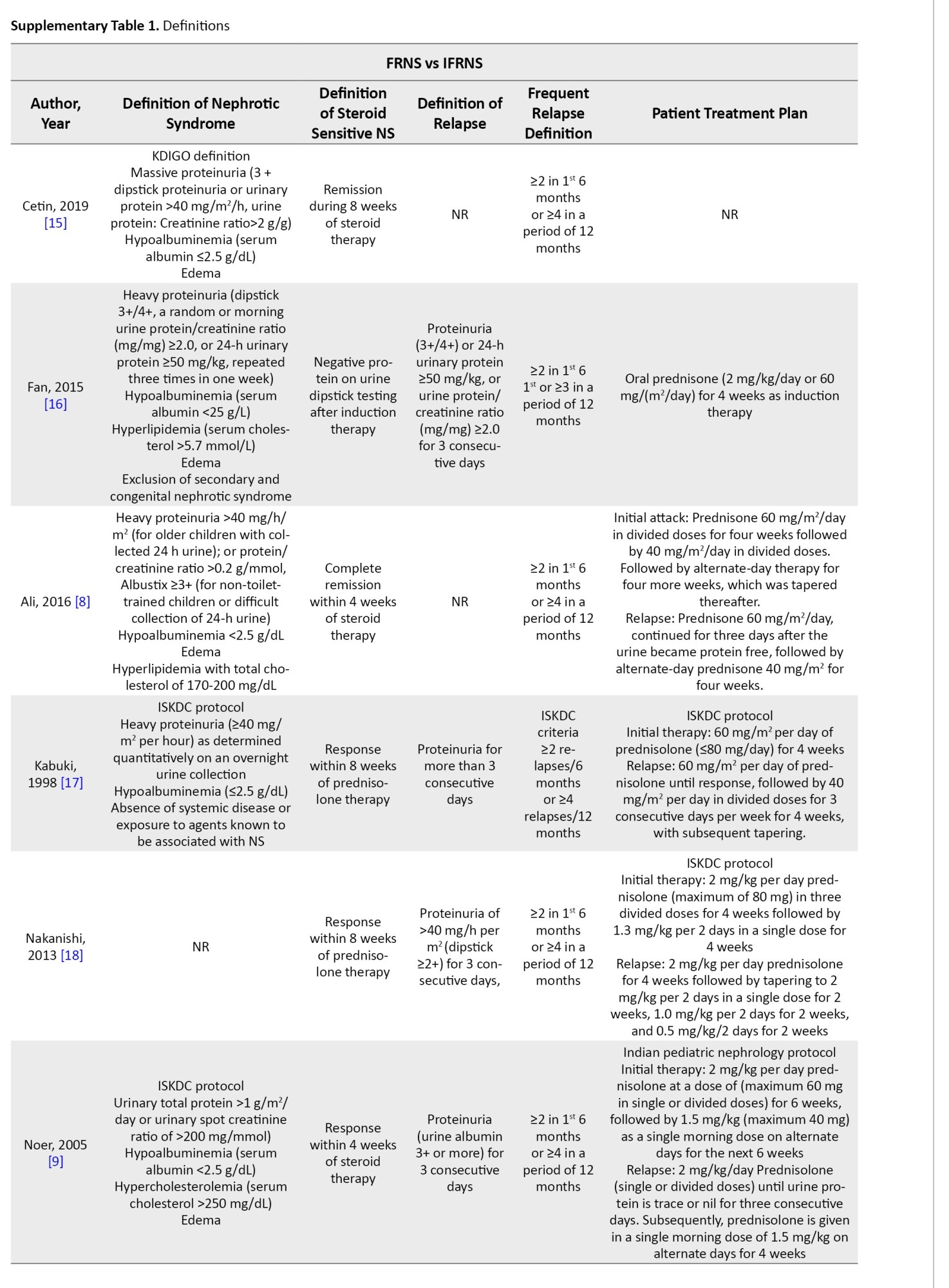
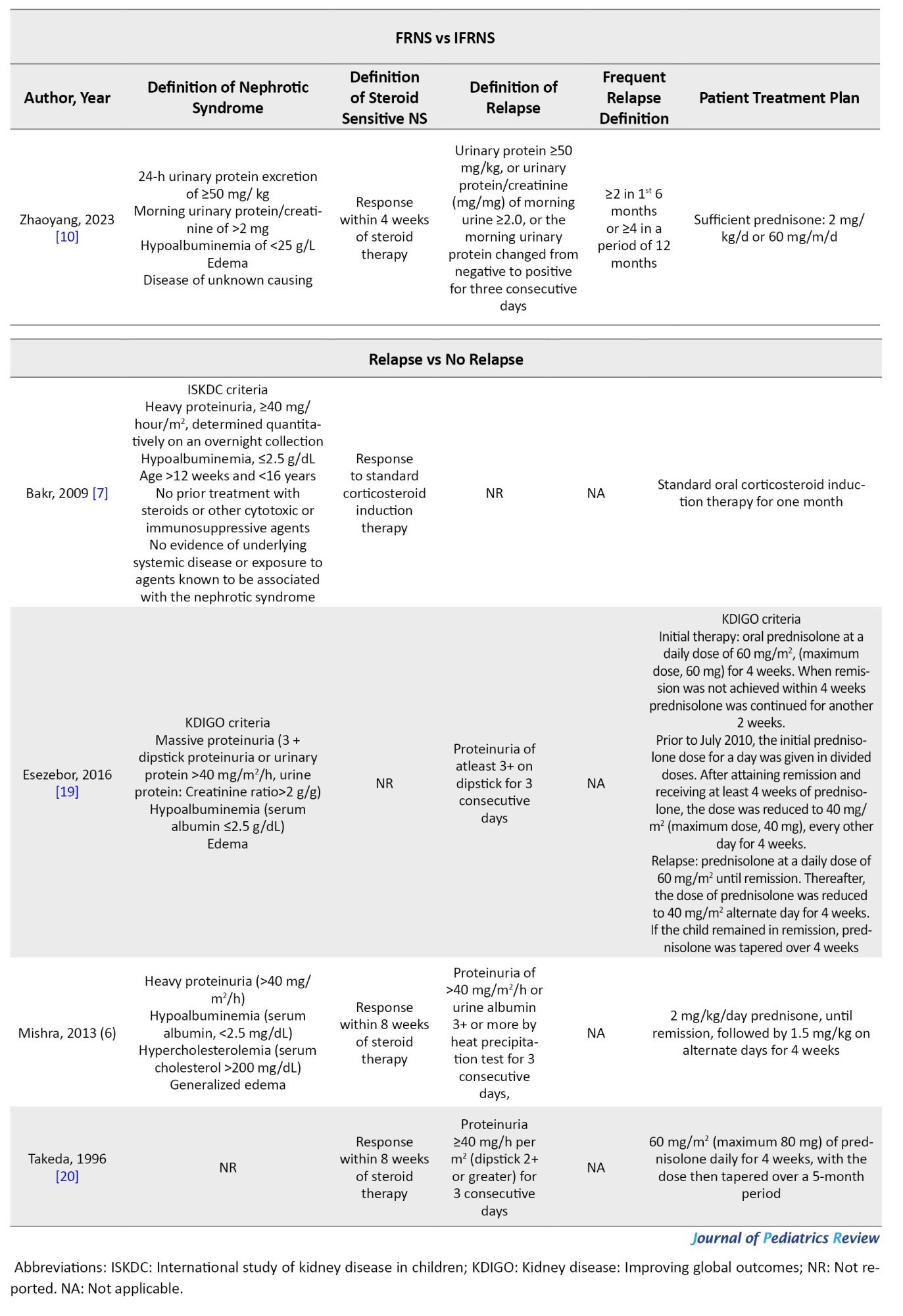
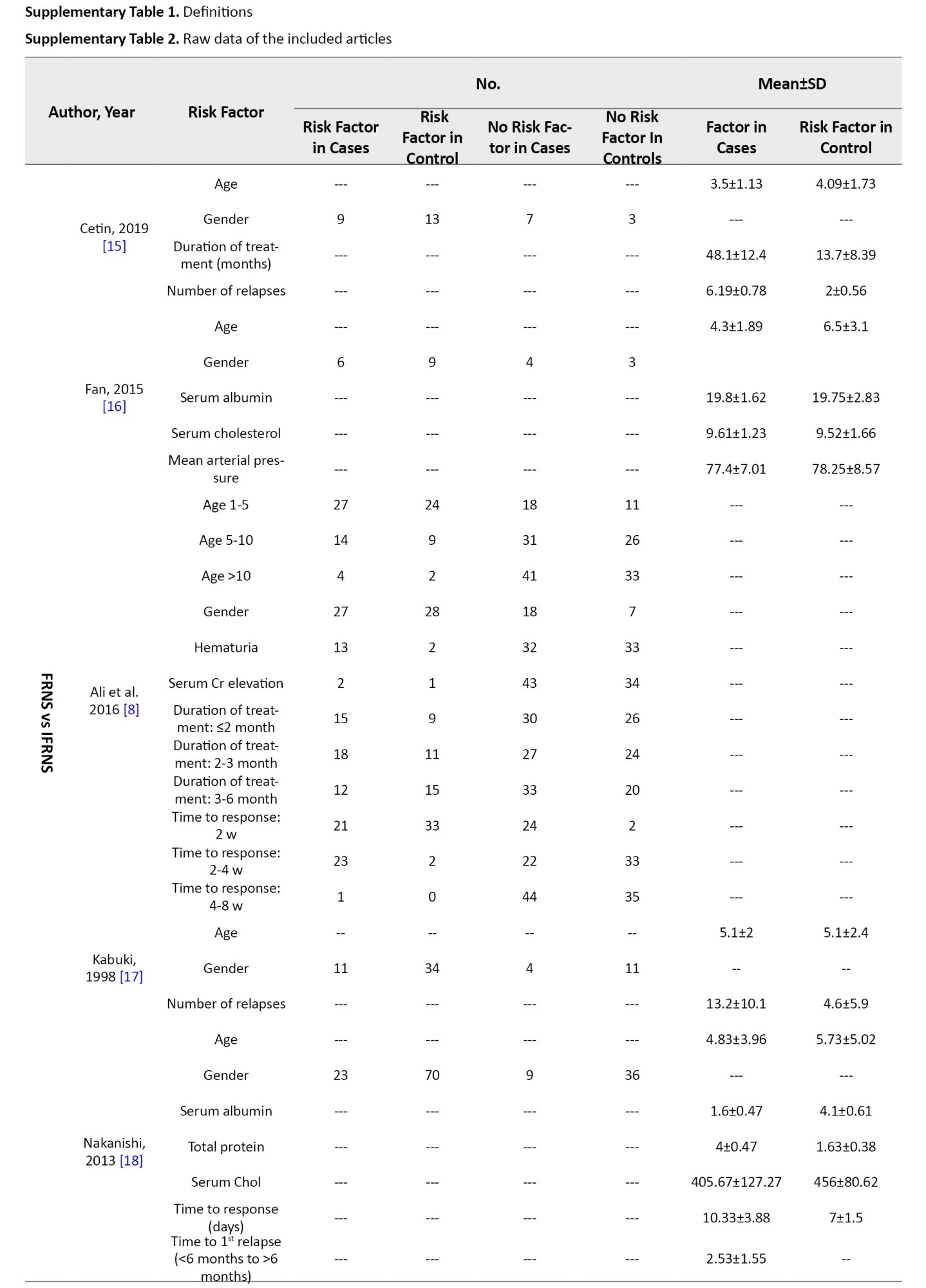
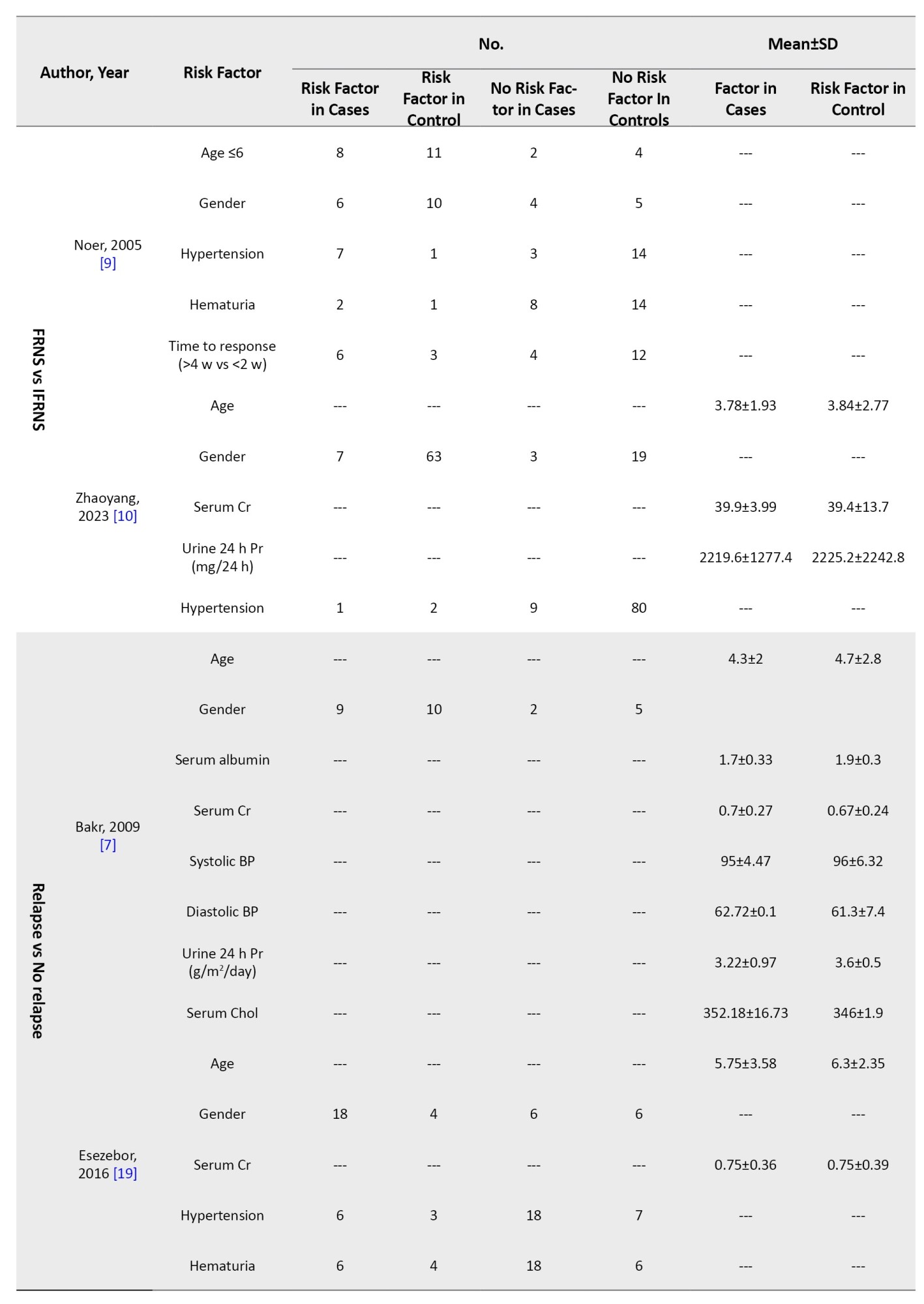
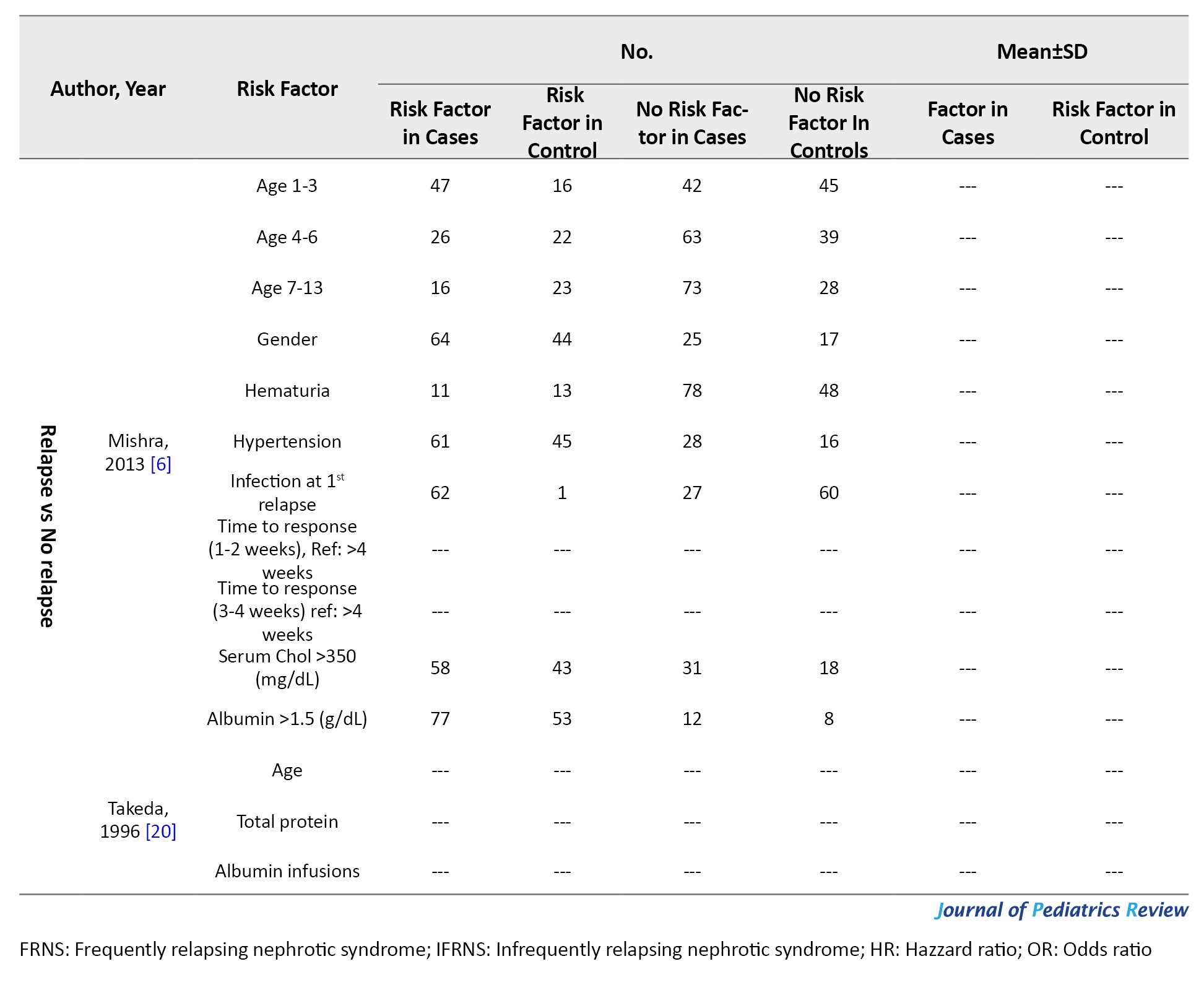
Nephrotic syndrome was defined as massive proteinuria, hypoalbuminemia, edema, and hypercholesterolemia. SSNS was defined as a response within 8 weeks of steroid therapy. Relapse was defined as proteinuria for more than 3 consecutive days. Frequent relapse was defined as ≥2 relapses in the first 6 months and ≥4 relapses in 12 months. Hematuria was microscopic (≥5 red blood cells/high power field). However, studies used different definitions and guidelines provided in Supplementary Tables 1 and 2.
Data synthesis
Studies have compared frequently relapsing nephrotic syndrome (FRNS) patients with IFRNS and reported risk factors of frequent relapse, or compared relapsing patients with non-relapsing patients and reported risk factors of relapse. Accordingly, this article’s results section separately reports each comparison’s results.
The reported data for qualitative variables, such as gender, were recorded as the number of patients with or without risk factors. All continuous variables, such as serum creatinine, were converted into Mean±SD to ease the comparison of study reports. Few studies have reported summary statistics, such as odds ratio (OR) and hazard ratio (HR). For studies without reports of summary statistics, OR and 95% confidence interval (95% CI) were calculated to ease the comparison of study reports.
Results
Study characteristics
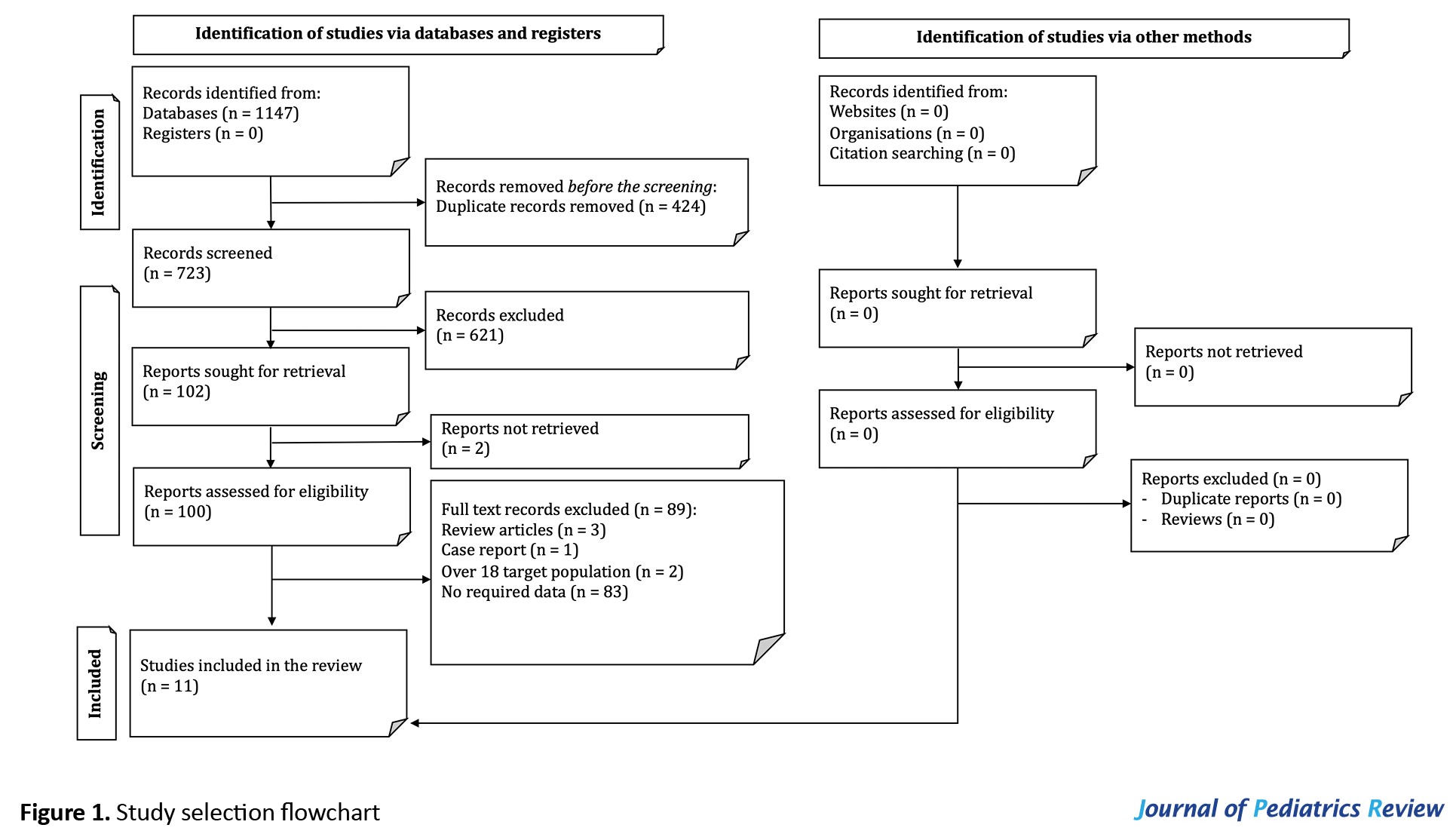
The systematic search resulted in 723 non-duplicate records. After the initial title and abstract screening, 102 articles were studied in more detail. Two articles were not retrieved [13, 14]. Finally, 11 articles were included in the present systematic review (Figure 1) [6, 7, 8, 9, 10, 15-20].
Nephrotic syndrome is a collection of symptoms caused by kidney injury. These symptoms include proteinuria, decreased serum albumin level, elevated serum lipids, and edema [1, 2]. The most efficient treatment for nephrotic syndrome is corticosteroid therapy, and the patients are divided into four groups steroid sensitive, steroid-resistant, steroid-tolerant, and steroid-dependent based on their response to treatment [3, 4].
Steroid-sensitive nephrotic syndrome (SSNS), which affects a large proportion of patients, is defined as the response to corticosteroid therapy within the first eight weeks of the treatment. Although patients with SSNS have a good prognosis, 40% to 50% of them experience multiple relapses or become steroid-dependent [1, 2, 3, 4]. These patients suffer from several complications, such as the Cushing symptoms, hypertension, elevated serum lipid level, hyperglycemia, severe infections, growth retardation, and osteopenia [5].
Identifying affecting and predictive factors of SSNS outcomes may greatly aid physicians in proper patient management. Several available studies have reported possible risk factors and predictive factors of adverse outcomes in patients with nephrotic syndrome. For instance, Mishra et al. [6] have shown that patients aged between 1 and 3 years old have a higher risk of relapse; however, Bakr et al. [7] reported no relation between patients’ age at debut and relapses. Ali et al. [8] have demonstrated that hematuria at debut may be a risk factor for relapse. However, Noer [9] have not reported a predictive value for hematuria. Discrepancies can also be seen in reports on the predictive value of hypertension [9, 10] and time from treatment to response [8, 9].
The currently existing evidence on the risk factors of SSNS relapse is inconsistent, and no conclusion has been reached in this regard. This study adopts a systematic approach and gathers available evidence on the risk factors of SSNS relapse in children and adolescents.
Methods
Study design
The current study was designed to investigate the risk factors of SSNS relapse in the pediatric and adolescent populations. The patient, exposure, comparison, and outcome framework in the present study was defined as follows: Patients (P) described children and adolescent patients with SSNS; exposure (E) showed risk factors of frequent relapse or relapse; comparison (C) demonstrated infrequently relapsing nephrotic syndrome (IFRNS) patients or non-relapsing patients; and outcome (O) showed SSNS frequent relapse or relapses. Two reviewers independently performed all study steps, and a third reviewer was consulted in case of disagreement.
Search strategy
A search was conducted on the electronic databases of Medline, Embase, Web of Science, and Scopus until February 18th, 2024. Relevant keywords were selected through Emtree terms of Embase database and MeSH terms of PubMed gateway, consultation with the experts, and review of related studies. Moreover, grey literature was addressed by a manual search in Google and Google Scholar search engines and forward (citation tracking) and backward (reference tracking) screening. Supplementary Material 1 shows the utilized search strategies.


Selection criteria
The observational studies investigating the value of patient baseline and clinical characteristics in predicting SSNS relapse were included. The target population was children and adolescents under 18 years old without language, location, racial or sexual limitations.
Articles not having a no-relapse group, not assessing relapse as an outcome, not reporting data collection protocol, articles without accurate definitions of the relapse, studies with mixed adult and pediatric populations with no stratification of the results by age group, case reports, and review articles were excluded from this study.
Data extraction
Two independent reviewers screened and selected articles based on the inclusion and exclusion criteria. Data from the included articles were summarized using a checklist based on preferred reporting items for systematic reviews and meta-analyses statement protocols [11]. The extracted data included study design, sample size, number of patients in relapse and non-relapse groups, follow-up, studied risk factors, reported data for each risk factor, and definitions of nephrotic syndrome, SSNS, relapse, and frequent relapse. Studied risk factors included patient characteristics (age at debut, gender), hematuria at debut, serum creatinine, serum albumin, serum cholesterol, serum protein, urine 24 h protein) blood pressure/pre-existing hypertension, and disease characteristics (number of relapses, time to response). Any disagreements in the screening or data extraction processes were resolved by consulting a third reviewer.
Quality assessment and certainty of evidence
The quality of the included studies was evaluated using National Heart, Lung, and Blood Institute (NHLBI) protocols. Two independent researchers performed the quality assessment, and disagreements were resolved using the third reviewer’s opinion. The NHLBI quality control checklists are available at NHLBI. Certainty of evidence was assessed using the grading of recommendations, assessment, development, and evaluation framework [12].
Terms definitions
Nephrotic syndrome was defined as massive proteinuria, hypoalbuminemia, edema, and hypercholesterolemia. SSNS was defined as a response within 8 weeks of steroid therapy. Relapse was defined as proteinuria for more than 3 consecutive days. Frequent relapse was defined as ≥2 relapses in the first 6 months and ≥4 relapses in 12 months. Hematuria was microscopic (≥5 red blood cells/high power field). However, studies used different definitions and guidelines provided in Supplementary Tables 1 and 2.





Data synthesis
Studies have compared frequently relapsing nephrotic syndrome (FRNS) patients with IFRNS and reported risk factors of frequent relapse, or compared relapsing patients with non-relapsing patients and reported risk factors of relapse. Accordingly, this article’s results section separately reports each comparison’s results.
The reported data for qualitative variables, such as gender, were recorded as the number of patients with or without risk factors. All continuous variables, such as serum creatinine, were converted into Mean±SD to ease the comparison of study reports. Few studies have reported summary statistics, such as odds ratio (OR) and hazard ratio (HR). For studies without reports of summary statistics, OR and 95% confidence interval (95% CI) were calculated to ease the comparison of study reports.
Results
Study characteristics
The systematic search resulted in 723 non-duplicate records. After the initial title and abstract screening, 102 articles were studied in more detail. Two articles were not retrieved [13, 14]. Finally, 11 articles were included in the present systematic review (Figure 1) [6, 7, 8, 9, 10, 15-20].
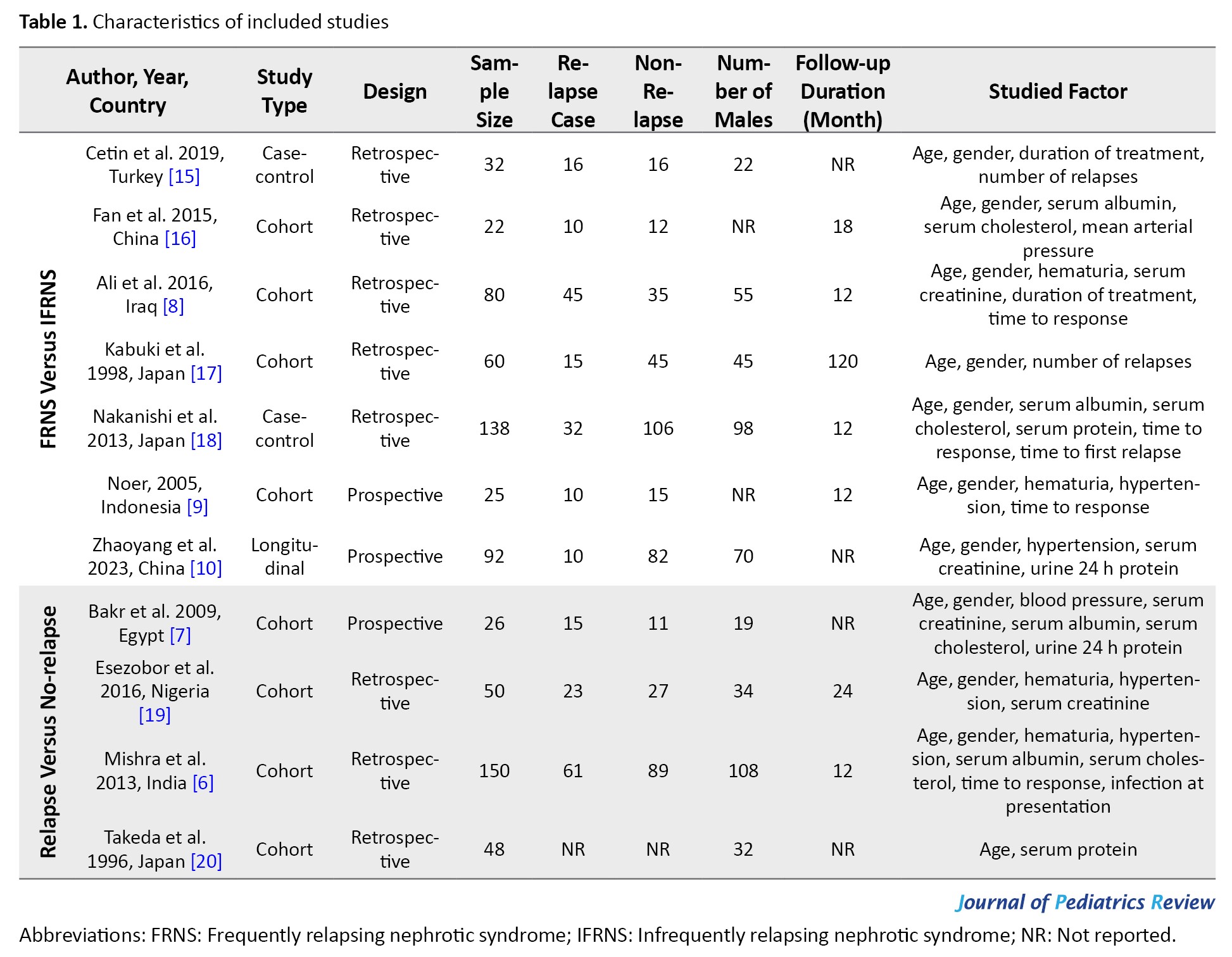
The included articles were designed as eight cohort studies and three longitudinal and case-control studies. Studies have investigated patients with FRNS vs IFRNS [8-10, 15, 16, 17, 18] and relapsers vs non-relapsers [6, 7, 19, 20]. Studies evaluated possible risk factors of relapse, such as patient characteristics including gender, age, hematuria, blood pressure, creatinine, albumin, serum protein, serum cholesterol, urine 24 h protein, and disease characteristics, including duration of treatment, time to response, and number of relapses. Table 1 presents the characteristics of the included studies.
FRNS versus IFRNS
Seven articles (449 patients, 30.7% of patients experienced frequent relapses) evaluated the risk factors of frequent relapse (Table 2).
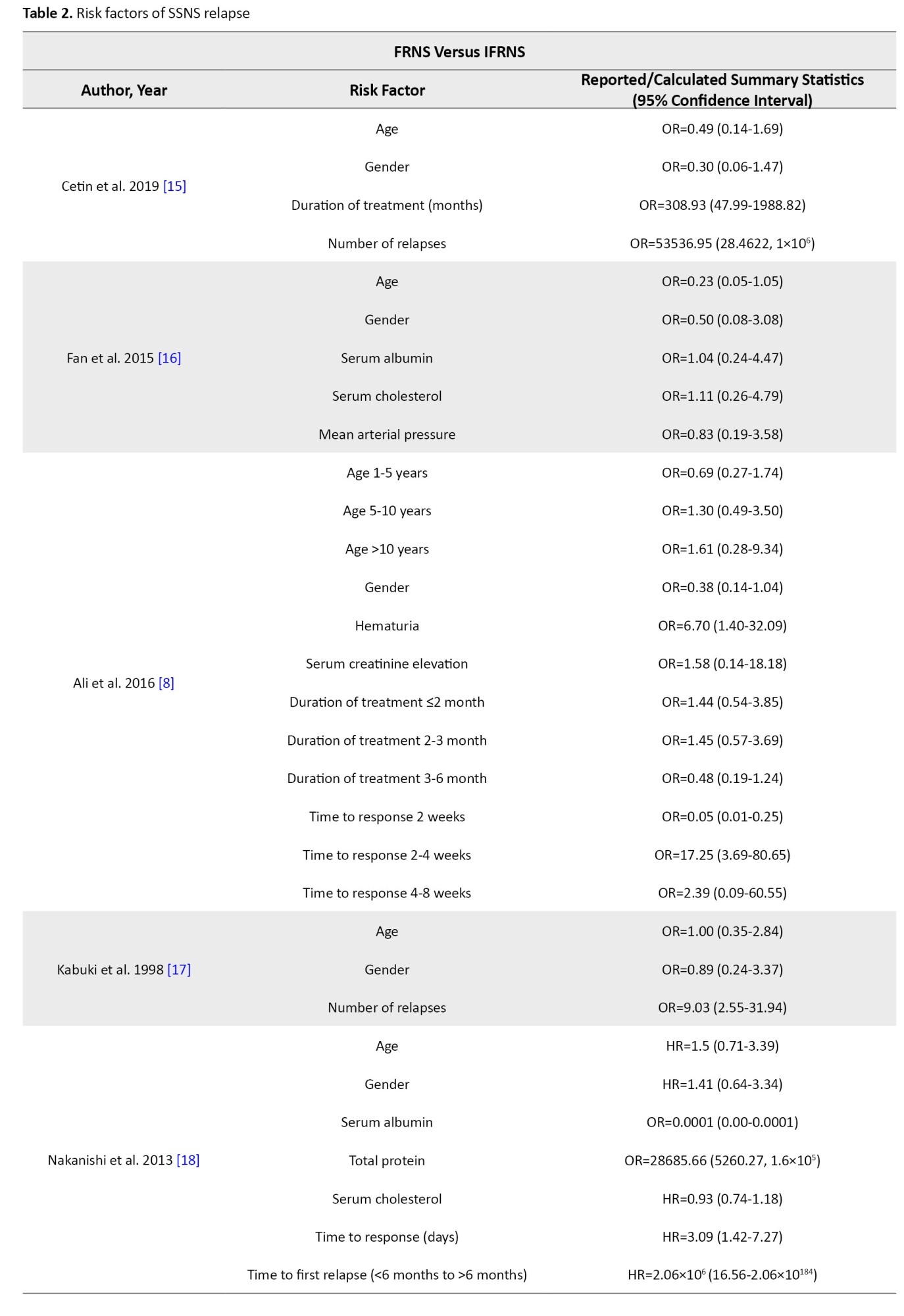
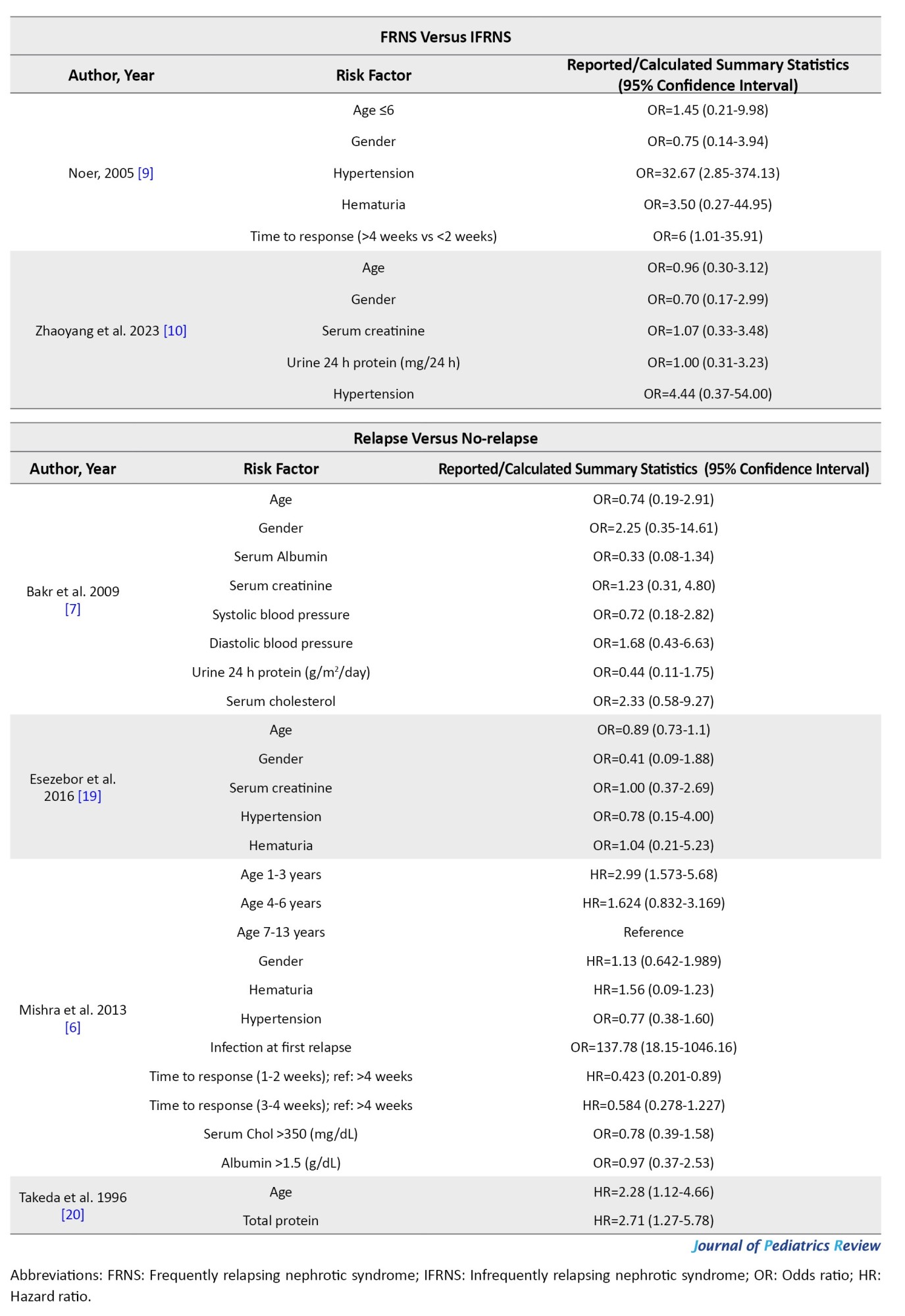
Studies showed that age at debut and gender are not risk factors for frequent relapse. Ali et al. [8] demonstrated that hematuria at debut could be a risk factor for frequent relapse (OR=6.70; 95% CI, 1.40%, 32.09%), while Noer [9] did not report the same findings (OR=3.50; 95% CI, 0.27%, 44.95%). Noer also demonstrated that hypertension can be a risk factor for frequent relapse (OR=32.67; 95% CI, 2.85%, 374.13%); however, the wide confidence interval is an important limitation. Zhaoyang et al. [10] did not report the same findings for hypertension (OR=4.44; 95% CI, 0.37%, 54.00%). Mean arterial blood pressure was not a risk factor for frequent relapse [16]. Serum creatinine, serum albumin, serum cholesterol, and serum protein at debut and urine 24 h protein were not shown to be risk factors for frequent relapse.
Cetin et al. [15] demonstrated that a longer duration of treatment can be a risk factor for frequent relapse (OR=308.93; 95% CI, 47.99%, 1988.82%) while Ali et al. [8] did not report the same findings. Studies demonstrated that a longer time from treatment to response could be a risk factor for frequent relapse, however, results had varying thresholds [8, 9, 18]. Nakanishi et al. [18] reported that time to the disappearance of proteinuria ≥9 days could be a risk factor for frequent relapse (HR=3.09; 95% CI, 1.42%, 7.27%), while Ali et al. [8] and Noer [9] demonstrated that a time to response between 2 to 4 weeks (OR=17.25; 95% CI, 3.69%, 80.65%, reference: <2 weeks) and a shorter time to response (OR=6; 95% CI, 1.01%, 35.91%) could be a risk factor of frequent relapse, respectively. Nakanishi et al. [18] also reported that time to first relapse can be a risk factor for frequent relapse (HR=2.09×106;
95% CI, 16.56%, 2.06%×10184). Cetin et al. [15] and Kabuki et al. [17] demonstrated that the number of relapses could be a risk factor for frequent relapse (OR=53536.95 and 9.03, respectively).
Relapse versus no-relapse
Four articles (274 patients, 43.8% of patients experienced relapse) evaluated risk factors of relapse (Table 2). Mishra et al. [6] demonstrated that ages between 1-3 years had a higher risk of relapse (HR=2.99; 95% CI, 1.57%, 5.68%). In studies done by Bakr et al. [7] and Esezobor et al. [19], age was not a risk factor for relapse. Gender, hematuria at debut, blood pressure, serum creatinine, albumin, cholesterol at debut, and urine 24 h protein were not shown to be a risk factor for relapse. Mishra et al. [6] reported that patients with a median time from treatment to response between 1-2 weeks had a lower risk of relapse (HR=0.42; 95% CI, 0.20%, 0.89%, reference: >4 weeks). In their study, infection at first relapse was shown to be a risk factor for relapse. However, infection at presentation was not shown to be a risk factor [6].
Risk of bias assessment
The NHLBI protocol was employed to assess the quality of included studies (Table 3).
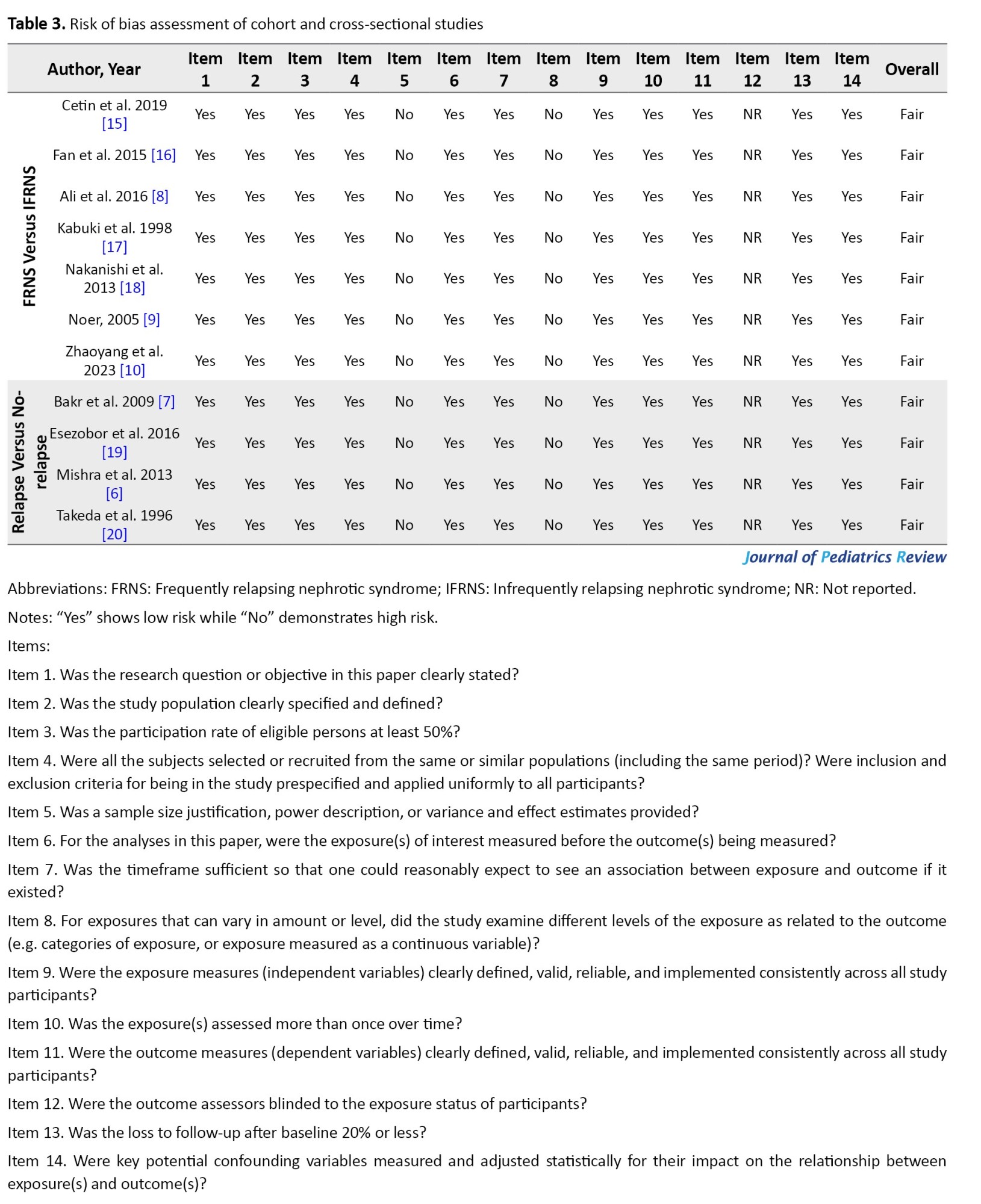
Fatal items for this study were considered as follows: 3, 4, 6, 7, 9, 11, 13, and 14 of the NHLBI checklist (Table 3). None of the included studies had concerns about fatal items. All the studies showed a high risk of bias in items 5, 8, and 14, concerning the sample size justification, power description, variance and effect estimation, and examination of different levels of exposure. None of the studies reported the blinding status of the outcome assessment (item 12); therefore, they had a high risk of bias regarding this matter. Studies had a low risk of bias in the remaining items.
Certainty of evidence
According to the grading of recommendations, assessment, development, and evaluation guidelines, the base level of evidence is set as low for observational studies. The level of evidence was reduced due to high heterogeneity between study designs and due to imprecision (widely reported CIs), and thus the level of evidence is low to very low for age and gender (Table 4).
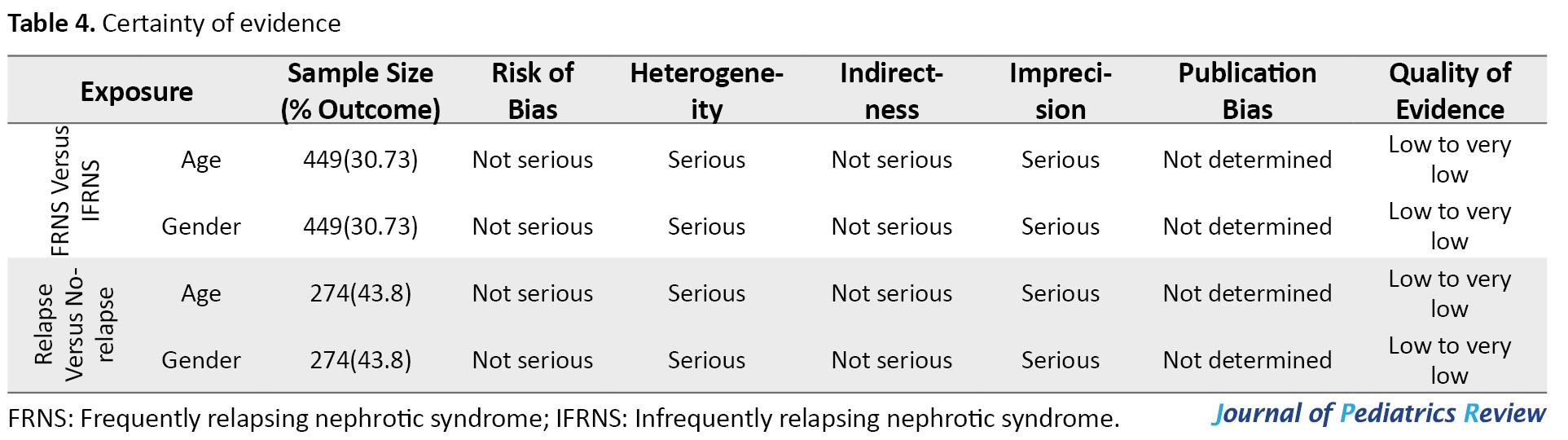
However, according to Centre for Evidence-Based Medicine recommendations, systematic reviews of observational studies should have a base evidence level of high [21]. Accordingly, the level of evidence of this study can be presented as low to moderate for outcomes of age and gender. Certainty of evidence could not be assessed for other variables due to the scarcity of included articles and is rated as very low.
Discussion
The present study was designed to evaluate the risk factors of relapse in children and adolescents with SSNS. Our results demonstrated that some studies proposed age, hematuria, hypertension, longer duration of treatment, longer time from treatment to response, and shorter time to first relapse as possible risk factors of relapse. While other studies did not confirm these findings. None of the reported laboratory variables, such as serum creatinine, albumin, and cholesterol, were a risk factor for relapse. Several relapses and time from treatment to response seem to be risk factors for frequent relapse, albeit a scarce number of studies have investigated these factors.
Mishra et al. [6] compared relapsers to non-relapsers and reported that children between 1 and 3 years old had a higher risk of relapse, while Bakr et al. [7] and Esezobor et al. [19] did not report similar findings for age as a continuous variable. Patients with lower age groups might have a higher risk of relapse while older patients are not at risk. Meanwhile, studies investigating FRNS vs IFRNS did not demonstrate a value for age as a risk factor [10, 15-18] even in lower age groups (ages between 1 to 5) [8].
The risk factor value of various laboratory variables at debut (such as serum creatinine, serum albumin, serum cholesterol, and serum protein) was evaluated, and none of the studies reported a relation between these values and relapse in SSNS patients. However, the scarce number of studies and their heterogeneity should be considered.
Studies have also investigated disease-specific variables, such as time from treatment to response, duration of treatment, and number of relapses. Accordingly, a longer time to respond and a higher number of relapses can be risk factors for relapse. The two studies with reports of the duration of treatment have shown conflicting results for its value in the prediction of relapse [8, 15]. Studies had different follow-ups which greatly affects the value of several relapses. The number of relapses per year could be a more concrete factor to investigate in future studies.
Conclusion
The present study aimed to evaluate possible risk factors for relapse of SSNS in children and adolescents. Low to very low evidence suggests that although factors such as hematuria, hypertension, time from treatment to response, and number of relapses have been proposed as possible risk factors of relapse, no conclusion can be reached due to the heterogeneity of studies. Future studies should have more conforming designs to make comparisons more reliable.
Study limitations
This study faced some limitations. Primarily, the included studies had reported various factors with differing comparison groups, making an overall conclusion difficult. We suggest that future studies be designed with attention to the current reported factors. The wide confidence interval reported for a few of the risk factors is also a limitation. Additionally, studies had variations in definitions of nephrotic syndrome, SSNS, relapse, frequent relapse, and their treatment regimens. Although the variations were minimal, they could still act as confounders. We suggest future studies conform more precisely to a uniform guideline.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS) (Code: IR.TUMS.CHMC.REC.1397.033).
Funding
This study has been funded and supported by Tehran University of Medical Sciences (Grant No.: 96-03-184-36538).
Authors contributions
Conceptualization and supervision: Mostafa Hosseini and Mahmoud Yousefifard; Methodology: Mostafa Hosseini, Mahmoud Yousefifard, Mohammed I M Gubari, Michael E. Jones, Nematollah Ataei and Mojtaba Fazel; Investigation and data curation: Arian Madani Neishaboori, Seyedeh Niloufar Rafiei Alavi, Koohyar Ahmadzadeh, Seyed Romina Rafiei Alavi, Hooman Ahmadzadeh and Amirmohammad Toloui; Formal analysis and validation: Mahmoud Yousefifard, Koohyar Ahmadzadeh and Mostafa Hosseini; Writing: All Authors.
Conflicts of interest
The authors declared no conflict of interest.
References

FRNS versus IFRNS
Seven articles (449 patients, 30.7% of patients experienced frequent relapses) evaluated the risk factors of frequent relapse (Table 2).


Studies showed that age at debut and gender are not risk factors for frequent relapse. Ali et al. [8] demonstrated that hematuria at debut could be a risk factor for frequent relapse (OR=6.70; 95% CI, 1.40%, 32.09%), while Noer [9] did not report the same findings (OR=3.50; 95% CI, 0.27%, 44.95%). Noer also demonstrated that hypertension can be a risk factor for frequent relapse (OR=32.67; 95% CI, 2.85%, 374.13%); however, the wide confidence interval is an important limitation. Zhaoyang et al. [10] did not report the same findings for hypertension (OR=4.44; 95% CI, 0.37%, 54.00%). Mean arterial blood pressure was not a risk factor for frequent relapse [16]. Serum creatinine, serum albumin, serum cholesterol, and serum protein at debut and urine 24 h protein were not shown to be risk factors for frequent relapse.
Cetin et al. [15] demonstrated that a longer duration of treatment can be a risk factor for frequent relapse (OR=308.93; 95% CI, 47.99%, 1988.82%) while Ali et al. [8] did not report the same findings. Studies demonstrated that a longer time from treatment to response could be a risk factor for frequent relapse, however, results had varying thresholds [8, 9, 18]. Nakanishi et al. [18] reported that time to the disappearance of proteinuria ≥9 days could be a risk factor for frequent relapse (HR=3.09; 95% CI, 1.42%, 7.27%), while Ali et al. [8] and Noer [9] demonstrated that a time to response between 2 to 4 weeks (OR=17.25; 95% CI, 3.69%, 80.65%, reference: <2 weeks) and a shorter time to response (OR=6; 95% CI, 1.01%, 35.91%) could be a risk factor of frequent relapse, respectively. Nakanishi et al. [18] also reported that time to first relapse can be a risk factor for frequent relapse (HR=2.09×106;
95% CI, 16.56%, 2.06%×10184). Cetin et al. [15] and Kabuki et al. [17] demonstrated that the number of relapses could be a risk factor for frequent relapse (OR=53536.95 and 9.03, respectively).
Relapse versus no-relapse
Four articles (274 patients, 43.8% of patients experienced relapse) evaluated risk factors of relapse (Table 2). Mishra et al. [6] demonstrated that ages between 1-3 years had a higher risk of relapse (HR=2.99; 95% CI, 1.57%, 5.68%). In studies done by Bakr et al. [7] and Esezobor et al. [19], age was not a risk factor for relapse. Gender, hematuria at debut, blood pressure, serum creatinine, albumin, cholesterol at debut, and urine 24 h protein were not shown to be a risk factor for relapse. Mishra et al. [6] reported that patients with a median time from treatment to response between 1-2 weeks had a lower risk of relapse (HR=0.42; 95% CI, 0.20%, 0.89%, reference: >4 weeks). In their study, infection at first relapse was shown to be a risk factor for relapse. However, infection at presentation was not shown to be a risk factor [6].
Risk of bias assessment
The NHLBI protocol was employed to assess the quality of included studies (Table 3).

Fatal items for this study were considered as follows: 3, 4, 6, 7, 9, 11, 13, and 14 of the NHLBI checklist (Table 3). None of the included studies had concerns about fatal items. All the studies showed a high risk of bias in items 5, 8, and 14, concerning the sample size justification, power description, variance and effect estimation, and examination of different levels of exposure. None of the studies reported the blinding status of the outcome assessment (item 12); therefore, they had a high risk of bias regarding this matter. Studies had a low risk of bias in the remaining items.
Certainty of evidence
According to the grading of recommendations, assessment, development, and evaluation guidelines, the base level of evidence is set as low for observational studies. The level of evidence was reduced due to high heterogeneity between study designs and due to imprecision (widely reported CIs), and thus the level of evidence is low to very low for age and gender (Table 4).

However, according to Centre for Evidence-Based Medicine recommendations, systematic reviews of observational studies should have a base evidence level of high [21]. Accordingly, the level of evidence of this study can be presented as low to moderate for outcomes of age and gender. Certainty of evidence could not be assessed for other variables due to the scarcity of included articles and is rated as very low.
Discussion
The present study was designed to evaluate the risk factors of relapse in children and adolescents with SSNS. Our results demonstrated that some studies proposed age, hematuria, hypertension, longer duration of treatment, longer time from treatment to response, and shorter time to first relapse as possible risk factors of relapse. While other studies did not confirm these findings. None of the reported laboratory variables, such as serum creatinine, albumin, and cholesterol, were a risk factor for relapse. Several relapses and time from treatment to response seem to be risk factors for frequent relapse, albeit a scarce number of studies have investigated these factors.
Mishra et al. [6] compared relapsers to non-relapsers and reported that children between 1 and 3 years old had a higher risk of relapse, while Bakr et al. [7] and Esezobor et al. [19] did not report similar findings for age as a continuous variable. Patients with lower age groups might have a higher risk of relapse while older patients are not at risk. Meanwhile, studies investigating FRNS vs IFRNS did not demonstrate a value for age as a risk factor [10, 15-18] even in lower age groups (ages between 1 to 5) [8].
The risk factor value of various laboratory variables at debut (such as serum creatinine, serum albumin, serum cholesterol, and serum protein) was evaluated, and none of the studies reported a relation between these values and relapse in SSNS patients. However, the scarce number of studies and their heterogeneity should be considered.
Studies have also investigated disease-specific variables, such as time from treatment to response, duration of treatment, and number of relapses. Accordingly, a longer time to respond and a higher number of relapses can be risk factors for relapse. The two studies with reports of the duration of treatment have shown conflicting results for its value in the prediction of relapse [8, 15]. Studies had different follow-ups which greatly affects the value of several relapses. The number of relapses per year could be a more concrete factor to investigate in future studies.
Conclusion
The present study aimed to evaluate possible risk factors for relapse of SSNS in children and adolescents. Low to very low evidence suggests that although factors such as hematuria, hypertension, time from treatment to response, and number of relapses have been proposed as possible risk factors of relapse, no conclusion can be reached due to the heterogeneity of studies. Future studies should have more conforming designs to make comparisons more reliable.
Study limitations
This study faced some limitations. Primarily, the included studies had reported various factors with differing comparison groups, making an overall conclusion difficult. We suggest that future studies be designed with attention to the current reported factors. The wide confidence interval reported for a few of the risk factors is also a limitation. Additionally, studies had variations in definitions of nephrotic syndrome, SSNS, relapse, frequent relapse, and their treatment regimens. Although the variations were minimal, they could still act as confounders. We suggest future studies conform more precisely to a uniform guideline.
Ethical Considerations
Compliance with ethical guidelines
The study was approved by the Ethics Committee of Tehran University of Medical Sciences (TUMS) (Code: IR.TUMS.CHMC.REC.1397.033).
Funding
This study has been funded and supported by Tehran University of Medical Sciences (Grant No.: 96-03-184-36538).
Authors contributions
Conceptualization and supervision: Mostafa Hosseini and Mahmoud Yousefifard; Methodology: Mostafa Hosseini, Mahmoud Yousefifard, Mohammed I M Gubari, Michael E. Jones, Nematollah Ataei and Mojtaba Fazel; Investigation and data curation: Arian Madani Neishaboori, Seyedeh Niloufar Rafiei Alavi, Koohyar Ahmadzadeh, Seyed Romina Rafiei Alavi, Hooman Ahmadzadeh and Amirmohammad Toloui; Formal analysis and validation: Mahmoud Yousefifard, Koohyar Ahmadzadeh and Mostafa Hosseini; Writing: All Authors.
Conflicts of interest
The authors declared no conflict of interest.
References
- Eddy AA, Symons JM. Nephrotic syndrome in childhood. Lancet. 2003; 362(9384):629-39. [DOI:10.1016/S0140-6736(03)14184-0] [PMID]
- Bagga A, Mantan M. Nephrotic syndrome in children. Indian J Med Res. 2005; 122(1):13-28. [PMID]
- Hodson EM, Knight JF, Willis NS, Craig JC. Corticosteroid therapy in nephrotic syndrome: A meta-analysis of randomised controlled trials. Arch Dis Child. 2000; 83(1):45-51. [DOI:10.1136/adc.83.1.45] [PMID]
- Jiang X, Shen W, Xu X, Shen X, Li Y, He Q. Immunosuppressive therapy for steroid-resistant nephrotic syndrome: A Bayesian network meta-analysis of randomized controlled studies. Clin Exp Nephrol. 2018; 22(3):562-9. [DOI:10.1007/s10157-017-1484-8] [PMID]
- Park SJ, Shin JI. Complications of nephrotic syndrome. Korean J Pediatr. 2011; 54(8):322-8. [DOI:10.3345/kjp.2011.54.8.322] [PMID]
- Mishra OP, Abhinay A, Mishra RN, Prasad R, Pohl M. Can we predict relapses in children with idiopathic steroid-sensitive nephrotic syndrome? J Trop Pediatr. 2013; 59(5):343-9.[DOI:10.1093/tropej/fmt029] [PMID]
- Bakr A, Hassan SA, Shoker M, Zaki M, Hassan R. Oxidant stress in primary nephrotic syndrome: Does it modulate the response to corticosteroids? Pediatr Nephrol. 2009; 24(12):2375-80. [DOI:10.1007/s00467-009-1246-2] [PMID]
- Ali SH, Ali AM, Najim AH. The predictive factors for relapses in children with steroid-sensitive nephrotic syndrome. Saudi J Kidney Dis Transpl. 2016; 27(1):67-72. [DOI:10.4103/1319-2442.174075] [PMID]
- Noer MS. Predictors of relapse in steroid-sensitive nephrotic syndrome. Southeast Asian J Trop Med Public Health. 2005; 36(5):1313-20. [PMID]
- Zhaoyang P, Wei L, Yanyan J, Wenqing X, Haidong F, Jianhua M. CCL22 and Leptin associated with steroid resistance in childhood idiopathic nephrotic syndrome. Front Pediatr. 2023; 11:1261034. [DOI:10.3389/fped.2023.1261034] [PMID]
- Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009; 151(4):264-9. [DOI:10.7326/0003-4819-151-4-200908180-00135] [PMID]
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for systematic reviews of interventions. New Jersey: John Wiley & Sons, Inc; 2019. [DOI:10.1002/9781119536604.ch14]
- Tu J, Chen CY, Yang HX, Jia Y, Geng HY, Li HR. [Clinical presentation and prognosis in children over 10-year-old with primary nephrotic syndrome (Chinese)]. Zhonghua Er Ke Za Zhi. 2023; 61(8):708-13. [PMID]
- Ying DJ, Jiang MJ, Chen LZ, Rong LP, Wu JY, Mo Y, et al. [Long-term outcomes of childhood steroid-sensitive nephrotic syndrome (Chinese)]. Zhonghua Er Ke Za Zhi. 2023; 61(7):620-625. [PMID]
- Cetin N, Gencler A, Sivrikoz IA. Bone mineral density and vitamin D status in children with remission phase of steroid-sensitive nephrotic syndrome. Saudi J Kidney Dis Transpl. 2019; 30(4):853-62. [DOI:10.4103/1319-2442.265461] [PMID]
- Fan A, Jiang X, Mo Y, Tan H, Jiang M, Li J. Plasma levels of oxidative stress in children with steroid-sensitive nephrotic syndrome and their predictive value for relapse frequency. Pediatr Nephrol. 2016; 31(1):83-8. [DOI:10.1007/s00467-015-3195-2] [PMID]
- Kabuki N, Okugawa T, Hayakawa H, Tomizawa S, Kasahara T, Uchiyama M. Influence of age at onset on the outcome of steroid-sensitive nephrotic syndrome. Pediatr Nephrol. 1998; 12(6):467-70. [DOI:10.1007/s004670050489] [PMID]
- Nakanishi K, Iijima K, Ishikura K, Hataya H, Nakazato H, Sasaki S, et al. Two-year outcome of the ISKDC regimen and frequent-relapsing risk in children with idiopathic nephrotic syndrome. Clin J Am Soc Nephrol. 2013; 8(5):756-62. [DOI:10.2215/CJN.09010912] [PMID]
- Esezobor CI, Ladapo TA, Lesi FE. Frequency of relapse among Nigerian children with steroidsensitive nephrotic syndrome. Niger J Clin Pract. 2016; 19(2):254-8. [DOI:10.4103/1119-3077.164326] [PMID]
- Takeda A, Matsutani H, Niimura F, Ohgushi H. Risk factors for relapse in childhood nephrotic syndrome. Pediatr Nephrol. 1996; 10(6):740-1. [DOI:10.1007/s004670050205] [PMID]
- Yousefifard M, Shafiee A. Should the reporting certainty of evidence for meta-analysis of observational studies using GRADE be revisited? Int J Surg. 2023; 109(2):129-30. [DOI:10.1097/JS9.0000000000000114] [PMID]
Type of Study: Systematic Review |
Subject:
Pediatric Nephrology
Received: 2024/01/17 | Accepted: 2024/03/16 | Published: 2024/04/1
Received: 2024/01/17 | Accepted: 2024/03/16 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |