Volume 12, Issue 2 (4-2024)
J. Pediatr. Rev 2024, 12(2): 125-142 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Hashemipour M, Yousofi J, Chegini R, Hovsepian S. Prevalence of Concurrent Congenital Disabilities in Infants With Congenital Hypothyroidism: A Systematic Review. J. Pediatr. Rev 2024; 12 (2) :125-142
URL: http://jpr.mazums.ac.ir/article-1-524-en.html
URL: http://jpr.mazums.ac.ir/article-1-524-en.html
1- Metabolic Liver Disease Research Center, Isfahan University of Medical Sciences, Isfahan, Iran.
2- Metabolic Liver Disease Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. ,silvahovsepsecret@gmail.com
2- Metabolic Liver Disease Research Center, Isfahan University of Medical Sciences, Isfahan, Iran. ,
Full-Text [PDF 602 kb]
(1198 Downloads)
| Abstract (HTML) (3362 Views)
Full-Text: (906 Views)
Introduction
Congenital hypothyroidism (CH) is one of the most common endocrine disorders in children, which can cause permanent mental retardation if untreated. Nowadays, CH is diagnosed through neonatal screening [1]. It affects nearly 1 in 3000 to 4000 newborns worldwide. It can be secondary or primary, and permanent or transient based on the laboratory findings. In permanent cases, the patient needs lifelong replacement of levothyroxine. Although in some cases, hypothyroidism is a part of a congenital syndrome, such as Pendred syndrome, anomalies related to the various systems are reported in CH patients, even in non-syndromic cases of this disorder [2]. In most cases, thyroid dysgenesis is the cause of CH [3].
In addition, a higher prevalence of CH was reported in Down syndrome. Many studies have shown that it is associated with an increased incidence of other congenital malformations [1, 3-5]. Teratogens and a few genes lead to CH and congenital malformations. However, the exact etiology of the high prevalence of congenital disabilities observed in CH infants is unknown [4, 6]. The presence of concurrent anomalies in CH patients raises the role of genetic components in this disorder. Based on available data, most of the reported anomalies are related to the cardiovascular system. However, environmental factors also may be related [6]. Mutations in TTF1, TTF2, FOXE1, PAX8, and TSHR are associated with thyroid dysgenesis and also anomalies, such as renal anomalies [7].
It is suggested that determining the rate of different concurrent congenital anomalies in these patients would help us in better understanding of the CH pathogenesis including both genetic and environmental factors as well as improving the protocol of CH screening in order to determine the disorders early in life and in accordance with routine screening program. A well designed screening program could help us in better management of the diseases. The aim of current study was to systematically review the papers in this field to provide us more applicable information for designing more effective CH screening program.
Methods
Search strategy, research question, and databases
This systematic review study was based on the preferred reporting items for systematic reviews and metaanalysis. The protocol of the study was approved by Isfahan University of Medical Sciences. The research question and population, intervention, comparison, and outcome framework of this study compared children with and without CH in terms of having extra-thyroidal congenital anomalies. A systematic literature search through PubMed, Science Direct, Scopus, and the Web of Science databases was performed from December 2021 until January 2022.
Inclusion and exclusion criteria
English language articles, including cross-sectional, cohort, and case-control studies, were included, and case reports, letters, and articles without an available full text were excluded. Studies that evaluated extra-thyroidal anomalies in CH patients were included. Duplicates, reviews, and unrelated and low-quality studies were excluded. Meeting abstracts were not included in this review.
Study selection and quality assessment process
After removing duplicates, the title and the abstracts were evaluated by two reviewers separately (Rojin Chegini and Jila Yousofi). Unrelated articles, reviews, letters, case reports, and articles published before 1990 were ignored. The full text of the remaining articles was separately reviewed by two reviewers, and unrelated items were removed. To find more related studies, references to the final articles were also reviewed. The final articles entered quality assessment using the strengthening of the reporting of observational studies in the epidemiology checklist and were done by two independent reviewers (Rojin Chegini and Jila Yousofi). Consult with an expert (Mahin Hashemipour) was considered in the case of disagreement.
Data extraction
Data extraction was done by two independent authors using a checklist with the following items: Name of the author, year of publication, country, sample size, gender, type of CH, inclusion and exclusion criteria, the number of CH patients with concurrent anomalies, and the type of anomalies and the number of each one. Data of the patients with cardiovascular, craniofacial, urogenital, gastrointestinal, and musculoskeletal anomalies and anomalies related to the nervous system are reported as numbers and percentages. Anomalies related to the other systems are reported as others. Considering the known association between Down syndrome and prematurity and congenital anomalies, data from the studies that have excluded these patients are reported separately.
Results
Characteristics of the studies
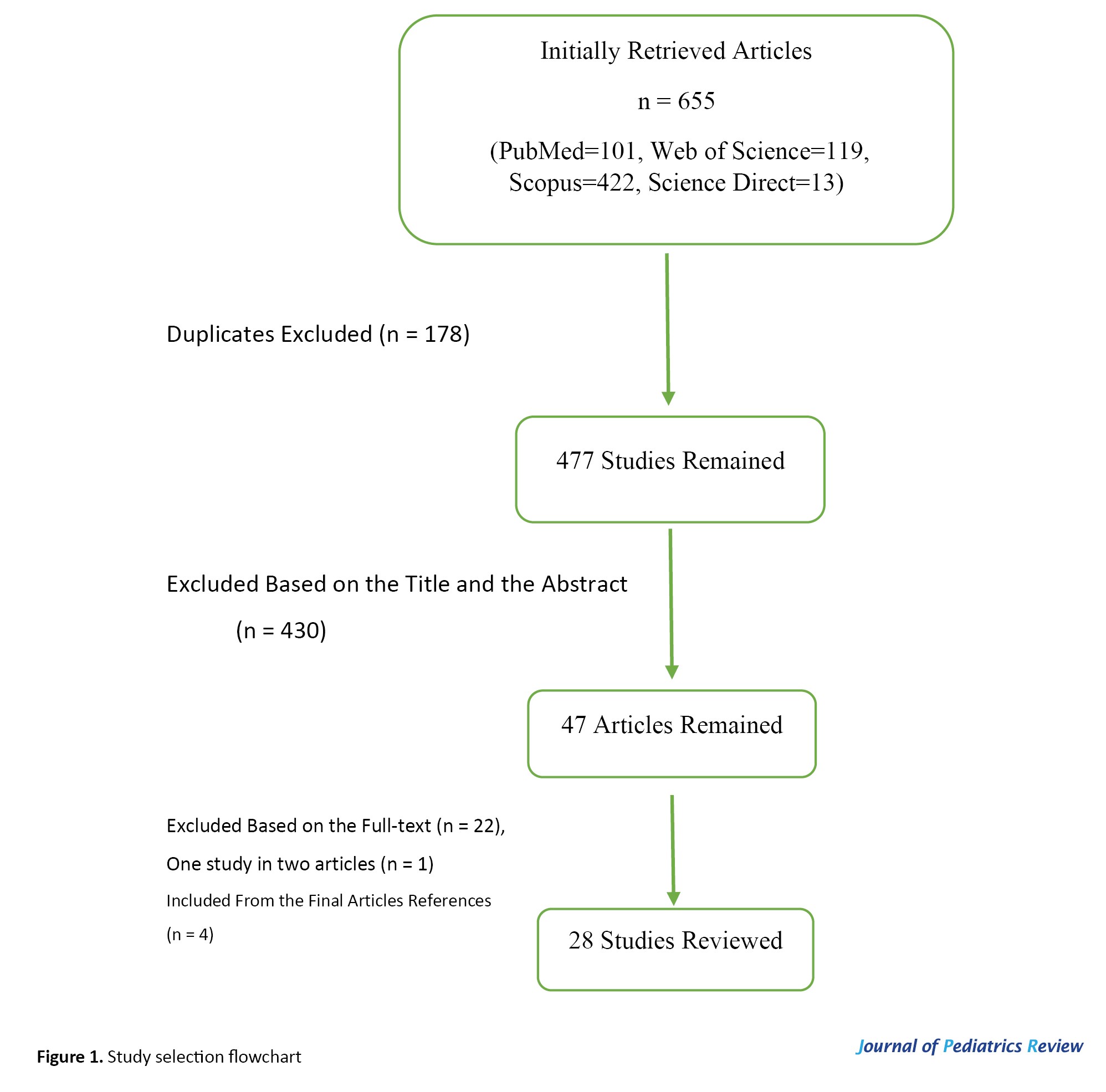
From the 655 initially retrieved articles, 178 were duplicates, and 430 were excluded based on the title and the abstract. Finally, among the remaining 47 articles, 24 articles entered the data extraction process, and 4 additional references were found by reviewing the references of the final articles. Finally, 28 articles were selected (Figure 1).
In total, 7401 patients (3067 females, 1987 males, others not reported) with CH were studied. The studied population in 12 studies were primary CH patients, 11 permanent CH patients, and in 4 studies, both permanent and transient cases.
In 5 studies, patients with known syndromes, such as Down syndrome were excluded, and 5 studies excluded pre-term newborns (Table 1).





In 2 studies, patients with Down syndrome and prematurity were not included.
Concurrent congenital anomalies from all studies (n=28) [1-28]
The prevalence of extra-thyroidal anomalies ranged from 5% to 50% in girls and from 4% to 80% in boys. Accordingly, 20% of the permanent CH patients and 13% of the patients with transient CH had non-thyroidal congenital malformations.
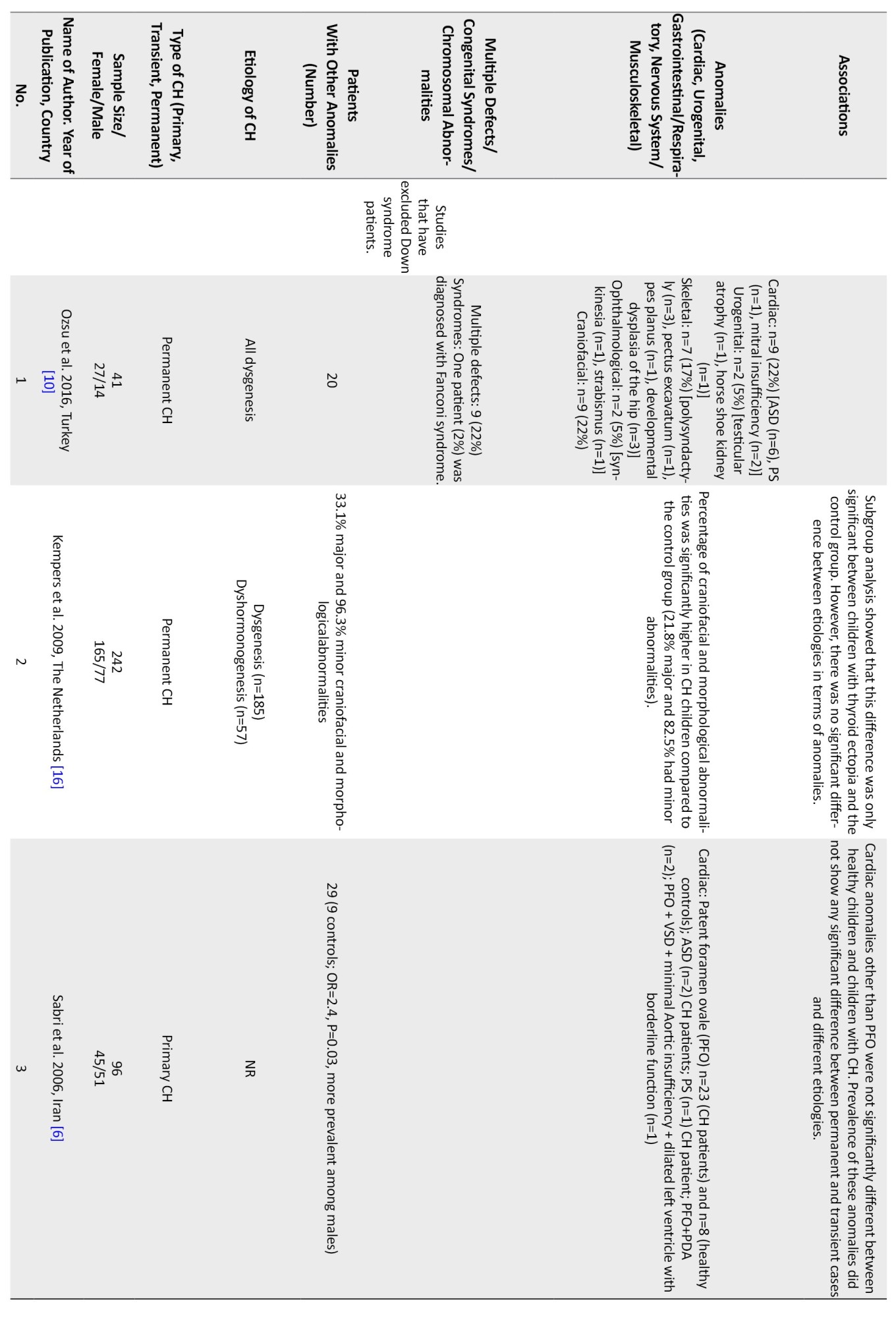
Multiple anomalies were reported in 1%-22% of the studied population [5, 9-12, 17, 18, 21-23, 25, 28]. Studies from Turkey (22%) [10] and India (12%) reported higher rates of multiple defects [9].
Chromosomal anomalies were reported in 1%-14% of the studied population [4, 5, 8, 17, 19, 22, 23, 27, 28]. The most common chromosomal anomaly was the Down syndrome. A higher rate of chromosomal anomalies was reported in Iran (14%) [4] and Saudi Arabia (10%) [27].
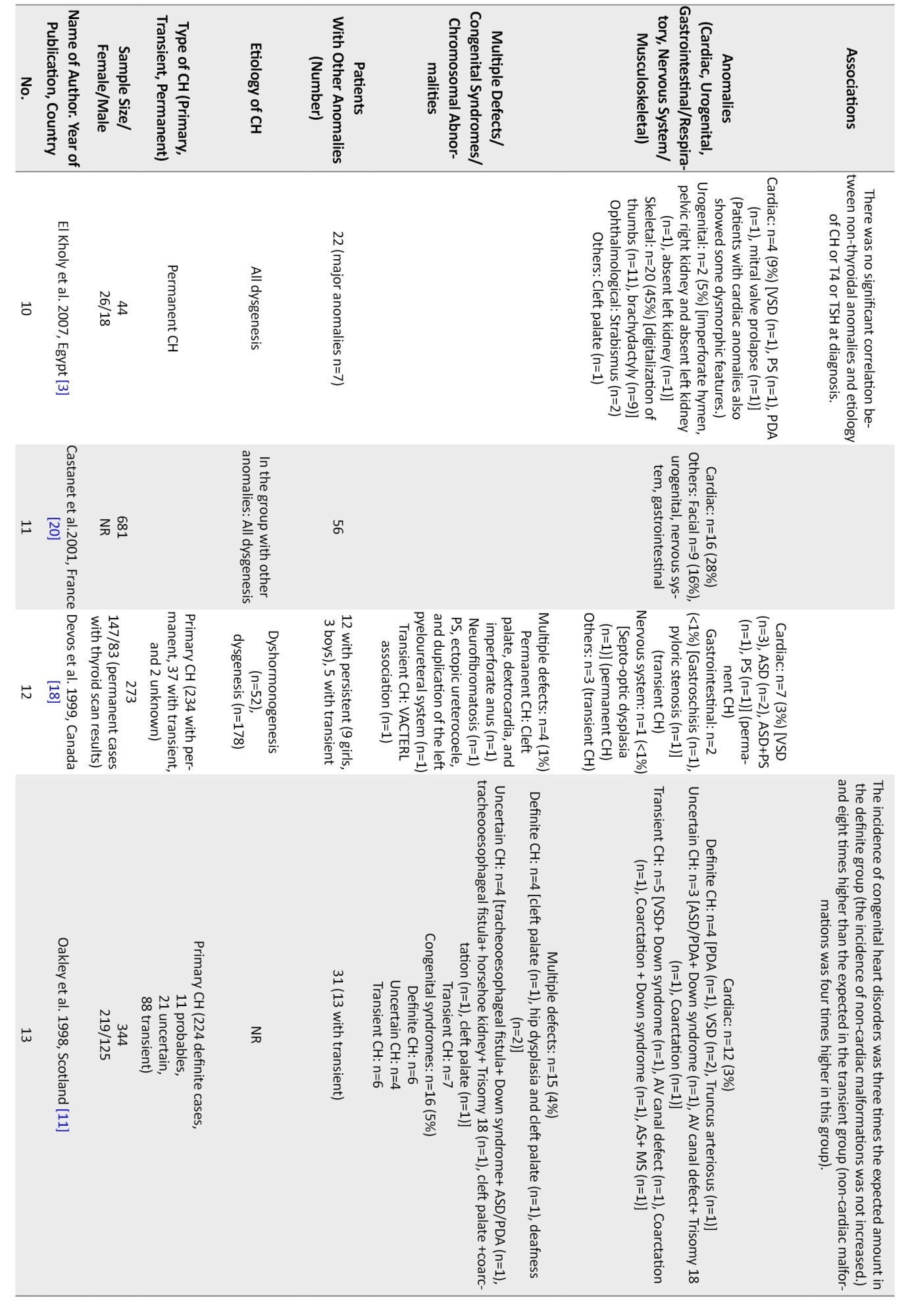
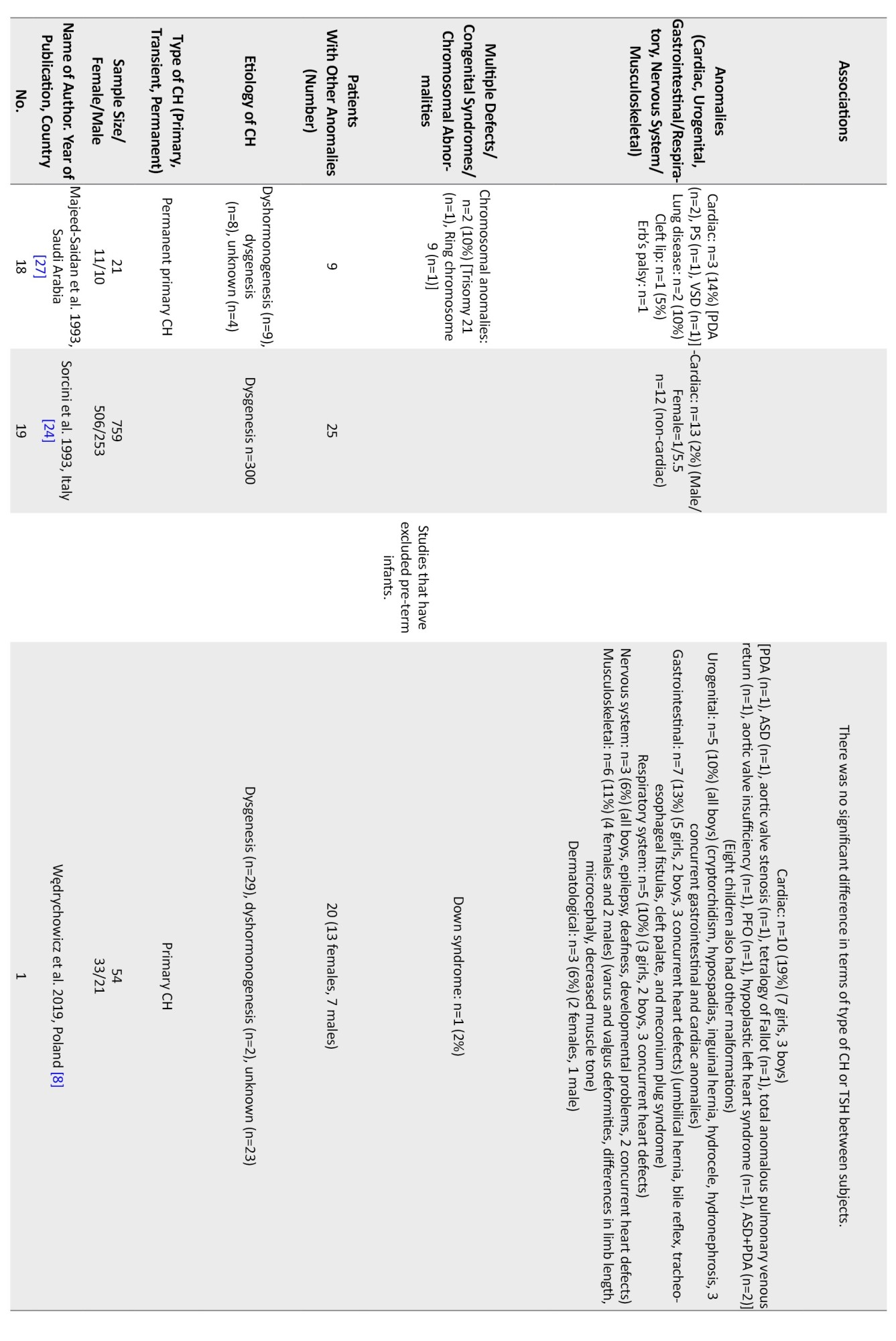
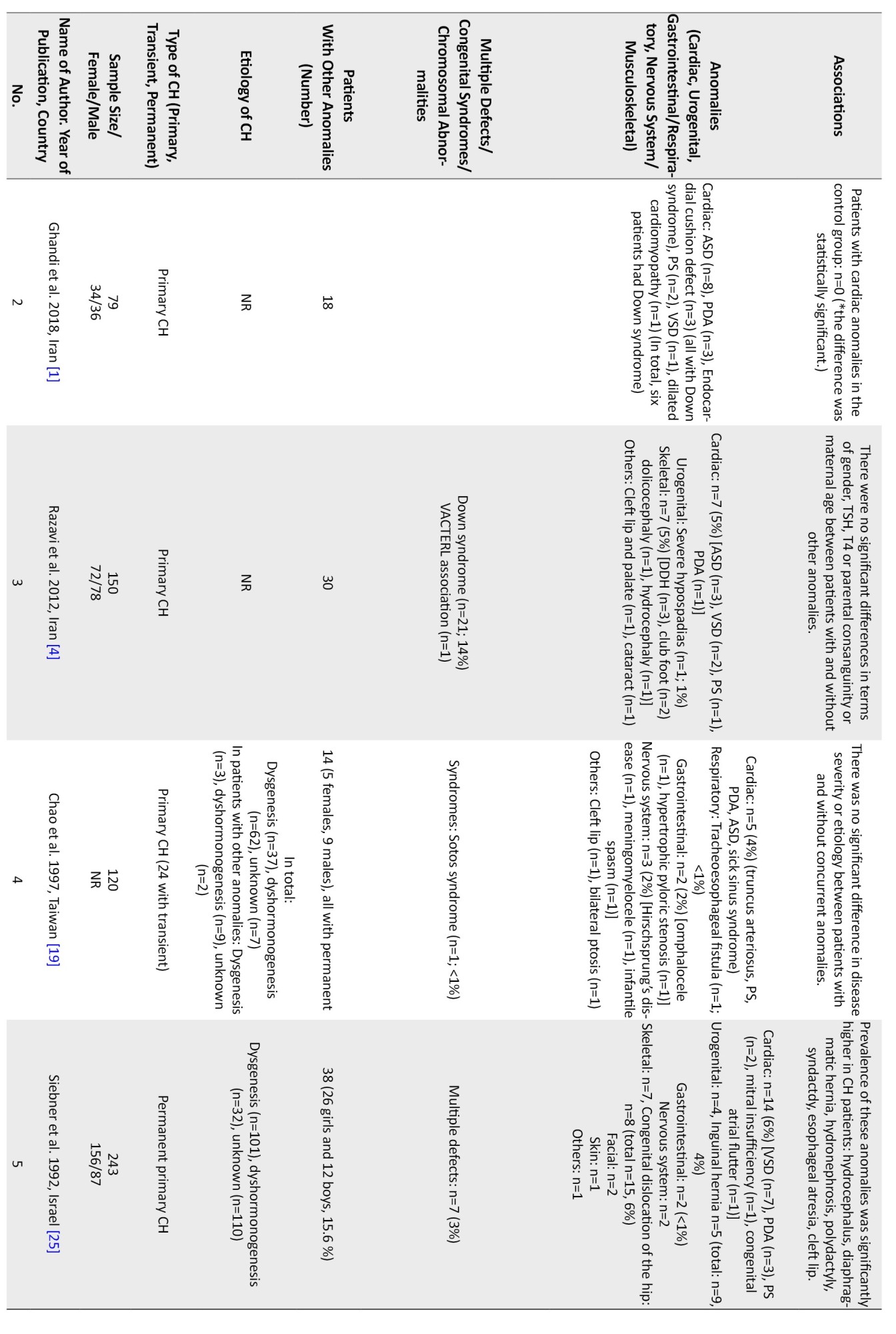
Regarding non-thyroidal congenital anomalies, most of the studies (18 out of 28) have reported cardiac anomalies as the most common non-thyroidal anomalies ranging from 2%-47% [1, 5, 6, 8, 11-15, 17-20, 22-24, 27, 28].In most European countries, the rate of cardiac anomalies was in the lower range. Higher rates of cardiac anomalies were reported from Turkey (47%) [13], India (29%) [9], Iran (22%-24%) [1], Poland (19%) [8], and Saudi Arabia (14%) [27].
Other more frequent non-thyroidal anomalies were urogenital, gastrointestinal, musculoskeletal, and nervous system. In one study from Iran, the rate of urogenital anomalies among CH patients has been reported at 32% [7]. Limb anomalies were more prevalent than cardiac ones in Italy [21]. In a study in India, spina bifida was more prevalent than cardiac ones (41% vs 29%) [9]. Meanwhile, in a study in Egypt, the rate of skeletal anomalies was higher than cardiac ones (45% vs 9%) [3]. In a study in Turkey, the rate of craniofacial anomalies was similar to cardiac ones (22%) [10]. The most common cardiac anomalies were atrial septal defect (ASD), ventricular septal defect (VSD), PDA, pulmonary stenosis, persistent foramen ovale (PFO), pulmonary valve dysplasia, and endocardial cushion defect, respectively. The female-to-male ratio ranged from 0.4 in one study from Georgia [26] to 4 in another study from India [2] Cardiac anomalies were more prevalent in girls (female to male ratio=1.6 [0.7 to 5.5]), and urogenital ones were more reported in boys. Among the four studies that have reported the gender of the patients with urogenital anomalies, in three studies, all of the patients were boys [7, 8, 23], and in the other one, out of the 24 patients with urogenital anomalies, 21 patients were male [17]. Among the patients with gastrointestinal or respiratory anomalies, 23 patients were male, and 24 were female. In one study, there were 15 female patients and 20 male patients with concurrent gastrointestinal or respiratory anomalies [17], and in the other one, there were 8 female patients and 4 male patients with these anomalies [8]. In three studies, the gender of the patients with nervous system anomalies is reported. In two of these, all of the patients were male [8, 23], and in one of them, there were 8 girls and 6 boys [17]. In total, there were 10 male patients and 8 female patients with CH and nervous system anomalies (female to male ratio=0.8). Musculoskeletal anomalies were reported in 11 girls and 6 boys (female to male ratio=1.8) [8, 17].
Consanguinity or its association with the anomalies has been evaluated in six studies. In one study, 57.14% (4 out of 7) of patients with two or more anomalies had parental consanguinity [25]. Ghandi et al., and Razavi et al. did not report any association between parental consanguinity and the presence of anomalies [1, 4]. Rather et al. [2] reported no consanguineous marriages between their patients. Gu et al. [17] also reported no consanguineous marriages in patients with other anomalies. In the study by Caiulo et al. [21], out of 22 patients with other malformations, 4 patients were born to consanguineous parents.
Concurrent anomalies form the studies that have excluded down syndrome patients (n=5) [6, 10, 14, 16]
In this group of studies, 590 CH patients were evaluated. Multiple anomalies were evaluated only in one study with a prevalence rate of 22% [10] and chromosomal anomalies in 1%-2% [5, 12]. The most common non-thyroidal anomaly was cardiac, ranging from 4%-24% [5, 6, 10, 12, 14, 15].
Other anomalies were craniofacial 22% and skeletal 17% [10]. Some studies evaluated only cardiac anomalies [6, 15]. A study from Iran reported a high rate of PFO in CH patients (24%) compared to other cardiac anomalies [6].
Concurrent anomalies from the studies that have excluded pre-term infants (n=5) [1, 4, 8, 19, 25]
In this group of studies, 646 CH patients were evaluated. Some of them were only evaluated for cardiac anomalies [1]. Multiple anomalies were reported only in one study [25], and chromosomal defects were reported in 1%-14% of the patients [4, 8]. The most common chromosomal case was the Down syndrome (14%) [4]. Cardiac anomalies with a range of 4%-22% were the most common anomalies. Other more frequent anomalies were gastrointestinal and skeletal. ASD and PDA were the most common cardiac malformations in this group.
Concurrent anomalies from the studies that have excluded the down syndrome patients and pre-term infants (n=2) [5, 12]
A total of 1617 patients were evaluated. In this group, the common non-thyroidal anomaly was cardiac anomalies ranging between 5% to 12%. The rate of multiple defects and chromosomal anomalies was reported in 2% to 6% and 1% to 2% of the studied population. The most common cardiac anomalies were PDA, ASD, VSD, and PS respectively.
Association between non-thyroidal anomalies with screening characteristics of CH patients
Most of the studies did not report an association between non-thyroidal anomalies and TSH, gender, etiology of CH, and transient and permanent CH [3-6, 8, 19, 28]. Few studies indicated that the anomalies are more prevalent in CH patients with dysgenesis of the thyroid gland [12, 15]. One study reported that the rate of cardiac and non-cardiac anomalies was 8 times and 4 times higher in CH patients with transient CH [11]. Some studies showed that the anomalies were significantly higher in CH patients with low T4 levels, prematurity, or low birth weight [5, 28]. One study from Iran reported that maternal age and parental consanguinity were not associated with anomalies [4].
Discussion
In this study, we review the studies that evaluated the rate of non-thyroidal anomalies in patients with CH. Our findings indicated that the most common anomalies were cardiac anomalies. Cardiac malformations were more prevalent in countries with a high rate of CH and in girls. Most of the studies did not report a significant association between screening TSH, gender, and etiology of CH. Some studies indicated that gestational age and screening T4 level were associated with the anomalies.
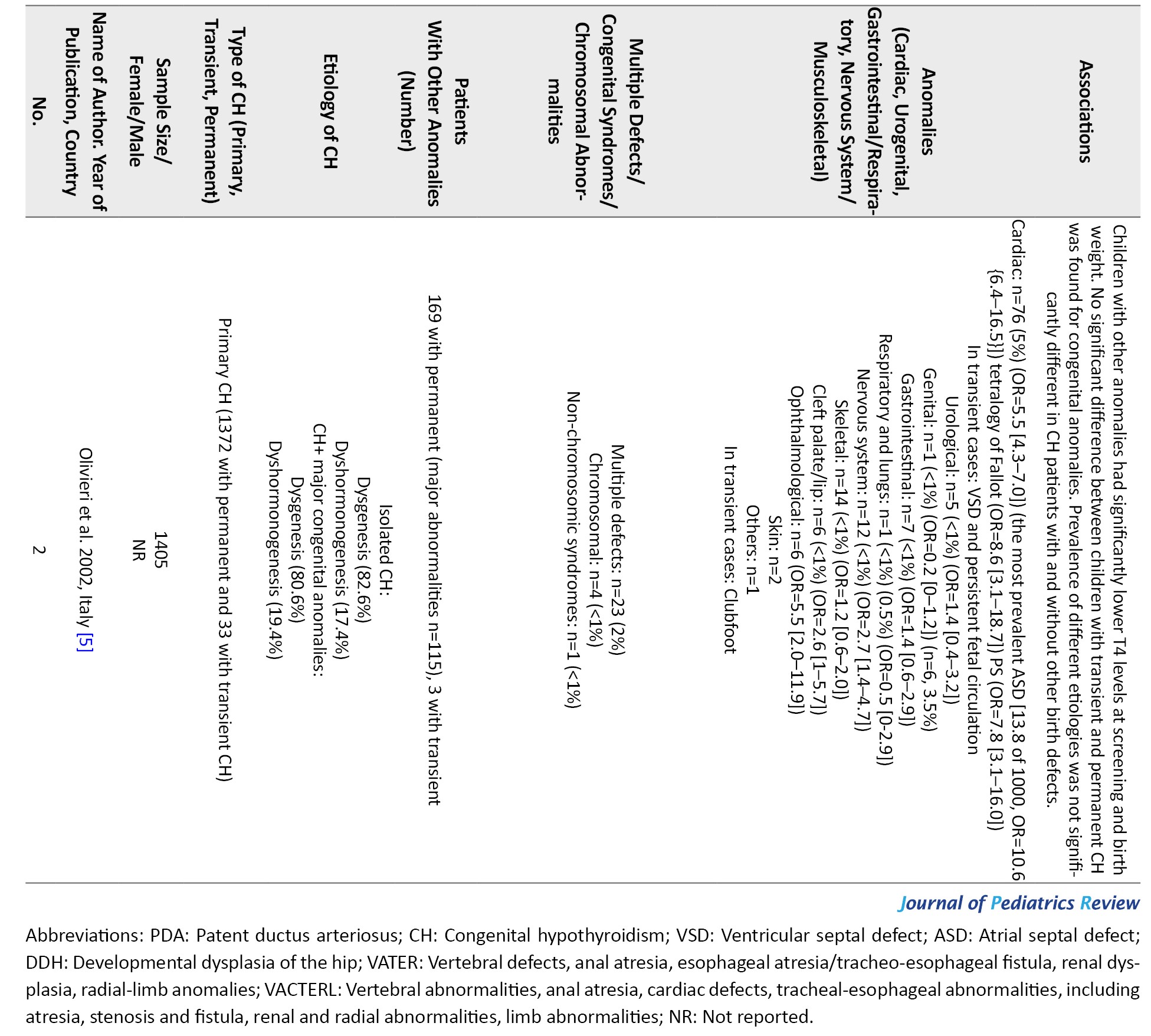
This study showed that although CH is more diagnosed among females [3], concurrent anomalies are more prevalent among male patients with permanent CH. The most common anomalies were cardiac, craniofacial, urogenital, and nervous system malformations. The most common cardiac anomalies were ASD, VSD, and PDA. Among the studies that have excluded Down syndrome patients, craniofacial anomalies were the most prevalent, and cardiac anomalies were the second one. The most common cardiac anomaly in this group was PFO, and ASD was the second most prevalent cardiac anomaly [5, 6, 10, 12, 14-16]. In the studies that have excluded pre-term infants and studies that have excluded both pre-term infants and Down syndrome patients, cardiac anomalies were the most common. ASD and PDA were the most common cardiac anomalies in these studies [1, 4, 5, 8, 12, 19, 25].
Congenital malformations occur in 3% to 4% of newborns [12]. CH affects nearly 1 in 4000 infants [3], and in the current study, the prevalence of congenital malformations in CH patients was found to be higher than the average population [12].
Patients with central CH may have midline facial anomalies [13, 21]. In patients with CH, congenital syndromes, such as Down syndrome are more prevalent than in the general population, and CH is more common among Down syndrome patients [4].
In the included studies, several syndromes, such as Jacobsen syndrome, Di George syndrome, Fanconi syndrome, Turner syndrome, Beckwith Wiedemann syndrome, VATER association, VACTERL association, Albright’s hereditary osteodystrophy, and Pierre Robin sequence were reported among the CH patients [4, 12, 17, 21].
The exact etiology of the higher incidence of congenital disabilities in CH patients is unclear [4]. Though, based on our review, congenital cardiac malformations were the most common concomitant anomaly in these patients, in different populations, the types of anomalies may be different from each other [17]. For example, in the study by El Kholy et al. in Egypt, the most common concomitant anomaly was musculoskeletal malformations [3]. During the embryonic period, several genes that are involved in the thyroid gland formation, are also involved in the other parts of organogenesis. For instance, the NKX2.5 gene is involved in the pathogenesis of congenital heart defects and is also involved in thyroidogenesis [6]. This may explain the high prevalence of cardiac malformations in patients with CH. The most common form of CH is thyroid dysgenesis, and mutations in genes, such as FOXE1, NKX2.1, and PAX8 are related to this disorder [2]. These mutations also lead to other malformations, such as renal, craniofacial, and nervous system anomalies [29]. The high prevalence of musculoskeletal anomalies also suggests the possible role of another genetic component, which should be investigated in future studies [3]. Although in most cases, CH is reported to be sporadic, Castanet et al. found a high frequency of positive family history of thyroid dysgenesis in these patients. All of these findings support the role of genetic components in this disorder [20].
Thyroid agenesis is associated with a higher rate of extra-thyroidal malformations [12]. However, Rather et al. showed a high incidence of anomalies in patients with dyshormonogenesis [2]. Considering the critical role of thyroid hormones in cellular growth and differentiation, the lack of sufficient amounts of thyroid hormones in the early stages of organogenesis is another hypothesis for the high incidence of congenital malformations observed in CH patients [12, 29].
It is recommended that in future studies the presence of different anomalies will be evaluated by genetic studies, to determine the possible association between CH-related genetic factors and the anomalies.
Recent evidence shows that environmental factors mainly, pollutants during the prenatal period, may have a role in fetal development. Considering that there are not many studies in this field, this issue is considered an essential issue in studying the pathogenesis of CH and its related anomalies [30].
The wide range of the prevalence of concomitant anomalies reported in patients with CH might be related to differences in the studied populations, or different study methods and criteria [15]. Overall, considering the high frequency of extra-thyroidal anomalies in CH infants, a complete evaluation of the child diagnosed with CH, especially for cardiac, renal, and nervous system anomalies, is needed.
Studies have shown that cardiac and musculoskeletal malformations are more prevalent in girls with CH, and nervous system and urogenital abnormalities are more common in boys [8, 17]. In the study by Gu et al., 1520 patients with CH were evaluated, and 222 patients showed concurrent anomalies. In this study, cardiac anomalies were 1.25 times more prevalent in girls than boys (10% of the girls and 8% of the boys showed cardiac anomalies, respectively), urogenital anomalies were 10 times more common in boys (3% of the boys and 0.3% of the girls showed urogenital anomalies, respectively), and nervous system anomalies were present in 8 girls and 6 boys (the prevalence of nervous system anomalies was near 1% in both genders) [17].
These findings would help plan the future screening protocol for evaluating non-thyroidal anomalies.
Studies from countries with high rates of CH indicated that parental consanguinity could be a potential risk factor for CH [31, 32].
It is suggested that consanguinity may also be a risk factor for the presence of the mentioned anomalies. In reviewed studies, this factor had not been investigated in most of the studies, and only in 1 study the rate of consanguinity was higher in CH patients with two or more anomalies than those without [25]. Ghandi et al. reported a high prevalence (22.7%) of congenital heart defects in CH infants. They observed that consanguinity marriage was not related to the occurrence of cardiac anomalies [1].
According to our findings, cardiac anomalies were higher in countries with a higher rate of CH than in European countries, where both rates of CH and consanguinity were not high. It is recommended to study the rate of different anomalies in association with consanguinity to find out the possible role of some genetic factors in the development of CH and its related anomalies.
Regarding the association between familial, demographic, and screening factors associations with CH-related anomalies, some studies indicated that low T4 level prematurity and low birth weight are associated with the anomalies [5, 28]. These findings confirm the role of thyroid hormone in fetal development and its possible role in CH-related anomalies. The association between prematurity and anomalies in CH patients is a challenging issue. However, prematurity itself is associated with a high rate of CH and also anomalies [33, 34].
Further studies are needed to investigate the role of the mentioned factors in this field.
Conclusion
Congenital anomalies are more common in CH patients compared with the general population, even in the absence of congenital syndromes or chromosomal abnormalities. The most common anomalies are cardiac, craniofacial, urogenital, and nervous system. Cardiac anomalies are more frequent among girls. The most common cardiac anomalies are septum defects. It is recommended that we use the data for revising the CH screening program to screen the most common anomalies.
Study limitations
This study faced several limitations. First, in a number of the included studies, male to female ratio, CH type (transient or permanent), and the type of anomalies are not reported. In patients with multiple anomalies, the type of each anomaly has not been reported. Also, due to the lack of complete information about the anomalies found in each patient, it is not possible to exclude all patients with Down syndrome and pre-term babies in all of the studies. The number of patients with concurrent anomalies, excluding patients with Down syndrome and pre-term infants, is only reported from the studies that have excluded these patients. In addition, included studies have not examined intervening variables such as race, consanguineous marriage, and family history. However, this study highlights the importance of at-birth screening in CH infants for other congenital anomalies considering the high frequency of extra-thyroidal malformations in these patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.MED.REC.1400.724).
Funding
This study was extracted from the pediatric endocrinology dissertation of Jila Yousofi, that was approved and funded by Department of Pediatric Endocrinology, School of Medicine, Isfahan University of Medical Sciences.
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
References
Congenital hypothyroidism (CH) is one of the most common endocrine disorders in children, which can cause permanent mental retardation if untreated. Nowadays, CH is diagnosed through neonatal screening [1]. It affects nearly 1 in 3000 to 4000 newborns worldwide. It can be secondary or primary, and permanent or transient based on the laboratory findings. In permanent cases, the patient needs lifelong replacement of levothyroxine. Although in some cases, hypothyroidism is a part of a congenital syndrome, such as Pendred syndrome, anomalies related to the various systems are reported in CH patients, even in non-syndromic cases of this disorder [2]. In most cases, thyroid dysgenesis is the cause of CH [3].
In addition, a higher prevalence of CH was reported in Down syndrome. Many studies have shown that it is associated with an increased incidence of other congenital malformations [1, 3-5]. Teratogens and a few genes lead to CH and congenital malformations. However, the exact etiology of the high prevalence of congenital disabilities observed in CH infants is unknown [4, 6]. The presence of concurrent anomalies in CH patients raises the role of genetic components in this disorder. Based on available data, most of the reported anomalies are related to the cardiovascular system. However, environmental factors also may be related [6]. Mutations in TTF1, TTF2, FOXE1, PAX8, and TSHR are associated with thyroid dysgenesis and also anomalies, such as renal anomalies [7].
It is suggested that determining the rate of different concurrent congenital anomalies in these patients would help us in better understanding of the CH pathogenesis including both genetic and environmental factors as well as improving the protocol of CH screening in order to determine the disorders early in life and in accordance with routine screening program. A well designed screening program could help us in better management of the diseases. The aim of current study was to systematically review the papers in this field to provide us more applicable information for designing more effective CH screening program.
Methods
Search strategy, research question, and databases
This systematic review study was based on the preferred reporting items for systematic reviews and metaanalysis. The protocol of the study was approved by Isfahan University of Medical Sciences. The research question and population, intervention, comparison, and outcome framework of this study compared children with and without CH in terms of having extra-thyroidal congenital anomalies. A systematic literature search through PubMed, Science Direct, Scopus, and the Web of Science databases was performed from December 2021 until January 2022.
Inclusion and exclusion criteria
English language articles, including cross-sectional, cohort, and case-control studies, were included, and case reports, letters, and articles without an available full text were excluded. Studies that evaluated extra-thyroidal anomalies in CH patients were included. Duplicates, reviews, and unrelated and low-quality studies were excluded. Meeting abstracts were not included in this review.
Study selection and quality assessment process
After removing duplicates, the title and the abstracts were evaluated by two reviewers separately (Rojin Chegini and Jila Yousofi). Unrelated articles, reviews, letters, case reports, and articles published before 1990 were ignored. The full text of the remaining articles was separately reviewed by two reviewers, and unrelated items were removed. To find more related studies, references to the final articles were also reviewed. The final articles entered quality assessment using the strengthening of the reporting of observational studies in the epidemiology checklist and were done by two independent reviewers (Rojin Chegini and Jila Yousofi). Consult with an expert (Mahin Hashemipour) was considered in the case of disagreement.
Data extraction
Data extraction was done by two independent authors using a checklist with the following items: Name of the author, year of publication, country, sample size, gender, type of CH, inclusion and exclusion criteria, the number of CH patients with concurrent anomalies, and the type of anomalies and the number of each one. Data of the patients with cardiovascular, craniofacial, urogenital, gastrointestinal, and musculoskeletal anomalies and anomalies related to the nervous system are reported as numbers and percentages. Anomalies related to the other systems are reported as others. Considering the known association between Down syndrome and prematurity and congenital anomalies, data from the studies that have excluded these patients are reported separately.
Results
Characteristics of the studies
From the 655 initially retrieved articles, 178 were duplicates, and 430 were excluded based on the title and the abstract. Finally, among the remaining 47 articles, 24 articles entered the data extraction process, and 4 additional references were found by reviewing the references of the final articles. Finally, 28 articles were selected (Figure 1).
In total, 7401 patients (3067 females, 1987 males, others not reported) with CH were studied. The studied population in 12 studies were primary CH patients, 11 permanent CH patients, and in 4 studies, both permanent and transient cases.
In 5 studies, patients with known syndromes, such as Down syndrome were excluded, and 5 studies excluded pre-term newborns (Table 1).










In 2 studies, patients with Down syndrome and prematurity were not included.
Concurrent congenital anomalies from all studies (n=28) [1-28]
The prevalence of extra-thyroidal anomalies ranged from 5% to 50% in girls and from 4% to 80% in boys. Accordingly, 20% of the permanent CH patients and 13% of the patients with transient CH had non-thyroidal congenital malformations.
Multiple anomalies were reported in 1%-22% of the studied population [5, 9-12, 17, 18, 21-23, 25, 28]. Studies from Turkey (22%) [10] and India (12%) reported higher rates of multiple defects [9].
Chromosomal anomalies were reported in 1%-14% of the studied population [4, 5, 8, 17, 19, 22, 23, 27, 28]. The most common chromosomal anomaly was the Down syndrome. A higher rate of chromosomal anomalies was reported in Iran (14%) [4] and Saudi Arabia (10%) [27].
Regarding non-thyroidal congenital anomalies, most of the studies (18 out of 28) have reported cardiac anomalies as the most common non-thyroidal anomalies ranging from 2%-47% [1, 5, 6, 8, 11-15, 17-20, 22-24, 27, 28].In most European countries, the rate of cardiac anomalies was in the lower range. Higher rates of cardiac anomalies were reported from Turkey (47%) [13], India (29%) [9], Iran (22%-24%) [1], Poland (19%) [8], and Saudi Arabia (14%) [27].
Other more frequent non-thyroidal anomalies were urogenital, gastrointestinal, musculoskeletal, and nervous system. In one study from Iran, the rate of urogenital anomalies among CH patients has been reported at 32% [7]. Limb anomalies were more prevalent than cardiac ones in Italy [21]. In a study in India, spina bifida was more prevalent than cardiac ones (41% vs 29%) [9]. Meanwhile, in a study in Egypt, the rate of skeletal anomalies was higher than cardiac ones (45% vs 9%) [3]. In a study in Turkey, the rate of craniofacial anomalies was similar to cardiac ones (22%) [10]. The most common cardiac anomalies were atrial septal defect (ASD), ventricular septal defect (VSD), PDA, pulmonary stenosis, persistent foramen ovale (PFO), pulmonary valve dysplasia, and endocardial cushion defect, respectively. The female-to-male ratio ranged from 0.4 in one study from Georgia [26] to 4 in another study from India [2] Cardiac anomalies were more prevalent in girls (female to male ratio=1.6 [0.7 to 5.5]), and urogenital ones were more reported in boys. Among the four studies that have reported the gender of the patients with urogenital anomalies, in three studies, all of the patients were boys [7, 8, 23], and in the other one, out of the 24 patients with urogenital anomalies, 21 patients were male [17]. Among the patients with gastrointestinal or respiratory anomalies, 23 patients were male, and 24 were female. In one study, there were 15 female patients and 20 male patients with concurrent gastrointestinal or respiratory anomalies [17], and in the other one, there were 8 female patients and 4 male patients with these anomalies [8]. In three studies, the gender of the patients with nervous system anomalies is reported. In two of these, all of the patients were male [8, 23], and in one of them, there were 8 girls and 6 boys [17]. In total, there were 10 male patients and 8 female patients with CH and nervous system anomalies (female to male ratio=0.8). Musculoskeletal anomalies were reported in 11 girls and 6 boys (female to male ratio=1.8) [8, 17].
Consanguinity or its association with the anomalies has been evaluated in six studies. In one study, 57.14% (4 out of 7) of patients with two or more anomalies had parental consanguinity [25]. Ghandi et al., and Razavi et al. did not report any association between parental consanguinity and the presence of anomalies [1, 4]. Rather et al. [2] reported no consanguineous marriages between their patients. Gu et al. [17] also reported no consanguineous marriages in patients with other anomalies. In the study by Caiulo et al. [21], out of 22 patients with other malformations, 4 patients were born to consanguineous parents.
Concurrent anomalies form the studies that have excluded down syndrome patients (n=5) [6, 10, 14, 16]
In this group of studies, 590 CH patients were evaluated. Multiple anomalies were evaluated only in one study with a prevalence rate of 22% [10] and chromosomal anomalies in 1%-2% [5, 12]. The most common non-thyroidal anomaly was cardiac, ranging from 4%-24% [5, 6, 10, 12, 14, 15].
Other anomalies were craniofacial 22% and skeletal 17% [10]. Some studies evaluated only cardiac anomalies [6, 15]. A study from Iran reported a high rate of PFO in CH patients (24%) compared to other cardiac anomalies [6].
Concurrent anomalies from the studies that have excluded pre-term infants (n=5) [1, 4, 8, 19, 25]
In this group of studies, 646 CH patients were evaluated. Some of them were only evaluated for cardiac anomalies [1]. Multiple anomalies were reported only in one study [25], and chromosomal defects were reported in 1%-14% of the patients [4, 8]. The most common chromosomal case was the Down syndrome (14%) [4]. Cardiac anomalies with a range of 4%-22% were the most common anomalies. Other more frequent anomalies were gastrointestinal and skeletal. ASD and PDA were the most common cardiac malformations in this group.
Concurrent anomalies from the studies that have excluded the down syndrome patients and pre-term infants (n=2) [5, 12]
A total of 1617 patients were evaluated. In this group, the common non-thyroidal anomaly was cardiac anomalies ranging between 5% to 12%. The rate of multiple defects and chromosomal anomalies was reported in 2% to 6% and 1% to 2% of the studied population. The most common cardiac anomalies were PDA, ASD, VSD, and PS respectively.
Association between non-thyroidal anomalies with screening characteristics of CH patients
Most of the studies did not report an association between non-thyroidal anomalies and TSH, gender, etiology of CH, and transient and permanent CH [3-6, 8, 19, 28]. Few studies indicated that the anomalies are more prevalent in CH patients with dysgenesis of the thyroid gland [12, 15]. One study reported that the rate of cardiac and non-cardiac anomalies was 8 times and 4 times higher in CH patients with transient CH [11]. Some studies showed that the anomalies were significantly higher in CH patients with low T4 levels, prematurity, or low birth weight [5, 28]. One study from Iran reported that maternal age and parental consanguinity were not associated with anomalies [4].
Discussion
In this study, we review the studies that evaluated the rate of non-thyroidal anomalies in patients with CH. Our findings indicated that the most common anomalies were cardiac anomalies. Cardiac malformations were more prevalent in countries with a high rate of CH and in girls. Most of the studies did not report a significant association between screening TSH, gender, and etiology of CH. Some studies indicated that gestational age and screening T4 level were associated with the anomalies.
This study showed that although CH is more diagnosed among females [3], concurrent anomalies are more prevalent among male patients with permanent CH. The most common anomalies were cardiac, craniofacial, urogenital, and nervous system malformations. The most common cardiac anomalies were ASD, VSD, and PDA. Among the studies that have excluded Down syndrome patients, craniofacial anomalies were the most prevalent, and cardiac anomalies were the second one. The most common cardiac anomaly in this group was PFO, and ASD was the second most prevalent cardiac anomaly [5, 6, 10, 12, 14-16]. In the studies that have excluded pre-term infants and studies that have excluded both pre-term infants and Down syndrome patients, cardiac anomalies were the most common. ASD and PDA were the most common cardiac anomalies in these studies [1, 4, 5, 8, 12, 19, 25].
Congenital malformations occur in 3% to 4% of newborns [12]. CH affects nearly 1 in 4000 infants [3], and in the current study, the prevalence of congenital malformations in CH patients was found to be higher than the average population [12].
Patients with central CH may have midline facial anomalies [13, 21]. In patients with CH, congenital syndromes, such as Down syndrome are more prevalent than in the general population, and CH is more common among Down syndrome patients [4].
In the included studies, several syndromes, such as Jacobsen syndrome, Di George syndrome, Fanconi syndrome, Turner syndrome, Beckwith Wiedemann syndrome, VATER association, VACTERL association, Albright’s hereditary osteodystrophy, and Pierre Robin sequence were reported among the CH patients [4, 12, 17, 21].
The exact etiology of the higher incidence of congenital disabilities in CH patients is unclear [4]. Though, based on our review, congenital cardiac malformations were the most common concomitant anomaly in these patients, in different populations, the types of anomalies may be different from each other [17]. For example, in the study by El Kholy et al. in Egypt, the most common concomitant anomaly was musculoskeletal malformations [3]. During the embryonic period, several genes that are involved in the thyroid gland formation, are also involved in the other parts of organogenesis. For instance, the NKX2.5 gene is involved in the pathogenesis of congenital heart defects and is also involved in thyroidogenesis [6]. This may explain the high prevalence of cardiac malformations in patients with CH. The most common form of CH is thyroid dysgenesis, and mutations in genes, such as FOXE1, NKX2.1, and PAX8 are related to this disorder [2]. These mutations also lead to other malformations, such as renal, craniofacial, and nervous system anomalies [29]. The high prevalence of musculoskeletal anomalies also suggests the possible role of another genetic component, which should be investigated in future studies [3]. Although in most cases, CH is reported to be sporadic, Castanet et al. found a high frequency of positive family history of thyroid dysgenesis in these patients. All of these findings support the role of genetic components in this disorder [20].
Thyroid agenesis is associated with a higher rate of extra-thyroidal malformations [12]. However, Rather et al. showed a high incidence of anomalies in patients with dyshormonogenesis [2]. Considering the critical role of thyroid hormones in cellular growth and differentiation, the lack of sufficient amounts of thyroid hormones in the early stages of organogenesis is another hypothesis for the high incidence of congenital malformations observed in CH patients [12, 29].
It is recommended that in future studies the presence of different anomalies will be evaluated by genetic studies, to determine the possible association between CH-related genetic factors and the anomalies.
Recent evidence shows that environmental factors mainly, pollutants during the prenatal period, may have a role in fetal development. Considering that there are not many studies in this field, this issue is considered an essential issue in studying the pathogenesis of CH and its related anomalies [30].
The wide range of the prevalence of concomitant anomalies reported in patients with CH might be related to differences in the studied populations, or different study methods and criteria [15]. Overall, considering the high frequency of extra-thyroidal anomalies in CH infants, a complete evaluation of the child diagnosed with CH, especially for cardiac, renal, and nervous system anomalies, is needed.
Studies have shown that cardiac and musculoskeletal malformations are more prevalent in girls with CH, and nervous system and urogenital abnormalities are more common in boys [8, 17]. In the study by Gu et al., 1520 patients with CH were evaluated, and 222 patients showed concurrent anomalies. In this study, cardiac anomalies were 1.25 times more prevalent in girls than boys (10% of the girls and 8% of the boys showed cardiac anomalies, respectively), urogenital anomalies were 10 times more common in boys (3% of the boys and 0.3% of the girls showed urogenital anomalies, respectively), and nervous system anomalies were present in 8 girls and 6 boys (the prevalence of nervous system anomalies was near 1% in both genders) [17].
These findings would help plan the future screening protocol for evaluating non-thyroidal anomalies.
Studies from countries with high rates of CH indicated that parental consanguinity could be a potential risk factor for CH [31, 32].
It is suggested that consanguinity may also be a risk factor for the presence of the mentioned anomalies. In reviewed studies, this factor had not been investigated in most of the studies, and only in 1 study the rate of consanguinity was higher in CH patients with two or more anomalies than those without [25]. Ghandi et al. reported a high prevalence (22.7%) of congenital heart defects in CH infants. They observed that consanguinity marriage was not related to the occurrence of cardiac anomalies [1].
According to our findings, cardiac anomalies were higher in countries with a higher rate of CH than in European countries, where both rates of CH and consanguinity were not high. It is recommended to study the rate of different anomalies in association with consanguinity to find out the possible role of some genetic factors in the development of CH and its related anomalies.
Regarding the association between familial, demographic, and screening factors associations with CH-related anomalies, some studies indicated that low T4 level prematurity and low birth weight are associated with the anomalies [5, 28]. These findings confirm the role of thyroid hormone in fetal development and its possible role in CH-related anomalies. The association between prematurity and anomalies in CH patients is a challenging issue. However, prematurity itself is associated with a high rate of CH and also anomalies [33, 34].
Further studies are needed to investigate the role of the mentioned factors in this field.
Conclusion
Congenital anomalies are more common in CH patients compared with the general population, even in the absence of congenital syndromes or chromosomal abnormalities. The most common anomalies are cardiac, craniofacial, urogenital, and nervous system. Cardiac anomalies are more frequent among girls. The most common cardiac anomalies are septum defects. It is recommended that we use the data for revising the CH screening program to screen the most common anomalies.
Study limitations
This study faced several limitations. First, in a number of the included studies, male to female ratio, CH type (transient or permanent), and the type of anomalies are not reported. In patients with multiple anomalies, the type of each anomaly has not been reported. Also, due to the lack of complete information about the anomalies found in each patient, it is not possible to exclude all patients with Down syndrome and pre-term babies in all of the studies. The number of patients with concurrent anomalies, excluding patients with Down syndrome and pre-term infants, is only reported from the studies that have excluded these patients. In addition, included studies have not examined intervening variables such as race, consanguineous marriage, and family history. However, this study highlights the importance of at-birth screening in CH infants for other congenital anomalies considering the high frequency of extra-thyroidal malformations in these patients.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Research Ethics Committee of Isfahan University of Medical Sciences (Code: IR.MUI.MED.REC.1400.724).
Funding
This study was extracted from the pediatric endocrinology dissertation of Jila Yousofi, that was approved and funded by Department of Pediatric Endocrinology, School of Medicine, Isfahan University of Medical Sciences.
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
References
- Ghandi Y, Sanatkar SA, Habibi D, Dorreh F, Sadeghizadeh B, Sharahee M. Frequency of congenital cardiac malformations in the neonates with congenital hypothyroidism. Iran J Neonatol. 2018; 9(2):66-70. [Link]
- Rather TA, Khan SH, Masoodi S, Alai MS. Thyroid dyshormonogenesis and associated non-thyroidal anomalies in a tertiary care hospital in India. Horm Res Paediatr. 2014; 81(5):314-8. [DOI:10.1159/000357843] [PMID]
- El Kholy M, Fahmi ME, Nassar AE, Selim S, Elsedfy HH. Prevalence of minor musculoskeletal anomalies in children with congenital hypothyroidism. Horm Res. 2007; 68(6):272-5. [DOI:10.1159/000104175] [PMID]
- Razavi Z, Yavarikia A, Torabian S. Congenital anomalies in infant with congenital hypothyroidism. Oman Med J. 2012; 27(5):364-7. [DOI:10.5001/omj.2012.92] [PMID]
- Olivieri A, Stazi MA, Mastroiacovo P, Fazzini C, Medda E, Spagnolo A, et al. A population-based study on the frequency of additional congenital malformations in infants with congenital hypothyroidism: data from the Italian Registry for Congenital Hypothyroidism (1991-1998). J Clin Endocrinol Metab. 2002; 87(2):557-62. [PMID]
- Sabri MR, Shahriari H, Hashemipour M. Congenital cardiac malformations in congenital hypothyroid patients in Isfahan. J Res Med Sci. 2006; 11(4):234-9. [Link]
- Yousefi Chaijan P, Dorreh F, Sharafkhah M, Amiri M, Ebrahimimonfared M, Rafeie M, et al. Congenital urogenital abnormalities in children with congenital hypothyroidism. Med J Islam Repub Iran. 2017; 31:7. [DOI:10.18869/mjiri.31.7] [PMID]
- Wędrychowicz A, Furtak A, Prośniak A, Żuberek M, Szczerkowska M, Pacut P, et al. Extrathyroidal congenital defects in children with congenital hypothyroidism - observations from a single paediatric centre in Central Europe with a review of literature. Pediatr Endocrinol Diabetes Metab. 2019; 25(3):114-21. [DOI:10.5114/pedm.2019.87178] [PMID]
- Reddy PA, Rajagopal G, Harinarayan CV, Vanaja V, Rajasekhar D, Suresh V, et al. High prevalence of associated birth defects in congenital hypothyroidism. Int J Pediatr Endocrinol. 2010; 2010:940980. [DOI:10.1155/2010/940980] [PMID]
- Ozsu E, Altun G, Çizmecioglu FM, Yildirim B, Akca A, Mutlu GY, et al. Extra-thyroid congenital abnormalities associated with thyroid dysgenesis in Turkey. HK J Paediatr. 2016; 21(1):3-6. [Link]
- Oakley GA, Muir T, Ray M, Girdwood RW, Kennedy R, Donaldson MD. Increased incidence of congenital malformations in children with transient thyroid-stimulating hormone elevation on neonatal screening. J Pediatr. 1998; 132(4):726-30. [DOI:10.1016/S0022-3476(98)70369-5] [PMID]
- Monroy-Santoyo S, Ibarra-González I, Fernández-Lainez C, Greenawalt-Rodríguez S, Chacón-Rey J, Calzada-León R, et al. Higher incidence of thyroid agenesis in Mexican newborns with congenital hypothyroidism associated with birth defects. Early Hum Dev. 2012; 88(1):61-4. [DOI:10.1016/j.earlhumdev.2011.07.009] [PMID]
- Kurtul BE, Ozer PA, Kabatas EU, Gürkan A, Aycan Z. Ophthalmic manifestations in children with congenital hypothyroidism. J Pediatr Ophthalmol Strabismus. 2016; 53(1):29-34. [DOI:10.3928/01913913-20160113-06] [PMID]
- Kurinczuk JJ, Bower C, Lewis B, Byrne G. Congenital hypothyroidism in Western Australia 1981-1998. J Paediatr Child Health. 2002; 38(2):187-91. [DOI:10.1046/j.1440-1754.2002.00812.x] [PMID]
- Kreisner E, Neto EC, Gross JL. High prevalence of extrathyroid malformations in a cohort of Brazilian patients with permanent primary congenital hypothyroidism. Thyroid. 2005; 15(2):165-9. [DOI:10.1089/thy.2005.15.165] [PMID]
- Kempers MJ, Ozgen HM, Vulsma T, Merks JH, Zwinderman KH, de Vijlder JJ, et al. Morphological abnormalities in children with thyroidal congenital hypothyroidism. Am J Med Genet A. 2009; 149A(5):943-51. [DOI:10.1002/ajmg.a.32777] [PMID]
- Gu YH, Harada S, Kato T, Inomata H, Aoki K, Hirahara F. Increased incidence of extrathyroidal congenital malformations in Japanese patients with congenital hypothyroidism and their relationship with Down syndrome and other factors. Thyroid. 2009; 19(8):869-79. [DOI:10.1089/thy.2008.0405] [PMID]
- Devos H, Rodd C, Gagné N, Laframboise R, Van Vliet G. A search for the possible molecular mechanisms of thyroid dysgenesis: Sex ratios and associated malformations. J Clin Endocrinol Metab. 1999; 84(7):2502-6. [DOI:10.1210/jcem.84.7.5831] [PMID]
- Chao T, Wang JR, Hwang B. Congenital hypothyroidism and concomitant anomalies. J Pediatr Endocrinol Metab. 1997; 10(2):217-21. [DOI:10.1515/JPEM.1997.10.2.217] [PMID]
- Castanet M, Polak M, Bonaïti-Pellié C, Lyonnet S, Czernichow P, Léger J, et al. Nineteen years of national screening for congenital hypothyroidism: Familial cases with thyroid dysgenesis suggest the involvement of genetic factors. J Clin Endocrinol Metab. 2001; 86(5):2009-14. [DOI:10.1210/jcem.86.5.7501] [PMID]
- Caiulo S, Corbetta C, Di Frenna M, Medda E, De Angelis S, Rotondi D, et al. Newborn screening for congenital hypothyroidism: The benefit of using differential TSH cutoffs in a 2-screen program. J Clin Endocrinol Metab. 2021; 106(1):E338-49. [DOI:10.1210/clinem/dgaa789] [PMID]
- Law WY, Bradley DM, Lazarus JH, John R, Gregory JW. Congenital hypothyroidism in Wales (1982-1993): Demographic features, clinical presentation and effects on early neurodevelopment. Clin Endocrinol (Oxf). 1998; 48(2):201-7. [DOI:10.1046/j.1365-2265.1998.3791206.x] [PMID]
- Al-Jurayyan NA, Al-Herbish AS, El-Desouki MI, Al-Nuaim AA, Abo-Bakr AM, Al-Husain MA. Congenital anomalies in infants with congenital hypothyroidism: Is it a coincidental or an associated finding? Hum Hered. 1997; 47(1):33-7. [DOI:10.1159/000154386] [PMID]
- Sorcini M, Balestrazzi P, Grandolfo ME, Carta S, Giovannelli G. The National Register of infants with congenital hypothyroidism detected by neonatal screening in Italy. J Endocrinol Invest. 1993; 16(8):573-7. [DOI:10.1007/BF03347672] [PMID]
- Siebner R, Merlob P, Kaiserman I, Sack J. Congenital anomalies concomitant with persistent primary congenital hypothyroidism. Am J Med Genet. 1992; 44(1):57-60. [DOI:10.1002/ajmg.1320440114] [PMID]
- Roberts HE, Moore CA, Fernhoff PM, Brown AL, Khoury MJ. Population study of congenital hypothyroidism and associated birth defects, Atlanta, 1979-1992. Am J Med Genet. 1997; 71(1):29-32. [PMID]
- Majeed-Saidan MA, Joyce B, Khan M, Hamam HD. Congenital hypothyroidism: The Riyadh military hospital experience. Clin Endocrinol (Oxf). 1993; 38(2):191-5. [DOI:10.1111/j.1365-2265.1993.tb00992.x] [PMID]
- Cassio A, Tatò L, Colli C, Spolettini E, Costantini E, Cacciari E. Incidence of congenital malformations in congenital hypothyroidism. Screening. 1994; 3(3):125-30. [DOI:10.1016/0925-6164(94)90020-5]
- Kumar J, Gordillo R, Kaskel FJ, Druschel CM, Woroniecki RP. Increased prevalence of renal and urinary tract anomalies in children with congenital hypothyroidism. J Pediatr. 2009; 154(2):263-6. [DOI:10.1016/j.jpeds.2008.08.023] [PMID]
- Street ME, Bernasconi S. Endocrine-disrupting chemicals in human fetal growth. Int J Mol Sci. 2020; 21(4):1430. [DOI:10.3390/ijms21041430] [PMID]
- Hashemipour M, Amini M, Talaie M, Kelishadi R, Hovespian S, Iranpour R, et al. Parental consanguinity among parents of neonates with congenital hypothyroidism in Isfahan. East Mediterr Health J. 2007; 13(3):567-74. [PMID]
- Mehran L, Azizi F, Mousapour P, Cheraghi L, Yarahmadi S, Amirshekari G, et al. Development of a risk prediction model for early discrimination between permanent and transient congenital hypothyroidism. Endocrine. 2021; 73(2):374-83. [DOI:10.1007/s12020-021-02641-0] [PMID]
- Kaluarachchi DC, Allen DB, Eickhoff JC, Dawe SJ, Baker MW. Increased congenital hypothyroidism detection in preterm infants with serial newborn screening. J Pediatr. 2019; 207:220-5. [DOI:10.1016/j.jpeds.2018.11.044] [PMID]
- Hashemipour M, Samei P, Kelishadi R, Hovsepian S, Hani Tabaei Zavareh N. A systematic review on the risk factors of congenital hypothyroidism. J Pediatr Rev. 2019; 7(4):199-210. [Link]
Type of Study: Review Article |
Subject:
Pediatric Endocrinology
Received: 2024/02/25 | Accepted: 2024/03/10 | Published: 2024/04/1
Received: 2024/02/25 | Accepted: 2024/03/10 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |