Volume 12, Issue 3 (7-2024)
J. Pediatr. Rev 2024, 12(3): 253-260 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Guo Y, Guo W, Zhang J, Lin L, Fu R, Zhang Y. Febrile Infection-related Epilepsy Syndrome Treated by Vagus Nerve Stimulation and Ketonic Diet. J. Pediatr. Rev 2024; 12 (3) :253-260
URL: http://jpr.mazums.ac.ir/article-1-656-en.html
URL: http://jpr.mazums.ac.ir/article-1-656-en.html
1- Department of Neonatology, The Second Hospital of Jilin University, Changchun, China.
2- Department of Neonatology, The Second Hospital of Jilin University, Changchun, China. ,zhangyunf@jlu.edu.cn
2- Department of Neonatology, The Second Hospital of Jilin University, Changchun, China. ,
Full-Text [PDF 960 kb]
(332 Downloads)
| Abstract (HTML) (1126 Views)
Full-Text: (253 Views)
Introduction
Febrile infection-related epilepsy syndrome (FIRES) is a refractory epilepsy syndrome that predominantly affects children, with a higher incidence in school-age children, and is associated with infectious factors [1]. Based on available reports, the incidence of FIRES in children is approximately 1 in 1,000,000 [1], with the peak onset occurring between the ages of four and nine years [2]. The incidence of FIRES is slightly higher in boys than in girls [3]. Currently, there are no effective treatments for FIRES. Diagnostic evaluations lack specificity and rely heavily on clinical manifestations for exclusionary diagnosis. This report describes the diagnostic and therapeutic course of a case of FIRES.
Case Presentation
Chief complaint
A 6-year-old boy was admitted to the Pediatric Intensive Care Unit (PICU) of Jilin University Second Hospital due to intermittent fever for five days, accompanied by intermittent seizures and loss of consciousness for two days.
History of present illness
Five days before admission, the patient developed a fever with no apparent cause, reaching a temperature of 38.0 °C. There were no symptoms of cough, phlegm, vomiting, diarrhea, or rash, and no signs of altered consciousness. The patient sought medical attention at a local hospital, where a single intravenous antiviral treatment was administered (specific medication details are unclear). Upon returning home, the patient was orally given anticold granules, but the fever persisted, reaching a peak temperature of 39.9 °C. Two days prior to admission, the patient experienced sudden seizures characterized by upward gaze of both eyes, head tilting backward, flexion of both upper limbs, frothing at the mouth, cyanosis of the lips, loss of consciousness, and failure to respond to attempts to awaken them by their parents. The seizure episode lasted approximately 2 minutes, after which the patient’s consciousness did not fully recover. Subsequently, there were three episodes of intermittent seizures with similar features. The patient was taken to a local hospital, where treatment included antiviral therapy, intracranial pressure reduction, and respiratory support (specific medication details are unknown). Despite these interventions, there was no improvement in symptoms. The patient was then urgently transferred to our hospital under the provisional diagnosis of seizures for further investigation.
Medical history
The patient has no history of epilepsy, illness exposure, immunization, travel, mosquito bites, or animal exposure.
Physical examination
The patient was admitted to our Department with tracheal intubation connected to synchronized intermittent mandatory ventilation (synchronized intermittent mandatory ventilation [SIMV]-assisted ventilation). Vital signs recorded were: Temperature 37.9 °C; heart rate 140 beats/min; respiratory rate 49 breaths/min; and blood pressure 88/55 mm Hg. The patient was in a coma, exhibiting bilateral pupil constriction of 1 mm in diameter, with diminished light reflex. No rash was observed. The neck was soft, without resistance, and there were negative signs of meningeal irritation. Bilateral lung breath sounds were consistent, with fine moist rales noted. No abnormalities were detected during cardiac and abdominal examinations. There was a paroxysmal increase in muscle tone in all four limbs, and the bilateral Babinski sign and Chvostek sign were negative. Other neurological examinations were not conducted due to the patient’s non-cooperation.
Laboratory examination
After admission, routine tests on blood, urine, and feces, as well as assessments of liver and kidney function, parathyroid hormone levels, lactate, humoral immunity, and coagulation function, showed no abnormalities.
A cerebrospinal fluid examination revealed a clear and colorless appearance, was negative for the Pandy test, and showed a total white blood cell count of 6×106/L. Biochemical analysis showed lactate dehydrogenase at 59.00 U/L, and chloride at 119.10 mmol/L. Pressure measurement was within normal limits. On the 20th day after admission, a follow-up cerebrospinal fluid examination also exhibited a clear and colorless appearance, was negative for the Pandy test, and showed a total white blood cell count of 1×106/L. Biochemical analysis showed lactate dehydrogenase at 147.00 U/L, glucose at 5.94 mmol/L, and lactate at 3.06 mmol/L. Autoimmune encephalitis-related antibodies (six items) and viral encephalitis-related antibodies (seven items) were all negative. During the course of treatment, blood and urine tandem mass spectrometry revealed no abnormalities. Genetic screening did not show any significant anomalies.
Imaging examination
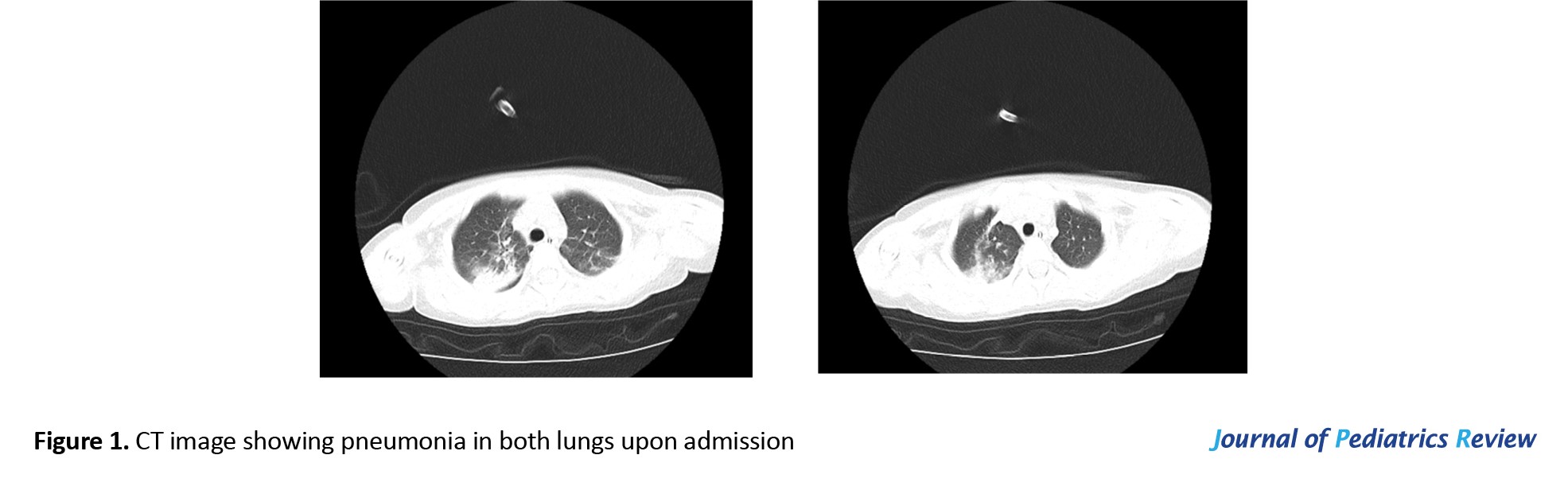
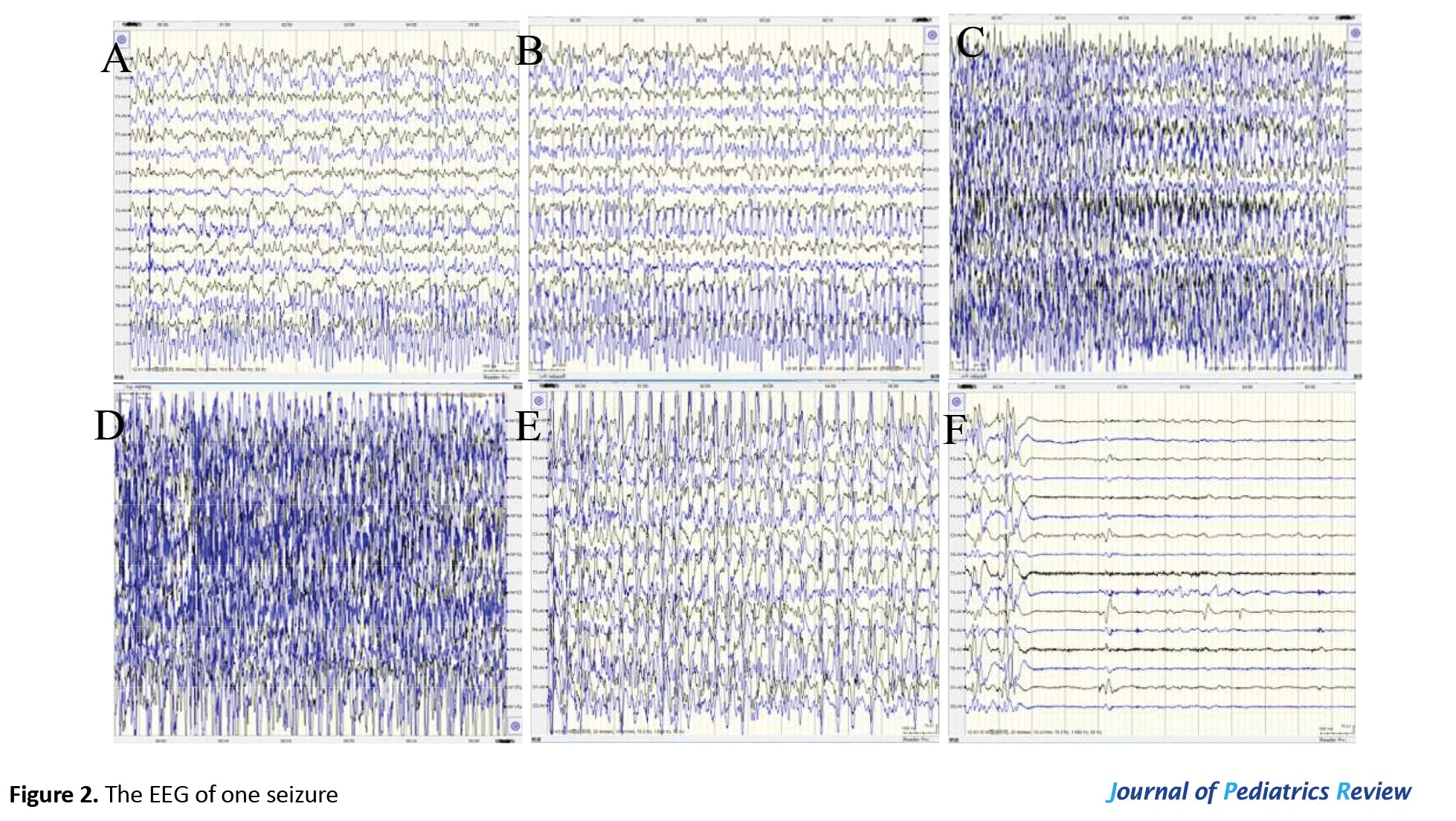
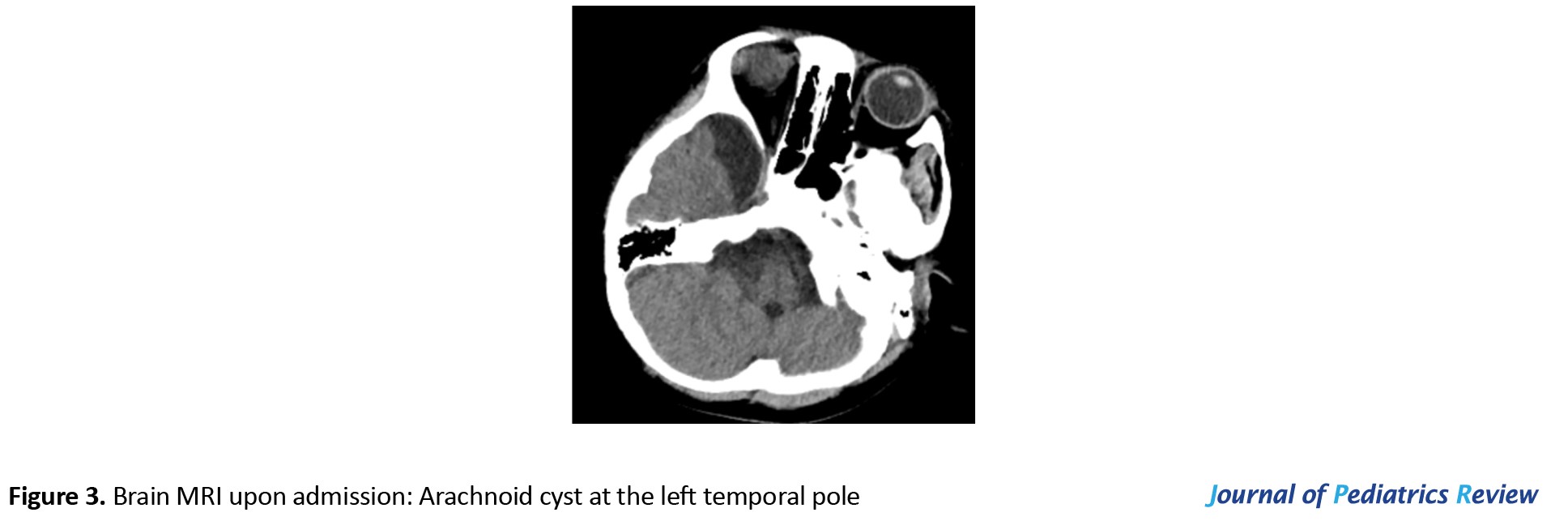
Upon admission, the CT scan revealed inflammation and pleural effusion in both lungs (Figure 1). After admission, the electroencephalogram (EEG) (video monitoring) showed that the child’s abnormal discharges originated in the right occipital region, characterized by high-amplitude (multi) spikes and (multi) spike slow waves. These discharges could affect adjacent leads and vary in duration, eventually diminishing to a transient resting state until the amplitudes in all leads returned to baseline levels (Figure 2). After multiple re-examinations of the EEGs, the starting locations and durations of abnormal discharges varied, but all exhibited diffuse slow wave activity. During the hospitalization, brain MRIs were monitored, revealing only a right temporal arachnoid cyst (Figure 3); no signs of brain atrophy or ventricular enlargement were noted. The cerebellar sulcus appeared slightly widened, along with bilateral mastoiditis and sphenoiditis.
Outcome and follow-up
Four months after the onset of the disease, the patient had not regained consciousness and experienced intermittent episodes of seizures along with severe intellectual impairment. Eleven months after the onset of the disease, the patient continued to experience occasional episodes of seizures and had a severe intellectual disorder.
Treatment
Upon transfer to our department, the patient received SIMV-assisted ventilation therapy. After 15 days of admission, non-invasive ventilation was initiated, and on the 45th day, the patient transitioned to nasal cannula oxygen therapy. Due to frequent convulsive seizures and anoxemia, a tracheostomy was performed on the 68th day of admission. Postoperatively, there was a gradual transition from non-invasive ventilation to low-flow oxygen inhalation via the endotracheal tube.
After admission, based on the clinical presentation and supplementary examination results, the child was diagnosed with bilateral pneumonia and respiratory failure. Treatments included vidarabine for antiviral therapy, ceftriaxone for infection control, and methylprednisolone injection (starting at 2 mg/kg/day) for anti-inflammation, as well as high-dose gamma globulin (2 g/kg, divided over 4 days) intravenous infusion for antibody blockade, along with symptomatic and supportive therapy. In addition, the child frequently experienced seizures accompanied by altered consciousness, and increased intracranial pressure could be ruled out. Mannitol (2.5 mg/kg/day) was administered to reduce intracranial pressure, and vital signs were closely monitored. After reviewing the lumbar puncture and finding the cerebrospinal fluid pressure to be normal, mannitol was discontinued.
Sputum culture results revealed infections with Staphylococcus aureus and Acinetobacter baumannii, and ceftriaxone was administered initially. Based on the drug sensitivity test results, vancomycin and meropenem were subsequently applied. As the patient’s condition improved, cefepime was administered. The blood culture results showed no bacterial growth.
The patient had recurrent convulsive seizures, indicative of status epilepticus (SE). Continuous intravenous infusion of midazolam (maximum dose of 20 μg/kg/min), intermittent intravenous injection of diazepam (0.5 mg/kg), phenobarbital (loading dose of 30 mg/kg, maintenance dose of 5 mg/kg), and chloral hydrate (25-50 mg/kg) were retained in the stomach; however, the anticonvulsant effect was inadequate, and the SE persisted. On the ninth day of admission, antiepileptic medication topiramate (starting dose 2 mg/kg, maximum dose 6 mg/kg) was administered, and on the 20th day of admission, it was combined with levetiracetam (starting dose 5 mg/kg, maximum dose 20 mg/kg), yet the effect remained unsatisfactory. Thus, chlorpromazine and promethazine were added (alternating every 6 hours at 1 mg/kg), rocuronium (initial dose of 0.6 mg/kg, maintenance dose of 10 μg/kg/min) as a muscle relaxant, and propofol (initial slow intravenous infusion of 2.5 mg/kg, followed by maintenance of 9-15 mg/kg/h); however, none yielded significant effects. On the 46th day of admission, oxcarbazepine was added (starting dose of 8 mg/kg, maximum dose of 12 mg/kg), but the antiepileptic effect remained inadequate. Ketogenic diet therapy was initiated on the 70th day of admission, consisting of five meals a day. For the first three days, ketone powder was combined with the milk powder, with a fat to (protein+carbohydrates) ratio of 2.5:1. On the fourth and fifth days, the ratio was increased to 3.5:1. From the sixth day onward, all meals consisted solely of ketogenic nutritional powder, with a fat to (protein+carbohydrates) ratio of 4:1.
On the 88th day of admission, the frequency of seizures was reduced slightly compared to before. However, the patient developed sudden cardiac arrest, and after resuscitation, electrocardiogram monitoring revealed frequent ventricular premature contractions and short runs of ventricular tachycardia, accompanied by upper gastrointestinal bleeding. Consequently, the ketogenic diet therapy was discontinued. On the 90th day of admission, the patient was transferred to Beijing Children’s Hospital for vagus nerve stimulation (VNS) therapy. Following the procedure, seizure episodes markedly decreased, but the patient remained unconscious and suffered from severe residual neurological sequelae.
Discussion and review of literature
FIRES typically is induced by fever with a sudden onset and progresses rapidly to SE within a short period. During the acute phase, the condition of patients is critical, and treatment efficacy is poor, often resulting in refractory seizures and severe cognitive impairment [4]. FIRES is now considered a subtype of new-onset refractory SE (NORSE), emphasizing the presence of fever-induced factors within 24 hours to two weeks before the onset of refractory SE [5, 6]. FIRES can occur across all age groups, but it is most common in school-age children [2, 7, 8, 9]. The patients often have a history of normal development, with no prior history of seizures or relevant family history. Detailed medical history inquiries confirm that this case fits this scenario.
The pathogenesis of FIRES has not been fully elucidated to date. Various hypotheses have been proposed, including inflammation, immune mechanisms, genetic factors, mitochondrial dysfunction and metabolic abnormalities, but none have clear evidence supporting them [10-12]. While potential etiology continues to expand, current views suggest that post-infection immune activation may be a significant contributor to FIRES, with inflammation activation in the central nervous system potentially leading to the persistent occurrence of seizures [13].
Up to date, FIRES still lacks specific diagnostic indicators. Wickstrom et al. [14] pointed out in the 2022 FIRES international consensus that there are currently relatively consistent opinions regarding the examination and diagnosis of FIRES, including: i) Early testing for autoimmune antibodies is recommended, as rapid acquisition of autoimmune antibody analysis is crucial, and the results may influence management decisions. ii) Serological testing should be conducted within the initial 48 hours of admission, including comprehensive rheumatological assessment, infection assessment, evaluation for congenital metabolic defects in infants, autoimmune and tumor antibody panels and storage of additional blood samples for future cytokine and gene analyses. iii) Cerebrospinal fluid-related examinations should be conducted within the initial 48 hours of admission, including comprehensive infectious disease assessment related to geography and season, evaluation for congenital metabolic defects in infants, autoimmune antibody panels, and storage of additional cerebrospinal fluid for future cytokine analyses. iv) Brain MRI should be completed within 48 hours of admission, with repeated MRIs during the course of the disease being significant for monitoring disease progression. If the etiology is unclear and MRI suggests a potential target lesion, brain biopsy should be considered. v) Genetic testing should be performed in infants as early as possible. vi) Continuous EEG monitoring is necessary for managing seizures in FIRES.
Currently, studies have found that early cerebrospinal fluid examination shows normal or slightly elevated lymphocyte counts, with glucose and protein levels mostly within normal ranges. Viral and autoimmune antibody tests in the cerebrospinal fluid have not revealed specific antibodies highly associated with the disease [15]. Although autoimmune encephalitis may be a rare cause of childhood FIRES, it is crucial to differentiate cases secondary to autoimmune encephalitis from those of cryptogenic NORSE, which may help guide treatment and determine prognosis [14]. EEG monitoring shows that discharges in FIRES are predominantly focal or evolve from focal to bilateral tonic-clonic seizures. During the acute phase of SE, burst-suppression patterns may be observed on EEG [16]. Brain MRI scans typically do not reveal any positive findings in the early stages of the disease. However, as the disease progresses, some patients may develop multiple lesions, and in the later stages, cerebral atrophy and ventricular enlargement are common, although specific changes are still lacking [17]. In this case, there were prodromal infectious symptoms, acute onset, and the rapid development of drug-resistant SE shortly after admission. Following comprehensive examinations and the exclusion of various infectious, immune, and genetic metabolic disorders, the diagnosis of FIRES was established.
Currently, there is no specific therapy for FIRES. According to the literature, inducing burst-suppression coma with high-dose barbiturates during the acute phase may be the only potentially effective approach against seizures [18]. In the course of treatment in this case, we initially selected phenobarbital for the treatment of epilepsy and gradually used a variety of anti-seizure medications (ASMs). Unfortunately, it was observed that despite the concurrent use of multiple ASMs with anesthetics during the acute phase, it remained challenging to terminate SE or shorten the duration of the acute phase. In the treatment of a FIRES patient, Xiao et al. attempted third-generation novel ASMs, lacosamide, and perampanel, demonstrating a favorable effect in terminating seizures during the acute phase of FIRES treatment [19]. However, large-sample, multicenter, long-term follow-up clinical controlled studies are still needed to further evaluate the exact efficacy and safety of novel ASMs in treating FIRES. Despite indications that autoimmune factors may be involved in the disease process, there was insufficient evidence prior to this case to support the efficacy of immunomodulators such as high-dose steroids, intravenous immunoglobulin (IVIG), or plasma exchange [2, 20]. Considering the child’s infection status, corticosteroid bolus therapy was not administered in this case to prevent uncontrollable infection.
Fortunately, we established new clinical consensus guidelines regarding FIRES in 2022, which suggest initiating antiepileptic drugs and anesthetics within the first 48 hours to treat seizures. Additionally, first-line immunotherapy, including corticosteroids, IVIG, or therapeutic plasma exchange, should be administered within the first 72 hours after seizure onset [14]. The consensus also indicates that ASMs typically have lower efficacy in FIRES, and there is no specific evidence recommending the preferential use of particular drugs. During the early stages of SE, the preferred approach is to administer an immediate loading dose and quickly reach therapeutic levels of ASMs. Once a clear diagnosis of FIRES is established, the ASM regimen should be escalated, and a ketogenic diet should be initiated. It is noteworthy that the guidelines recommend using second-line immunotherapy within one week following the onset of SE [21], including rituximab [22] (anti-CD20 monoclonal antibody), anakinra [23] (an interleukin-1 [IL-1] receptor antagonist) and tocilizumab (an IL-6 receptor antagonist). We hope that future research can better clarify the impact of these treatments on patient outcomes.
As understanding of FIRES deepens, reports have suggested that the ketogenic diet is not only effective in treating SE during the acute phase of FIRES but also helps improve long-term cognitive function. This may be attributed to its dual effects of anti-inflammatory and counteracting autoimmune responses [24]. In our case, despite aggressive use of various ASMs, anesthetics, and sedatives during the acute phase, the control of SE was inadequate. Although there was slight improvement in seizure activity after initiating the ketogenic diet, treatment had to be discontinued due to intolerance to enteral ketogenic diet. To minimize brain damage resulting from prolonged seizures, the patient underwent VNS therapy. VNS is the most commonly used neuromodulation technique for treating drug-resistant epilepsy and has been approved for use in children aged four and above [25]. VNS does not require craniotomy and, without impairing important neurological functions, interrupts the pathways of electrical conduction during epileptic seizures through surgical intervention. This helps reduce or control seizures, thereby lowering the risk of secondary brain damage from epileptic discharges and reducing the side effects associated with antiepileptic drug use. Révész et al. found that in a follow-up of 130 patients undergoing VNS therapy (with an average implantation age of 16.5 years), there was a gradual reduction in the number of ASMs and a 50% decrease in seizure frequency one-year post-operation [26]. Ji et al. discovered from a multicenter randomized double-blind prospective trial that VNS effectively reduced seizure occurrence in children aged three to six with drug-resistant epilepsy. Additionally, the earlier the implementation of VNS implantation surgery, the greater the benefits for the children [27]. In addition to seizure control, retrospective studies have also found that some children experienced improvements in social functioning, emotional well-being, and behavioral issues following VNS treatment [28-30]. In our case, the patient showed a significant reduction in seizure frequency following VNS therapy. Upon discharge, the patient continued treatment with ketogenic diet and antiepileptic drugs. However, during follow-up visits, the patient occasionally experienced seizures, resulting in unconsciousness, and demonstrated a noticeable decline in intellectual functioning compared to prior to the onset of FIRES.
Conclusion
FIRES is a severe form of refractory epilepsy syndrome with unclear etiology, pathogenesis and treatment options. It is associated with high mortality and disability rates, with the majority of the patients experiencing severe cognitive impairments and drug-resistant seizures. Early implementation of ketogenic diet therapy and VNS may be effective during the acute phase; however, further evaluation through large-sample, prospective, or randomized controlled studies is needed to assess the safety and exact efficacy of these treatments.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Second Hospital of Jilin University.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and study design: Yongjing Guo and Yunfeng Zhang; Data analysis and interpretation: Wanxu Guo, Jinpu Zhang, Lifang Lin and Rong Fu; Writing: Yongjing Guo and Yunfeng Zhang; Final approval: All authors.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors would like to thank Yuxian Gao for her guidance with the case management.
References
Febrile infection-related epilepsy syndrome (FIRES) is a refractory epilepsy syndrome that predominantly affects children, with a higher incidence in school-age children, and is associated with infectious factors [1]. Based on available reports, the incidence of FIRES in children is approximately 1 in 1,000,000 [1], with the peak onset occurring between the ages of four and nine years [2]. The incidence of FIRES is slightly higher in boys than in girls [3]. Currently, there are no effective treatments for FIRES. Diagnostic evaluations lack specificity and rely heavily on clinical manifestations for exclusionary diagnosis. This report describes the diagnostic and therapeutic course of a case of FIRES.
Case Presentation
Chief complaint
A 6-year-old boy was admitted to the Pediatric Intensive Care Unit (PICU) of Jilin University Second Hospital due to intermittent fever for five days, accompanied by intermittent seizures and loss of consciousness for two days.
History of present illness
Five days before admission, the patient developed a fever with no apparent cause, reaching a temperature of 38.0 °C. There were no symptoms of cough, phlegm, vomiting, diarrhea, or rash, and no signs of altered consciousness. The patient sought medical attention at a local hospital, where a single intravenous antiviral treatment was administered (specific medication details are unclear). Upon returning home, the patient was orally given anticold granules, but the fever persisted, reaching a peak temperature of 39.9 °C. Two days prior to admission, the patient experienced sudden seizures characterized by upward gaze of both eyes, head tilting backward, flexion of both upper limbs, frothing at the mouth, cyanosis of the lips, loss of consciousness, and failure to respond to attempts to awaken them by their parents. The seizure episode lasted approximately 2 minutes, after which the patient’s consciousness did not fully recover. Subsequently, there were three episodes of intermittent seizures with similar features. The patient was taken to a local hospital, where treatment included antiviral therapy, intracranial pressure reduction, and respiratory support (specific medication details are unknown). Despite these interventions, there was no improvement in symptoms. The patient was then urgently transferred to our hospital under the provisional diagnosis of seizures for further investigation.
Medical history
The patient has no history of epilepsy, illness exposure, immunization, travel, mosquito bites, or animal exposure.
Physical examination
The patient was admitted to our Department with tracheal intubation connected to synchronized intermittent mandatory ventilation (synchronized intermittent mandatory ventilation [SIMV]-assisted ventilation). Vital signs recorded were: Temperature 37.9 °C; heart rate 140 beats/min; respiratory rate 49 breaths/min; and blood pressure 88/55 mm Hg. The patient was in a coma, exhibiting bilateral pupil constriction of 1 mm in diameter, with diminished light reflex. No rash was observed. The neck was soft, without resistance, and there were negative signs of meningeal irritation. Bilateral lung breath sounds were consistent, with fine moist rales noted. No abnormalities were detected during cardiac and abdominal examinations. There was a paroxysmal increase in muscle tone in all four limbs, and the bilateral Babinski sign and Chvostek sign were negative. Other neurological examinations were not conducted due to the patient’s non-cooperation.
Laboratory examination
After admission, routine tests on blood, urine, and feces, as well as assessments of liver and kidney function, parathyroid hormone levels, lactate, humoral immunity, and coagulation function, showed no abnormalities.
A cerebrospinal fluid examination revealed a clear and colorless appearance, was negative for the Pandy test, and showed a total white blood cell count of 6×106/L. Biochemical analysis showed lactate dehydrogenase at 59.00 U/L, and chloride at 119.10 mmol/L. Pressure measurement was within normal limits. On the 20th day after admission, a follow-up cerebrospinal fluid examination also exhibited a clear and colorless appearance, was negative for the Pandy test, and showed a total white blood cell count of 1×106/L. Biochemical analysis showed lactate dehydrogenase at 147.00 U/L, glucose at 5.94 mmol/L, and lactate at 3.06 mmol/L. Autoimmune encephalitis-related antibodies (six items) and viral encephalitis-related antibodies (seven items) were all negative. During the course of treatment, blood and urine tandem mass spectrometry revealed no abnormalities. Genetic screening did not show any significant anomalies.
Imaging examination
Upon admission, the CT scan revealed inflammation and pleural effusion in both lungs (Figure 1). After admission, the electroencephalogram (EEG) (video monitoring) showed that the child’s abnormal discharges originated in the right occipital region, characterized by high-amplitude (multi) spikes and (multi) spike slow waves. These discharges could affect adjacent leads and vary in duration, eventually diminishing to a transient resting state until the amplitudes in all leads returned to baseline levels (Figure 2). After multiple re-examinations of the EEGs, the starting locations and durations of abnormal discharges varied, but all exhibited diffuse slow wave activity. During the hospitalization, brain MRIs were monitored, revealing only a right temporal arachnoid cyst (Figure 3); no signs of brain atrophy or ventricular enlargement were noted. The cerebellar sulcus appeared slightly widened, along with bilateral mastoiditis and sphenoiditis.
Outcome and follow-up
Four months after the onset of the disease, the patient had not regained consciousness and experienced intermittent episodes of seizures along with severe intellectual impairment. Eleven months after the onset of the disease, the patient continued to experience occasional episodes of seizures and had a severe intellectual disorder.
Treatment
Upon transfer to our department, the patient received SIMV-assisted ventilation therapy. After 15 days of admission, non-invasive ventilation was initiated, and on the 45th day, the patient transitioned to nasal cannula oxygen therapy. Due to frequent convulsive seizures and anoxemia, a tracheostomy was performed on the 68th day of admission. Postoperatively, there was a gradual transition from non-invasive ventilation to low-flow oxygen inhalation via the endotracheal tube.
After admission, based on the clinical presentation and supplementary examination results, the child was diagnosed with bilateral pneumonia and respiratory failure. Treatments included vidarabine for antiviral therapy, ceftriaxone for infection control, and methylprednisolone injection (starting at 2 mg/kg/day) for anti-inflammation, as well as high-dose gamma globulin (2 g/kg, divided over 4 days) intravenous infusion for antibody blockade, along with symptomatic and supportive therapy. In addition, the child frequently experienced seizures accompanied by altered consciousness, and increased intracranial pressure could be ruled out. Mannitol (2.5 mg/kg/day) was administered to reduce intracranial pressure, and vital signs were closely monitored. After reviewing the lumbar puncture and finding the cerebrospinal fluid pressure to be normal, mannitol was discontinued.
Sputum culture results revealed infections with Staphylococcus aureus and Acinetobacter baumannii, and ceftriaxone was administered initially. Based on the drug sensitivity test results, vancomycin and meropenem were subsequently applied. As the patient’s condition improved, cefepime was administered. The blood culture results showed no bacterial growth.
The patient had recurrent convulsive seizures, indicative of status epilepticus (SE). Continuous intravenous infusion of midazolam (maximum dose of 20 μg/kg/min), intermittent intravenous injection of diazepam (0.5 mg/kg), phenobarbital (loading dose of 30 mg/kg, maintenance dose of 5 mg/kg), and chloral hydrate (25-50 mg/kg) were retained in the stomach; however, the anticonvulsant effect was inadequate, and the SE persisted. On the ninth day of admission, antiepileptic medication topiramate (starting dose 2 mg/kg, maximum dose 6 mg/kg) was administered, and on the 20th day of admission, it was combined with levetiracetam (starting dose 5 mg/kg, maximum dose 20 mg/kg), yet the effect remained unsatisfactory. Thus, chlorpromazine and promethazine were added (alternating every 6 hours at 1 mg/kg), rocuronium (initial dose of 0.6 mg/kg, maintenance dose of 10 μg/kg/min) as a muscle relaxant, and propofol (initial slow intravenous infusion of 2.5 mg/kg, followed by maintenance of 9-15 mg/kg/h); however, none yielded significant effects. On the 46th day of admission, oxcarbazepine was added (starting dose of 8 mg/kg, maximum dose of 12 mg/kg), but the antiepileptic effect remained inadequate. Ketogenic diet therapy was initiated on the 70th day of admission, consisting of five meals a day. For the first three days, ketone powder was combined with the milk powder, with a fat to (protein+carbohydrates) ratio of 2.5:1. On the fourth and fifth days, the ratio was increased to 3.5:1. From the sixth day onward, all meals consisted solely of ketogenic nutritional powder, with a fat to (protein+carbohydrates) ratio of 4:1.
On the 88th day of admission, the frequency of seizures was reduced slightly compared to before. However, the patient developed sudden cardiac arrest, and after resuscitation, electrocardiogram monitoring revealed frequent ventricular premature contractions and short runs of ventricular tachycardia, accompanied by upper gastrointestinal bleeding. Consequently, the ketogenic diet therapy was discontinued. On the 90th day of admission, the patient was transferred to Beijing Children’s Hospital for vagus nerve stimulation (VNS) therapy. Following the procedure, seizure episodes markedly decreased, but the patient remained unconscious and suffered from severe residual neurological sequelae.
Discussion and review of literature
FIRES typically is induced by fever with a sudden onset and progresses rapidly to SE within a short period. During the acute phase, the condition of patients is critical, and treatment efficacy is poor, often resulting in refractory seizures and severe cognitive impairment [4]. FIRES is now considered a subtype of new-onset refractory SE (NORSE), emphasizing the presence of fever-induced factors within 24 hours to two weeks before the onset of refractory SE [5, 6]. FIRES can occur across all age groups, but it is most common in school-age children [2, 7, 8, 9]. The patients often have a history of normal development, with no prior history of seizures or relevant family history. Detailed medical history inquiries confirm that this case fits this scenario.
The pathogenesis of FIRES has not been fully elucidated to date. Various hypotheses have been proposed, including inflammation, immune mechanisms, genetic factors, mitochondrial dysfunction and metabolic abnormalities, but none have clear evidence supporting them [10-12]. While potential etiology continues to expand, current views suggest that post-infection immune activation may be a significant contributor to FIRES, with inflammation activation in the central nervous system potentially leading to the persistent occurrence of seizures [13].
Up to date, FIRES still lacks specific diagnostic indicators. Wickstrom et al. [14] pointed out in the 2022 FIRES international consensus that there are currently relatively consistent opinions regarding the examination and diagnosis of FIRES, including: i) Early testing for autoimmune antibodies is recommended, as rapid acquisition of autoimmune antibody analysis is crucial, and the results may influence management decisions. ii) Serological testing should be conducted within the initial 48 hours of admission, including comprehensive rheumatological assessment, infection assessment, evaluation for congenital metabolic defects in infants, autoimmune and tumor antibody panels and storage of additional blood samples for future cytokine and gene analyses. iii) Cerebrospinal fluid-related examinations should be conducted within the initial 48 hours of admission, including comprehensive infectious disease assessment related to geography and season, evaluation for congenital metabolic defects in infants, autoimmune antibody panels, and storage of additional cerebrospinal fluid for future cytokine analyses. iv) Brain MRI should be completed within 48 hours of admission, with repeated MRIs during the course of the disease being significant for monitoring disease progression. If the etiology is unclear and MRI suggests a potential target lesion, brain biopsy should be considered. v) Genetic testing should be performed in infants as early as possible. vi) Continuous EEG monitoring is necessary for managing seizures in FIRES.
Currently, studies have found that early cerebrospinal fluid examination shows normal or slightly elevated lymphocyte counts, with glucose and protein levels mostly within normal ranges. Viral and autoimmune antibody tests in the cerebrospinal fluid have not revealed specific antibodies highly associated with the disease [15]. Although autoimmune encephalitis may be a rare cause of childhood FIRES, it is crucial to differentiate cases secondary to autoimmune encephalitis from those of cryptogenic NORSE, which may help guide treatment and determine prognosis [14]. EEG monitoring shows that discharges in FIRES are predominantly focal or evolve from focal to bilateral tonic-clonic seizures. During the acute phase of SE, burst-suppression patterns may be observed on EEG [16]. Brain MRI scans typically do not reveal any positive findings in the early stages of the disease. However, as the disease progresses, some patients may develop multiple lesions, and in the later stages, cerebral atrophy and ventricular enlargement are common, although specific changes are still lacking [17]. In this case, there were prodromal infectious symptoms, acute onset, and the rapid development of drug-resistant SE shortly after admission. Following comprehensive examinations and the exclusion of various infectious, immune, and genetic metabolic disorders, the diagnosis of FIRES was established.
Currently, there is no specific therapy for FIRES. According to the literature, inducing burst-suppression coma with high-dose barbiturates during the acute phase may be the only potentially effective approach against seizures [18]. In the course of treatment in this case, we initially selected phenobarbital for the treatment of epilepsy and gradually used a variety of anti-seizure medications (ASMs). Unfortunately, it was observed that despite the concurrent use of multiple ASMs with anesthetics during the acute phase, it remained challenging to terminate SE or shorten the duration of the acute phase. In the treatment of a FIRES patient, Xiao et al. attempted third-generation novel ASMs, lacosamide, and perampanel, demonstrating a favorable effect in terminating seizures during the acute phase of FIRES treatment [19]. However, large-sample, multicenter, long-term follow-up clinical controlled studies are still needed to further evaluate the exact efficacy and safety of novel ASMs in treating FIRES. Despite indications that autoimmune factors may be involved in the disease process, there was insufficient evidence prior to this case to support the efficacy of immunomodulators such as high-dose steroids, intravenous immunoglobulin (IVIG), or plasma exchange [2, 20]. Considering the child’s infection status, corticosteroid bolus therapy was not administered in this case to prevent uncontrollable infection.
Fortunately, we established new clinical consensus guidelines regarding FIRES in 2022, which suggest initiating antiepileptic drugs and anesthetics within the first 48 hours to treat seizures. Additionally, first-line immunotherapy, including corticosteroids, IVIG, or therapeutic plasma exchange, should be administered within the first 72 hours after seizure onset [14]. The consensus also indicates that ASMs typically have lower efficacy in FIRES, and there is no specific evidence recommending the preferential use of particular drugs. During the early stages of SE, the preferred approach is to administer an immediate loading dose and quickly reach therapeutic levels of ASMs. Once a clear diagnosis of FIRES is established, the ASM regimen should be escalated, and a ketogenic diet should be initiated. It is noteworthy that the guidelines recommend using second-line immunotherapy within one week following the onset of SE [21], including rituximab [22] (anti-CD20 monoclonal antibody), anakinra [23] (an interleukin-1 [IL-1] receptor antagonist) and tocilizumab (an IL-6 receptor antagonist). We hope that future research can better clarify the impact of these treatments on patient outcomes.
As understanding of FIRES deepens, reports have suggested that the ketogenic diet is not only effective in treating SE during the acute phase of FIRES but also helps improve long-term cognitive function. This may be attributed to its dual effects of anti-inflammatory and counteracting autoimmune responses [24]. In our case, despite aggressive use of various ASMs, anesthetics, and sedatives during the acute phase, the control of SE was inadequate. Although there was slight improvement in seizure activity after initiating the ketogenic diet, treatment had to be discontinued due to intolerance to enteral ketogenic diet. To minimize brain damage resulting from prolonged seizures, the patient underwent VNS therapy. VNS is the most commonly used neuromodulation technique for treating drug-resistant epilepsy and has been approved for use in children aged four and above [25]. VNS does not require craniotomy and, without impairing important neurological functions, interrupts the pathways of electrical conduction during epileptic seizures through surgical intervention. This helps reduce or control seizures, thereby lowering the risk of secondary brain damage from epileptic discharges and reducing the side effects associated with antiepileptic drug use. Révész et al. found that in a follow-up of 130 patients undergoing VNS therapy (with an average implantation age of 16.5 years), there was a gradual reduction in the number of ASMs and a 50% decrease in seizure frequency one-year post-operation [26]. Ji et al. discovered from a multicenter randomized double-blind prospective trial that VNS effectively reduced seizure occurrence in children aged three to six with drug-resistant epilepsy. Additionally, the earlier the implementation of VNS implantation surgery, the greater the benefits for the children [27]. In addition to seizure control, retrospective studies have also found that some children experienced improvements in social functioning, emotional well-being, and behavioral issues following VNS treatment [28-30]. In our case, the patient showed a significant reduction in seizure frequency following VNS therapy. Upon discharge, the patient continued treatment with ketogenic diet and antiepileptic drugs. However, during follow-up visits, the patient occasionally experienced seizures, resulting in unconsciousness, and demonstrated a noticeable decline in intellectual functioning compared to prior to the onset of FIRES.
Conclusion
FIRES is a severe form of refractory epilepsy syndrome with unclear etiology, pathogenesis and treatment options. It is associated with high mortality and disability rates, with the majority of the patients experiencing severe cognitive impairments and drug-resistant seizures. Early implementation of ketogenic diet therapy and VNS may be effective during the acute phase; however, further evaluation through large-sample, prospective, or randomized controlled studies is needed to assess the safety and exact efficacy of these treatments.
Ethical Considerations
Compliance with ethical guidelines
This study was approved by the Ethics Committee of the Second Hospital of Jilin University.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or non-profit sectors.
Authors contributions
Conceptualization and study design: Yongjing Guo and Yunfeng Zhang; Data analysis and interpretation: Wanxu Guo, Jinpu Zhang, Lifang Lin and Rong Fu; Writing: Yongjing Guo and Yunfeng Zhang; Final approval: All authors.
Conflicts of interest
The authors declared no conflicts of interest.
Acknowledgements
The authors would like to thank Yuxian Gao for her guidance with the case management.
References
- van Baalen A, Häusler M, Plecko-Startinig B, Strautmanis J, Vlaho S, Gebhardt B, et al. Febrile infection-related epilepsy syndrome without detectable autoantibodies and response to immunotherapy: A case series and discussion of epileptogenesis in FIRES. Neuropediatrics. 2012; 43(04):209-16. [DOI:10.1055/s-0032-1323848]
- Kramer U, Chi CS, Lin KL, Specchio N, Sahin M, Olson H, Nabbout R, Kluger G, Lin JJ, van Baalen A. Febrile infection–related epilepsy syndrome (FIRES): pathogenesis, treatment, and outcome: A multicenter study on 77 children. Epilepsia. 2011; 52(11):1956-65. [DOI:10.1111/j.1528-1167.2011.03250.x]
- Gaspard N, Hirsch LJ, Sculier C, Loddenkemper T, van Baalen A, Lancrenon J, et al. New-onset refractory status epilepticus (NORSE) and febrile infection-related epilepsy syndrome (FIRES): State of the art and perspectives. Epilepsia. 2018; 59(4):745-752. [DOI:10.1111/epi.14022]
- Chung VC, Hillier S, Lau CH, Wong SY, Yeoh EK, Griffiths SM. Referral to and attitude towards traditional Chinese medicine amongst western medical doctors in postcolonial Hong Kong. Soc Sci Med. 2011; 72(2):247-55. [PMID] [DOI:10.1016/j.socscimed.2010.10.021]
- Collaborative Group for Fever-induced Refractory Epileptic Encephalopathy in School-aged Children. [Fever-induced refractory epileptic encephalopathy in school-aged children: clinical features and outcome-a multicenter study on 13 children (Chinese)]. Zhonghua Er Ke Za Zhi. 2012; 50(8):575-9. [PMID]
- Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018; 59:739-44 [DOI:10.1111/epi.14016]
- Nabbout R. FIRES and IHHE: Delineation of the syndromes. Epilepsia. 2013; 54(Suppl 6):54-6. [DOI:10.1111/epi.12278] [PMID]
- Körtvelyessy P, Lerche H, Weber Y. FIRES and NORSE are distinct entities. Epilepsia. 2012; 53(7):1276. [DOI:10.1111/j.1528-1167.2012.03517.x]
- Costello DJ, Kilbride RD, Cole AJ. Cryptogenic new onset refractory status epilepticus (NORSE) in adults-Infectious or not? J Neurol Sci. 2009; 277(1):26-31. [DOI:10.1016/j.jns.2008.10.007]
- Mikaeloff Y, Jambaqué I, Hertz-Pannier L, Zamfirescu A, Adamsbaum C, Plouin P, et al. Devastating epileptic encephalopathy in school-aged children (DESC): A pseudo encephalitis. Epilepsy Res. 2006; 69(1):67-79. [DOI:10.1016/j.eplepsyres.2006.01.002]
- Wheless JW. Treatment of refractory convulsive status epilepticus In children: Other therapies. Semin Pediatr Neurol 2010; 17(3):190- 4. [DOI:10.1016/j.spen.2010.06.007]
- Pardo CA, Nabbout R, Galanopoulou AS. Mechanisms of epileptogenesis in pediatric epileptic syndromes: Rasmussen encephalitis, infantile spasms, and febrile infection-related epilepsy syndrome (FIRES). Neurotherapeutics. 2014; 11(2):297-310. [DOI:10.1007/s13311-014-0265-2]
- Sculier C, Gaspard N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Curr Opin Neurol. 2023; 36(2):110-6. [DOI:10.1097/WCO.0000000000001137]
- Wickstrom R, Taraschenko O, Dilena R, Payne ET, Specchio N, Nabbout R, et al. International NORSE consensus group. international consensus recommendations for management of new onset refractory status epilepticus (NORSE) including febrile infection-related epilepsy syndrome (FIRES): Summary and clinical tools. Epilepsia. 2022; 63(11):2827-39. [DOI:10.1111/epi.17391]
- Iizuka T, Kanazawa N, Kaneko J, Tominaga N, Nonoda Y, Hara A, et al. Cryptogenic NORSE: Its distinctive clinical features and response to immunotherapy. Neurol Neuroimmunol Neuroinflamm. 2017; 4(6):e396. [DOI:10.1212/NXI.0000000000000396]
- Fox K, Wells ME, Tennison M, Vaughn B. Febrile infection-related epilepsy syndrome (FIRES): A literature review and case study. Neurodiagn J. 2017; 57(3):224-33. [DOI:10.1080/21646821.2017.1355181] [PMID]
- Wang GL, Deng XL, Peng J, Wang X, Wu LW, Zhang CL et al. Diagnosis and treatment of 12 cases of febrile infection-related epilepsy syndrome. Chin Physician J. 2019, 21(9):1297-301. [Link]
- Specchio N, Pietrafusa N. New-onset refractory status epilepticus and febrile infection-related epilepsy syndrome. Dev Med Child Neurol. 2020; 62(8): 897-905. [DOI:10.1111/dmcn.14553]
- Xiao X, Tang JH, Wang ML, Shao YH, Zhao DJ, Zhang BB. Febrile infection-related epilepsy syndrome: One case. J Epilepsy. 2021; 7(2): 183-6.
- Sakuma H, Awaya Y, Shiomi M, Yamanouchi H, Takahashi Y, Saito Y, et al. Acute encephalitis with refractory, repetitive partial seizures (AERRPS): A peculiar form of childhood encephalitis. Acta Neurol Scand. 2010; 121(4):251-6. [DOI:10.1111/j.1600-0404.2009.01198.x]
- Wickstrom R, Taraschenko O, Dilena R, Payne ET, Specchio N, Nabbout R, et al. International consensus recommendations for management of new onset refractory status epilepticus including febrile infection-related epilepsy syndrome: Statements and supporting evidence. Epilepsia. 2022; 63(11):2840-64. [DOI:10.1111/epi.17397]
- Jun JS, Lee ST, Kim R, Chu K, Lee SK. Tocilizumab treatment for new onset refractory status epilepticus. Ann Neurol. 2018; 84(6):940-5. [DOI:10.1002/ana.25374]
- Lai YC, Muscal E, Wells E, Shukla N, Eschbach K, Hyeong Lee K, et al. Anakinra usage in febrile infection related epilepsy syndrome: An international cohort. Ann Clin Transl Neurol. 2020; 7(12):2467-74. [DOI:10.1002/acn3.51229]
- Wells J, Swaminathan A, Paseka J, Hanson C. Efficacy and safety of a ketogenic diet in children and adolescents with refractory epilepsy-A review. Nutrients. 2020; 12(6):1809. [DOI:10.3390/nu12061809] [PMID]
- Serdaroglu A, Arhan E, Kurt G, Erdem A, Hirfanoglu T, Aydin K, et al. Long term effect of vagus nerve stimulation in pediatric intractable epilepsy: An extended follow-up. Child Nerv Syst. 2016; 32(4):641-6. [DOI:10.1007/s00381-015-3004-z]
- Révész D, Fröjd V, Rydenhag B, Ben-Menachem E. Estimating long-term vagus nerve stimulation effectiveness: Accounting for antiepileptic drug treatment changes. Neuromodulation. 2018; 21(8):797-804. [DOI:10.1111/ner.12775]
- Ji T, Yang Z, Liu Q, Liao J, Yin F, Chen Y, et al. Vagus nerve stimulation for pediatric patients with intractable epilepsy between 3 and 6 years of age: Study protocol for a double-blind, randomized control trial. Trials. 2019; 20(1):44. [DOI:10.1186/s13063-018-3087-4]
- Kavčič A, Kajdič N, Rener-Primec Z, Krajnc N, Žgur T. Efficacy and tolerability of vagus nerve stimulation therapy (VNS) in Slovenian epilepsy patients: Younger age and shorter duration of epilepsy might result in better outcome. Acta Clin Croat. 2019; 58(2):255-64. [DOI:10.20471/acc.2019.58.02.08] [PMID]
- Pindrik J, Hoang N, Smith L, Halverson M, Wojnaroski M, McNally K, et al. Preoperative evaluation and surgical management of infants and toddlers with drug-resistant epilepsy. Neurosurg Focus. 2018; 45(3):E3. [DOI:10.3171/2018.7.FOCUS18220]
- Moro-De Faes G, Serrano-Moyano B, Cantarin-Extremera V, Moreno-Vinues B, Garcia-Fernandez M, Perez-Jimenez MA, et al. [Ten years’ experience with vagus nerve stimulation in a paediatric population (Spanish)]. Rev Neurol. 2018; 67(10):382-6. [DOI:10.33588/rn.6710.2018267]
Type of Study: Case Report and Review of Literature |
Subject:
Pediatric Infectious Diseases
Received: 2024/07/24 | Accepted: 2024/09/1 | Published: 2024/07/31
Received: 2024/07/24 | Accepted: 2024/09/1 | Published: 2024/07/31
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |