Volume 11, Issue 3 (7-2023)
J. Pediatr. Rev 2023, 11(3): 245-250 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fazeli F, Asgari Jafarabadi E, Zardast A H, Joodi M, Azarkar G. Solid Pseudopapillary Tumor of Pancreas in a 14-year-old Adolescent Presenting With Melena: A Case Report. J. Pediatr. Rev 2023; 11 (3) :245-250
URL: http://jpr.mazums.ac.ir/article-1-484-en.html
URL: http://jpr.mazums.ac.ir/article-1-484-en.html
Fateme Fazeli1 

 , Emad Asgari Jafarabadi *2
, Emad Asgari Jafarabadi *2 

 , Amir Hossein Zardast3
, Amir Hossein Zardast3 
 , Marjan Joodi4
, Marjan Joodi4 
 , Ghodsiyeh Azarkar5
, Ghodsiyeh Azarkar5 




 , Emad Asgari Jafarabadi *2
, Emad Asgari Jafarabadi *2 

 , Amir Hossein Zardast3
, Amir Hossein Zardast3 
 , Marjan Joodi4
, Marjan Joodi4 
 , Ghodsiyeh Azarkar5
, Ghodsiyeh Azarkar5 


1- Department of Pediatrics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
2- Department of Pediatrics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran. ,dr.askary@hotmail.com
3- Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Pediatric Surgery, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Department of Radiology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
2- Department of Pediatrics, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran. ,
3- Student Research Committee, Mashhad University of Medical Sciences, Mashhad, Iran.
4- Department of Pediatric Surgery, School of Medicine, Mashhad University of Medical Sciences, Mashhad, Iran.
5- Department of Radiology, School of Medicine, Birjand University of Medical Sciences, Birjand, Iran.
Full-Text [PDF 781 kb]
(1374 Downloads)
| Abstract (HTML) (2595 Views)
Full-Text: (845 Views)
Background
Pancreatic neoplasms rarely occur in infants and children. The North American population-based Surveillance, Epidemiology, and End Results (SEER) registry examination from 1973-2004 reported an incidence of 1.8 cases per 1000000 for pediatric pancreatic tumors in the United States. Three of children’s most common primary pancreatic neoplasms are pancreatoblastoma, solid pseudopapillary neoplasm of the pancreas, and pancreatic endocrine neoplasms [1].
The pancreas’s solid pseudopapillary neoplasm (SPN) is a low-grade malignant tumor composed of poorly cohesive epithelial cells, forming solid and pseudopapillary structures and lacking a specific line of pancreatic epithelial differentiation [2]. The first case of SPN was reported by Virginia Frantz in 1959. With its different names, the World Health Organization (WHO) designated this neoplasm as SPN in 1996 [3]. Solid pseudopapillary neoplasms occur most classically in young female patients (mean age of 22 years) and are well-reported in children and adolescents [1]. About 20%-25% of the cases are seen in pediatric patients [4]. Though aggressive behavior has been reported, it generally behaves as a low-grade neoplasm with a good prognosis. Children with SPN often present differently than adult patients. In the adult group, the diagnosis is usually made accidentally during screening with the detection of a mass. By contrast, all of the children are symptomatic [1]. Solid pseudopapillary neoplasms in children are presented with a palpable mass (60%), followed by abdominal pain (33.3%) [4]. Although duodenal invasion frequently occurs in patients with pancreatic cancer, massive gastrointestinal bleeding is seldom encountered. Pancreatic cancer accounts for only 0.35%–1.9% of upper gastrointestinal bleeding cases. About 2.6% of patients with pancreatic cancer presented with gastrointestinal bleeding as the initial manifestation. On the other hand, few researchers have reported serious hemorrhage as an initial manifestation of pancreatic cancer [5, 6].
The mean diameter of the tumors based on pathological examination was 6.0 cm (range, 1.5-14 cm) in adults and 8.0 cm (range, 3.5-14 cm) in children. In adults, the pancreatic body or tail was the most common location of the tumor. However, in children, the pancreatic head was the most common site [1]. Tumor location frequency rates in children are as follows: Tail (35.9%-44%), head (30%-34%), body, and tail (10.3%-13%) [4]. These tumors are also often texturally fairly soft; this accounts for the rarity of ductal dilatation and jaundice even for tumors arising in the pancreatic head [7].
