Volume 12, Issue 2 (4-2024)
J. Pediatr. Rev 2024, 12(2): 109-124 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Fakhri M, Azadbakht M, Farhadi R. Effects of Purgative Manna on the Serum Bilirubin Level of Newborns: A Systematic Review and Meta-analysis. J. Pediatr. Rev 2024; 12 (2) :109-124
URL: http://jpr.mazums.ac.ir/article-1-590-en.html
URL: http://jpr.mazums.ac.ir/article-1-590-en.html
1- Traditional and Complementary Medicine Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran. , mmfir@yahoo.com
2- Traditional and Complementary Medicine Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
2- Traditional and Complementary Medicine Research Center, Addiction Institute, Mazandaran University of Medical Sciences, Sari, Iran.
3- Pediatric Infectious Diseases Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
Keywords: Purgative manna, Billinaster, Shir-khesht, Cotoneaster, Rosaceae, Jaundice, Icterus, Hyperbilirubinemia, Bilirubinemia, Infant, Newborn, Neonatal
Full-Text [PDF 623 kb]
(1105 Downloads)
| Abstract (HTML) (2967 Views)
Full-Text: (1088 Views)
Introduction
The majority of newborns experience jaundice in the early stages of their lives [1]. The incidence of neonatal jaundice and the associated healthcare costs are increasing worldwide [2].
Phototherapy is the most common intervention for the prevention and treatment of severe hyperbilirubinemia [3]. However, many infants require blood transfusions if phototherapy fails to reduce serum bilirubin levels effectively [4]. On the other hand, retinal degeneration, diarrhea, dehydration, skin rashes [5], and oxidative stress-related diseases such as necrotizing enterocolitis and patent ductus arteriosus (PDA) are among the side effects of phototherapy [6]. In China, infants exposed to phototherapy for hyperbilirubinemia receive combined treatment with traditional Chinese herbal medicines, which have been shown to reduce serum bilirubin levels and improve jaundice [7].
Accordingly, the development of traditional and complementary medicine for neonatal jaundice is an important and potentially safe step in primary healthcare [8, 9]. Before phototherapy became commonplace, complementary medicine, such as Cotoneaster was used in Iran to treat neonatal jaundice [10]. Cotoneaster, or Purgative manna, is a white and slightly yellowish, sweet substance derived from the plant genus Cotoneaster spp. which belongs to the Rosaceae family. Its most important compounds include carbohydrates, such as mannitol, fructose, glucose, and sucrose, which induce osmotic diarrhea and may thus contribute to a reduction in bilirubin levels [11].
Numerous studies have examined the effects of P. manna on neonatal jaundice. A systematic review and meta-analysis demonstrated that Manna could reduce the duration of phototherapy by decreasing bilirubin levels in jaundiced neonates [12]. A randomized clinical trial compared the use of P. manna drops with phototherapy and demonstrated that P. manna resulted in a greater reduction in serum bilirubin concentration [13]. Another intervention study found that Cotoneaster administration had no significant impact on neonatal jaundice compared to a placebo [14]. Contradictory results from previous studies highlight the necessity for a systematic review and meta-analysis in this area. Accordingly, this study investigates the effect of Cotoneaster consumption on serum bilirubin levels in neonates worldwide, with no geographical location restrictions. However, all published studies were conducted in Iran. Since this plant is native to Iran and East Asian countries, it is not utilized in many other countries, and consequently, studies in this regard have not been conducted.
Materials and Methods
The present research was a systematic review and meta-analysis designed based on the preferred reporting items for systematic reviews and meta-analysis guidelines [15].
Search strategy
In this meta-analysis, an online search was conducted on Iranian databases, including Barakat Gostar, Scientific Information Database (SID), Magiran, IranDoc and international databases, such as PubMed, Scopus, Web of Science, Cochrane and the Google Scholar search engine, without time restrictions up to July 20, 2023. Language restriction was not performed during the search. The search was performed using standard keywords and MeSH terms, including “Purgative manna,” “billinaster,” “shir-khesht,” “Cotoneaster,” “rosaceae,” “jaundice,” “icterus,” “hyperbilirubinemia,” “bilirubinemia,” “infant,” and “newborn.” The keyword combinations were explored using logical operators (AND, OR) within the mentioned databases. For manual searching, a list of eligible primary studies was also examined. A sample of the search strategy in the PubMed database is provided below: ([Purgative manna OR billinaster OR shir-khesht OR Cotoneaster OR Rosaceae] AND [jaundice OR Icterus OR hyperbilirubinemia OR bilirubinemia]) AND (infant, newborn OR neonatal).
Population, intervention, comparison, and outcome components
The components of population, intervention, comparison and outcome for this study were as follows: The population included clinical trial studies, involving infants as participants; the intervention comprised an intervention group that could either solely use a Cotoneaster-derived product or receive Cotoneaster in conjunction with phototherapy; the comparison group could include several scenarios, namely no intervention (one-group, before-after, trial), placebo, or phototherapy; and the outcome of the studies under investigation should assess at least the serum bilirubin level.
Inclusion criteria
The inclusion criteria for this study were clinical trial studies that evaluated the effect of P. manna consumption on infant jaundice.
Exclusion criteria
The exclusion criteria comprised observational studies, review studies, studies with no access to the full text, protocol papers, conference studies, studies with low quality, studies lacking necessary data for data analysis, and duplicate studies
Quality assessment of primary studies
After identifying the included studies, two independent reviewers assessed the quality of clinical trials using the Cochrane collaboration’s checklist for assessing the risk of bias in randomized trials [16]. This checklist comprises seven questions, each addressing a critical aspect of bias in clinical trials. Each question has three response options, namely high risk of bias, low risk of bias, and unclear. After completing the risk of bias assessment for all studies, discrepancies in responses to the questions in each study were evaluated. Any disagreements were resolved through consensus or agreement between the two assessors, resulting in a unified response.
Data extraction
Two independent researchers conducted data extraction from the included studies. The extracted data were entered into a checklist that included the following information: First author’s name, publication year of the study, total number of infants, number of female and male infants, infant weight, infant age, type of maternal delivery (cesarean or vaginal), study location, Cotoneaster dosage, number of infants in the intervention and control groups, and Mean±SD of serum bilirubin levels before and after the intervention in the intervention and control groups. A third researcher reviewed the extracted data from the previous two researchers to resolve any discrepancies.
Statistical analysis
All included studies had both intervention and control groups and repeatedly measured serum bilirubin levels at baseline and at multiple time points (at regular intervals of 12 h). This allowed for the calculation of within-group mean differences and between-group mean difference indices. The standardized mean difference (SMD) was estimated using the sample size, Mean±SD of serum bilirubin levels before and after the intervention in the intervention and control groups. The SMD indicates the strength of the relationship, with values closer to zero representing a weaker relationship and values closer to one or higher indicating a stronger relationship [17]. To assess heterogeneity, the Cochrane Q test and I2 index were used. Subgroup analysis and meta-regression were used to investigate the sources of heterogeneity, and the Funnel plot was used to assess publication bias [18]. The I2 index has three classifications (<25% indicates low heterogeneity, between 25% and 75% shows moderate heterogeneity and >75% indicates high heterogeneity) [19]. In this study, a random-effects model was used for data analysis. Data analysis was performed using the STATA software, version 14, and the significance level was considered P<0.05.
Results
Study selection
In the initial search, a total of 206 articles were found. Upon reviewing the titles of the studies, 49 duplicate studies were removed. The abstracts of the remaining 157 articles were examined, and of these, 24 articles were excluded due to the unavailability of their full texts. Among the remaining 133 articles, 6 were excluded because they lacked the necessary data for data analysis. Out of the remaining 127 articles, an additional 115 articles were excluded based on other exclusion criteria, leaving 12 articles for qualitative evaluation, all of which were of acceptable quality (Figure 1).
Summary of reviewed studies
In this study, 12 clinical trial articles with a total of 1557 infants (781 infants in the control group and 776 infants in the intervention group) were examined. Out of these, 2 studies investigated the effect of Cotoneaster on preventing neonatal jaundice [20, 21], and 10 studies investigated the effect of Cotoneaster on the treatment of neonatal jaundice [4, 10, 13, 14, 22–27]. There were no statistically significant differences between the intervention and control groups in terms of the number of participants, age, weight, gender and serum bilirubin levels of the infants. Other information from the reviewed articles is presented in Table 1.




Analysis of the primary outcome
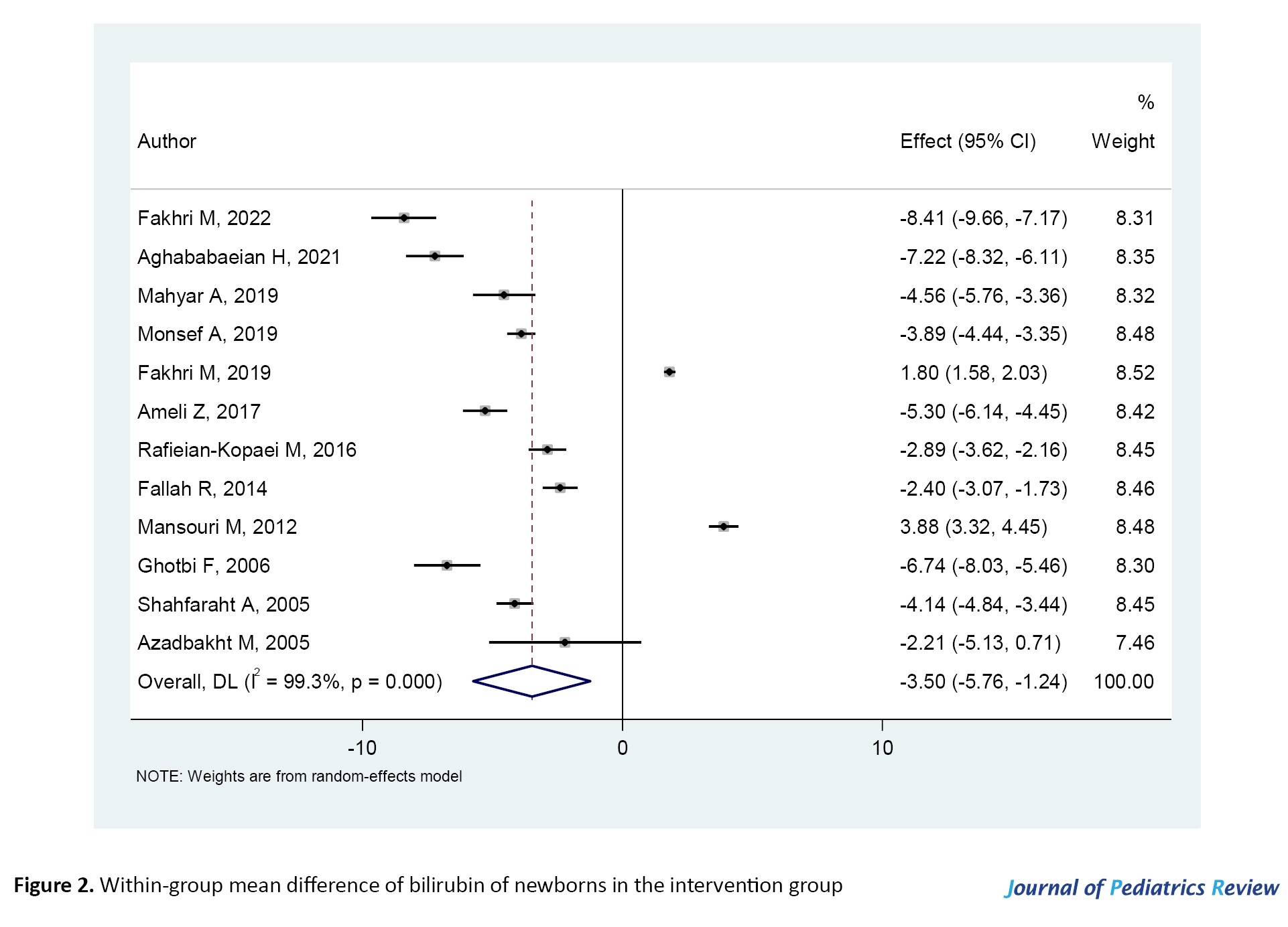
According to Figure 2, in the intervention group, after the administration of Cotoneaster, the serum bilirubin levels of infants significantly decreased (SMD=-3.50; 95% CI, -5.76%, -1.24%; P=0.000). Figure 3 shows that in the control group, after phototherapy, the serum bilirubin levels of infants significantly decreased (SMD=-2.14; 95% CI, -4.01%, -0.27%; P=0.000). Meanwhile, phototherapy is currently the most common method for the treatment of jaundice and the reduction of bilirubin levels in infants.
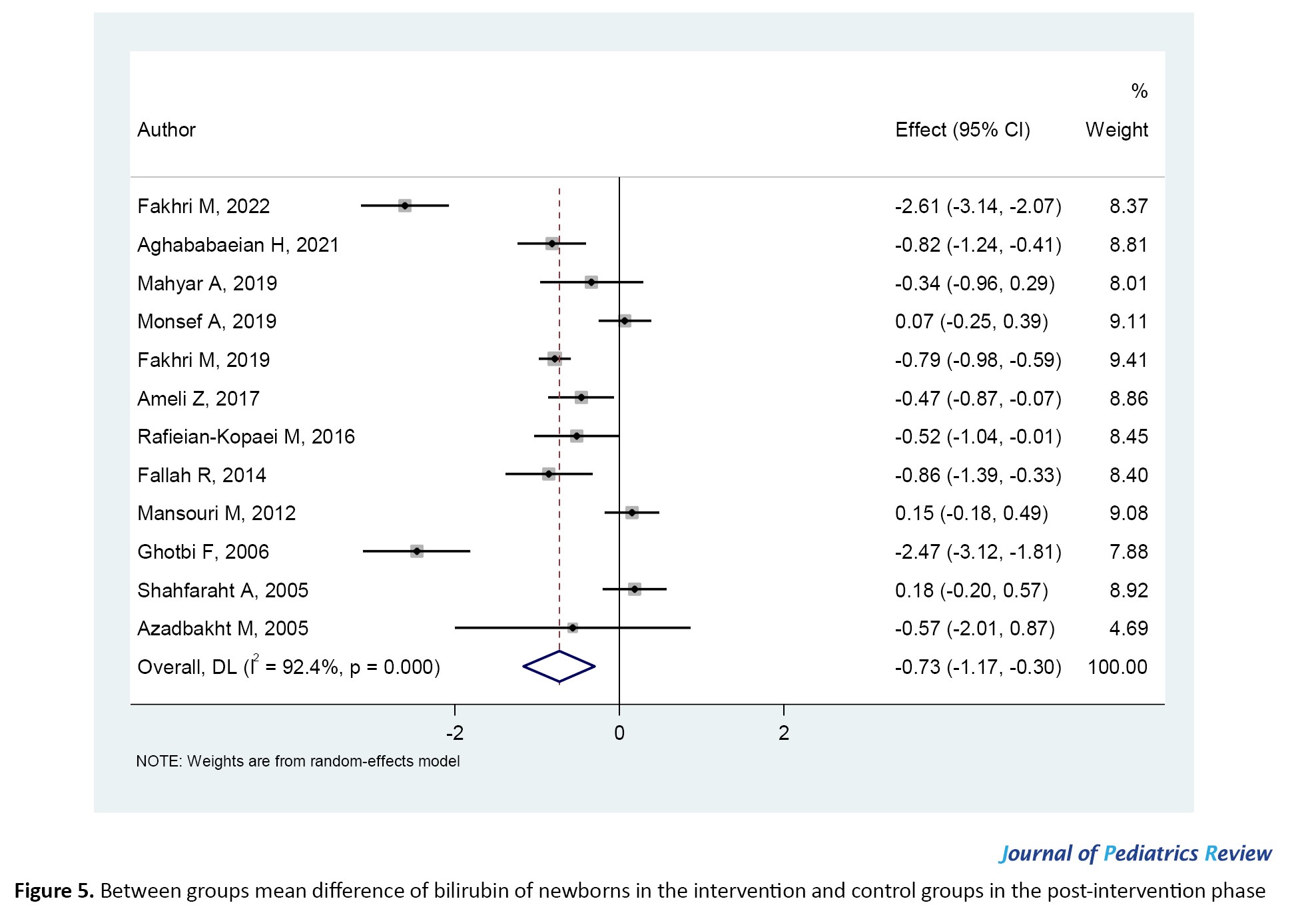
Before the intervention and in the baseline state, there was no statistically significant difference in serum bilirubin levels between the two groups (intervention and control; SMD=-0.02; 95% CI, -0.12%, 0.09%; P=0.473). This is expected since infants were evaluated upon entry into the study and did not receive any treatment (Figure 4). Figure 5 demonstrates that infants who received Cotoneaster had significantly lower bilirubin levels compared to the control group, which received phototherapy alone (SMD=-0.73; 95% CI, -1.17%, -0.30%; P=0.000).
Subgroup analysis
In the intervention group, at time intervals of 12 h, 24 h, 36 h, 48 h, 60 h and 96 h after Cotoneaster administration, the serum bilirubin levels of infants showed a noticeable reduction, reaching their peak at 96 h after the intervention. However, at 72 h and 84 h after Cotoneaster administration, there was no effect on the bilirubin levels of infants.
Conversely, in the control group, at time intervals of 12 h, 24 h, 36 hours, 48 h, 60 h, 84 hours, and 96 h after phototherapy, the bilirubin levels of infants significantly decreased. However, no statistically significant effect on the bilirubin levels of infants was observed at 72 h after phototherapy. As seen in both the intervention and control groups, bilirubin levels in infants did not decrease 72 h after the intervention (Table 2).

In Table 3, by comparing the scores between the two intervention and control groups, in time intervals of 12 h, 24 h, 36 h, 48 h, and 72 h after the intervention, the serum bilirubin levels of the Cotoneaster group were lower than those of the control group.

However, at time intervals of 60 h, 84 h, and 96 h after the intervention, no statistically significant difference in bilirubin levels in infants between the two groups was observed. Overall, in all the studied time intervals, either the effect of Cotoneaster was better than phototherapy or it was equivalent to phototherapy (Table 3).
Additional analyses
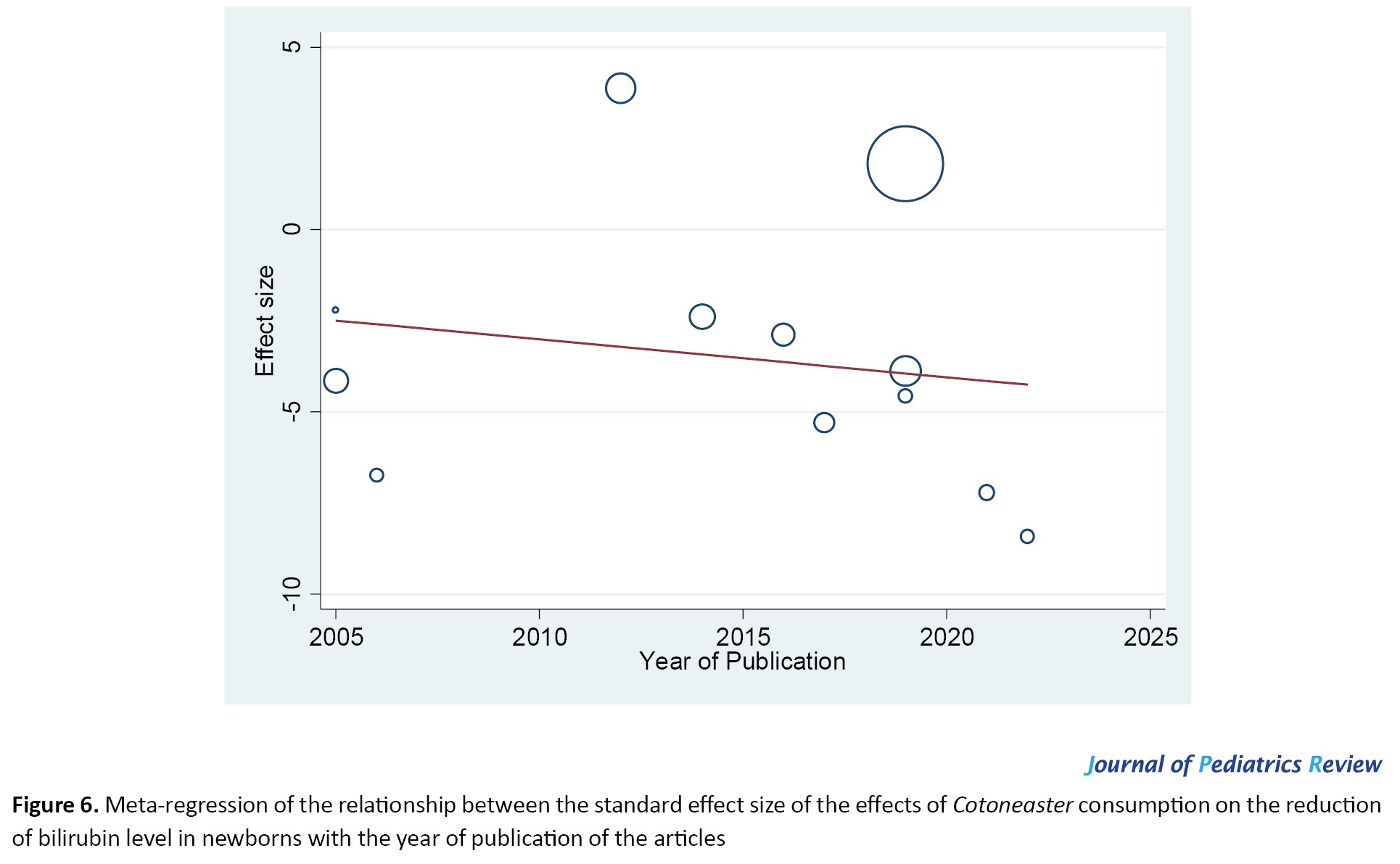
Meta-regression in Figure 6 showed no statistically significant correlation between the effect of Cotoneaster on reducing the bilirubin levels of infants and the publication year of the studies (P=0.582). In other words, the effectiveness of Cotoneaster in reducing the bilirubin levels of infants did not decrease over time from 2005 to 2022.
Meta-regression indicated no statistically significant relationship between the effect of Cotoneaster on reducing the bilirubin levels of infants and the sample size of the studies (P=0.104). Accordingly, Cotoneaster’s effect on reducing the bilirubin levels of infants was not reported more in studies with larger sample sizes and vice versa (Figure 7). The funnel plot for publication bias was statistically significant (P=0.004) and indicated that studies reporting the effectiveness of Cotoneaster in reducing the bilirubin levels of infants had a higher chance of being published (Figure 8).
Discussion
Based on the results of our study, overall, the use of Cotoneaster is more effective than phototherapy alone in reducing the serum bilirubin levels of infants, and jaundice in the Cotoneaster group significantly improved compared to the control group.
In a systematic review by Khedmat et al. (2021), Cotoneaster manna was identified as the most important herbal remedy for the treatment of neonatal hyperbilirubinemia. The administration of Cotoneaster drops, pomegranate paste and chicory extract by mothers led to a reduction in serum bilirubin and the duration of hospitalization [28]. In a meta-analysis conducted by Fakhri et al. (2018), involving eight clinical trials with a total of 862 infants and aiming to evaluate the effects of C. manna on neonatal jaundice, C. manna was more effective than phototherapy in the treatment of neonatal jaundice [29]. Sajedi et al. (2019), in a meta-analysis comprising seven controlled randomized clinical trials with 812 infants and investigated the effect of P. manna on non-conjugated hyperbilirubinemia in neonates, showed that the levels of bilirubin at 12 h (weighted mean difference [WMD]=-1.48; 95% CI, -2.31%, -0.65%), 24 h (WMD=-2.47; 95% CI, -3.22%, -1.71%), 36 h (WMD:-2.83; 95% CI, -4.87%, -0.80%), 48 h (WMD=-1.49; 95% CI, -2.36%, -0.63%) and 72 h (WMD=-0.68; 95% CI, -1.28%, -0.08%) after the intervention were significantly lower in the P. manna group [12]. In a meta-analysis conducted by Salehi et al. (2018), involving seven studies with 804 participants, the overall plasma bilirubin levels at 0, 12, 24, 36, and 48 h were examined. Furthermore, the effects of Cotoneaster on reducing neonatal jaundice were demonstrated (odd ratio (OR)=0.242; 95% CI, 0.147%, 0.399%; P<0.0001) [30]. The results of the current meta-analysis were consistent with the findings of these studies. The advantage of our study over previous meta-analyses was that it was more up-to-date and included a larger number of studies (12 clinical trials) for investigation. The number of neonatal samples in our meta-analysis (1557 neonates) was nearly twice the number of neonatal samples in previous meta-analyses. The results obtained from a larger population have greater reliability.
In a study by Shah Farhat in 2002, the serum bilirubin level after treatment with Cotoneaster did not show a statistically significant difference between the intervention and control groups. This study demonstrated that the consumption of 6 grams of Cotoneaster for the treatment of neonatal jaundice is not more effective than a placebo [14]. Nabavizadeh et al. conducted an in vitro study in which they aimed to investigate the effects of herbal medicines on neonatal hyperbilirubinemia. They reported that although jujube, Cotoneaster, and manna of Hedysarum are mild laxatives and can reduce the enterohepatic circulation of bilirubin in the intestine and promote intestinal bilirubin excretion, only chicory can effectively reduce bilirubin levels outside the body and without the influence of internal factors [31]. The results of the study by Rahani showed no statistically significant difference in the rate of bilirubin reduction in serum between the field massage group and the group receiving synthetic oral Cotoneaster drops (bilineaster) and the control group. Despite traditional beliefs in the efficacy of Cotoneaster, it did not affect reducing jaundice in neonates undergoing phototherapy [32]. These studies indicated that the consumption of Cotoneaster does not have a statistically significant effect on neonatal bilirubin levels and is not effective in reducing neonatal jaundice. Therefore, the results of these studies were not in line with the findings of the current meta-analysis. However, factors, such as differences in the type of study, sample size, and variations in the weight, age, and gender of the infants under investigation may have contributed to these differences in results. Of course, other issues are involved in the jaundice of babies, a randomized clinical trial study was conducted on 88 infants with jaundice. Both groups received standard conventional phototherapy and the intervention group received 5 drops of probiotic until hospital discharge and the comparison group received a placebo then the results showed that the probiotic group had a significantly lower hospitalization stay in comparison to the placebo group [33]. And in another study, there was a significant relationship between maternal normal serum vitamin D levels with neonatal 14th day jaundice [34].
Conclusion
Following the intervention, the bilirubin levels of neonates who received Cotoneaster were generally lower than those in the control group, which only received phototherapy. As the control group received phototherapy, it was natural that their bilirubin levels also decreased, and in some phases of the study, no significant difference was observed between the two groups. However, in most stages of the study, the condition of jaundiced neonates in the Cotoneaster group was better than that of the control group. In the future, healthcare professionals may consider using Cotoneaster alongside phototherapy to reduce neonatal bilirubin levels further, potentially reducing the duration of phototherapy and hospitalization and associated costs.
Study limitations
Since the studies included in our analysis did not report the effect of Cotoneaster on neonatal bilirubin levels based on variables, such as the gender of the neonates or the type of maternal delivery (cesarean section vs vaginal delivery), we were unable to compare the effect of Cotoneaster on bilirubin levels between male and female neonates or between neonates born through cesarean section and those born through vaginal delivery. Meanwhile, in the studies examined, the age and weight ranges of the neonates were such that they could not be categorized effectively. Therefore, in subgroup analyses, we were unable to assess the effect of Cotoneaster on neonatal bilirubin levels based on variables such as the age and weight of the neonates. Similarly, it was not possible to evaluate the effect of Cotoneaster on neonatal bilirubin levels based on the dosage of Cotoneaster consumed. It is recommended that these limitations be addressed in future studies.
Ethical Considerations
Compliance with ethical guidelines
This protocol was registered by the PROSPERO registry (Code: CRD42023457218).
Funding
This study was financially supported by Mazandaran University of Medical Sciences (Grant No.: IR.MAZUMS..REC.1402.18565).
Authors contributions
Conceptualization, study design, data analysis and results interpretation: Moloud Fakhri; Review, editing and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Mazandaran University of Medical Sciences for the financial support of the research project in this article.
References
The majority of newborns experience jaundice in the early stages of their lives [1]. The incidence of neonatal jaundice and the associated healthcare costs are increasing worldwide [2].
Phototherapy is the most common intervention for the prevention and treatment of severe hyperbilirubinemia [3]. However, many infants require blood transfusions if phototherapy fails to reduce serum bilirubin levels effectively [4]. On the other hand, retinal degeneration, diarrhea, dehydration, skin rashes [5], and oxidative stress-related diseases such as necrotizing enterocolitis and patent ductus arteriosus (PDA) are among the side effects of phototherapy [6]. In China, infants exposed to phototherapy for hyperbilirubinemia receive combined treatment with traditional Chinese herbal medicines, which have been shown to reduce serum bilirubin levels and improve jaundice [7].
Accordingly, the development of traditional and complementary medicine for neonatal jaundice is an important and potentially safe step in primary healthcare [8, 9]. Before phototherapy became commonplace, complementary medicine, such as Cotoneaster was used in Iran to treat neonatal jaundice [10]. Cotoneaster, or Purgative manna, is a white and slightly yellowish, sweet substance derived from the plant genus Cotoneaster spp. which belongs to the Rosaceae family. Its most important compounds include carbohydrates, such as mannitol, fructose, glucose, and sucrose, which induce osmotic diarrhea and may thus contribute to a reduction in bilirubin levels [11].
Numerous studies have examined the effects of P. manna on neonatal jaundice. A systematic review and meta-analysis demonstrated that Manna could reduce the duration of phototherapy by decreasing bilirubin levels in jaundiced neonates [12]. A randomized clinical trial compared the use of P. manna drops with phototherapy and demonstrated that P. manna resulted in a greater reduction in serum bilirubin concentration [13]. Another intervention study found that Cotoneaster administration had no significant impact on neonatal jaundice compared to a placebo [14]. Contradictory results from previous studies highlight the necessity for a systematic review and meta-analysis in this area. Accordingly, this study investigates the effect of Cotoneaster consumption on serum bilirubin levels in neonates worldwide, with no geographical location restrictions. However, all published studies were conducted in Iran. Since this plant is native to Iran and East Asian countries, it is not utilized in many other countries, and consequently, studies in this regard have not been conducted.
Materials and Methods
The present research was a systematic review and meta-analysis designed based on the preferred reporting items for systematic reviews and meta-analysis guidelines [15].
Search strategy
In this meta-analysis, an online search was conducted on Iranian databases, including Barakat Gostar, Scientific Information Database (SID), Magiran, IranDoc and international databases, such as PubMed, Scopus, Web of Science, Cochrane and the Google Scholar search engine, without time restrictions up to July 20, 2023. Language restriction was not performed during the search. The search was performed using standard keywords and MeSH terms, including “Purgative manna,” “billinaster,” “shir-khesht,” “Cotoneaster,” “rosaceae,” “jaundice,” “icterus,” “hyperbilirubinemia,” “bilirubinemia,” “infant,” and “newborn.” The keyword combinations were explored using logical operators (AND, OR) within the mentioned databases. For manual searching, a list of eligible primary studies was also examined. A sample of the search strategy in the PubMed database is provided below: ([Purgative manna OR billinaster OR shir-khesht OR Cotoneaster OR Rosaceae] AND [jaundice OR Icterus OR hyperbilirubinemia OR bilirubinemia]) AND (infant, newborn OR neonatal).
Population, intervention, comparison, and outcome components
The components of population, intervention, comparison and outcome for this study were as follows: The population included clinical trial studies, involving infants as participants; the intervention comprised an intervention group that could either solely use a Cotoneaster-derived product or receive Cotoneaster in conjunction with phototherapy; the comparison group could include several scenarios, namely no intervention (one-group, before-after, trial), placebo, or phototherapy; and the outcome of the studies under investigation should assess at least the serum bilirubin level.
Inclusion criteria
The inclusion criteria for this study were clinical trial studies that evaluated the effect of P. manna consumption on infant jaundice.
Exclusion criteria
The exclusion criteria comprised observational studies, review studies, studies with no access to the full text, protocol papers, conference studies, studies with low quality, studies lacking necessary data for data analysis, and duplicate studies
Quality assessment of primary studies
After identifying the included studies, two independent reviewers assessed the quality of clinical trials using the Cochrane collaboration’s checklist for assessing the risk of bias in randomized trials [16]. This checklist comprises seven questions, each addressing a critical aspect of bias in clinical trials. Each question has three response options, namely high risk of bias, low risk of bias, and unclear. After completing the risk of bias assessment for all studies, discrepancies in responses to the questions in each study were evaluated. Any disagreements were resolved through consensus or agreement between the two assessors, resulting in a unified response.
Data extraction
Two independent researchers conducted data extraction from the included studies. The extracted data were entered into a checklist that included the following information: First author’s name, publication year of the study, total number of infants, number of female and male infants, infant weight, infant age, type of maternal delivery (cesarean or vaginal), study location, Cotoneaster dosage, number of infants in the intervention and control groups, and Mean±SD of serum bilirubin levels before and after the intervention in the intervention and control groups. A third researcher reviewed the extracted data from the previous two researchers to resolve any discrepancies.
Statistical analysis
All included studies had both intervention and control groups and repeatedly measured serum bilirubin levels at baseline and at multiple time points (at regular intervals of 12 h). This allowed for the calculation of within-group mean differences and between-group mean difference indices. The standardized mean difference (SMD) was estimated using the sample size, Mean±SD of serum bilirubin levels before and after the intervention in the intervention and control groups. The SMD indicates the strength of the relationship, with values closer to zero representing a weaker relationship and values closer to one or higher indicating a stronger relationship [17]. To assess heterogeneity, the Cochrane Q test and I2 index were used. Subgroup analysis and meta-regression were used to investigate the sources of heterogeneity, and the Funnel plot was used to assess publication bias [18]. The I2 index has three classifications (<25% indicates low heterogeneity, between 25% and 75% shows moderate heterogeneity and >75% indicates high heterogeneity) [19]. In this study, a random-effects model was used for data analysis. Data analysis was performed using the STATA software, version 14, and the significance level was considered P<0.05.
Results
Study selection
In the initial search, a total of 206 articles were found. Upon reviewing the titles of the studies, 49 duplicate studies were removed. The abstracts of the remaining 157 articles were examined, and of these, 24 articles were excluded due to the unavailability of their full texts. Among the remaining 133 articles, 6 were excluded because they lacked the necessary data for data analysis. Out of the remaining 127 articles, an additional 115 articles were excluded based on other exclusion criteria, leaving 12 articles for qualitative evaluation, all of which were of acceptable quality (Figure 1).
Summary of reviewed studies
In this study, 12 clinical trial articles with a total of 1557 infants (781 infants in the control group and 776 infants in the intervention group) were examined. Out of these, 2 studies investigated the effect of Cotoneaster on preventing neonatal jaundice [20, 21], and 10 studies investigated the effect of Cotoneaster on the treatment of neonatal jaundice [4, 10, 13, 14, 22–27]. There were no statistically significant differences between the intervention and control groups in terms of the number of participants, age, weight, gender and serum bilirubin levels of the infants. Other information from the reviewed articles is presented in Table 1.




Analysis of the primary outcome
According to Figure 2, in the intervention group, after the administration of Cotoneaster, the serum bilirubin levels of infants significantly decreased (SMD=-3.50; 95% CI, -5.76%, -1.24%; P=0.000). Figure 3 shows that in the control group, after phototherapy, the serum bilirubin levels of infants significantly decreased (SMD=-2.14; 95% CI, -4.01%, -0.27%; P=0.000). Meanwhile, phototherapy is currently the most common method for the treatment of jaundice and the reduction of bilirubin levels in infants.
Before the intervention and in the baseline state, there was no statistically significant difference in serum bilirubin levels between the two groups (intervention and control; SMD=-0.02; 95% CI, -0.12%, 0.09%; P=0.473). This is expected since infants were evaluated upon entry into the study and did not receive any treatment (Figure 4). Figure 5 demonstrates that infants who received Cotoneaster had significantly lower bilirubin levels compared to the control group, which received phototherapy alone (SMD=-0.73; 95% CI, -1.17%, -0.30%; P=0.000).
Subgroup analysis
In the intervention group, at time intervals of 12 h, 24 h, 36 h, 48 h, 60 h and 96 h after Cotoneaster administration, the serum bilirubin levels of infants showed a noticeable reduction, reaching their peak at 96 h after the intervention. However, at 72 h and 84 h after Cotoneaster administration, there was no effect on the bilirubin levels of infants.
Conversely, in the control group, at time intervals of 12 h, 24 h, 36 hours, 48 h, 60 h, 84 hours, and 96 h after phototherapy, the bilirubin levels of infants significantly decreased. However, no statistically significant effect on the bilirubin levels of infants was observed at 72 h after phototherapy. As seen in both the intervention and control groups, bilirubin levels in infants did not decrease 72 h after the intervention (Table 2).

In Table 3, by comparing the scores between the two intervention and control groups, in time intervals of 12 h, 24 h, 36 h, 48 h, and 72 h after the intervention, the serum bilirubin levels of the Cotoneaster group were lower than those of the control group.

However, at time intervals of 60 h, 84 h, and 96 h after the intervention, no statistically significant difference in bilirubin levels in infants between the two groups was observed. Overall, in all the studied time intervals, either the effect of Cotoneaster was better than phototherapy or it was equivalent to phototherapy (Table 3).
Additional analyses
Meta-regression in Figure 6 showed no statistically significant correlation between the effect of Cotoneaster on reducing the bilirubin levels of infants and the publication year of the studies (P=0.582). In other words, the effectiveness of Cotoneaster in reducing the bilirubin levels of infants did not decrease over time from 2005 to 2022.
Meta-regression indicated no statistically significant relationship between the effect of Cotoneaster on reducing the bilirubin levels of infants and the sample size of the studies (P=0.104). Accordingly, Cotoneaster’s effect on reducing the bilirubin levels of infants was not reported more in studies with larger sample sizes and vice versa (Figure 7). The funnel plot for publication bias was statistically significant (P=0.004) and indicated that studies reporting the effectiveness of Cotoneaster in reducing the bilirubin levels of infants had a higher chance of being published (Figure 8).
Discussion
Based on the results of our study, overall, the use of Cotoneaster is more effective than phototherapy alone in reducing the serum bilirubin levels of infants, and jaundice in the Cotoneaster group significantly improved compared to the control group.
In a systematic review by Khedmat et al. (2021), Cotoneaster manna was identified as the most important herbal remedy for the treatment of neonatal hyperbilirubinemia. The administration of Cotoneaster drops, pomegranate paste and chicory extract by mothers led to a reduction in serum bilirubin and the duration of hospitalization [28]. In a meta-analysis conducted by Fakhri et al. (2018), involving eight clinical trials with a total of 862 infants and aiming to evaluate the effects of C. manna on neonatal jaundice, C. manna was more effective than phototherapy in the treatment of neonatal jaundice [29]. Sajedi et al. (2019), in a meta-analysis comprising seven controlled randomized clinical trials with 812 infants and investigated the effect of P. manna on non-conjugated hyperbilirubinemia in neonates, showed that the levels of bilirubin at 12 h (weighted mean difference [WMD]=-1.48; 95% CI, -2.31%, -0.65%), 24 h (WMD=-2.47; 95% CI, -3.22%, -1.71%), 36 h (WMD:-2.83; 95% CI, -4.87%, -0.80%), 48 h (WMD=-1.49; 95% CI, -2.36%, -0.63%) and 72 h (WMD=-0.68; 95% CI, -1.28%, -0.08%) after the intervention were significantly lower in the P. manna group [12]. In a meta-analysis conducted by Salehi et al. (2018), involving seven studies with 804 participants, the overall plasma bilirubin levels at 0, 12, 24, 36, and 48 h were examined. Furthermore, the effects of Cotoneaster on reducing neonatal jaundice were demonstrated (odd ratio (OR)=0.242; 95% CI, 0.147%, 0.399%; P<0.0001) [30]. The results of the current meta-analysis were consistent with the findings of these studies. The advantage of our study over previous meta-analyses was that it was more up-to-date and included a larger number of studies (12 clinical trials) for investigation. The number of neonatal samples in our meta-analysis (1557 neonates) was nearly twice the number of neonatal samples in previous meta-analyses. The results obtained from a larger population have greater reliability.
In a study by Shah Farhat in 2002, the serum bilirubin level after treatment with Cotoneaster did not show a statistically significant difference between the intervention and control groups. This study demonstrated that the consumption of 6 grams of Cotoneaster for the treatment of neonatal jaundice is not more effective than a placebo [14]. Nabavizadeh et al. conducted an in vitro study in which they aimed to investigate the effects of herbal medicines on neonatal hyperbilirubinemia. They reported that although jujube, Cotoneaster, and manna of Hedysarum are mild laxatives and can reduce the enterohepatic circulation of bilirubin in the intestine and promote intestinal bilirubin excretion, only chicory can effectively reduce bilirubin levels outside the body and without the influence of internal factors [31]. The results of the study by Rahani showed no statistically significant difference in the rate of bilirubin reduction in serum between the field massage group and the group receiving synthetic oral Cotoneaster drops (bilineaster) and the control group. Despite traditional beliefs in the efficacy of Cotoneaster, it did not affect reducing jaundice in neonates undergoing phototherapy [32]. These studies indicated that the consumption of Cotoneaster does not have a statistically significant effect on neonatal bilirubin levels and is not effective in reducing neonatal jaundice. Therefore, the results of these studies were not in line with the findings of the current meta-analysis. However, factors, such as differences in the type of study, sample size, and variations in the weight, age, and gender of the infants under investigation may have contributed to these differences in results. Of course, other issues are involved in the jaundice of babies, a randomized clinical trial study was conducted on 88 infants with jaundice. Both groups received standard conventional phototherapy and the intervention group received 5 drops of probiotic until hospital discharge and the comparison group received a placebo then the results showed that the probiotic group had a significantly lower hospitalization stay in comparison to the placebo group [33]. And in another study, there was a significant relationship between maternal normal serum vitamin D levels with neonatal 14th day jaundice [34].
Conclusion
Following the intervention, the bilirubin levels of neonates who received Cotoneaster were generally lower than those in the control group, which only received phototherapy. As the control group received phototherapy, it was natural that their bilirubin levels also decreased, and in some phases of the study, no significant difference was observed between the two groups. However, in most stages of the study, the condition of jaundiced neonates in the Cotoneaster group was better than that of the control group. In the future, healthcare professionals may consider using Cotoneaster alongside phototherapy to reduce neonatal bilirubin levels further, potentially reducing the duration of phototherapy and hospitalization and associated costs.
Study limitations
Since the studies included in our analysis did not report the effect of Cotoneaster on neonatal bilirubin levels based on variables, such as the gender of the neonates or the type of maternal delivery (cesarean section vs vaginal delivery), we were unable to compare the effect of Cotoneaster on bilirubin levels between male and female neonates or between neonates born through cesarean section and those born through vaginal delivery. Meanwhile, in the studies examined, the age and weight ranges of the neonates were such that they could not be categorized effectively. Therefore, in subgroup analyses, we were unable to assess the effect of Cotoneaster on neonatal bilirubin levels based on variables such as the age and weight of the neonates. Similarly, it was not possible to evaluate the effect of Cotoneaster on neonatal bilirubin levels based on the dosage of Cotoneaster consumed. It is recommended that these limitations be addressed in future studies.
Ethical Considerations
Compliance with ethical guidelines
This protocol was registered by the PROSPERO registry (Code: CRD42023457218).
Funding
This study was financially supported by Mazandaran University of Medical Sciences (Grant No.: IR.MAZUMS..REC.1402.18565).
Authors contributions
Conceptualization, study design, data analysis and results interpretation: Moloud Fakhri; Review, editing and final approval: All authors.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank Mazandaran University of Medical Sciences for the financial support of the research project in this article.
References
- Badiehneshin L, Saghafi Z, Asadollahi Z, Moghadari M, Derakhshan R, Sadeghi T. The effects of chicory extract consumption by mothers on the frequency of icterus and the serum bilirubin level in neonates. Int J Pediatr. 2022; 10(3):15601-8. [DOI:10.22038/ijp.2022.62842.4800]
- Olusanya BO, Kaplan M, Hansen TWR. Neonatal hyperbilirubinaemia: A global perspective. Lancet Child Adolesc Health. 2018; 2(8):610-20. [DOI:10.1016/S2352-4642(18)30139-1] [PMID]
- Hamidi M, Aliakbari F. Comparison of Phototherapy with light-editing diodes (LED) and Conventional Phototherapy (fluorescent lamps) in reducing Jaundice in Term and Preterm Newborns. Middle East J Fam Med. 2018; 7(10):123. [DOI:10.5742/MEWFM.2018.93319]
- Ghotbi F, Nahidi S, Zangi M. [Surveying the effect of cotoneaster spp. (shir khesht) on neonatal jaundice (Persian)]. Res Med. 2006; 30(4):353-61. [Link]
- Karakukcu C, Ustdal M, Ozturk A, Baskol G, Saraymen R. Assessment of DNA damage and plasma catalase activity in healthy term hyperbilirubinemic infants receiving phototherapy. Mutat Res. 2009; 680(1-2):12-6. [DOI:10.1016/j.mrgentox.2009.07.016] [PMID]
- Gathwala G, Sharma S. Oxidative stress, phototherapy and the neonate. Indian J Pediatr. 2000; 67(11):805-8. [DOI:10.1007/BF02726223] [PMID]
- Wu R, Feng S, Han M, Caldwell P, Liu S, Zhang J, et al. Yinzhihuang oral liquid combined with phototherapy for neonatal jaundice: A systematic review and meta-analysis of randomized clinical trials. BMC Complement Altern Med. 2018; 18(1):228. [DOI:10.1186/s12906-018-2290-x] [PMID] [PMCID]
- Tavoli Z, Mohammadi M, Tavoli A, Moini A, Effatpanah M, Khedmat L, et al. Quality of life and psychological distress in women with recurrent miscarriage: A comparative study. Health Qual Life Outcomes. 2018; 16(1):150. [DOI:10.1186/s12955-018-0982-z] [PMID] [PMCID]
- Tavakolizadeh R, Izadi A, Seirafi G, Khedmat L, Mojtahedi SY. Maternal risk factors for neonatal jaundice: A hospital-based cross-sectional study in Tehran. Eur J Transl Myol. 2018; 28(3):7618. [DOI:10.4081/ejtm.2018.7618] [PMID] [PMCID]
- Mahyar A, Mehrpisheh S, Khajeh B, Ayazi P, Oveisi S, Mahyar S, et al. The effect of purgative manna and clofibrate on neonatal unconjugated hyperbilirubinemia. Acta Med Iran. 2019; 57(6):368-73. [DOI:10.18502/acta.v57i6.1882]
- Khoshdel A, Khayeri S. Surveying the effect of use of cotoneaster spp (shirkhesht) by mother or neonatal on neonatal jaundice (Persian)]. J Shahrecord Univ Med Sci. 2009; 13(4):67-73. [Link]
- Sajedi F, Fatollahierad S. Effect of purgative manna on neonatal hyperbilirubinemia: A systematic review and meta-analysis. Iran J Pharm Res. 2019; 18(2):1020-31. [PMID]
- Monsef A, Eghbalian F, Rahimi N. Comparison of purgative manna drop and phototherapy with phototherapy treatment of neonatal jaundice: A randomized double-blind clinical trial. Osong Public Health Res Perspect. 2019; 10(3):152-7. [DOI:10.24171/j.phrp.2019.10.3.06] [PMID] [PMCID]
- Shah Farhat A, Mohammadzadeh A, Ramezani M, Amiri M. [The effect of shirkhesht on newborns' indirect hyperbilirobinemia (Persian)]. Razi J Med Sci. 2005; 12(47):93-8. [Link]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev. 2015; 4(1):1. [DOI:10.1186/2046-4053-4-1]
- Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928. [DOI:10.1136/bmj.d5928] [PMID] [PMCID]
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in Meta-Analysis. In: Egger M, Smith GD, Altman DG, editors. Systematic reviews in health care: Meta-analysis in context. London: BMJ Publishing Group; 2001. [DOI:10.1002/9780470693926.ch15]
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315(7109):629-34. [DOI:10.1136/bmj.315.7109.629] [PMID] [PMCID]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21(11):1539-58. [DOI:10.1002/sim.1186] [PMID]
- Fakhri M, Farhadi R, Azadbakht M, Akbari J, Yousefi S, Mousavinasab N, et al. Cotoneaster manna oral drop for the management of neonatal hyperbilirubinemia; a randomized, double-blinded and placebo controlled clinical trial. Immunopathol Persa. 2022; 8(1):e10. [DOI:10.34172/ipp.2022.10]
- Aghababaeian H, Nirouzad F, Kiarsi M, Amirgholami N, Kalani L, Beiranvand R, et al. The effect of Bilineaster "Cotoneaster" on reducing bilirubin in neonates with jaundice-a triple-blind randomized clinical trial. J Adv Pharm Educ Res. 2021; 11(1):23-8. [DOI:10.51847/qABgSKl]
- Fakhri M, Farhadi R, Mousavinasab N, Hosseinimehr SJ, Yousefi SS, Davoodi A, et al. Preventive effect of purgative manna on neonatal jaundice: A double blind randomized controlled clinical trial. J Ethnopharmacol. 2019; 236:240-9. [DOI:10.1016/j.jep.2019.03.009] [PMID]
- Ameli Z, Assarroudi A, Akrami R. Effect of bilineaster drop on neonatal hyperbilirubinemia. Evid Based Care j. 2017; 6(4):66-73. [DOI:10.22038/EBCJ.2016.7982]
- Rafieian-Kopaei M, Khoshdel A, Kheiri S, Shemian R. Cotoneaster: A safe and easy way to reduce neonatal jaundice. J Clin Diagn Res. 2016; 10(4):SC01-3. [DOI:10.7860/JCDR/2016/17084.7574] [PMID] [PMCID]
- Fallah R, Ali Fallahzadeh M, Noori-Shadkam M. Evaluation of safety and efficacy of purgative manna (billinaster drop) and glycerin suppository in icterus of healthy term newborns. Curr Drug Saf. 2014; 9(1):29-33. [DOI:10.2174/15748863113086660052] [PMID]
- Mansouri M, Ghotbi N, Bahadorbeigi L. [Evaluation of the preventive effects of purgative manna on neonatal icterus in Sanandaj (Persian)]. Sci J Kurdistsn Univ Med Sci. 2012; 17(2):30-5. [Link]
- Azadbakht M, Pishva N, Mohammadi samani S, Alinejad F. [Effect of Manna from Cotoneaster discolor on infant Jaundice (effect on blood bilirubin level) (Persian)]. J Med Plants 2005; 14(4):36-44. [Link]
- Khedmat L, Mojtahedi S, Moienafshar A. Recent clinical evidence in the herbal therapy of neonatal jaundice in Iran: A review. J Herb Med. 2021; 29:100457. [DOI:10.1016/j.hermed.2021.100457]
- Fakhri M, Davoodi A, Hamzegardeshi Z, Farhadi R, Mousavinasab N, Keshtkar A, et al. Is cotoneaster manna improving the treatment of neonatal jaundice? Bangladesh J Pharmacol. 2018; 13(2):168-78. [DOI:10.3329/bjp.v13i2.36017]
- Salehi A, Ostovar M, Marzban M. A systematic review and Meta-Analysis on the effect of Cotoneaster manna on Neonatal Jaundice. J Herb Drugs. 2018; 9(1):47-54. [Link]
- Nabavizadeh S, Safari M, Khoshnevisan F. The effect of herbal drugs on neonatal jaundice. Iran J Pediatr. 2005; 15(2):133-8. [Link]
- Rahani T, Boskabadi H, Sadeghi T, Boskabadi MH, Gharaei R, Pasban F. Comparison of the effect of cotoneaster manna drop (Bilineaster) and massage on bilirubin in neonates under phototherapy. J Babol Univ Med Sci. 2017; 19(11):21-7. [Link]
- Mojabi SH, Mohammadkhaniha F, Mohammadi N, Mohammadhoseini M. Phototherapy with probiotics supplementation therapy and phototherapy alone in neonates with jaundice: A randomized clinical trial. Immunopathol Persa. 2022; 8(1):e02. [DOI:10.34172/ ipp.2022.02]
- Sadr Z, Nourbakhsh M, Bakhshizade M, Mokhtari F, Biglari Abhari M. Relationship between vitamin D levels in mother’s blood and neonatal umbilical cord with jaundice in neonates. Immunopathol Persa. 2022; 8(2):e24237. [DOI: 10.34172/ ipp.2022.24237]
Type of Study: Review Article |
Subject:
Neonatology
Received: 2024/01/1 | Accepted: 2024/03/17 | Published: 2024/04/1
Received: 2024/01/1 | Accepted: 2024/03/17 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |