Volume 12, Issue 2 (4-2024)
J. Pediatr. Rev 2024, 12(2): 199-204 |
Back to browse issues page
Download citation:
BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:



BibTeX | RIS | EndNote | Medlars | ProCite | Reference Manager | RefWorks
Send citation to:
Nikkhah A, Nasehi M M, Moosazadeh M, Saket S, Afshari M, Alizadeh-Navaei R. Investigating the Profile of Multiple Sclerosis Registry Among Iranian Children: Background, Aims, Design, and Preliminary Results. J. Pediatr. Rev 2024; 12 (2) :199-204
URL: http://jpr.mazums.ac.ir/article-1-457-en.html
URL: http://jpr.mazums.ac.ir/article-1-457-en.html
Ali Nikkhah1 

 , Mohammad Mahdi Nasehi *2
, Mohammad Mahdi Nasehi *2 

 , Mahmood Moosazadeh3
, Mahmood Moosazadeh3 

 , Sasan Saket1
, Sasan Saket1 

 , Mahdi Afshari4
, Mahdi Afshari4 

 , Reza Alizadeh-Navaei4
, Reza Alizadeh-Navaei4 




 , Mohammad Mahdi Nasehi *2
, Mohammad Mahdi Nasehi *2 

 , Mahmood Moosazadeh3
, Mahmood Moosazadeh3 

 , Sasan Saket1
, Sasan Saket1 

 , Mahdi Afshari4
, Mahdi Afshari4 

 , Reza Alizadeh-Navaei4
, Reza Alizadeh-Navaei4 


1- Department of Pediatric Neurology, Pediatric Neurology Research Center, School of Medicine, Mofid Children’s Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2- Department of Pediatric Neurology, School of Medicine, Mofid Children’s Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,mmnasehi@gmail.com
3- Gastrointestinal Cancer Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
4- Pediatric Gastroenterology and Hepatology Research Center, Zabol University of Medical Sciences, Zabol, Iran.
2- Department of Pediatric Neurology, School of Medicine, Mofid Children’s Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran. ,
3- Gastrointestinal Cancer Research Center, Non-communicable Diseases Institute, Mazandaran University of Medical Sciences, Sari, Iran.
4- Pediatric Gastroenterology and Hepatology Research Center, Zabol University of Medical Sciences, Zabol, Iran.
Full-Text [PDF 381 kb]
(468 Downloads)
| Abstract (HTML) (1775 Views)
Full-Text: (379 Views)
Introduction
Multiple sclerosis (MS) is one of the most common morbidities of central nervous system in young adults [1, 2]. It was pathologically diagnosed for the first time in the 19th century [3]. This disorder is defined as inflammation and destruction of myelin in the central nervous system, characterized by small and large, single/multiple plaques leading to various complications, such as visual impairment, spastic paralysis, impairment of sphincters control, sexual dysfunction, especially in men, imbalance, speech dysfunction, convulsion, action tremor, and depression [4, 5].
The main cause of MS is unknown and previous studies suggested different etiologies. Genetic, ethnic, environmental, infectious, toxic, and immunologic factors are responsible for the development of MS [6, 7, 8, 9]. The disease is characterized by various clinical manifestations from mild to severe and from transient to chronic [2, 3, 5]. It is difficult to predict the future course of MS, but evidence shows that 50% of patients will need assistance 15 years after diagnosis [2]. The disease is classified into relapsing-remitting, primary progressive, and secondary progressive types [2, 10, 11]. Prevalence of MS in women is 2-4 folds compared to men [2]. The disease occurs in young and middle-aged adults; however, it can affect children 2 years of age [2, 3, 8, 12]. The clinical pattern of MS in children is similar to that of adults and causes adverse impacts on different aspects of child activities [3, 8, 12].
In 2002, all countries were classified into three zones according to the prevalence of MS, including high prevalence (>30 per 100,000), moderate prevalence (5-29 per 100,000), and low prevalence (<5 per 100,000) [2]. European and American countries and New Zealand and Australia have high rates of MS and are considered high-prevalence countries [13]. Abedini et al. estimated a moderate prevalence for Iran, while according to Kurtzke’s three-zone scale, the country is in a low prevalence zone [2, 14].
The age of MS in children is incompatible with the common age of this disorder and is often misdiagnosed. Therefore, it is crucial to distinguish between pediatric MS and other neurological disorders in children. MS is usually ignored in most cases and raises further mental and economic challenges in families. Therefore, designing a comprehensive survey can provide a suitable structure for investigating different aspects of pediatric MS. Based on our investigations, there is a scarcity of information about MS in children. The current survey can provide a reasonable infrastructure for the promotion of medical, educational, and research activities in Iran and the world. This registry investigates the clinical and epidemiological aspects of MS among Iranian children to provide diagnostic criteria and regional patterns for policymakers and physicians. This registry by providing an appropriate registration system, in addition to the mentioned objectives, provides a suitable platform for conducting interventional and observational studies. Accordingly, the objectives of the study are as follows; investigating the clinical and paraclinical characteristics of MS in Iranian children (onset, frequency of sensory, motor, visual, cerebellar, urinary, and brain stem disorders, range of signs and symptoms, time of diagnostic delay, clinical trend, treatment response, degree of disability, level of consciousness, muscular power, tonicity, deep tendon reflexes, pyramidal assessment, ophthalmoscopic results, and visual evoked potentials); examining the diagnostic criteria for Iranian children with MS; investigating the epidemiological characteristics of Iranian children with MS (sex, age, residence area, socio-economic status, education, seasonal and geographical situation of the disease incidence, familial history in first-degree and second-degree relatives, race and ethnicity, previous history of infectious and immunologic diseases, history of drug use, stress, quality of life considering physical, mental, social, and environmental health); investigating the risk of relapse in Iranian children with MS (time gap between the first attack and first relapse, the time gap between second and third relapses, and also between third and fourth relapses).
Methods
The registry system of pediatric MS in Iran was submitted to the National Institute for Medical Research Development (NIMAD) in 2016 and approved with a cohort approach in 2017. The study began in 2017 and is predicted to end in 2022. The location of the MS registry in Iran is Mofid Children’s Hospital affiliated with Shahid Beheshti University of Medical Sciences. The decision to continue the registry from 2022 will be made after evaluating the effectiveness of the registration system.
To achieve the objectives of this study, two phases were considered. Phase 1 includes designing software for the registration of Iranian children with MS. First, a software engineer who was highly experienced in disease registry programs was employed. Then, a list of variables and methods of the registry were provided and an entry strategy of the software was developed. In parallel, a pilot phase was carried out in Mofid Hospital, to assess the efficacy and probable deficiencies. At the end of each week, the research team investigated the software function. After the complete implementation of MS registry software, final reports were presented.
The second phase was considered to achieve the goals of the registry in Iran. At first, a list of relevant research centers, pediatric hospitals, MS support associations, and names of specialists was provided. All of these people were interviewed. A representative for the data registry was introduced in each province with an official tie. Monthly reports of the pediatric MS registry were provided. In addition, several relevant programs were designed to manage the software, hardware, and technical challenges.
Variables observed at this phase included sex, age, residence area, socio-economic status, education, the seasonal and geographical incidence of the disease, familial history among first-degree and second-degree relatives, race and ethnicity, previous history of infectious and immunologic diseases, history of drug use, stress, quality of life considering physical, mental, social and environmental health, onset, frequency of sensory, motor, visual, cerebellar, urinary and brain stem disorders, range of signs and symptoms, diagnostic delay, clinical trend, treatment response, degree of disability, level of consciousness, muscular power, tonicity, deep tendon reflexes, pyramidal assessment, ophthalmoscopic results, visual evoked potential, magnetic resonance imaging and cerebrospinal fluid results, and definite and probable clinical diagnosis. This information will be presented as total and regional data in the Excel software and then will be transferred to the STATA software, version 14. After data cleaning, descriptive indicators will be presented. In addition, comparing these indicators between different groups will be conducted using different statistical tests, including univariate and multivariate regression models and time series. The spatial analysis will be performed using a geographic information system.
In the present article, as an introduction to the protocol, primary data were reported using percentage, Mean±standard deviation and were compared between different groups using an independent t-test and chi-square test.
Results
Following the approval of the Pediatric MS registry in Iran in 2017, a total of 932 children (0-18 years) were registered, of whom 74.4% were girls. The mean ages at diagnosis of MS for boys, girls, and total were 10.96, 11.68, and 11.50 years, respectively. There was no significant difference in mean age between girls and boys (Mean difference=0.73, P=0.133) (Table 1).
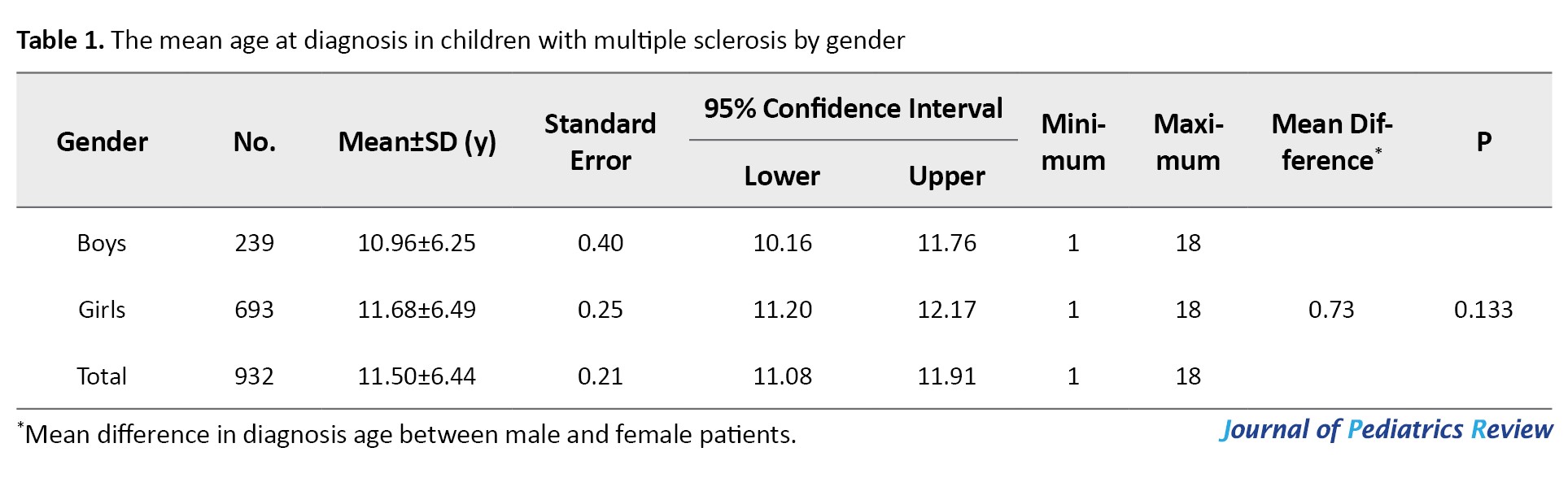
Most cases were 15-18 years at diagnosis (52.4%).
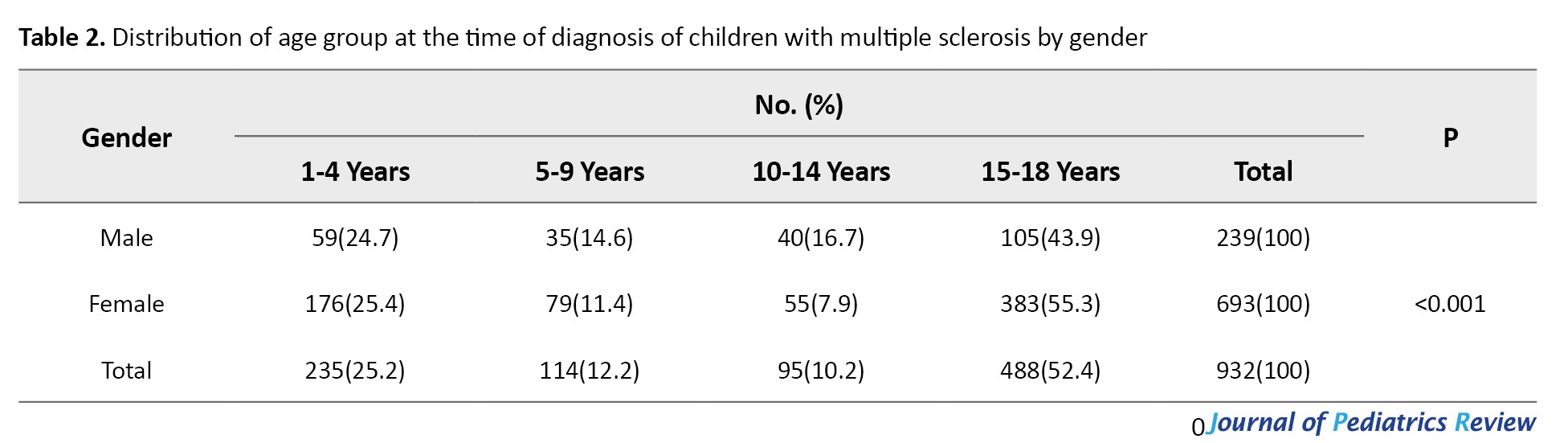
The age group distribution of the time at diagnosis by gender is presented in Table 2, where the differences were statistically significant.
Discussion
The pediatric MS registry was submitted to the NIMAD and received approval. The registry process began in 2017 and will continue until 2022. After designing the software, 932 MS cases aged 0-18 years with a female-male ratio of 2.90 were registered. The mean age of onset of MS in children was 11.50 years.
In a longitudinal study in Colombia, 116 cases were diagnosed with MS under the age of 16 years. Their mean age was 12.73, of whom 3.6% had early MS [15]. The prevalence of early onset of MS is reported at 19.8% in Jordan [10] and 3.6% in Russia [16]. In another study in Isfahan City, Iran, the mean, minimum, and maximum ages of MS patients were 33.4, 13, and 73 years, respectively [17]. According to previous studies, 2% to 5% of patients with MS show the first evidence of the disease before the age of 16 years [18]. The prevalence of early MS in different parts of the world varies greatly from 0.4% to 10.5% [19].
The age group of MS patients being recorded in this registry is 0-18 years. According to a review study about cohort studies of pediatric MS in different parts of the world, the age ranges of patients were 0-18 years in three cohorts, 1-18 years in one cohort, under 18 in two cohorts, and 15 or more in two cohorts [20]. Another review study reported a female-male ratio of the cases from 1.2 in children under 12 to 2.8 in children over 12 years [21].
About 4%-5% of MS patients are children [22]. Therefore, the pediatric MS registry is of great importance. A population-based study carried out in Ontario, Canada reported that administrative data provides a practical and reliable tool for estimating the prevalence and incidence of MS in children [23].
MS is a rare condition in children; however, long-time clinical consequences of the disease can lead to cognitive and physical disabilities during adulthood. Achieving no evidence of disease activity is the final goal of MS treatment in children. Therefore, early diagnosis of MS could help to achieve this goal [24].
Benefits and expected results from this registry include the following items: Implementing a suitable infrastructure for the registration of MS in Iranian children; providing a platform for regional and international research; designing a cohort study based on the MS population in children; designing a nested case-control study to investigate the risk factors; designing a case-control study to investigate the risk factors; designing interventional studies in the context of a registration system created for MS cases in children; detecting the prevalence and incidence of MS among Iranian children in different regions; detecting the first and most common manifestations of MS in children; improving the knowledge of physicians for early diagnosis of the disease; decreasing the economic and mental burden of the disease through early diagnosis; and having an effective role in promoting scientific production in Iran and the world.
As an innovation, this study will be performed more extensively in the country and will comprehensively investigate all necessary factors that were not addressed during previous studies. There is not enough identification for MS patients in all parts of Iran.
Conclusion
Although the pediatric MS registry is in its early stages, the initial results showed that this system could be of great benefit in making early diagnosis of more cases of the disease.
Registration of MS in children in Iran can lead to accurate awareness of health policymakers about the incidence of pediatric MS throughout the country and different geographical areas, the first and most common symptoms in this population, raising physicians’ awareness and early diagnosis of the illness and consequently lead to reduction of economic, psychological and psychological burden of the disease.
Limitations
Several limitations may have influenced the results obtained in this study. First, we were unable to access study samples which would be managed using different communication channels. Another limitation was some unexpected deficiencies in diagnostic tools during the study that will be controlled by quality control programs.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the National Institute for Medical Research Development (NIMAD) Ethics Committee (Code: IR.NIMAD.REC.1396.091). All ethical principles of the Helsinki Ethical Declaration have been met and informed consent has been obtained from all participants.
Funding
This survey was supported by the Researcher Grant Committee from the National Institute for Medical Research Development (NIMAD), Tehran, Iran (Grant No.: 958107).
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the National Institute for Medical Research Development (NIMAD) for the support.
References
Multiple sclerosis (MS) is one of the most common morbidities of central nervous system in young adults [1, 2]. It was pathologically diagnosed for the first time in the 19th century [3]. This disorder is defined as inflammation and destruction of myelin in the central nervous system, characterized by small and large, single/multiple plaques leading to various complications, such as visual impairment, spastic paralysis, impairment of sphincters control, sexual dysfunction, especially in men, imbalance, speech dysfunction, convulsion, action tremor, and depression [4, 5].
The main cause of MS is unknown and previous studies suggested different etiologies. Genetic, ethnic, environmental, infectious, toxic, and immunologic factors are responsible for the development of MS [6, 7, 8, 9]. The disease is characterized by various clinical manifestations from mild to severe and from transient to chronic [2, 3, 5]. It is difficult to predict the future course of MS, but evidence shows that 50% of patients will need assistance 15 years after diagnosis [2]. The disease is classified into relapsing-remitting, primary progressive, and secondary progressive types [2, 10, 11]. Prevalence of MS in women is 2-4 folds compared to men [2]. The disease occurs in young and middle-aged adults; however, it can affect children 2 years of age [2, 3, 8, 12]. The clinical pattern of MS in children is similar to that of adults and causes adverse impacts on different aspects of child activities [3, 8, 12].
In 2002, all countries were classified into three zones according to the prevalence of MS, including high prevalence (>30 per 100,000), moderate prevalence (5-29 per 100,000), and low prevalence (<5 per 100,000) [2]. European and American countries and New Zealand and Australia have high rates of MS and are considered high-prevalence countries [13]. Abedini et al. estimated a moderate prevalence for Iran, while according to Kurtzke’s three-zone scale, the country is in a low prevalence zone [2, 14].
The age of MS in children is incompatible with the common age of this disorder and is often misdiagnosed. Therefore, it is crucial to distinguish between pediatric MS and other neurological disorders in children. MS is usually ignored in most cases and raises further mental and economic challenges in families. Therefore, designing a comprehensive survey can provide a suitable structure for investigating different aspects of pediatric MS. Based on our investigations, there is a scarcity of information about MS in children. The current survey can provide a reasonable infrastructure for the promotion of medical, educational, and research activities in Iran and the world. This registry investigates the clinical and epidemiological aspects of MS among Iranian children to provide diagnostic criteria and regional patterns for policymakers and physicians. This registry by providing an appropriate registration system, in addition to the mentioned objectives, provides a suitable platform for conducting interventional and observational studies. Accordingly, the objectives of the study are as follows; investigating the clinical and paraclinical characteristics of MS in Iranian children (onset, frequency of sensory, motor, visual, cerebellar, urinary, and brain stem disorders, range of signs and symptoms, time of diagnostic delay, clinical trend, treatment response, degree of disability, level of consciousness, muscular power, tonicity, deep tendon reflexes, pyramidal assessment, ophthalmoscopic results, and visual evoked potentials); examining the diagnostic criteria for Iranian children with MS; investigating the epidemiological characteristics of Iranian children with MS (sex, age, residence area, socio-economic status, education, seasonal and geographical situation of the disease incidence, familial history in first-degree and second-degree relatives, race and ethnicity, previous history of infectious and immunologic diseases, history of drug use, stress, quality of life considering physical, mental, social, and environmental health); investigating the risk of relapse in Iranian children with MS (time gap between the first attack and first relapse, the time gap between second and third relapses, and also between third and fourth relapses).
Methods
The registry system of pediatric MS in Iran was submitted to the National Institute for Medical Research Development (NIMAD) in 2016 and approved with a cohort approach in 2017. The study began in 2017 and is predicted to end in 2022. The location of the MS registry in Iran is Mofid Children’s Hospital affiliated with Shahid Beheshti University of Medical Sciences. The decision to continue the registry from 2022 will be made after evaluating the effectiveness of the registration system.
To achieve the objectives of this study, two phases were considered. Phase 1 includes designing software for the registration of Iranian children with MS. First, a software engineer who was highly experienced in disease registry programs was employed. Then, a list of variables and methods of the registry were provided and an entry strategy of the software was developed. In parallel, a pilot phase was carried out in Mofid Hospital, to assess the efficacy and probable deficiencies. At the end of each week, the research team investigated the software function. After the complete implementation of MS registry software, final reports were presented.
The second phase was considered to achieve the goals of the registry in Iran. At first, a list of relevant research centers, pediatric hospitals, MS support associations, and names of specialists was provided. All of these people were interviewed. A representative for the data registry was introduced in each province with an official tie. Monthly reports of the pediatric MS registry were provided. In addition, several relevant programs were designed to manage the software, hardware, and technical challenges.
Variables observed at this phase included sex, age, residence area, socio-economic status, education, the seasonal and geographical incidence of the disease, familial history among first-degree and second-degree relatives, race and ethnicity, previous history of infectious and immunologic diseases, history of drug use, stress, quality of life considering physical, mental, social and environmental health, onset, frequency of sensory, motor, visual, cerebellar, urinary and brain stem disorders, range of signs and symptoms, diagnostic delay, clinical trend, treatment response, degree of disability, level of consciousness, muscular power, tonicity, deep tendon reflexes, pyramidal assessment, ophthalmoscopic results, visual evoked potential, magnetic resonance imaging and cerebrospinal fluid results, and definite and probable clinical diagnosis. This information will be presented as total and regional data in the Excel software and then will be transferred to the STATA software, version 14. After data cleaning, descriptive indicators will be presented. In addition, comparing these indicators between different groups will be conducted using different statistical tests, including univariate and multivariate regression models and time series. The spatial analysis will be performed using a geographic information system.
In the present article, as an introduction to the protocol, primary data were reported using percentage, Mean±standard deviation and were compared between different groups using an independent t-test and chi-square test.
Results
Following the approval of the Pediatric MS registry in Iran in 2017, a total of 932 children (0-18 years) were registered, of whom 74.4% were girls. The mean ages at diagnosis of MS for boys, girls, and total were 10.96, 11.68, and 11.50 years, respectively. There was no significant difference in mean age between girls and boys (Mean difference=0.73, P=0.133) (Table 1).

Most cases were 15-18 years at diagnosis (52.4%).
The age group distribution of the time at diagnosis by gender is presented in Table 2, where the differences were statistically significant.

Discussion
The pediatric MS registry was submitted to the NIMAD and received approval. The registry process began in 2017 and will continue until 2022. After designing the software, 932 MS cases aged 0-18 years with a female-male ratio of 2.90 were registered. The mean age of onset of MS in children was 11.50 years.
In a longitudinal study in Colombia, 116 cases were diagnosed with MS under the age of 16 years. Their mean age was 12.73, of whom 3.6% had early MS [15]. The prevalence of early onset of MS is reported at 19.8% in Jordan [10] and 3.6% in Russia [16]. In another study in Isfahan City, Iran, the mean, minimum, and maximum ages of MS patients were 33.4, 13, and 73 years, respectively [17]. According to previous studies, 2% to 5% of patients with MS show the first evidence of the disease before the age of 16 years [18]. The prevalence of early MS in different parts of the world varies greatly from 0.4% to 10.5% [19].
The age group of MS patients being recorded in this registry is 0-18 years. According to a review study about cohort studies of pediatric MS in different parts of the world, the age ranges of patients were 0-18 years in three cohorts, 1-18 years in one cohort, under 18 in two cohorts, and 15 or more in two cohorts [20]. Another review study reported a female-male ratio of the cases from 1.2 in children under 12 to 2.8 in children over 12 years [21].
About 4%-5% of MS patients are children [22]. Therefore, the pediatric MS registry is of great importance. A population-based study carried out in Ontario, Canada reported that administrative data provides a practical and reliable tool for estimating the prevalence and incidence of MS in children [23].
MS is a rare condition in children; however, long-time clinical consequences of the disease can lead to cognitive and physical disabilities during adulthood. Achieving no evidence of disease activity is the final goal of MS treatment in children. Therefore, early diagnosis of MS could help to achieve this goal [24].
Benefits and expected results from this registry include the following items: Implementing a suitable infrastructure for the registration of MS in Iranian children; providing a platform for regional and international research; designing a cohort study based on the MS population in children; designing a nested case-control study to investigate the risk factors; designing a case-control study to investigate the risk factors; designing interventional studies in the context of a registration system created for MS cases in children; detecting the prevalence and incidence of MS among Iranian children in different regions; detecting the first and most common manifestations of MS in children; improving the knowledge of physicians for early diagnosis of the disease; decreasing the economic and mental burden of the disease through early diagnosis; and having an effective role in promoting scientific production in Iran and the world.
As an innovation, this study will be performed more extensively in the country and will comprehensively investigate all necessary factors that were not addressed during previous studies. There is not enough identification for MS patients in all parts of Iran.
Conclusion
Although the pediatric MS registry is in its early stages, the initial results showed that this system could be of great benefit in making early diagnosis of more cases of the disease.
Registration of MS in children in Iran can lead to accurate awareness of health policymakers about the incidence of pediatric MS throughout the country and different geographical areas, the first and most common symptoms in this population, raising physicians’ awareness and early diagnosis of the illness and consequently lead to reduction of economic, psychological and psychological burden of the disease.
Limitations
Several limitations may have influenced the results obtained in this study. First, we were unable to access study samples which would be managed using different communication channels. Another limitation was some unexpected deficiencies in diagnostic tools during the study that will be controlled by quality control programs.
Ethical Considerations
Compliance with ethical guidelines
The present study was approved by the National Institute for Medical Research Development (NIMAD) Ethics Committee (Code: IR.NIMAD.REC.1396.091). All ethical principles of the Helsinki Ethical Declaration have been met and informed consent has been obtained from all participants.
Funding
This survey was supported by the Researcher Grant Committee from the National Institute for Medical Research Development (NIMAD), Tehran, Iran (Grant No.: 958107).
Authors contributions
All authors equally contributed to preparing this article.
Conflicts of interest
The authors declared no conflict of interest.
Acknowledgements
The authors thank the National Institute for Medical Research Development (NIMAD) for the support.
References
- Ascherio A, Munger K. Epidemiology of multiple sclerosis: From risk factors to prevention. Semin Neurol. 2008; 28(1):17-28. [DOI:10.1055/s-2007-1019126] [PMID]
- Abedidni M, Habibi Saravi R, Zarvani A, Farahmand M. [Epidemiologic study of multiple sclerosis in Mazandaran, Iran, 2007 (Persian)]. J Mazandaran Univ Med Sci. 2008; 18(66):82-6. [Link]
- Nasehi M, Sahraian MA, Moghadasi A, Ghofrani M, Ashtari F, Taghdiri MM, et al. Clinical and epidemiological aspects of multiple sclerosis in children. Iranian Journal of Child Neurology. 2017; 11(2):37-43. [Link]
- Benito-Leon J, Martínez-Martín P. [Health-related quality of life in multiple sclerosis (Spanish)]. Neurologia (Barcelona, Spain). 2003; 18(4):210-7. [PMID]
- Nedjat S, Montazeri A, Mohammad K, Majdzadeh R, Nabavi N, Nedjat F et al. [Quality of life in multiple sclerosis compared to the healthy population in Tehran (Persian)]. Iran J Epidemiol. 2006; 2(3-4):19-24. [Link]
- Marrie RA. Environmental risk factors in multiple sclerosis aetiology. Lancet Neurol. 2004; 3(12):709-18. [DOI:10.1016/S1474-4422(04)00933-0] [PMID]
- Milo R, Kahana E. Multiple sclerosis: Geoepidemiology, genetics and the environment. Autoimmun Rev. 2010; 9(5):A387-94. [DOI:10.1016/j.autrev.2009.11.010] [PMID]
- Banwell B, Ghezzi A, Bar-Or A, Mikaeloff Y, Tardieu M. Multiple sclerosis in children: Clinical diagnosis, therapeutic strategies, and future directions. Lancet Neurol. 2007; 6(10):887-902. [DOI:10.1016/S1474-4422(07)70242-9] [PMID]
- Ebers GC. Environmental factors and multiple sclerosis. Lancet Neurol. 2008; 7(3):268-77. [DOI:10.1016/S1474-4422(08)70042-5] [PMID]
- Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000; 343(13):938-52. [DOI:10.1056/NEJM200009283431307] [PMID]
- Rudick RA, Cohen JA, Weinstock-Guttman B, Kinkel RP, Ransohoff RM. Management of multiple sclerosis. N Engl J Med. 1997; 337(22):1604-11. [DOI:10.1056/NEJM199711273372207] [PMID]
- Greer JM, McCombe PA. Role of gender in multiple sclerosis: clinical effects and potential molecular mechanisms. J Neuroimmunol. 2011; 234(1-2):7-18. [DOI:10.1016/j.jneuroim.2011.03.003] [PMID]
- Nabavi SM, Poorfarzam Sh, Ghassemi H. [Clinical course and prognosis of 203 patients with MS in shahid Mostafa Khomeini Hospital, Tehran 2002 (Persian)]. Tehran Univ Med J. 2006; 64(7):90-7. [Link]
- Kurtzke JF. Epidemiology of multiple sclerosis. Does this really point toward an etiology? Lectio Doctoralis. Neurol Sci. 2000; 21(6):383-403. [DOI:10.1007/s100720070055] [PMID]
- Boiko A, Vorobeychik G, Paty D, Devonshire V, Sadovnick D; University of British Columbia MS Clinic Neurologists.Early onset multiple sclerosis: A longitudinal study. Neurology. 2002; 59(7):1006-10. [DOI:10.1212/WNL.59.7.1006] [PMID]
- Mazaheri S, Fazlian M, Hosseinzadeh A. [Clinical and epidemiological features of early and adult onset multiple sclerosis in Hamedan, Iran, 2004-2005 (Persian)]. Yafteh. 2008; 9(4):39-44. [Link]
- Ashtari F, Shaygannejad V, Heidari F, Akbari M. Prevalence of Familial Multiple Sclerosis in Isfahan, Iran. J Isfahan Med Sch. 2011; 29(138):1. [Link]
- Ness JM, Chabas D, Sadovnick AD, Pohl D, Banwell B, Weinstock-Guttman B. Clinical features of children and adolescents with multiple sclerosis. Neurology. 2007; 68(16 Suppl 2):S37-45. [DOI:10.1212/01.wnl.0000259447.77476.a9]
- Renoux C, Vukusic S, Mikaeloff Y, Edan G, Clanet M, Dubois B, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007; 356(25):2603-13. [DOI:10.1056/NEJMoa067597] [PMID]
- Alroughani R, Boyko A. Pediatric multiple sclerosis: A review. BMC Neurol. 2018; 18(1):27. [DOI:10.1186/s12883-018-1026-3] [PMID]
- Jeong A, Oleske DM, Holman J. Epidemiology of pediatric-onset multiple sclerosis: A systematic review of the literature. J Child Neurol. 2019; 34(12):705-12. [DOI:10.1177/0883073819845827] [PMID]
- Baker E. Multiple sclerosis in children: A systematic review. J Mult Scler. 2021; 8(2):1. [Link]
- Marrie RA, O’Mahony J, Maxwell C, Ling V, Yeh EA, Arnold DL, et al. Incidence and prevalence of MS in children: A population-based study in Ontario, Canada. Neurology. 2018; 91(17):e1579-e90. [DOI:10.1212/WNL.0000000000006395]
- Fisher KS, Cuascut FX, Rivera VM, Hutton GJ. Current advances in pediatric onset multiple sclerosis. Biomedicines. 2020; 8(4):71. [DOI:10.3390/biomedicines8040071] [PMID]
Type of Study: Original Article |
Subject:
Pediatric Neurology
Received: 2022/02/17 | Accepted: 2023/01/10 | Published: 2024/04/1
Received: 2022/02/17 | Accepted: 2023/01/10 | Published: 2024/04/1
Send email to the article author
| Rights and permissions | |
 |
This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License. |






