Volume 9, Issue 3 (7-2021)
J. Pediatr. Rev 2021, 9(3): 255-262 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Golbabaei A, Memarian S, Naemi M, Rastgar A, Gharib B, Majnoon M T. Cardiac Involvement in a Fetus With Vein of Galen Aneurysmal Malformation, Diagnosis, and Treatment: A Case Report. J. Pediatr. Rev 2021; 9 (3) :255-262
URL: http://jpr.mazums.ac.ir/article-1-306-en.html
URL: http://jpr.mazums.ac.ir/article-1-306-en.html
Alireza Golbabaei1 

 , Sara Memarian2
, Sara Memarian2 

 , Mahsa Naemi3
, Mahsa Naemi3 

 , Azade Rastgar4
, Azade Rastgar4 
 , Behdad Gharib2
, Behdad Gharib2 

 , Mohammad Taghi Majnoon *5
, Mohammad Taghi Majnoon *5 




 , Sara Memarian2
, Sara Memarian2 

 , Mahsa Naemi3
, Mahsa Naemi3 

 , Azade Rastgar4
, Azade Rastgar4 
 , Behdad Gharib2
, Behdad Gharib2 

 , Mohammad Taghi Majnoon *5
, Mohammad Taghi Majnoon *5 


1- Department of Perinatology and Fetal Cardiology, Shariati Hospital, Tehran University of Medical Sciences, Iran.
2- Department of Pediatrics, Children’s Medical Center, Tehran University of Medical Sciences, Iran.
3- Department of Gynecology, Shariati Hospital, Tehran University of Medical Sciences, Iran.
4- Department of Gynecology, Shariati Hospital, Karaj, Iran.
5- Department of Pediatrics, Children’s Medical Center, Tehran University of Medical Sciences, Iran. ,107majnoon@gmail.com
2- Department of Pediatrics, Children’s Medical Center, Tehran University of Medical Sciences, Iran.
3- Department of Gynecology, Shariati Hospital, Tehran University of Medical Sciences, Iran.
4- Department of Gynecology, Shariati Hospital, Karaj, Iran.
5- Department of Pediatrics, Children’s Medical Center, Tehran University of Medical Sciences, Iran. ,
Full-Text [PDF 1858 kb]
(1601 Downloads)
| Abstract (HTML) (4102 Views)
Full-Text: (1576 Views)
1. Introduction
Vein of Galen Aneurysmal Malformations (VGAMs) are rare congenital vasculature abnormalities and the most common form of symptomatic cerebrovascular anomaly in neonates and infants. VGAMs prevalence is less than 1 in 25000 live deliveries, which can cause high morbidity and mortality, particularly in neonates and then infants and older children. This abnormality was first described in 1895. The exact incidence is unknown, but the prevalence is 1% of all neonatal intracranial vasculature malformations. This congenital malformation develops during weeks 6th–11th of gestational age. This anomaly is characterized by the connection between intracranial vessels (typically the posterior choroid arteries or the anterior cerebral arteries) and the deep venous system (the Vein of Galen), which leads to a communication between the cerebral arteries and the deep veins of the brain. The direct shunting of cerebral arterial flow into the draining vein (arteriovenous shunting of blood) makes it markedly enlarged and aneurysmal. Compression of the developing brain by the enlarged vessel may lead to cerebral hypoplasia, atrophy, or hydrocephalus. Developmentally, the dilated vein of Galen arises from the persistence of the embryonic median prosencephalic vein of Markowski [1].
The prognosis of neonates and infants with the vein of Galen malformations is poor, with a postnatal mortality rate of 50% and a high risk of neurologic complications. Pathophysiologically, the size of the fistula determines the amount of blood shunting and the time of presentation. Also, it presents in several different ways, and the most common feature includes the high output cardiac failure and cyanosis in the newborn that can be mistaken for a cyanotic congenital heart anomaly. In a neonate with congestive heart failure and a structurally normal heart, the diagnosis of an intracranial arteriovenous malformation should be noticed [1, 2, 3, 4, 5, 6, 7, 8]. Diagnosis of this anomaly is possible by ultrasonography; however, there is a paucity of information regarding the specific findings of VGAMs [1].
There is often a delay in diagnosis as the clinical presentation can mimic cyanotic congenital heart disease or persistent pulmonary hypertension of the newborn. Treatment in the neonatal period includes supportive care in neonatal intensive care. Without intervention, the mortality rate among infants with VGAM is close to 100%. Endovascular embolization revolutionized the management of VGAM. Endovascular treatment is the treatment of choice for VGAM presenting in infancy with heart failure and significantly reduces its morbidity and mortality. Embolization, both of feeding arteries and draining veins, can considerably reduce aneurysmal blood flow. Overall prognosis in the short and the long term has improved but is still not ideal for this group of neonates [2, 4, 7, 8, 9, 10].
This study highlights the identification of this anomaly in the pregnancy period. We report a severe case of VGAM with significant secondary cardiac involvement. It eventually led to the death of the baby after birth.
2. Case Presentation
A 37-year-old pregnant female (Gravid 4, para 0, abort 2, living child 1) was referred to our perinatology clinic at her 28th week of pregnancy with an ultrasound report of a vasculature aneurysmal dilation in the brain of the fetus, which suggested a malformation of Galen, and cardiomegaly. No additional maternal risk factors were detected.
In the fetal echocardiography, evidence of skin edema and pleural effusion was detected, which indicated the diagnosis of hydrops fetalis and orientation and position of the heart was normal (levocardia and levoposition). The apical 4-chamber view showed right ventricular and atrial enlargement (Figure 1).
.png)
Short axis and 3-vessel view showed dilation of the pulmonary artery (Figure 2).
.png)
In the axial view, a high flow and prominent superior vena cava and left innominate vein were detected (Figures 3 & 4), and the bicaval view showed superior vena cava dilation (Figure 5).
.png)
.png)
.png)
The pregnancy continued until the 38th week of gestation, and soon after the birth, the baby was intubated because of significant cyanosis. Transthoracic echocardiography showed right heart (right atrium and ventricle) enlargement (Figure 6) and prominent superior vena cava with high flow and right to left shunt through the foramen oval (Figure 7).
.png)
.png)
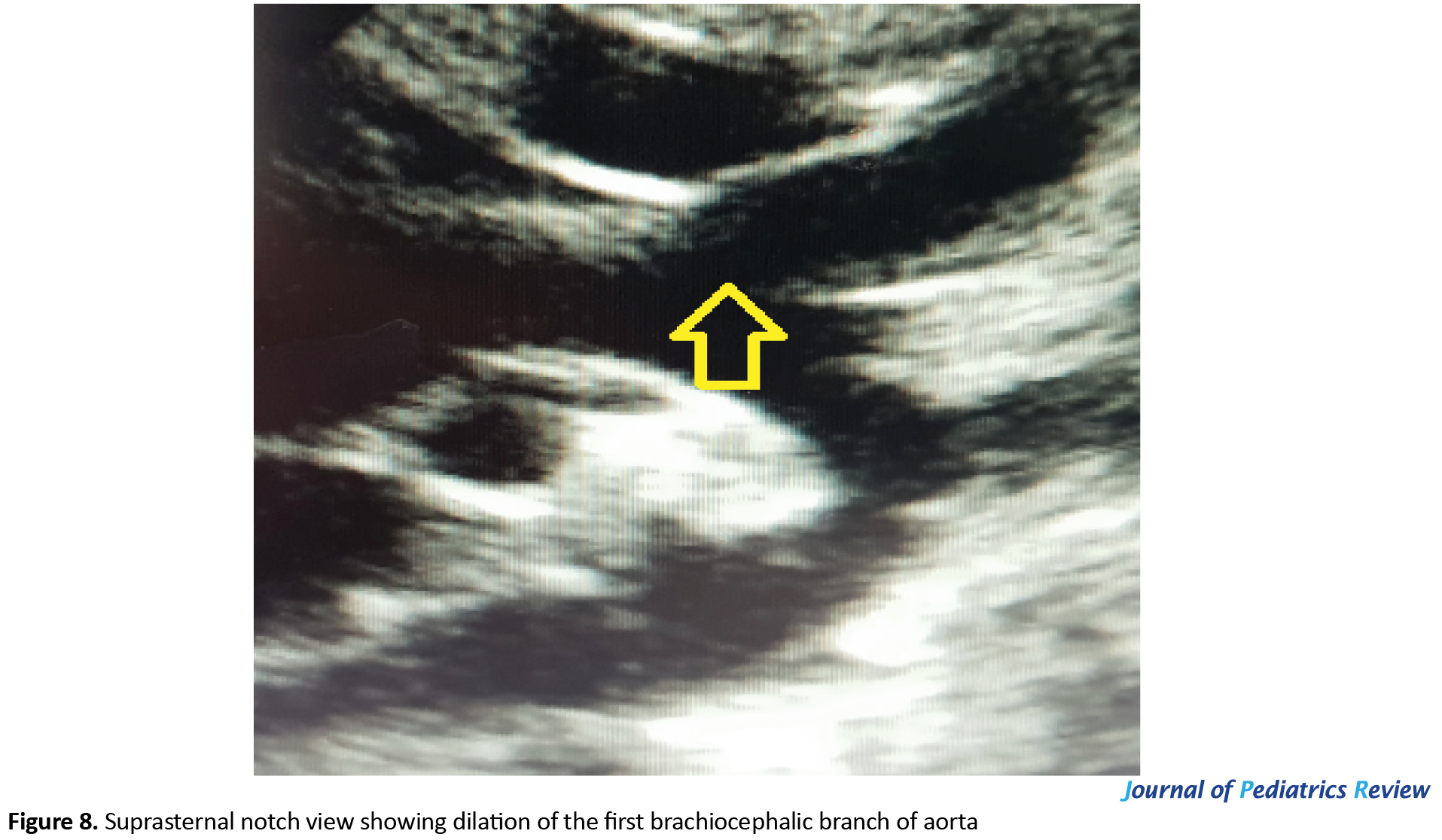
Brachiocephalic arterial branches in suprasternal notch view were enlarged (Figure 8).
The neonate was treated with medical treatment, including milrinone (as a pulmonary vasodilator) and dopamine infusions (with renal dose to support renal blood flow). The patient was taken to another hospital for cerebral vascular embolization, and the embolization of this brain vascular anomaly was performed, but the patient became ill and died immediately after this procedure.
3. Discussion
Vein of Galen aneurysmal malformations are the most frequent arteriovenous malformations in infants and fetuses that result in significant dilation of the great vein of Galen. It commonly presents in the neonatal period, although it may present in early childhood. The clinical presentation of VGM varies with age. Neonates usually have multiple fistulae and present with high-output heart failure. Usually, the signs of severe heart failure develop shortly after birth. Often cyanosis is present, which is also suggestive of persistent pulmonary hypertension. Infants and younger children with a single fistula are presented with seizures, hydrocephalus, distention of scalp veins, and failure to thrive. Older children and adults often present with neurological symptoms, including headaches and seizures. VGAM occurs when the vein of Galen receives (directly or indirectly) an arterial communication from one or more major intracranial arteries [2, 5, 7, 9].
VGAM often results in high-output heart failure. The increased preload caused by the intracranial vasculature anomaly leads to the significant dilation of the superior vena cava. With the progression of the disease, heart failure, as a usual finding, may develop, and the fetal right-side heart will be dilated. Heart failure is due to the size of the arteriovenous shunt, which can steal 80% or more of the cardiac output. It is also the most common cause of death in such patients. Tricuspid regurgitation, cardiomegaly, and fetal hydrops may ensue [1, 2, 5, 9]. Dilation of superior vena cava and right heart enlargement was prominent in our patient.
The treatment of vein of Galen aneurysmal malformation includes initial cardiovascular stabilization. This is achieved by reducing systemic and pulmonary vasculature resistance and improving systemic perfusion and myocardial function. In most cases, a vasodilator agent, either alone or in combination with inotropic agents, is needed. Milrinone and inhaled nitric oxide have been found beneficial. Some patients can benefit from treatment with prostaglandin E1. Without interventional neuroradiology and embolization, the prognosis is poor for neonates with early severe cardiac failure. Severe heart failure rapidly progresses to multiorgan failure, severe lactic acidosis, and death. Before new imaging technologies and endovascular treatment, Galen malformations were fatal in 90% of patients under 1 month of age, and half of those were between 1 month and 1 year old. Nowadays, treatment of this lesion has been revolutionized by transcatheter embolization techniques, with 70%–80% survival, and surgical intervention does not have an important role. Endovascular embolization is the preferred treatment modality, preferably after the age of 5 to 6 months. A good outcome is to be expected when treatment is performed before the significant brain injury. A recent systematic meta-analysis of 667 patients performed by Yan j et al. revealed that 68% of the patients, including neonates (44%), infants (41%), children, and adults (12%), had a good outcome after endovascular embolization [11]. Similar results were found in a review by Khullar et al., who reported 84% of good to the fair outcome and a 15% mortality rate among 337 patients undergoing endovascular treatment during 2001 and 2010 [12]. The development of intravascular occlusion devices has shown to be safer and more effective. A study by Ciricillo et al. showed that all six neonates with VGAM who underwent direct microsurgery died while six of the eight who were treated with endovascular embolization survived [13]. Lasjaunias et al. [14] reported a mortality rate of 13% in 36 cases of VGAMs treated with endovascular embolization. However, endovascular treatment of neonates and young infants has not been without complications, including perforation of the thin-walled aneurysm or the feeding vessels [3, 4, 7, 9].
In the uterus, heart failure is rare because the low resistance of the cerebral Arteriovenous Malformation (AVM) is balanced by the low resistance of the uteroplacental unit, allowing perfusion of the peripheries. With the loss of the placenta at birth, up to 70% of cardiac output is directed to the cerebral circulation. Pulmonary arterial pressure remains high, and the ductus arteriosus remains open, directing right ventricular output through the patent ductus arteriosus and into the descending aorta. Therefore the right ventricle and pulmonary artery have become dilated. Severe heart failure associated with VGAM appears to be more severe than heart failure associated with intracardiac anomalies. As a result, stabilization of these neonates before neuro-intervention or neurosurgery is difficult, and cardiac failure is often resistant to treatment. In critically ill patients, medical treatment with milrinone improves myocardial function and produces systemic and pulmonary vasodilatation [4, 5, 9].
Pulmonary hypertension of the newborn associated with Galen malformations will be dramatically decreased following endovascular embolization, which could be a significant factor in the mortality and morbidity of these patients [2, 3]. Holden et al. have found in the neonates and infants with intracranial arteriovenous malformations and congestive heart failure that pulmonary hypertension is invariably present and is at or above the systemic levels in some of the patients [15]. They showed that pulmonary artery pressure decreased in some patients in response to oxygen inhalation. Our patient did not respond to oxygen therapy and mechanical ventilation, and we had to perform a transcatheter embolization procedure.
4. Conclusion
VGAM is a rare congenital anomaly and, in general, has a poor prognosis. It is also called “median prosencephalic arteriovenous fistula”, and its development occurs between 6 and 11 weeks of the fetus’s development. Most cases are diagnosed after birth, and the prenatal diagnosis is made by fetal echocardiography and prenatal magnetic resonance imaging [16]. When an aneurysmal lesion is seen in the fetus’s brain, VGAM is a differential diagnosis.
The prenatal diagnosis of VGAMs is possible with high accuracy by fetal ultrasound findings. Therefore, early fetal screening by ultrasonography (and fetal echocardiography when a diagnosis is suspected) should be considered.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We would like to appreciate the Pernatology and Fetal Cardiology of Shariati Hospital, Tehran University of Medical Sciences
References
Vein of Galen Aneurysmal Malformations (VGAMs) are rare congenital vasculature abnormalities and the most common form of symptomatic cerebrovascular anomaly in neonates and infants. VGAMs prevalence is less than 1 in 25000 live deliveries, which can cause high morbidity and mortality, particularly in neonates and then infants and older children. This abnormality was first described in 1895. The exact incidence is unknown, but the prevalence is 1% of all neonatal intracranial vasculature malformations. This congenital malformation develops during weeks 6th–11th of gestational age. This anomaly is characterized by the connection between intracranial vessels (typically the posterior choroid arteries or the anterior cerebral arteries) and the deep venous system (the Vein of Galen), which leads to a communication between the cerebral arteries and the deep veins of the brain. The direct shunting of cerebral arterial flow into the draining vein (arteriovenous shunting of blood) makes it markedly enlarged and aneurysmal. Compression of the developing brain by the enlarged vessel may lead to cerebral hypoplasia, atrophy, or hydrocephalus. Developmentally, the dilated vein of Galen arises from the persistence of the embryonic median prosencephalic vein of Markowski [1].
The prognosis of neonates and infants with the vein of Galen malformations is poor, with a postnatal mortality rate of 50% and a high risk of neurologic complications. Pathophysiologically, the size of the fistula determines the amount of blood shunting and the time of presentation. Also, it presents in several different ways, and the most common feature includes the high output cardiac failure and cyanosis in the newborn that can be mistaken for a cyanotic congenital heart anomaly. In a neonate with congestive heart failure and a structurally normal heart, the diagnosis of an intracranial arteriovenous malformation should be noticed [1, 2, 3, 4, 5, 6, 7, 8]. Diagnosis of this anomaly is possible by ultrasonography; however, there is a paucity of information regarding the specific findings of VGAMs [1].
There is often a delay in diagnosis as the clinical presentation can mimic cyanotic congenital heart disease or persistent pulmonary hypertension of the newborn. Treatment in the neonatal period includes supportive care in neonatal intensive care. Without intervention, the mortality rate among infants with VGAM is close to 100%. Endovascular embolization revolutionized the management of VGAM. Endovascular treatment is the treatment of choice for VGAM presenting in infancy with heart failure and significantly reduces its morbidity and mortality. Embolization, both of feeding arteries and draining veins, can considerably reduce aneurysmal blood flow. Overall prognosis in the short and the long term has improved but is still not ideal for this group of neonates [2, 4, 7, 8, 9, 10].
This study highlights the identification of this anomaly in the pregnancy period. We report a severe case of VGAM with significant secondary cardiac involvement. It eventually led to the death of the baby after birth.
2. Case Presentation
A 37-year-old pregnant female (Gravid 4, para 0, abort 2, living child 1) was referred to our perinatology clinic at her 28th week of pregnancy with an ultrasound report of a vasculature aneurysmal dilation in the brain of the fetus, which suggested a malformation of Galen, and cardiomegaly. No additional maternal risk factors were detected.
In the fetal echocardiography, evidence of skin edema and pleural effusion was detected, which indicated the diagnosis of hydrops fetalis and orientation and position of the heart was normal (levocardia and levoposition). The apical 4-chamber view showed right ventricular and atrial enlargement (Figure 1).
.png)
Short axis and 3-vessel view showed dilation of the pulmonary artery (Figure 2).
.png)
In the axial view, a high flow and prominent superior vena cava and left innominate vein were detected (Figures 3 & 4), and the bicaval view showed superior vena cava dilation (Figure 5).
.png)
.png)
.png)
The pregnancy continued until the 38th week of gestation, and soon after the birth, the baby was intubated because of significant cyanosis. Transthoracic echocardiography showed right heart (right atrium and ventricle) enlargement (Figure 6) and prominent superior vena cava with high flow and right to left shunt through the foramen oval (Figure 7).
.png)
.png)
Brachiocephalic arterial branches in suprasternal notch view were enlarged (Figure 8).

The neonate was treated with medical treatment, including milrinone (as a pulmonary vasodilator) and dopamine infusions (with renal dose to support renal blood flow). The patient was taken to another hospital for cerebral vascular embolization, and the embolization of this brain vascular anomaly was performed, but the patient became ill and died immediately after this procedure.
3. Discussion
Vein of Galen aneurysmal malformations are the most frequent arteriovenous malformations in infants and fetuses that result in significant dilation of the great vein of Galen. It commonly presents in the neonatal period, although it may present in early childhood. The clinical presentation of VGM varies with age. Neonates usually have multiple fistulae and present with high-output heart failure. Usually, the signs of severe heart failure develop shortly after birth. Often cyanosis is present, which is also suggestive of persistent pulmonary hypertension. Infants and younger children with a single fistula are presented with seizures, hydrocephalus, distention of scalp veins, and failure to thrive. Older children and adults often present with neurological symptoms, including headaches and seizures. VGAM occurs when the vein of Galen receives (directly or indirectly) an arterial communication from one or more major intracranial arteries [2, 5, 7, 9].
VGAM often results in high-output heart failure. The increased preload caused by the intracranial vasculature anomaly leads to the significant dilation of the superior vena cava. With the progression of the disease, heart failure, as a usual finding, may develop, and the fetal right-side heart will be dilated. Heart failure is due to the size of the arteriovenous shunt, which can steal 80% or more of the cardiac output. It is also the most common cause of death in such patients. Tricuspid regurgitation, cardiomegaly, and fetal hydrops may ensue [1, 2, 5, 9]. Dilation of superior vena cava and right heart enlargement was prominent in our patient.
The treatment of vein of Galen aneurysmal malformation includes initial cardiovascular stabilization. This is achieved by reducing systemic and pulmonary vasculature resistance and improving systemic perfusion and myocardial function. In most cases, a vasodilator agent, either alone or in combination with inotropic agents, is needed. Milrinone and inhaled nitric oxide have been found beneficial. Some patients can benefit from treatment with prostaglandin E1. Without interventional neuroradiology and embolization, the prognosis is poor for neonates with early severe cardiac failure. Severe heart failure rapidly progresses to multiorgan failure, severe lactic acidosis, and death. Before new imaging technologies and endovascular treatment, Galen malformations were fatal in 90% of patients under 1 month of age, and half of those were between 1 month and 1 year old. Nowadays, treatment of this lesion has been revolutionized by transcatheter embolization techniques, with 70%–80% survival, and surgical intervention does not have an important role. Endovascular embolization is the preferred treatment modality, preferably after the age of 5 to 6 months. A good outcome is to be expected when treatment is performed before the significant brain injury. A recent systematic meta-analysis of 667 patients performed by Yan j et al. revealed that 68% of the patients, including neonates (44%), infants (41%), children, and adults (12%), had a good outcome after endovascular embolization [11]. Similar results were found in a review by Khullar et al., who reported 84% of good to the fair outcome and a 15% mortality rate among 337 patients undergoing endovascular treatment during 2001 and 2010 [12]. The development of intravascular occlusion devices has shown to be safer and more effective. A study by Ciricillo et al. showed that all six neonates with VGAM who underwent direct microsurgery died while six of the eight who were treated with endovascular embolization survived [13]. Lasjaunias et al. [14] reported a mortality rate of 13% in 36 cases of VGAMs treated with endovascular embolization. However, endovascular treatment of neonates and young infants has not been without complications, including perforation of the thin-walled aneurysm or the feeding vessels [3, 4, 7, 9].
In the uterus, heart failure is rare because the low resistance of the cerebral Arteriovenous Malformation (AVM) is balanced by the low resistance of the uteroplacental unit, allowing perfusion of the peripheries. With the loss of the placenta at birth, up to 70% of cardiac output is directed to the cerebral circulation. Pulmonary arterial pressure remains high, and the ductus arteriosus remains open, directing right ventricular output through the patent ductus arteriosus and into the descending aorta. Therefore the right ventricle and pulmonary artery have become dilated. Severe heart failure associated with VGAM appears to be more severe than heart failure associated with intracardiac anomalies. As a result, stabilization of these neonates before neuro-intervention or neurosurgery is difficult, and cardiac failure is often resistant to treatment. In critically ill patients, medical treatment with milrinone improves myocardial function and produces systemic and pulmonary vasodilatation [4, 5, 9].
Pulmonary hypertension of the newborn associated with Galen malformations will be dramatically decreased following endovascular embolization, which could be a significant factor in the mortality and morbidity of these patients [2, 3]. Holden et al. have found in the neonates and infants with intracranial arteriovenous malformations and congestive heart failure that pulmonary hypertension is invariably present and is at or above the systemic levels in some of the patients [15]. They showed that pulmonary artery pressure decreased in some patients in response to oxygen inhalation. Our patient did not respond to oxygen therapy and mechanical ventilation, and we had to perform a transcatheter embolization procedure.
4. Conclusion
VGAM is a rare congenital anomaly and, in general, has a poor prognosis. It is also called “median prosencephalic arteriovenous fistula”, and its development occurs between 6 and 11 weeks of the fetus’s development. Most cases are diagnosed after birth, and the prenatal diagnosis is made by fetal echocardiography and prenatal magnetic resonance imaging [16]. When an aneurysmal lesion is seen in the fetus’s brain, VGAM is a differential diagnosis.
The prenatal diagnosis of VGAMs is possible with high accuracy by fetal ultrasound findings. Therefore, early fetal screening by ultrasonography (and fetal echocardiography when a diagnosis is suspected) should be considered.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors' contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We would like to appreciate the Pernatology and Fetal Cardiology of Shariati Hospital, Tehran University of Medical Sciences
References
- Doyle NM, Mastrobattista JM, Thapar MK, Lantin-Hermoso MR. Perinatal pseudocoarctation: Echocardiographic findings in vein of Galen malformation. Journal of Ultrasound in Medicine. 2005; 24(1):93-8. [DOI:10.7863/jum.2005.24.1.93] [PMID]
- Hendson L, Emery DJ, Phillipos EZ, Bhargava R, Olley PM, Lemke RP. Persistent pulmonary hypertension of the newborn presenting as the primary manifestation of intracranial arteriovenous malformation of the vein of Galen. American Journal of Perinatology. 2000; 17(8):405-10. [DOI:10.1055/s-2000-13456] [PMID]
- McElhinney DB, Halbach VV, Silverman NH, Dowd CF, Hanley FL. Congenital cardiac anomalies with vein of Galen malformations in infants. Archives of Disease in Childhood. 1998; 78(6):548-51. [DOI:10.1055/s-2000-13456] [PMID]
- Frawley GP, Dargaville PA, Mitchell PJ, Tress BM, Loughnan P. Clinical course and medical management of neonates with severe cardiac failure related to vein of Galen malformation. Archives of Disease in Childhood - Fetal and Neonatal Edition. 2002; 87(2):F144-9. [DOI:10.1055/s-2000-13456] [PMID]
- Darji PJ, Gandhi VS, Banker H, Chaudhari H. Antenatal diagnosis of aneurysmal malformation of the vein of Galen.BMJ Case Reports. 2015; 2015:bcr2015213785. [DOI:10.1136/bcr-2015-213785] [PMID]
- Gerards FA, Engels MA, Barkhof F, van den Dungen FA, Vermeulen RJ, van Vugt JM. Prenatal diagnosis of aneurysms of the vein of Galen (vena magna cerebri) with conventional sonography, three-dimensional sonography, and magnetic resonance imaging: Report of 2 cases. Journal of Ultrasound in Medicine. 2003; 22(12):1363-8. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Aly AM, Garcia CY, von Ritschl R. Successful embolization of a large vein of galen malformation in a premature infant presenting with congestive heart failure and persistent pulmonary hypertension. American Journal of Perinatology Reports. 2012; 2(1):19-22. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Giesinger RE, Elsayed YN, Castaldo MP, McNamara PJ. Targeted neonatal echocardiography-guided therapy in vein of Galen aneurysmal malformation: A report of two cases with a review of physiology and approach to management. American Journal of Perinatology Reports. 2019; 9(2):e172-6. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Madhuban A, van den Heuvel F, van Stuijvenberg M. Vein of Galen aneurysmal malformation in neonates presenting with congestive heart failure. Child Neurology Open. 2016; 3. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Paladini D, Deloison B, Rossi A, Chalouhi GE, Gandolfo C, Sonigo P, et al. Vein of Galen Aneurysmal Malformation (VGAM) in the fetus: Retrospective analysis of perinatal prognostic indicators in a two-center series of 49 cases. Ultrasound in Obstetrics & Gynecology. 2017; 50(2):192-9. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Yan J, Wen J, Gopaul R, Zhang CY, Xiao SW. Outcome and complications of endovascular embolization for vein of Galen malformations: A systematic review and meta-analysis. Journal of Neurosurgery. 2015; 123(4):872-90. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Khullar D, Andeejani AM, Bulsara KR. Evolution of treatment options for vein of Galen malformations. Journal of Neurosurgery. Pediatrics. 2010; 6(5):444-51. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Ciricillo SF, Edwards MS, Schmidt KG, Hieshima GB, Silverman NH, Higashida RT, et al. Interventional neuroradiological management of vein of Galen malformations in the neonate. Neurosurgery. 1990; 27(1):22-7. [PMID]
- Lasjaunias P, Rodesch G, Terbrugge K, Pruvost Ph, Devictor D, Comoy J, et al. Vein of Galen aneurismal malformations. Report of 36 cases managed between 1982 and 1988. Acta Neurochirurgica. 1989; 99(1-2):26-37. [DOI:10.3171/2014.12.JNS141249] [PMID]
- Holden AM, Fyler DC, Shillito Jr J, Nadas AS. Congestive heart failure from intracranial arteriovenous fistula in infancy. Clinical and physiologic considerations in eight patients. Pediatrics. 1972; 49(1):30-9. [PMID]
- Calheiros-Trigo F, Cadilhe A, Reis J, Silva N, Pereira A. Vein of Galen malformation: Prenatal diagnosis, postnatal monitoring and treatment. Journal Pediatric Neonatal Individual ized Medicine. 2020; 9(2):e090217. [DOI:10.3171/2014.12.JNS141249]
Type of Study: Case & Review |
Subject:
Pediatric Cardiology
Received: 2020/02/11 | Accepted: 2020/12/15 | Published: 2021/07/28
Received: 2020/02/11 | Accepted: 2020/12/15 | Published: 2021/07/28
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






