Volume 12, Issue 2 (4-2024)
J. Pediatr. Rev 2024, 12(2): 171-182 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Sobhanian P, Karami S, Saffar H, Shafizad M, Baradaran M, Shahbaznejad L. Investigating Legg-calve-perthes Disease: A Comprehensive Review of Diagnosis, Management and Current Treatment Options. J. Pediatr. Rev 2024; 12 (2) :171-182
URL: http://jpr.mazums.ac.ir/article-1-606-en.html
URL: http://jpr.mazums.ac.ir/article-1-606-en.html
Pooria Sobhanian1 

 , Shaghayegh Karami2
, Shaghayegh Karami2 
 , Homina Saffar1
, Homina Saffar1 

 , Misagh Shafizad3
, Misagh Shafizad3 

 , Mansoureh Baradaran4
, Mansoureh Baradaran4 

 , Leila Shahbaznejad *5
, Leila Shahbaznejad *5 




 , Shaghayegh Karami2
, Shaghayegh Karami2 
 , Homina Saffar1
, Homina Saffar1 

 , Misagh Shafizad3
, Misagh Shafizad3 

 , Mansoureh Baradaran4
, Mansoureh Baradaran4 

 , Leila Shahbaznejad *5
, Leila Shahbaznejad *5 


1- Student Research Committee, Mazandaran University of Medical Sciences, Sari, Iran.
2- School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Neurosurgery, Faculty of Medicine, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Radiology, Imam Ali Hospital, North Khorasan University of Medical Science, Bojnurd, Iran.
5- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,Leilashahbaznezhad@yahoo.com
2- School of Medicine, Tehran University of Medical Sciences, Tehran, Iran.
3- Department of Neurosurgery, Faculty of Medicine, Orthopedic Research Center, Mazandaran University of Medical Sciences, Sari, Iran.
4- Department of Radiology, Imam Ali Hospital, North Khorasan University of Medical Science, Bojnurd, Iran.
5- Pediatric Infectious Diseases Research Center, Communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran. ,
Keywords: Legg-calve-perthes disease (LCPD), Prognosis, Steinberg classification, Pathophysiology, Joint diagnosis, Treatment outcome
Full-Text [PDF 1595 kb]
(681 Downloads)
| Abstract (HTML) (2216 Views)
Full-Text: (357 Views)
Introduction
Legg-calve-perthes disease (LCPD) is an idiopathic condition affecting children’s hip joints. The characteristic feature of LCPD is the cutting off of the blood supply to the femoral head epiphysis, which ultimately results in the death of bone tissue (aseptic osteonecrosis) and the deformity of the femoral head (FH). LCPD typically manifests clinically between the ages of 2 and 15 years, with males afflicted five times more often than females [1].
Initial symptoms of LCPD may be subtle, such as mild discomfort or limping, which may intensify with physical activity (primarily in the anterior hip and midthigh) [1]. As the disease progresses, limited hip range of motion, atrophy of the thigh muscles, decreased internal rotation, hip abduction, and Trendelenburg gait can occur [2, 3].
The pathogenesis of LCPD is characterized by various stages as follows: 1) Necrosis phase, 2) Fragmentation and 3) Re-ossification and remodeling. In the early phases of LCPD, x-rays are frequently used to determine the disease’s stage, FH position and FH involvement. However, in cases where radiographic findings are not diagnostic, magnetic resonance imaging (MRI) is recommended [3, 4].
The therapy of LCPD is determined in part by several parameters, including the age and disability of the patient at the time of diagnosis, the lateral pillar classification and the stage of the disease. Conservative treatments, such as rest, physical therapy, and braces or casts can be combined with surgical procedures, such as osteotomy to preserve the hip joint. Treatment maintains normal hip joint function, prevents deformity, and decreases the likelihood of long-term complications [5, 6].
Despite advances in knowledge and treatment options, managing LCPD remains complicated. These may result from the disease’s varied manifestations, the inability to predict outcomes precisely and the lack of consensus regarding the most effective treatment strategies. Hence, this paper is based on recent data in the literature to review the etiology, imaging, and treatment of LCPD.
Clinical manifestations and physical examination
Patients in the early stages of the disease experience mild and nonspecific symptoms, such as pain or limping [3, 7]. Due to stimulation of the sensory branch of the obturator nerve, the patient often localizes the pain in the thigh or knee. The patient’s examination revealed a reduction in internal rotation and abduction of the hip, an occasional antalgic gait, and a reduction in the range of motion of the hip joint, all of which are caused by muscle spasm, joint effusion and FH collapse [2, 3, 8]. Significant FH collapse can result in a leg length discrepancy and Trendelenburg gait in patients. In addition, shortening of the ipsilateral gluteal musculature can exacerbate the patient’s limping [1, 2, 8].
Etiology
Despite numerous hypotheses, the exact cause of LCPD is still uncertain. A disruption in the blood supply to the developing FH is the hallmark of LCPD resulting in ischemic necrosis and subsequent structural alterations in the developing FH. Genetic and environmental factors contribute to the condition’s development. Genetic factors may influence susceptibility to interruption of blood supply to the FH. LCPD has been linked to mutations of the COL2A1 gene on the 12q13 chromosome and the COL1A1 gene, which is associated with osteogenesis imperfecta [9, 10]. In contrast, environmental factors, such as repetitive trauma or mechanical excess, increased blood viscosity [11] and coagulation disorders may initiate the disease. In addition, passive smoking exposure, and high-dose and long-term glucocorticoids have been linked to an increased risk of LCPD [12, 13]. Furthermore, sickle-cell anemia, thalassemia, trichorhinophalangeal syndrome, and hemophilia can be a risk factor for LCPD [6]. Sickle-cell anemia patients with osteonecrosis had substantially higher hospitalization rates (mostly due to vaso-occlusive crisis) compared to subjects without osteonecrosis, according to Adekile [14]. Meanwhile, according to Leandro et al. polymorphisms in the BMP6, Klotho and ANXA2 genes may be related to LCPD in sickle-cell anemia patients [15].
Pathogenesis
The progression of LCPD consists of various stages: 1) In the initial phase, the FH’s blood supply (the subchondral cortical bone mostly) is cut off, resulting in bone and cartilage death (necrosis phase); 2) In the revascularization stage, new blood vessels form, and new bone gradually supplants necrotic bone (fragmentation); 3) During this phase, osteoblasts reactivate, resulting in remodeling, which might be malformed or asymmetrical, leading to adult-onset degenerative osteoarthritis (re-ossification and remodeling).
The pathological progression of LCPD affects multiple parts of the FH, including the articular cartilage, epiphysis, physis, and metaphysis, leading to the destruction of trabecular bone microarchitecture and necrosis in the bone marrow. The disruption of blood supply can result in growth cessation of the epiphysis, necrosis of the deep layer of articular cartilage, an increase in vascular endothelial growth factor (VEGF), a rise in vascularization, and deformity. In addition, serum VEGF may serve as a useful disease follow-up marker [16, 17].
Following revascularization, the primary reparative response is osteoclastic bone resorption which causes the normal tissue of the FH to be replaced by fibrovascular tissue. The inability of the growth plate that surrounds the osseous epiphysis to continue forming is another factor that leads to the degeneration of the FH. VEGF and bone morphogenetic protein (BMP)-2 are involved in this process due to their respective roles in angiogenesis and endochondral ossification [18].
The blood supply to the FH depends on several number of anastomoses that contribute to its vascularization. Several anastomoses contribute to the FH’s blood supply: 1) The anastomosis between the medial and lateral circumflex branches of the profunda femoris is the most essential; 2) Cruciate anastomosis of the inferior gluteal artery to the medial circumflex femoral artery; 3) An anastomosis between the superior gluteal artery and the medial/lateral circumflex femoral arteries at the trochanter. In addition, the foveal artery, which passes through the ligamentum teres, supplies blood to the FH, which is especially significant in children. Due to insufficient collateral circulation in the FH, ischemia and necrosis may develop, if the blood supply is cut off.
Neutrophils and macrophages are the most prevalent inflammatory cells involved in the pathogenesis of osteonecrosis, resulting in macroscopic subchondral collapse and subsequent joint deterioration. Disruption in blood supply causes ischemia of the covering cartilage and flattening of the head articulates with the acetabulum, which causes secondary osteoarthritis [19-21].
Classification
LCPD is classified into the following stages based on symptoms, as well as plain radiographs, bone scans and MRI findings (Table 1) [22].

Imaging
The majority of LCPD diagnosis and staging is performed using conventional radiography as the imaging modality. The epiphyseal involvement and the height of the lateral pillar are associated with FH deformity and can be used to assist in classification, prognostic evaluation, and surveillance of disease progression on conventional radiographs [6, 23]. If the FH involvement is not evident on standard x-rays, lateral x-rays may be necessary to detect alterations in the anterolateral aspect of the FH [4].
In cases where hip radiographs are normal or inconclusive, the MRI is essential for diagnosing LCPD (Figure 1). For effective joint preservation treatments to be initiated, early diagnosis is essential. Hence, using non-enhanced and contrast-enhanced imaging sequences, MRI can confidently diagnose LCPD and facilitate the initiation of appropriate therapies. Contrast-enhanced MRI using gadolinium has proved to be superior to radiography and can detect FH perfusion early at an early stage and aid in early prognostication [3]. Hypoperfusion is one of the early alterations visible in LCPD and can be demonstrated on T1-weighted fat-suppressed imaging performed 2–5 min after contrast administration [24]. Furthermore, bone scintigraphy and diffusion-weighted MRI can be employed to evaluate the severity of perfusion abnormalities in LCPD may be a significant prognostic factor [23]. Dynamic contrast-enhanced subtraction MRI has demonstrated high sensitivity, excellent correlation with bone scintigraphy, and the ability to provide specific information about blood flow to the FH, thereby facilitating the early detection of ischemia [24].
MRI is also beneficial for accurate disease early staging, assessing associated complications, and distinguishing LCPD from other epiphyseal lesions [25]. It allows for the differentiation of LCPD from multiple epiphyseal dysplasia, spondyloepiphyseal dysplasia and Meyer dysplasia. In the early phases of LCPD, contrast-enhanced MRI better defines the extent of FH necrosis than non-contrast MRI. Radiographic measurements of FH deformity correlate strongly with perfusion measurements obtained from contrast-enhanced subtraction MRI, specifically the MRI perfusion index, after at least two years of follow-up [26].
Treatment
The objective of LCPD treatment is to achieve a spherical FH contour with excellent congruency during the disease process [27]. Depending on the onset age, disease stage, and severity of FH involvement LCPD is treated differently. Current LCPD treatment emphasizes protecting the FH mechanically to prevent future hip deformity and degeneration [1]. The following treatment options and factors can be considered.
Conservative treatment
LCPD can be treated conservatively with non-weight bearing casts, abduction casts, orthotics, and rehabilitation, and are better suited to younger patients (Table 2).
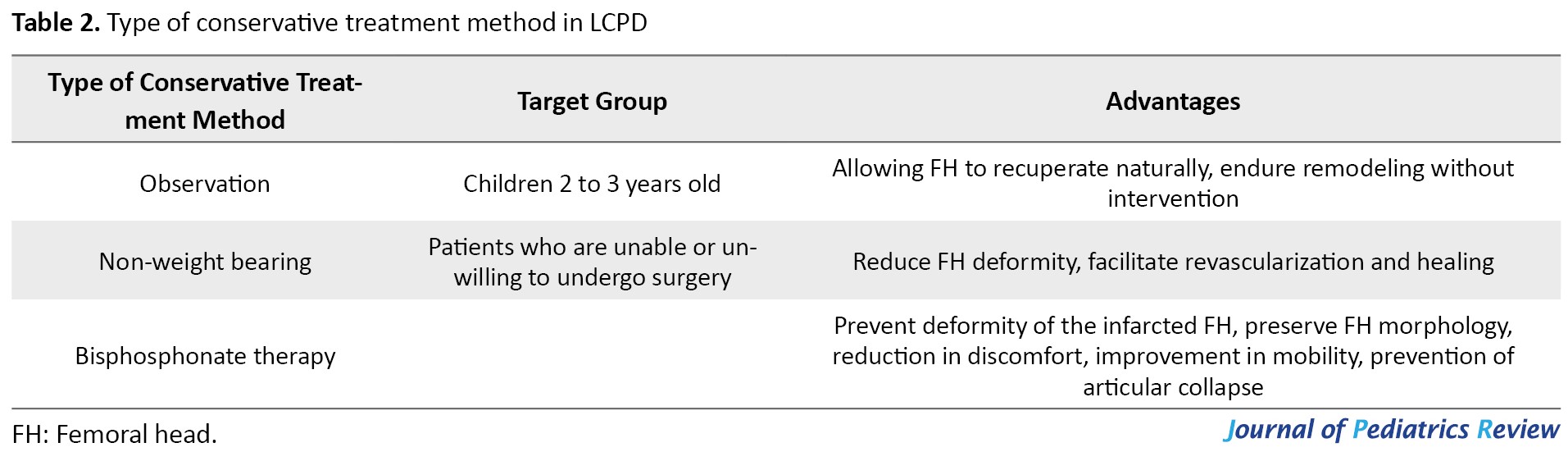
The goal is to decrease deformity, promote revascularization and resorption of the infarcted epiphysis, and increase the range of motion. However, the effectiveness of conservative treatment has been contested, and results can vary. Those who are younger than 6 years at the time of diagnosis or who have necrosis of less than 50% of the FH should be treated symptomatically, according to Wiig et al. [28].
Observation
The observation method may be advised in children aged 2 to 3 years and permits the FH to recuperate naturally and endure remodeling without intervention [28].
Non-weight bearing
In the treatment of LCPD, the non-weight bearing has demonstrated contradictory results. Several studies indicate that early weight-bearing restrictions can reduce FH deformity and facilitate revascularization and healing. It could be considered a treatment option for patients who are unable or unwilling to undergo surgery. According to Peck et al., prolonged non-weight-bearing should be regarded as a treatment option for patients unwilling to undergo operative treatment in early-stage LCPD with significant hypoperfusion [29]. Protective weight bearing improved collapse ratings. Protective weight-bearing helps most pre-collapse and post-collapse patients delay total hip arthroplasty or hip-preserving surgery [30].
Bisphosphonate therapy
Due to the lack of consistent patient groups and pharmacological protocols, the clinical evidence for bisphosphonate therapy to reduce FH deformity in LCPD is inconclusive. Studies suggest that bisphosphonate therapy (systemic and local) can prevent deformity of the infarcted FH in LCPD and preserve FH morphology. This strategy targets the pathological processes that contribute to FH collapse, particularly the imbalance of osteoclast resorption and the delay in new bone formation [31]. Some authors reported that bisphosphonate can reduce discomfort, improve mobility, and prevent articular collapse [32].
FH deformation can be prevented or greatly reduced when BMP2 is administered locally [33]. In a pig model of ischemic osteonecrosis, administration of BMP-2 decreased bone resorption and increased new bone formation in an animal study [34]. According to Vandermeer et al. in an immature animal model of ischemic osteonecrosis, the combination of ibandronate and BMP-2 reduced FH deformity and stimulated bone formation [35].
Bisphosphonate dose may depend on therapy timing. Preventing deformity may require starting therapy early. Anabolic stimulation is needed to facilitate healing and new bone formation, even if bisphosphonates and other anti-resorptive medicines can preserve FH’s spherical shape. Weight loss and activity modifications may be required during recovery due to the necrotic FH’s impaired mechanical characteristics [36].
Surgical treatment
In certain cases of LCPD, surgery may be considered a treatment option (Table 3).

Typically, the decision to conduct surgery is influenced by age, the extent of FH involvement (Catterall classification), and prognosis. In patients with 6 years of age or higher with FH necrosis exceeding 50%, surgical intervention should be considered [28]. In surgical procedures, femoral osteotomy, salter osteotomy, lateral shelf acetabuloplasty, and so on may be performed. Surgical treatment in patients older than 6 years has outstanding outcomes for Herring B and B/C hips and poor outcomes for Herring C hips, with a modest advantage for patients aged 6 to 8 years [37].
A-frame orthoses
According to Rich et al. restoring and sustaining a hip range of motion in conjunction with the use of an A-frame orthosis led to a high proportion of spherically congruent hips in patients of all ages and disease severity. This method has proven effective for preserving joint function and improving hip congruency [38].
Arthrodiastasis
Arthodiastasis is a relatively new method of treating LCPD. Using an external fixator, an articulated distraction is conducted on the hip joint. The purpose of the procedure is to restore joint function, make room for the FH, alleviate physical pressure, and maintain synovial fluid circulation. In several studies, improvements in range of motion and joint congruence have been observed [39]. Aguado-Maestro et al. asserted that arthrodiastasis is a minimally invasive treatment for late-onset LCPD and, early application enhances clinical outcomes and maintains cephalic shape. However, complications can occur in as many as eight out of ten patients who undergo the procedure [40].
Rotational open-wedge osteotomy
Rotational open-wedge osteotomy is performed to correct the FH deformity and enhance the alignment of the hip joint. The surgical process entails making a carefully controlled incision in the femur and rotating the FH to achieve a more favorable position within the hip socket.
The aims of rotational open-wedge osteotomy in LCPD encompass the following items: 1) Improving the position of the FH within the hip socket by rotating it and achieving realignment; 2) Realignment of the FH; 3) Prevention of long-term complications, including the development of hip joint arthritis and degenerative changes. Nakamura discovered that combining rotational open-wedge osteotomy with non-weight-bearing treatment produced superior results in elder children with severe LCPD than in non-weight bearing treatment alone [41].
The salter or innominate osteotomy
The salter or innominate osteotomy surgical method is a form of femoral osteotomy that is frequently used to treat LCPD. This osteotomy’s primary advantage is its influence on FH remodeling during remaining growth. This osteotomy also moves the acetabulum one to one and a half centimeters to the medial side, which results in a reduction in the number of biomechanical compression pressures across the hip joint [42]. Kitakoji et al. asserted that salter innominate osteotomy is the superior treatment for patients with LCPD to reduce residual complications, such as coxa vara, trochanteric prominence, inadequate acetabular coverage and surgical incision [43]. Kacki et al. hypothesized that surgical treatment with salter osteotomy could facilitate spherical FH reconstruction, and patients in Catterall categories III and IV may benefit from this surgical procedure [44]. The salter pelvic osteotomy is an effective surgical treatment for LCPD in children aged 6 to 8 years [45]. salter osteotomy can be used to treat older patients who manifest with lateral pillar stages B, B/C and C [46].
The primary benefit of the salter osteotomy is its ability to ensure adequate coverage of the FH by the acetabulum, which is crucial for maintaining joint stability and preventing further deformity [47]. The procedure restores the normal contour and alignment of the FH, leading to improved long-term outcomes for LCPD patients.
The results of a salter osteotomy are permanent and independent of the length of follow-up. This indicates that the positive effects of the surgery are anticipated to be maintained throughout the patient’s life without the need for further interventions related to the LCPD condition. This operation seeks to restore proper alignment and improve the condition of the FH [1].
Femoral varus osteotomy
Femoral varus osteotomy is a common surgical procedure used to treat LCPD. The primary goal of surgical procedures, such as femoral varus osteotomy is to prevent the deformation of the FH and the subsequent onset of hip osteoarthritis. This procedure involves repositioning the femur to enhance hip joint alignment and correct deformities. It alleviates symptoms, enhances joint function, and encourages healthy joint development.
During the procedure, a controlled incision is made in the femur bone, typically near the hip joint. By meticulously repositioning the bone fragments, the surgeon seeks to center the FH within the acetabulum and correct any flexion and rotational deformities that may be present. The hip joint must have an adequate range of motion, congruency, and the capacity to contain the FH during abduction.
Studies have shown that femoral varus osteotomy provides greater benefits than non-operative interventions for adolescents older than 6 years. This procedure provides for hip joint realignment, joint congruity restoration, reduction of femoroacetabular impingement, correct deformities, and prevent the onset of hip osteoarthritis.
Without any FH deformity and flatness, proximal femoral varus osteotomy produces satisfactory outcomes in children aged 6–10 years, especially with adequate containment in abduction [48]. According to Kim et al., when performing proximal femoral varus osteotomy on hips with early stages of LCPD, it is recommended to achieve 10° to 15° of varus correction. This is because greater varus angulation does not necessarily produce better preservation of the FH following the procedure [49].
Triple innominate osteotomy
Triple innominate osteotomy is an additional containment method for LCPD. In severe instances, salter osteotomy may not provide sufficient acetabular rotation to encompass the FH, leading to iatrogenic hinge abduction. For more severe cases, sophisticated containment techniques, such as triple innominate osteotomy, have been devised due to the limitations of these two procedures. Multiple studies indicate that advanced age and extensive involvement of the FH are risk factors for inferior outcomes. Prior research revealed unsatisfactory surgical outcomes for patients older than 10 years at onset. It is anticipated that triple innominate osteotomy will accomplish greater FH containment than salter osteotomy alone and prevent the leg length disparity induced by femoral varus osteotomy. In all circumstances, this is one of the most effective methods for FH containment. However, excessive coverage may cause pincer impingement. It is not recommended to correct the enter-edge angle further than 44° to prevent pincer impingement. As expected, triple innominate osteotomy appears to be more effective in younger patients with less severe disease, but favorable outcomes are not wholly dependent on the stage of disease in LCPD when triple innominate osteotomy is used for containment [50]. Rosello et al. asserted that triple pelvic osteotomy provided superior radiologic and clinical outcomes in comparison to Chiari osteotomy, particularly in terms of the final Stulberg classification [51].
Triple pelvic osteotomy
The triple pelvic osteotomy surgery is typically used in older adolescents in whom hip containment is difficult to achieve with salter pelvic osteotomy. This procedure is at end-stage disease, when a large, inadequately covered FH remained, necessitating surgical coverage by those who rejected other types of osteotomies. Bernese triple pelvic osteotomy (a modified anterior Smith-Peterson technique) is an alternative treatment for LCPD in skeletally underdeveloped older children. In addition to providing a substantial acetabular correction, it also accomplishes biomechanical stability [52]. Jishuitan (another simplified approach for triple pelvic osteotomy) is a safe, more effective method that can achieve satisfactory correction [53].
Lateral shelf acetabuloplasty
When hip subluxation is reduced with shelf acetabuloplasty, the FH is better able to undergo biological remodeling inside the acetabulum [54]. In severe cases of LCPD in patients older than 8 years, the surgical results of combined shelf acetabuloplasty with femoral varus osteotomy are on par with those of other cutting-edge techniques [55].
The primary goals of lateral shelf acetabuloplasty are as follows: 1) Enhance coverage of the FH by creating a shelf on the outer side of the acetabulum, thereby increasing the supportive surface area available; 2) Improve joint stability by reshaping the acetabulum, promoting better alignment and reducing the chances of hip subluxation or dislocation; and 3) Facilitate the remodeling process during the healing of LCPD disease by providing a more favorable environment through the reshaping of the acetabulum [56].
Total hip arthroplasty
The incidence of total hip arthroplasty among LCPD patients was relatively low. According to Zhi et al. the incidence of THA increased with age at disease onset and duration of follow-up, but the Stulberg classification was not explicitly associated with the incidence of total hip arthroplasty [57]. According to the findings of Froberg and colleagues, people who have LCPD have a higher risk of developing radiographic hip osteoarthritis than a control group does. Those who are classified as Class III/IV/V appear to be at a greater risk of developing total hip arthroplasty and osteoarthritis compared to individuals who have FH classified as Class I/II [58].
Conclusion
The outcomes of this review underscore the significance of enhancing the knowledge of physicians and healthcare professionals regarding LCPD. This condition arises from a combination of genetic and environmental factors impacting the hip joints of children, leading to deformities and morbidity. While conventional radiography serves as the primary imaging modality for this disease, MRI contributes to enhanced diagnostic accuracy, particularly in the initial phases, thereby optimizing patient prognoses. Treatment strategies for affected individuals encompass conservative and surgical interventions aimed at preserving joint functionality, averting deformities and mitigating long-term complications.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to preparing all parts of the research.
Conflicts of interest
The author declared no conflict of interest.
References
Legg-calve-perthes disease (LCPD) is an idiopathic condition affecting children’s hip joints. The characteristic feature of LCPD is the cutting off of the blood supply to the femoral head epiphysis, which ultimately results in the death of bone tissue (aseptic osteonecrosis) and the deformity of the femoral head (FH). LCPD typically manifests clinically between the ages of 2 and 15 years, with males afflicted five times more often than females [1].
Initial symptoms of LCPD may be subtle, such as mild discomfort or limping, which may intensify with physical activity (primarily in the anterior hip and midthigh) [1]. As the disease progresses, limited hip range of motion, atrophy of the thigh muscles, decreased internal rotation, hip abduction, and Trendelenburg gait can occur [2, 3].
The pathogenesis of LCPD is characterized by various stages as follows: 1) Necrosis phase, 2) Fragmentation and 3) Re-ossification and remodeling. In the early phases of LCPD, x-rays are frequently used to determine the disease’s stage, FH position and FH involvement. However, in cases where radiographic findings are not diagnostic, magnetic resonance imaging (MRI) is recommended [3, 4].
The therapy of LCPD is determined in part by several parameters, including the age and disability of the patient at the time of diagnosis, the lateral pillar classification and the stage of the disease. Conservative treatments, such as rest, physical therapy, and braces or casts can be combined with surgical procedures, such as osteotomy to preserve the hip joint. Treatment maintains normal hip joint function, prevents deformity, and decreases the likelihood of long-term complications [5, 6].
Despite advances in knowledge and treatment options, managing LCPD remains complicated. These may result from the disease’s varied manifestations, the inability to predict outcomes precisely and the lack of consensus regarding the most effective treatment strategies. Hence, this paper is based on recent data in the literature to review the etiology, imaging, and treatment of LCPD.
Clinical manifestations and physical examination
Patients in the early stages of the disease experience mild and nonspecific symptoms, such as pain or limping [3, 7]. Due to stimulation of the sensory branch of the obturator nerve, the patient often localizes the pain in the thigh or knee. The patient’s examination revealed a reduction in internal rotation and abduction of the hip, an occasional antalgic gait, and a reduction in the range of motion of the hip joint, all of which are caused by muscle spasm, joint effusion and FH collapse [2, 3, 8]. Significant FH collapse can result in a leg length discrepancy and Trendelenburg gait in patients. In addition, shortening of the ipsilateral gluteal musculature can exacerbate the patient’s limping [1, 2, 8].
Etiology
Despite numerous hypotheses, the exact cause of LCPD is still uncertain. A disruption in the blood supply to the developing FH is the hallmark of LCPD resulting in ischemic necrosis and subsequent structural alterations in the developing FH. Genetic and environmental factors contribute to the condition’s development. Genetic factors may influence susceptibility to interruption of blood supply to the FH. LCPD has been linked to mutations of the COL2A1 gene on the 12q13 chromosome and the COL1A1 gene, which is associated with osteogenesis imperfecta [9, 10]. In contrast, environmental factors, such as repetitive trauma or mechanical excess, increased blood viscosity [11] and coagulation disorders may initiate the disease. In addition, passive smoking exposure, and high-dose and long-term glucocorticoids have been linked to an increased risk of LCPD [12, 13]. Furthermore, sickle-cell anemia, thalassemia, trichorhinophalangeal syndrome, and hemophilia can be a risk factor for LCPD [6]. Sickle-cell anemia patients with osteonecrosis had substantially higher hospitalization rates (mostly due to vaso-occlusive crisis) compared to subjects without osteonecrosis, according to Adekile [14]. Meanwhile, according to Leandro et al. polymorphisms in the BMP6, Klotho and ANXA2 genes may be related to LCPD in sickle-cell anemia patients [15].
Pathogenesis
The progression of LCPD consists of various stages: 1) In the initial phase, the FH’s blood supply (the subchondral cortical bone mostly) is cut off, resulting in bone and cartilage death (necrosis phase); 2) In the revascularization stage, new blood vessels form, and new bone gradually supplants necrotic bone (fragmentation); 3) During this phase, osteoblasts reactivate, resulting in remodeling, which might be malformed or asymmetrical, leading to adult-onset degenerative osteoarthritis (re-ossification and remodeling).
The pathological progression of LCPD affects multiple parts of the FH, including the articular cartilage, epiphysis, physis, and metaphysis, leading to the destruction of trabecular bone microarchitecture and necrosis in the bone marrow. The disruption of blood supply can result in growth cessation of the epiphysis, necrosis of the deep layer of articular cartilage, an increase in vascular endothelial growth factor (VEGF), a rise in vascularization, and deformity. In addition, serum VEGF may serve as a useful disease follow-up marker [16, 17].
Following revascularization, the primary reparative response is osteoclastic bone resorption which causes the normal tissue of the FH to be replaced by fibrovascular tissue. The inability of the growth plate that surrounds the osseous epiphysis to continue forming is another factor that leads to the degeneration of the FH. VEGF and bone morphogenetic protein (BMP)-2 are involved in this process due to their respective roles in angiogenesis and endochondral ossification [18].
The blood supply to the FH depends on several number of anastomoses that contribute to its vascularization. Several anastomoses contribute to the FH’s blood supply: 1) The anastomosis between the medial and lateral circumflex branches of the profunda femoris is the most essential; 2) Cruciate anastomosis of the inferior gluteal artery to the medial circumflex femoral artery; 3) An anastomosis between the superior gluteal artery and the medial/lateral circumflex femoral arteries at the trochanter. In addition, the foveal artery, which passes through the ligamentum teres, supplies blood to the FH, which is especially significant in children. Due to insufficient collateral circulation in the FH, ischemia and necrosis may develop, if the blood supply is cut off.
Neutrophils and macrophages are the most prevalent inflammatory cells involved in the pathogenesis of osteonecrosis, resulting in macroscopic subchondral collapse and subsequent joint deterioration. Disruption in blood supply causes ischemia of the covering cartilage and flattening of the head articulates with the acetabulum, which causes secondary osteoarthritis [19-21].
Classification
LCPD is classified into the following stages based on symptoms, as well as plain radiographs, bone scans and MRI findings (Table 1) [22].

Imaging
The majority of LCPD diagnosis and staging is performed using conventional radiography as the imaging modality. The epiphyseal involvement and the height of the lateral pillar are associated with FH deformity and can be used to assist in classification, prognostic evaluation, and surveillance of disease progression on conventional radiographs [6, 23]. If the FH involvement is not evident on standard x-rays, lateral x-rays may be necessary to detect alterations in the anterolateral aspect of the FH [4].
In cases where hip radiographs are normal or inconclusive, the MRI is essential for diagnosing LCPD (Figure 1). For effective joint preservation treatments to be initiated, early diagnosis is essential. Hence, using non-enhanced and contrast-enhanced imaging sequences, MRI can confidently diagnose LCPD and facilitate the initiation of appropriate therapies. Contrast-enhanced MRI using gadolinium has proved to be superior to radiography and can detect FH perfusion early at an early stage and aid in early prognostication [3]. Hypoperfusion is one of the early alterations visible in LCPD and can be demonstrated on T1-weighted fat-suppressed imaging performed 2–5 min after contrast administration [24]. Furthermore, bone scintigraphy and diffusion-weighted MRI can be employed to evaluate the severity of perfusion abnormalities in LCPD may be a significant prognostic factor [23]. Dynamic contrast-enhanced subtraction MRI has demonstrated high sensitivity, excellent correlation with bone scintigraphy, and the ability to provide specific information about blood flow to the FH, thereby facilitating the early detection of ischemia [24].
MRI is also beneficial for accurate disease early staging, assessing associated complications, and distinguishing LCPD from other epiphyseal lesions [25]. It allows for the differentiation of LCPD from multiple epiphyseal dysplasia, spondyloepiphyseal dysplasia and Meyer dysplasia. In the early phases of LCPD, contrast-enhanced MRI better defines the extent of FH necrosis than non-contrast MRI. Radiographic measurements of FH deformity correlate strongly with perfusion measurements obtained from contrast-enhanced subtraction MRI, specifically the MRI perfusion index, after at least two years of follow-up [26].
Treatment
The objective of LCPD treatment is to achieve a spherical FH contour with excellent congruency during the disease process [27]. Depending on the onset age, disease stage, and severity of FH involvement LCPD is treated differently. Current LCPD treatment emphasizes protecting the FH mechanically to prevent future hip deformity and degeneration [1]. The following treatment options and factors can be considered.
Conservative treatment
LCPD can be treated conservatively with non-weight bearing casts, abduction casts, orthotics, and rehabilitation, and are better suited to younger patients (Table 2).

The goal is to decrease deformity, promote revascularization and resorption of the infarcted epiphysis, and increase the range of motion. However, the effectiveness of conservative treatment has been contested, and results can vary. Those who are younger than 6 years at the time of diagnosis or who have necrosis of less than 50% of the FH should be treated symptomatically, according to Wiig et al. [28].
Observation
The observation method may be advised in children aged 2 to 3 years and permits the FH to recuperate naturally and endure remodeling without intervention [28].
Non-weight bearing
In the treatment of LCPD, the non-weight bearing has demonstrated contradictory results. Several studies indicate that early weight-bearing restrictions can reduce FH deformity and facilitate revascularization and healing. It could be considered a treatment option for patients who are unable or unwilling to undergo surgery. According to Peck et al., prolonged non-weight-bearing should be regarded as a treatment option for patients unwilling to undergo operative treatment in early-stage LCPD with significant hypoperfusion [29]. Protective weight bearing improved collapse ratings. Protective weight-bearing helps most pre-collapse and post-collapse patients delay total hip arthroplasty or hip-preserving surgery [30].
Bisphosphonate therapy
Due to the lack of consistent patient groups and pharmacological protocols, the clinical evidence for bisphosphonate therapy to reduce FH deformity in LCPD is inconclusive. Studies suggest that bisphosphonate therapy (systemic and local) can prevent deformity of the infarcted FH in LCPD and preserve FH morphology. This strategy targets the pathological processes that contribute to FH collapse, particularly the imbalance of osteoclast resorption and the delay in new bone formation [31]. Some authors reported that bisphosphonate can reduce discomfort, improve mobility, and prevent articular collapse [32].
FH deformation can be prevented or greatly reduced when BMP2 is administered locally [33]. In a pig model of ischemic osteonecrosis, administration of BMP-2 decreased bone resorption and increased new bone formation in an animal study [34]. According to Vandermeer et al. in an immature animal model of ischemic osteonecrosis, the combination of ibandronate and BMP-2 reduced FH deformity and stimulated bone formation [35].
Bisphosphonate dose may depend on therapy timing. Preventing deformity may require starting therapy early. Anabolic stimulation is needed to facilitate healing and new bone formation, even if bisphosphonates and other anti-resorptive medicines can preserve FH’s spherical shape. Weight loss and activity modifications may be required during recovery due to the necrotic FH’s impaired mechanical characteristics [36].
Surgical treatment
In certain cases of LCPD, surgery may be considered a treatment option (Table 3).

Typically, the decision to conduct surgery is influenced by age, the extent of FH involvement (Catterall classification), and prognosis. In patients with 6 years of age or higher with FH necrosis exceeding 50%, surgical intervention should be considered [28]. In surgical procedures, femoral osteotomy, salter osteotomy, lateral shelf acetabuloplasty, and so on may be performed. Surgical treatment in patients older than 6 years has outstanding outcomes for Herring B and B/C hips and poor outcomes for Herring C hips, with a modest advantage for patients aged 6 to 8 years [37].
A-frame orthoses
According to Rich et al. restoring and sustaining a hip range of motion in conjunction with the use of an A-frame orthosis led to a high proportion of spherically congruent hips in patients of all ages and disease severity. This method has proven effective for preserving joint function and improving hip congruency [38].
Arthrodiastasis
Arthodiastasis is a relatively new method of treating LCPD. Using an external fixator, an articulated distraction is conducted on the hip joint. The purpose of the procedure is to restore joint function, make room for the FH, alleviate physical pressure, and maintain synovial fluid circulation. In several studies, improvements in range of motion and joint congruence have been observed [39]. Aguado-Maestro et al. asserted that arthrodiastasis is a minimally invasive treatment for late-onset LCPD and, early application enhances clinical outcomes and maintains cephalic shape. However, complications can occur in as many as eight out of ten patients who undergo the procedure [40].
Rotational open-wedge osteotomy
Rotational open-wedge osteotomy is performed to correct the FH deformity and enhance the alignment of the hip joint. The surgical process entails making a carefully controlled incision in the femur and rotating the FH to achieve a more favorable position within the hip socket.
The aims of rotational open-wedge osteotomy in LCPD encompass the following items: 1) Improving the position of the FH within the hip socket by rotating it and achieving realignment; 2) Realignment of the FH; 3) Prevention of long-term complications, including the development of hip joint arthritis and degenerative changes. Nakamura discovered that combining rotational open-wedge osteotomy with non-weight-bearing treatment produced superior results in elder children with severe LCPD than in non-weight bearing treatment alone [41].
The salter or innominate osteotomy
The salter or innominate osteotomy surgical method is a form of femoral osteotomy that is frequently used to treat LCPD. This osteotomy’s primary advantage is its influence on FH remodeling during remaining growth. This osteotomy also moves the acetabulum one to one and a half centimeters to the medial side, which results in a reduction in the number of biomechanical compression pressures across the hip joint [42]. Kitakoji et al. asserted that salter innominate osteotomy is the superior treatment for patients with LCPD to reduce residual complications, such as coxa vara, trochanteric prominence, inadequate acetabular coverage and surgical incision [43]. Kacki et al. hypothesized that surgical treatment with salter osteotomy could facilitate spherical FH reconstruction, and patients in Catterall categories III and IV may benefit from this surgical procedure [44]. The salter pelvic osteotomy is an effective surgical treatment for LCPD in children aged 6 to 8 years [45]. salter osteotomy can be used to treat older patients who manifest with lateral pillar stages B, B/C and C [46].
The primary benefit of the salter osteotomy is its ability to ensure adequate coverage of the FH by the acetabulum, which is crucial for maintaining joint stability and preventing further deformity [47]. The procedure restores the normal contour and alignment of the FH, leading to improved long-term outcomes for LCPD patients.
The results of a salter osteotomy are permanent and independent of the length of follow-up. This indicates that the positive effects of the surgery are anticipated to be maintained throughout the patient’s life without the need for further interventions related to the LCPD condition. This operation seeks to restore proper alignment and improve the condition of the FH [1].
Femoral varus osteotomy
Femoral varus osteotomy is a common surgical procedure used to treat LCPD. The primary goal of surgical procedures, such as femoral varus osteotomy is to prevent the deformation of the FH and the subsequent onset of hip osteoarthritis. This procedure involves repositioning the femur to enhance hip joint alignment and correct deformities. It alleviates symptoms, enhances joint function, and encourages healthy joint development.
During the procedure, a controlled incision is made in the femur bone, typically near the hip joint. By meticulously repositioning the bone fragments, the surgeon seeks to center the FH within the acetabulum and correct any flexion and rotational deformities that may be present. The hip joint must have an adequate range of motion, congruency, and the capacity to contain the FH during abduction.
Studies have shown that femoral varus osteotomy provides greater benefits than non-operative interventions for adolescents older than 6 years. This procedure provides for hip joint realignment, joint congruity restoration, reduction of femoroacetabular impingement, correct deformities, and prevent the onset of hip osteoarthritis.
Without any FH deformity and flatness, proximal femoral varus osteotomy produces satisfactory outcomes in children aged 6–10 years, especially with adequate containment in abduction [48]. According to Kim et al., when performing proximal femoral varus osteotomy on hips with early stages of LCPD, it is recommended to achieve 10° to 15° of varus correction. This is because greater varus angulation does not necessarily produce better preservation of the FH following the procedure [49].
Triple innominate osteotomy
Triple innominate osteotomy is an additional containment method for LCPD. In severe instances, salter osteotomy may not provide sufficient acetabular rotation to encompass the FH, leading to iatrogenic hinge abduction. For more severe cases, sophisticated containment techniques, such as triple innominate osteotomy, have been devised due to the limitations of these two procedures. Multiple studies indicate that advanced age and extensive involvement of the FH are risk factors for inferior outcomes. Prior research revealed unsatisfactory surgical outcomes for patients older than 10 years at onset. It is anticipated that triple innominate osteotomy will accomplish greater FH containment than salter osteotomy alone and prevent the leg length disparity induced by femoral varus osteotomy. In all circumstances, this is one of the most effective methods for FH containment. However, excessive coverage may cause pincer impingement. It is not recommended to correct the enter-edge angle further than 44° to prevent pincer impingement. As expected, triple innominate osteotomy appears to be more effective in younger patients with less severe disease, but favorable outcomes are not wholly dependent on the stage of disease in LCPD when triple innominate osteotomy is used for containment [50]. Rosello et al. asserted that triple pelvic osteotomy provided superior radiologic and clinical outcomes in comparison to Chiari osteotomy, particularly in terms of the final Stulberg classification [51].
Triple pelvic osteotomy
The triple pelvic osteotomy surgery is typically used in older adolescents in whom hip containment is difficult to achieve with salter pelvic osteotomy. This procedure is at end-stage disease, when a large, inadequately covered FH remained, necessitating surgical coverage by those who rejected other types of osteotomies. Bernese triple pelvic osteotomy (a modified anterior Smith-Peterson technique) is an alternative treatment for LCPD in skeletally underdeveloped older children. In addition to providing a substantial acetabular correction, it also accomplishes biomechanical stability [52]. Jishuitan (another simplified approach for triple pelvic osteotomy) is a safe, more effective method that can achieve satisfactory correction [53].
Lateral shelf acetabuloplasty
When hip subluxation is reduced with shelf acetabuloplasty, the FH is better able to undergo biological remodeling inside the acetabulum [54]. In severe cases of LCPD in patients older than 8 years, the surgical results of combined shelf acetabuloplasty with femoral varus osteotomy are on par with those of other cutting-edge techniques [55].
The primary goals of lateral shelf acetabuloplasty are as follows: 1) Enhance coverage of the FH by creating a shelf on the outer side of the acetabulum, thereby increasing the supportive surface area available; 2) Improve joint stability by reshaping the acetabulum, promoting better alignment and reducing the chances of hip subluxation or dislocation; and 3) Facilitate the remodeling process during the healing of LCPD disease by providing a more favorable environment through the reshaping of the acetabulum [56].
Total hip arthroplasty
The incidence of total hip arthroplasty among LCPD patients was relatively low. According to Zhi et al. the incidence of THA increased with age at disease onset and duration of follow-up, but the Stulberg classification was not explicitly associated with the incidence of total hip arthroplasty [57]. According to the findings of Froberg and colleagues, people who have LCPD have a higher risk of developing radiographic hip osteoarthritis than a control group does. Those who are classified as Class III/IV/V appear to be at a greater risk of developing total hip arthroplasty and osteoarthritis compared to individuals who have FH classified as Class I/II [58].
Conclusion
The outcomes of this review underscore the significance of enhancing the knowledge of physicians and healthcare professionals regarding LCPD. This condition arises from a combination of genetic and environmental factors impacting the hip joints of children, leading to deformities and morbidity. While conventional radiography serves as the primary imaging modality for this disease, MRI contributes to enhanced diagnostic accuracy, particularly in the initial phases, thereby optimizing patient prognoses. Treatment strategies for affected individuals encompass conservative and surgical interventions aimed at preserving joint functionality, averting deformities and mitigating long-term complications.
Ethical Considerations
Compliance with ethical guidelines
There were no ethical considerations to be considered in this research.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
All authors contributed equally to preparing all parts of the research.
Conflicts of interest
The author declared no conflict of interest.
References
- Maleki A, Qoreishy SM, Bahrami MN. Surgical treatments for Legg-Calvé-Perthes disease: Comprehensive review. Interact J Med Res. 2021; 10(2):e27075. [DOI:10.2196/27075] [PMID] [PMCID]
- Mills S, Burroughs KE. Legg calve perthes disease. 2023. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PMID]
- Mazloumi SM, Ebrahimzadeh MH, Kachooei AR. Evolution in diagnosis and treatment of Legg-Calve-Perthes disease. Arch Bone Jt Surg. 2014; 2(2):86-92. [PMID]
- Westhoff B, Zilkens C, Reith A, Jelinek E, Martiny F, Willers R, et al. Correlation of functional outcome and X-ray findings after Perthes disease. Int Orthop. 2011; 35(12):1833-7. [DOI:10.1007/s00264-011-1254-2] [PMID] [PMCID]
- Tripathy SK, Goyal T, Sen RK. Management of femoral head osteonecrosis: Current concepts. Indian J Orthop. 2015; 49(1):28-45. [DOI:10.4103/0019-5413.143911] [PMID] [PMCID]
- Nelitz M, Lippacher S, Krauspe R, Reichel H. Perthes disease: Current principles of diagnosis and treatment. Dtsch Arztebl Int. 2009; 106(31-32):517-23. [DOI:10.3238/arztebl.2009.0517] [PMID] [PMCID]
- Laine JC, Novotny SA, Tis JE, Sankar WN, Martin BD, Kelly DM, et al. Demographics and clinical presentation of early-stage Legg-Calvé-Perthes disease: A prospective, multicenter, international study. J Am Acad Orthop Surg. 2021; 29(2):e85-91. [DOI:10.5435/JAAOS-D-19-00379] [PMID]
- Divi SN, Bielski RJ. Legg-calvé-perthes disease. Pediatr Ann. 2016; 45(4):e144-9. [DOI:10.3928/00904481-20160310-03] [PMID]
- Hong P, Zhao X, Liu R, Rai S, Song Y, Xu R, et al. Perthes disease in a child with osteogenesis imperfecta from a rare genetic variant: A case report. Front Genet. 2022; 13:920950. [DOI:10.3389/fgene.2022.920950] [PMID] [PMCID]
- Al-Omran AK, Sadat-Ali M. Legg-Calve-Perthes disease in two generations of male family members: A case report. J Orthop Surg (Hong Kong). 2013; 21(2):258-61. [DOI:10.1177/230949901302100230] [PMID]
- Mukisi-Mukaza M, Saint Martin C, Etienne-Julan M, Donkerwolcke M, Burny ME, Burny F. Risk factors and impact of orthopaedic monitoring on the outcome of avascular necrosis of the femoral head in adults with sickle cell disease: 215 patients case study with control group. Orthop Traumatol Surg Res. 2011; 97(8):814-20. [DOI:10.1016/j.otsr.2011.09.011] [PMID]
- Gao H, Huang Z, Jia Z, Ye H, Fu F, Song M, et al. Influence of passive smoking on the onset of Legg-Calvè-Perthes disease: A systematic review and meta-analysis. J Pediatr Orthop B. 2020; 29(6):556-66. [DOI:10.1097/BPB.0000000000000725] [PMID]
- Chen G, Chen T, Zhang P, Zhang Z, Huang R, Chen T, et al. Can large doses of glucocorticoids lead to Perthes? A case report and review of the literature. BMC Pediatr. 2021; 21(1):339. [DOI:10.1186/s12887-021-02755-4] [PMID] [PMCID]
- Adekile A, Gupta R, Yacoub F, Sinan T, Al-Bloushi M, Haider M. Avascular necrosis of the hip in children with sickle cell disease and high Hb F: Magnetic resonance imaging findings and influence of α-thalassemia trait. Acta Haematol. 2001; 105(1):27-31. [DOI:10.1159/000046529] [PMID]
- Leandro MP, Almeida ND, Hocevar LS, Sá CKCd, Souza AJd, Matos MA. Polymorphisms and avascular necrosis in patients with sickle cell disease-A systematic review. Rev Paul Pediatr. 2022; 40. [DOI:10.1590/1984-0462/2022/40/2021013]
- Tiwari V, Poudel R, Khan S, Mehra S, Chauhan S, Raje A. Is VEGF under-expressed in Indian children with Perthes disease? Musculoskelet Surg. 2018; 102:81-5. [DOI:10.1007/s12306-017-0502-z]
- Sezgin H, Gulman B, Cirakli A, Bedir A, Usta D, Coskun S, et al. Effects of circulating endothelial progenitor cells, serum vascular endothelial growth factor and hypogammaglobulinemia in Perthes disease. Acta Orthop Traumatol Turc. 2014; 48(6):628-34. [DOI:10.3944/AOTT.2014.13.0084] [PMID]
- Wu H, Yin G, Pu X, Wang J, Liao X, Huang Z. Inhibitory effects of combined bone morphogenetic protein 2, vascular endothelial growth factor, and basic fibroblast growth factor on osteoclast differentiation and activity. Tissue Eng Part A. 2021; 27(21-22):1387-98. [DOI:10.1089/ten.tea.2020.0325] [PMID]
- de Camargo FP, de Godoy Jr RM, Tovo R. Angiography in perthes’ disease. Clin Orthop Relat Res. 1984; 191:216-20. [DOI:10.1097/00003086-198412000-00028]
- Moreno Grangeiro P, Rodrigues JC, de Angeli LRA, Leão Filho H, Montenegro NB, Guarniero R, et al. Feasibility of magnetic resonance angiography in patients with Legg-Calvé-Perthes disease. J Pediatr Orthop. 2021; 41(9):e774-9. [DOI:10.1097/BPO.0000000000001910] [PMID]
- Adapala NS, Yamaguchi R, Phipps M, Aruwajoye O, Kim HK. Necrotic bone stimulates proinflammatory responses in macrophages through the activation of toll-like receptor 4. Am J Pathol. 2016; 186(11):2987-99. [DOI:10.1016/j.ajpath.2016.06.024] [PMID]
- Sultan AA, Mohamed N, Samuel LT, Chughtai M, Sodhi N, Krebs VE, et al. Classification systems of hip osteonecrosis: An updated review. Int Orthop. 2019; 43(5):1089-95. [DOI:10.1007/s00264-018-4018-4] [PMID]
- Yoo WJ, Kim YJ, Menezes NM, Cheon JE, Jaramillo D. Diffusion-weighted MRI reveals epiphyseal and metaphyseal abnormalities in Legg-Calvé-Perthes disease: A pilot study. Clin Orthop Relat Res. 2011; 469(10):2881-8. [DOI:10.1007/s11999-011-1931-x] [PMID] [PMCID]
- Jamil K, Walker T, Onikul E, Munns CF, Little DG. A comparison of subtraction MRI with the standard contrast-enhanced imaging in Perthes’ disease. J Child Orthop. 2019; 13(1):82-8. [DOI:10.1302/1863-2548.13.180136] [PMID] [PMCID]
- Banu NR, Kamal MZ, Uddin MS, Ruly RA, Ferdaus AM, Islam FA, et al. Legg-Calve-Perthes Disease: Correlation between computed radiography and magnetic resonance imaging. Mymensingh Med J. 2020; 29(1):55-9. [PMID]
- Dimeglio A, Canavese F. Imaging in legg-calve-perthes disease. Orthop Clin North Am. 2011; 42(3):297-302, v. [DOI:10.1016/j.ocl.2011.04.003] [PMID]
- Atsumi T, Yoshiwara S. Rotational open wedge osteotomy in a patient aged older than 7 years with Perthes’ disease-a preliminary report. Arch Orthop Trauma Surg. 2002; 122(6):346-9. [DOI:10.1007/s00402-002-0408-5] [PMID]
- Wiig O, Svenningsen S, Terjesen T. [Legg-Calvé-Perthes disease (Norwegian)]. Tidsskr Nor Laegeforen. 2011; 131(9-10):946-9. [DOI:10.4045/tidsskr.10.0456] [PMID]
- Peck JB, Greenhill DA, Morris WZ, Do DH, McGuire MF, Kim HKW. Prolonged non-weightbearing treatment decreases femoral head deformity compared to symptomatic treatment in the initial stage of Legg-Calvé-Perthes disease. J Pediatr Orthop B. 2022; 31(3):209-15. [DOI:10.1097/BPB.0000000000000873] [PMID]
- Chen WH, Guo WX, Li JX, Wei QS, Li ZQ, He W. Application of protective weight-bearing in osteonecrosis of the femoral head: A systematic review and meta-analysis of randomized controlled trials and observational studies. Front Surg. 2022; 9:1000073. [DOI:10.3389/fsurg.2022.1000073] [PMID] [PMCID]
- Young ML, Little DG, Kim HK. Evidence for using bisphosphonate to treat Legg-Calvé-Perthes disease. Clin Orthop Relat Res. 2012; 470(9):2462-75. [DOI:10.1007/s11999-011-2240-0] [PMID] [PMCID]
- Cardozo JB, Andrade DM, Santiago MB. The use of bisphosphonate in the treatment of avascular necrosis: A systematic review. Clin Rheumatol. 2008; 27(6):685-8. [DOI:10.1007/s10067-008-0861-9] [PMID]
- Kim H, Ma C, Park M, Monte F, Gokani V, Aruwajoye O, Ren Y, et al. Local administration of bone morphogenetic protein-2 using a hydrogel carrier for robust bone regeneration in a large animal model of legg-calvé-perthes disease. 2023 [Unpublished]. [DOI:10.21203/rs.3.rs-2465423/v1]
- Kim HK, Aruwajoye O, Du J, Kamiya N. Local administration of bone morphogenetic protein-2 and bisphosphonate during non-weight-bearing treatment of ischemic osteonecrosis of the femoral head: An experimental investigation in immature pigs. J Bone Joint Surg Am. 2014; 96(18):1515-24. [DOI:10.2106/JBJS.M.01361] [PMID]
- Vandermeer JS, Kamiya N, Aya-ay J, Garces A, Browne R, Kim HK. Local administration of ibandronate and bone morphogenetic protein-2 after ischemic osteonecrosis of the immature femoral head: A combined therapy that stimulates bone formation and decreases femoral head deformity. J Bone Joint Surg Am. 2011; 93(10):905-13. [DOI:10.2106/JBJS.J.00716] [PMID]
- Little DG, Kim HK. Potential for bisphosphonate treatment in Legg-Calve-Perthes disease. J Pediatr Orthop. 2011; 31(2 Suppl):S182-8. [DOI:10.1097/BPO.0b013e318223b541] [PMID]
- Caldaci A, Testa G, Dell'Agli E, Sapienza M, Vescio A, Lucenti L, et al. Mid-long-term outcomes of surgical treatment of legg-calvè-perthes disease: A systematic review. Children. 2022; 9(8):1121. [DOI:10.3390/children9081121] [PMID] [PMCID]
- Rich MM, Schoenecker PL. Management of Legg-Calve-Perthes disease using an A-frame orthosis and hip range of motion: A 25-year experience. J Pediatr Orthop. 2013; 33(2):112-9. [DOI:10.1097/BPO.0b013e318281ab44] [PMID]
- Ibrahim YH, Kersh MAAL, Fahmy H. Arthrodiastasis in the management of Perthes disease: A systematic review. J Pediatr Orthop B. 2020; 29(6):550-5. [DOI:10.1097/BPB.0000000000000690] [PMID]
- Aguado-Maestro I, Abril JC, Diaz AB, Alonso MG. Hip arthrodiastasis in legg-calvé-perthes disease. Rev Esp Cir Ortop Traumatol. 2016; 60(4):243-50. [DOI:10.1016/j.recot.2016.03.002]
- Nakamura N, Inaba Y, Machida J, Saito T. Rotational open-wedge osteotomy improves treatment outcomes for patients older than eight years with Legg-Calve-Perthes disease in the modified lateral pillar B/C border or C group. Int Orthop. 2015; 39(7):1359-64. [DOI:10.1007/s00264-015-2729-3] [PMID]
- Thompson GH. Salter osteotomy in Legg-Calve-Perthes disease. J Pediatr Orthop. 2011; 31(2 Suppl):S192-7. [DOI:10.1097/BPO.0b013e318223b59d] [PMID]
- Kitakoji T, Hattori T, Kitoh H, Katoh M, Ishiguro N. Which is a better method for Perthes’ disease: Femoral varus or Salter osteotomy? Clin Orthop Relat Res. 2005; (430):163-70. [DOI:10.1097/01.blo.0000137549.60694.63] [PMID]
- Kacki W, Zarzycka M, Zarzycki D, Kaliciński M, Sienkiel W, Jasiewicz B, et al. Comparison of radiological results of conservative and operative treatment by Salter osteotomy in severe cases of Perthes’ disease. Ortop Traumatol Rehabil. 2004; 6(6):740-7. [PMID]
- Bulut M, Demirtş A, Uçar BY, Azboy I, Alemdar C, Karakurt L. Salter pelvic osteotomy in the treatment of Legg-Calve-Perthes disease: The medium-term results. Acta Orthop Belg. 2014; 80(1):56-62. [PMID]
- Yavuz U, Demir B, Yildirim T, Beng K, Karakas ES. Salter innominate osteotomy in the treatment of late presentation Perthes disease. Hip Int. 2014; 24(1):39-43. [DOI:10.5301/hipint.5000086] [PMID]
- Venkatadass K, Durga Prasad V, Al Ahmadi NMM, Rajasekaran S. Pelvic osteotomies in hip dysplasia: Why, when and how? EFORT Open Rev. 2022; 7(2):153-63. [DOI:10.1530/EOR-21-0066] [PMID] [PMCID]
- Elzohairy MM. Short follow-up evaluation of proximal femoral varus osteotomy for treatment of Legg-Calvé-Perthes disease. J Orthop Traumatol. 2016; 17(4):345-51. [DOI:10.1007/s10195-016-0412-0] [PMID] [PMCID]
- Kim HKW, da Cunha AM, Browne R, Kim HT, Herring JA. How much varus is optimal with proximal femoral osteotomy to preserve the femoral head in legg-calvé-perthes disease? J Bone Joint Surg Am. 2011; 93(4):341-7. [DOI:10.2106/JBJS.J.00830] [PMID]
- Stepanovich M, Upasani VV, Bomar JD, Wenger DR. Advanced containment with triple innominate osteotomy in Legg-Calve-Perthes disease: A viable option even in severe cases. J Pediatr Orthop. 2017; 37(8):563-9. [DOI:10.1097/BPO.0000000000000714] [PMID]
- Rosello O, Solla F, Oborocianu I, Chau E, ElHayek T, Clement J-L, et al. Advanced containment methods for Legg-Calvé-Perthes disease: Triple pelvic osteotomy versus Chiari osteotomy. Hip Int. 2018; 28(3):297-301. [DOI:10.5301/hipint.5000569] [PMID]
- Li Y, Xu H, Slongo T, Zhou Q, Liu Y, Chen W, et al. Bernese-type triple pelvic osteotomy through a single incision in children over five years: A retrospective study of twenty eight cases. Int Orthop. 2018; 42(12):2961-8. [DOI:10.1007/s00264-018-3946-3] [PMID]
- Lyu X, Yang Z, Wang Y, Zhang T, Lu M, Bian Z. Novel minimally-invasive triple pelvic osteotomy: JiShuiTan Minimally-Invasive Approach. J Pediatr Orthop. 2022; 42(2):e154-62. [DOI:10.1097/BPO.0000000000002019] [PMID]
- Li WC, Xu RJ. Lateral shelf acetabuloplasty for severe Legg-Calvé-Perthes disease in patients older than 8 years: A mean eleven-year follow-up. Medicine (Baltimore). 2016; 95(45):e5272. [DOI:10.1097/MD.0000000000005272] [PMID] [PMCID]
- Lim KS, Shim JS. Outcomes of combined shelf acetabuloplasty with femoral varus osteotomy in severe Legg-Calve-Perthes (LCP) Disease: Advanced containment method for severe LCP Disease. Clin Orthop Surg. 2015; 7(4):497-504.[DOI:10.4055/cios.2015.7.4.497] [PMID] [PMCID]
- Bicanic G, Barbaric K, Bohacek I, Aljinovic A, Delimar D. Current concept in dysplastic hip arthroplasty: Techniques for acetabular and femoral reconstruction. World J Orthop. 2014; 5(4):412-24. [DOI:10.5312/wjo.v5.i4.412] [PMID] [PMCID]
- Zhi X, Wu H, Xiang C, Wang J, Tan Y, Zeng C, et al. Incidence of total hip arthroplasty in patients with Legg-Calve-Perthes disease after conservative or surgical treatment: A meta-analysis. Int Orthop. 2023; 47(6):1449-64. [DOI:10.1007/s00264-023-05770-5] [PMID]
- Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in perthes disease: A long-term study. Clin Orthop Relat Res. 2011; 469(4):1134-40. [DOI:10.1007/s11999-010-1566-3] [PMID] [PMCID]
Type of Study: Review Article |
Subject:
Pediatrics
Received: 2024/02/16 | Accepted: 2024/03/27 | Published: 2024/04/1
Received: 2024/02/16 | Accepted: 2024/03/27 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |







