Volume 13, Issue 4 (10-2025)
J. Pediatr. Rev 2025, 13(4): 281-288 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Talebi Anaraki K, Yazdi M, Moalem S, Heidari-Beni M, Khademian M, Kelishadi R. Association Between Zinc and Pediatric Metabolic Syndrome: A Systematic Review and Meta-analysis. J. Pediatr. Rev 2025; 13 (4) :281-288
URL: http://jpr.mazums.ac.ir/article-1-732-en.html
URL: http://jpr.mazums.ac.ir/article-1-732-en.html
Kasra Talebi Anaraki1 

 , Maryam Yazdi1
, Maryam Yazdi1 

 , Sepideh Moalem2
, Sepideh Moalem2 

 , Motahar Heidari-Beni *3
, Motahar Heidari-Beni *3 

 , Majid Khademian1
, Majid Khademian1 

 , Roya Kelishadi1
, Roya Kelishadi1 




 , Maryam Yazdi1
, Maryam Yazdi1 

 , Sepideh Moalem2
, Sepideh Moalem2 

 , Motahar Heidari-Beni *3
, Motahar Heidari-Beni *3 

 , Majid Khademian1
, Majid Khademian1 

 , Roya Kelishadi1
, Roya Kelishadi1 


1- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Department of Pediatrics, School of Medicine, Imam Hossein Children’s Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran. ,heidari.motahar@gmail.com
2- Department of Pediatrics, School of Medicine, Imam Hossein Children’s Hospital, Isfahan University of Medical Sciences, Isfahan, Iran.
3- Child Growth and Development Research Center, Research Institute for Primordial Prevention of Non-Communicable Disease, Isfahan University of Medical Sciences, Isfahan, Iran. ,
Full-Text [PDF 507 kb]
(145 Downloads)
| Abstract (HTML) (257 Views)
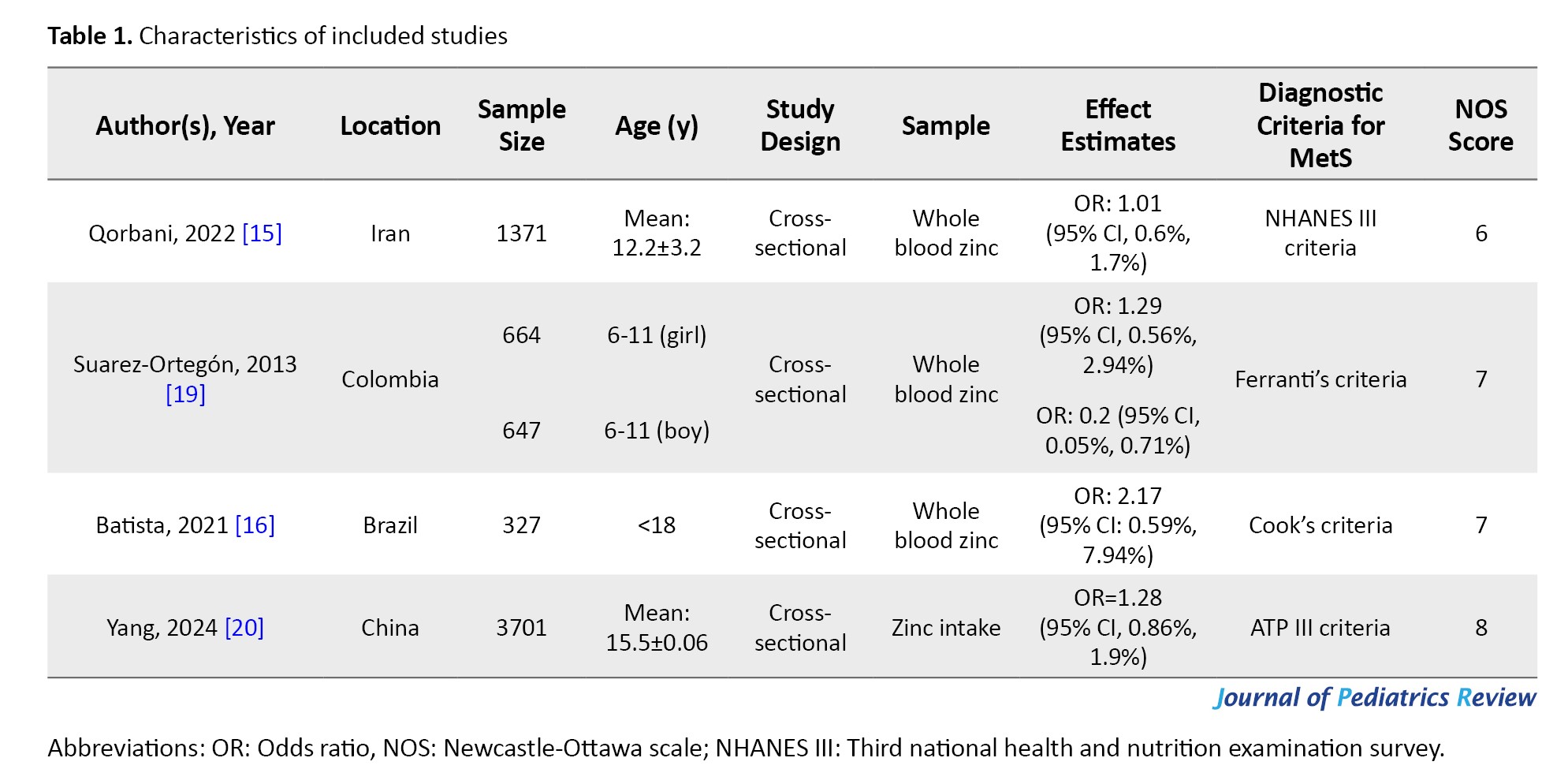
These studies, published between 2013 and 2024, were cross-sectional studies. The studies were conducted in Iran [15], China [20], Colombia [19], and Brazil [16]. The sample size ranged from 327 to 3701, for a total of 6710 participants.
Qorbani et al. [15] in a cross-sectional study on Iranian children and adolescents, revealed no significant association between serum zinc levels and MetS. Batista et al. [16] in a cross-sectional study on 327 adolescents demonstrated a significant association between MetS and its components with the intake of antioxidant nutrients in Brazilian adolescents. Suarez-Ortegon et al. [19] in a cross-sectional study among 1311 adolescents, showed a negative correlation between the highest quartile of zinc intake and MetS in males. They found no significant association in females. Yang et al. [20] in a cross-sectional study on 3701 adolescents in China found no significant association between levels of zinc intake and MetS risk.
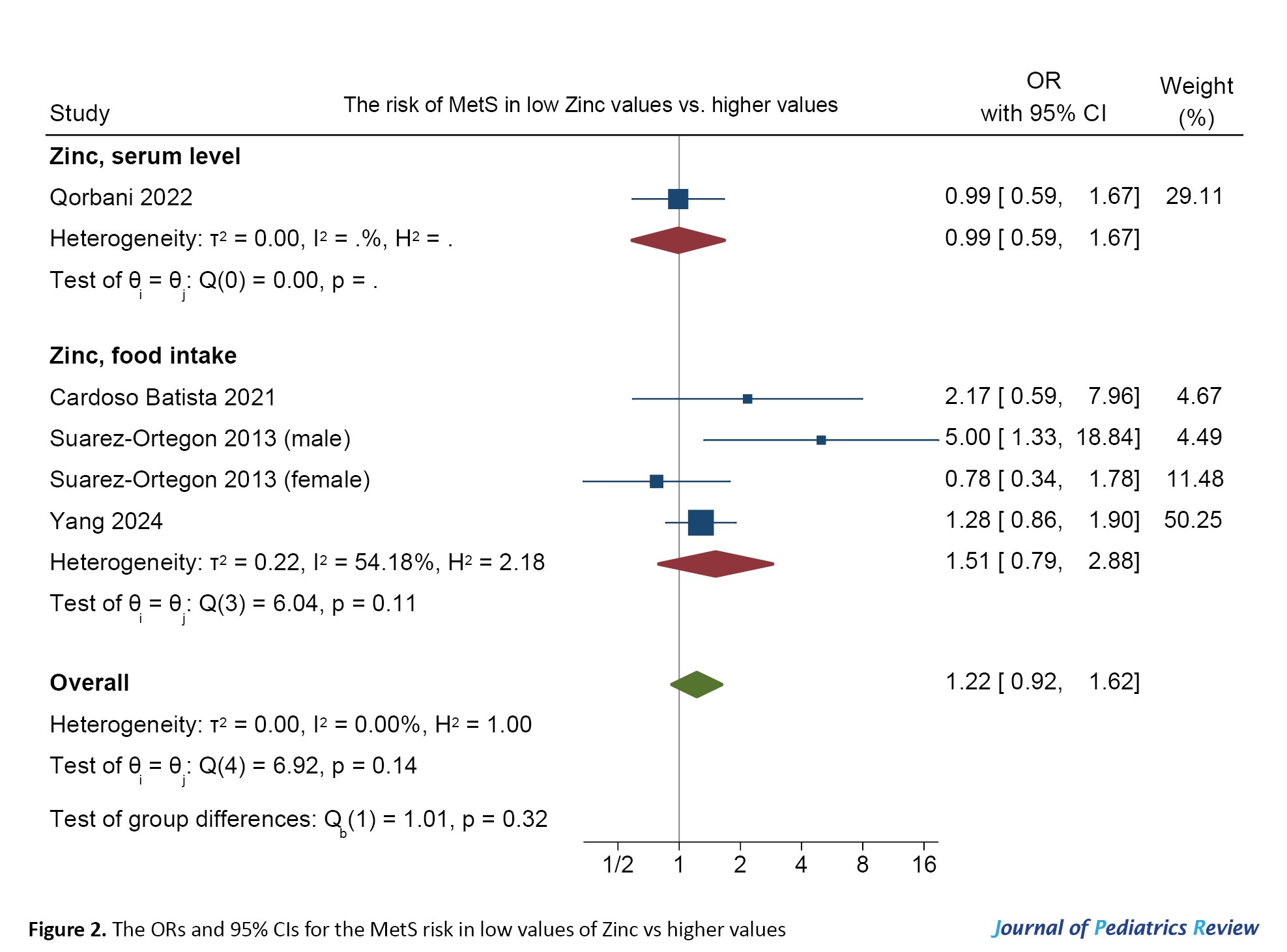
Figure 2 shows the ORs and 95% CIs of the MetS risk in low values of zinc vs higher values.
References
Full-Text: (49 Views)
Introduction
Metabolic syndrome (MetS) is a known medical condition. It is characterized by the presence of abdominal obesity, insulin resistance, high blood pressure, and elevated lipid levels, thereby increasing the risk of cardiovascular diseases [1, 2]. It is characterized by at least three of the following conditions: increased waist circumference, elevated triglyceride levels, reduced HDL cholesterol levels, elevated blood sugar levels, and increased blood pressure [3, 4]. The global prevalence of MetS ranges from 11.6% to 62.5% [5].
While the precise etiology remains incompletely elucidated, some trace elements, including zinc, have been postulated to play a pivotal role. Emerging evidence has suggested an association between zinc deficiency and metabolic disorders [6, 7]. Trace elements are essential cofactors in various metabolic processes, facilitating enzyme reactions and supporting overall cellular function [8, 9]. Zinc ranks as the second most prevalent trace metal in the human body, playing a crucial role in DNA functions, protein synthesis, cell division, and preserving cellular and endothelial integrity. It can reduce oxidative stress, chronic inflammation, and insulin resistance [10, 11]. Epidemiological investigations have indicated an inverse relationship between dietary zinc intake and MetS-related conditions such as diabetes [12, 13]. It was assumed that higher dietary zinc intake could have an inverse effect on the occurrence of MetS [14]. However, the findings of some observational studies have shown an inconsistent association between dietary zinc intake and MetS in children and adolescents [15, 16]. This study aims to review the studies that examined the association of dietary zinc intake and serum zinc levels with MetS among children and adolescents.
Methods
This is a systematic review and meta-analysis study conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) 2020 guidelines [17]. We conducted searches in online databases, including PubMed, Web of Science, and Scopus, for related studies published up to May 2024. Search terms related to MetS (“metabolic syndrome,” “metabolism syndrome”) and zinc (“zinc,” “Zn”), targeting children and adolescents were used for the search. Initially, the titles and abstracts of articles were screened to find relevant studies, followed by an in-depth review of the full texts to select eligible studies. Furthermore, we examined the reference lists of included articles to find additional relevant studies. Two authors (Kasra Talebi Anaraki and Motahar Heidari-Beni) independently screened the titles, abstracts, and full texts. Any discrepancies were settled through discussion and mutual consultation. Observational studies that investigated the association between serum zinc levels or dietary zinc intake with MetS in children and adolescents were included. Only English-language articles and human studies were included. Irrelevant studies, review studies, letters to the editor, case reports, randomized controlled clinical trials; and animal studies were excluded.
Data extraction was performed independently by two authors (Motahar Heidari-Beni and Sepideh Moalem), with any discrepancies addressed through discussion and mutual agreement. The information extracted included the first author's name, year of publication, study location, age, gender, sample size, study design, adjustments made, serum zinc assessment, dietary zinc intake, effect estimates, and the diagnostic criteria for MetS.
For quality assessment, we used the Newcastle-Ottawa scale (NOS), applicable to non-randomized studies [18]. This tool has three main elements: The process of selecting study cohorts, the comparability of various cohorts, and the determination of exposure or outcome in the study cohorts. Any disputes concerning methodological quality were addressed through dialogue and collaborative consultation between authors.
Odds ratios (ORs) and 95% confidence intervals (CIs) comparing the risk of MetS in people with lower levels of zinc versus those with higher levels were extracted from each study. The subgroup analysis was done based on dietary intake or serum zinc level. A fixed-effects meta-analysis was employed to derive effect estimates due to the inherent study design and varied methodologies. The assessment of population bias was not conducted due to the insufficient number of studies available. The heterogeneity between studies was assessed by the I2 statistic. All statistical analyses were carried out in Stata software, version 17 (StataCorp LP), with a significance level set at 0.05 for all tests.
Results
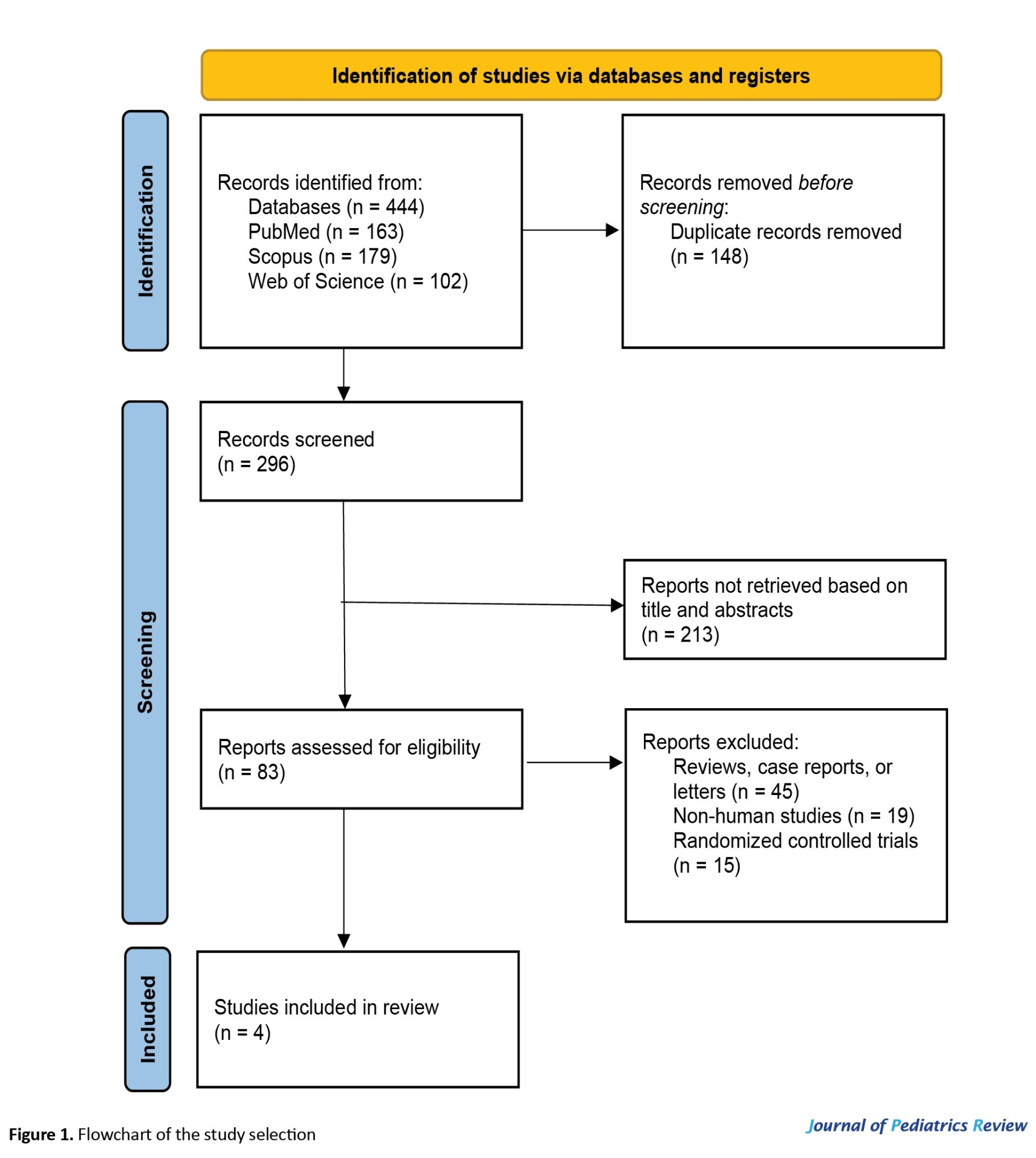
During the initial search, 444 records were identified. After removing 148 duplicates, the remaining 296 articles underwent screening based on titles and abstracts, which led to the exclusion of 213 irrelevant studies. Subsequently, 79 studies, including review studies, case reports, letters to the editor, animal studies, or randomized controlled clinical trials, were excluded. Finally, four articles [15, 16, 19, 20] were included in this meta-analysis. Figure 1 illustrates a comprehensive diagram of these articles and Table 1 presents their key characteristics.
Metabolic syndrome (MetS) is a known medical condition. It is characterized by the presence of abdominal obesity, insulin resistance, high blood pressure, and elevated lipid levels, thereby increasing the risk of cardiovascular diseases [1, 2]. It is characterized by at least three of the following conditions: increased waist circumference, elevated triglyceride levels, reduced HDL cholesterol levels, elevated blood sugar levels, and increased blood pressure [3, 4]. The global prevalence of MetS ranges from 11.6% to 62.5% [5].
While the precise etiology remains incompletely elucidated, some trace elements, including zinc, have been postulated to play a pivotal role. Emerging evidence has suggested an association between zinc deficiency and metabolic disorders [6, 7]. Trace elements are essential cofactors in various metabolic processes, facilitating enzyme reactions and supporting overall cellular function [8, 9]. Zinc ranks as the second most prevalent trace metal in the human body, playing a crucial role in DNA functions, protein synthesis, cell division, and preserving cellular and endothelial integrity. It can reduce oxidative stress, chronic inflammation, and insulin resistance [10, 11]. Epidemiological investigations have indicated an inverse relationship between dietary zinc intake and MetS-related conditions such as diabetes [12, 13]. It was assumed that higher dietary zinc intake could have an inverse effect on the occurrence of MetS [14]. However, the findings of some observational studies have shown an inconsistent association between dietary zinc intake and MetS in children and adolescents [15, 16]. This study aims to review the studies that examined the association of dietary zinc intake and serum zinc levels with MetS among children and adolescents.
Methods
This is a systematic review and meta-analysis study conducted according to the PRISMA (preferred reporting items for systematic reviews and meta-analyses) 2020 guidelines [17]. We conducted searches in online databases, including PubMed, Web of Science, and Scopus, for related studies published up to May 2024. Search terms related to MetS (“metabolic syndrome,” “metabolism syndrome”) and zinc (“zinc,” “Zn”), targeting children and adolescents were used for the search. Initially, the titles and abstracts of articles were screened to find relevant studies, followed by an in-depth review of the full texts to select eligible studies. Furthermore, we examined the reference lists of included articles to find additional relevant studies. Two authors (Kasra Talebi Anaraki and Motahar Heidari-Beni) independently screened the titles, abstracts, and full texts. Any discrepancies were settled through discussion and mutual consultation. Observational studies that investigated the association between serum zinc levels or dietary zinc intake with MetS in children and adolescents were included. Only English-language articles and human studies were included. Irrelevant studies, review studies, letters to the editor, case reports, randomized controlled clinical trials; and animal studies were excluded.
Data extraction was performed independently by two authors (Motahar Heidari-Beni and Sepideh Moalem), with any discrepancies addressed through discussion and mutual agreement. The information extracted included the first author's name, year of publication, study location, age, gender, sample size, study design, adjustments made, serum zinc assessment, dietary zinc intake, effect estimates, and the diagnostic criteria for MetS.
For quality assessment, we used the Newcastle-Ottawa scale (NOS), applicable to non-randomized studies [18]. This tool has three main elements: The process of selecting study cohorts, the comparability of various cohorts, and the determination of exposure or outcome in the study cohorts. Any disputes concerning methodological quality were addressed through dialogue and collaborative consultation between authors.
Odds ratios (ORs) and 95% confidence intervals (CIs) comparing the risk of MetS in people with lower levels of zinc versus those with higher levels were extracted from each study. The subgroup analysis was done based on dietary intake or serum zinc level. A fixed-effects meta-analysis was employed to derive effect estimates due to the inherent study design and varied methodologies. The assessment of population bias was not conducted due to the insufficient number of studies available. The heterogeneity between studies was assessed by the I2 statistic. All statistical analyses were carried out in Stata software, version 17 (StataCorp LP), with a significance level set at 0.05 for all tests.
Results
During the initial search, 444 records were identified. After removing 148 duplicates, the remaining 296 articles underwent screening based on titles and abstracts, which led to the exclusion of 213 irrelevant studies. Subsequently, 79 studies, including review studies, case reports, letters to the editor, animal studies, or randomized controlled clinical trials, were excluded. Finally, four articles [15, 16, 19, 20] were included in this meta-analysis. Figure 1 illustrates a comprehensive diagram of these articles and Table 1 presents their key characteristics.

These studies, published between 2013 and 2024, were cross-sectional studies. The studies were conducted in Iran [15], China [20], Colombia [19], and Brazil [16]. The sample size ranged from 327 to 3701, for a total of 6710 participants.
Qorbani et al. [15] in a cross-sectional study on Iranian children and adolescents, revealed no significant association between serum zinc levels and MetS. Batista et al. [16] in a cross-sectional study on 327 adolescents demonstrated a significant association between MetS and its components with the intake of antioxidant nutrients in Brazilian adolescents. Suarez-Ortegon et al. [19] in a cross-sectional study among 1311 adolescents, showed a negative correlation between the highest quartile of zinc intake and MetS in males. They found no significant association in females. Yang et al. [20] in a cross-sectional study on 3701 adolescents in China found no significant association between levels of zinc intake and MetS risk.
Figure 2 shows the ORs and 95% CIs of the MetS risk in low values of zinc vs higher values.
There was no significant association between zinc (dietary intake or serum level) and MetS (OR=1.22, 95% CI, 0.92%, 1.62%). Heterogeneity was not substantial (I2=0.00).
Discussion
A meta-analysis conducted on adults revealed that elevated serum zinc levels were linked to an increased risk of MetS. The findings indicated that participants diagnosed with MetS exhibited higher serum zinc levels compared to the control group [21]. Zinc content has been assessed in various tissues like serum, blood cells, hair, and nails [22]. Factors such as stress, infection, and hormonal changes can influence serum zinc levels. Thus, serum zinc is considered the best gauge of zinc status [23]. The presence of phytates in foods like nuts and grains may affect the absorption of dietary zinc [24]. Therefore, randomized controlled trials using zinc supplements are necessary. Dietary sources, such as meat, nuts, and whole grains, contain zinc. However, modern nutritional patterns are often deficient in this essential nutrient [25]. Zinc, an indispensable trace metal in the human body, has critical roles in DNA replication, transcription, protein synthesis, and various cellular processes. It serves as a cofactor in enzymatic reactions associated with superoxide dismutase, catalase, and glutathione peroxidase [26, 27].
Childhood obesity has adverse effects on erythroid antioxidant levels. This accentuates the role of oxidative stress in MetS [28]. There are significant variations in zinc levels among children with MetS. This indicates a potential imbalance that may exacerbate metabolic dysregulation. Some studies investigated the role of zinc concentrations in the morbidity of obese children, with or without MetS [29, 30]. Their findings showed that zinc levels correlated with the health status of these children. Chronic inflammation is a potential underlying factor for MetS [31]. Zinc acts as a cofactor for antioxidant enzymes, thereby diminishing the production of inflammatory cytokines [32].
The connection between serum zinc and MetS is debated. The connection has been reported in the adult population. Two prospective cohort studies have shown a significant association between higher serum zinc levels and an increased risk of hypertension [33] and diabetes [34]. Two meta-analysis studies on adults suggested a significant inverse association between zinc dietary intake and MetS. However, results in adolescents were non-significant [21, 35]. This association may underscore the importance of collaboration between physicians and nutritionists in managing metabolic disorders. Of course, the adverse effects of excess zinc intake should not be ignored.
Zinc status should be considered in the broader context of metabolic regulation, as it affects insulin signaling, lipid metabolism, and inflammatory pathways. Zinc plays a key role in insulin crystallization and secretion via transporters such as ZnT8 (critical for pancreatic β-cell function), and modulates the PI3K/Akt pathway, enhancing insulin sensitivity and glucose uptake [36]. Additionally, zinc influences lipid metabolism by regulating adipokines (e.g. leptin, ZAG), PPARγ activation, and antioxidant defenses, thereby mitigating oxidative stress and inflammation [12]. Clarifying these mechanisms in pediatric populations may open new avenues for MetS prevention and treatment.
The findings of the present study, however, revealed no significant correlation between zinc levels and the risk of MetS in youths. The high degree of heterogeneity between reviewed studies suggests that the studies may not be enough to draw a definitive conclusion. Therefore, well-designed longitudinal studies are needed. This is the first meta-analysis study on the association between serum zinc levels and dietary intake with MetS among children and adolescents. However, the small number of included papers was a disadvantage. It highlights the necessity for further research with larger sample sizes and standardized zinc measurement techniques in the pediatric population. Future studies should also explore the impact of dietary patterns and nutritional interventions.
Conclusion
The current study does not confirm a definitive association of serum zinc levels or dietary zinc intake with pediatric MetS. This study provides a comprehensive understanding and evidence-based guidance for clinical practice in pediatric MetS and public health strategies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran (Code: IR.MUI.MED.REC.1402.276).
Funding
This study was funded by Isfahan University of Medical Sciences, Isfahan, Iran.
Authors contributions
Conceptualization: Motahar Heidari-Beni and Majid Khademian; Investigation and project administration: Roya Kelishadi; Data curation: Sepideh Moalem and Kasra Talebi Anaraki; Formal analysis: Maryam Yazdi; Methodology: Kasra Talebi Anaraki and Motahar Heidari-Beni; Writing the original draft: Kasra Talebi Anaraki, Sepideh Moalem, and Maryam Yazdi; Review and editing: Motahar Heidari-Beni, Majid Khademian, and Roya Kelishadi.
Conflicts of interest
The authors declared no conflict of interest
Discussion
A meta-analysis conducted on adults revealed that elevated serum zinc levels were linked to an increased risk of MetS. The findings indicated that participants diagnosed with MetS exhibited higher serum zinc levels compared to the control group [21]. Zinc content has been assessed in various tissues like serum, blood cells, hair, and nails [22]. Factors such as stress, infection, and hormonal changes can influence serum zinc levels. Thus, serum zinc is considered the best gauge of zinc status [23]. The presence of phytates in foods like nuts and grains may affect the absorption of dietary zinc [24]. Therefore, randomized controlled trials using zinc supplements are necessary. Dietary sources, such as meat, nuts, and whole grains, contain zinc. However, modern nutritional patterns are often deficient in this essential nutrient [25]. Zinc, an indispensable trace metal in the human body, has critical roles in DNA replication, transcription, protein synthesis, and various cellular processes. It serves as a cofactor in enzymatic reactions associated with superoxide dismutase, catalase, and glutathione peroxidase [26, 27].
Childhood obesity has adverse effects on erythroid antioxidant levels. This accentuates the role of oxidative stress in MetS [28]. There are significant variations in zinc levels among children with MetS. This indicates a potential imbalance that may exacerbate metabolic dysregulation. Some studies investigated the role of zinc concentrations in the morbidity of obese children, with or without MetS [29, 30]. Their findings showed that zinc levels correlated with the health status of these children. Chronic inflammation is a potential underlying factor for MetS [31]. Zinc acts as a cofactor for antioxidant enzymes, thereby diminishing the production of inflammatory cytokines [32].
The connection between serum zinc and MetS is debated. The connection has been reported in the adult population. Two prospective cohort studies have shown a significant association between higher serum zinc levels and an increased risk of hypertension [33] and diabetes [34]. Two meta-analysis studies on adults suggested a significant inverse association between zinc dietary intake and MetS. However, results in adolescents were non-significant [21, 35]. This association may underscore the importance of collaboration between physicians and nutritionists in managing metabolic disorders. Of course, the adverse effects of excess zinc intake should not be ignored.
Zinc status should be considered in the broader context of metabolic regulation, as it affects insulin signaling, lipid metabolism, and inflammatory pathways. Zinc plays a key role in insulin crystallization and secretion via transporters such as ZnT8 (critical for pancreatic β-cell function), and modulates the PI3K/Akt pathway, enhancing insulin sensitivity and glucose uptake [36]. Additionally, zinc influences lipid metabolism by regulating adipokines (e.g. leptin, ZAG), PPARγ activation, and antioxidant defenses, thereby mitigating oxidative stress and inflammation [12]. Clarifying these mechanisms in pediatric populations may open new avenues for MetS prevention and treatment.
The findings of the present study, however, revealed no significant correlation between zinc levels and the risk of MetS in youths. The high degree of heterogeneity between reviewed studies suggests that the studies may not be enough to draw a definitive conclusion. Therefore, well-designed longitudinal studies are needed. This is the first meta-analysis study on the association between serum zinc levels and dietary intake with MetS among children and adolescents. However, the small number of included papers was a disadvantage. It highlights the necessity for further research with larger sample sizes and standardized zinc measurement techniques in the pediatric population. Future studies should also explore the impact of dietary patterns and nutritional interventions.
Conclusion
The current study does not confirm a definitive association of serum zinc levels or dietary zinc intake with pediatric MetS. This study provides a comprehensive understanding and evidence-based guidance for clinical practice in pediatric MetS and public health strategies.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of Isfahan University of Medical Sciences, Isfahan, Iran (Code: IR.MUI.MED.REC.1402.276).
Funding
This study was funded by Isfahan University of Medical Sciences, Isfahan, Iran.
Authors contributions
Conceptualization: Motahar Heidari-Beni and Majid Khademian; Investigation and project administration: Roya Kelishadi; Data curation: Sepideh Moalem and Kasra Talebi Anaraki; Formal analysis: Maryam Yazdi; Methodology: Kasra Talebi Anaraki and Motahar Heidari-Beni; Writing the original draft: Kasra Talebi Anaraki, Sepideh Moalem, and Maryam Yazdi; Review and editing: Motahar Heidari-Beni, Majid Khademian, and Roya Kelishadi.
Conflicts of interest
The authors declared no conflict of interest
References
- Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018; 20(2):12. [DOI:10.1007/s11906-018-0812-z] [PMID]
- Kelishadi R, Qorbani M, Heshmat R, Motamed-Gorji N, Motlagh ME, Ziaodini H, et al. Association of alanine aminotransferase concentration with cardiometabolic risk factors in children and adolescents: The CASPIAN-V cross-sectional study. Sao Paulo Med J. 2018; 136(6):511-9. [DOI:10.1590/1516-3180.2018.0161161118] [PMID]
- Lee MK, Han K, Kim MK, Koh ES, Kim ES, Nam GE, et al. Changes in metabolic syndrome and its components and the risk of type 2 diabetes: A nationwide cohort study. Sci Rep. 2020; 10(1):2313. [DOI:10.1038/s41598-020-59203-z] [PMID]
- Arefian M, Mazaheri-Tehrani S, Yazdi M, Kelishadi R. Caveolin Gene, a Possible Risk Factor for Metabolic Syndrome in Humans: A systematic review and meta-analysis. Int J Prev Med. 2025; 16:7. [DOI:10.4103/ijpvm.ijpvm_216_24] [PMID]
- Hirode G, Wong RJ. Trends in the prevalence of metabolic syndrome in the United States, 2011-2016. JAMA. 2020; 323(24):2526-8. [DOI:10.1001/jama.2020.4501] [PMID]
- Lu CW, Lee YC, Kuo CS, Chiang CH, Chang HH, Huang KC. Association of Serum Levels of Zinc, Copper, and Iron with Risk of Metabolic Syndrome. Nutrients. 2021; 13(2):548. [DOI:10.3390/nu13020548] [PMID]
- Aslani Z, Sadeghi O, Heidari-Beni M, Zahedi H, Baygi F, Shivappa N, et al. Association of dietary inflammatory potential with cardiometabolic risk factors and diseases: A systematic review and dose-response meta-analysis of observational studies. Diabetol Metab Syndr. 2020; 12:86. [DOI:10.1186/s13098-020-00592-6] [PMID]
- Fakhrolmobasheri M, Mazaheri-Tehrani S, Kieliszek M, Zeinalian M, Abbasi M, Karimi F, et al. COVID-19 and Selenium Deficiency: A systematic review. Biol Trace Elem Res. 2022; 200(9):3945-56. [DOI:10.1007/s12011-021-02997-4] [PMID]
- Mazaheri-Tehrani S, Abhari AP, Ostadsharif N, Shekarian A, Vali M, Saffari E, et al. Serum selenium levels and lipid profile: A systematic review and meta-analysis of observational studies. Biol Trace Elem Res. 2025; 203(5):2517-38. [DOI:10.1007/s12011-024-04365-4] [PMID]
- Atazadegan MA, Heidari-Beni M, Riahi R, Kelishadi R. Association of selenium, zinc and copper concentrations during pregnancy with birth weight: A systematic review and meta-analysis. J Trace Elem Med Biol. 2022; 69:126903. [DOI:10.1016/j.jtemb.2021.126903] [PMID]
- Mazaheri-Tehrani S, Haghighatpanah MA, Abhari AP, Fakhrolmobasheri M, Shekarian A, Kieliszek M. Dynamic changes of serum trace elements following cardiac surgery: A systematic review and meta-analysis. J Trace Elem Med Biol. 2024; 81:127331. [DOI:10.1016/j.jtemb.2023.127331] [PMID]
- Olechnowicz J, Tinkov A, Skalny A, Suliburska J. Zinc status is associated with inflammation, oxidative stress, lipid, and glucose metabolism. J Physiol Sci. 2018; 68(1):19-31. [DOI:10.1007/s12576-017-0571-7] [PMID]
- Fathi M, Alavinejad P, Haidari Z, Amani R. The effects of zinc supplementation on metabolic profile and oxidative stress in overweight/obese patients with non-alcoholic fatty liver disease: A randomized, double-blind, placebo-controlled trial. J Trace Elem Med Biol. 2020; 62:126635. [DOI:10.1016/j.jtemb.2020.126635] [PMID]
- Zhu Z, He Y, Wu F, Zhao L, Wu C, Lu Y, et al. The associations of dietary iron, zinc and magnesium with metabolic syndrome in China’s Mega Cities. Nutrients. 2020; 12(3):659. [DOI:10.3390/nu12030659] [PMID]
- Qorbani M, Movasaghi N, Mohammadian Khonsari N, Daneshzad E, Shafiee G, Ashraf H, et al. Association of zinc serum level with metabolic syndrome in iranian children and adolescents: The CASPIAN-V study. Front Nutr. 2022; 9:932746. [DOI:10.3389/fnut.2022.932746] [PMID]
- Batista CC, Nascimento LM, Lustosa LCRS, Rodrigues BGM, Campelo V, Frota KMG. Metabolic syndrome in adolescents and antioxidant nutrient intake: A cross-sectional study. Rev Assoc Med Bras (1992). 2021; 67(7):918-25. [DOI:10.1590/1806-9282.20200733] [PMID]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ. 2021; 372:n71. [DOI:10.1136/bmj.n71] [PMID]
- Lo CKL, Mertz D, Loeb M. Newcastle-Ottawa Scale: Comparing reviewers’ to authors’ assessments. BMC Med Res Methodol. 2014; 14:45. [DOI:10.1186/1471-2288-14-45] [PMID]
- Suarez-Ortegón MF, Ordoñez-Betancourth JE, Aguilar-de Plata C. Dietary zinc intake is inversely associated to metabolic syndrome in male but not in female urban adolescents. Am J Hum Biol. 2013; 25(4):550-4. [DOI:10.1002/ajhb.22408] [PMID]
- Yang S, Chen Q, Wang L. Association of zinc intake, tobacco smoke exposure, with metabolic syndrome: Evidence from NHANES 2007-2018. Biol Trace Elem Res. 2024; 202(12):5429-37. [DOI:10.1007/s12011-024-04120-9] [PMID]
- Zhang Y, Zhang DZ. Relationship between serum zinc level and metabolic syndrome: A meta-analysis of observational studies. J Am Coll Nutr. 2018; 37(8):708-15. [DOI:10.1080/07315724.2018.1463876] [PMID]
- Gibson RS, Hess SY, Hotz C, Brown KH. Indicators of zinc status at the population level: A review of the evidence. Br J Nutr. 2008; 99(Suppl 3):S14-23. [DOI:10.1017/S0007114508006818] [PMID]
- Hess SY, Peerson JM, King JC, Brown KH. Use of serum zinc concentration as an indicator of population zinc status. Food Nutr Bull. 2007; 28(3 Suppl):S403-29. [DOI:10.1177/15648265070283S303] [PMID]
- Shkembi B, Huppertz T. Influence of dairy products on bioavailability of zinc from other food products: A review of complementarity at a meal level. Nutrients. 2021; 13(12):4253. [DOI:10.3390/nu13124253] [PMID]
- Ceballos-Rasgado M, Lowe NM, Moran VH, Clegg A, Mallard S, Harris C, et al. Toward revising dietary zinc recommendations for children aged 0 to 3 years: A systematic review and meta-analysis of zinc absorption, excretion, and requirements for growth. Nutr Rev. 2023; 81(8):967-87. [DOI:10.1093/nutrit/nuac098] [PMID]
- Costa MI, Sarmento-Ribeiro AB, Gonçalves AC. Zinc: From biological functions to therapeutic potential. Int J Mol Sci. 2023; 24(5):4822. [DOI:10.3390/ijms24054822] [PMID]
- Yusuf AP, Abubakar MB, Malami I, Ibrahim KG, Abubakar B, Bello MB, et al. Zinc metalloproteins in epigenetics and their crosstalk. Life (Basel). 2021; 11(3):186. [DOI:10.3390/life11030186] [PMID]
- González-Domínguez Á, Visiedo F, Domínguez-Riscart J, Ruiz-Mateos B, Saez-Benito A, Lechuga-Sancho AM, et al. Blunted reducing power generation in erythrocytes contributes to oxidative stress in prepubertal obese children with insulin resistance. Antioxidants (Basel). 2021; 10(2):244. [DOI:10.3390/antiox10020244] [PMID]
- Vivek SM, Dayal D, Khaiwal R, Bharti B, Bhalla A, Singh S, et al. Low serum copper and zinc concentrations in North Indian children with overweight and obesity. Pediatr Endocrinol Diabetes Metab. 2020; 26(2):79-83. [DOI:10.5114/pedm.2020.95627] [PMID]
- Kelishadi R, Hashemipour M, Adeli K, Tavakoli N, Movahedian-Attar A, Shapouri J, et al. Effect of zinc supplementation on markers of insulin resistance, oxidative stress, and inflammation among prepubescent children with metabolic syndrome. Metab Syndr Relat Disord. 2010; 8(6):505-10. [DOI:10.1089/met.2010.0020] [PMID]
- Mazaheri-Tehrani S, Rezaei F, Heidari-Hasanabadi S, Malakoutikhah M, Amani-Beni R, Arefian M, et al. Serum lipopolysaccharide binding protein (LBP) and metabolic syndrome: A systematic review and meta-analysis. Diabetol Metab Syndr. 2025; 17(1):268. [DOI:10.1186/s13098-025-01847-w] [PMID]
- Seo JA, Song SW, Han K, Lee KJ, Kim HN. The associations between serum zinc levels and metabolic syndrome in the Korean population: Findings from the 2010 Korean National Health and Nutrition Examination Survey. PLoS One. 2014; 9(8):e105990. [DOI:10.1371/journal.pone.0105990] [PMID]
- Kunutsor SK, Laukkanen JA. Serum zinc concentrations and incident hypertension: New findings from a population-based cohort study. J Hypertens. 2016; 34(6):1055-61. [DOI:10.1097/HJH.0000000000000923] [PMID]
- Yary T, Virtanen JK, Ruusunen A, Tuomainen TP, Voutilainen S. Serum zinc and risk of type 2 diabetes incidence in men: The Kuopio Ischaemic Heart Disease Risk Factor Study. J Trace Elem Med Biol. 2016; 33:120-4. [DOI:10.1016/j.jtemb.2015.11.001] [PMID]
- Ding J, Liu Q, Liu Z, Guo H, Liang J, Zhang Y. Association Between Dietary Zinc Intake and Metabolic Syndrome. A Meta-Analysis of Observational Studies. Front Nutr. 2022; 9:825913. [DOI:10.3389/fnut.2022.825913]
- Chen B, Yu P, Chan WN, Xie F, Zhang Y, Liang L, et al. Cellular zinc metabolism and zinc signaling: From biological functions to diseases and therapeutic targets. Signal Transduct Target Ther. 2024; 9(1):6. [DOI:10.1038/s41392-023-01679-y] [PMID]
Type of Study: Meta-analysis Review |
Subject:
Pediatrics
Received: 2025/03/2 | Accepted: 2025/10/18 | Published: 2025/10/18
Received: 2025/03/2 | Accepted: 2025/10/18 | Published: 2025/10/18
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |