Volume 7, Issue 3 (7-2019)
J. Pediatr. Rev 2019, 7(3): 129-140 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Masoumi B, Eslami G, Alizadeh-Navaei R, Mondal P, Rezai M S. Safety Profile of Using Ciprofloxacin in Paediatrics: A Systematic Review and Meta-Analysis. J. Pediatr. Rev 2019; 7 (3) :129-140
URL: http://jpr.mazums.ac.ir/article-1-203-en.html
URL: http://jpr.mazums.ac.ir/article-1-203-en.html
Baraneh Masoumi1 

 , Gohar Eslami2
, Gohar Eslami2 

 , Reza Alizadeh-Navaei3
, Reza Alizadeh-Navaei3 

 , Pritish Mondal4
, Pritish Mondal4 

 , Mohammad Sadegh Rezai *5
, Mohammad Sadegh Rezai *5 




 , Gohar Eslami2
, Gohar Eslami2 

 , Reza Alizadeh-Navaei3
, Reza Alizadeh-Navaei3 

 , Pritish Mondal4
, Pritish Mondal4 

 , Mohammad Sadegh Rezai *5
, Mohammad Sadegh Rezai *5 


1- Paediatric Infectious Diseases Research Centre, Mazandaran University of Medical Sciences, Sari, Iran.
2- Department of Clinical Pharmacy, Faculty of Pharmacy, Cardiovascular Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
3- Gastrointestinal Cancer Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Paediatrics, Division of Pulmonary, Penn State College of Medicine, Hershey, Pennsylvania, USA.
5- Paediatric Infectious Diseases Research Centre, Mazandaran University of Medical Sciences, Sari, Iran. ,rezai@mazums.ac.ir
2- Department of Clinical Pharmacy, Faculty of Pharmacy, Cardiovascular Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
3- Gastrointestinal Cancer Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Paediatrics, Division of Pulmonary, Penn State College of Medicine, Hershey, Pennsylvania, USA.
5- Paediatric Infectious Diseases Research Centre, Mazandaran University of Medical Sciences, Sari, Iran. ,
Full-Text [PDF 471 kb]
(10130 Downloads)
| Abstract (HTML) (10335 Views)
Full-Text: (24231 Views)
1. Context
Quinolones have been used for many years for treatment of various infectious diseases. Nalidixic acid is the first quinolone that was discovered in 1962 (1). However, due to its limitations, such as toxicity issues, the narrow spectrum of antibacterial activity and low serum bioavailability, it is no longer used in practice (1, 2). The fluorination of quinolones resulted in the production of fluoroquinolones. Ciprofloxacin is a second-generation fluoroquinolone, which was discovered in 1987 (1). Ciprofloxacin and other second-generation fluoroquinolones have superior properties compared to nalidixic acid, such as the broad-spectrum bactericidal activity, and appropriate tissue penetration (2). Considering the above-mentioned properties, these antibiotics are more suitable to be used in practice (1, 3).
1.1. Mechanism of action
Although the antimicrobial resistance is increasing, many bacteria are still sensitive to fluoroquinolones (4). Based on the evidence, ciprofloxacin is the safest and most effective antibiotic in children compared to other fluoroquinolones (3, 5). It is effective in a wide range of Gram-positive and Gram-negative pathogens and acts by interfering with DNA functioning and inhibiting DNA gyrase and topoisomerase IV in bacteria. These enzymes are essential for DNA replication, transcription, repair, and recombination (5, 6).
Fluoroquinolones have many favorable properties in the treatment of bacterial infections in adults and children (2, 4, 5). Despite their effectiveness, the use of these antibiotics in growing children were limited due to the debatable safety concerns over the risk of joint toxicity and other adverse side effects (2). In vitro study of juvenile animals of different species, such as dogs, mice, rats, and rabbits, have illustrated that the use of ciprofloxacin and other quinolones can cause arthropathy in weight-bearing joints (2, 3). No evidence has proved the definite joint injury induced by ciprofloxacin in children (5). Since adverse effects are inevitable, fluoroquinolones like ciprofloxacin are recommended when other options are not available or effective (5).
1.2. Pharmacokinetics
The pharmacological data available on the use of ciprofloxacin to treat hospitalized pediatric patients are scarce. The literature review suggests that the half-life of ciprofloxacin is significantly longer in infants (under 1 year old) than toddlers (1 to 5 years old) (3). However, there is a very slight difference in maximum serum concentration levels (3, 7). Therefore, it is important to consider the age of the child at prescription. Most research on ciprofloxacin pharmacology has been performed on children with Cystic Fibrosis (CF).
Aradottir et al. (1999) found the Mean±SD scores of peak serum concentration and peak time following oral administration as 3.7±1.4 mg/L and 2.5±1.8 hours, respectively. The same records after intravenous administration were 5.0±1.5 mg/L and 1.0±0.3 h (7). The mean oral bioavailability of ciprofloxacin was obtained 76% in the same study. Younger patients (about 68%) absorb ciprofloxacin less compared to older patients (95%). Due to the higher clearance of this drug in adults, smaller doses are recommended in children. Ciprofloxacin is eliminated through renal and hepatic pathways (2).
1.3. Indications
Some guidelines approve the use of fluoroquinolones in pediatrics when the first line of treatment is not effective and no alternative is available (8, 9). Normally, the fluoroquinolone of choice is ciprofloxacin, due to its higher safety profile in comparison with other fluoroquinolones. Ciprofloxacin is prescribed in children with serious infections when its benefit outweighs the risks (3). The potential indications in pediatrics that several European and American guidelines agreed on include respiratory infections with Pseudomonas (P) aeruginosa in cystic fibrosis, multidrug-resistant infections, particularly in immunocompromised patients and those under chemotherapy, multidrug-resistant Shigella and Salmonella infections, and complicated urinary tract infections (5, 8, 9).
The mentioned infections are the main indications of ciprofloxacin in pediatrics. Furthermore, in the model list of essential medicines for children by the World Health Organization (WHO) (2010), ciprofloxacin (250 mg tablets) is only indicated for Shigella infection treatment in children aged 1 to 17 years, but not neonates (5, 6). While there are newer generations of fluoroquinolones available in the first world countries and developing countries, the shortage of these antibiotics has remained an issue (10). However, ciprofloxacin is the most cost-effective, cost-benefit, and available antibiotic in fluoroquinolones that can be used in practice in such countries (3, 10). Furthermore, upon discharging the patients on intravenous antibiotics from the hospital, the regimen must change to an oral preparation when appropriate, which is more convenient for the patients and their parents (11).
2. Objective
Ciprofloxacin is an appropriate oral antibiotic applicable in many serious infections when there is no available alternative. There is no recent systemic review available to confirm the safety of this antibiotic in children. The present review aimed to collect evidence from clinical trials to evaluate the safety of using ciprofloxacin, focusing on arthropathy in children on a short-term basis (2 to 3 weeks).
3. Data Sources
The reviewed databases were Cochrane library, Trip database, ScienceDirect, PubMed, and Google Scholar as well as hand searching.
4. Study Selection
We have only considered clinical trials and the applied keywords that have been used were “ciprofloxacin”, “fluoroquinolones”, “pediatrics”, “arthropathy”, and “children under 18 years”.
5. Data Extraction
A pediatrician and a pharmacist screened the collected data. We only included the full text English clinical trials and those focusing on pediatrics. We have considered ciprofloxacin treatment, as well as prophylaxis for different conditions. The factors that were included in this study were patients’ age, underlying conditions, administration route, treatment duration, and potential side effects, with a special focus on arthropathy or cartilage toxicity. The studies that only involved adults were excluded from our research. Inclusion criteria consisted of the studies with the study patients aged ≥18 years and children with underlying conditions such as CF and malignancies.
Quinolones have been used for many years for treatment of various infectious diseases. Nalidixic acid is the first quinolone that was discovered in 1962 (1). However, due to its limitations, such as toxicity issues, the narrow spectrum of antibacterial activity and low serum bioavailability, it is no longer used in practice (1, 2). The fluorination of quinolones resulted in the production of fluoroquinolones. Ciprofloxacin is a second-generation fluoroquinolone, which was discovered in 1987 (1). Ciprofloxacin and other second-generation fluoroquinolones have superior properties compared to nalidixic acid, such as the broad-spectrum bactericidal activity, and appropriate tissue penetration (2). Considering the above-mentioned properties, these antibiotics are more suitable to be used in practice (1, 3).
1.1. Mechanism of action
Although the antimicrobial resistance is increasing, many bacteria are still sensitive to fluoroquinolones (4). Based on the evidence, ciprofloxacin is the safest and most effective antibiotic in children compared to other fluoroquinolones (3, 5). It is effective in a wide range of Gram-positive and Gram-negative pathogens and acts by interfering with DNA functioning and inhibiting DNA gyrase and topoisomerase IV in bacteria. These enzymes are essential for DNA replication, transcription, repair, and recombination (5, 6).
Fluoroquinolones have many favorable properties in the treatment of bacterial infections in adults and children (2, 4, 5). Despite their effectiveness, the use of these antibiotics in growing children were limited due to the debatable safety concerns over the risk of joint toxicity and other adverse side effects (2). In vitro study of juvenile animals of different species, such as dogs, mice, rats, and rabbits, have illustrated that the use of ciprofloxacin and other quinolones can cause arthropathy in weight-bearing joints (2, 3). No evidence has proved the definite joint injury induced by ciprofloxacin in children (5). Since adverse effects are inevitable, fluoroquinolones like ciprofloxacin are recommended when other options are not available or effective (5).
1.2. Pharmacokinetics
The pharmacological data available on the use of ciprofloxacin to treat hospitalized pediatric patients are scarce. The literature review suggests that the half-life of ciprofloxacin is significantly longer in infants (under 1 year old) than toddlers (1 to 5 years old) (3). However, there is a very slight difference in maximum serum concentration levels (3, 7). Therefore, it is important to consider the age of the child at prescription. Most research on ciprofloxacin pharmacology has been performed on children with Cystic Fibrosis (CF).
Aradottir et al. (1999) found the Mean±SD scores of peak serum concentration and peak time following oral administration as 3.7±1.4 mg/L and 2.5±1.8 hours, respectively. The same records after intravenous administration were 5.0±1.5 mg/L and 1.0±0.3 h (7). The mean oral bioavailability of ciprofloxacin was obtained 76% in the same study. Younger patients (about 68%) absorb ciprofloxacin less compared to older patients (95%). Due to the higher clearance of this drug in adults, smaller doses are recommended in children. Ciprofloxacin is eliminated through renal and hepatic pathways (2).
1.3. Indications
Some guidelines approve the use of fluoroquinolones in pediatrics when the first line of treatment is not effective and no alternative is available (8, 9). Normally, the fluoroquinolone of choice is ciprofloxacin, due to its higher safety profile in comparison with other fluoroquinolones. Ciprofloxacin is prescribed in children with serious infections when its benefit outweighs the risks (3). The potential indications in pediatrics that several European and American guidelines agreed on include respiratory infections with Pseudomonas (P) aeruginosa in cystic fibrosis, multidrug-resistant infections, particularly in immunocompromised patients and those under chemotherapy, multidrug-resistant Shigella and Salmonella infections, and complicated urinary tract infections (5, 8, 9).
The mentioned infections are the main indications of ciprofloxacin in pediatrics. Furthermore, in the model list of essential medicines for children by the World Health Organization (WHO) (2010), ciprofloxacin (250 mg tablets) is only indicated for Shigella infection treatment in children aged 1 to 17 years, but not neonates (5, 6). While there are newer generations of fluoroquinolones available in the first world countries and developing countries, the shortage of these antibiotics has remained an issue (10). However, ciprofloxacin is the most cost-effective, cost-benefit, and available antibiotic in fluoroquinolones that can be used in practice in such countries (3, 10). Furthermore, upon discharging the patients on intravenous antibiotics from the hospital, the regimen must change to an oral preparation when appropriate, which is more convenient for the patients and their parents (11).
2. Objective
Ciprofloxacin is an appropriate oral antibiotic applicable in many serious infections when there is no available alternative. There is no recent systemic review available to confirm the safety of this antibiotic in children. The present review aimed to collect evidence from clinical trials to evaluate the safety of using ciprofloxacin, focusing on arthropathy in children on a short-term basis (2 to 3 weeks).
3. Data Sources
The reviewed databases were Cochrane library, Trip database, ScienceDirect, PubMed, and Google Scholar as well as hand searching.
4. Study Selection
We have only considered clinical trials and the applied keywords that have been used were “ciprofloxacin”, “fluoroquinolones”, “pediatrics”, “arthropathy”, and “children under 18 years”.
5. Data Extraction
A pediatrician and a pharmacist screened the collected data. We only included the full text English clinical trials and those focusing on pediatrics. We have considered ciprofloxacin treatment, as well as prophylaxis for different conditions. The factors that were included in this study were patients’ age, underlying conditions, administration route, treatment duration, and potential side effects, with a special focus on arthropathy or cartilage toxicity. The studies that only involved adults were excluded from our research. Inclusion criteria consisted of the studies with the study patients aged ≥18 years and children with underlying conditions such as CF and malignancies.
5.1. Statistical analysis
The obtained data were analyzed by Comprehensive Meta-Analysis software (CMA.2). We used random or fixed-effect methods base on the heterogeneity of the results. Heterogeneity was checked by I2 index and Tau-squared. The standard continuity correction of 0.5 was applied to zero events. Publication bias was evaluated by the Begg’s test.
6. Results
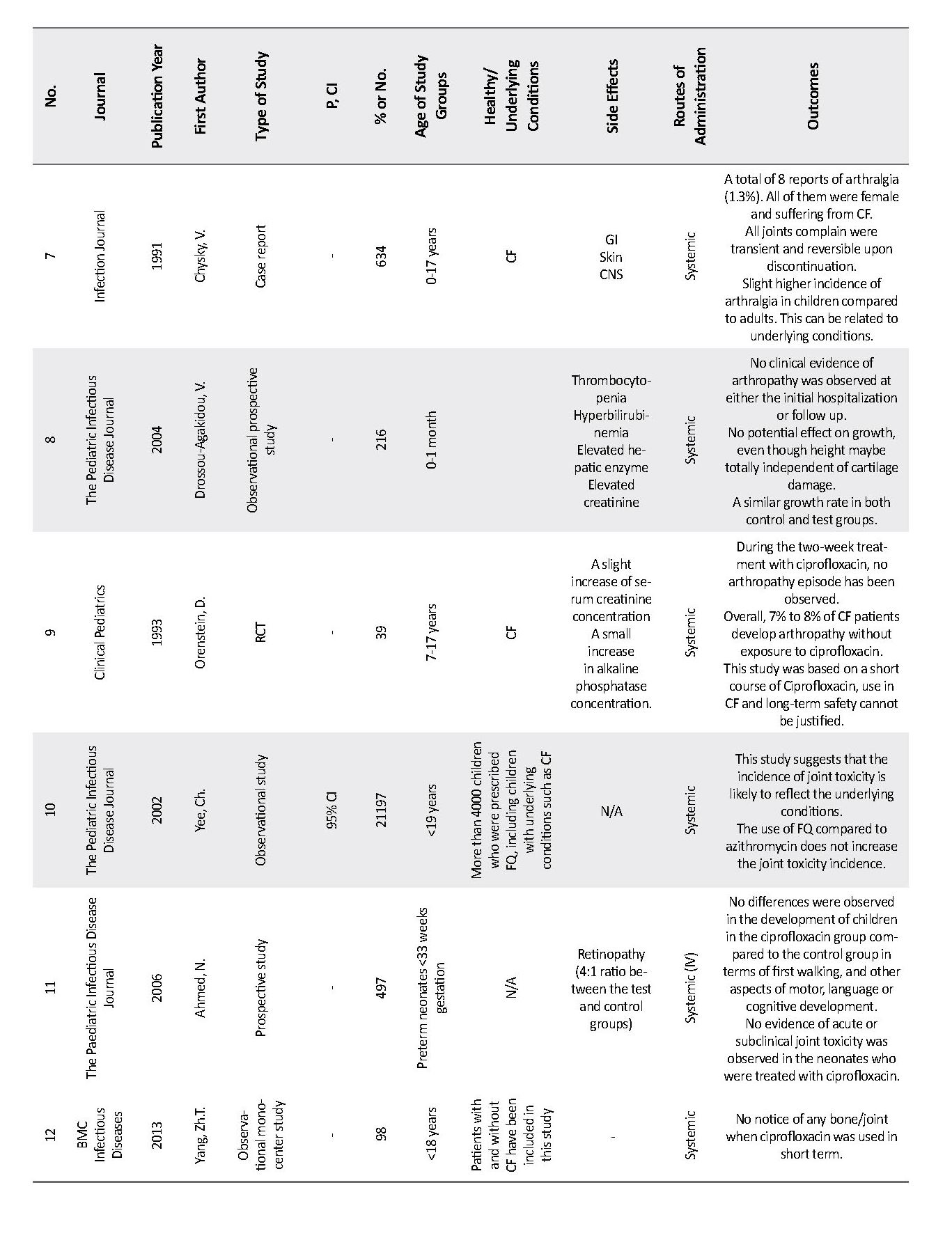
We identified a total of 109 studies (Figure 1). From those, 26 studies investigated the safety of ciprofloxacin use in children and most of them were randomized clinical trials and observational studies. Appendix 1 presents the details of these 26 studies. We collected data from clinical trials published from 1990 to 2018. In total, there were 16155 pediatric study patients who were exposed to ciprofloxacin as a treatment for a range of indications. Among those, there were 82 reports of musculoskeletal adverse reactions.
The obtained data were analyzed by Comprehensive Meta-Analysis software (CMA.2). We used random or fixed-effect methods base on the heterogeneity of the results. Heterogeneity was checked by I2 index and Tau-squared. The standard continuity correction of 0.5 was applied to zero events. Publication bias was evaluated by the Begg’s test.
6. Results
We identified a total of 109 studies (Figure 1). From those, 26 studies investigated the safety of ciprofloxacin use in children and most of them were randomized clinical trials and observational studies. Appendix 1 presents the details of these 26 studies. We collected data from clinical trials published from 1990 to 2018. In total, there were 16155 pediatric study patients who were exposed to ciprofloxacin as a treatment for a range of indications. Among those, there were 82 reports of musculoskeletal adverse reactions.
Some studies suggest the risk of arthropathy associated with the use of ciprofloxacin equal to less than 1% (about 0.82%), and some stated that the odds could be as high as 3.3% (3, 5). Appendix 1 presents that the ciprofloxacin use in neonates (under 1 month old) is less likely to cause arthropathy; however, there exists a possibility of under-diagnosis in this age group. The obtained data suggest that the youngest age of the child’s ciprofloxacin prescription was 3 days old (12, 13) (Tables 1 and 2).
7. Discussion
This systematic and meta-analysis review was conducted to evaluate the safety of using ciprofloxacin in children. We have pooled together and analyzed all the relevant articles, for drawing a conclusion in this regard. The studied reports in this review were clinical trials on the safety of ciprofloxacin and its suspected adverse events, especially arthropathy in children. Ciprofloxacin is not the first line recommended antibiotic in children (except for limited indications), based on joint toxicity reported by juvenile animal studies (1, 3). Ciprofloxacin is widely used in practice despite being contraindicated in this age category (3). We have investigated the arthropathy risk associated with ciprofloxacin and the effect of underlying conditions, treatment duration, age, and its dosage.
This systematic and meta-analysis review was conducted to evaluate the safety of using ciprofloxacin in children. We have pooled together and analyzed all the relevant articles, for drawing a conclusion in this regard. The studied reports in this review were clinical trials on the safety of ciprofloxacin and its suspected adverse events, especially arthropathy in children. Ciprofloxacin is not the first line recommended antibiotic in children (except for limited indications), based on joint toxicity reported by juvenile animal studies (1, 3). Ciprofloxacin is widely used in practice despite being contraindicated in this age category (3). We have investigated the arthropathy risk associated with ciprofloxacin and the effect of underlying conditions, treatment duration, age, and its dosage.
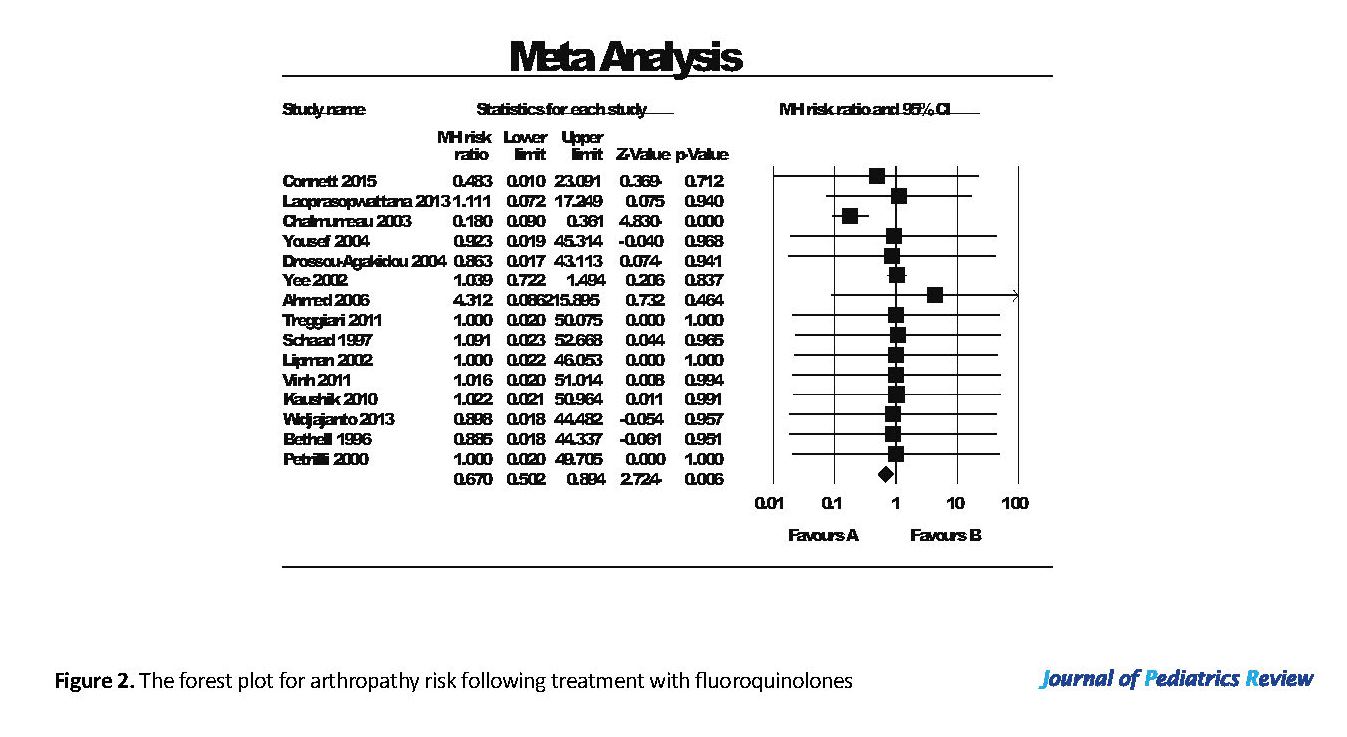
Fifteen out of 26 studies compared ciprofloxacin use to a control group; with 5 using a placebo in the control group. The rest were compared to other antibiotics (Figure 2). As shown in Figure 2, the decrease in arthropathy after treatment with fluoroquinolones compared to other antibiotic or placebo was 0.67 (95% CI: 0.5-0.89), which was statistically significant. The heterogeneity I2 index and tau-squared were 32.2% and 0.36, respectively. Egger’s test suggested no publication bias in the studies (P=0.729).
In conclusion, arthropathy has occurred either less frequently or equally in three studies with ciprofloxacin use, compared to placebo or other antibiotics (Figure 2). Laoprasopwattana et al. (2013) compared the use of ciprofloxacin to other antibiotics. They illustrated that arthropathy occurrence was the same in the ciprofloxacin-treated and control (placebo) groups (14).
Study number 17 reported that the incidence of joint dysfunction associated with the use of ciprofloxacin and ofloxacin was 0.82% (13 of 1539), compared to 0.78% for azithromycin (118 of 15073) (15). However, azithromycin usually has no adverse effects on joint, tendon or cartilage; thus, the incidence of arthropathy could be related to the underlying conditions in the studied children rather than the antibiotics use (15, 16).
Underlying conditions, therefore, can be an important factor in the occurrence of arthropathy in children. Studies number 7, 14 and 26, listed in Appendix 1 revealed a higher number of children with joint-related side effects after ciprofloxacin exposure. However, all those children had CF and two studies have reported that arthropathy occurred in 7% to 8% of pediatric patients with CF even without ciprofloxacin exposure (17, 18). Consequently, having CF as an underlying condition can increase the risk of arthralgia development in children (17-19). Ciprofloxacin is often used in children with CF compared to other diseases. Therefore, a higher incidence of ciprofloxacin-related arthropathy is observed in this group of patients.
The follow-up time for investigating the arthropathy occurrence or any other joint toxicity after ciprofloxacin exposure in these studies ranged from two weeks to three years (2, 19). Arthralgia occurred in about 50% of the musculoskeletal complications and ranged from the mild pain of joints to inflammation and severe pain in patients. In total, only 9 children out of 82 who developed musculoskeletal problems discontinued their treatment with ciprofloxacin. All cases of arthralgia were reversible and patients remained asymptomatic on long-term follow-up (2, 8, 12, 20, 21).
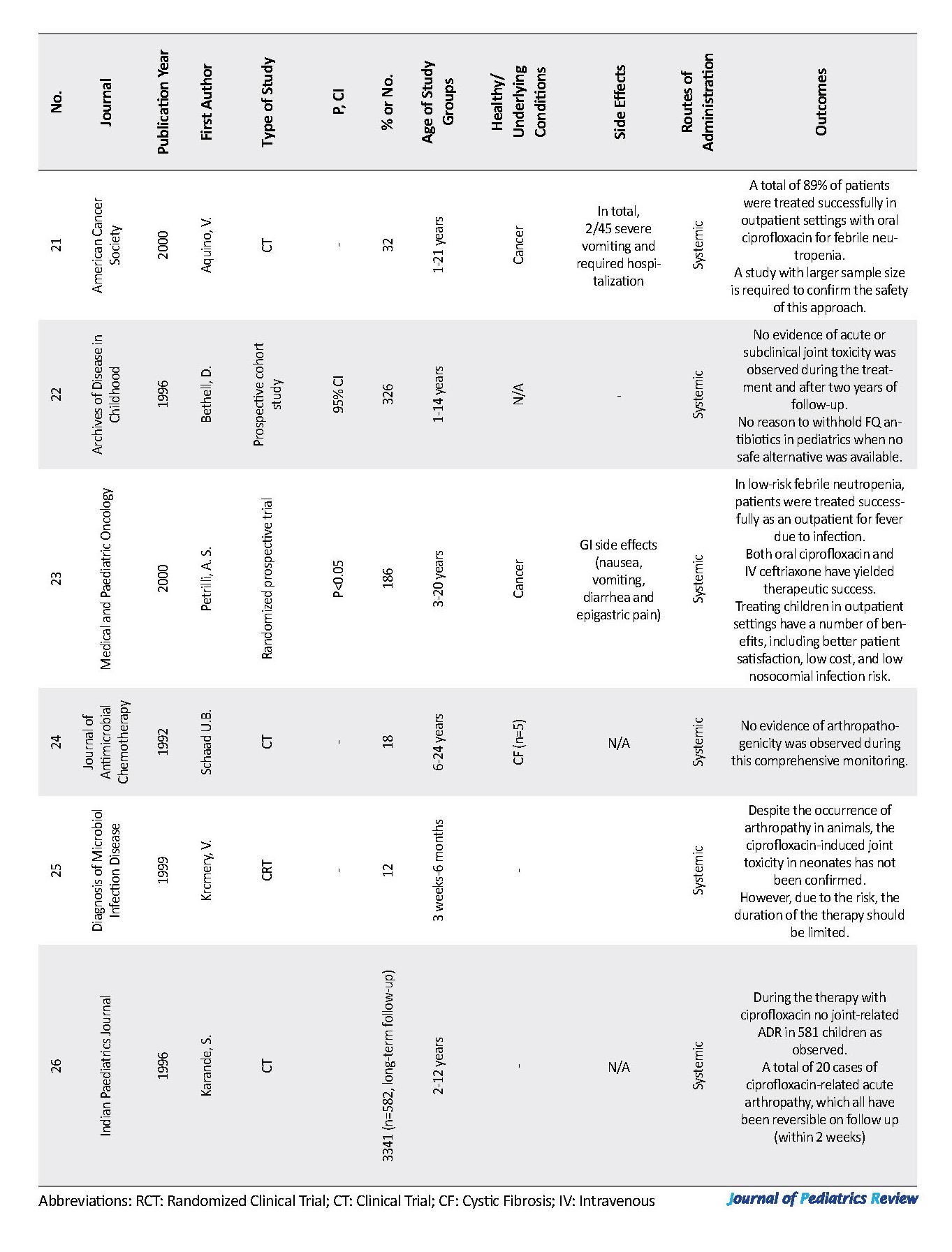
We have also investigated the potential link between the treatment duration and arthropathy risk. In 7 studies ciprofloxacin has been used on a short-term (2 to 3 weeks) basis and the reported joint toxicity was either very small or none, which could indicate the possible effect of treatment duration on the risk of developing arthropathy (2, 14, 15, 21-23). In most studies, the short-term use of ciprofloxacin has not been associated with arthropathy even after a long-term follow-up of the patients (15, 16, 21, 24-31).
Only two studies have observed arthropathy occurrence in short-term ciprofloxacin usage. However, the incidence rate was either similar (study No. 2) or smaller (study No. 3) compared to the control groups. This could indicate that ciprofloxacin exposure was not the only factor causing arthropathy in those patients (2, 3). Contrary to these findings, which recognized the treatment duration as a risk factor in arthropathy occurrence, the finding of only one study was not compatible with the others. Faghihi (2017) study suggests that the risk of joint toxicity or arthropathy is not related to the dose or duration of treatment (32). However, this study had some limitations, including small sample size, no control group, and missing the effect of underlying conditions on arthropathy occurrence in pediatrics.
Furthermore, we have studied the potential relationship between the children’s age and incidence of arthropathy. Of total 16155 children who studied in our dataset, 82 children developed joint problems after or during the ciprofloxacin treatment. The majority of these children were ≥6 years old. Only 7 of 82 affected children were under 6 years old, which indicates a much lower percentage compared to the older ones (8.5% vs. 91.5%) (8, 12, 15, 20). In addition, the use of ciprofloxacin in neonates up to one-year-old has not been associated with an increase in arthropathy incidence (16, 19). Therefore, ciprofloxacin use may be safe in younger ages, especially neonates.
In general, ciprofloxacin was administrated either orally or intravenously. The doses have been calculated according to the patient’ weight and ranged from 3.1 to 93.8 mg/kg/day and were normally prescribed in two divided doses. We failed to find any relationship between the ciprofloxacin dose and arthropathy occurrence. This finding is similar to the review by Adefurin et al. (2011) where they could not confirm this relationship (1). We have considered ciprofloxacin treatment as well as prophylaxis use in this study. According to the collected data, the prophylactic use of ciprofloxacin has not been associated with an increased risk of arthropathy (9, 14). There were no major limitations associated with this study. However, we excluded some articles in this study from Embase due to the access limitations.
8. Conclusions
Our systemic review identified a relatively low and reversible risk of arthropathy associated with ciprofloxacin use in children compared to other fluoroquinolones. This adverse event is less likely to occur in short-term use and the incidence could also be related to other factors such as patients’ underlying conditions and other medications used. Therefore, we suggest that when needed, this antibiotic can be prescribed safely in most children without the risk of joint toxicity. We suggest that future research is performed on patients with underlying conditions, especially those with CF to understand the effect of these conditions among children, with respect to arthropathy occurrence.
In conclusion, arthropathy has occurred either less frequently or equally in three studies with ciprofloxacin use, compared to placebo or other antibiotics (Figure 2). Laoprasopwattana et al. (2013) compared the use of ciprofloxacin to other antibiotics. They illustrated that arthropathy occurrence was the same in the ciprofloxacin-treated and control (placebo) groups (14).
Study number 17 reported that the incidence of joint dysfunction associated with the use of ciprofloxacin and ofloxacin was 0.82% (13 of 1539), compared to 0.78% for azithromycin (118 of 15073) (15). However, azithromycin usually has no adverse effects on joint, tendon or cartilage; thus, the incidence of arthropathy could be related to the underlying conditions in the studied children rather than the antibiotics use (15, 16).
Underlying conditions, therefore, can be an important factor in the occurrence of arthropathy in children. Studies number 7, 14 and 26, listed in Appendix 1 revealed a higher number of children with joint-related side effects after ciprofloxacin exposure. However, all those children had CF and two studies have reported that arthropathy occurred in 7% to 8% of pediatric patients with CF even without ciprofloxacin exposure (17, 18). Consequently, having CF as an underlying condition can increase the risk of arthralgia development in children (17-19). Ciprofloxacin is often used in children with CF compared to other diseases. Therefore, a higher incidence of ciprofloxacin-related arthropathy is observed in this group of patients.
The follow-up time for investigating the arthropathy occurrence or any other joint toxicity after ciprofloxacin exposure in these studies ranged from two weeks to three years (2, 19). Arthralgia occurred in about 50% of the musculoskeletal complications and ranged from the mild pain of joints to inflammation and severe pain in patients. In total, only 9 children out of 82 who developed musculoskeletal problems discontinued their treatment with ciprofloxacin. All cases of arthralgia were reversible and patients remained asymptomatic on long-term follow-up (2, 8, 12, 20, 21).
We have also investigated the potential link between the treatment duration and arthropathy risk. In 7 studies ciprofloxacin has been used on a short-term (2 to 3 weeks) basis and the reported joint toxicity was either very small or none, which could indicate the possible effect of treatment duration on the risk of developing arthropathy (2, 14, 15, 21-23). In most studies, the short-term use of ciprofloxacin has not been associated with arthropathy even after a long-term follow-up of the patients (15, 16, 21, 24-31).
Only two studies have observed arthropathy occurrence in short-term ciprofloxacin usage. However, the incidence rate was either similar (study No. 2) or smaller (study No. 3) compared to the control groups. This could indicate that ciprofloxacin exposure was not the only factor causing arthropathy in those patients (2, 3). Contrary to these findings, which recognized the treatment duration as a risk factor in arthropathy occurrence, the finding of only one study was not compatible with the others. Faghihi (2017) study suggests that the risk of joint toxicity or arthropathy is not related to the dose or duration of treatment (32). However, this study had some limitations, including small sample size, no control group, and missing the effect of underlying conditions on arthropathy occurrence in pediatrics.
Furthermore, we have studied the potential relationship between the children’s age and incidence of arthropathy. Of total 16155 children who studied in our dataset, 82 children developed joint problems after or during the ciprofloxacin treatment. The majority of these children were ≥6 years old. Only 7 of 82 affected children were under 6 years old, which indicates a much lower percentage compared to the older ones (8.5% vs. 91.5%) (8, 12, 15, 20). In addition, the use of ciprofloxacin in neonates up to one-year-old has not been associated with an increase in arthropathy incidence (16, 19). Therefore, ciprofloxacin use may be safe in younger ages, especially neonates.
In general, ciprofloxacin was administrated either orally or intravenously. The doses have been calculated according to the patient’ weight and ranged from 3.1 to 93.8 mg/kg/day and were normally prescribed in two divided doses. We failed to find any relationship between the ciprofloxacin dose and arthropathy occurrence. This finding is similar to the review by Adefurin et al. (2011) where they could not confirm this relationship (1). We have considered ciprofloxacin treatment as well as prophylaxis use in this study. According to the collected data, the prophylactic use of ciprofloxacin has not been associated with an increased risk of arthropathy (9, 14). There were no major limitations associated with this study. However, we excluded some articles in this study from Embase due to the access limitations.
8. Conclusions
Our systemic review identified a relatively low and reversible risk of arthropathy associated with ciprofloxacin use in children compared to other fluoroquinolones. This adverse event is less likely to occur in short-term use and the incidence could also be related to other factors such as patients’ underlying conditions and other medications used. Therefore, we suggest that when needed, this antibiotic can be prescribed safely in most children without the risk of joint toxicity. We suggest that future research is performed on patients with underlying conditions, especially those with CF to understand the effect of these conditions among children, with respect to arthropathy occurrence.
Ethical Considerations
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, Methodology, investigation and analysis: All authors; Writing-original draft: Baraneh Masoumi; and Supervision: Mohammad Sadegh Rezai, Gohar Eslami.
Conflict of interest
The authors declared no conflict of interest.
References
Adefurin A, Sammons H, Jacqz-Aigrain E, Choonara I. Ciprofloxacin safety in pediatrics: A systematic review. Archives of Disease in Childhood. 2011; 96(9):874-80. [DOI:10.1136/adc.2010.208843] [PMID] [PMCID]
Meesters K, Mauel R, Dhont E, Walle JV, De Bruyne P. Systemic fluoroquinolone prescriptions for hospitalized children in Belgium, results of a multicenter retrospective drug utilization study. BMC Infectious Diseases. 2018; 18(1):89. [DOI:10.1186/s12879-018-2994-z] [PMID] [PMCID]
Gendrel D, Moulin F. Fluoroquinolones in pediatrics. Pediatric Drugs. 2001; 3(5):365-77. [DOI:10.2165/00128072-200103050-00005] [PMID]
Bradley JS, Jackson MA, Committee on Infectious Diseases, American Academy of Pediatrics. The use of systemic and topical fluoroquinolones. Pediatrics. 2011; 128(4):e1034-45. [DOI:10.1542/peds.2011-1496] [PMID]
World Health Organization. Second meeting of the subcommittee of the expert committee on the selection and use of essential medicines. Geneva: World Health Organization; 2018.
Bourgeois T, Delezoide AL, Zhao W, Guimiot F, Adle-Biassette H, Durand E, et al. Safety study of Ciprofloxacin in newborn mice. Regulatory Toxicology and Pharmacology. 2016; 74:161-9. [DOI:10.1016/j.yrtph.2015.11.002] [PMID]
Aradottir E, Yogev R. The use of fluoroquinolones in paediatrics- A reassessment. Seminars in Pediatric Infectious Diseases 1999; 10(1):31-7. [DOI:10.1016/S1045-1870(99)80007-7]
Jung C, Shamdasani S. Antimicrobial agents and related therapy. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: Report of the Committee on Infectious Diseases. Itasca, Illinois: American Academy of Pediatrics; 2009.
Newland JG, Hersh AL. Purpose and design of antimicrobial stewardship programs in pediatrics. The Pediatric Infectious Disease Journal. 2010; 29(9):862-3. [DOI:10.1097/INF.0b013e3181ef2507] [PMID]
Orenstein DM, Pattishall EN, Noyes BE, Kurland G, Hartigan ER, Yu VL. Safety of ciprofloxacin in children with cystic fibrosis. Clinical Pediatrics. 1993; 32(8):504-6. [DOI:10.1177/000992289303200811] [PMID]
Connett GJ, Pike KC, Legg JP, Cathie K, Dewar A, Foote K, et al. Ciprofloxacin during upper respiratory tract infections to reduce Pseudomonas aeruginosa infection in pediatric cystic fibrosis: A pilot study. Therapeutic Advances in Respiratory Disease. 2015; 9(6):272-80. [DOI:10.1177/1753465815601571] [PMID]
Drossou-Agakidou V, Roilides E, Papakyriakidou-Koliouska P, Agakidis C, Nikolaides N, Sarafidis K, et al. Use of ciprofloxacin in neonatal sepsis: Lack of adverse effects up to one year. The Pediatric Infectious Disease Journal. 2004; 23(4):346-9. [DOI:10.1097/00006454-200404000-00014] [PMID]
Yang ZT, Zahar JR, Méchaï F, Postaire M, Blanot S, Balfagon-Viel S, et al. Current ciprofloxacin usage in children hospitalized in a referral hospital in Paris. BMC Infectious Diseases. 2013; 13(1):245-8. [DOI:10.1186/1471-2334-13-245] [PMID] [PMCID]
Laoprasopwattana K, Khwanna T, Suwankeeree P, Sujjanunt T, Tunyapanit W, Chelae S. Ciprofloxacin reduces occurrence of fever in children with acute leukemia who develop neutropenia during chemotherapy. The Pediatric Infectious Disease Journal. 2013; 32(3):e94-8. [PMID]
Lipman J, Gous A, Mathivha L, Tshukutsoane S, Scribante J, Hon H, et al. Ciprofloxacin pharmacokinetic profiles in pediatric sepsis: How much ciprofloxacin is enough. Intensive Care Medicine. 2002; 28(4):493-500. [DOI:10.1007/s00134-002-1212-y] [PMID]
Treggiari M, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kronmal R, Ramsey B, et al. Comparative efficacy and safety of four randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Archives of Pediatrics and Adolescent Medicine. 2011; 165(9):847-56. [DOI:10.1001/archpediatrics.2011.136] [PMID] [PMCID]
Black A, Redmond AO, Steen HJ, Oborska IT. Tolerance and safety of ciprofloxacin in pediatric patients. Journal of Antimicrobial Chemotherapy. 1990; 26(suppl. F):25-9. [DOI:10.1093/jac/26.suppl_F.25] [PMID]
Schaad UB, Sander E, Wedgwood J, Schaffner T. Morphologic studies for skeletal toxicity after prolonged ciprofloxacin therapy in two juvenile cystic fibrosis patients. The Pediatric Infectious Disease Journal. 1992; 11(12):1047-9. [DOI:10.1097/00006454-199211120-00011] [PMID]
Ahmed AN, Khan NZ, Saha SK, Chowdhury MA, Muslima H, Law P, et al. Ciprofloxacin treatment in preterm neonates in Bangladesh: Lack of effects on growth and development. The Pediatric Infectious Disease Journal. 2006; 25(12):1137-41. [DOI:10.1097/01.inf.0000245105.99934.5f] [PMID]
Yee CL, Duffy C, Gerbino PG, Stryker S, Noel GJ. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. The Pediatric Infectious Disease Journal. 2002; 21(6):525-9. [DOI:10.1097/00006454-200206000-00009] [PMID]
Schaad UB. Use of quinolones in pediatrics. European Journal of Clinical Microbiology and Infectious Diseases. 1991; 10(4):355-60. [DOI:10.1007/BF01967011] [PMID]
Chalumeau M, Tonnelier S, d’Athis P, Tréluyer JM, Gendrel D, Bréart G, et al. Fluoroquinolone safety in pediatric patients: A prospective, multicenter, comparative cohort study in France. Pediatrics. 2003; 111(6):e714-9. [DOI:10.1542/peds.111.6.e714] [PMID]
Yousef AA, Fryer CJ, Chedid FD, Abbas AA, Felimban SK, Khattab TM. A pilot study of prophylactic ciprofloxacin during delayed intensification in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2004; 43(6):637-43. [DOI:10.1002/pbc.20065] [PMID]
Singh UK, Sinha RK, Prasad B, Chakrabarti B, Sharma SK. Ciprofloxacin in children: Is arthropathy a limitation. The Indian Journal of Pediatrics. 2000; 67(5):386-7. [DOI:10.1007/BF02820695] [PMID]
Vinh H, Anh VT, Anh ND, Campbell JI, Hoang NV, Nga TV, et al. A multi-center randomized trial to assess the efficacy of gatifloxacin versus ciprofloxacin for the treatment of shigellosis in Vietnamese children. PLoS Neglected Tropical Diseases. 2011; 5(8):e1264. [DOI:10.1371/journal.pntd.0001264] [PMID] [PMCID]
Kaushik JS, Gupta P, Faridi MM, Das S. Single dose azithromycin versus ciprofloxacin for cholera in children: A randomized controlled trial. Indian Pediatrics. 2010; 47(4):309-15. [DOI:10.1007/s13312-010-0059-5] [PMID]
Widjajanto PH, Sumadiono S, Cloos J, Purwanto I, Sutaryo S, Veerman AJ. Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia. Journal of Blood Medicine. 2013; 4:1-9. [DOI:10.2147/JBM.S33906] [PMID] [PMCID]
Aquino VM, Herrera L, Sandler ES, Buchanan GR. Feasibility of oral ciprofloxacin for the outpatient management of febrile neutropenia in selected children with cancer. Cancer. 2000; 88(7):1710-4. [DOI:10.1002/(SICI)1097-0142(20000401)88:73.0.CO;2-1]
Bethell DB, Hien TT, Phi LT, Day NP, Vinh H, Duong NM, et al. Effects on growth of single short courses of fluoroquinolones. Archives of Disease in Childhood. 1996; 74(1):44-6. [DOI:10.1136/adc.74.1.44] [PMID] [PMCID]
Krcmery Jr V, Filka J, Uher J, Kurak H, Sagat T, Tuharsky J, et al. Ciprofloxacin in treatment of nosocomial meningitis in neonates and in infants: Report of 12 Cases and. Diagnostic Microbiology and Infectious Disease. 1999; 35(1):75-80. [DOI:10.1016/S0732-8893(99)00052-8]
Karande S, Kshirsagar NA. Ciprofloxacin use: Acute arthropathy and long-term follow up. Indian Pediatrics. 1996; 33(11):910-6. [PMID]
Faghihi T, Tekmehdash LY, Radfar M, Gholami K. Ciprofloxacin use in hospitalized children: Approved or off-label. Journal of Research in Pharmacy Practice. 2017; 6(4):193-8. [DOI:10.4103/jrpp.JRPP_17_27] [PMID] [PMCID]
Compliance with ethical guidelines
All ethical principles were considered in this article. The participants were informed about the purpose of the research and its implementation stages; they were also assured about the confidentiality of their information; Moreover, They were allowed to leave the study whenever they wish, and if desired, the results of the research would be available to them.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Authors contributions
Conceptualization, Methodology, investigation and analysis: All authors; Writing-original draft: Baraneh Masoumi; and Supervision: Mohammad Sadegh Rezai, Gohar Eslami.
Conflict of interest
The authors declared no conflict of interest.
References
Adefurin A, Sammons H, Jacqz-Aigrain E, Choonara I. Ciprofloxacin safety in pediatrics: A systematic review. Archives of Disease in Childhood. 2011; 96(9):874-80. [DOI:10.1136/adc.2010.208843] [PMID] [PMCID]
Meesters K, Mauel R, Dhont E, Walle JV, De Bruyne P. Systemic fluoroquinolone prescriptions for hospitalized children in Belgium, results of a multicenter retrospective drug utilization study. BMC Infectious Diseases. 2018; 18(1):89. [DOI:10.1186/s12879-018-2994-z] [PMID] [PMCID]
Gendrel D, Moulin F. Fluoroquinolones in pediatrics. Pediatric Drugs. 2001; 3(5):365-77. [DOI:10.2165/00128072-200103050-00005] [PMID]
Bradley JS, Jackson MA, Committee on Infectious Diseases, American Academy of Pediatrics. The use of systemic and topical fluoroquinolones. Pediatrics. 2011; 128(4):e1034-45. [DOI:10.1542/peds.2011-1496] [PMID]
World Health Organization. Second meeting of the subcommittee of the expert committee on the selection and use of essential medicines. Geneva: World Health Organization; 2018.
Bourgeois T, Delezoide AL, Zhao W, Guimiot F, Adle-Biassette H, Durand E, et al. Safety study of Ciprofloxacin in newborn mice. Regulatory Toxicology and Pharmacology. 2016; 74:161-9. [DOI:10.1016/j.yrtph.2015.11.002] [PMID]
Aradottir E, Yogev R. The use of fluoroquinolones in paediatrics- A reassessment. Seminars in Pediatric Infectious Diseases 1999; 10(1):31-7. [DOI:10.1016/S1045-1870(99)80007-7]
Jung C, Shamdasani S. Antimicrobial agents and related therapy. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, editors. Red Book: Report of the Committee on Infectious Diseases. Itasca, Illinois: American Academy of Pediatrics; 2009.
Newland JG, Hersh AL. Purpose and design of antimicrobial stewardship programs in pediatrics. The Pediatric Infectious Disease Journal. 2010; 29(9):862-3. [DOI:10.1097/INF.0b013e3181ef2507] [PMID]
Orenstein DM, Pattishall EN, Noyes BE, Kurland G, Hartigan ER, Yu VL. Safety of ciprofloxacin in children with cystic fibrosis. Clinical Pediatrics. 1993; 32(8):504-6. [DOI:10.1177/000992289303200811] [PMID]
Connett GJ, Pike KC, Legg JP, Cathie K, Dewar A, Foote K, et al. Ciprofloxacin during upper respiratory tract infections to reduce Pseudomonas aeruginosa infection in pediatric cystic fibrosis: A pilot study. Therapeutic Advances in Respiratory Disease. 2015; 9(6):272-80. [DOI:10.1177/1753465815601571] [PMID]
Drossou-Agakidou V, Roilides E, Papakyriakidou-Koliouska P, Agakidis C, Nikolaides N, Sarafidis K, et al. Use of ciprofloxacin in neonatal sepsis: Lack of adverse effects up to one year. The Pediatric Infectious Disease Journal. 2004; 23(4):346-9. [DOI:10.1097/00006454-200404000-00014] [PMID]
Yang ZT, Zahar JR, Méchaï F, Postaire M, Blanot S, Balfagon-Viel S, et al. Current ciprofloxacin usage in children hospitalized in a referral hospital in Paris. BMC Infectious Diseases. 2013; 13(1):245-8. [DOI:10.1186/1471-2334-13-245] [PMID] [PMCID]
Laoprasopwattana K, Khwanna T, Suwankeeree P, Sujjanunt T, Tunyapanit W, Chelae S. Ciprofloxacin reduces occurrence of fever in children with acute leukemia who develop neutropenia during chemotherapy. The Pediatric Infectious Disease Journal. 2013; 32(3):e94-8. [PMID]
Lipman J, Gous A, Mathivha L, Tshukutsoane S, Scribante J, Hon H, et al. Ciprofloxacin pharmacokinetic profiles in pediatric sepsis: How much ciprofloxacin is enough. Intensive Care Medicine. 2002; 28(4):493-500. [DOI:10.1007/s00134-002-1212-y] [PMID]
Treggiari M, Retsch-Bogart G, Mayer-Hamblett N, Khan U, Kronmal R, Ramsey B, et al. Comparative efficacy and safety of four randomized regimens to treat early Pseudomonas aeruginosa infection in children with cystic fibrosis. Archives of Pediatrics and Adolescent Medicine. 2011; 165(9):847-56. [DOI:10.1001/archpediatrics.2011.136] [PMID] [PMCID]
Black A, Redmond AO, Steen HJ, Oborska IT. Tolerance and safety of ciprofloxacin in pediatric patients. Journal of Antimicrobial Chemotherapy. 1990; 26(suppl. F):25-9. [DOI:10.1093/jac/26.suppl_F.25] [PMID]
Schaad UB, Sander E, Wedgwood J, Schaffner T. Morphologic studies for skeletal toxicity after prolonged ciprofloxacin therapy in two juvenile cystic fibrosis patients. The Pediatric Infectious Disease Journal. 1992; 11(12):1047-9. [DOI:10.1097/00006454-199211120-00011] [PMID]
Ahmed AN, Khan NZ, Saha SK, Chowdhury MA, Muslima H, Law P, et al. Ciprofloxacin treatment in preterm neonates in Bangladesh: Lack of effects on growth and development. The Pediatric Infectious Disease Journal. 2006; 25(12):1137-41. [DOI:10.1097/01.inf.0000245105.99934.5f] [PMID]
Yee CL, Duffy C, Gerbino PG, Stryker S, Noel GJ. Tendon or joint disorders in children after treatment with fluoroquinolones or azithromycin. The Pediatric Infectious Disease Journal. 2002; 21(6):525-9. [DOI:10.1097/00006454-200206000-00009] [PMID]
Schaad UB. Use of quinolones in pediatrics. European Journal of Clinical Microbiology and Infectious Diseases. 1991; 10(4):355-60. [DOI:10.1007/BF01967011] [PMID]
Chalumeau M, Tonnelier S, d’Athis P, Tréluyer JM, Gendrel D, Bréart G, et al. Fluoroquinolone safety in pediatric patients: A prospective, multicenter, comparative cohort study in France. Pediatrics. 2003; 111(6):e714-9. [DOI:10.1542/peds.111.6.e714] [PMID]
Yousef AA, Fryer CJ, Chedid FD, Abbas AA, Felimban SK, Khattab TM. A pilot study of prophylactic ciprofloxacin during delayed intensification in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer. 2004; 43(6):637-43. [DOI:10.1002/pbc.20065] [PMID]
Singh UK, Sinha RK, Prasad B, Chakrabarti B, Sharma SK. Ciprofloxacin in children: Is arthropathy a limitation. The Indian Journal of Pediatrics. 2000; 67(5):386-7. [DOI:10.1007/BF02820695] [PMID]
Vinh H, Anh VT, Anh ND, Campbell JI, Hoang NV, Nga TV, et al. A multi-center randomized trial to assess the efficacy of gatifloxacin versus ciprofloxacin for the treatment of shigellosis in Vietnamese children. PLoS Neglected Tropical Diseases. 2011; 5(8):e1264. [DOI:10.1371/journal.pntd.0001264] [PMID] [PMCID]
Kaushik JS, Gupta P, Faridi MM, Das S. Single dose azithromycin versus ciprofloxacin for cholera in children: A randomized controlled trial. Indian Pediatrics. 2010; 47(4):309-15. [DOI:10.1007/s13312-010-0059-5] [PMID]
Widjajanto PH, Sumadiono S, Cloos J, Purwanto I, Sutaryo S, Veerman AJ. Randomized double blind trial of ciprofloxacin prophylaxis during induction treatment in childhood acute lymphoblastic leukemia in the WK-ALL protocol in Indonesia. Journal of Blood Medicine. 2013; 4:1-9. [DOI:10.2147/JBM.S33906] [PMID] [PMCID]
Aquino VM, Herrera L, Sandler ES, Buchanan GR. Feasibility of oral ciprofloxacin for the outpatient management of febrile neutropenia in selected children with cancer. Cancer. 2000; 88(7):1710-4. [DOI:10.1002/(SICI)1097-0142(20000401)88:73.0.CO;2-1]
Bethell DB, Hien TT, Phi LT, Day NP, Vinh H, Duong NM, et al. Effects on growth of single short courses of fluoroquinolones. Archives of Disease in Childhood. 1996; 74(1):44-6. [DOI:10.1136/adc.74.1.44] [PMID] [PMCID]
Krcmery Jr V, Filka J, Uher J, Kurak H, Sagat T, Tuharsky J, et al. Ciprofloxacin in treatment of nosocomial meningitis in neonates and in infants: Report of 12 Cases and. Diagnostic Microbiology and Infectious Disease. 1999; 35(1):75-80. [DOI:10.1016/S0732-8893(99)00052-8]
Karande S, Kshirsagar NA. Ciprofloxacin use: Acute arthropathy and long-term follow up. Indian Pediatrics. 1996; 33(11):910-6. [PMID]
Faghihi T, Tekmehdash LY, Radfar M, Gholami K. Ciprofloxacin use in hospitalized children: Approved or off-label. Journal of Research in Pharmacy Practice. 2017; 6(4):193-8. [DOI:10.4103/jrpp.JRPP_17_27] [PMID] [PMCID]
Type of Study: Meta-analysis Review |
Subject:
Pediatric infection disease
Received: 2018/08/28 | Accepted: 2018/10/17 | Published: 2019/07/1
Received: 2018/08/28 | Accepted: 2018/10/17 | Published: 2019/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |