Volume 11, Issue 3 (7-2023)
J. Pediatr. Rev 2023, 11(3): 221-230 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fakhri M, Hosseini A, Farhadi R, Moosazadeh M, Azadbakht M, Berneti V. The Effect of Chicory on Bilirubin Level in Newborns Suffering From Jaundice: A Systematic Review. J. Pediatr. Rev 2023; 11 (3) :221-230
URL: http://jpr.mazums.ac.ir/article-1-510-en.html
URL: http://jpr.mazums.ac.ir/article-1-510-en.html
Moloud Fakhri *1 

 , Amirsaeed Hosseini2
, Amirsaeed Hosseini2 

 , Roya Farhadi3
, Roya Farhadi3 

 , Mahmood Moosazadeh4
, Mahmood Moosazadeh4 

 , Mohammad Azadbakht5
, Mohammad Azadbakht5 

 , Vahidreza Berneti6
, Vahidreza Berneti6 




 , Amirsaeed Hosseini2
, Amirsaeed Hosseini2 

 , Roya Farhadi3
, Roya Farhadi3 

 , Mahmood Moosazadeh4
, Mahmood Moosazadeh4 

 , Mohammad Azadbakht5
, Mohammad Azadbakht5 

 , Vahidreza Berneti6
, Vahidreza Berneti6 


1- Traditional and Complementary Medicine Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran. , mmfir@yahoo.com
2- Complementary and Alternative Medicine Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
4- Gastrointestinal Cancer Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
5- Traditional and Complementary Medicine Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
6- School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
2- Complementary and Alternative Medicine Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
4- Gastrointestinal Cancer Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
5- Traditional and Complementary Medicine Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
6- School of Medicine, Mazandaran University of Medical Sciences, Sari, Iran.
Full-Text [PDF 543 kb]
(1656 Downloads)
| Abstract (HTML) (2843 Views)
Full-Text: (1953 Views)
Introduction
Jaundice is a prevalent complication from which 60% of term newborns and 80% of premature newborns suffer over the first week of their lives [1, 2]. The largest number of severe neonatal hyperbilirubinemia incidences has been reported in Asia. Neonatal hyperbilirubinemia incidence has been reported to cause one-third of infant hospital admissions in Iran [3].
Although phototherapy is the primary treatment for hyperbilirubinemia, it exposes newborns to serious complications such as dehydration, retinal damage, diarrhea, and bronze-baby syndrome [4]. Hence, traditional drugs—especially herbal medicine—are used to reduce blood exchange frequency and phototherapy duration or as an alternative for these treatments [5]. Fumitory, jujube, chicory, mana, and cotoneaster have also been mentioned in our culture as treatments for this complication. These plants reduce bilirubin levels due to their laxative effects, increasing bowel movement frequency and intestinal bilirubin excretion [6].
Chicory (Chicorium intybus) is a perennial herbaceous plant from the Asteraceae family. It is among the most common plants in the Iranian diet in concentrate, infusion, etc. This plant is rich in inulin, a member of the fructan family, and a good water-soluble agent. Human digestive enzymes barely hydrolyze it, and thus it has many health benefits for cardiovascular and gastrointestinal problems [7, 8, 9]. Chicory is a 30- to 120-cm-high perennial plant from the composite family with reported anti-oxidant, anti-diabetic, anti-allergic, laxative, anti-inflammatory, diuretic, and anti-jaundice properties [10, 11]. Given its cold temperament, chicory is reported to have several medicinal benefits, such as protection against jaundice, reduced bile concentration, and improved liver function [12, 13].
Thanks to its flavonoid content, including quercetin, apigenin, and luteolin, which stimulate the UDP-glucuronosyltransferase enzyme, chicory accelerates bilirubin excretion [14]. The aforementioned is important in newborns given their premature liver function, UDP-glucuronosyltransferase deficiency at birth, and hepatic intestinal blood circulation due to high β-glucuronidase in the intestines concentration in full term and preterm infants. These are newborns’ main causes of icterus [1, 15, 16]. However, given the contradictory results of previous studies on the effect of chicory on bilirubin levels in newborns [5, 14, 17-20], the present study seeks to examine the influence of chicory on bilirubin levels in newborns who have jaundice through a systematic review. It must be noted that the present study is the first to perform a systematic review in this field.
Methods
Study design
The present study is a systematic review examining the influence of chicory on bilirubin levels in newborns with jaundice. The study has been compiled based on the PRISMA (preferred reporting items for systematic reviews and meta-analyses) checklist [21].
Research population
The research population in the present study included all newborns who have jaundice.
Outcome
The primary outcome of the present study was determining the influence of chicory on bilirubin levels in newborns with jaundice.
Search strategy
Two authors carried out the reference search process. Iranian databases, including Baerkat Gostar, Scientific Information Database (SID), Irandoc, and Magiran, as well as international databases, including Cochrane, Web of Science, Scopus, PubMed, and Google Scholar search engines, were explored without language and time limitation using relevant MesH terms of “bilirubin”, “chicory”, “infant”, “jaundice”, and “systematic review” and their Persian equivalents to retrieve pertinent studies (updated as of April 2022). Combinations of the keywords were also searched on the mentioned databases using “AND” and “OR” operators. The initially retrieved studies were entered into EndNote 7 at this stage to detect duplicate studies quickly by referring to the software, and only one study was kept from each group of duplicate studies. The list of the references mentioned in all initial studies remaining by the end of the PRISMA flowchart was then used for a manual search. Table 1 contains an example of the search strategy in some databases.
PICO components
PICO comprised population (infants with jaundice), intervention (chicory), comparison (a group of infants used sterile water instead of chicory and underwent phototherapy), and outcome (bilirubin level in infants who have jaundice).
Inclusion criteria
We entered into this systematic review in vitro and randomized controlled trials examining the influence of chicory on bilirubin levels in infants with jaundice. The intervention group received chicory, while the control or comparison group included infants undergoing phototherapy. Chicory was administered in the intervention group orally, through bathing with chicory extract, and by adding chicory extract to infants’ blood samples under laboratory conditions.
Exclusion criteria
The exclusion criteria were Low-quality studies based on the quality assessment checklist, case studies or case report studies, full-text unavailability, and studies investigating the influence of chicory and other drugs on bilirubin levels in infants simultaneously.
Qualitative assessment
To assess the quality of RCT studies, two researchers used the Cochrane collaboration’s checklist to evaluate the risk of bias in randomized trials, including 7 items, each examining one important dimension or type of bias in clinical trials. Each item on the checklist had 3 options “low risk”, “high risk”, or “unclear risk” [22] . JBI (The Joanna Briggs Institute) checklist for assessing the quality of experimental studies was also used to assess the quality of in vitro studies. The checklist included 9 questions, each with the 4 options of yes, no, unclear, and non-applicable, where “yes” gets one score and “no”, “unclear”, and “non-applicable” get zero scores [23]. After the risk of bias was assessed in all studies, the inconsistencies between the options of items were examined in each study, and all inconsistencies were resolved by reaching an agreement between the two assessors (Appendix 1, 2).


Data extraction
Two researchers extracted data from the studies separately to minimize the risk of bias in reports and errors in data collection. Researchers entered the data into a checklist including the name of the researcher, type of study, study title, execution year, execution location, mean of the infant age, mean of the infant weight, sample size, number of girls and boys, chicory administration type, and general study output. The third researcher examined the extracted data to resolve inconsistencies.
Data analysis
Since the study was a systematic review, qualitative data analysis was performed, and the results were reported qualitatively.
Results
Study selection process
A total of 175 articles were initially retrieved by searching the databases mentioned before. The titles of the articles were examined, and 79 duplicate studies were eliminated. The abstracts of the remaining 96 studies were examined, and 6 studies were excluded since their full texts were unavailable. Out of the remaining 90 articles, 83 were excluded due to the exclusion criteria, and 7 studies ultimately entered the quality assessment stage, all of which were of acceptable quality and entered the systematic review process (Figure 1).
Infants profile
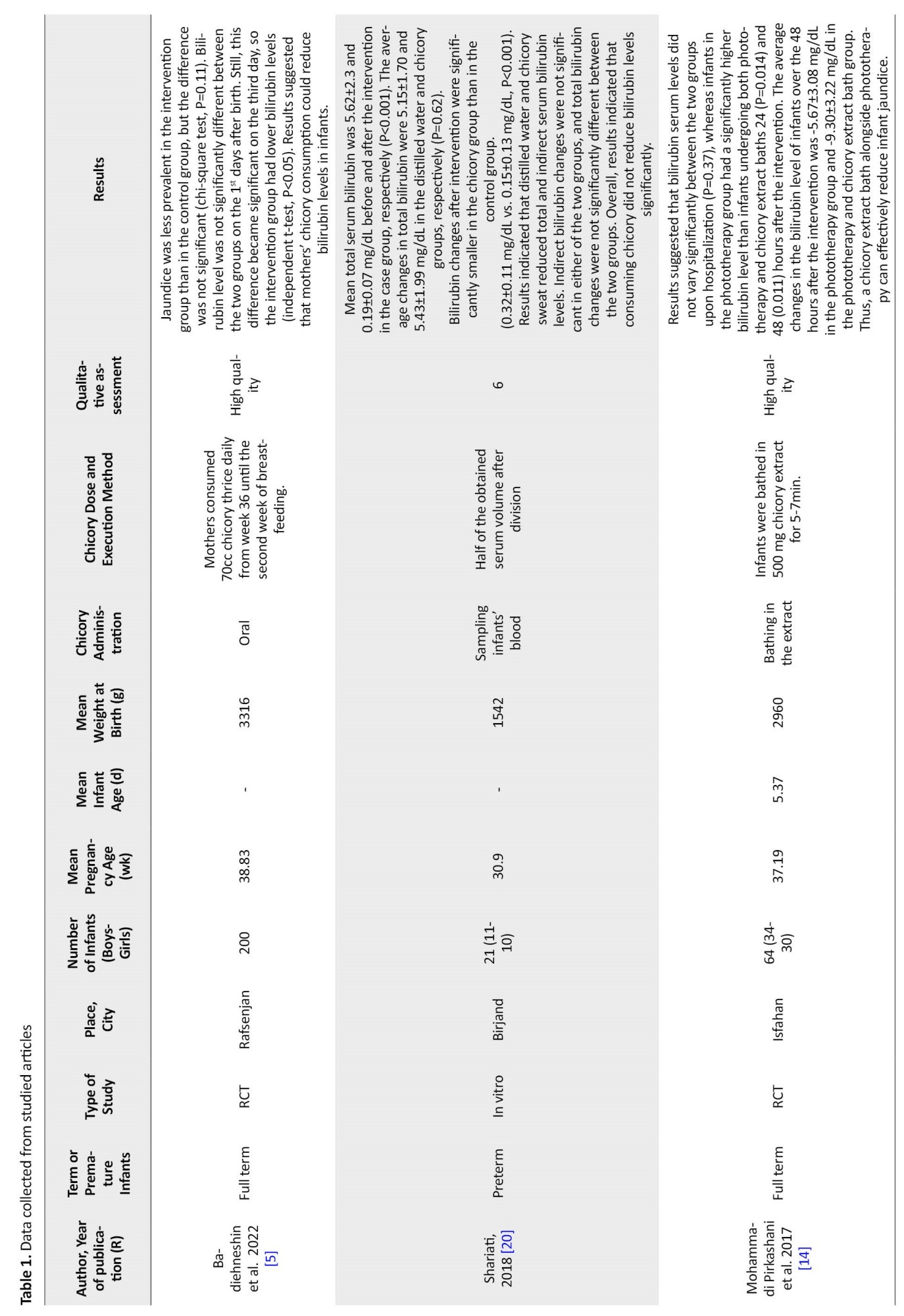
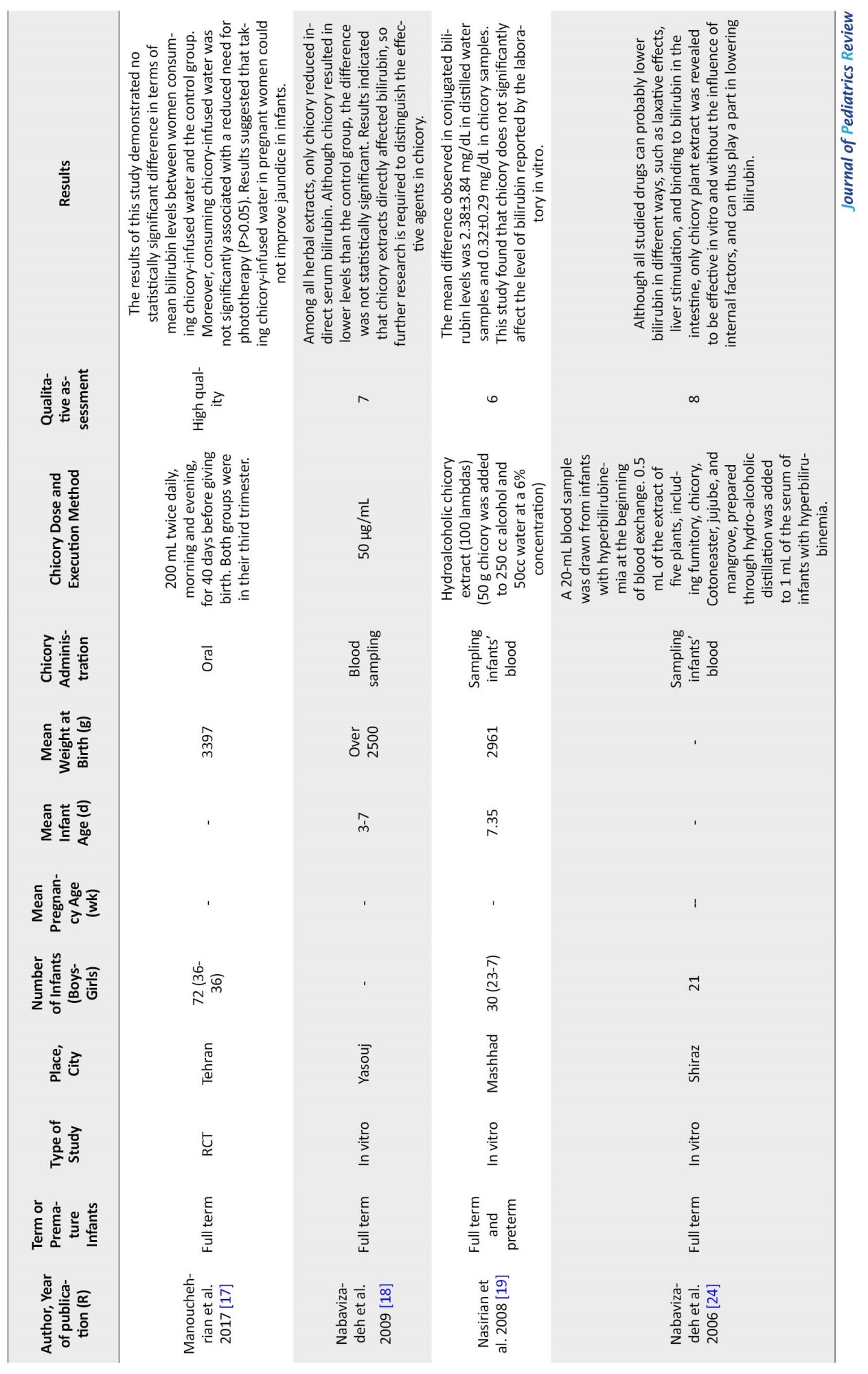
Among the 7 studied articles with a sample size of 408 (204 infants in the intervention group and 204 in the control group) from 2006 to 2020, 4 studies were in vitro and 3 RCTs. Among these studies, mothers’ pregnancy duration ranged between 30.9 and 38.8 weeks, infants’ age ranged between 3 and 7 days, and their weight ranged between 1544 and 3316 g. Four studies examined infants’ blood samples in vitro and compared serum bilirubin levels before and after the intervention; two studies administered chicory sweat orally by mothers. They examined its impact on jaundice in infants, and one study used chicory extract to bathe the infants and investigated its effect through dermal absorption. All the reviewed studies were performed in Iran, although no filter was used regarding location when exploring studies (Table 1).
Discussion
The results of previous research on the influence of chicory on reducing bilirubin levels in infants are still uncertain. However, the contradictions between the effects of various studies examined in the present systematic review may have stemmed from their differences in terms of chicory administration and dose and the type of study. It should also be noted that factors such as infants’ gender and their weight at birth also affect the results. However, the limited number of studies prevented us from classifying them and comparing those under similar conditions.
Several studies reported chicory effectiveness in reducing infant bilirubin levels. Nabavizadeh et al. (2009) examined the influence of common herbal drugs on reducing jaundice in infants using in vitro techniques. They reported that only chicory extract indirectly reduced serum bilirubin levels among all herbal extracts. Still, no significant difference was observed between the direct bilirubin levels of the two groups, although the chicory group had a lower bilirubin level than the control group [18]. Badiehneshin et al. (2022) examined the impact of chicory extract administration by mothers on the frequency of jaundice and serum bilirubin level in infants. They reported that the intervention group had a lower bilirubin level than the control group. Still, the difference was not significant (P=0.11). Moreover, the mean bilirubin level difference between the two groups was not significant on the first day of birth. Still, the intervention group had a significantly lower bilirubin level than the control group by the third day after birth (independent t-test, P<0.05). These results suggest that chicory administration by mothers can reduce bilirubin levels in infants effectively [5].
Mohammadi Pirkashani et al. (2017) conducted a randomized double-blinded clinical trial on 64 infants with jaundice in a selection of Isfahan hospitals. They examined the impact of bathing in chicory extract in these infants. Their results suggest that mean bilirubin levels were not significantly different between the two groups at admission (P=0.37). In contrast, the mean bilirubin level of infants in the phototherapy group was significantly higher than infants that underwent phototherapy and were bathed in chicory extract 24 h (P=0.014) and 48 h (P=0.011) after the intervention. Mean±SD bilirubin level variation in the infants in the 48-h phase after the intervention was -5.67±3.08 mg/dL in the phototherapy group and -9.30±3.22 mg/dL in the chicory extract bath group. Thus, bathing in chicory extract could reduce jaundice in infants [14]. Given its laxative effects, chicory reduced bilirubin levels in infants with jaundice by increasing their number of stools.
On the contrary, several studies reported chicory to be ineffective in reducing bilirubin levels. Shariati et al. (2018) conducted a study to examine the effects of phototherapy and chicory extract on serum bilirubin in premature infants hospitalized in a neonatal intensive care unit. Their results suggested that distilled water and chicory sweat reduced the total and indirect bilirubin levels. Indirect bilirubin variation was not significantly different between the two groups, and total bilirubin variation was the same in both groups. Overall, chicory extract did not significantly reduce bilirubin levels in premature infants [20]. Manouchehrian et al. (2017) aimed to examine whether 40 days of taking chicory extract was associated with fewer jaundice symptoms. The result indicated that consuming chicory extract by pregnant women did not improve their infants’ jaundice [17]. The study of Nasirian et al. (2008) seeking to examine in vitro effects of chicory on bilirubin levels in 30 infants in a neonatal intensive care unit suffering from jaundice found the Mean±SD difference in conjugated bilirubin levels to be 2.38±3.84 mg/dL in samples with distilled water and 0.32±0.29 mg/dL in samples with chicory. This study found that chicory did not influence reported bilirubin levels in vitro [19]. It would appear that further clinical trials are required to make definitive conclusions in this regard.
Conclusion
Out of the 7 examined studies, 4 reported that chicory had a positive impact on reducing bilirubin levels and improving jaundice in infants, while the other 3 studies reported no statistically significant influence of chicory on reducing infants’ bilirubin. According to the results of this study, Iran was the only country that had evaluated the effect of chicory on neonatal jaundice in the form of several research studies. Still, due to the limited number of studies and the different dosages of chicory, the way of consuming chicory, the type of studies, and the age and weight of babies, we could not make a general and accurate conclusion on the efficacy of chicory. Thus, the effectiveness of chicory in reducing jaundice in infants is still debatable, and future researchers are recommended to perform further studies in this regard.
Study limitations
Some articles’ full texts were unavailable. Also, the articles published in this regard were exclusively conducted in Iran. No information was available from other countries, and no data on chicory’s influence on infants’ jaundice was measured in relation to variables such as weight at birth, infant gender, and infant age.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was registered on The International Prospective Register of Systematic Reviews (PROSPERO) (Code: CRD42022328982).
Funding
This study has been conducted with the financial support of the Mazandaran University of Medical Sciences.
Authors contributions
Conceptualisation and study design: Moloud Fakhri, Roya Farhadi, Mahmood Moosazadeh, Amirsaeed Hosseini, and Mohammad Azadbakht; Data analysis: Roya Farhadi, Amirsaeed Hosseini, and Moloud Fakhri; Results interpretation: Mahmood Moosazadeh and Vahidreza Berneti; Review and editing: Amirsaeed Hosseini and Moloud Fakhri; Final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We thank Mazandaran University of Medical Sciences for the financial support of the research project of this article.
References
Jaundice is a prevalent complication from which 60% of term newborns and 80% of premature newborns suffer over the first week of their lives [1, 2]. The largest number of severe neonatal hyperbilirubinemia incidences has been reported in Asia. Neonatal hyperbilirubinemia incidence has been reported to cause one-third of infant hospital admissions in Iran [3].
Although phototherapy is the primary treatment for hyperbilirubinemia, it exposes newborns to serious complications such as dehydration, retinal damage, diarrhea, and bronze-baby syndrome [4]. Hence, traditional drugs—especially herbal medicine—are used to reduce blood exchange frequency and phototherapy duration or as an alternative for these treatments [5]. Fumitory, jujube, chicory, mana, and cotoneaster have also been mentioned in our culture as treatments for this complication. These plants reduce bilirubin levels due to their laxative effects, increasing bowel movement frequency and intestinal bilirubin excretion [6].
Chicory (Chicorium intybus) is a perennial herbaceous plant from the Asteraceae family. It is among the most common plants in the Iranian diet in concentrate, infusion, etc. This plant is rich in inulin, a member of the fructan family, and a good water-soluble agent. Human digestive enzymes barely hydrolyze it, and thus it has many health benefits for cardiovascular and gastrointestinal problems [7, 8, 9]. Chicory is a 30- to 120-cm-high perennial plant from the composite family with reported anti-oxidant, anti-diabetic, anti-allergic, laxative, anti-inflammatory, diuretic, and anti-jaundice properties [10, 11]. Given its cold temperament, chicory is reported to have several medicinal benefits, such as protection against jaundice, reduced bile concentration, and improved liver function [12, 13].
Thanks to its flavonoid content, including quercetin, apigenin, and luteolin, which stimulate the UDP-glucuronosyltransferase enzyme, chicory accelerates bilirubin excretion [14]. The aforementioned is important in newborns given their premature liver function, UDP-glucuronosyltransferase deficiency at birth, and hepatic intestinal blood circulation due to high β-glucuronidase in the intestines concentration in full term and preterm infants. These are newborns’ main causes of icterus [1, 15, 16]. However, given the contradictory results of previous studies on the effect of chicory on bilirubin levels in newborns [5, 14, 17-20], the present study seeks to examine the influence of chicory on bilirubin levels in newborns who have jaundice through a systematic review. It must be noted that the present study is the first to perform a systematic review in this field.
Methods
Study design
The present study is a systematic review examining the influence of chicory on bilirubin levels in newborns with jaundice. The study has been compiled based on the PRISMA (preferred reporting items for systematic reviews and meta-analyses) checklist [21].
Research population
The research population in the present study included all newborns who have jaundice.
Outcome
The primary outcome of the present study was determining the influence of chicory on bilirubin levels in newborns with jaundice.
Search strategy
Two authors carried out the reference search process. Iranian databases, including Baerkat Gostar, Scientific Information Database (SID), Irandoc, and Magiran, as well as international databases, including Cochrane, Web of Science, Scopus, PubMed, and Google Scholar search engines, were explored without language and time limitation using relevant MesH terms of “bilirubin”, “chicory”, “infant”, “jaundice”, and “systematic review” and their Persian equivalents to retrieve pertinent studies (updated as of April 2022). Combinations of the keywords were also searched on the mentioned databases using “AND” and “OR” operators. The initially retrieved studies were entered into EndNote 7 at this stage to detect duplicate studies quickly by referring to the software, and only one study was kept from each group of duplicate studies. The list of the references mentioned in all initial studies remaining by the end of the PRISMA flowchart was then used for a manual search. Table 1 contains an example of the search strategy in some databases.


PICO components
PICO comprised population (infants with jaundice), intervention (chicory), comparison (a group of infants used sterile water instead of chicory and underwent phototherapy), and outcome (bilirubin level in infants who have jaundice).
Inclusion criteria
We entered into this systematic review in vitro and randomized controlled trials examining the influence of chicory on bilirubin levels in infants with jaundice. The intervention group received chicory, while the control or comparison group included infants undergoing phototherapy. Chicory was administered in the intervention group orally, through bathing with chicory extract, and by adding chicory extract to infants’ blood samples under laboratory conditions.
Exclusion criteria
The exclusion criteria were Low-quality studies based on the quality assessment checklist, case studies or case report studies, full-text unavailability, and studies investigating the influence of chicory and other drugs on bilirubin levels in infants simultaneously.
Qualitative assessment
To assess the quality of RCT studies, two researchers used the Cochrane collaboration’s checklist to evaluate the risk of bias in randomized trials, including 7 items, each examining one important dimension or type of bias in clinical trials. Each item on the checklist had 3 options “low risk”, “high risk”, or “unclear risk” [22] . JBI (The Joanna Briggs Institute) checklist for assessing the quality of experimental studies was also used to assess the quality of in vitro studies. The checklist included 9 questions, each with the 4 options of yes, no, unclear, and non-applicable, where “yes” gets one score and “no”, “unclear”, and “non-applicable” get zero scores [23]. After the risk of bias was assessed in all studies, the inconsistencies between the options of items were examined in each study, and all inconsistencies were resolved by reaching an agreement between the two assessors (Appendix 1, 2).


Data extraction
Two researchers extracted data from the studies separately to minimize the risk of bias in reports and errors in data collection. Researchers entered the data into a checklist including the name of the researcher, type of study, study title, execution year, execution location, mean of the infant age, mean of the infant weight, sample size, number of girls and boys, chicory administration type, and general study output. The third researcher examined the extracted data to resolve inconsistencies.
Data analysis
Since the study was a systematic review, qualitative data analysis was performed, and the results were reported qualitatively.
Results
Study selection process
A total of 175 articles were initially retrieved by searching the databases mentioned before. The titles of the articles were examined, and 79 duplicate studies were eliminated. The abstracts of the remaining 96 studies were examined, and 6 studies were excluded since their full texts were unavailable. Out of the remaining 90 articles, 83 were excluded due to the exclusion criteria, and 7 studies ultimately entered the quality assessment stage, all of which were of acceptable quality and entered the systematic review process (Figure 1).
Infants profile
Among the 7 studied articles with a sample size of 408 (204 infants in the intervention group and 204 in the control group) from 2006 to 2020, 4 studies were in vitro and 3 RCTs. Among these studies, mothers’ pregnancy duration ranged between 30.9 and 38.8 weeks, infants’ age ranged between 3 and 7 days, and their weight ranged between 1544 and 3316 g. Four studies examined infants’ blood samples in vitro and compared serum bilirubin levels before and after the intervention; two studies administered chicory sweat orally by mothers. They examined its impact on jaundice in infants, and one study used chicory extract to bathe the infants and investigated its effect through dermal absorption. All the reviewed studies were performed in Iran, although no filter was used regarding location when exploring studies (Table 1).
Discussion
The results of previous research on the influence of chicory on reducing bilirubin levels in infants are still uncertain. However, the contradictions between the effects of various studies examined in the present systematic review may have stemmed from their differences in terms of chicory administration and dose and the type of study. It should also be noted that factors such as infants’ gender and their weight at birth also affect the results. However, the limited number of studies prevented us from classifying them and comparing those under similar conditions.
Several studies reported chicory effectiveness in reducing infant bilirubin levels. Nabavizadeh et al. (2009) examined the influence of common herbal drugs on reducing jaundice in infants using in vitro techniques. They reported that only chicory extract indirectly reduced serum bilirubin levels among all herbal extracts. Still, no significant difference was observed between the direct bilirubin levels of the two groups, although the chicory group had a lower bilirubin level than the control group [18]. Badiehneshin et al. (2022) examined the impact of chicory extract administration by mothers on the frequency of jaundice and serum bilirubin level in infants. They reported that the intervention group had a lower bilirubin level than the control group. Still, the difference was not significant (P=0.11). Moreover, the mean bilirubin level difference between the two groups was not significant on the first day of birth. Still, the intervention group had a significantly lower bilirubin level than the control group by the third day after birth (independent t-test, P<0.05). These results suggest that chicory administration by mothers can reduce bilirubin levels in infants effectively [5].
Mohammadi Pirkashani et al. (2017) conducted a randomized double-blinded clinical trial on 64 infants with jaundice in a selection of Isfahan hospitals. They examined the impact of bathing in chicory extract in these infants. Their results suggest that mean bilirubin levels were not significantly different between the two groups at admission (P=0.37). In contrast, the mean bilirubin level of infants in the phototherapy group was significantly higher than infants that underwent phototherapy and were bathed in chicory extract 24 h (P=0.014) and 48 h (P=0.011) after the intervention. Mean±SD bilirubin level variation in the infants in the 48-h phase after the intervention was -5.67±3.08 mg/dL in the phototherapy group and -9.30±3.22 mg/dL in the chicory extract bath group. Thus, bathing in chicory extract could reduce jaundice in infants [14]. Given its laxative effects, chicory reduced bilirubin levels in infants with jaundice by increasing their number of stools.
On the contrary, several studies reported chicory to be ineffective in reducing bilirubin levels. Shariati et al. (2018) conducted a study to examine the effects of phototherapy and chicory extract on serum bilirubin in premature infants hospitalized in a neonatal intensive care unit. Their results suggested that distilled water and chicory sweat reduced the total and indirect bilirubin levels. Indirect bilirubin variation was not significantly different between the two groups, and total bilirubin variation was the same in both groups. Overall, chicory extract did not significantly reduce bilirubin levels in premature infants [20]. Manouchehrian et al. (2017) aimed to examine whether 40 days of taking chicory extract was associated with fewer jaundice symptoms. The result indicated that consuming chicory extract by pregnant women did not improve their infants’ jaundice [17]. The study of Nasirian et al. (2008) seeking to examine in vitro effects of chicory on bilirubin levels in 30 infants in a neonatal intensive care unit suffering from jaundice found the Mean±SD difference in conjugated bilirubin levels to be 2.38±3.84 mg/dL in samples with distilled water and 0.32±0.29 mg/dL in samples with chicory. This study found that chicory did not influence reported bilirubin levels in vitro [19]. It would appear that further clinical trials are required to make definitive conclusions in this regard.
Conclusion
Out of the 7 examined studies, 4 reported that chicory had a positive impact on reducing bilirubin levels and improving jaundice in infants, while the other 3 studies reported no statistically significant influence of chicory on reducing infants’ bilirubin. According to the results of this study, Iran was the only country that had evaluated the effect of chicory on neonatal jaundice in the form of several research studies. Still, due to the limited number of studies and the different dosages of chicory, the way of consuming chicory, the type of studies, and the age and weight of babies, we could not make a general and accurate conclusion on the efficacy of chicory. Thus, the effectiveness of chicory in reducing jaundice in infants is still debatable, and future researchers are recommended to perform further studies in this regard.
Study limitations
Some articles’ full texts were unavailable. Also, the articles published in this regard were exclusively conducted in Iran. No information was available from other countries, and no data on chicory’s influence on infants’ jaundice was measured in relation to variables such as weight at birth, infant gender, and infant age.
Ethical Considerations
Compliance with ethical guidelines
The study protocol was registered on The International Prospective Register of Systematic Reviews (PROSPERO) (Code: CRD42022328982).
Funding
This study has been conducted with the financial support of the Mazandaran University of Medical Sciences.
Authors contributions
Conceptualisation and study design: Moloud Fakhri, Roya Farhadi, Mahmood Moosazadeh, Amirsaeed Hosseini, and Mohammad Azadbakht; Data analysis: Roya Farhadi, Amirsaeed Hosseini, and Moloud Fakhri; Results interpretation: Mahmood Moosazadeh and Vahidreza Berneti; Review and editing: Amirsaeed Hosseini and Moloud Fakhri; Final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
We thank Mazandaran University of Medical Sciences for the financial support of the research project of this article.
References
- Boskabadi H, Maamouri G, Mafinejad S, Rezagholizadeh F. Clinical course and prognosis of hemolytic jaundice in neonates in north east of Iran. Maced J Med Sci. 2011; 4(4):403-7. [DOI:10.3889/MJMS.1857-5773.2011.0177]
- Ramy N, Ghany EA, Alsharany W, Nada A, Darwish RK, Rabie WA, et al. Jaundice, phototherapy and DNA damage in full-term neonates. J Perinatol. 2016; 36(2):132-6.[DOI:10.1038/jp.2015.166] [PMID]
- Mohammadzadeh A, Farhat ASh, Iranpour R. Effect of clofibrate in jaundiced term newborns. Indian J Pediatr. 2005; 72(2):123-6. [DOI:10.1007/BF02760695] [PMID]
- Kliegman R, Stanton B, W. St Geme J, Felice Schor N, E. Behrman R, Nelson WE,. Nelson Textbook of Pediatrics, Edition:20th edition. PhiladelphiaL Elsevier; 2016. [Link]
- Badiehneshin L, Saghafi Z, Asadollahi Z, Moghadari M, Derakhshan R, Sadeghi T. [The effects of chicory extract consumption by mothers on the frequency of icterus and the serum bilirubin level in neonates (Persian)]. Int J Pediatr. 2022; 10(3):15601-8. [DOI:10.22038/ijp.2022.62842.4800]
- Najib KS, Saki F, Hemmati F, Inaloo S. Incidence, risk factors and causes of severe neonatal hyperbilirubinemia in the South of Iran (Fars Province). Iran Red Crescent Med J. 2013; 15(3):260-3. [DOI:10.5812/ircmj.3337] [PMID] [PMCID]
- Kim M, Shin HK. The water-soluble extract of chicory influences serum and liver lipid concentrations, cecal short-chain fatty acid concentrations and fecal lipid excretion in rats. J Nutr. 1998; 128(10):1731-6. [DOI:10.1093/jn/128.10.1731] [PMID]
- Shoorideh H, Peighambari S, Omidi M, Naghavi MR, Maroufi A. Assessing potential of iranian chicory genotypes for industrial application. Int J Hortic Sci Tech. 2016; 3(1):59-68. [Link]
- Mohammadi Q, Minae MB, Somi MH, Mosaddegh M, Kamalinejad M. Novel use of chicory for the treatment of hiccups in liver obstruction: In Iranian traditional medicine. Iran Red Cres Med J. 15(11):e6647. [DOI:10.5812/ircmj.6647]
- Emami A, Khalili H, Mehregan I, Fasihi Sh, Taleb AM. [References for herbal medicines (Persian)]. Aligoudarz: Kalam Hagh; 2011. [Link]
- Ghannadi AR, Minaiyan M, Abed AR. Kasni (Cichorium intybus L.). J Islam Iran Tradit Med. 2011; 1(4):365-72. [Link]
- Aghili Shirazi M, Mohammadhadi MH. [Makhzan-al-advia (Persian)]. Tehran: Rah-e-Kamal; 2009. [Link]
- Aghili M. [Kholase Al-Hekmah (Persian)]. Qom: Esmailian; 2006. [Link]
- Mohammadi Pirkashani L, Asghari G, Marofi M, Barekatain B. [Effect of chicory extract bath on neonatal bilirubin levels: A randomized clinical trial study (Persian)]. Int J Pediatr. 2017; 5(12):6679-88. [DOI:10.22038/ijp.2017.26069.2226]
- Watchko JF. Neonatal indirect hyperbilirubinemia and kernicterus. In: Gleason CA, Juul SE, editors. Avery’s diseases of the newborn. Amsterdam: Elsevier; 2018. [DOI:10.1016/B978-0-323-40139-5.00084-X]
- Stanton B, Geme JWS, Schor NF. Nelson textbook of pediatrics. Philadelphia: Elsevier; 2016. [Link]
- Manouchehrian M, Shakiba M, Shariat M, Kamalinejad M, Pasalar M, Jafarian A, et al. Chicory aroma water for neonatal jaundice: A randomized clinical trial. Galen Med J. 2017; 6(4):312-8. [DOI:10.31661/gmj.v6i4.973]
- Nabavizadeh SH, Majid OB, Anushiravani A. Direct ex vivo effects of herbal extracts on serum bilirubin in neonatal blood samples. Afr J Biochem Res. 2009; 3(5):226-8. [Link]
- Nassirian H, Eslami ST. Effects of chichorium intybus on bilirubin. Indian J Pediatr. 2008; 75(4):331-3. [DOI:10.1007/s12098-008-0033-6] [PMID]
- Shariati M. [In vitro evaluation of the extract of Chicory on Serum bilirubin preterm infants admitted to the NICU (Persian)] [PhD dissertation]. Birjand: Birjand University of Medical Sciences; 2018.
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4(1):1. [DOI:10.1186/2046-4053-4-1] [PMID] [PMCID]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.[DOI:10.1136/bmj.d5928] [PMID] [PMCID]
- The Joanna Briggs Institute. The Joanna Briggs Institute critical appraisal tools for use in jbi systematic reviews: Checklist for systematic reviews and research syntheses. Adelaide: The Joanna Briggs Institute; 2017. [Link]
- Nabavizadeh S, Safari M, Khoshnevisan F. The effect of herbal drugs on neonatal jaundice. Iran J Pediatr. 2005; 15(2):133-8. [Link]
Type of Study: Review Article |
Subject:
Neonatology
Received: 2022/11/15 | Accepted: 2023/07/5 | Published: 2023/07/24
Received: 2022/11/15 | Accepted: 2023/07/5 | Published: 2023/07/24
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







