Volume 11, Issue 4 (10-2023)
J. Pediatr. Rev 2023, 11(4): 363-372 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Baryar Langroudi F, Mohammadjafari H, Rostami Rad H, Momeni M, Guran M, Navaeifar M R. Clinical and Laboratory Characteristics of Acute Kidney Injury in Critically Ill Children: A Single Center Study. J. Pediatr. Rev 2023; 11 (4) :363-372
URL: http://jpr.mazums.ac.ir/article-1-525-en.html
URL: http://jpr.mazums.ac.ir/article-1-525-en.html
Faeghe Baryar Langroudi1 

 , Hamid Mohammadjafari2
, Hamid Mohammadjafari2 

 , Hani Rostami Rad3
, Hani Rostami Rad3 
 , Mohaddeseh Momeni3
, Mohaddeseh Momeni3 

 , Maedeh Guran3
, Maedeh Guran3 

 , Mohammad Reza Navaeifar
, Mohammad Reza Navaeifar 

 4
4


 , Hamid Mohammadjafari2
, Hamid Mohammadjafari2 

 , Hani Rostami Rad3
, Hani Rostami Rad3 
 , Mohaddeseh Momeni3
, Mohaddeseh Momeni3 

 , Maedeh Guran3
, Maedeh Guran3 

 , Mohammad Reza Navaeifar
, Mohammad Reza Navaeifar 

 4
4
1- Department of Pediatric, Faculty of Medicine, Bou Ali-Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
2- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
3- Bou Ali-Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
4- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. , dr.navaifar@gmail.com
2- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
3- Bou Ali-Sina Hospital, Mazandaran University of Medical Sciences, Sari, Iran.
4- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. , dr.navaifar@gmail.com
Full-Text [PDF 435 kb]
(259 Downloads)
| Abstract (HTML) (848 Views)
Full-Text: (65 Views)
Introduction
Acute kidney injury (AKI) is associated with a sudden decrease in kidney function, leading to hemodynamic and electrolyte disturbances [1]. AKI can develop in 2%-5% of patients admitted to a tertiary pediatric hospital [2, 3].
AKI has become one of the common complications seen in pediatric intensive care units (PICUs) [4 5, 6]. The prevalence of AKI reported up to 27% in critically ill children [7, 8]. Recently, AKI prevalence was reported as 22% to 25% in children admitted to the hospital for severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) [9, 10]. In addition, AKI could be a disturbing complication in children with underlying diseases [8, 11].
Clinical presentations showed diminished urine output and increased blood urea or creatinine levels. One of the most common definitions of AKI is recommended by KDIGO (kidney disease improving global guidelines) [12]. However, we employed the pRIFLE (pediatric risk, injury, failure, loss, end-stage renal disease) classification to define AKI in children (Table 1) [13, 14].
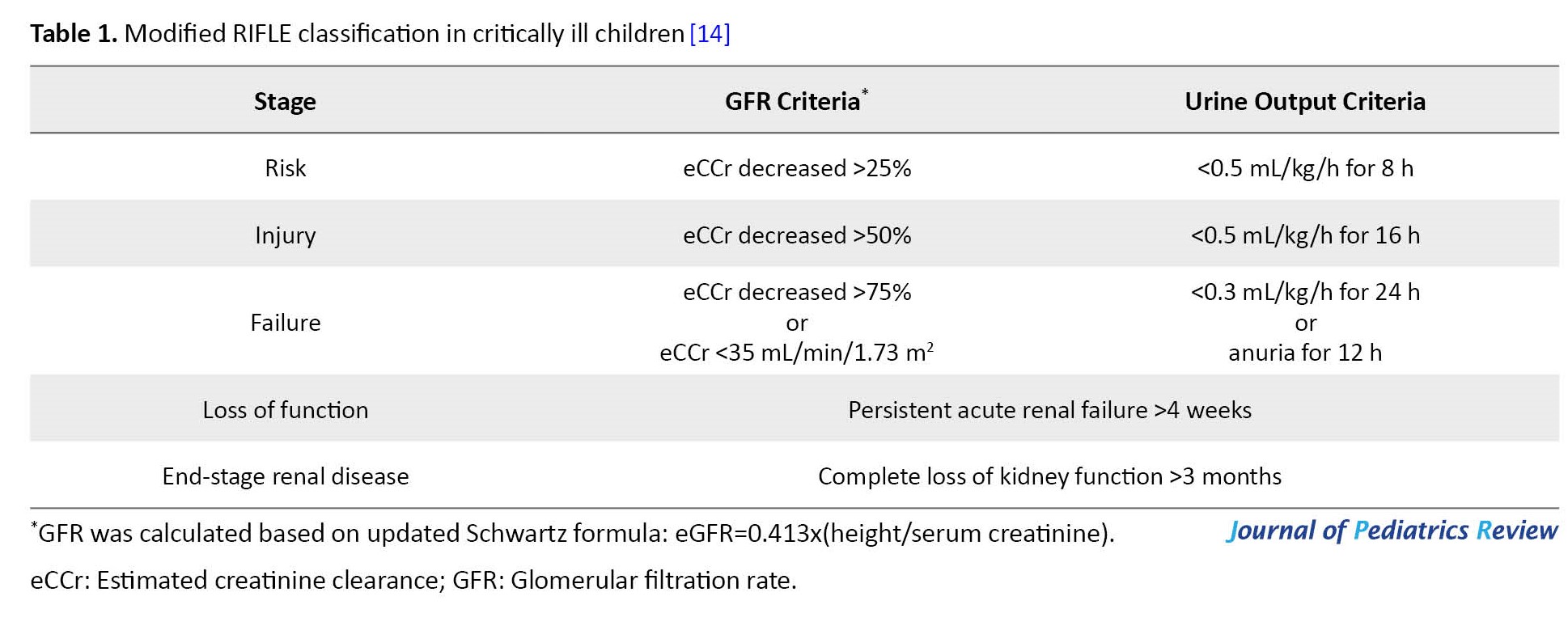
Based on the pRIFLE classification, AKI is divided into 3 stages in the acute phase, i.e. risk, injury, failure, and another 2 stages in a more chronic course, i.e. loss of function and end-stage renal disease.
The etiology of AKI is divided into prerenal, renal, and postrenal causes. Prerenal AKI (the most common form) results from decreased kidney blood perfusion. Prerenal AKI commonly results from hypovolemic states, heart failure, and conditions with increased third space. Renal AKI is a condition with renal damage from toxic, inflammatory, or immunologic insults, while postrenal AKI causes consist of obstructing lesions, such as congenital anomalies and acquired states [15-17].
Due to the scarcity of data in our region, we assessed the prevalence of AKI, clinical and laboratory characteristics, etiologies, and course in children admitted to a tertiary training PICU with average annual admission of 350 children, focusing on nephrotoxins and comorbidities.
Methods
Study population
This cross-sectional study was performed in a 12-bed mixed medical and surgical PICU in northern Iran between September 2017 and April 2019.
We studied all 1 month to 18 years old patients admitted to PICU and remained there for at least 48 hours. The exclusion criteria included a lack of complete information or diagnosis of AKI before the first 48 hours of hospitalization.
Study measurements
The demographic data, medical history, and pediatric risk of mortality (PRISM) III were obtained in the first 12 hours of admission. The clinical and laboratory determinants were assessed and recorded meticulously. Age, sex, family history of kidney diseases, and hematologic and biochemistry tests were also recorded.
The PRISM III score is a widely used scoring system to quantify critical illness in pediatric patients. The components comprised cardiovascular (heart rate, systolic blood pressure, and temperature), neurologic (pupillary reactivity and mental status), respiratory (arterial PO2, pH, PCO2, and total bicarbonate), chemical (glucose, potassium, blood urea nitrogen, and creatinine), and hematologic (WBC count, platelet count, prothrombin, and partial thromboplastin time) profiles.
AKI was defined based on the pRIFLE definition. The urine was collected and measured for 8 hours. The measurement was performed in 3 forms. For cooperative ambulatory patients, urine was collected in measured bottles and recorded as mL/kg/min. For immobile patients with a urinary catheter, the urine was stored in urine bags and recorded at least every 8 hours. Moreover, for immobile or non-cooperative children without a urinary catheter, the diapers were scaled before and after urination with an electrical scale with a 1-g error. The Jaffe method was used to measure serum creatinine.
In the AKI group, the variables likely to contribute to renal failure were selected for statistical analysis up to 48 hours before the onset of renal failure. This method prevented the possible effect of kidney failure on these parameters as much as possible. The AKI group followed for 4 weeks to define the loss phase of pRIFLE.
When a patient received at least 2 nephrotoxic agents, it was recorded as exposure to multiple nephrotoxic agents. Anemia was defined based on lower hemoglobin limits in Nathan and Oski’s hematology of infancy and childhood [18]. Sepsis and organ dysfunction (except for renal dysfunction) were defined based on the international pediatric sepsis consensus conference criteria [19].
The estimated glomerular filtration rate (GFR) was calculated based on the Schwartz formula [20] for patients under two years old and based on the updated Schwartz formula for older cases [21].
The etiology of AKI was assessed based on the clinical and laboratory findings. The relation between clinical or laboratory disorders and the occurrence or severity of AKI was assessed, too.
Statistical analysis
The categorical variables were presented as numbers and percentages, and the non-categorical ones were Mean±SD. Univariate analysis was undertaken to evaluate the relationship between clinical and demographic variables and AKI and death. Statistical analysis of the difference between the groups was determined using the t-test or Fisher exact test to compare the two groups.
Analysis of possible variables contributing to AKI (Table 2, Table 3 and Table 4) was made using data up to 48 hours before the onset of renal failure.

Nonparametric tests (such as the Mann-Whitney and Kruskal-Wallis tests) were used to analyze the variables without normal distributions.
Multivariate logistic regression was undertaken to evaluate the independent association with risk factors of AKI, which were significant in the univariate analysis. When more than one organ failure occurred in a patient, the first organ failure entered the multivariate analysis. A P<0.05 was considered statistically significant. All statistical analyses were performed in SPSS software, version 16 (SPSS Inc., Chicago, USA).
Results
A total of 263 patients were admitted and remained in the PICU for more than 48 hours. Eight cases were excluded because of incomplete data. Of the 255 enrolled children, 123(48.2%) were female. Their median age was 18 (interquartile range [IQR], 6-60) months. Their median PRISM III score was 4 (IQR, 0-8), whereas 43 patients (16.9%) had a PRISM III score of more than 10. The causes of admission were infectious diseases in 128 cases (50.2%), neurologic disorders in 66(25.9%), trauma surgery in 22(8.6%), respiratory diseases in 19(7.5%), endocrine disorders in 13(5.1%), and gastrointestinal diseases in 7(2.7%).
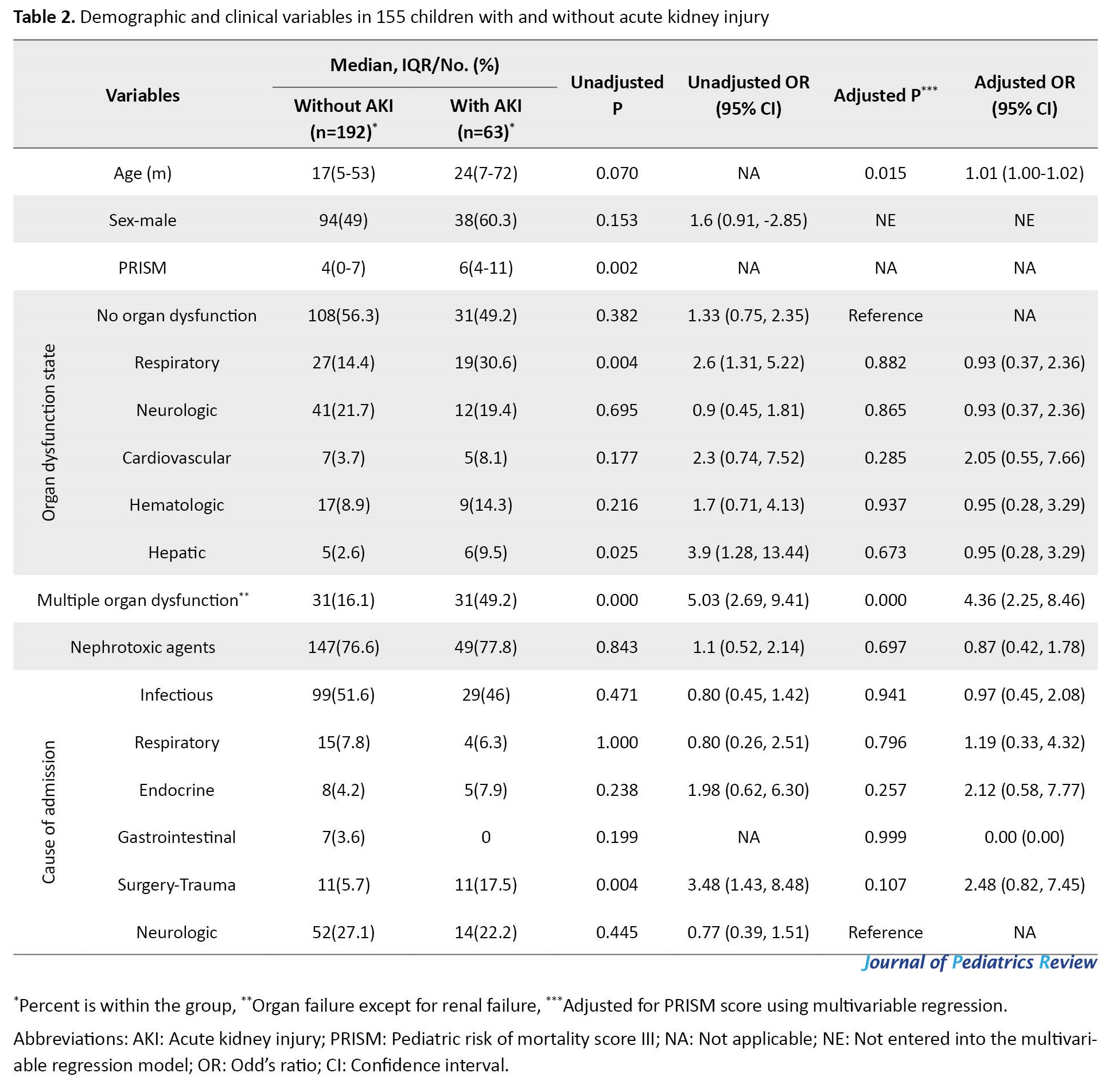
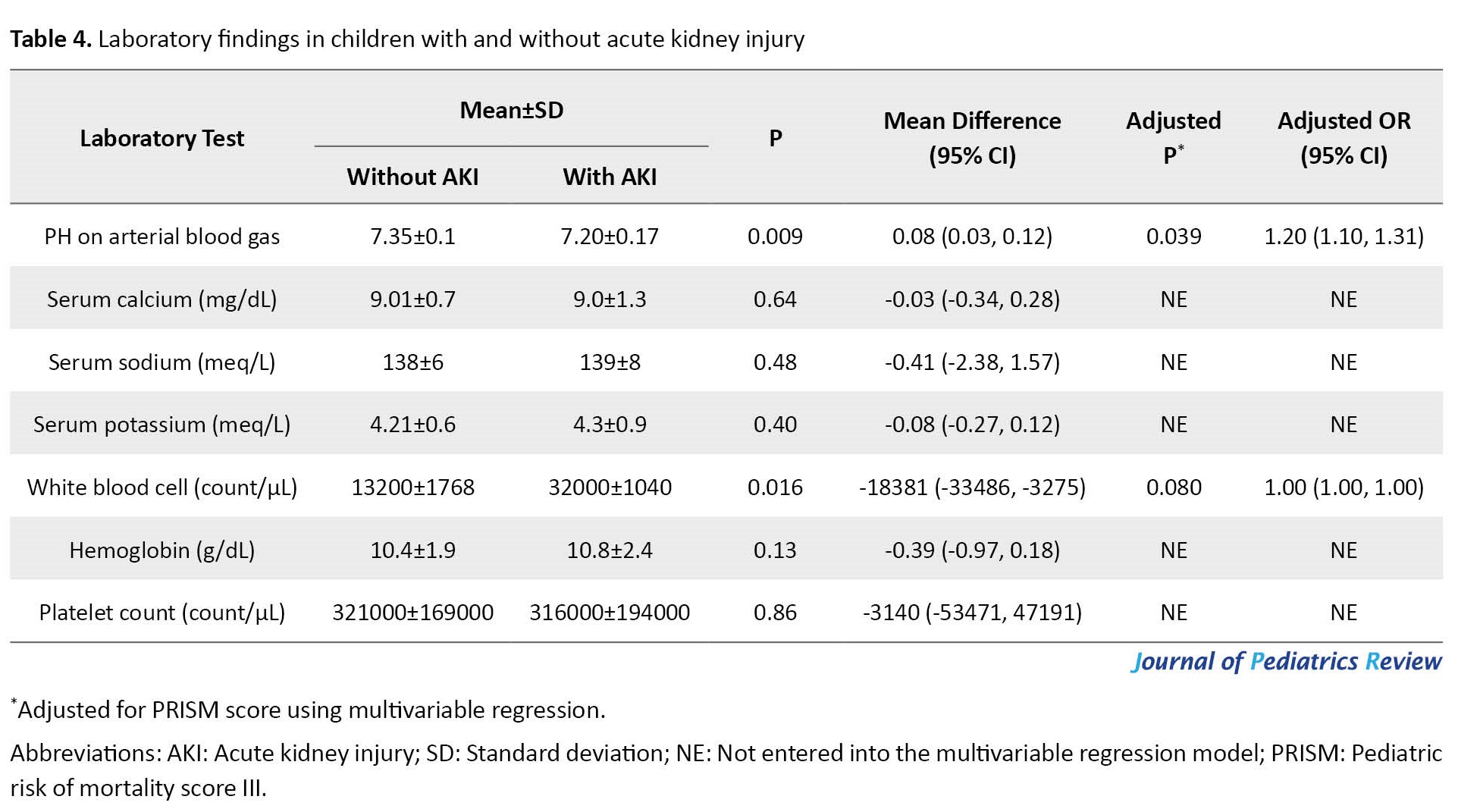
AKI occurred in 63 patients (24.7%). The mean serum creatinine level in patients with and without AKI was 1.13±0.96 and 0.5±0.12 mg/dL, respectively. (Table 2, Table 3 and Table 4) show the analysis of demographic variables, laboratory, and clinical data in patients with and without AKI.
Etiologically, 43 patients (68.3%) had prerenal AKI, 11(17.5%) had renal, 2(3.2%) had postrenal, and 7(11.1%) had unspecified AKI. The numbers of patients within the risk, injury, failure, and loss stages of the pRIFLE definition were 40(63.5%), 14(22.2%), 6(9.5%), and 3(4.8%), respectively. Twenty-six children (10.2%) died, of whom 11(42.3%) had AKI.
As shown in Table 2, in logistic regression adjusted for PRISM score, the occurrence of AKI was statistically higher in younger patients or with multiple organ dysfunction.
Using logistic regression adjustment for the PRISM score, we examined the abnormal laboratory variables with a significant relationship in the univariate analysis with the occurrence of AKI. Leukocytosis, hypernatremia, and acidosis showed an independent relationship with AKI (Table 3). However, as shown in Table 4, only lower arterial PH had an independent relation with AKI in multivariable regression (P=0.01).
The average length of hospital stay (LOS) in patients with and without AKI was 8 (IQR 4-17) and 5 (IQR 4-9) days, respectively. The univariate analysis showed a relationship between LOS and AKI (P=0.000). This relationship remained significant after controlling for the PRISM score (P=0.041; OR, 1.01; 95% CI, 1.20%-1.46%).
As seen in Table 5, the presence of AKI was not associated with mortality after adjusting the results for the PRISM score.
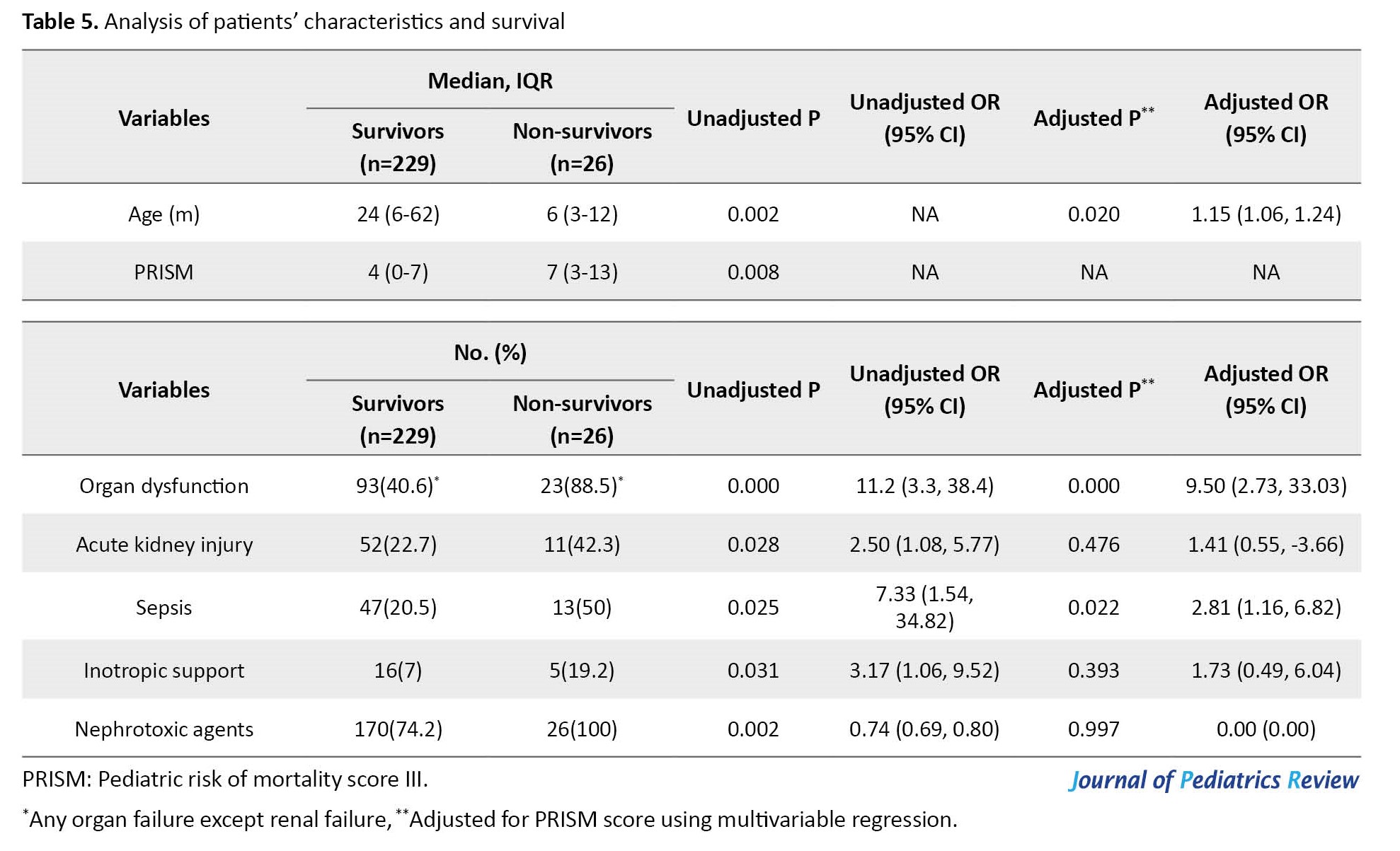
In variables that showed a significant relationship with mortality in univariate tests, the relationship between lower age, presence of organ dysfunction, and sepsis with death remained statistically significant in multivariable analysis adjusted for PRISM score.
In non-survivors, the patients with the risk, injury, failure, and loss stages were 6(54.5%), 4(36.4%), 1(9.1%), and 0(0%), respectively. There was no significant relationship between different stages of AKI and death before and after adjustment for PRISM score.
Discussion
We assessed the prevalence and severity of AKI in a tertiary academic PICU, as well as the correlation of risk factors with AKI. Based on the pRIFLE definition, the prevalence of AKI in PICU patients was 24.7%.
During the study period, more than three-quarters of our patients received nephrotoxic agents, although there was no relationship between the use of nephrotoxic agents and AKI. Although AKI was significantly correlated with increased children’s hospital stay, it was not associated with mortality after adjusting for other risk factors.
Consistent with the results of this study, the prevalence of AKI in PICU was reported to be 22% to 40% in different studies [22-27]. Although the criteria for the definition of AKI differ in various studies, the reported frequency of AKI is not so different. In some specified cases (such as post-cardiac arrest, post-cardiac surgery, and in children with acute respiratory distress syndrome), the rate of AKI is higher—up to 80% [28-30].
Similar to our findings, Srinivasa et al. studied 680 pediatric patients admitted to PICU. Using pRIFLE criteria, they found that AKI occurred in 26.1% of patients. The severity of AKI constituted a risk for 60.7%, injury for 28.6%, and failure for 10.6%. Etiologically, 68% had prerenal and 32% had intrinsic renal damage [26].
In a prospective national cohort study, Holmes et al. collected all data on pediatric AKI cases identified by an electronic AKI reporting system based on the KDIGO (kidney disease: Improving global outcomes) definition of AKI. They found that most cases presented as AKI stage 1(76.1%), with 15% classified as AKI stage 2, and only 8.9% classified as AKI stage 3 [1]. Kari et al., in a prospective cohort study at three tertiary care hospitals, used the KDIGO definition to diagnose AKI. They found that AKI affected 37.4% of their patients, with 17.8% classified as stage 1, 12.3% as stage 2, and 7.3% classified as stage 3 [31].
Our study shows that most children develop a mild form of the disease. Regardless of the classifications used to diagnose AKI in PICU, most children appear to have a mild form of AKI. A retrospective analysis of risk factors for AKI in critically ill children reported that age >2 months, serum creatinine at admission >0.5 mg/dL, presence of at least one comorbidity, use of at least 2 inotropes, use of diuretics, exposure to nephrotoxic drugs, multi-organ failure, and coagulopathy were independent risk factors for the development of AKI [32].
In Kaushik et al. study on AKI in children with acute respiratory distress syndrome, they reported the need for inotropes, diuretics, higher positive end-expiratory pressure, and lower PaO2/FiO2 ratio were associated with the occurrence of AKI. Still, no factors reached statistical significance in the multivariable model [29].
It seems that when patients need more medical attention and intensive care support, the risk for AKI is higher [4]. In our study, the lower age, higher PRISM III scores, and multiple organ dysfunction were significantly associated with AKI. Moreover, leukocytosis, hypernatremia, and acidosis in laboratory tests showed an independent relationship with AKI.
In patients with AKI, the LOS is longer than in patients without AKI. In our study, consistent with De Zan et al.’s and Kaushik et al.’s studies, the LOS of these patients was significantly longer than other patients [29, 32].
Xu et al. reported longer hospital stay in patients with AKI; they found that community-acquired AKI and hospital-acquired AKI were associated with 10.9% and 16.1% longer LOS when adjusted for age, sex, and comorbidities, compared with those without AKI [33]. The different mortality rates of pediatric AKI were reported in previous studies; the mortality was 2 to 10 times more than non-AKI children [1, 2, 25, 32].
In our study, although it was not statistically significant when controlled for other risk factors, the mortality of AKI patients was 42.3%, versus 22.7% in non-AKI. Kari et al. reported that mortality was 6 times more likely among patients with AKI compared to patients with normal renal function (OR: 6.5). In De Zan’s study, the mortality was tenfold higher in AKI [31, 32]. Although in some mentioned studies, the mortality was not controlled for other risk factors, it seems that the other reasons for the relatively low mortality of AKI patients in our study compared with other studies were early detection and treatment of AKI, the small number of patients with high mortality score, and also the absence of some critical patients such as children undergoing heart surgery, immunodeficiency, and organ transplant cases.
Other risk factors for death in our study were younger age, higher PRISM score, presence of organ dysfunction, and sepsis. In the present study, the severity of AKI was not associated with increased mortality.
Several studies have shown that a higher PRISM III score is associated with a higher mortality rate and higher risk for AKI [34-36]. Similarly, our study’s higher PRISM III score was associated with AKI and mortality.
A considerable number of our patients had received at least one nephrotoxic agent. In this study, prescribing a nephrotoxic agent was not correlated with AKI. Nephrotoxins (such as antimicrobial agents) are frequently used in ICUs. Soler et al. reported that only aminoglycosides significantly increased the incidence of AKI [25].
Although in Srinivasa et al.’s study, the administration of nephrotoxins was mistakenly reported as a risk factor for AKI, according to the statistical analysis published in their research, it is clear that the administration of nephrotoxins was not associated with an increase in the rate of kidney failure. In Srinivasa et al.’s research, nephrotoxins were used in 42.1% of children with AKI versus 58% of patients without AKI; this difference was significant [26].
Bresolin et al., in a prospective study on 126 PICU patients, reported that the use of nephrotoxic agents was significantly higher in children with AKI than in those without AKI (39.7% vs 14.7%; P<0.001) [37].
Overall, 76.9% of our patients received at least one nephrotoxic agent. Nephrotoxic agents did not influence the occurrence of AKI. Frequent administration of nephrotoxic drugs in both AKI and non-AKI children may be one of the reasons for hiding their nephrotoxic effect.
The current study had several limitations. Although our study period was two years, more accurate results would have been obtained if a study had been conducted with a larger sample size. In this study, surgery and trauma patients were a small group. The presence of such patients in a study may lead to different results depending on their specific problems. Many children in our study needed more than one nephrotoxic drug. This fact prevented proper evaluation of the effect of nephrotoxic medications on the frequency of AKI in statistical tests.
Conclusion
This research results support that AKI is a significant concern in critically ill children, which is associated with prolonged LOS. Special attention is required for renal function in PICU, especially in children with lower age, higher PRISM III scores, multiple organ dysfunction, leukocytosis, hypernatremia, and acidosis.
Ethical Considerations
Compliance with ethical guidelines
This cross-sectional study was approved by the Ethical Research Committee of Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.95.2841). All parents provided signed written informed consent before enrollment.
Funding
This research results from a residency dissertation of Faeghe Baryar Langroudi, approved by the Research Deputy of Mazandaran University of Medical Sciences (No.: 2841).
Authors contributions
Conceptualization: Mohammad Reza Navaeifar; Study design, critical revision of the manuscript: Mohammad Reza Navaeifar and Hamid Mohammadjafari; Data collection, drafting the manuscript: Faeghe Baryar Langroudi, Hani Rostami Rad and Maedeh Guran; Critically revising the manuscript, and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Elham Motevalian, the head nurse of our PICU, and her nursing team.
References
Acute kidney injury (AKI) is associated with a sudden decrease in kidney function, leading to hemodynamic and electrolyte disturbances [1]. AKI can develop in 2%-5% of patients admitted to a tertiary pediatric hospital [2, 3].
AKI has become one of the common complications seen in pediatric intensive care units (PICUs) [4 5, 6]. The prevalence of AKI reported up to 27% in critically ill children [7, 8]. Recently, AKI prevalence was reported as 22% to 25% in children admitted to the hospital for severe acute respiratory distress syndrome coronavirus 2 (SARS-CoV-2) [9, 10]. In addition, AKI could be a disturbing complication in children with underlying diseases [8, 11].
Clinical presentations showed diminished urine output and increased blood urea or creatinine levels. One of the most common definitions of AKI is recommended by KDIGO (kidney disease improving global guidelines) [12]. However, we employed the pRIFLE (pediatric risk, injury, failure, loss, end-stage renal disease) classification to define AKI in children (Table 1) [13, 14].

Based on the pRIFLE classification, AKI is divided into 3 stages in the acute phase, i.e. risk, injury, failure, and another 2 stages in a more chronic course, i.e. loss of function and end-stage renal disease.
The etiology of AKI is divided into prerenal, renal, and postrenal causes. Prerenal AKI (the most common form) results from decreased kidney blood perfusion. Prerenal AKI commonly results from hypovolemic states, heart failure, and conditions with increased third space. Renal AKI is a condition with renal damage from toxic, inflammatory, or immunologic insults, while postrenal AKI causes consist of obstructing lesions, such as congenital anomalies and acquired states [15-17].
Due to the scarcity of data in our region, we assessed the prevalence of AKI, clinical and laboratory characteristics, etiologies, and course in children admitted to a tertiary training PICU with average annual admission of 350 children, focusing on nephrotoxins and comorbidities.
Methods
Study population
This cross-sectional study was performed in a 12-bed mixed medical and surgical PICU in northern Iran between September 2017 and April 2019.
We studied all 1 month to 18 years old patients admitted to PICU and remained there for at least 48 hours. The exclusion criteria included a lack of complete information or diagnosis of AKI before the first 48 hours of hospitalization.
Study measurements
The demographic data, medical history, and pediatric risk of mortality (PRISM) III were obtained in the first 12 hours of admission. The clinical and laboratory determinants were assessed and recorded meticulously. Age, sex, family history of kidney diseases, and hematologic and biochemistry tests were also recorded.
The PRISM III score is a widely used scoring system to quantify critical illness in pediatric patients. The components comprised cardiovascular (heart rate, systolic blood pressure, and temperature), neurologic (pupillary reactivity and mental status), respiratory (arterial PO2, pH, PCO2, and total bicarbonate), chemical (glucose, potassium, blood urea nitrogen, and creatinine), and hematologic (WBC count, platelet count, prothrombin, and partial thromboplastin time) profiles.
AKI was defined based on the pRIFLE definition. The urine was collected and measured for 8 hours. The measurement was performed in 3 forms. For cooperative ambulatory patients, urine was collected in measured bottles and recorded as mL/kg/min. For immobile patients with a urinary catheter, the urine was stored in urine bags and recorded at least every 8 hours. Moreover, for immobile or non-cooperative children without a urinary catheter, the diapers were scaled before and after urination with an electrical scale with a 1-g error. The Jaffe method was used to measure serum creatinine.
In the AKI group, the variables likely to contribute to renal failure were selected for statistical analysis up to 48 hours before the onset of renal failure. This method prevented the possible effect of kidney failure on these parameters as much as possible. The AKI group followed for 4 weeks to define the loss phase of pRIFLE.
When a patient received at least 2 nephrotoxic agents, it was recorded as exposure to multiple nephrotoxic agents. Anemia was defined based on lower hemoglobin limits in Nathan and Oski’s hematology of infancy and childhood [18]. Sepsis and organ dysfunction (except for renal dysfunction) were defined based on the international pediatric sepsis consensus conference criteria [19].
The estimated glomerular filtration rate (GFR) was calculated based on the Schwartz formula [20] for patients under two years old and based on the updated Schwartz formula for older cases [21].
The etiology of AKI was assessed based on the clinical and laboratory findings. The relation between clinical or laboratory disorders and the occurrence or severity of AKI was assessed, too.
Statistical analysis
The categorical variables were presented as numbers and percentages, and the non-categorical ones were Mean±SD. Univariate analysis was undertaken to evaluate the relationship between clinical and demographic variables and AKI and death. Statistical analysis of the difference between the groups was determined using the t-test or Fisher exact test to compare the two groups.
Analysis of possible variables contributing to AKI (Table 2, Table 3 and Table 4) was made using data up to 48 hours before the onset of renal failure.



Nonparametric tests (such as the Mann-Whitney and Kruskal-Wallis tests) were used to analyze the variables without normal distributions.
Multivariate logistic regression was undertaken to evaluate the independent association with risk factors of AKI, which were significant in the univariate analysis. When more than one organ failure occurred in a patient, the first organ failure entered the multivariate analysis. A P<0.05 was considered statistically significant. All statistical analyses were performed in SPSS software, version 16 (SPSS Inc., Chicago, USA).
Results
A total of 263 patients were admitted and remained in the PICU for more than 48 hours. Eight cases were excluded because of incomplete data. Of the 255 enrolled children, 123(48.2%) were female. Their median age was 18 (interquartile range [IQR], 6-60) months. Their median PRISM III score was 4 (IQR, 0-8), whereas 43 patients (16.9%) had a PRISM III score of more than 10. The causes of admission were infectious diseases in 128 cases (50.2%), neurologic disorders in 66(25.9%), trauma surgery in 22(8.6%), respiratory diseases in 19(7.5%), endocrine disorders in 13(5.1%), and gastrointestinal diseases in 7(2.7%).
AKI occurred in 63 patients (24.7%). The mean serum creatinine level in patients with and without AKI was 1.13±0.96 and 0.5±0.12 mg/dL, respectively. (Table 2, Table 3 and Table 4) show the analysis of demographic variables, laboratory, and clinical data in patients with and without AKI.
Etiologically, 43 patients (68.3%) had prerenal AKI, 11(17.5%) had renal, 2(3.2%) had postrenal, and 7(11.1%) had unspecified AKI. The numbers of patients within the risk, injury, failure, and loss stages of the pRIFLE definition were 40(63.5%), 14(22.2%), 6(9.5%), and 3(4.8%), respectively. Twenty-six children (10.2%) died, of whom 11(42.3%) had AKI.
As shown in Table 2, in logistic regression adjusted for PRISM score, the occurrence of AKI was statistically higher in younger patients or with multiple organ dysfunction.
Using logistic regression adjustment for the PRISM score, we examined the abnormal laboratory variables with a significant relationship in the univariate analysis with the occurrence of AKI. Leukocytosis, hypernatremia, and acidosis showed an independent relationship with AKI (Table 3). However, as shown in Table 4, only lower arterial PH had an independent relation with AKI in multivariable regression (P=0.01).
The average length of hospital stay (LOS) in patients with and without AKI was 8 (IQR 4-17) and 5 (IQR 4-9) days, respectively. The univariate analysis showed a relationship between LOS and AKI (P=0.000). This relationship remained significant after controlling for the PRISM score (P=0.041; OR, 1.01; 95% CI, 1.20%-1.46%).
As seen in Table 5, the presence of AKI was not associated with mortality after adjusting the results for the PRISM score.

In variables that showed a significant relationship with mortality in univariate tests, the relationship between lower age, presence of organ dysfunction, and sepsis with death remained statistically significant in multivariable analysis adjusted for PRISM score.
In non-survivors, the patients with the risk, injury, failure, and loss stages were 6(54.5%), 4(36.4%), 1(9.1%), and 0(0%), respectively. There was no significant relationship between different stages of AKI and death before and after adjustment for PRISM score.
Discussion
We assessed the prevalence and severity of AKI in a tertiary academic PICU, as well as the correlation of risk factors with AKI. Based on the pRIFLE definition, the prevalence of AKI in PICU patients was 24.7%.
During the study period, more than three-quarters of our patients received nephrotoxic agents, although there was no relationship between the use of nephrotoxic agents and AKI. Although AKI was significantly correlated with increased children’s hospital stay, it was not associated with mortality after adjusting for other risk factors.
Consistent with the results of this study, the prevalence of AKI in PICU was reported to be 22% to 40% in different studies [22-27]. Although the criteria for the definition of AKI differ in various studies, the reported frequency of AKI is not so different. In some specified cases (such as post-cardiac arrest, post-cardiac surgery, and in children with acute respiratory distress syndrome), the rate of AKI is higher—up to 80% [28-30].
Similar to our findings, Srinivasa et al. studied 680 pediatric patients admitted to PICU. Using pRIFLE criteria, they found that AKI occurred in 26.1% of patients. The severity of AKI constituted a risk for 60.7%, injury for 28.6%, and failure for 10.6%. Etiologically, 68% had prerenal and 32% had intrinsic renal damage [26].
In a prospective national cohort study, Holmes et al. collected all data on pediatric AKI cases identified by an electronic AKI reporting system based on the KDIGO (kidney disease: Improving global outcomes) definition of AKI. They found that most cases presented as AKI stage 1(76.1%), with 15% classified as AKI stage 2, and only 8.9% classified as AKI stage 3 [1]. Kari et al., in a prospective cohort study at three tertiary care hospitals, used the KDIGO definition to diagnose AKI. They found that AKI affected 37.4% of their patients, with 17.8% classified as stage 1, 12.3% as stage 2, and 7.3% classified as stage 3 [31].
Our study shows that most children develop a mild form of the disease. Regardless of the classifications used to diagnose AKI in PICU, most children appear to have a mild form of AKI. A retrospective analysis of risk factors for AKI in critically ill children reported that age >2 months, serum creatinine at admission >0.5 mg/dL, presence of at least one comorbidity, use of at least 2 inotropes, use of diuretics, exposure to nephrotoxic drugs, multi-organ failure, and coagulopathy were independent risk factors for the development of AKI [32].
In Kaushik et al. study on AKI in children with acute respiratory distress syndrome, they reported the need for inotropes, diuretics, higher positive end-expiratory pressure, and lower PaO2/FiO2 ratio were associated with the occurrence of AKI. Still, no factors reached statistical significance in the multivariable model [29].
It seems that when patients need more medical attention and intensive care support, the risk for AKI is higher [4]. In our study, the lower age, higher PRISM III scores, and multiple organ dysfunction were significantly associated with AKI. Moreover, leukocytosis, hypernatremia, and acidosis in laboratory tests showed an independent relationship with AKI.
In patients with AKI, the LOS is longer than in patients without AKI. In our study, consistent with De Zan et al.’s and Kaushik et al.’s studies, the LOS of these patients was significantly longer than other patients [29, 32].
Xu et al. reported longer hospital stay in patients with AKI; they found that community-acquired AKI and hospital-acquired AKI were associated with 10.9% and 16.1% longer LOS when adjusted for age, sex, and comorbidities, compared with those without AKI [33]. The different mortality rates of pediatric AKI were reported in previous studies; the mortality was 2 to 10 times more than non-AKI children [1, 2, 25, 32].
In our study, although it was not statistically significant when controlled for other risk factors, the mortality of AKI patients was 42.3%, versus 22.7% in non-AKI. Kari et al. reported that mortality was 6 times more likely among patients with AKI compared to patients with normal renal function (OR: 6.5). In De Zan’s study, the mortality was tenfold higher in AKI [31, 32]. Although in some mentioned studies, the mortality was not controlled for other risk factors, it seems that the other reasons for the relatively low mortality of AKI patients in our study compared with other studies were early detection and treatment of AKI, the small number of patients with high mortality score, and also the absence of some critical patients such as children undergoing heart surgery, immunodeficiency, and organ transplant cases.
Other risk factors for death in our study were younger age, higher PRISM score, presence of organ dysfunction, and sepsis. In the present study, the severity of AKI was not associated with increased mortality.
Several studies have shown that a higher PRISM III score is associated with a higher mortality rate and higher risk for AKI [34-36]. Similarly, our study’s higher PRISM III score was associated with AKI and mortality.
A considerable number of our patients had received at least one nephrotoxic agent. In this study, prescribing a nephrotoxic agent was not correlated with AKI. Nephrotoxins (such as antimicrobial agents) are frequently used in ICUs. Soler et al. reported that only aminoglycosides significantly increased the incidence of AKI [25].
Although in Srinivasa et al.’s study, the administration of nephrotoxins was mistakenly reported as a risk factor for AKI, according to the statistical analysis published in their research, it is clear that the administration of nephrotoxins was not associated with an increase in the rate of kidney failure. In Srinivasa et al.’s research, nephrotoxins were used in 42.1% of children with AKI versus 58% of patients without AKI; this difference was significant [26].
Bresolin et al., in a prospective study on 126 PICU patients, reported that the use of nephrotoxic agents was significantly higher in children with AKI than in those without AKI (39.7% vs 14.7%; P<0.001) [37].
Overall, 76.9% of our patients received at least one nephrotoxic agent. Nephrotoxic agents did not influence the occurrence of AKI. Frequent administration of nephrotoxic drugs in both AKI and non-AKI children may be one of the reasons for hiding their nephrotoxic effect.
The current study had several limitations. Although our study period was two years, more accurate results would have been obtained if a study had been conducted with a larger sample size. In this study, surgery and trauma patients were a small group. The presence of such patients in a study may lead to different results depending on their specific problems. Many children in our study needed more than one nephrotoxic drug. This fact prevented proper evaluation of the effect of nephrotoxic medications on the frequency of AKI in statistical tests.
Conclusion
This research results support that AKI is a significant concern in critically ill children, which is associated with prolonged LOS. Special attention is required for renal function in PICU, especially in children with lower age, higher PRISM III scores, multiple organ dysfunction, leukocytosis, hypernatremia, and acidosis.
Ethical Considerations
Compliance with ethical guidelines
This cross-sectional study was approved by the Ethical Research Committee of Mazandaran University of Medical Sciences (Code: IR.MAZUMS.REC.95.2841). All parents provided signed written informed consent before enrollment.
Funding
This research results from a residency dissertation of Faeghe Baryar Langroudi, approved by the Research Deputy of Mazandaran University of Medical Sciences (No.: 2841).
Authors contributions
Conceptualization: Mohammad Reza Navaeifar; Study design, critical revision of the manuscript: Mohammad Reza Navaeifar and Hamid Mohammadjafari; Data collection, drafting the manuscript: Faeghe Baryar Langroudi, Hani Rostami Rad and Maedeh Guran; Critically revising the manuscript, and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors would like to thank Elham Motevalian, the head nurse of our PICU, and her nursing team.
References
- Holmes J, Roberts G, May K, Tyerman K, Geen J, Williams JD, et al. The incidence of pediatric acute kidney injury is increased when identified by a change in a creatinine-based electronic alert. Kidney Int. 2017; 92(2):432-9. [DOI:10.1016/j.kint.2017.03.009] [PMID]
- Esezobor CI, Ladapo TA, Osinaike B, Lesi FE. Paediatric acute kidney injury in a tertiary hospital in Nigeria: Prevalence, causes and mortality rate. PloS One. 2012; 7(12):e51229. [DOI:10.1371/journal.pone.0051229] [PMID]
- McGregor TL, Jones DP, Wang L, Danciu I, Bridges BC, Fleming GM, et al. Acute kidney injury incidence in noncritically ill hospitalized children, adolescents, and young adults: A retrospective observational study.Am J Kidney Dis. 2016; 67(3):384-90. [DOI:10.1053/j.ajkd.2015.07.019] [PMID]
- Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021; 7(1):52. [DOI:10.1038/s41572-021-00284-z] [PMID]
- Farrar A. Acute Kidney Injury. Nurs Clin North Am. 2018; 53(4):499-510. [DOI:10.1016/j.cnur.2018.07.001] [PMID]
- Sutherland SM, Kwiatkowski DM. Acute kidney injury in children. Adv Chronic Kidney Dis. 2017; 24(6):380-7. [DOI:10.1053/j.ackd.2017.09.007] [PMID]
- Bernardo EO, Cruz AT, Buffone GJ, Devaraj S, Loftis LL, Arikan AA. Community-acquired acute kidney injury among children seen in the Pediatric Emergency Department. Acad Emerg Med. 2018; 25(7):758-68. [DOI:10.1111/acem.13421] [PMID]
- Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators. Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med. 2017; 376(1):11-20. [DOI:10.1056/NEJMoa1611391] [PMID]
- Chopra S, Saha A, Kumar V, Thakur A, Pemde H, Kapoor D, et al. Acute kidney injury in hospitalized children with covid19. J Trop Pediatr. 2021; 67(2):fmab037. [DOI:10.1093/tropej/fmab037] [PMID]
- Kari JA, Shalaby MA, Albanna AS, Alahmadi TS, Alherbish A, Alhasan KA. Acute kidney injury in children with COVID-19: A retrospective study. BMC Nephrol. 2021; 22(1):202. [DOI:10.1186/s12882-021-02389-9] [PMID]
- Lebel A, Teoh CW, Zappitelli M. Long-term complications of acute kidney injury in children. Curr Opin Pediatr. 2020; 32(3):367-75. [DOI:10.1097/MOP.0000000000000906] [PMID]
- Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clinical Practice. 2012; 120(4):c179–84. [DOI:10.1159/000339789]
- Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007; 71(10):1028-35. [DOI:10.1038/sj.ki.5002231] [PMID]
- Ciccia E, Devarajan P. Pediatric acute kidney injury: Prevalence, impact and management challenges. Int J Nephrol Renovasc Dis. 2017; 10:77-84. [DOI:10.2147/IJNRD.S103785] [PMID]
- Higaki M, Tanemoto M, Shiraishi T, Taniguchi K, Fujigaki Y, Uchida S. Acute kidney injury facilitates hypocalcemia by exacerbating the hyperphosphatemic effect of muscle damage in rhabdomyolysis. Nephron. 2015; 131(1):11-6. [DOI:10.1159/000437391] [PMID]
- Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its use in practice. Kidney Int. 2015; 87(1):62-73. [DOI:10.1038/ki.2014.328] [PMID]
- Goldstein SL, Kirkendall E, Nguyen H, Schaffzin JK, Bucuvalas J, Bracke T, et al. Electronic health record identification of nephrotoxin exposure and associated acute kidney injury. Pediatrics. 2013; 132(3):e756-67. [DOI:10.1542/peds.2013-0794] [PMID]
- Orkin SH, Nathan DG. Nathan and Oski’s hematology of infancy and childhood. Philadelphia: Saunders/Elsevier; 2009. [Link]
- Weiss SL, Peters MJ, Alhazzani W, Agus MSD, Flori HR, Inwald DP, et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020; 46(Suppl 1):10-67. [DOI:10.1007/s00134-019-05878-6] [PMID]
- Schwartz GJ, Brion LP, Spitzer A. The use of plasma creatinine concentration for estimating glomerular filtration rate in infants, children, and adolescents. Pediatr Clin North Am. 1987; 34(3):571-90. [DOI:10.1016/S0031-3955(16)36251-4] [PMID]
- Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009; 20(3):629-37. [DOI:10.1681/ASN.2008030287] [PMID]
- Krishnamurthy S, Mondal N, Narayanan P, Biswal N, Srinivasan S, Soundravally R. Incidence and etiology of acute kidney injury in southern India. Indian J Pediatr. 2013; 80(3):183-9. [DOI:10.1007/s12098-012-0791-z] [PMID]
- Macedo E, Cerdá J, Hingorani S, Hou J, Bagga A, Burdmann EA, et al. Recognition and management of acute kidney injury in children: The ISN 0by25 Global Snapshot study. PloS One. 2018; 13(5):e0196586. [DOI:10.1371/journal.pone.0196586] [PMID]
- Mohkam M, Tabatabaii S, Bashardoost B, Alaii S. [Prevalence and risk factors of acute renal failure in PICU based on RIFLE scoring system (Persian]. Razi J Med Sci. 2010; 17(4):62-8. [Link]
- Soler YA, Nieves-Plaza M, Prieto M, García-De Jesús R, Suárez-Rivera M. Pediatric risk, injury, failure, loss, end-stage renal disease score identifies acute kidney injury and predicts mortality in critically ill children: A prospective study. Pediatr Crit Care Med. 2013; 14(4):e189-95. [DOI:10.1097/PCC.0b013e3182745675] [PMID]
- Srinivasa S, Reshmavathi V. Incidence and etiology of acute kidney injury in children admitted to PICU using pRIFLE criteria. Curr Pediatr Res. 2016; 20(1). [Link]
- Ostermann M, Bellomo R, Burdmann EA, Doi K, Endre ZH, Goldstein SL, et al. Controversies in acute kidney injury: Conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Conference. Kidney Int. 2020; 98(2):294-309. [DOI:10.1016/j.kint.2020.04.020] [PMID]
- Uber AM, Sutherland SM. Acute kidney injury in hospitalized children: Consequences and outcomes. Pediatr Nephrol. 2020; 35(2):213-20. [DOI:10.1007/s00467-018-4128-7] [PMID]
- Kaushik S, Villacres S, Eisenberg R, Medar SS. Acute Kidney Injury in pediatric acute respiratory distress syndrome. J Intensive Care Med. 2021; 36(9):1084-90. [DOI:10.1177/0885066620944042] [PMID]
- Zappitelli M, Parikh CR, Kaufman JS, Go AS, Kimmel PL, Hsu CY, et al. Acute Kidney Injury and Risk of CKD and Hypertension after Pediatric Cardiac Surgery. Clin J Am Soc Nephrol. 2020; 15(10):1403-12. [DOI:10.2215/CJN.00150120] [PMID]
- Kari JA, Alhasan KA, Shalaby MA, Khathlan N, Safdar OY, Al Rezgan SA, et al. Outcome of pediatric acute kidney injury: A multicenter prospective cohort study. Pediatr Nephrol. 2018; 33(2):335-40. [DOI:10.1007/s00467-017-3786-1] [PMID]
- De Zan F, Amigoni A, Pozzato R, Pettenazzo A, Murer L, Vidal E. Acute kidney injury in critically Ill children: A retrospective analysis of risk factors. Blood Purif. 2020; 49(1-2):1-7. [DOI:10.1159/000502081] [PMID]
- Xu X, Nie S, Zhang A, Mao J, Liu HP, Xia H, et al. Acute kidney injury among hospitalized children in China. Clin J Am Soc Nephrol. 2018; 13(12):1791-800. [DOI:10.2215/CJN.00800118] [PMID]
- Al-Otaibi NG, Zeinelabdin M, Shalaby MA, Khathlan N, Mashat GD, Zahrani AA, et al. Impact of acute kidney injury on long-term mortality and progression to chronic kidney disease among critically ill children. Saudi Med J. 2017; 38(2):138-42. [DOI:10.15537/smj.2017.2.16012] [PMID]
- Nickavar A, Safaeian B, Valavi E. Evaluation and comparison of urinary cytokines for the diagnosis of acute pyelonephritis. Arch Pediatr Infect Dis. 2016; 4(4):e38877. [DOI:10.5812/pedinfect.38877]
- Rustagi RS, Arora K, Das RR, Pooni PA, Singh D. Incidence, risk factors and outcome of acute kidney injury in critically ill children - a developing country perspective. Paediatr Int Child Health. 2017; 37(1):35-41. [DOI:10.1080/20469047.2015.1120409] [PMID]
- Bresolin N, Bianchini AP, Haas CA. Pediatric acute kidney injury assessed by pRIFLE as a prognostic factor in the intensive care unit. Pediatr Nephrol. 2013; 28(3):485-92. [DOI:10.1007/s00467-012-2357-8] [PMID]
Type of Study: Original Article |
Subject:
Pediatrics
Received: 2023/03/25 | Accepted: 2023/08/22 | Published: 2023/10/4
Received: 2023/03/25 | Accepted: 2023/08/22 | Published: 2023/10/4
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |





