Volume 11, Issue 1 (1-2023)
J. Pediatr. Rev 2023, 11(1): 11-24 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Toopchizadeh V, Barzegar M, Taleschian-Tabrizi N, Pashazadeh F, Rashedi N, Chahvechi-Akbari M et al . Long-term Disability and Poor Outcome Predictors of Guillain-Barre Syndrome in Children: A Systematic Review. J. Pediatr. Rev 2023; 11 (1) :11-24
URL: http://jpr.mazums.ac.ir/article-1-487-en.html
URL: http://jpr.mazums.ac.ir/article-1-487-en.html
Vahideh Toopchizadeh1 

 , Mohammad Barzegar2
, Mohammad Barzegar2 
 , Negar Taleschian-Tabrizi
, Negar Taleschian-Tabrizi 

 3, Fariba Pashazadeh4
3, Fariba Pashazadeh4 

 , Nasim Rashedi1
, Nasim Rashedi1 

 , Masood Chahvechi-Akbari5
, Masood Chahvechi-Akbari5 

 , Ozra Noori4
, Ozra Noori4 



 , Mohammad Barzegar2
, Mohammad Barzegar2 
 , Negar Taleschian-Tabrizi
, Negar Taleschian-Tabrizi 

 3, Fariba Pashazadeh4
3, Fariba Pashazadeh4 

 , Nasim Rashedi1
, Nasim Rashedi1 

 , Masood Chahvechi-Akbari5
, Masood Chahvechi-Akbari5 

 , Ozra Noori4
, Ozra Noori4 

1- Physical Medicine and Rehabilitation Research Center, Tabriz University of Medical Science, Tabriz, Iran.
2- Pediatric Health Research Center, Tabriz University of Medical Science, Tabriz, Iran.
3- Physical Medicine and Rehabilitation Research Center, Tabriz University of Medical Science, Tabriz, Iran. , n.t.tabrizi@gmail.com
4- Evidence Based Medicine Research Center, Tabriz University of Medical Science, Tabriz, Iran.
5- Department of Physical Medicine and Rehabilitation, Children’s Medical Center, Tehran University of Medical Science, Tehran, Iran.
2- Pediatric Health Research Center, Tabriz University of Medical Science, Tabriz, Iran.
3- Physical Medicine and Rehabilitation Research Center, Tabriz University of Medical Science, Tabriz, Iran. , n.t.tabrizi@gmail.com
4- Evidence Based Medicine Research Center, Tabriz University of Medical Science, Tabriz, Iran.
5- Department of Physical Medicine and Rehabilitation, Children’s Medical Center, Tehran University of Medical Science, Tehran, Iran.
Full-Text [PDF 560 kb]
(572 Downloads)
| Abstract (HTML) (1067 Views)
Full-Text: (393 Views)
Introduction
Guillan-Barre syndrome (GBS) is an acute neurological disorder that is a common etiology for acute flaccid paralysis in children. Some patients step into remitting phase 7-14 days after disease onset, but it can result in life-threatening situations and long-term disabilities [1]. The age group of the affected patients lies between 3 to 6 years old [2]. The estimated incidence of disease per year is 1.1-1.8/100000 in 2009, which increases up to 3.3/100000 after age 50 and the prognosis is worse than younger ages [3].
The most common clinical presentation of this syndrome is a bilateral ascending weakness with the absence of deep tendon reflexes [4]. Impairment in the autonomic systems, such as cardiac dysrhythmias, instability of blood pressure, disability of controlling bowel and bladder, and paralysis of bulbar muscles results in dysarthria, absent gag reflex, and dysphagia; motor and sensory involvement leading to bilateral ascending paralysis and reduced reflexes are the other complications caused by this disease [5, 6, 7].
Based on the clinical presentation and electrophysiological studies, Guillan-Barre has some subtypes, acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN) and Miller-Fischer syndrome [8]. The incidence of these subtypes is different in every geographical area. For example, AIDP is the most common variant in North America; however, AMAN is mainly seen in South America, China, and Japan [7, 9]. Prognosis and outcomes vary between subtypes, the majority of AMAN patients have a history of diarrhea before experiencing GBS symptoms and their period of hospitalization is more than AIDP type [10]. Infectious viral agents, such as Campylobacter jejuni, Epstein-Barr virus, Varicella Zoster virus and more recently Zika and COVID-19 viruses were the most prevalent agents causing GBS [11, 12, 13].
Although treatment modalities help patients recover from acute phase and serious complications, some disabilities, and residual motor and sensory signs and symptoms may remain and cause psychosocial problems that can greatly affect the patient’s quality of life causing mobility difficulties, pain, fatigue, and depression [14]. The prognosis in patients with GBS differs in adults and children. Since the clinical feature is more severe and long-term outcomes are poorer in adults. Thus, the studies of the course and prognosis of childhood GBS should be considered independently from adult studies [15]. Even if children recover faster and better than adults, long-term disability and the impact of childhood are significant and poorly defined [16]. That is why this systematic review aimed to include children suffering from GBS.
Considering long-term disabilities caused by GBS and its psychosocial effects, we conducted a systematic review to evaluate pediatric patients with GBS in terms of long-term disabilities caused by the disease and predictive factors of poor outcomes. Long-term functional outcomes included disability score, walking ability, need for mechanical ventilation, mortality rate, relapse rate, and residual disability.
Review question
What are the long-term outcomes of Guillan-Barre syndrome in children?
What are the poor outcome predictors of Guillan-Barre syndrome in children?
Inclusion criteria
Condition
This review considered studies that included long-term (>1 year) functional outcomes of GBS in children, long-term functional outcomes, including disability score, mortality rate, relapse rate, walking ability and residual disability, and predictors of poor outcome of GBS in children.
Context
This review considered studies that evaluated long-term (>1 year) functional outcomes of GBS in hospitalized children.
Types of studies
This review considered all observational studies, including prospective/retrospective cohort studies, and analytical cross-sectional studies were considered for inclusion. This review also considered descriptive observational study designs, including case series, individual case reports, and descriptive cross-sectional studies for inclusion.
Studies published in English and published up to April 2022 were considered for inclusion in this review.
Methods
This systematic review was conducted by the Joanna Briggs Institute (JBI) methodology for the systematic review of prevalence studies [17, 18]. The previous protocol of this review has been registered in PROSPERO (CRD42019124452).
Population
This review considered studies that included children with GBS.
Search strategy
The search strategy aimed to find both published and unpublished studies conducted by a reviewer (FP). A three-step search strategy was utilized in this review. An initial limited search of PubMed was undertaken followed by an analysis of the text words in the title and abstract and the index terms used to describe the articles. A second search was performed using all identified keywords and index terms up to 1 April 2022 across the following databases, Medline (via PubMed), Cochrane library, Embase, Web of Science, and Scopus. The search for unpublished studies and gray literature included ProQuest (dissertation and thesis), Google Scholar, and grey.net. Finally, the reference lists of all reports and articles selected for critical appraisal were searched for additional studies. Appendix I presents the full search strategy for Embase.
Study selection
Following the search, all identified citations were loaded into Endnote software, version 7.2, and duplicates were removed. Titles and abstracts were screened by two independent reviewers to assess the review against the inclusion criteria. The full text of potentially eligible studies was retrieved and assessed in detail against the inclusion criteria by two independent reviewers (VT & MG). Any disagreements between the reviewers were resolved through discussion, or with a third reviewer.
Assessment of methodological quality
Eligible studies were critically appraised by two independent reviewers (MG & NTT) at the study level using standardized critical appraisal instruments from the Joanna Briggs Institute for cross-sectional studies, and prospective and retrospective cohort studies (Table 1) [19].
.jpg)
Any disagreements between the reviewers were resolved by discussion, or with a third reviewer (MB).
Data extraction
Data were extracted from studies included in the review by two independent reviewers (VT & NR), using the Modified standardized Joanna Briggs Institute (JBI) data extraction tool. The data extracted included specific details about the populations (sample size, mean age, gender), study methods (study designs, follow-up duration), treatments, GBS subtypes, and outcomes of significance to the review question, poor outcome predictors at 1-year follow up, and other outcomes, including mortality rate, relapse rate. Any disagreements between the reviewers were resolved through discussion, or with a third reviewer (MB). Authors of papers were contacted to request missing or additional data.
Data synthesis
Stata software, version 16. was used to report descriptive frequencies and their 95% confidence intervals.
Results
Studies characteristics
The flow diagram of study selection process was shown in Figure 1.
.jpg)
Fourteen studies were included in our meta-analysis consisting of 1141 patients (children suffering from GBS), 955 of whom were followed up for at least one year. Follow-up duration varied from one year to 11 years. Participants included 647 males, 466 females, and 28 unclassified patients.
Among 14 studies, 9 were retrospective, 3 were prospective and two were with cross-sectional design. Table 2 presents the characteristics of each study.
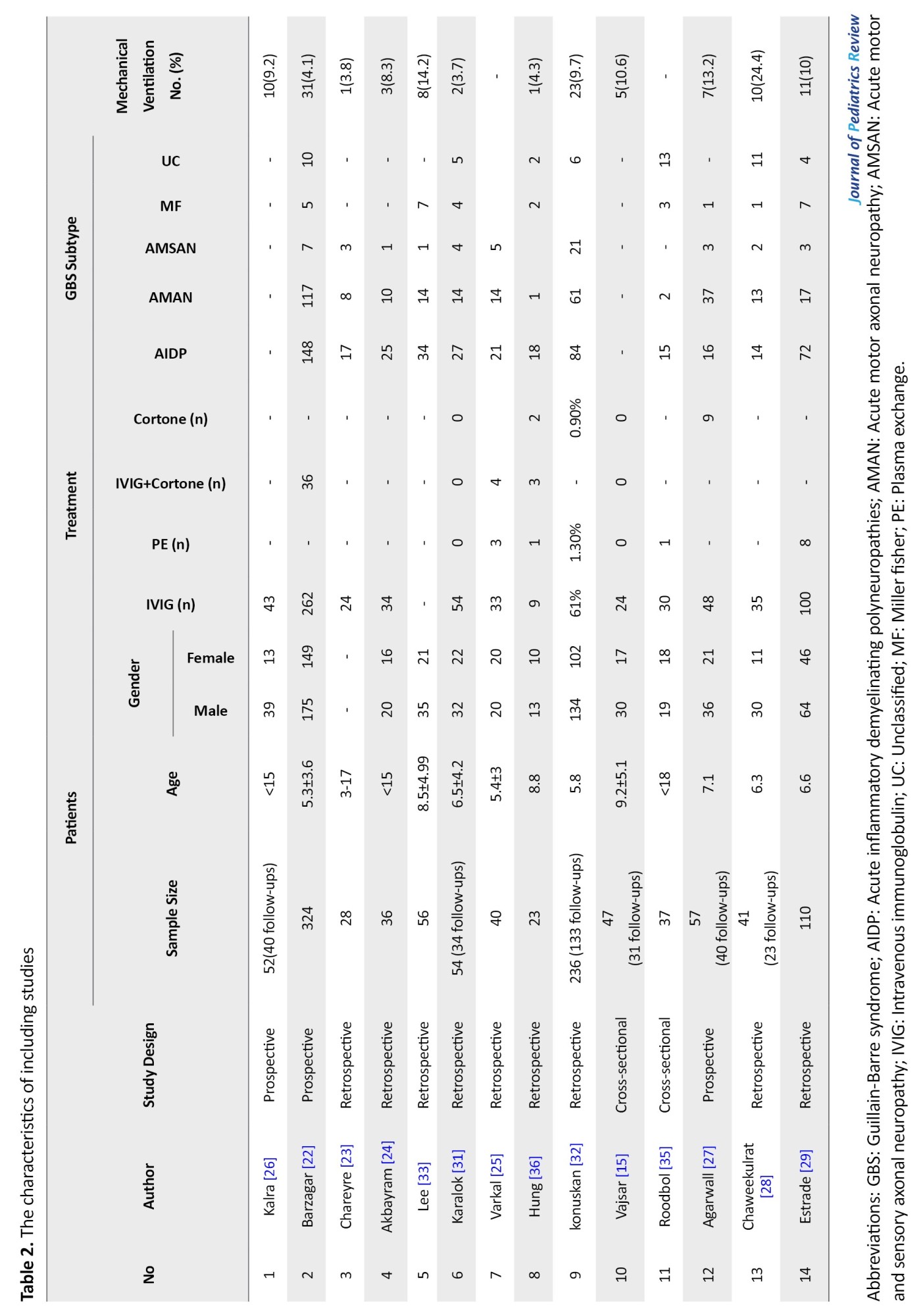
Almost all patients received the treatment of intravenous immunoglobulin (IVIG)±corticosteroid or in limited cases plasmapheresis or corticosteroid alone.
Among the studies that classified GBS subtypes, the prevalence of GBS subtype were as follows, AIDP 46.6% [95% CI: 0.432-0.501], AMAN 30.2% [95% CI: 0.271-0.335], AMSAN 6.8% [95% CI: 0.050-0.090], Miller Fisher (MF) 6.1% [95% CI: 0.040-0.093], and unclassified 7.9% [95% CI: 0.057-0.109].
Poor outcomes and sequels
The most commonly poor outcome after at least one year of follow-up was walking disabilities and gait disorders. Motor deficits and weakness, sensory complaints, including pain or paresthesia and fatigue were other prevalent residual symptoms which are reported in detail in Table 3.
.jpg)
Hughes disability score
Seven studies (630 patients) reported Hughes’s disability score after one year of follow-up. GBS disability scale ≥2 which means the patient can walk at least 10 meters but was unable to run, ranging the overall rate of GBS disability scale ≥2 was 3% for cross-sectional, 3% for prospective, and 8% for retrospective studies. The rate of disability scores equal/under 2 in patients was 4% [CI 95%: 2-8%, P<0.0001], and the rate of disability score of 3 was 4% [CI 95%: 0–13%, P<0.0001]
Mortality and relapse rate
Eleven studies reported mortality rates ranging from zero to six patients (Table 3). The overall mortality rate of 756 patients with up to eleven years of follow-up was 1.98% [CI 95%, 1–5%, P=0.03]. Of 14 studies, 8 studies were included to assign relapse rates among 613 patients. The overall number of relapses was 12 ranging from 0 to 6 (Table 3).
Need for mechanical ventilation
Among 12 studies, including 1064 patients, 123 underwent mechanical ventilation ranging from 3.7% to 24.4% in different studies.
Predictors of poor outcome
Twelve studies reported predictors of poor outcomes, which were extracted from each paper and depicted in Table 4 in detail.
.jpg)
Discussion
Electrodiagnostic findings
GBS subtypes are diagnosed according to clinical and electro-diagnostic features [20]. The most prevalent subtype in this review was AIDP with a prevalence of 46.6%, while previous studies estimated that AIDP was even more prevalent up to 85% [8]. Compared to western countries, AMAN is more common in the Far East [21]. Electrodiagnostic features can be correlated with the long-term prognosis of GBS, which was assessed in eleven studies, in which 8 had axonal type as a poor predictive factor, while 3 studies showed no significant difference between demyelinating and axonal forms in their long-term function.
Among 14 studies, 8 studies reported that the axonal variant of GBS was associated with poor outcomes [22, 23, 24, 25, 26, 27, 28, 29]. Chareyre et al retrospectively studied 28 French children (17 AIDP, 8 AMAN, 3 AMSAN) and compared their short-term and long-term evolution with clinical and electro-diagnostic studies in the intervals of 3,6, and 12 months after diagnosis. Axonal forms had a more severe evolution than demyelinating forms (P<0.05). Axonal forms of GBS in children have a more severe global outcome than demyelinating forms and the axonal type is a poor outcome predictor [23]. Akbayram et al. reported that patients with axonal involvement showed more severe clinical progression than patinas with AIDP. Three (8.3%) patients died; one patient had AIDP and two patients had axonal involvement. Due to the low number of participants, a significant difference was observed between subtypes [24]. Incecik et al. studied 46 GBS patients and retrospectively grouped them to full or partial recovery after two months and concluded that the axonal form subtype was related to poor outcomes (60.4% of patients with partial recovery) [30]. Varkal et al. studied 40 patients (21 AIDP, 14 AMAN, 5 AMSAN) and concluded that the following factors were significantly longer in the axonal type compared to demyelinating type, time until response to treatment (P=0.001), time until aided (P=0.001) and unaided (P=0.002) walking, and time until complete recovery (P=0.002) [25]. Chaweekurat et al. stated demyelinating type is the only predictive factor of independent walking after 1 year in multivariate analysis. It was reported that all 14 patients with AIDP in follow-up could walk independently, while two (8%) of axonal type could not walk independently after a year of follow-up [28]. Estrade et al. reported sequelae in 29 % of children with the axonal form, compared to 5% in those suffering demyelinating form, and indicated it as the first predictive factor of poor prognosis [29].
Three studies [31, 32] stated no difference in the axonal or demyelinating form in predicting the prognosis, Karalok et al. compared the demyelinating and axonal forms, and all of the groups had a favorable outcome with no significant difference, also Cranial nerve involvement rates were similar; however, residual symptoms were only observed in AIDP group in 3 patients. Nevertheless, sensorial and autonomic dysfunction symptoms were highly observed in the axonal forms (AMAN, AMSAN) (P=0.006) [31]. Konuskan et al. enrolled 84 AIDP, 61 AMAN, and 21 AMSAN Clinical scale scores between axonal and demyelinating subgroups did not show statistically significant difference except for admission (P<0.05) and did not affect the prognosis; however, the duration of weakness, duration of hospitalization, and need for mechanical ventilation can negatively affect prognosis [32]. Lee et al. studied 56 patients and divided them according to electrodiagnsotic characteristics (14 AMAN and 34 AIDP) and functional status (disability scale) at nadir and followed them for up to 2 years. They stated that functional status was not different in axonal and demyelinating forms and all of them achieved good functional outcomes for walking but the function at the nadir was the more crucial prognostic factor in the long term [33].
Hughes disability score
Hughes et al. described a disability score for the functional status of patients with GBS. It is a widely accepted score ranging from 0 (healthy) to 6 (dead) and was used to indicate the functional state of patients several studies we included, which considered a score of 2 or more a poor predictive outcome after at least one year of follow up. You can find the classification below [34]:
0. A healthy state
1. Minor symptom and able to run
2. Able to walk 10 m or more without assistance but unable to run
3. Able to walk 10 m across an open space with the help
4. Bedridden or chair bound
5. Requiring assisted ventilation for at least part of the day
6. Dead
Lee et al. stated that functional status at nadir calculated by the Hughes scale (3.56±1.11 in the AIDP group, 3.86±1.17 in the AMAN group with no significant difference), was a more critical factor than electrophysiological subtypes in predicting long-term outcome [33]. According to the long-term (11 years of follow-up) study of Roodbol, most untreated patients were mildly affected (GBS disability score ≤2). No difference was observed in outcomes between treated and untreated patients [35].
Time to independent walking
Three studies reported time to independent walking [22, 27, 28]. A retrospective cohort by Chaweekulrat et al. with about 8 years of follow-up evaluated factors affecting time to independent walking in GBS children and produced a prediction model. They developed a prognostic scoring system in which a score of 5 required a mean of 34 days to gain independent walking. Conversely, patients with a score of zero needed a mean of 158 days to achieve the ability to walk independently (P=0.008) [28]. Barzegar et al’s study on 342 children with GBS showed that the mean time to independent walking was 2.97±3.02 months and, eventually 96% achieved this goal within one year [22]. Agrawal et al. calculated the time to independent walking with the maximum period of follow-up of one year to be 68.2±16.8 days ranging from 9 to 305 days [27].
Poor outcomes and sequels of Guillain-Barre syndrome (GBS)
The clinical course of GBS is highly variable both regarding the severity and the speed of recovery. Even if most children lose the ability to walk in the acute phase and become worse in nadir, most of them recover (almost) completely after a few months, while still suffering from long-term ambulatory problems, they have even completely lost their ambulation as Chaweekulrat et al. [28]stated 4.3% of patients became bedridden. In addition to walking disabilities other symptoms also remain as residual deficits in these patients as follows. According to Karalok’s retrospective study, among 31 patients who completed one-year follow-up, 27 patients had a full recovery, one died, one had a relapse and 3 patients had residual symptoms of night pain or paresthesia, seen in AIDP patients [31].
In a two-year follow-up of 47 patients, Vasjar stated that persisting long-term weakness at least in one muscle was observed in 23% of cases, nevertheless, it had minimal impact on function. Weakness was predicted by young age (P=0.03) and a rapid progression to maximal weakness (P=0.03) predicted long-term poor outcomes [15].
Roodbol et al. conducted a cross-sectional cohort to study long-term outcomes of GBS, among 37 participating patients, 23 were now adults, with a median age of 20 years (range 4 to 39 years), and a median follow-up time of 11 years. Residual complaints were reported by 24 (65%) patients, including paresthesias (38%), unsteadiness of gait in the dark (37%), painful hands or feet (24%), and severe fatigue (22%). Four patients had severe neurological deficits, including facial diplegia and limb weakness [35]. Barzegar et al. studied 324 GBS patients with intervals of 2 and 6 months to determine predictive factors of independent walking. Disability scores of >3 (P=0.03), autonomic involvement (P=0.003), cranial involvement (P=0.008), and absent CMAP (P=0.048) were significantly associated with poor walking outcomes [22]. Hung et al. studied 23 patients and at 1 year or more follow-up, 20 patients (87%) recovered and three (13%) had long-term deficits. Of these three people, two could not walk independently [36]. According to the study conducted by Konuskan at a year follow-up, 85.6% of children had a normal neurological examination; 9% were able to walk 5 meters without aid, 3.8% with aid and 1.5% were bedridden [32].
Estrade et al concluded that of GBS patients, ten had sequels. Among six patients with a GBS disability score of 2, three had a motor deficit, two had fatigue and one had peripheral facial paralysis. Among four with a GBS score of 1, each had motor deficits, oculomotor paralysis, residual pain, or pyramidal syndrome [29].
Need for respiratory support
It is estimated that mechanical ventilation (MV) is required in about 20% to 30% of children suffering GBS as a risk factor for poor outcomes. Recovery in mechanically ventilated GBS patients may be prolonged [37, 38, 39]. Cole et al. found that among 11 children with GBS who were undergone MV, two died in the acute phase and nine made an excellent recovery, and the need for MV may not necessarily be a bad prognostic factor for neurological recovery in children [39].
Patients requiring MV had higher disability scores at admission, discharge, and 6 and 12 months after discharge (P<0.001) [32].
In our meta-analysis, 123 cases needed respiratory support and were intubated (about 10% of patients). Fletcher et al. compared the outcomes of mechanically ventilated and non-ventilated GBS patients (60 vs. 56). MV was required in 81% of patients with a poor outcome, but those who survived were well able to gain ambulation. Predictors of poor recovery were increased age (P=0.001), upper limb paralysis (P=0.004), and duration of ventilation (P=0.006) [37].
Kalra et al. studied 52 children with one-year follow-up, 95% of children had fully recovered or had minimal symptoms. The vital prognostic factor was the need for artificial ventilation and other factors were inevitable nerves in the nerve conduction test and delay in independent walking [26].
Mortality and relapse rate
The overall mortality rate with up to eleven years of follow-up was 2.6%. Nine studies reported mortality rates ranging from zero to six patients. The highest mortality rate in the acute phase was 11.5% (6 out of 52 patients) [26].
Acute relapse was defined as the worsening of the clinical condition after the initial improvement for at least a week following treatment [40]. The relapse rate in the included studies was 1%- 2%. The relapse rate between the groups was not delayed at any type of treatment [41].
Treatment
First-line treatment for GBS includes IVIG and plasmapheresis which have beneficial effects in recovering symptoms and preventing severe complications to occur. The results of a clinical trial on the IVIG showed a significant improvement in the IVIG group, the rate of intubation and mechanical ventilation was significantly lower in the IVIG group and the period of staying in the ICU was shorter compared to the control group [42]. Of the 10 untreated patients in the study of Estrade, 8 had lost their ability to walk and just 2 were recovering [29]
Conclusion
This review evaluated the long-term outcomes of GBS among children using data from the English language literature. The main limitation of this review was the retrospective nature of most included studies, also variations in outcomes were evaluated in different studies despite the good prognosis of GBS in children compared to adults, significant sequels exist especially in the walking ability and gait of patients. The main outcome evaluated in most studies included disability score. The most predictive factors for poor outcomes in included studies were the axonal form of GBS, disability score and function at nadir, and the need for mechanical ventilation.
However, further investigations are recommended to determine outcome predictors and evaluate the role of rehabilitation in reducing long-term disabilities of GBS in children.
It is also recommended to develop and validate a model to predict the risk of poor outcomes and long-term disability among children with GBS.
Ethical Considerations
Compliance with ethical guidelines
This article is a systematic review with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and Supervision: Vahdieh Toopchizadeh and Mohammad Barzegar; Search strategy and analysis: Fariba Pashazadeh; Study selection and appraise: Vahideh Toopchizadeh, Masood Ghahvechi-Akbari, Nasim Rashedi, Negar Taleschian-Tabrizi; Data extraction: Nasim Rashedi, Vahideh Toopchizadeh; Drafting the review: Negar Taleschian-Tabrizi, Fariba Pashazadeh; Ozra nouri.
Conflicts of interest
The authors declared no conflict of interest.
References
Guillan-Barre syndrome (GBS) is an acute neurological disorder that is a common etiology for acute flaccid paralysis in children. Some patients step into remitting phase 7-14 days after disease onset, but it can result in life-threatening situations and long-term disabilities [1]. The age group of the affected patients lies between 3 to 6 years old [2]. The estimated incidence of disease per year is 1.1-1.8/100000 in 2009, which increases up to 3.3/100000 after age 50 and the prognosis is worse than younger ages [3].
The most common clinical presentation of this syndrome is a bilateral ascending weakness with the absence of deep tendon reflexes [4]. Impairment in the autonomic systems, such as cardiac dysrhythmias, instability of blood pressure, disability of controlling bowel and bladder, and paralysis of bulbar muscles results in dysarthria, absent gag reflex, and dysphagia; motor and sensory involvement leading to bilateral ascending paralysis and reduced reflexes are the other complications caused by this disease [5, 6, 7].
Based on the clinical presentation and electrophysiological studies, Guillan-Barre has some subtypes, acute inflammatory demyelinating polyneuropathy (AIDP), acute motor axonal neuropathy (AMAN), acute motor and sensory axonal neuropathy (AMSAN) and Miller-Fischer syndrome [8]. The incidence of these subtypes is different in every geographical area. For example, AIDP is the most common variant in North America; however, AMAN is mainly seen in South America, China, and Japan [7, 9]. Prognosis and outcomes vary between subtypes, the majority of AMAN patients have a history of diarrhea before experiencing GBS symptoms and their period of hospitalization is more than AIDP type [10]. Infectious viral agents, such as Campylobacter jejuni, Epstein-Barr virus, Varicella Zoster virus and more recently Zika and COVID-19 viruses were the most prevalent agents causing GBS [11, 12, 13].
Although treatment modalities help patients recover from acute phase and serious complications, some disabilities, and residual motor and sensory signs and symptoms may remain and cause psychosocial problems that can greatly affect the patient’s quality of life causing mobility difficulties, pain, fatigue, and depression [14]. The prognosis in patients with GBS differs in adults and children. Since the clinical feature is more severe and long-term outcomes are poorer in adults. Thus, the studies of the course and prognosis of childhood GBS should be considered independently from adult studies [15]. Even if children recover faster and better than adults, long-term disability and the impact of childhood are significant and poorly defined [16]. That is why this systematic review aimed to include children suffering from GBS.
Considering long-term disabilities caused by GBS and its psychosocial effects, we conducted a systematic review to evaluate pediatric patients with GBS in terms of long-term disabilities caused by the disease and predictive factors of poor outcomes. Long-term functional outcomes included disability score, walking ability, need for mechanical ventilation, mortality rate, relapse rate, and residual disability.
Review question
What are the long-term outcomes of Guillan-Barre syndrome in children?
What are the poor outcome predictors of Guillan-Barre syndrome in children?
Inclusion criteria
Condition
This review considered studies that included long-term (>1 year) functional outcomes of GBS in children, long-term functional outcomes, including disability score, mortality rate, relapse rate, walking ability and residual disability, and predictors of poor outcome of GBS in children.
Context
This review considered studies that evaluated long-term (>1 year) functional outcomes of GBS in hospitalized children.
Types of studies
This review considered all observational studies, including prospective/retrospective cohort studies, and analytical cross-sectional studies were considered for inclusion. This review also considered descriptive observational study designs, including case series, individual case reports, and descriptive cross-sectional studies for inclusion.
Studies published in English and published up to April 2022 were considered for inclusion in this review.
Methods
This systematic review was conducted by the Joanna Briggs Institute (JBI) methodology for the systematic review of prevalence studies [17, 18]. The previous protocol of this review has been registered in PROSPERO (CRD42019124452).
Population
This review considered studies that included children with GBS.
Search strategy
The search strategy aimed to find both published and unpublished studies conducted by a reviewer (FP). A three-step search strategy was utilized in this review. An initial limited search of PubMed was undertaken followed by an analysis of the text words in the title and abstract and the index terms used to describe the articles. A second search was performed using all identified keywords and index terms up to 1 April 2022 across the following databases, Medline (via PubMed), Cochrane library, Embase, Web of Science, and Scopus. The search for unpublished studies and gray literature included ProQuest (dissertation and thesis), Google Scholar, and grey.net. Finally, the reference lists of all reports and articles selected for critical appraisal were searched for additional studies. Appendix I presents the full search strategy for Embase.

Study selection
Following the search, all identified citations were loaded into Endnote software, version 7.2, and duplicates were removed. Titles and abstracts were screened by two independent reviewers to assess the review against the inclusion criteria. The full text of potentially eligible studies was retrieved and assessed in detail against the inclusion criteria by two independent reviewers (VT & MG). Any disagreements between the reviewers were resolved through discussion, or with a third reviewer.
Assessment of methodological quality
Eligible studies were critically appraised by two independent reviewers (MG & NTT) at the study level using standardized critical appraisal instruments from the Joanna Briggs Institute for cross-sectional studies, and prospective and retrospective cohort studies (Table 1) [19].
.jpg)
Any disagreements between the reviewers were resolved by discussion, or with a third reviewer (MB).
Data extraction
Data were extracted from studies included in the review by two independent reviewers (VT & NR), using the Modified standardized Joanna Briggs Institute (JBI) data extraction tool. The data extracted included specific details about the populations (sample size, mean age, gender), study methods (study designs, follow-up duration), treatments, GBS subtypes, and outcomes of significance to the review question, poor outcome predictors at 1-year follow up, and other outcomes, including mortality rate, relapse rate. Any disagreements between the reviewers were resolved through discussion, or with a third reviewer (MB). Authors of papers were contacted to request missing or additional data.
Data synthesis
Stata software, version 16. was used to report descriptive frequencies and their 95% confidence intervals.
Results
Studies characteristics
The flow diagram of study selection process was shown in Figure 1.
.jpg)
Fourteen studies were included in our meta-analysis consisting of 1141 patients (children suffering from GBS), 955 of whom were followed up for at least one year. Follow-up duration varied from one year to 11 years. Participants included 647 males, 466 females, and 28 unclassified patients.
Among 14 studies, 9 were retrospective, 3 were prospective and two were with cross-sectional design. Table 2 presents the characteristics of each study.

Almost all patients received the treatment of intravenous immunoglobulin (IVIG)±corticosteroid or in limited cases plasmapheresis or corticosteroid alone.
Among the studies that classified GBS subtypes, the prevalence of GBS subtype were as follows, AIDP 46.6% [95% CI: 0.432-0.501], AMAN 30.2% [95% CI: 0.271-0.335], AMSAN 6.8% [95% CI: 0.050-0.090], Miller Fisher (MF) 6.1% [95% CI: 0.040-0.093], and unclassified 7.9% [95% CI: 0.057-0.109].
Poor outcomes and sequels
The most commonly poor outcome after at least one year of follow-up was walking disabilities and gait disorders. Motor deficits and weakness, sensory complaints, including pain or paresthesia and fatigue were other prevalent residual symptoms which are reported in detail in Table 3.
.jpg)
Hughes disability score
Seven studies (630 patients) reported Hughes’s disability score after one year of follow-up. GBS disability scale ≥2 which means the patient can walk at least 10 meters but was unable to run, ranging the overall rate of GBS disability scale ≥2 was 3% for cross-sectional, 3% for prospective, and 8% for retrospective studies. The rate of disability scores equal/under 2 in patients was 4% [CI 95%: 2-8%, P<0.0001], and the rate of disability score of 3 was 4% [CI 95%: 0–13%, P<0.0001]
Mortality and relapse rate
Eleven studies reported mortality rates ranging from zero to six patients (Table 3). The overall mortality rate of 756 patients with up to eleven years of follow-up was 1.98% [CI 95%, 1–5%, P=0.03]. Of 14 studies, 8 studies were included to assign relapse rates among 613 patients. The overall number of relapses was 12 ranging from 0 to 6 (Table 3).
Need for mechanical ventilation
Among 12 studies, including 1064 patients, 123 underwent mechanical ventilation ranging from 3.7% to 24.4% in different studies.
Predictors of poor outcome
Twelve studies reported predictors of poor outcomes, which were extracted from each paper and depicted in Table 4 in detail.
.jpg)
Discussion
Electrodiagnostic findings
GBS subtypes are diagnosed according to clinical and electro-diagnostic features [20]. The most prevalent subtype in this review was AIDP with a prevalence of 46.6%, while previous studies estimated that AIDP was even more prevalent up to 85% [8]. Compared to western countries, AMAN is more common in the Far East [21]. Electrodiagnostic features can be correlated with the long-term prognosis of GBS, which was assessed in eleven studies, in which 8 had axonal type as a poor predictive factor, while 3 studies showed no significant difference between demyelinating and axonal forms in their long-term function.
Among 14 studies, 8 studies reported that the axonal variant of GBS was associated with poor outcomes [22, 23, 24, 25, 26, 27, 28, 29]. Chareyre et al retrospectively studied 28 French children (17 AIDP, 8 AMAN, 3 AMSAN) and compared their short-term and long-term evolution with clinical and electro-diagnostic studies in the intervals of 3,6, and 12 months after diagnosis. Axonal forms had a more severe evolution than demyelinating forms (P<0.05). Axonal forms of GBS in children have a more severe global outcome than demyelinating forms and the axonal type is a poor outcome predictor [23]. Akbayram et al. reported that patients with axonal involvement showed more severe clinical progression than patinas with AIDP. Three (8.3%) patients died; one patient had AIDP and two patients had axonal involvement. Due to the low number of participants, a significant difference was observed between subtypes [24]. Incecik et al. studied 46 GBS patients and retrospectively grouped them to full or partial recovery after two months and concluded that the axonal form subtype was related to poor outcomes (60.4% of patients with partial recovery) [30]. Varkal et al. studied 40 patients (21 AIDP, 14 AMAN, 5 AMSAN) and concluded that the following factors were significantly longer in the axonal type compared to demyelinating type, time until response to treatment (P=0.001), time until aided (P=0.001) and unaided (P=0.002) walking, and time until complete recovery (P=0.002) [25]. Chaweekurat et al. stated demyelinating type is the only predictive factor of independent walking after 1 year in multivariate analysis. It was reported that all 14 patients with AIDP in follow-up could walk independently, while two (8%) of axonal type could not walk independently after a year of follow-up [28]. Estrade et al. reported sequelae in 29 % of children with the axonal form, compared to 5% in those suffering demyelinating form, and indicated it as the first predictive factor of poor prognosis [29].
Three studies [31, 32] stated no difference in the axonal or demyelinating form in predicting the prognosis, Karalok et al. compared the demyelinating and axonal forms, and all of the groups had a favorable outcome with no significant difference, also Cranial nerve involvement rates were similar; however, residual symptoms were only observed in AIDP group in 3 patients. Nevertheless, sensorial and autonomic dysfunction symptoms were highly observed in the axonal forms (AMAN, AMSAN) (P=0.006) [31]. Konuskan et al. enrolled 84 AIDP, 61 AMAN, and 21 AMSAN Clinical scale scores between axonal and demyelinating subgroups did not show statistically significant difference except for admission (P<0.05) and did not affect the prognosis; however, the duration of weakness, duration of hospitalization, and need for mechanical ventilation can negatively affect prognosis [32]. Lee et al. studied 56 patients and divided them according to electrodiagnsotic characteristics (14 AMAN and 34 AIDP) and functional status (disability scale) at nadir and followed them for up to 2 years. They stated that functional status was not different in axonal and demyelinating forms and all of them achieved good functional outcomes for walking but the function at the nadir was the more crucial prognostic factor in the long term [33].
Hughes disability score
Hughes et al. described a disability score for the functional status of patients with GBS. It is a widely accepted score ranging from 0 (healthy) to 6 (dead) and was used to indicate the functional state of patients several studies we included, which considered a score of 2 or more a poor predictive outcome after at least one year of follow up. You can find the classification below [34]:
0. A healthy state
1. Minor symptom and able to run
2. Able to walk 10 m or more without assistance but unable to run
3. Able to walk 10 m across an open space with the help
4. Bedridden or chair bound
5. Requiring assisted ventilation for at least part of the day
6. Dead
Lee et al. stated that functional status at nadir calculated by the Hughes scale (3.56±1.11 in the AIDP group, 3.86±1.17 in the AMAN group with no significant difference), was a more critical factor than electrophysiological subtypes in predicting long-term outcome [33]. According to the long-term (11 years of follow-up) study of Roodbol, most untreated patients were mildly affected (GBS disability score ≤2). No difference was observed in outcomes between treated and untreated patients [35].
Time to independent walking
Three studies reported time to independent walking [22, 27, 28]. A retrospective cohort by Chaweekulrat et al. with about 8 years of follow-up evaluated factors affecting time to independent walking in GBS children and produced a prediction model. They developed a prognostic scoring system in which a score of 5 required a mean of 34 days to gain independent walking. Conversely, patients with a score of zero needed a mean of 158 days to achieve the ability to walk independently (P=0.008) [28]. Barzegar et al’s study on 342 children with GBS showed that the mean time to independent walking was 2.97±3.02 months and, eventually 96% achieved this goal within one year [22]. Agrawal et al. calculated the time to independent walking with the maximum period of follow-up of one year to be 68.2±16.8 days ranging from 9 to 305 days [27].
Poor outcomes and sequels of Guillain-Barre syndrome (GBS)
The clinical course of GBS is highly variable both regarding the severity and the speed of recovery. Even if most children lose the ability to walk in the acute phase and become worse in nadir, most of them recover (almost) completely after a few months, while still suffering from long-term ambulatory problems, they have even completely lost their ambulation as Chaweekulrat et al. [28]stated 4.3% of patients became bedridden. In addition to walking disabilities other symptoms also remain as residual deficits in these patients as follows. According to Karalok’s retrospective study, among 31 patients who completed one-year follow-up, 27 patients had a full recovery, one died, one had a relapse and 3 patients had residual symptoms of night pain or paresthesia, seen in AIDP patients [31].
In a two-year follow-up of 47 patients, Vasjar stated that persisting long-term weakness at least in one muscle was observed in 23% of cases, nevertheless, it had minimal impact on function. Weakness was predicted by young age (P=0.03) and a rapid progression to maximal weakness (P=0.03) predicted long-term poor outcomes [15].
Roodbol et al. conducted a cross-sectional cohort to study long-term outcomes of GBS, among 37 participating patients, 23 were now adults, with a median age of 20 years (range 4 to 39 years), and a median follow-up time of 11 years. Residual complaints were reported by 24 (65%) patients, including paresthesias (38%), unsteadiness of gait in the dark (37%), painful hands or feet (24%), and severe fatigue (22%). Four patients had severe neurological deficits, including facial diplegia and limb weakness [35]. Barzegar et al. studied 324 GBS patients with intervals of 2 and 6 months to determine predictive factors of independent walking. Disability scores of >3 (P=0.03), autonomic involvement (P=0.003), cranial involvement (P=0.008), and absent CMAP (P=0.048) were significantly associated with poor walking outcomes [22]. Hung et al. studied 23 patients and at 1 year or more follow-up, 20 patients (87%) recovered and three (13%) had long-term deficits. Of these three people, two could not walk independently [36]. According to the study conducted by Konuskan at a year follow-up, 85.6% of children had a normal neurological examination; 9% were able to walk 5 meters without aid, 3.8% with aid and 1.5% were bedridden [32].
Estrade et al concluded that of GBS patients, ten had sequels. Among six patients with a GBS disability score of 2, three had a motor deficit, two had fatigue and one had peripheral facial paralysis. Among four with a GBS score of 1, each had motor deficits, oculomotor paralysis, residual pain, or pyramidal syndrome [29].
Need for respiratory support
It is estimated that mechanical ventilation (MV) is required in about 20% to 30% of children suffering GBS as a risk factor for poor outcomes. Recovery in mechanically ventilated GBS patients may be prolonged [37, 38, 39]. Cole et al. found that among 11 children with GBS who were undergone MV, two died in the acute phase and nine made an excellent recovery, and the need for MV may not necessarily be a bad prognostic factor for neurological recovery in children [39].
Patients requiring MV had higher disability scores at admission, discharge, and 6 and 12 months after discharge (P<0.001) [32].
In our meta-analysis, 123 cases needed respiratory support and were intubated (about 10% of patients). Fletcher et al. compared the outcomes of mechanically ventilated and non-ventilated GBS patients (60 vs. 56). MV was required in 81% of patients with a poor outcome, but those who survived were well able to gain ambulation. Predictors of poor recovery were increased age (P=0.001), upper limb paralysis (P=0.004), and duration of ventilation (P=0.006) [37].
Kalra et al. studied 52 children with one-year follow-up, 95% of children had fully recovered or had minimal symptoms. The vital prognostic factor was the need for artificial ventilation and other factors were inevitable nerves in the nerve conduction test and delay in independent walking [26].
Mortality and relapse rate
The overall mortality rate with up to eleven years of follow-up was 2.6%. Nine studies reported mortality rates ranging from zero to six patients. The highest mortality rate in the acute phase was 11.5% (6 out of 52 patients) [26].
Acute relapse was defined as the worsening of the clinical condition after the initial improvement for at least a week following treatment [40]. The relapse rate in the included studies was 1%- 2%. The relapse rate between the groups was not delayed at any type of treatment [41].
Treatment
First-line treatment for GBS includes IVIG and plasmapheresis which have beneficial effects in recovering symptoms and preventing severe complications to occur. The results of a clinical trial on the IVIG showed a significant improvement in the IVIG group, the rate of intubation and mechanical ventilation was significantly lower in the IVIG group and the period of staying in the ICU was shorter compared to the control group [42]. Of the 10 untreated patients in the study of Estrade, 8 had lost their ability to walk and just 2 were recovering [29]
Conclusion
This review evaluated the long-term outcomes of GBS among children using data from the English language literature. The main limitation of this review was the retrospective nature of most included studies, also variations in outcomes were evaluated in different studies despite the good prognosis of GBS in children compared to adults, significant sequels exist especially in the walking ability and gait of patients. The main outcome evaluated in most studies included disability score. The most predictive factors for poor outcomes in included studies were the axonal form of GBS, disability score and function at nadir, and the need for mechanical ventilation.
However, further investigations are recommended to determine outcome predictors and evaluate the role of rehabilitation in reducing long-term disabilities of GBS in children.
It is also recommended to develop and validate a model to predict the risk of poor outcomes and long-term disability among children with GBS.
Ethical Considerations
Compliance with ethical guidelines
This article is a systematic review with no human or animal sample.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and Supervision: Vahdieh Toopchizadeh and Mohammad Barzegar; Search strategy and analysis: Fariba Pashazadeh; Study selection and appraise: Vahideh Toopchizadeh, Masood Ghahvechi-Akbari, Nasim Rashedi, Negar Taleschian-Tabrizi; Data extraction: Nasim Rashedi, Vahideh Toopchizadeh; Drafting the review: Negar Taleschian-Tabrizi, Fariba Pashazadeh; Ozra nouri.
Conflicts of interest
The authors declared no conflict of interest.
References
- Willison HJ, Jacobs BC, Van Doorn PA. Guillain-Barré syndrome. Lancet. 2016; 388(10045):717-27.[DOI:10.1016/S0140-6736(16)00339-1] [PMID]
- Hughes RA, Rees JH. Clinical and epidemiologic features of Guillain-Barré syndrome. J Infect Dis. 1997; 176(Suppl 2):S92-8. [DOI:10.1086/513793] [PMID]
- Mc Grogan A, Madle GC, Seaman HE, De Vries CS. The epidemiology of Guillain-Barré syndrome worldwide. A systematic literature review. Neuroepidemiology. 2009; 32(2):150-63. [DOI:10.1159/000184748] [PMID]
- Dhadke SV, Dhadke VN, Bangar SS, Korade MB. Clinical profile of Guillain Barre syndrome. J Assoc Physicians India. 2013; 61(3):168-72. [PMID]
- Korinthenberg R, Mönting JS. Natural history and treatment effects in Guillain-Barré syndrome: A multicentre study. Arch Dis Child. 1996; 74(4):281-7.[DOI:10.1136/adc.74.4.281] [PMID] [PMCID]
- Rantala H, Uhari M, Niemelä M. Occurrence, clinical manifestations, and prognosis of Guillain-Barré syndrome. Arch Dis Child. 1991; 66(6):706-8. [DOI:10.1136/adc.66.6.706] [PMID] [PMCID]
- Asahina M, Kuwabara S, Suzuki A, Hattori T. Autonomic function in demyelinating and axonal subtypes of Guillain-Barré syndrome. Acta Neurol Scand. 2002; 105(1):44-50. [DOI:10.1034/j.1600-0404.2002.00099.x] [PMID]
- Pithadia AB, Kakadia N. Guillain-Barré syndrome (GBS). Pharmacol Rep. 2010; 62(2):220-32. [DOI:10.1016/S1734-1140(10)70261-9] [PMID]
- Zhang G, Li Q, Zhang R, Wei X, Wang J, Qin X. Subtypes and prognosis of Guillain-Barré Syndrome in southwest China. Plos One. 2015; 10(7):e0133520. [DOI:10.1371/journal.pone.0133520] [PMID] [PMCID]
- Tian J, Cao C, Li T, Zhang K, Li P, Liu Y, et al. Electrophysiological subtypes and prognostic factors of Guillain-Barre syndrome in northern China. Front Neurol. 2019; 10:714. [DOI:10.3389/fneur.2019.00714] [PMID] [PMCID]
- Yuki N, Hartung HP. Guillain-Barré syndrome. N Engl J Med. 2012; 366(24):2294-304. [DOI:10.1056/NEJMra1114525] [PMID]
- Rozé B, Najioullah F, Fergé JL, Dorléans F, Apetse K, Barnay JL, et al. Guillain-Barré syndrome associated with zika virus infection in martinique in 2016: A prospective study. Clin Infect Dis. 2017; 65(9):1462-8. [DOI:10.1093/cid/cix588] [PMID]
- Eslamian F, Taleschian-Tabrizi N, Izadseresht B, Shakouri SK, Gholian S, Rahbar M. Electrophysiologic findings in patients with COVID-19 and quadriparesia in the northwest of Iran: A case series study and literature review. Caspian J Intern Med. 2021; 12(Suppl 2):S451-9. [DOI:10.22088/cjim.12.0.451] [PMID] [PMCID]
- Darweesh SK, Polinder S, Mulder MJ, Baena CP, van Leeuwen N, Franco OH, et al. Health-related quality of life in Guillain-Barré syndrome patients: A systematic review. J Peripher Nerv Syst. 2014; 19(1):24-35. [DOI:10.1111/jns5.12051] [PMID]
- Vajsar J, Fehlings D, Stephens D. Long-term outcome in children with Guillain-Barré syndrome. J Pediatr. 2003; 142(3):305-9. [DOI:10.1067/mpd.2003.115] [PMID]
- Juurlink DN, Stukel TA, Kwong J, Kopp A, McGeer A, Upshur RE, et al. Guillain-Barré syndrome after influenza vaccination in adults: A population-based study. Arch Intern Med. 2006; 166(20):2217-21. [DOI:10.1001/archinte.166.20.2217] [PMID]
- Peters MD, Godfrey CM, McInerney P, Soares CB, Khalil H, Parker D. Methodology for JBI scoping reviews. In: Aromataris E, Editor. The Joanna Briggs Institute Reviewers' Manual 2015. Adelaide: The Joanna Briggs Institute; 2015. [Link]
- Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015; 13(3):147-53. [DOI:10.1097/XEB.0000000000000054] [PMID]
- Joanna Briggs Institute. Critical Appraisal tools for use in JBI Systematic reviiews. [Internet]. 2023 [Updated 2022 April 20] Available from: [Link]
- Hughes RA, Cornblath DR. Guillain-Barré syndrome. Lancet. 2005; 366(9497):1653-66. [DOI:10.1016/S0140-6736(05)67665-9] [PMID]
- Hiraga A, Mori M, Ogawara K, Hattori T, Kuwabara S. Differences in patterns of progression in demyelinating and axonal Guillain-Barré syndromes. Neurology. 2003; 61(4):471-4. [DOI:10.1212/01.WNL.0000081231.08914.A1] [PMID]
- Barzegar M, Toopchizadeh V, Maher MHK, Sadeghi P, Jahanjoo F, Pishgahi A. Predictive factors for achieving independent walking in children with Guillain-Barre syndrome. Pediatr Res. 2017; 82(2):333-9. [DOI:10.1038/pr.2017.67] [PMID]
- Chareyre J, Hully M, Simonnet H, Musset L, Barnerias C, Kossorotoff M, et al. Acute axonal neuropathy subtype of Guillain Barré syndrome in a French pediatric series: Adequate follow-up may require repetitive electrophysiological studies. Eur J Paediatr Neurol. 2017; 21(6):891-7. [DOI:10.1016/j.ejpn.2017.07.005] [PMID]
- Akbayram S, Doğan M, Akgün C, Peker E, Sayιn R, Aktar F, et al. Clinical features and prognosis with Guillain-Barré syndrome. Ann Indian Acad Neurol. 2011; 14(2):98-102. [DOI:10.4103/0972-2327.82793] [PMID] [PMCID]
- Varkal MA, Uzunhan TA, Aydınlı N, Ekici B, Çalışkan M, Özmen M. Pediatric Guillain-Barré syndrome: Indicators for a severe course. Ann Indian Acad Neurol. 2015; 18(1):24-8. [DOI:10.4103/0972-2327.144274] [PMID] [PMCID]
- Kalra V, Sankhyan N, Sharma S, Gulati S, Choudhry R, Dhawan B. Outcome in childhood Guillain-Barré syndrome. Indian J Pediatr. 2009; 76(8):795-9. [DOI:10.1007/s12098-009-0125-y] [PMID]
- Agarwal E, Bhagat A, Srivastava K, Thakore B, Jagtap S, Kalane U, et al. Clinical and electrophysiological factors predicting prolonged recovery in children with Guillain-Barré syndrome. Indian J Pediatr. 2022; 89(5):452-8. [DOI:10.1007/s12098-021-03804-7] [PMID]
- Chaweekulrat P, Sanmaneechai O. Prognostic model for time to achieve independent walking in children with Guillain-Barré syndrome. Pediatr Res. 2022; 92(5):1417-22. [DOI:10.1038/s41390-021-01919-3] [PMID] [PMCID]
- Estrade S, Guiomard C, Fabry V, Baudou E, Cances C, Chaix Y, et al. Prognostic factors for the sequelae and severity of Guillain-Barré syndrome in children. Muscle Nerve. 2019; 60(6):716-23. [DOI:10.1002/mus.26706] [PMID]
- Incecik F, Ozlem Hergüner M, Altunbasak S. Guillain-Barré syndrome in children. Neurol Sci. 2011; 32(3):381-5. [DOI:10.1007/s10072-010-0434-y] [PMID]
- Karalok ZS, Taskin BD, Yanginlar ZB, Gurkas E, Guven A, Degerliyurt A, et al. Guillain-Barré syndrome in children: Subtypes and outcome. Childs Nerv Syst. 2018; 34(11):2291-7. [DOI:10.1007/s00381-018-3856-0] [PMID]
- Konuşkan B, Okuyaz Ç, Taşdelen B, Kurul SH, Anlar B, Turkish Childhood Guillan-Barre Syndrome Study Group. Electrophysiological subtypes and prognostic factors of childhood Guillain-Barré syndrome. Noro Psikiyatr Ars. 2018; 55(3):199-204. [DOI:10.5152/npa.2017.16996] [PMID] [PMCID]
- Lee JH, Sung IY, Rew IS. Clinical presentation and prognosis of childhood Guillain-Barré syndrome. J Paediatr Child Health. 2008; 44(7-8):449-54. [DOI:10.1111/j.1440-1754.2008.01325.x] [PMID]
- Hughes RA, Newsom-Davis JM, Perkin GD, Pierce JM. Controlled trial prednisolone in acute polyneuropathy. Lancet. 1978; 2(8093):750-3. [DOI:10.1016/S0140-6736(78)92644-2] [PMID]
- Roodbol J, De Wit MC, Aarsen FK, Catsman-Berrevoets CE, Jacobs BC. Long-term outcome of Guillain-Barré syndrome in children. J Peripher Nerv Syst. 2014; 19(2):121-6. [DOI:10.1111/jns5.12068] [PMID]
- Hung PL, Chang WN, Huang LT, Huang SC, Chang YC, Chang CJ, et al. A clinical and electrophysiologic survey of childhood Guillain-Barré syndrome. Pediatr Neurol. 2004; 30(2):86-91. [DOI:10.1016/S0887-8994(03)00403-X] [PMID]
- Fletcher DD, Lawn ND, Wolter TD, Wijdicks EF. Long-term outcome in patients with Guillain-Barré syndrome requiring mechanical ventilation. Neurology. 2000; 54(12):2311-5. [DOI:10.1212/WNL.54.12.2311] [PMID]
- Lawn ND, Wijdicks EF. Fatal Guillain-Barré syndrome. Neurology. 1999; 52(3):635-8. [DOI:10.1212/WNL.52.3.635] [PMID]
- Cole GF, Matthew DJ. Prognosis in severe Guillain-Barré syndrome. Arch Dis Child. 1987; 62(3):288-91. [DOI:10.1136/adc.62.3.288] [PMID] [PMCID]
- Koul RL, Alfutaisi A. Prospective study of children with Guillain-Barre syndrome. Indian J Pediatr. 2008; 75(8):787-90. [DOI:10.1007/s12098-008-0099-1] [PMID]
- Newswanger DL, Warren CR. Guillain-Barré syndrome. Am Fam Physician. 2004; 69(10):2405-10. [PMID]
- Walgaard C, Jacobs BC, Lingsma HF, Steyerberg EW, Cornblath DR, van Doorn PA, et al. Second IVIg course in Guillain-Barré syndrome patients with poor prognosis (SID-GBS trial): Protocol for a double-blind randomized, placebo-controlled clinical trial. J Peripher Nerv Syst. 2018; 23(4):210-5. [DOI:10.1111/jns.12286] [PMID]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann Intern Med. 2009; 151(4):264-9. [DOI:10.7326/0003-4819-151-4-200908180-00135] [PMID]
Type of Study: Systematic Review |
Subject:
Pediatric Neurology
Received: 2022/07/25 | Accepted: 2022/11/26 | Published: 2023/01/1
Received: 2022/07/25 | Accepted: 2022/11/26 | Published: 2023/01/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