The origin and histogenesis of SPN are still unknown, although it is thought to originate from totipotent stem cells with the capacity for both endocrine and exocrine differentiation [7]. The gross appearance of SPN is a well-circumscribed encapsulated cystic mass, which may be hemorrhagic and necrotic. Histologically, SPN is a cellular neoplasm with cells often in several layers around fibrovascular stalks, appearing papillary. A predominantly solid or microcystic pattern is also identified. Solid pseudopapillary neoplasms comprise loosely-cohesive uniform cells with grooved nuclei and eosinophilic or clear cytoplasm. Intracytoplasmic PAS+ hyaline globules may also be found [1].
Pancreatic enzymes and common serum tumor markers (α-fetoprotein, carcinoembryonic antigen, and β-human chorionic gonadotrophin) are consistently negative, as seen in our patient. There are no known SPN-specific serum markers [4].
According to studies using whole-exome genomic sequencing, the most notable finding in their assessment of SPNs was the paucity of genetic alterations. While all tumors contained mutations of CTNNB1, only 1 out of the 8 tumors exhibited any LOH (loss of heterozygosity) [8].
Ultrasonographically, SPNs may appear as hypoechoic solid masses containing cystic areas or cystic masses. The fibrous capsule may be visualized as an echogenic or, less commonly, hypoechoic rim [4].
The most helpful imaging technique is the CT scan [5]. Regarding CT scan imaging findings, SPNs tend to be large, well-defined, isodense to normal pancreas on unenhanced images and heterogeneously hypoenhancing on postcontrast images with predominantly (about 80%) mixed solid-cystic morphology. Nearly half have a demonstrable pseudocapsule, about 40% have internal calcification, while 30% show splenic invasion. Meanwhile, the occasional smaller tumors are more homogeneous and solid, with no pseudocapsule identified at the CT scan. In an appropriate clinical context, these imaging findings reasonably suggest the diagnosis, though not pathognomonic [7].
The immunohistochemical study is very useful, revealing a diffuse positivity for certain histochemical markers like neuron-specific enolase (NSE), vimentin, CD-10, and β-catenin, which is not specific. α1-Antitrypsin and α1-antichymotrypsin are intensely positive in a small group of cells. Immunostaining for estrogen receptors, especially progesterone receptors, is sometimes positive; this marking evokes a possible hormone sensitivity of SPNs and could explain the female predominance. Immunostaining for chromogranin is negative, eliminating an endocrine tumor that is the main differential diagnosis. According to recently published data, a particular dot-like intracytoplasmic expression of CD99 is highly unique for SPNs. To date, this cytoplasmic paranuclear “dot-like” pattern has not been described in any other type of endocrine or exocrine pancreatic tumors included in the differential diagnosis of SPNs [4].
Solid pseudopapillary neoplasms lack alterations in genes commonly found in ductal adenocarcinomas, such as KRAS, TP53, P16/CDKN2A, and SMAD4, and show a low prevalence of abnormalities in chromosomes 11q, 13q, 17q, 1q, and 8q. The molecular hallmark of SPNs is represented by point mutations in exon 3 of the CTNNB1 gene, which is involved in the Wnt/β-catenin signaling pathway. This genetic alteration is observed in over 90% of cases [2].
Surgical resection is the treatment of choice for SPN, with the procedural type based on the location of the mass. Complete resection with negative margins typically proves curative. Despite large tumor size and the ability to extend locally, children and adolescents with SPNs do very well with surgical resection [1]. The optimal surgical strategy for solid pseudopapillary neoplasms in children is still debatable. Instead of radical resections, limited pancreatic resections, such as enucleations with negative surgical margins, should be attempted. For unresectable or recurrent tumors, cisplatin and 5-FU-based chemotherapy might be considered [9].
Predicting aggressive behavior is difficult, and investigators have differently defined the clinically relevant criteria [1]. There is limited data on the incidence of splenic invasion in SPNs. However, it has been described as an infrequent feature, which may be associated with an increased potential for malignant behavior. Angioinvasion, perineural invasion, deep invasion of the surrounding pancreatic parenchyma as well as large size, cellular or nuclear atypia, high mitotic rate, and extensive necrosis have been reported to increase the malignant potential of SPNs. However, without these histologic features, metastases can still rarely occur. Thus, SPNs are classified as uncertain malignant potential lesions in the latest World Health Organization (WHO) classification [2].
In summary, the pediatric pancreatic neoplasms presented here are rare, with morphologic features that can overlap with each other or with metastatic entities. Due to their rarity, our understanding of biological behavior and treatment of the lesions in children has been limited. With greater knowledge of their syndromic associations and or cellular origin, we can better categorize and treat these entities.
Case Presentation
A 14-year-old male adolescent was referred to our pediatric emergency department with fatigue, dizziness, fever, and vomiting. He had melena 5 days before admission, and his symptoms began afterward. He was alert, and vital signs were as follows: Heart rate, 120 beats per minute; respiratory rate, 20 per minute; blood pressure, 110 over 70 mm Hg in the supine position and 95 over 50 mm Hg in the sitting position; axillary temperature, 38.5°C; and peripheral oxygen saturation, 95% while breathing room air. Height, weight, and developmental values were normal. A detailed physical exam only revealed pallor suggestive of anemia. He was a healthy adolescent before the start of recent symptoms.
Two intravenous lines were placed immediately after the initial evaluation, and blood samples were taken for necessary laboratory tests. To stabilize the patient, crystalloids and pantoprazole were administered, but packed red blood cells were reserved. Packed red blood cells were transfused as the initial serum hemoglobin level was 5.3 g/dL. Serum lipase was 82 U/L and higher than the upper normal limit. Other items of the initial laboratory investigations, including liver enzymes and liver function tests, renal function tests, coagulation studies, and serum ions, were within normal limits. Chest radiography was normal.
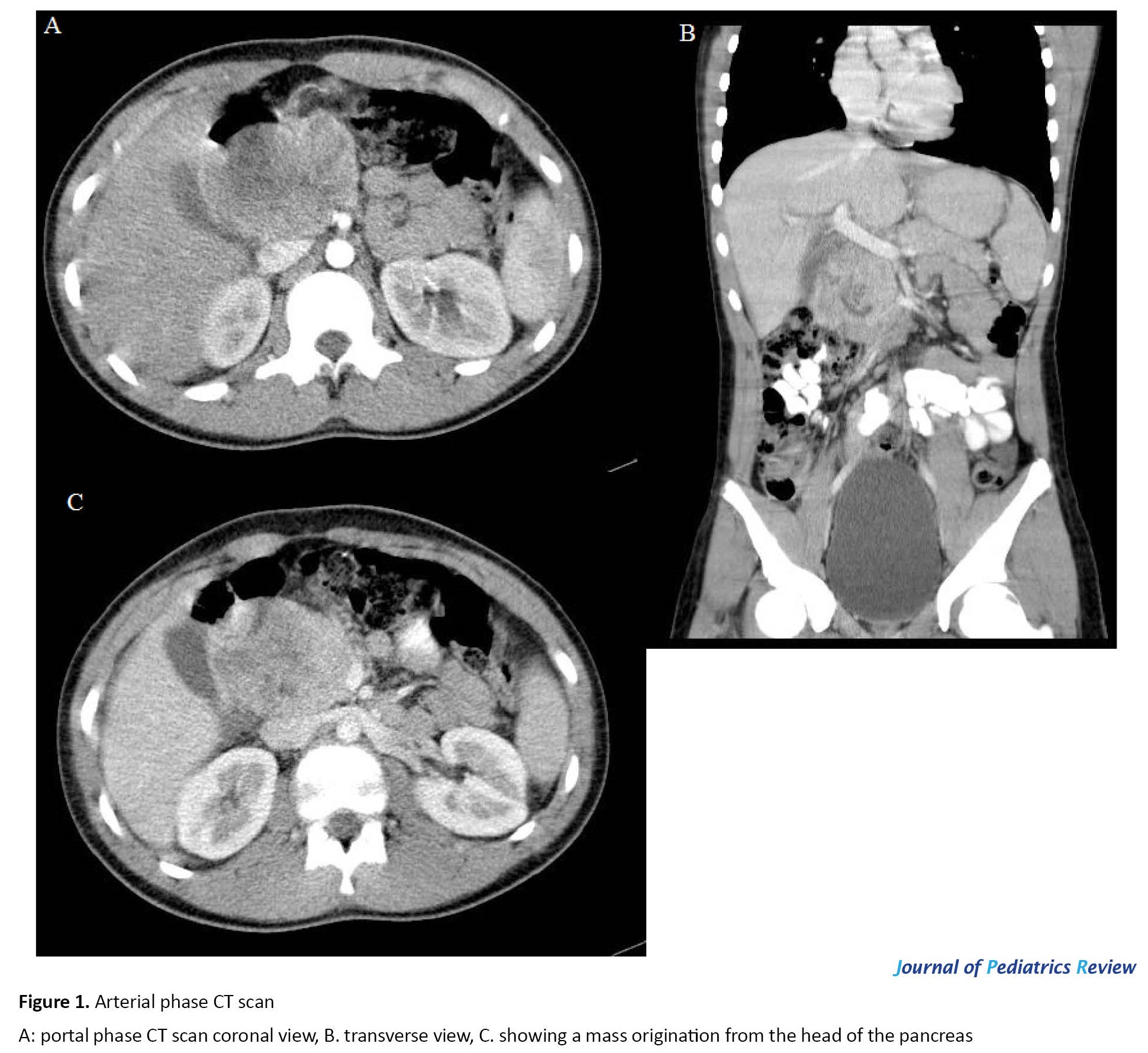
As the initial resuscitation measures stabilized the patient, endoscopic gastroduodenoscopy was performed; the esophagus, stomach, and bulb were normal, but a vascular lesion measuring 60×70 mm was noted in the second part of the duodenum. Tissues surrounding the lesion were biopsied. Abdominal ultrasonography revealed a solid mass containing cystic parts measuring 85×51 mm in the head of the pancreas. CT scan of the abdomen with intravenous and oral contrast showed a mass with solid and cystic components measuring 75×52 mm between the head of the pancreas and gallbladder origination from the head of the pancreas; no other abnormal element was noted (Figure 1). The result of the pathologic evaluation of the duodenal biopsy taken during gastroduodenoscopy was mild chronic erosive duodenitis with minimal activity with no dysplasia or malignancy.
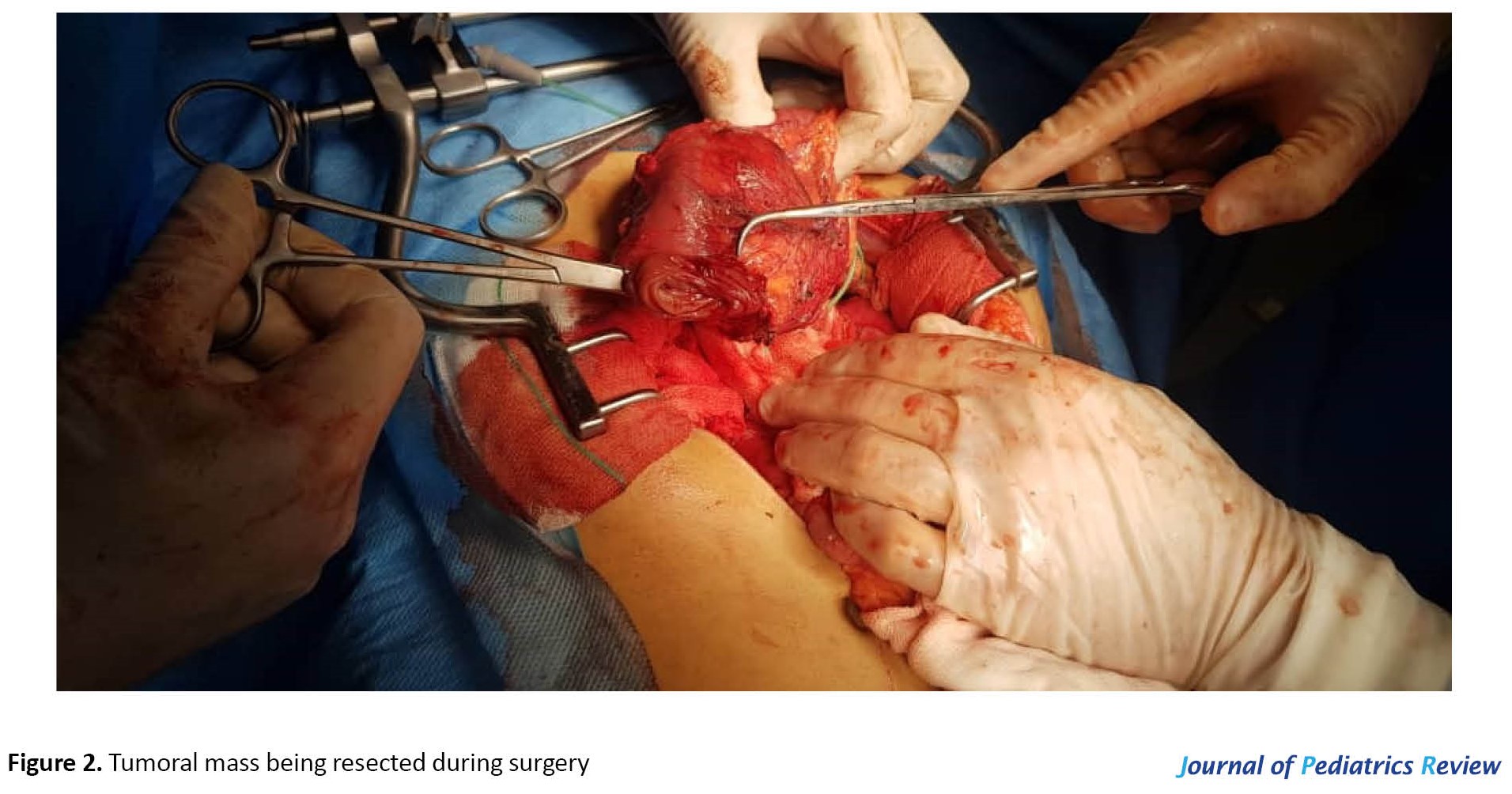
The patient was hospitalized for a total duration of 4 days in our center. The last hemoglobin value was 9.9 g/dL. As no pediatric surgeon was in our facility, the patient was transferred in stable condition to the nearest pediatric surgery center. The patient underwent Whipple surgery by a pediatric surgeon (Figure 2). A pathologist evaluated the specimen, and the results were as follows. The resected mass was a round-oval large brown mass weighing 185 g measuring 10×8×6 cm, surrounded by fat with an attached segment of duodenum measuring 10×2 cm. There were segments of the pancreas and gastric wall, gallbladder measuring 5×2 cm containing bile with intact velvety mucosa and wall thickness of 0.2 cm. Also, there were 4 lymph nodes, the largest measuring 1 cm. On cross-sectioning, the mass consisted of creamy solid homogenous material. Microscopic evaluation revealed a neoplasm including monotonous round-oval tumor cells with folded nuclei in a solid growth pattern, few foamy cells, focal microcystic areas, and a delicate vascular network. The final diagnosis of the pathologic evaluations was as follows: morphologic features are mostly in favor of solid pseudopapillary tumor of the pancreas; anterior margins were positive for tumor involvement; duodenal and gastric serosa were involved by tumoral tissue; gall bladder was tumor free; and paraceliac lymph nodes were tumor free.
A pediatric oncologist was also involved in further workup and treatment.
Discussion and Conclusions
As pancreatic neoplasms are uncommon in pediatric populations, most clinicians do not encounter this clinical scenario in their daily clinical practice. Case reports describing these tumors could inform clinicians about the possible clinical presentations of these tumors and enhance the diagnosis of them.
Most pediatric pseudopapillary tumors of the pancreas present with a palpable mass and abdominal pain, and gastrointestinal bleeding is a rare presentation not mentioned in previous case reports. On the other hand, since gastrointestinal bleeding has been the main presentation of our case report, some clinical aspects of this tumor might be unknown to us. Although this is a rare tumor, clinicians must be aware of the critical and life-threatening factors of these tumors. We suggest clinicians report this tumor’s clinical, imaging, and laboratory aspects not mentioned in the literature review.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and or national research committee and with the 1975 Helsinki Declaration.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
The initial resuscitation and gastroduodenoscopy: Fateme Fazeli; Performing surgery: Marjan Joodi; CT scan findings report: Ghodsiyeh Azarkar; Data collection and manuscript writing: Emad Asgari Jafarabadi; Literature review: Amir Hossein Zardast.
Conflicts of interest
The authors declared no conflicts of interest.
References
Pancreatic neoplasms rarely occur in infants and children. The North American population-based Surveillance, Epidemiology, and End Results (SEER) registry examination from 1973-2004 reported an incidence of 1.8 cases per 1000000 for pediatric pancreatic tumors in the United States. Three of children’s most common primary pancreatic neoplasms are pancreatoblastoma, solid pseudopapillary neoplasm of the pancreas, and pancreatic endocrine neoplasms [1].
The pancreas’s solid pseudopapillary neoplasm (SPN) is a low-grade malignant tumor composed of poorly cohesive epithelial cells, forming solid and pseudopapillary structures and lacking a specific line of pancreatic epithelial differentiation [2]. The first case of SPN was reported by Virginia Frantz in 1959. With its different names, the World Health Organization (WHO) designated this neoplasm as SPN in 1996 [3]. Solid pseudopapillary neoplasms occur most classically in young female patients (mean age of 22 years) and are well-reported in children and adolescents [1]. About 20%-25% of the cases are seen in pediatric patients [4]. Though aggressive behavior has been reported, it generally behaves as a low-grade neoplasm with a good prognosis. Children with SPN often present differently than adult patients. In the adult group, the diagnosis is usually made accidentally during screening with the detection of a mass. By contrast, all of the children are symptomatic [1]. Solid pseudopapillary neoplasms in children are presented with a palpable mass (60%), followed by abdominal pain (33.3%) [4]. Although duodenal invasion frequently occurs in patients with pancreatic cancer, massive gastrointestinal bleeding is seldom encountered. Pancreatic cancer accounts for only 0.35%–1.9% of upper gastrointestinal bleeding cases. About 2.6% of patients with pancreatic cancer presented with gastrointestinal bleeding as the initial manifestation. On the other hand, few researchers have reported serious hemorrhage as an initial manifestation of pancreatic cancer [5, 6].
The mean diameter of the tumors based on pathological examination was 6.0 cm (range, 1.5-14 cm) in adults and 8.0 cm (range, 3.5-14 cm) in children. In adults, the pancreatic body or tail was the most common location of the tumor. However, in children, the pancreatic head was the most common site [1]. Tumor location frequency rates in children are as follows: Tail (35.9%-44%), head (30%-34%), body, and tail (10.3%-13%) [4]. These tumors are also often texturally fairly soft; this accounts for the rarity of ductal dilatation and jaundice even for tumors arising in the pancreatic head [7].
The origin and histogenesis of SPN are still unknown, although it is thought to originate from totipotent stem cells with the capacity for both endocrine and exocrine differentiation [7]. The gross appearance of SPN is a well-circumscribed encapsulated cystic mass, which may be hemorrhagic and necrotic. Histologically, SPN is a cellular neoplasm with cells often in several layers around fibrovascular stalks, appearing papillary. A predominantly solid or microcystic pattern is also identified. Solid pseudopapillary neoplasms comprise loosely-cohesive uniform cells with grooved nuclei and eosinophilic or clear cytoplasm. Intracytoplasmic PAS+ hyaline globules may also be found [1].
Pancreatic enzymes and common serum tumor markers (α-fetoprotein, carcinoembryonic antigen, and β-human chorionic gonadotrophin) are consistently negative, as seen in our patient. There are no known SPN-specific serum markers [4].
According to studies using whole-exome genomic sequencing, the most notable finding in their assessment of SPNs was the paucity of genetic alterations. While all tumors contained mutations of CTNNB1, only 1 out of the 8 tumors exhibited any LOH (loss of heterozygosity) [8].
Ultrasonographically, SPNs may appear as hypoechoic solid masses containing cystic areas or cystic masses. The fibrous capsule may be visualized as an echogenic or, less commonly, hypoechoic rim [4].
The most helpful imaging technique is the CT scan [5]. Regarding CT scan imaging findings, SPNs tend to be large, well-defined, isodense to normal pancreas on unenhanced images and heterogeneously hypoenhancing on postcontrast images with predominantly (about 80%) mixed solid-cystic morphology. Nearly half have a demonstrable pseudocapsule, about 40% have internal calcification, while 30% show splenic invasion. Meanwhile, the occasional smaller tumors are more homogeneous and solid, with no pseudocapsule identified at the CT scan. In an appropriate clinical context, these imaging findings reasonably suggest the diagnosis, though not pathognomonic [7].
The immunohistochemical study is very useful, revealing a diffuse positivity for certain histochemical markers like neuron-specific enolase (NSE), vimentin, CD-10, and β-catenin, which is not specific. α1-Antitrypsin and α1-antichymotrypsin are intensely positive in a small group of cells. Immunostaining for estrogen receptors, especially progesterone receptors, is sometimes positive; this marking evokes a possible hormone sensitivity of SPNs and could explain the female predominance. Immunostaining for chromogranin is negative, eliminating an endocrine tumor that is the main differential diagnosis. According to recently published data, a particular dot-like intracytoplasmic expression of CD99 is highly unique for SPNs. To date, this cytoplasmic paranuclear “dot-like” pattern has not been described in any other type of endocrine or exocrine pancreatic tumors included in the differential diagnosis of SPNs [4].
Solid pseudopapillary neoplasms lack alterations in genes commonly found in ductal adenocarcinomas, such as KRAS, TP53, P16/CDKN2A, and SMAD4, and show a low prevalence of abnormalities in chromosomes 11q, 13q, 17q, 1q, and 8q. The molecular hallmark of SPNs is represented by point mutations in exon 3 of the CTNNB1 gene, which is involved in the Wnt/β-catenin signaling pathway. This genetic alteration is observed in over 90% of cases [2].
Surgical resection is the treatment of choice for SPN, with the procedural type based on the location of the mass. Complete resection with negative margins typically proves curative. Despite large tumor size and the ability to extend locally, children and adolescents with SPNs do very well with surgical resection [1]. The optimal surgical strategy for solid pseudopapillary neoplasms in children is still debatable. Instead of radical resections, limited pancreatic resections, such as enucleations with negative surgical margins, should be attempted. For unresectable or recurrent tumors, cisplatin and 5-FU-based chemotherapy might be considered [9].
Predicting aggressive behavior is difficult, and investigators have differently defined the clinically relevant criteria [1]. There is limited data on the incidence of splenic invasion in SPNs. However, it has been described as an infrequent feature, which may be associated with an increased potential for malignant behavior. Angioinvasion, perineural invasion, deep invasion of the surrounding pancreatic parenchyma as well as large size, cellular or nuclear atypia, high mitotic rate, and extensive necrosis have been reported to increase the malignant potential of SPNs. However, without these histologic features, metastases can still rarely occur. Thus, SPNs are classified as uncertain malignant potential lesions in the latest World Health Organization (WHO) classification [2].
In summary, the pediatric pancreatic neoplasms presented here are rare, with morphologic features that can overlap with each other or with metastatic entities. Due to their rarity, our understanding of biological behavior and treatment of the lesions in children has been limited. With greater knowledge of their syndromic associations and or cellular origin, we can better categorize and treat these entities.
Case Presentation
A 14-year-old male adolescent was referred to our pediatric emergency department with fatigue, dizziness, fever, and vomiting. He had melena 5 days before admission, and his symptoms began afterward. He was alert, and vital signs were as follows: Heart rate, 120 beats per minute; respiratory rate, 20 per minute; blood pressure, 110 over 70 mm Hg in the supine position and 95 over 50 mm Hg in the sitting position; axillary temperature, 38.5°C; and peripheral oxygen saturation, 95% while breathing room air. Height, weight, and developmental values were normal. A detailed physical exam only revealed pallor suggestive of anemia. He was a healthy adolescent before the start of recent symptoms.
Two intravenous lines were placed immediately after the initial evaluation, and blood samples were taken for necessary laboratory tests. To stabilize the patient, crystalloids and pantoprazole were administered, but packed red blood cells were reserved. Packed red blood cells were transfused as the initial serum hemoglobin level was 5.3 g/dL. Serum lipase was 82 U/L and higher than the upper normal limit. Other items of the initial laboratory investigations, including liver enzymes and liver function tests, renal function tests, coagulation studies, and serum ions, were within normal limits. Chest radiography was normal.
As the initial resuscitation measures stabilized the patient, endoscopic gastroduodenoscopy was performed; the esophagus, stomach, and bulb were normal, but a vascular lesion measuring 60×70 mm was noted in the second part of the duodenum. Tissues surrounding the lesion were biopsied. Abdominal ultrasonography revealed a solid mass containing cystic parts measuring 85×51 mm in the head of the pancreas. CT scan of the abdomen with intravenous and oral contrast showed a mass with solid and cystic components measuring 75×52 mm between the head of the pancreas and gallbladder origination from the head of the pancreas; no other abnormal element was noted (Figure 1). The result of the pathologic evaluation of the duodenal biopsy taken during gastroduodenoscopy was mild chronic erosive duodenitis with minimal activity with no dysplasia or malignancy.
The patient was hospitalized for a total duration of 4 days in our center. The last hemoglobin value was 9.9 g/dL. As no pediatric surgeon was in our facility, the patient was transferred in stable condition to the nearest pediatric surgery center. The patient underwent Whipple surgery by a pediatric surgeon (Figure 2). A pathologist evaluated the specimen, and the results were as follows. The resected mass was a round-oval large brown mass weighing 185 g measuring 10×8×6 cm, surrounded by fat with an attached segment of duodenum measuring 10×2 cm. There were segments of the pancreas and gastric wall, gallbladder measuring 5×2 cm containing bile with intact velvety mucosa and wall thickness of 0.2 cm. Also, there were 4 lymph nodes, the largest measuring 1 cm. On cross-sectioning, the mass consisted of creamy solid homogenous material. Microscopic evaluation revealed a neoplasm including monotonous round-oval tumor cells with folded nuclei in a solid growth pattern, few foamy cells, focal microcystic areas, and a delicate vascular network. The final diagnosis of the pathologic evaluations was as follows: morphologic features are mostly in favor of solid pseudopapillary tumor of the pancreas; anterior margins were positive for tumor involvement; duodenal and gastric serosa were involved by tumoral tissue; gall bladder was tumor free; and paraceliac lymph nodes were tumor free.
A pediatric oncologist was also involved in further workup and treatment.
Discussion and Conclusions
As pancreatic neoplasms are uncommon in pediatric populations, most clinicians do not encounter this clinical scenario in their daily clinical practice. Case reports describing these tumors could inform clinicians about the possible clinical presentations of these tumors and enhance the diagnosis of them.
Most pediatric pseudopapillary tumors of the pancreas present with a palpable mass and abdominal pain, and gastrointestinal bleeding is a rare presentation not mentioned in previous case reports. On the other hand, since gastrointestinal bleeding has been the main presentation of our case report, some clinical aspects of this tumor might be unknown to us. Although this is a rare tumor, clinicians must be aware of the critical and life-threatening factors of these tumors. We suggest clinicians report this tumor’s clinical, imaging, and laboratory aspects not mentioned in the literature review.
Ethical Considerations
Compliance with ethical guidelines
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and or national research committee and with the 1975 Helsinki Declaration.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
The initial resuscitation and gastroduodenoscopy: Fateme Fazeli; Performing surgery: Marjan Joodi; CT scan findings report: Ghodsiyeh Azarkar; Data collection and manuscript writing: Emad Asgari Jafarabadi; Literature review: Amir Hossein Zardast.
Conflicts of interest
The authors declared no conflicts of interest.
References
- Tucker SM. Pediatric neoplasms of the pancreas: A review. Ann Clin Pathol. 2014; 2(2):1017. [Link]
- La Rosa S, Bongiovanni M. Pancreatic solid pseudopapillary neoplasm: Key pathologic and genetic features. Arch Pathol Lab Med. 2020; 144(7):829-37. [PMID]
- Chung EM, Travis MD, Conran RM. Pancreatic tumors in children: Radiologic-pathologic correlation. RadioGraphics. 2006; 26(4):1211-38. [DOI:10.1148/rg.264065012] [PMID]
- Berrada G, Belaaroussi S, Chbani K, Salam S, Laoudiyi D, Ouzidane L, et al. Solid pseudopapillary tumor of the pancreas: A rare entity in children. Pan Afr Med J. 2020; 35:137. [DOI:10.11604/pamj.2020.35.137.22404] [PMID] [PMCID]
- Carreño Toro L, Smok Sahid G, Villarroel Perez MA, Sanhueza Linares V. [Solid pseudopapillary pancreatic neoplasm: report of five cases (Persian)]. Gastroenterol Hepatol. 2011; 34(4):266-70. [DOI:10.1016/j.gastrohep.2011.01.013] [PMID]
- Morita Y, Sakaguchi T, Kitajima R, Furuhashi S, Kiuchi R, Takeda M, et al. Lethal bleeding from a duodenal cancerous ulcer communicating with the superior mesenteric artery in a patient with pancreatic head cancer. Case Rep Gastroenterol. 2018; 12(2):479-86. [DOI:10.1159/000492207] [PMID] [PMCID]
- Anil G, Zhang J, Al Hamar NE, Nga ME. Solid pseudopapillary neoplasm of the pancreas: CT imaging features and radiologic-pathologic correlation. Diagn Interv Radiol. 2017; 23(2):94-99. [DOI:10.5152/dir.2016.16104] [PMID] [PMCID]
- Wu J, Jiao Y, Dal Molin M, Maitra A, de Wilde RF, Wood LD, et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A. 2011; 108(52):21188-93. [DOI:10.1073/pnas.1118046108] [PMID] [PMCID]
- Yalçın B, Yağcı-Küpeli B, Ekinci S, Orhan D, Oğuz B, Varan A, et al. Solid pseudopapillary neoplasm of the pancreas in children: Hacettepe experience. ANZ J Surg. 2019; 89(6):E236-40. [DOI:10.1111/ans.15111] [PMID]
Type of Study: Case & Review |
Subject:
Pediatrics
Received: 2022/07/6 | Accepted: 2023/07/3 | Published: 2023/07/1
Received: 2022/07/6 | Accepted: 2023/07/3 | Published: 2023/07/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |